?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
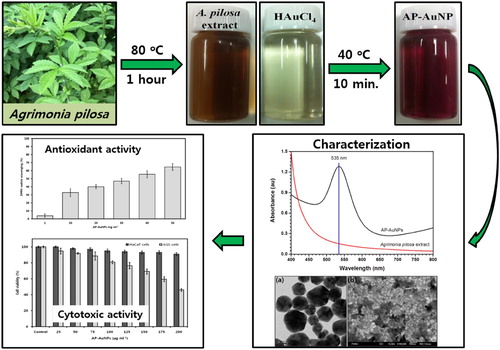
The aim of the present study is to develop an eco-friendly, simple, cost-effective and single-step method for the synthesis of gold nanoparticles using Agrimonia pilosa (AP-AuNPs) aerial parts extract and evaluation of their antioxidant and cytotoxic activity. Synthesis of AP-AuNPs was observed by a color change from light yellow to wine-red, and confirmed by the UV–VIS absorbance peak at 535 nm. From transmission electron microscopic analysis it is observed that the AP-AuNPs were hexagonal with size of about 20–50 nm. The field emission-scanning electron microscopy image revealed the synthesized AP-AuNPs to have a hexagonal shape. The energy-dispersive X-ray and X-ray diffraction analyses confirmed the metal gold and crystalline nature of AP-AuNPs. A. pilosa extract-mediated AP-AuNPs were found to be non-toxic on normal cells (HaCaT, Keratinocyte), exhibited a concentration-dependent cytotoxic effect on cancer cells (AGS, gastric adenocarcinoma) and antioxidant potential. The AP-AuNPs are synthesized using the A. pilosa aerial part extract using a simple and eco-friendly method. The synthesized AP-AuNPs can be useful in therapeutics.
GRAPHICAL ABSTRACT
1. Introduction
Green synthesis of metallic nanoparticles (NPs) is growing vigorously in recent years, and most of the researchers focus on this area due to simple, eco-friendly methods and their significant applications (Citation1, Citation2). The outcome from the recent literature survey indicates that the synthesis of metallic NPs through the green synthesis method is one of the best and safer methods that can be useful for human welfare (Citation3). This method utilizes an eco-friendly approach for the manipulated synthesis of NPs (Citation4, Citation5).
The synthesis of NPs by conventional methods includes physical and chemical methods and are not safe to living things and the environment because of production of toxic substances and by-products (Citation6). On the other hand, toxic-free, eco-friendly and cost-effective AuNPs synthesis methods lead to the use of living microorganisms such as bacteria (Citation7), fungi (Citation8), yeast (Citation9), algae (Citation10), and plants (Citation4, Citation5). Among these, the medicinal plant sources are highlighted, as different parts of plants such as galls (Citation4) leaf (Citation5, Citation11), seeds (Citation12), fruit (Citation13), flower (Citation14), and root (Citation15) can be used. The use of plant extract in the green method for the synthesis of NPs is more preferred in comparison with the use of microorganisms. Microbes need additional efforts such as isolation, identification, culture preservation, growing in specific medium and need specific temperature for growth (Citation7–10). Size-controlled synthesis of NPs can be achieved by varying different parameters of reaction, including, reaction pH, temperature, and metal salt concentration (Citation1–3, Citation7).
The plant extract-mediated synthesis of the AuNPs has been reported for human wellness; applications of AuNPs include as antibacterial (Citation12, Citation14), antifungal (Citation16), anticancer (Citation4, Citation5, Citation11), and antioxidant (Citation12, Citation13). Cancer is a major disease with a dominant death rate that affects peoples from all around the world. For curing cancer, there is a need to develop a new strategic drug. It has been reported that medicinal plant mediated AuNPs are good candidate for controlling the growth of different cancerous cells without affecting normal cells (Citation4, Citation5, Citation11). Bearing this aspect, we are reporting here the synthesis of AuNPs using the medicinal plant Agrimonia pilosa and their biological activity. A. pilosa has been recently reported for the synthesis of silver–silver chloride nanoparticles’ synthesis and their antibacterial activity against human pathogens (Citation17). A. pilosa is a herb with 30–120 cm long stem, belonging to family Rosaceae, a medicinal plant having significant properties such as anti-inflammatory and anti-allergic (Citation18, Citation19). Phytochemical composition of A. pilosa includes triterpenoids, flavonoids, triterpenes, agrimonolide, coumarin, and tannins (Citation20, Citation21).
In the present study, we proposed the synthesis of gold nanoparticles using A. pilosa (AP-AuNPs) without the addition of any other reducing or capping agent. This is the first reporting for the synthesis of AP-AuNPs using the aerial part extract of A. pilosa and their biological applications. The synthesized AP-AuNPs were characterized using different techniques including ultraviolet–visible spectroscopy (UV–VIS), field emission-scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), Fourier transform infra-red spectroscopy (FT-IR), electrophoretic light scattering (ELS), X-ray diffraction (XRD), energy-dispersive X-ray (EDX) analysis. Synthesized AP-AuNPs were tested for antioxidant activity and cytotoxic effect on normal cells and cancer cells.
2. Material and methods
2.1. Materials
Gold(III) chloride trihydrate (HAuCl4·3H2O) and DPPH (2,2-diphenyl-1-picrylhydrazyl) were purchased from Sigma-Aldrich, St. Louis, MO, USA. Phosphate buffer solution (PBS) pH 7.0 was obtained from Biosesang, Seongnam, South Korea. Cell viability reagent WST-1® (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) was purchased from Daeil Lab Services, Seoul, South Korea. The 96-well plate and cell culture flasks were supplied by SPL Life-Sciences, Gyeonggi, South Korea. The dried sample of A. pilosa aerial parts was obtained from Jirisan Herb Food supplier, Sancheong, South Korea. Tripled distilled water with 0.2 µm filtration was used for plant extract preparation and in all steps of AP-AuNPs synthesis. Cell cultures were obtained from the American Type Culture Collection (ATCC) Manassas, VA, USA. HaCaT (Human keratinocyte) cells, routinely cultured in Dulbecco's Modified Eagle Medium (DMEM) and AGS (Human gastric adenocarcinoma) cells were cultured in RPMI-1640 with l-glutamine medium (RPMI). DMEM and RPMI were purchased from Cellgro, Manassas, VA, USA, and both media were supplemented with 10% heat-inactivated fetal bovine serum (FBS; Cellgro) and 1% antibiotics (100 U mL−1 penicillin and 10 µg mL−1 streptomycin; Hyclone, Logan, UT, USA).
2.2. A. pilosa extract preparation
A. pilosa aerial parts were washed with triple distilled water and dried at room temperature, the powder was prepared using an electric grinder. The extraction process was followed as described by Patil et al. (Citation17). Briefly, 10 g of the prepared plant powder was boiled in 100 mL of distilled water at 80°C. After 60 min, the supernatant was centrifuged by high-speed centrifugation (3500 rpm, 30 min, and 4oC) to remove particulate matters and after that filtered by 0.2 µm filter (Minisart® syringe filter, Sartorius, Germany). The prepared extract was stored in a refrigerator until needed.
2.3. Synthesis of AP-AuNPs
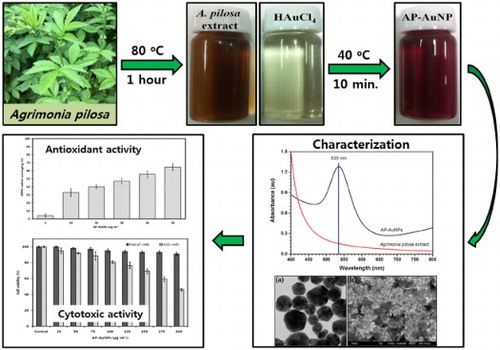
For the eco-friendly synthesis of AP-AuNPs, Gold(III) chloride trihydrate (1 mM, 30 mL) solution in tripled distilled water was used as a gold source and 1 mL of the prepared A. pilosa extract was added as a reducing agent. The synthesis was carried out in a water bath at 40°C for 10 min with continuous stirring. The synthesized AP-AuNPs were subjected to high-speed centrifugation (10,000 rpm, 20 min) for the removal of unwanted materials and non-reactive residues present in NPs suspension. The pellet obtained finally were carefully collected from the bottom of centrifuge tubes and lyophilized for the preparation of the powder. The obtained powder form of AP-AuNPs was stored in a refrigerator until required. The overall workflow of AP-AuNPs’ synthesis, characterization, and their biological applications are presented in Scheme 1.
2.4. Structural characterization of AP-AuNPs
A. pilosa extract-mediated AP-AuNPs were observed visually and absorbance spectra were monitored by UV–VIS spectrophotometer (Jasco V-670, Japan) in the range of 300–800 nm. The obtained powder form of AP-AuNPs was further subjected to different analyses in order to confirm the anticipated NPs formation. The morphology of the synthesized AP-AuNPs was monitored by JEOL JSM-6700F (Tokyo, Japan) FE-SEM and the presence of elemental gold was analyzed by EDX. The shape, size, and morphology were monitored by TEM analysis; the freshly prepared NPs were washed with triple distilled water and placed on carbon grid copper disc and dried at room temperature. TEM (Hitachi, H7500, Japan) images were captured at 120 kV. For the Zeta potential study, ELS (OTSUKA, ELS-8000, Japan) were analyzed to know total surface charges on AP-AuNPs; triple distilled water was used for the sample preparation. To know the crystalline nature of AP-AuNPs XRD (Philips X'Pert-MPD Diffractometer, Netherland) analysis was performed; the XRD pattern was obtained using CuKα radiation (1.540 Å) at 40 kV acceleration voltage. Finally, the involvement of functional groups of A. pilosa in AP-AuNPs formation was analyzed by FT-IR (Nicolet iS10, USA). FT-IR spectra of A. pilosa extract and synthesized AP-AuNPs were obtained in the range of 400–4000 cm−1 by preparing pellet of samples in dry KBr.
2.5. Antioxidant potential analysis
2.5.1. DPPH free radical scavenging assay
The DPPH free radical scavenging assay was performed as per the method of Manivasagan et al. (Citation22) with minor modifications. Briefly, 1 mL of solution of synthesized AP-AuNPs in ethanol with different concentrations (1, 10, 20, 30, 40, and 50 mg mL−1) was added to 4 mL of 0.07 mM DPPH (in ethanol) solution. The reaction mixtures were shaken vigorously for few seconds and incubated in the dark for 30 min (at room temperature). After completion of incubation ethanol was used as a blank, the reaction solution without AP-AuNPs as a control and sample with different concentration were observed for absorbance at 517 nm. All measurements repeated in triplicates and the mean ± standard deviation (SD) represented for each value measurement. The less absorbance of reaction mixture indicates a higher percentage of scavenging activity. The percentage of inhibition / free radical scavenging was calculated by the following formula:
2.6. Cytotoxic activity
The cell viability assay was performed as per the method of Patil et al. (Citation4). Briefly, HaCaT and AGS cells were cultured and seeded into 96-well plates with a density of 1X104 cells per well and incubated at 37°C for 24 h in 5% CO2 (humidified atmosphere). After incubation, media from each well replaced by fresh media and treated with different concentration of synthesized AP-AuNPs such as 25, 50, 75, 100, 125, 150, 175, and 200 µg mL−1. Plates were further incubated for 24 h at 37°C in 5% CO2. At the end of incubation media from each well were replaced by fresh media (100 µL) in addition with the 10 µL of cell viability reagent WST-1® solution and followed by incubation for additional 4 h at the same incubation conditions mentioned above. Controls were prepared without AP-AuNPs treatment, and 50% cell inhibition at a concentration of AP-AuNPs was expressed as IC50. The OD was observed at 460 nm in a multi-well ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA). Percentage of cell viability was calculated with the following formula:
3. Results and discussion
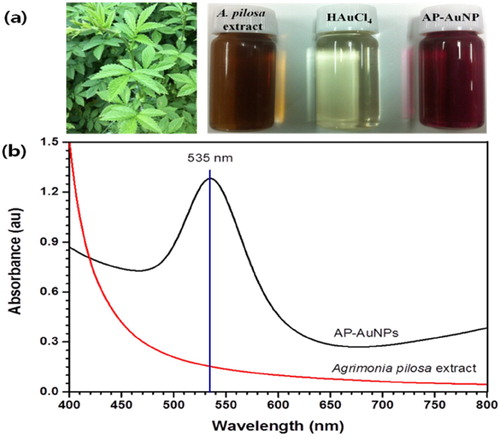
The color of reaction mixture after addition of A. pilosa aerial part-extract to the Gold (III) chloride trihydrate solution (1 mM) changed within 10 min from pale yellow to wine red ((a)). According to Mie theory (Citation23) color change in the gold nanoparticles are due to excitation of surface plasmon vibration. The surface plasmon resonance (SPR) spectra bandwidth depends on parameters such as shape, size, dielectric constant and surrounding environment (Citation24). In this study, the maximum absorption peak for AP-AuNPs was observed at 535 nm under UV–VIS spectroscopy, which is presented in (b). The narrow UV–VIS spectrum peak indicates synthesized NPs are monodispersed and there is no specific peak for A. pilosa extract, which means this extract not interfere in absorption. The color change of reaction mixture is the primary indication of AuNPs formation and SPR peak around 500–550 nm were reported for AuNPs synthesized using medicinal plants extract (Citation4, Citation5). Present study results are in good agreement with previous studies (Citation8–10).
Figure 1. (a) From left to right, aerial part of A. pilosa and its extract, 1 mM tetrachloroauric acid, and synthesized AP-AuNPs, (b) UV–visible spectrum of synthesized AP-AuNPs and extract of plant.
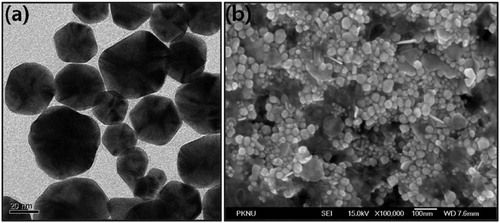
The morphology, shape and size of the AP-AuNPs synthesized using A. pilosa aerial part-extract was monitored by TEM. In the (a), the TEM image of AP-AuNPs demonstrating the polydisperse nature of NPs. The AP-AuNPs from TEM image were observed hexagonal shaped and particles size in the range of 20–50 nm. Similarly, from (b), the FE-SEM image revealed the few synthesized AP-AuNPs size is around 100 nm and mostly hexagonal shape. The exact mechanism behind formation of different sized NPs is unclear (Citation5, Citation8). As per previous reports, the formation of polydisperse nature of AuNPs is might be due to the involvement of different reducing agents those present in plant extract (Citation11–13).
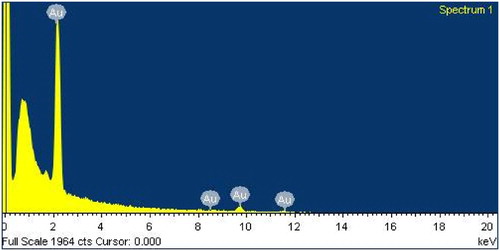
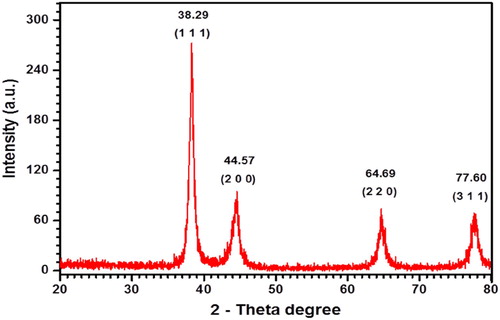
The EDX and XRD analyses were performed for determination of elemental composition and crystalline nature of AP-AuNPs, respectively. EDX spectrum of A. pilosa extract-mediated AP-AuNPs were observed on FE-SEM. Moreover, elemental gold can be seen in the EDX spectrum at 2.3 keV (see ). Similar observations for elemental gold were reported previously (Citation11, Citation14) and our results were in the same line. shows the XRD pattern of AP-AuNPs, where 4 diffraction peaks was observed. The obtained peaks pattern was compared with reference pure crystalline gold structure have been published by Joint Committee on Powder Diffraction Standards (JCPDS) file no. 65–8601. The AP-AuNPs diffraction peaks at 2θ values of 38.29°, 44.57°, 64.69°, and 77.60° that corresponds to the (111), (200), (220), and (311) planes of the face-centered cubic structure (FCC), respectively. The crystalline nature confirmed by XRD and previously reported FCC planes results are compatible with these results (Citation12–14). The results of EDX and XRD taken together confirm the AP-AuNPs contains elemental gold.
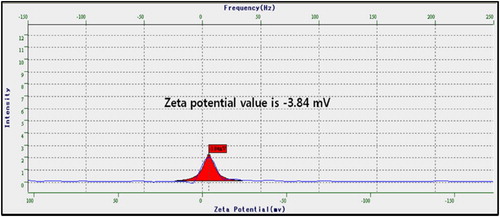
The overall surface charges on NPs determine stability and dispersion of the NPs. In the zeta potential measurement of AP-AuNPs shows –3.84 mV, the negative charges on NPs might due to covering of A. pilosa extract containing phytochemicals. Distilled water was the medium used for measurement. The positive or negative charges on the surface of NPs provide stability and make dispersive to the NPs by electrostatic repulsion of similar charges. Similar observations were reported for AuNPs synthesized using extract of Sasa borealis (Citation5) and marine algae (Citation10) and the results of present study are in good agreement with previous reports (Citation16).
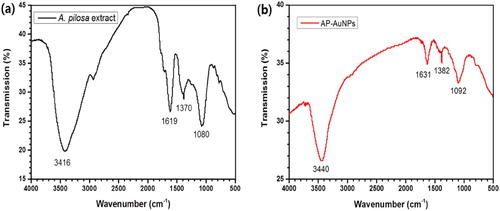
The functional group of active biomolecules was ascertained using FT-IR spectroscopy. The FT-IR spectra of A. pilosa aerial-parts extract and AP-AuNPs is given in the . The A. pilosa extract, major vibration stretches were observed at 3416, 1619, 1370, and 1080 cm−1. Similarly, major vibration stretches were observed on synthesized AP-AuNPs at 3440, 1631, 1382, and 1092 cm−1. The shifting of peaks indicates involvement of biomolecules in formation of AP-AuNPs by the reduction process. The transmittance at 3416 cm−1 corresponds to O–H stretch or H-bonded of alcohols and phenol groups. The medium vibration stretch at 1619 cm−1 is attributed to N–H bending and assigned to 1° amines. The band at 1370 cm−1 corresponds to the C–H rock, assigned to alkanes. The strong vibration stretch at 1080 cm−1 is attributed to C–O of alcohols, carboxylic acid, ether, or ester. The consistent vibrational bands such as O–H, N–H, and C–N are derived from the compounds proteins, tannins, phenols, terpenoids, and flavonoids (Citation25, Citation26). Another research reported that the tannins and other phenolic compounds were oxidized in the presence of AuCl4– and thus the hydroxyl group was converted to carboxyl groups, and carbonyl groups are involved in formation of NPs (Citation27). The FT-IR spectrum indicates the involvement of different functional groups in AP-AuNPs formation, the involvement functional groups such as O–H, C–H is also reported for the synthesis of NPs using different plant extracts (Citation4, Citation5, Citation17).
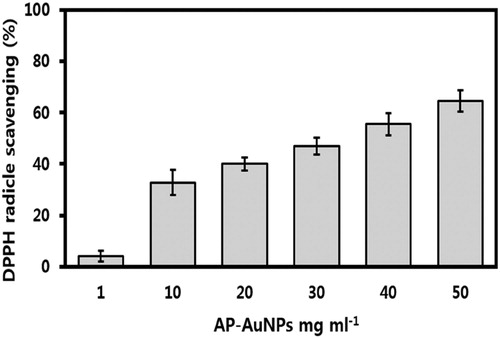
The DPPH reducing ability of AP-AuNPs was assessed calorimetrically from purple to yellow at 517 nm. The synthesized AP-AuNPs indicate concentration-dependent activity, and the observed results are shown in . The DPPH scavenging effect was 4.05%, 37.71%, 39.98%, 46.94%, 55.45%, and 64.59% at the concentration of 1, 10, 20, 30, 40, and 50 mg mL−1, respectively. Synthesized AP-AuNPs showed good antioxidant activity, different biomolecules of A. pilosa extract might be covered on NPs and take part in antioxidant activity. The high surface area to volume ratio of AuNPs synthesized using the Sumac extract reported excellent antioxidant activity by DPPH free radical scavenging (Citation27). Similarly, concentration-dependent antioxidant activity of AuNPs synthesized using microorganisms and plant extract has been reported and our results are in good agreement with these reports (Citation7, Citation22, Citation27). However, no report is available on the AP-AuNPs synthesis using the A. pilosa extract and their antioxidant activity, here we report that AP-AuNPs possess good antioxidant activity and demonstrate potentials as free radical scavenger in therapeutic applications.
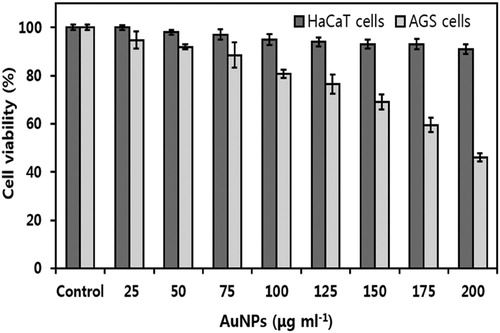
Anticancer activity of plant extract-mediated AuNPs are the center of attraction and are considered as a novel therapeutic drug (Citation2, Citation3). A WST-1® assay was performed to analyze the cytotoxic effect of AP-AuNPs on normal cells (HaCaT) and cancer cells (AGS). As shown in , HaCaT and AGS cell lines showed cell viability after 24 h treatment with different concentrations of AP-AuNPs. We found dose-dependent cells growth inhibition activity of AP-AuNPs on AGS cells, cell viability significantly decreased by 94.79%, 91.91%, 88.51%, 80.72%, 76.53%, 69.19%, 59.54%, and 46.03% at 25, 50, 75, 100, 125, 150, 175, and 200 µg mL−1 doses, respectively. There is no significant growth inhibition activity of AP-AuNPs on HaCaT cells, which support the biocompatibility of AuNPs in the cancer cell lines. Similarly, biocompatibility of plant extract-mediated and microorganism mediated green synthesized AuNPs was previously reported (Citation5, Citation7). Jayraj et al., reported that the plant extract mediated AuNPs for anticancer potential against HeLa cells; DNA damage leads to the activation of caspase and causes cells growth arrest in the G2/M phase (Citation29). AuNPs could be novel agent in cancer treatment (Citation2–7). Likewise, cancer cells’ death due to induction of apoptosis or DNA damage were reported previously (Citation4, Citation5). Biomolecules of A. pilosa extract may serve as protective non-toxic coating on AP-AuNPs. The AuNPs without cytotoxic effect can be the best candidate for safe nano-drug carrier in cancer therapeutics, diagnosis, biosensing, biomedical imaging, and photothermal therapy (Citation3, Citation28).
4. Conclusion
We successfully developed an eco-friendly route for the synthesis of colloid AP-AuNPs through the aqueous aerial parts’ extract of A. pilosa as a potential reducing and capping agent. The synthesized AP-AuNPs were analyzed for their morphological characteristics by TEM and FE-SEM and the crystalline nature and elemental composition by XRD and EDX, respectively. The biomolecules present in the plant extract and AP-AuNPs were determined by FT-IR. The synthesized AP-AuNPs were hexagonal in shape and their average particle size is about 20–50 nm. Synthesized AP-AuNPs showed antioxidant activity by DPPH free radical scavenging and it was also observed that AP-AuNPs showed biocompatibility on normal cells (HaCaT, keratinocyte) and exhibited dose-dependent cytotoxic effect on cancer cells (AGS, gastric adenocarcinoma). All results taken together indicate that the A. pilosa extract-mediated AP-AuNPs can be the good candidate as a therapeutic agent. Further studies are needed to find out the biomolecules of A. pilosa involved in NPs formation and their cytotoxic effect on cancer cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr Maheshkumar Prakash Patil has completed PhD (2017) in Microbiology from Pukyong National University, Busan, Republic of Korea, and currently working as a Post-Doctoral Fellow in Research Institute for Basic Sciences at Pukyong National University. His major interests focus on development of green, eco-friendly and single-step methods for metallic nanoparticles synthesis and their applications in different fields including microbiology and cell biology.
Dr Yong Bae Seo has completed his PhD (2009) in Microbiology from Pukyong National University, Busan, Republic of Korea, and currently working as a Post-Doctoral Fellow in Institute of Marine Microbiology at Pukyong National University. His main research interests include microbial pigment isolation and identification, gene cloning and development of environmental-friendly methods for biologically important biomolecules’ isolation and production.
Prof. Han Kyu Lim is a Professor in Department of Marine and Fisheries Resources at Mokpo National University, Republic of Korea. His main research focused on reproductive biology and cryopreservation, also interested in isolation and characterization of biologically important biomolecules or peptides from fishes for biomedical applications such as anti-cancer agents.
Prof. Gun-Do Kim has completed his PhD (1996) in Molecular Pharmacology and Cell Biology from University of Glasgow, UK. Since 2004 he is working as a Professor in Department of Microbiology, College of Natural Sciences at Pukyong National University, Busan, Republic of Korea. His teaching is related to cell biology and microbiology. His major research is related to finding anti-cancer agents from natural resources.
ORCID
Maheshkumar Prakash Patil http://orcid.org/0000-0001-5303-5985
Yong Bae Seo http://orcid.org/0000-0002-1526-9403
Gun-Do Kim http://orcid.org/0000-0001-5965-0495
Additional information
Funding
References
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13 (10), 2638–2650.
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials. 2015, 8 (11), 7278–7308.
- Patil, M.P.; Kim, G.-D. Eco-friendly Approach for Nanoparticles Synthesis and Mechanism Behind Antibacterial Activity of Silver and Anticancer Activity of Gold Nanoparticles. Appl. Microbial. Biotechnol. 2017, 101 (1), 79–92.
- Patil, M.P.; Ngabire, D.; Thi, H.H.P.; Kim, M.D.; Kim, G.-D. Eco-friendly Synthesis of Gold Nanoparticles and Evaluation of Their Cytotoxic Activity on Cancer Cells. J. Cluster Sci. 2017, 28 (1), 119–132.
- Patil, M.P.; Jin, X.; Simeon, N.C.; Palma, J.; Kim, D.; Ngabire, D.; Kim, N.H.; Tarte, N.H.; Kim, G.-D. Anticancer Activity of Sasa Borealis Leaf Extract-mediated Gold Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46 (1), 82–88.
- Hutchison, J.E. Greener Nanoscience: A Proactive Approach to Advancing Applications and Reducing Implications of Nanotechnology. ACS Nano. 2008, 2, 395–402.
- Patil, M.P.; Kim, G.-D. Marine Microorganisms for Synthesis of Metallic Nanoparticles and Their Biomedical Applications. Colloids Surf. B. 2018, 172, 487–495.
- Roy, S.; Das, T.K.; Maiti, G.P.; Basu, U. Microbial Biosynthesis of Nontoxic Gold Nanoparticles. Mater. Sci. Eng. B. 2016, 203, 41–51.
- Agnihotri, M.; Joshi, S.; Kumar, A.R.; Zinjarde, S.; Kulkarni, S. Biosynthesis of Gold Nanoparticles by the Tropical Marine Yeast Yarrowia Lipolytica NCIM 3589. Mater. Lett. 2009, 63 (15), 1231–1234.
- Ramakrishna, M.; Babu, D.R.; Gengan, R.M.; Chandra, S.; Rao, G.N. Green Synthesis of Gold Nanoparticles Using Marine Algae and Evaluation of Their Catalytic Activity. J. Nanostruct. Chem. 2016, 6 (1), 1–13.
- Wang, C.; Mathiyalagan, R.; Kim, Y.J.; Castro-Aceituno, V.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid Green Synthesis of Silver and Gold Nanoparticles Using Dendropanax Morbifera Leaf Extract and Their Anticancer Activities. Int. J. Nanomed. 2016, 11, 3691–3701.
- Rajan, A.; Rajan, A.R.; Philip, D. Elettaria Cardamomum Seed Mediated Rapid Synthesis of Gold Nanoparticles and its Biological Activities. OpenNano. 2017, 2, 1–8.
- Kumar, B.; Smita, K.; Cumbal, L.; Camacho, J.; Hernández-Gallegos, E.; de Guadalupe Chávez-López, M.; Grijalva, M.; Andrade, K. One Pot Phytosynthesis of Gold Nanoparticles Using Genipa Americana Fruit Extract and its Biological Applications. Mater. Sci. Eng. C. 2016, 62, 725–731.
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-synthesized Gold Nanoparticles From Plumeria Alba Flower Extract to Augment Catalytic Degradation of Organic Dyes and Inhibit Bacterial Growth. Particuology. 2016, 24, 78–86.
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; El-Agamy Farh, M.; Yang, D.C. Biogenic Silver and Gold Nanoparticles Synthesized Using Red Ginseng Root Extract, and Their Applications. Artif. Cells, Nanomed. Biotechnol. 2016, 44 (3), 811–816.
- Swain, S.; Barik, S.K.; Behera, T.; Nayak, S.K.; Sahoo, S.K.; Mishra, S.S.; Swain, P. Green Synthesis of Gold Nanoparticles Using Root and Leaf Extracts of Vetiveria Zizanioides and Cannabis Sativa and Its Antifungal Activities. Bionanoscience. 2016, 6 (3), 205–213.
- Patil, M.P.; Seo, Y.B.; Kim, G.D. Morphological Changes of Bacterial Cells upon Exposure of Silver-silver Chloride Nanoparticles Synthesized Using Agrimonia Pilosa. Microb. Pathog. 2018, 116, 84–90.
- Kim, J.J.; Jiang, J.; Shim, D.W.; Kwon, S.C.; Kim, T.J.; Ye, S.K.; Kim, M.K.; Shin, Y.K.; Koppula, S.; Kang, T.B.; Choi, D.K.; Lee, K.H. Anti-inflammatory and Anti-allergic Effects of Agrimonia Pilosa Ledeb Extract on Murine Cell Lines and OVA-induced Airway Inflammation. J. Ethnopharmacol. 2012, 140 (2), 213–221.
- Chen, L.; Teng, H.; Fang, T.; Xiao, J. Agrimonolide from Agrimonia Pilosa Suppresses Inflammatory Responses Through Down-regulation of COX-2/iNOS and Inactivation of NF-κB in Lipopolysaccharide-stimulated Macrophages. Phytomedicine 2016, 23 (8), 846–855.
- Liu, W.J.; Hou, X.Q.; Chen, H.; Liang, J.Y.; Sun, J.B. Chemical Constituents from Agrimonia Pilosa Ledeb. and Their Chemotaxonomic Significance. Nat. Prod. Res 2016, 30 (21), 2495–2499.
- Kim, H.W.; Park, J.; Kang, K.B.; Kim, T.B.; Oh, W.K.; Kim, J.; Sung, S.H. Acylphloroglucinolated Catechin and Phenylethyl Isocoumarin Derivatives from Agrimonia Pilosa. J. Nat. Prod. 2016, 79 (9), 2376–2383.
- Manivasagan, P.; Alam, M.S.; Kang, K.H.; Kwak, M.; Kim, S.K. Extracellular Synthesis of Gold Bionanoparticles by Nocardiopsis sp. and Evaluation of its Antimicrobial, Antioxidant and Cytotoxic Activities. Bioprocess Biosyst. Eng. 2015, 38 (6), 1167–1177.
- Mie, G. Contributions to the Optics of Turbid Media, Particularly of Colloidal Metal Solutions. Ann. Phys. 1908, 25 (3), 377–445.
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12 (3), 788–800.
- Zhou, Y.; Lin, W.; Huang, J.; Wang, W.; Gao, Y.; Lin, L.; Li, Q.; Lin, L.; Du, M. Biosynthesis of Gold Nanoparticles by Foliar Broths: Roles of Biocompounds and Other Attributes of the Extracts. Nanoscale Res. Lett. 2010, 5 (8), 1351–1359.
- Seetharaman, P.; Chandrasekaran, R.; Gnanasekar, S.; Mani, I.; Sivaperumal, S. Biogenic Gold Nanoparticles Synthesized Using Crescentia Cujete L. and Evaluation of Their Different Biological Activities. Biocatal. Agric. Biotechnol. 2017, 11, 75–82.
- Shabestarian, H.; Homayouni-Tabrizi, M.; Soltani, M.; Namvar, F.; Azizi, S.; Mohamad, R.; Shabestarian, H. Green Synthesis of Gold Nanoparticles Using Sumac Aqueous Extract and Their Antioxidant Activity. Mater. Res. 2017, 20 (1), 264–270.
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1 (1), 13–28.
- Jayraj, M.; Arun, R.; Sathishkumar, G.; MubarakAli, D.; Rajesh, M.; Sivanandhan, G.; Manickavasagam, M.; Thanjuddin, N.; Ganapathi, A. An Evidence on G2/M Arrest, DNA Damage and Caspase Mediated Apoptotic Effect of Biosynthesized Gold Nanoparticles on Human Cervical Carcinoma Cells (HeLa). Mater. Res. 2014, 52, 15–24.