ABSTRACT
This study focuses on time-dependent microwave-assisted biosynthesis of silver nanoparticles using aqueous peel extracts of Citrus paradisi as a reducing, stabilizing and capping agent with emphasis on its antibacterial property. Optical, structural and morphological properties of the synthesized Citrus paradisi peel extract silver nanoparticle (CPAgNp) were characterized using UV-visible spectrophotometer, transmission electron microscope (TEM), scanning electron microscope (SEM), energy dispersive spectroscopy (EDS), Brunauer–Emmett–Teller (BET) and X-ray diffractometer (XRD). The antimicrobial activity was evaluated using the well- and disc-diffusion methods. Characteristic surface plasmon resonance (SPR) wavelength in the range of 420–440 nm at an optimized intensity growth rate typical of silver nanoparticles was obtained. The synthesized CPAgNp are spherical in shape having the size range of 14.84 ± 5 nm and possessing fcc unit phase structure. The Citrus paradisi peel extract mediated AgNp were found to possess a broad-spectrum antimicrobial activity against water-borne pathogenic microbes.
GRAPHICAL ABSTRACT
Introduction
In the last decades, efforts are aiming at developing environmental benign, efficient, new energy transfer methods and cost-effective route in the synthesis of nanomaterial paramount for the survival of human life, environmental protection and recovery. The application of nanomaterials has been rapidly gaining importance in a number of areas such as environmental health, biomedical sciences, and water purification among other biotechnological applications (Citation1, Citation2).
The importance of choosing a proper synthetic route in designing nanostructured materials has been a driving force for the development of new methodologies for several decades (Citation3). Moreover, the need for an efficient, sustainable and conservation of energy methods to process chemical reactions has led to the search for an alternative power source over conventional heating routes in recent times. Microwave-assisted synthesis methods provide a much faster way not only to prepare materials but also to create smaller particulate materials, thus contributing to energy savings and satisfying the demands for more environmentally benign materials and procedures (Citation4).
In nano-structuring of material; the size, shape, stability as well as application of the nanomaterials during synthesis depends upon the choice of the reductants, capping and stabilizing agent. The use of material of biological entities in the formation of nanomaterial with significantly novel, improved structural and biological properties are progressively associated with modern developments in the field of material and nanoscience. Currently, plant-mediated synthesis of nanoparticles is fast gaining interest due to the vast distribution of plants and plant-parts extracts consisting of a wide range of metabolites, which favorably contributes to the exceptional stability and overall property of these materials in their area of applications (Citation5, Citation6).
Several reports had elucidated the choice of silver as the metal of choice among the noble metals due to its broad-spectrum antibacterial properties at very low concentrations, good biocompatibility in potential applications in the field of biological systems, medicine, water purification, industrial processes and many sectors of life (Citation6–11).
Researchers have documented successful biosynthetic routes for silver nanoparticle using wide varieties of plant extracts over other methods because of its reliability, eco-friendliness and reproducibility as well as in preventing agglomeration of the nanoparticles without interfering with their biomedical activities (Citation6, Citation12). Furthermore, it has also been reported that the different phytochemicals of polyol, nitrogen-containing and water-soluble heterocyclic plant constituents in these plants play a significant role in the reduction and stabilization of silver nanoparticles free from toxic chemicals (Citation13, Citation14).
Citrus is one of the major commercial fruit crops that is widely consumed both as fresh fruit or juice and most often the peel is discarded. This represents a huge waste, which often creates harmful effects on the environment. However, this is sometimes seen as an economic loss because citrus peels have been reported to possess the highest amounts of polyphenolic compounds, ascorbic acid, flavonoids, etc. compared to other segments of the fruits, these phytochemical components are responsible for various bioactivities properties, including antioxidant, antiproliferation, anti-fungal, antibacterial and antiviral activities (Citation15–19).
This work focuses on the biosynthesis of silver nanoparticles using C. paradisi peel extracts as a reductant, stabilizing and capping supportive agent. The biosynthesized material undergoes microwave irradiation as a heating medium to generate a porous nanoparticle structure. The growth rate optimization phenomenon and surface morphologies of the silver nanoparticle were evaluated and reported. The as-synthesized nanoparticle was studied for their antibacterial activity against Escherichia coli, Staphylococcus aureus and Klebsiella pneumonia.
Materials and methods
Chemicals used were of analytical reagent grade. AgNO3 used was purchased from Rochelle chemicals, South Africa and used directly without further purification. Deionized water from a Millipore water (18.2 MΩ/cm) was used for the preparation and dilution of standards throughout the experiment.
Preparation of Citrus paradisi peel extract
The fruit peels of Citrus paradisi (Grapefruit red) were removed, cleaned thoroughly using ultrapure (18.2 MΩ/cm) water to remove any dust particles adhering to the surface and cut into small pieces. 30 g of the peel was added to 100 mL of Ultrapure water and boiled for 20 min at 70°C. The extract was cooled and filtered through Whatmann No.1 filter paper and stored at 4°C for further used in the subsequent experiments.
Microwave-assisted synthesis of silver nanoparticles using Citrus paradisi peel extract (CPAgNp)
Biosynthesized Silver nanoparticles (AgNp) was obtained via microwave assisted sol–gel method. In a typical experiment, 20 mL of the filtrate of the Citrus paradisi peel was added to 80 mL of 1 mM AgNO3 in 250 mL Erlenmeyer flasks at the ratio (1:4 v/v) for the reduction of Ag+ to Ag0. The mixture was then placed on a turntable domestic microwave oven (Russell Hobbs 20L (RHEM 21L)) operating at a power of 700 W and frequency 2450 MHz at different time interval (30–150) seconds for complete bio-reduction. The formation of silver nanoparticle (AgNp) was monitored using UV-vis. Spectrophotometer (220–600 nm). The solution was centrifuged at 6000 rpm for 15 min and washed with ultrapure water (18.2 MΩ/cm) severally to remove any residue. The obtained nanoparticle was subjected to oven-drying at 40°C for 1 hr to obtain the CPAgNp. The procedure was repeated at a different mixing ratio (C. paradisi Peel extracts/1 mM AgNO3) (1:4, 2:3, 3:2, 1:1 and 4:1) for optimization of the nucleation intensity of the nanoparticle. Time-resolved absorption spectra of the UV–Vis spectroscopy was used in monitoring the periodic bioprocess growth kinetics of AgNp through color variation. The silver nanoparticles obtained were characterized.
Characterization of the biosynthesized silver nanoparticle (CPAgNp)
The synthesized silver nanoparticle absorption range was monitored using UV-Visible Spectrophotometers (SPECTROstar Nano/ BMG LABTECH). Fourier transform infrared (FTIR-ATR) spectra was recorded using Bruker: ALPHA FT-IR Spectrophotometer (4000 – 400 cm−1). The determination of the crystalline phases present in materials was identified by X-ray diffraction (X’Pert Pro, Cu-K radiation, wavelength 1.54443 Å). Surface morphology examination and particle distribution analysis of the nanoparticles were characterized using SEM with an FEI Nova NanoSEM 230 with the field emission gun equipped with an Oxford Xmax SDD detector operating at an accelerating voltage of 20Kv for the EDS detector (Oxford X-Max with INCA software). Transmission electron microscopy (TEM) image was taken using an FEI Tecnai20 equipped with a LaB6 emitter, operating at 200 KV and fitted with a Gatan Tridiem GIF with a 2kX2k CCD camera. Images were collected using the Digital Micrograph suite of programs in relation to size and shape. The surface area, pore area and pore volume of the synthesized nanoparticle were measured using nitrogen adsorption Brunauer–Emmett–Teller (BET) surface area and porosity analyzer (Micromeritics ASAP2020). The adsorption–desorption plots were used to calculate the specific surface area.
Antibacterial evaluation of the nanoparticles
The bacterial resistance of the biosynthesized Ag nanoparticle was determined from the observed zone of inhibition (mm) using the standard Agar-Well disc diffusion methods (Kirby Bauer disk diffusion test) and Microdilution assay. The indicator strains used were Escherichia coli (ATCC 35218), Staphylococcus aureus (ATCC 33591) and a clinical isolate of Klebsiella pneumonia. Bacterial suspensions were prepared with the turbidity of 0.5 McFarland. Wells with a diameter of 6 mm were cut using a sterile cork borer and filled with 50 μL 1 mg/ml of CPAgNPs solution on the Macconkey nutrient agar plates already inoculated with the bacterial cell suspension (50 μL). Plates were incubated at 37°C for 24 h. After incubation, the growth inhibition zone diameters were measured in millimeter (mm) and compared. Minimum inhibitory concentration (MIC) of biosynthesized Ag was also determined by using a 96-well microtitre plate broth-dilution method as described by Samie et al. (Citation20).
To determine the release of silver ions in solution, 1 mg/ml of the biosynthesized CPAgNPs was left to stand at room temperature for 3 h. At different time intervals, the suspension was collected and filtered through a membrane filter. The concentrations of Ag ions in the filtrate (20 μL) were measured on a PinAAcle 900 T Atomic Absorption Spectrometers (AAS, PerkinElmer Inc).
Results and discussion
Physicochemical and mineralogical characterization of Ag nanoparticles
UV-visible analysis
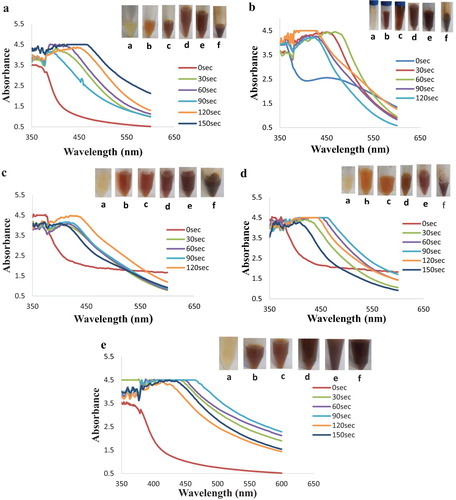
Understanding the growth rate of the biosynthesized silver nanoparticles towards integration into a specific application is critical for optimizing performance. (a–e) show the different mixing ratio (C. paradisi Peel extracts: 1 mM AgNO3) bioreduction process obtained from the time-resolved UV–Visible analysis. The first evidence in the formation of CPAgNPs from the silver ion precursor (AgNO3) is obtained from the color variation of the reaction mixture as the reaction time increases. As shown in the photographic representations ((a–e)), the nucleation and growth of the CPAgNps displayed an outright bioreduction property within 30 s of reaction with plasmon resonance bands between the wavelength range of 390–470 nm across the different time intervals. The color variations from pale yellow to brown were observed across the different reaction mixing ratio upon microwave irradiation. The final color deepened with an increase in time of reaction. The changes associated with these color variations are due to characteristic vibrations due to changes in electronic energy levels, which arise from the coupling between the electron cloud on the CPAgNps surface with the incident electromagnetic radiation typical of the surface plasmon resonance phenomenon (Citation21–23). Furthermore, reports showed that an absorption band can exhibit a shift towards either the red end (increase in wavelength) or the blue end (decrease in wavelength) of an absorption spectrum depending on the particle size, shape, state of aggregation and the surrounding dielectric medium (Citation24,Citation25). As shown in the optical bands, increasing the volume of CP extracts and the silver nitrate solution increases both the yield and the concentration of reductant in the reaction mixture, consequently shifting the bands and affecting the formation as well as the CPAgNPs sizes (Citation26). Both band shifts were observed across the spectra as the time increases with respect to the mixing solution ratios. Spectra in (d,e) show broader resonance bands with a maximum shift in absorption maxima observed in 1: 4 ratio at 470 nm. Consequently, the more the time of exposure, the more the bandwidth and increase in absorption maxima resulting in polydispersity and size increment of the CPAgNPs (Citation27, Citation28). In addition, the bioreduction process leading to the formation of CPAgNPs occurred due to the availability and interaction of the phytochemical constituents in C. paradisi peel extracts creating the capping and stability effects on the synthesized nanoparticles from aggregation (Citation12–Citation29).
Figure 1. UV–Visible spectra and photographic representation of silver nanoparticle synthesis by aqueous extract of C. Paradisi peel extract at mixing ratio (a) 1:1; (b) 3:2; (c) 2:3; (d) 4:1; (e) 1:4 at different time interval.
Finally, a significant improvement in the overall rate through a bioreduction process of CPAgNPs is observed during the interaction between the reaction mixture and the microwave heating method. Contrary to the conventional heating method at ambient temperature, exposing the reaction mixture to microwave irradiation accelerates the reaction medium providing lower energy usage, uniform nucleation and growth conditions for nanoparticles (Citation30, Citation31). In addition, the rapid heating created by the irradiation facilitates ease of releasing the phytochemical constituents in the peel through the disruption of the cell wall into the reaction mixture (Citation29).
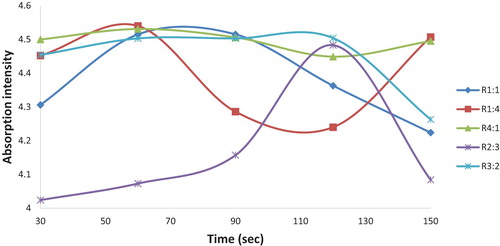
Furthermore, the surface plasmon intensity relative to the maximum reaction time of microwave exposure for all the bio-reduction process across the different reaction mixture ratios from the respective UV–Vis graph was monitored and is shown in . The influence of the reaction mixtures is observed to affect the SPR patterns on the different nucleation, stability and nanoparticle growth using the microwave induced method. As shown in the graph, a rapid growth rate of CPAgNPs occurred in mixing ratio 1:1, 3:2, 4:1 and 1:4 with maximum intensity at the reaction time of 60 s. The fast particle growth may have been attributed to the C. paradisi peel extracts possessing optimum phytochemical agents for the bio-reduction (Citation12). However, the optimized mixing ratio at 2:3 exhibited a steady growth synthesis of CPAgNPs with the highest plasmon intensity at 120 s with significance growth control on the size of the particles. Although, this occurred at a higher time, the steady and slow growth may be attributed to strong interactions between the C. paradisi peel biomolecules and the growing isotropic silver particles as it is time-dependent as well as the dependency of the surface plasmon resonance on interparticle interactions (Citation32–34). Conclusively, increasing the volumes of both the AgNO3 and CP peel extracts ultimately increases bio-reduction of more silver ions on the surface of the particles leading to the formation of a stable CPAgNp. Therefore, the mixing ratio of 2:3 was chosen as the optimum reaction mixing ratio relative to the bio-reduction process contributing to the particle size growth of CPAgNps.
Fourier-transform infrared spectroscopy
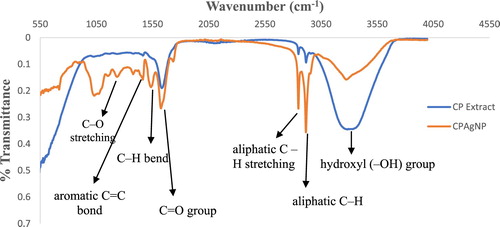
FTIR spectroscopic study of the green synthesized Ag nanoparticle (CPAgNp) was obtained to show the interaction of the different phytochemical constituents of the C. paradisi peel extract with the nanoparticle. shows the comparative FTIR spectra of the aqueous extract of the peel and CPAgNp. In the spectrum, the broader band appearing in the range 3282–3300 cm−1 is attributed to the stretching vibrations of the –OH group, N–H stretch of various metabolites present in the peel extract in form of an alcohol, N–H stretch of 1° and 2° amines or amides, carboxylic acid, ester and ether (Citation35). The broadness and shapes of these bands in both spectra depend on the hydrogen bonding systems interacting with the phytochemicals resulting in the formation of the nanoparticles through bio-reduction stages. The sharp peaks observed between 2915 and 2850 cm−1 in the spectra could be assigned to the symmetric and asymmetric C–H stretching vibrations of methyl, methylene, and methoxy groups (Citation36,Citation37). The presence of C = O is found in both spectrums at around 1662 cm−1 in the extracts which shifted to 1626 cm−1 in the CPAgNps. This may be attributed to the presence of carboxylate ion and an amide functional group. In both spectra, different characteristic sharp peaks were also observed between 1538 and 1377 cm−1 which may be assigned to the −C = C in the aromatic rings, asymmetric stretching (C–N) mode in addition to the bending vibrations of the methyl and methylene groups of fatty acids. The C–O–C, C–C and carboxylic group (C–O) stretching vibrations were identified at 1238, 1156 and 1032 cm−1 band respectively (Citation35). The presence, interaction and shifting of the above bands in C. paradisi peel extract indicate that bioactive compounds are presumed to act as reducing, capping and stabilizing agents of silver ions to the biosynthesized CPAgNps.
Surface morphological analysis
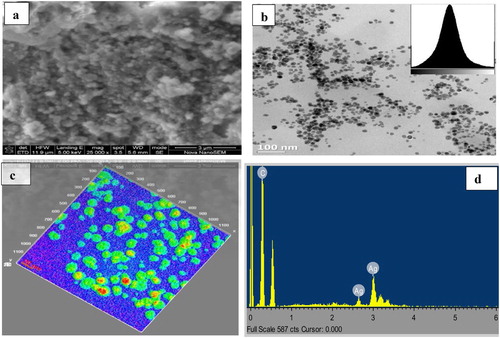
The shape, size and overall surface morphology of the synthesized silver nanoparticles using Citrus paradisi peel extracts (CPAgNps) were examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis. The SEM micrograph ((a)) showed traces of congregation and polydispersity on the synthesized CPAgNps. The observed array of biomass could be attributed to the presence of the plant phytochemicals responsible for the bio-reducing and capping the nanoparticles resulting in their stabilization. The TEM micrograph (x100 nm) with the histogram image (inset) ((b)) together with the corresponding surface interactive 3D Image (x20 nm) ((c)) reveal the particles by their morphological size and circularity. As shown in (b), the CPAgNp are homogeneous, well dispersed and majorly small and sphere-like in shape with an average particle size of 14.84 ± 5 nm. The energy dispersive X-ray spectroscopy (EDS) ((d)) revealed absorption peak showing the presence of typical elemental silver around 3 keV without any form of impurity.
XRD study
The crystalline phase of the CPAgNp was analyzed through the XRD spectroscopy as shown in and . XRD pattern of CPAgNp showed major diffraction peaks at 38.26°, 44.47° and 64.71°, 77.76° and 81.91° which indexed the planes (1 1 1), (2 0 0), (2 2 0), (3 1 1) and (2 2 2) respectively typical of a cubical crystal system with an Fm-3 m space group face-centred silver (Citation38,Citation39). The lattice constant calculated from the observed pattern was a = 4.0710 Å (calculated density 10.62 g/cm3) matched with the database Cross-References code ICSD: 064996. The X-ray diffraction studies clearly indicate that the silver nanoparticles synthesized from C. paradisi peel extract are crystalline in nature.
Figure 5. XRD pattern of the silver nanoparticle synthesized from an aqueous extract of C. paradisi peel.
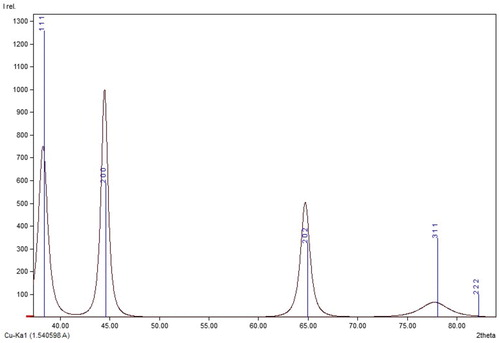
Table 1. XRD data of CPAgNp.
Brunauer–Emmett–Teller (BET) surface area and porosity analysis
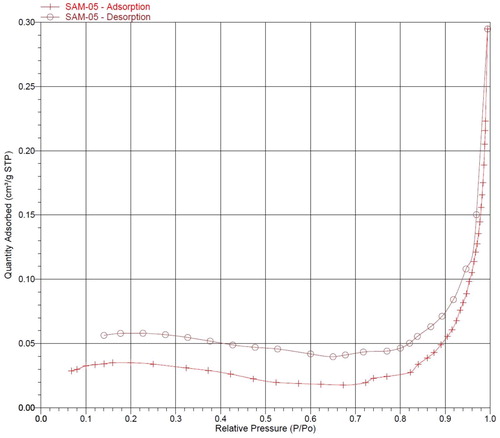
shows the results of the BET specific surface areas, pore volume and pore width measurements of the optimized CPAgNp (2:3) at an ambient temperature of 22°C. As shown in the table, the particle size obtained from the BET conforms with TEM result. Furthermore, the N2 adsorption–desorption and pore size distribution isotherm curve of the CPAgNp () classified the isotherm to be type IV with the obtained P/Po within the range 0.5–1.0 (Citation40, Citation41). This is a typical characterization of mesoporous materials; therefore, the biosynthesized CPAgNp is mesoporous in nature with a BET pore diameter of 14.313 nm.
Table 2. BET Surface analysis parameters of CPAgNp (2:3).
Antimicrobial properties
The antimicrobial activity of CPAgNPs was examined by the well-diffusion and Microdilution assay method against both the Gram-positive bacterium of Staphylococcus aureus (ATCC 33591) and the Gram-negative bacteria of Escherichia coli (ATCC 35218) as well as Klebsiella pneumonia respectively. summarized the comparative zone of inhibition and the viability to different concentration surrounding the CPAgNPs against all the test bacteria. As shown, it is evident from the results, that CPAgNPs possessed the ability to inhibit the growth of these pathogenic waterborne bacteria with maximum inhibition against Gram-negative E. coli (13 mm) in comparison to Klebsiella pneumoniae (Citation4) and Gram-positive S. aureus (9 mm). It is important to note that the susceptibility of these bacterial to CPAgNp was subject to different concentration. Minimum Inhibitory Concentration (MIC) values of 20, 40 and 40 μg/mL of CPAgNp was recorded for S. aureus and Klebsiella pneumonia and E. coli respectively (). Studies have shown that the toxicity effects at different MICs values depend on the type of bacterial strains, precursor concentration, methods of preparation and the capping agents (Citation42–44).
Table 3. Antibacterial activity of CPAgNPs.
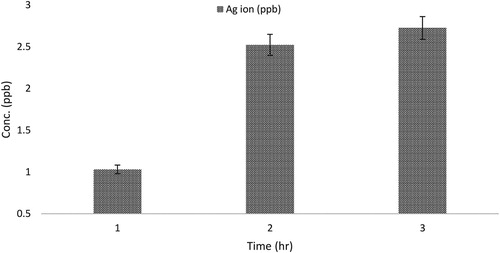
As illustrated in , the time-dependent releases of metal ions from C. paradisi peel extracts biosynthesized Ag nanoparticle was also examined. It was observed that the Ag ions release was affected by time and rate of dissolution; metal ions release increases as time progresses. The concentration at the highest time interval (Ag ion (2.7 ppb)) is lower than the observed minimum inhibitory concentration (MIC) (). These results suggested that MIC value does not correlate with the mode of action of the antimicrobial activity of CPAgNPs and the release of these metal ions against the bacteria.
Although there is controversy about the antibacterial mechanism of silver nanoparticles, ability in producing intracellular reactive oxygen species ROS production (Citation45,Citation46, Citation46), contact action, size, as well as the reducing and capping agents used in the synthesis route (Citation12–Citation47) between the nanoparticles and cells are widely believed to be some of the main factors responsible for the antibacterial activity. Consequently, these factors were considered as prominent agents that contributed to the potency of CPAgNPs targetting multiple sites in the bacterial cell leading to their inactivation.
Conclusion
This study demonstrated the successful rapid green synthesis of an eco-friendly, cost-effective AgNPs using aqueous peel extract of Citrus paradisi and its antibacterial potency against some pathogenic water strains. The optical properties showed the participation of the metabolites in the peel extracts to be responsible for the bioreduction process with a steady growth rate at an optimized mixing ration of 2:3 through the bioreduction process. The CPAgNp morphological analysis suggests a spherical, cubical crystalline phase with an average size of 14.84 nm without any impurity was obtained. The CPAgNp demonstrated potential antibacterial potency against common gram-positive and gram-negative water pathogens.
Acknowledgment
Authors would like to acknowledge financial support from University of Venda, South Africa (RPC grant number: SES/17/ERM/03) and Prof. Gitari DHET Research Incentive funds.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
W. B. Ayinde (PhD) is a member of the Environmental Remediation and Nanoscience (EnviReN), Department of Ecology and Resources Management at the School of Environmental Sciences, University of Venda, Thohoyandou, South Africa. His area of research are in Water Quality Management, Water and waste water treatment, Nanomaterial and nanoparticle synthesis, Environmental Chemistry, and Material Characterization. He is currently working as a Research assistant (USAID PEER Cycle 6 project) on development of functional materials for household water treatment.
W. M. Gitari is a full Professor and Group Leader. Environmental Remediation and Nanoscience (EnviReN), School of Environmental Sciences, Department of Ecology and Resources Management professor of Environmental/Analytical chemistry at School of Environmental Sciences, University of Venda, South Africa. His research interests are environmental remediation through beneficial and innovative utilization of industrial and mining waste products, development of functional materials for household water treatment and water quality monitoring and management.
Amidou Samie currently works at the Department of Microbiology, University of Venda as an Associate Professor. His primary research interest is infectious diseases from diagnostics to control. He also conduct research on medicinal plants and genetic susceptibility to infectious diseases and more especially to opportunistic infections. Their current project is ‘NIH birth cohort’.
ORCID
W. B. Ayinde http://orcid.org/0000-0002-2930-9728
Additional information
Funding
References
- Ananya, S.; Makwana, B.A. Am. J. Nanosci. Nanotechnol. 2014, 4, 84–92.
- Soloviev, A.; Mikhail, D.J. Nanobiotechnol. 2007, 5 (11), 62–78.
- Bang, J.H.; Suslick, K.S. Adv. Mater. 2010, 22, 1039–1059.
- Genuino, H.; Huang, H.; Njagi, E.; Stafford, L.; Suib, S.L. Handb. Green Chem. 2012, 8, 217–244.
- Kulkarni, N.; Muddapur, U. J. Nanotechnol. 2014, 2014, 1–8.
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S.J. Adv. Res. 2016, 7 (1), 17–28.
- Jaina, D.; Daimab, H.K.; Kachhwahaa, S.; Kotharia, S.L. Dig. J. Nanomater. Biostruct. 2009, 4, 723–727.
- El-Kheshen, A.A.; Gad El-Rab, S.F. Der. Pharma Chemical. 2012, 4 (1), 53–65.
- Jain, P.; Pradeep, T. Biotechnol. Bioengg. 2005, 90, 59–63.
- Fewtrell, L. Water Disinfection and Toxicity; Aberystwyth University: Aberystwyth, 2014.
- Babu, R.; Zhang, J.; Beckman, E.J.; Virji, M.; Pasculle, W.A.; Wells, A. Biomaterials. 2006, 27 (24), 4304–4314.
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Saudi Pharm. J. 2016, 24 (4), 473–484.
- Dare, E.O.; Oseghale, C.O.; Labulo, A.H.; Adesuji, E.T.; Elemike, E.E.; Onwuka, J.C.; Bamgbose, J.T. J. Nanostruct Chem. 2015, 5 (1), 85–94.
- Leela, A.; Vivekanandan, M. Afr. J. Biotech. 2008, 7, 3162.
- Manthey, J.A.; Grohman, K.J. Agric. Food Chem. 2001, 49, 3268–3273.
- Gorinstein, S.; Martın-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Ciz, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Food Chem. 2001, 74, 309–315.
- Yusof, S.; Mohd Ghazali, H.; Swee King, G. Food Chem. 1990, 37, 113–121.
- Aruoma, O.I.; Landes, B.; Ramful-Baboolall, D.; Bourdon, E.; Neergheen-Bhujun, V.; Wagner, K.H.; Bahorun, T. Prev. Med. 2012, 54, S12–S16.
- Dillard, C.J.; German, J.B. J. Sci. Food Agric. 2000, 80, 1744–1756.
- Samie, A.; Obi, C.L.; Bessong, P.O.; Namrita, L. Afri. J. Biotech. 2005, 4 (12), 1443–1451.
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: New York, 2008.
- Link, S.; El-Sayed, M.A. Annu. Rev. Phys. Chem. 2003, 54 (1), 331–366.
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Colloid. Surface B. 2010, 76 (1), 50–56.
- Smitha, S.L.; Nissamudeen, K.M.; Philip, D.; Gopchandran, K.G. Spectrochim. Acta A. 2008, 71 (1), 186–190.
- Hao, E.; Schatz, G.C. J. Phys. Chem. 2004, 120, 357–366.
- Prathna, T.C.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Colloid. Surfaces B. 2011, 82, 152–159.
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Asian Pac. J. Trop. Med. 2012, 2, 574–580.
- He, R.X.; Liang, R.; Peng, P.; Zhou, Y.N. J. Nanopart. Res. 2017, 19 (8), 267.
- Sökmen, M.; Alomar, S.Y.; Albay, C.; Serdar, G. J. Alloys Compd. 2017, 725, 190–198.
- Siby, J.; Mathew, B. Res. J. Recent Sci. 2013, 3, 185–191.
- Meng, L.Y.; Wang, B.; Ma, M.G.; Lin, K.L. Mater. Today. 2016, 1-2, 63–83.
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; Hong, J.; Chen, C. Nanotechnology. 2007, 18 (10), 105104.
- Su, K.H.; Wei, Q.H.; Zhang, X.; Mock, J.J.; Smith, D.R.; Schultz, S. Nano Lett. 2003, 3 (8), 1087–1090.
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonics. 2007, 2 (3), 107–118.
- Kalsi, P.S. Spectroscopy of Organic Compounds; New Age International: New Delhi, 2007.
- Li, J.; Barron, A.R. OpenStax-CNX module: m3 4660, 2010.
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. J. Saudi Chem. Soc. 2014, 18 (4), 364–369.
- Owen, E.A.; Williams, G.I. J. Sci. Instrum. 1954, 31 (2), 49–54.
- Zuas, O.; Hamim, N.; Sampora, Y. Mater. Lett. 2014, 123, 156–159.
- Hwang, N.; Barron, A.R. The Connexions Project 2011, 1–11.
- Ma, J.; Wang, L.; Wu, Y.; Dong, X.; Ma, Q.; Qiao, C.; Zhang, Q.; Zhang, J. Mater. Trans. 2014, 55 (12), 1900–1902.
- Velazquez-Meza, M.E.; Hernández-Salgado, M.; Sánchez-Alemán, M.A. Microb. Drug Resist. 2015, 21 (4), 367–372.
- Mittal, J.; Jain, R.; Sharma, M.M. Adv. Nat. Sci: Nanosci. Nanotechnol. 2017, 8 (2), 025011.
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Nanotechnology. 2007, 18 (28), 285604.
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Nanomed. Nanotechnol. 2016, 12 (3), 789–799.
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Toxicol. In Vitro. 2016, 36, 216–223.
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Langmuir. 2011, 27 (7), 4020–4028.