?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Biosynthetic procedure is the best alternative to the preparation of nonmaterials as the challenges faced by the scientist from the last few decades. Present research reports cost-effective, inexpensive eco-friendly synthesis of titanium dioxide nanoparticles using a methanolic extract of fruits peel agro-waste. X-ray diffraction spectrum of identified TiO2NPs found noncrystalline in nature. Fourier transform infrared showed O–H, C = O, C–O, and C–H functional groups present in the fruit peel involved in the biosynthesis of TiO2NPs. The prominent peak at 1708, 1720 and 1700 cm−1 observed in the spectrum attributed to O–Ti–O stretching in Plum, Kiwi and Peach mediated TiO2NPs. The SEM images of all three TiO2NPs revealed cylindrical in shape. The size of TiO2NPs synthesized from Plum, Kiwi and Peach were found to be 47.1 and 63.2, 54.1 and 85.1 and 200 nm, respectively. All the TiO2NPs exhibited size- and dose-dependent antibacterial and antioxidant activities.
GRAPHICAL ABSTRACT
1. Introduction
Nanotechnology is essentially associated with the synthesis of nanoparticles of variable shapes and sizes, chemical compositions for varieties of human applications. Nanotechnology is getting a higher response in the field of modern research with plenty of applications in electronics and medicine (Citation1). Nanobiotechnology is such a new branch, where nanotechnological advancement was used in biotechnology by combining the physical and chemical methods to generate nano-sized particles with specialized functions.
Several physical and chemical methods of synthesizing metal nanoparticles are a costly process and also raised an environmental risk question due to the involvement of toxic, hazardous materials (Citation2). Utilization of green chemistry via biological process towards the synthesis of desired metal nanoparticles has led to the development of environmentally acceptable method (Citation3). Under the normal air pressure and temperature, these biological processes reduced energy and cost together with high-yield low cost (Citation4). The most important basic requirements of nanotechnology are the development of clean, non-toxic and environmentally friendly green methods (Citation2), instead of traditional physical and chemical methods (Citation2,Citation5).
Exploring the waste materials in different area of human applications has given much attention in recent years. Use of plant waste materials (e.g. fruit peel) for value-added discovery for the identification of major biological potentials is one such area where scientists are focusing on zeal (Citation6–8).
Recently, titanium dioxide nanoparticles of different sizes have been explored for their different applications. Titanium dioxide (TiO2) environmentally acceptable due to photocatalyst, high chemical stability and nontoxicity (Citation9,Citation10). Titanium dioxide nanoparticle is said to be most valuable materials for cosmetics, pharmaceuticals (Citation8) and most importantly to protect skin from ultraviolet (UV) rays, in papers inks, food colorants and toothpaste (Citation9). TiO2 NPs produce significant antibacterial activity (Citation12, Citation13). Antibacterial and antioxidant activities of TiO2 nanoparticles were studied so far is slightly different as other known metal nanoparticles (Ag, ZnO, Au NPs) due to their unique behavior as a photocatalyst (Citation10). It is believed that the antioxidant potential of TiO2 nanoparticles characterized by the production of reactive oxygen species (ROS). Upon activation through photocatalysis TiO2, nanoparticles undergo oxidative stress and produce ROS, which disrupt the balance between antioxidant defense as well as oxidative pressure. Typical ROS (hydroxyl radical, superoxide, etc.) produce not only by photo-activation of TiO2 also by the interaction of some chemicals on the NP surface or between particles and cellular components (Citation11). The production of these ROS also cause damage to protein, lipids and DNA in cells, which confirm the TiO2 nanoparticles act as a good antibacterial agent (Citation11).
Due to their semiconductor properties used as natural purification of aqueous systems and enhance purification as a result of the redox process under the influence of sunlight (Citation14).
Researcher (Citation15) claimed that the TiO2 generates reactive oxygen species when exposed to UV radiation, nanoparticulate TiO2 used in antibacterial coatings and wastewater disinfection has been identified as an anti-cancer agent (Citation16). Hu et al. (Citation17) indicated that TiO2 nanoparticles could induce significant damage to earthworms due to their antioxidant effects.
Besides TiO2, WO3–TiO2 composite was reported to be used in the removal of acetylsalicylate and methyl-theobromine from the aqueous environment (Citation18) and WO3 photocatalysts in the waste water treatment (Citation19). Moreover, extensive research on other metal nanoparticles such Silver (Ag), Zinc oxide (ZnO) and Gold (Au) with their eco-friendly approach of synthesis and plenty of biological applications were reported (Citation20–22).
Titanium dioxide nanoparticles are biosynthesized by different natural sources for their biological uses. Earlier authors reported that the TiO2 NPs were synthesized from microbes and plant extracts, Propionibacterium (Citation23), Aeromonas hydrophilia (Citation24), Planomicrobium sp. (Citation25), Bacterium Bacillus subtilis (Citation26), Yeast (Citation27), Aspergillusflavus (Citation24). Plant-mediated titanium dioxide nanoparticles: Nyctanthes arbor-tristis leaf (Citation24), Solanumtrilo batum (Citation28) Annona squamosal (Citation29), Catharanthus roseus leaf (Citation30), Eclipta prostrate (Citation28), Jatropha curcas (Citation31), Calotropis gigantean (Citation32).
The present research is devoted to the biosynthesis of titanium dioxide nanoparticles from the extract of some rosaceous fruits (Prunus domestica L. (Plum), Prunus Persia L. (Peach) and Actinidia deliciosa (Kiwi)) peel as agro-waste material to fulfill the requirements of green chemistry approach. Further, the synthesized nanoparticles were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) study and scanning electron microscopy (SEM) for the morphological attributes to confirm the structure. Finally, nanoparticles were evaluated for antibacterial and antioxidant adopting the standard protocol.
2. Materials and methods
2.1. Materials
Chemicals and necessary reagents used in these experiments were procured from Sigma Aldrich (USA) with no extra purification employed. FTIR spectra of four rosaceous fruits peel (P. domestica L.(Plum), P. Persia L.(Peach) and A. deliciosa (Kiwi)) extract and titanium dioxide nanoparticles were identified by potassium bromide (KBr) discs using FT/IR – 4100 JASKO model in the ratio of 1:100 and Thermo Scientific, Model-Nicollet 6700. XRD study was performed on a Rigaku Ultima IV X-ray diffractometer between angles 2° and 80°, to the scan rate of 2°/min and PANALYTICAL, Model-Netherlands X’pert 3. SEM was done using Carl Zeiss EVO LS10 (Oberkochen, Germany) and Model-UK &SIGMA. UV spectrophotometer (Cary, 60, Agilent Technologies, Model- G 6860A, USA) was used to confirm the bioreduction of titanium dioxide metal ions using a scan range of 200–800 nm.
2.2. Preparation of rosaceous fruits (P. domestica L. (Plum), P. Persia L. (Peach) and A. deliciosa (Kiwi)) peel extract
Titanium dioxide nanoparticles were synthesized using rosaceous fruits (Prunusdomestica L.(Plum), Prunus Persia L. (Peach) and Actinidia deliciosa (Kiwi)) peel extract which performed as reducing and capping agent as similar as mentioned in literatures (Citation20–22). Selected rosaceous fruits were purchased from the local market. Peels were separated from the flesh and washed with water many times till the removal of dust particles. Peels were then spread in full sunlight to remove any moisture residue. It was further cut into pieces and ground to a small size using mixer grinder. Small size peels powder was then passing through a mesh of fixed size. About, 50 g of peels powder was taken in a 250 mL glass beaker containing 200 mL methanol. The solution was boiled around 1 h so that its color changes from colorless to different visible colors. The methanolic extract was cooled to room temperature and filter using Whatman filter paper. All the extracts so prepared stored in a refrigerator for the synthesis of titanium dioxide (Metal nanoparticles).
2.3. Fruits peels extract-mediated titanium dioxide nanoparticles
To the synthesis of titanium dioxide nanoparticles, 50 mL of fruits P. domestica L.(Plum), P. Persia L. (Peach) and A. deliciosa (Kiwi) peel extract were boiled for 70–80°C using heating stirrer. To the extract solution, 5 g of titanium dioxide was added as the boiling progresses to 70°C. The mixture continuously boiled until it reduced to some color change with a paste-type texture. The obtained paste was placed in a ceramic crucible and heated in furnace around 400–500°C for 4 h. As a result, a light colored powder was obtained and mashed in a pestle-mortar to receive a fine powder, which was collected and packed for necessary characterization.
2.3.1. Characterization of prepared titanium dioxide nanoparticles
Various characterizations were carried out for the synthesized titanium dioxide nanoparticles. XRD analysis of prepared powder nanoparticles was used by X-ray diffractometer to confirm and analyze amorphous and crystalline nature of NPs together with size by Brags equation.
FTIR spectra of rosaceous fruits (P. domestica L.(Plum), P. Persia L. (Peach), A. deliciosa (Kiwi)) peels extract and metal nanoparticles (TiO2NPs) were identified to check the formation of titanium dioxide nanoparticles through observation of spectrum of extract and individual TiO2NPs. It was performed by pressed pellet technique utilizing potassium bromide to make a thin film disc to 1:100 ratio of sample to KBr. SEM analysis was done to characterize the morphology of the metal nanoparticles. The sample is coated with gold onto the surface and put it to SEM machine. UV-spectral analysis of all the synthesized TiO2NPs were carried out to confirm the characteristics of each nanoparticle at a specific wavelength.
2.4. Biological activities
2.4.1. Evaluation of antibacterial activity of fruits peels extract-mediated titanium dioxide nanoparticles
The antibacterial activity of the synthesized fruits peels (P. domestica L. (Plum), P. Persia L. (Peach) and A. deliciosa (Kiwi)) extract-mediated titanium dioxide nanoparticles was studied by disc diffusion method as reported previously (Citation33). The antimicrobial activity was evaluated against some selected pathogenic bacterial strains such as Escherichia coli (NCIM 2079), Staphylococcus aureus (NCIM 2079), Pseudomonas aeruginosa (ATCC 10145) and Bacillus substilis (NCIM2439). The sterile discs (6 mm) were kept at the core of plate and different concentrations of metal nanoparticles (12.5, 25, 50 and100 μg/mL) were added on the disc. Gentamycin disc was used as the reference standard (50 μg/mL) and fruits peel extract was used as a control. The plate was incubated in the upright position at 37°C for 24 h. After incubation, the antimicrobial evaluation of nanoparticles was estimated as a zone of inhibition in mm.
2.4.2. Evaluation of in-vitro antioxidant activity of fruits peels extract-mediated titanium dioxide nanoparticles
Antioxidant activity of the fruits peels extract-mediated metal nanoparticles were determined by ,1-Diphenyl-2-picrylhydrazyl (DPPH), hydrogen peroxide, nitric oxide radical scavenging and reducing power assays.
2.4.2.1. DPPH free radical scavenging assay
The free radical scavenging ability of the fruits peels extract-mediated metal nanoparticles against DPPH free radical was evaluated as per literature (Citation34). The fruits peels extract-mediated metal nanoparticles at different concentrations (10, 20, 30, 40, 50, 75 and100 μg/mL) and standard butylatedhydroxyltoluene (BHT) was added to test tubes labeled accordingly. To the above test tubes, 1 mL methanolic solution of DPPH (1 mM) was added and thoroughly mixed. All the test tubes were incubated in a dark place for 30 min. The absorbance of the resultant solution was measured at 517 nm. The DPPH solution without sample or standard was used as a blank. The free radical scavenging activity was calculated by the following equation and results expressed as % inhibition.where AC is the absorbance of blank, i.e. absorbance of methanolic DPPH radical; AS is the absorbance of sample + methanolic DPPH radical.
2.4.2.2. Hydrogen peroxide scavenging assay
The H2O2 scavenging potential of metal nanoparticles was studied as described earlier (Citation34). Various concentrations of metal nanoparticles and ascorbic acid (reference standard) were taken in test tubes and mixed with 50 μL of 5 mM H2O2 solution. Blank consisted of H2O2 solution devoid of any sample. The mixture was incubated for 20 min at room temperature. The absorbance was measured spectrophotometrically at 610 nm. The percentage of H2O2 scavenging was calculated using a control (blank) and sample (nanoparticle treated) absorbance.
2.4.2.3. Nitric oxide scavenging activity
Nitric oxide scavenging activity was estimated by the use of Griess reaction (Citation35). In the present study, 10 mM solution of NaNO2 prusside buffered in phosphate saline was poured with various concentrations of metal NPs and incubated at 30°C for 2 h. Control was also prepared devoid of any treated samples. On completion of incubation, 0.5 ml of Griesse agent was transferred to each tube. The absorbance of the resulting chromophore formed during the diazotization reaction was measured at 550 nm. The antioxidant ascorbic acid was used as a reference standard.
2.4.2.4. Reducing power assay
The antioxidant activity of metal nanoparticles can be measured by using reducing power assay. In this assay, antioxidant compounds and related samples convert the oxidation form of iron (Fe+3) in ferric chloride to ferrous (Fe+2). The reducing power was determined by the reported method (Citation33). Different concentrations of metal nanoparticles were mixed with 2.5 mL of Phosphate Buffer (200 mM, pH 6.6), and to this 2.5 mL of 1%, potassium ferric cyanide was added. The resulting mixture was heated at 50°C for 20 min and then cooled rapidly. Then, the mixture was precipitated with the addition of 2.5 mL of 10% TCA solution and centrifuged at 3000 rpm for 8 min. The supernatant was collected and diluted (1:1) with an equal volume of Milli-Q water. To this, 1 mL offer chloride solution (0.1%) was added and the absorbance was measured spectrophotometrically at 700 nm. Samples of nanoparticles serve as a control. BHT was used as a standard.
3. Results and discussion
A total of three titanium dioxide nanoparticles has been synthesized from three rosaceous fruits peels extract namely (P. domestica L. (Plum), P. Persia L. (Peach) and A. deliciosa (Kiwi)). To the extract solution, titanium dioxide was added up to boiling until some color change with paste-type texture was the first sign of nanoparticle formation. Further, nanoparticles were characterized by different analytical techniques such as XRD, FTIR and SEM to demonstrate the morphological attributes of synthesized nanoparticles.
3.1. Characterization of fruit peels extract-mediated titanium dioxide nanoparticles
3.1.1. X-ray diffraction
XRD analysis of prepared powder nanoparticle was used by X-ray diffractometer to confirm and analyze all-metal nanoparticles synthesized here.
Powder XRD pattern of biosynthesized titanium dioxide nanoparticles using P. domestica L. (Plum), A. deliciosa (Kiwi) and P. Persia L. (Peach) are shown in (A–C).
Figure 1. XRD pattern of biosynthesized TiO2 NPs with Miller indices (hkl) from (A) Plum, (B) Kiwi and (C) Peach peels extract.
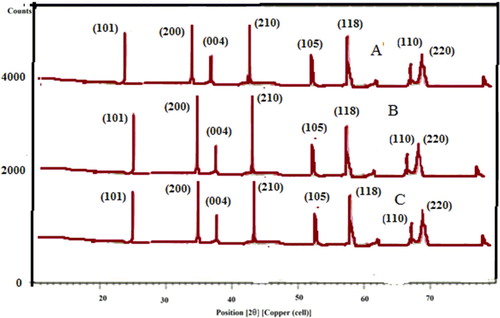
XRD-diffraction peak clearly emerged as a sharp peak and all are alike. From XRD pattern, it is clear that TiO2 nanoparticles appear as Anatase form and 2θ values with their corresponding (hkl) planes are 25.5° (101), 35.5° (200), 44.5° (004), 52.5° (210), 57.5° (105), 66.5° (118), 68.5° (110) and 69.5° (220) which reflects crystallographic nature of TiO2 as nanocrystalline. The result was the reflection of crystal planes (JCPDS files 21-1272) which was again confirmed by similar results of some reported work (Citation36,Citation37). All the above XRD peaks were found with similar 2θ despite of different sizes of TiO2 nanoparticles, which might be confirmed by SEM morphological analysis.
3.1.2. FTIR analysis
Biomolecules present in the selected fruits (P. domestica L. (Plum), P.Persia L. (Peach) and A. deliciosa (Kiwi)) peels are involved in the reduction and stabilization to the conversion of titanium dioxide to nano range. FTIR absorption spectra of methanolic peels extract of all the fruits before and after the reduction of TiO2 NPs were discussed separately in .
Table 1. FTIR values of fruits peel extract and biosynthesized TiO2 nanoparticles (TiO NPs).
FTIR spectra of all the above fruits peel extract contains necessary functional groups which are required to convert TiO2 to TiO2 NPs. The IR spectrum of peels extract showed intense bands around 3300–3400 cm−1 corresponds to strong stretching vibrations due to a hydroxyl group (−OH) of phenolic compounds. Peaks at 2900, 2800 cm−1 can be demonstrated due to the –OH– and C = O stretching vibrations, which are confirmed as for aromatic and carbonyl groups of the proteins and metabolites present in the fruits peels extract which was certainly responsible for reduction process (Citation34). The peak at 1600–1700c m−1 justified to C = O of an amide. The rest of peaks around 1000 cm−1 for –C–O– stretching vibrations of carboxylic, ethyl, ester groups present in biomolecules. Bands at 1200–1300 cm−1 were attributed to –CH– groups. Thus, the presence of these major organic moieties in biomolecules of chosen fruits peels extract might contribute to the bioreduction as well as stabilization of biosynthesized titanium dioxide nanoparticles.
Biosynthesized titanium dioxide nanoparticles have been formed from Plum, Kiwi and Peach peels extract and FTIR spectrum showed a bioreduction (, ). The FTIR values of plum fruits peels were discussed in our previous work (Citation38).
Figure 2. FTIR pattern of biosynthesized TiO2 NPs from (A) Plum, (B) Kiwi and (C) Peach peels extract.
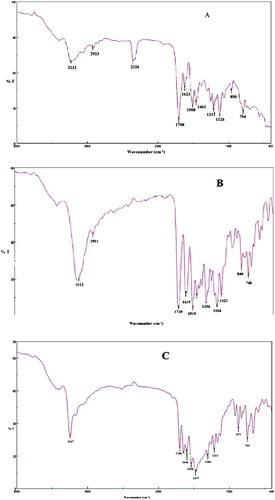
Peaks at 1708, 1720 and 1700 cm−1 attributed to the O–Ti–O prominent band for Plum (A), Kiwi (B) and Peach (C) mediated TiO2 NPs, which were also justified by some reported research (Citation39). Peaks around 1400 cm−1 indicate Ti–O stretching and 3000 cm−1 confirmed for the presence of OH stretching which was again in line with similar results (Citation40). The rest of the peaks at 1000–1300 cm−1 indicate the existence of some aliphatic amines and nitro compounds present in the titanium dioxide nanoparticles.
3.1.3. SEM analysis
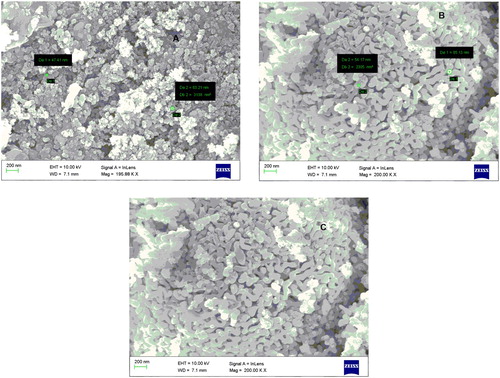
SEM is an important technique to characterize size and shape of the metal nanoparticles. Titanium dioxide nanoparticles were biosynthesized from P. domestica L. (Plum) (A), A. deliciosa (Kiwi) (B) and P. Persia L. (Peach) (C) peels extract found to be different size and shape as presented in SEM micrograph in .
Above SEM micrograph of TiO2 synthesized by different fruits peels appeared in size and shape differently. Titanium dioxide synthesized from plum peels showed particle size of 47.1 and 63.21 nm (A) with an overall size 200 nm and cylindrical shape. In another way, Kiwi peels-mediated biosynthesized TiO2 was observed 54.17 and 85.13 nm size with the average size of 200 nm with cylindrical shape (B). Another TiO2 NPs was prepared from Peach peels with uniform size of 200 nm having cylindrical in shape(C). Different shapes of nanoparticles were identified due to the capping of TiO2 with the compounds present in peels. Morphological differences of TiO2 nanopartcles is also affected by the nature of biomolecules present in individual extract, pH of extract, storage condition of extract, etc. Therefore, the polydispersed nanoparticle of titanium dioxide with varying size was investigated through the use of green methodology utilizing agro-waste of selected fruits peels.
3.1.4. UV spectroscopy
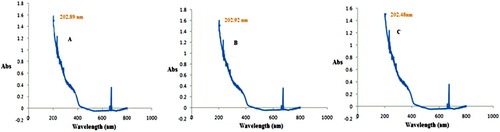
UV spectroscopy is one of the valuable techniques to identify various types of synthesized nanoparticles. At a specified wavelength of light, metal nanoparticles showed different peaks. The UV absorption spectrum for TiO2 NPs synthesized from Plum (A), Kiwi (B) and Peach (C) were identified at 202.89, 202.92 and 202.48 nm (), respectively. The appearance of UV spectrum of numerous titanium dioxide nanoparticles was also evaluated at around 200 nm by many reported works (Citation40, Citation41).
3.2. Biological activities
3.2.1. Antibacterial activities
3.2.1.1. Evaluation of antibacterial activity of fruits peels extract-mediated titanium dioxide nanoparticles
We studied the effect of three titanium dioxide nanoparticles biosynthesized from Plum, Kiwi and Peach peels extract, on selected pathogens. We have found that TiO2 NPs exhibited good antibacterial activity against all grams (−) types with E. coli being most affected (). The zone of inhibition against E. coli was in the range of 4–20 mm with extract of Plum peels – TiO2 NPs, 5–20 mm with Kiwi peels extract – TiO2 NPs and 3–18 mm with Peach peels extract – TiO2 NPs when tested at 12.5–100 µg/mL concentration. The zone of inhibition against P. aeruginosa was in the range of 4–19 mm with Plum peels – TiO2 NPs, 2–16 mm with Kiwi peels extract – TiO2 NPs and 4–19 mm with Peach peels extract – TiO2 NPs when tested at 12.5–100 µg/mL concentration.
Figure 5. Antibacterial activities of TiO2 NPs synthesized using peels extract of Plum, Kiwi and Peach against E.coli, S. aureus, P. aerugenosa and Bacillus Subtilis.
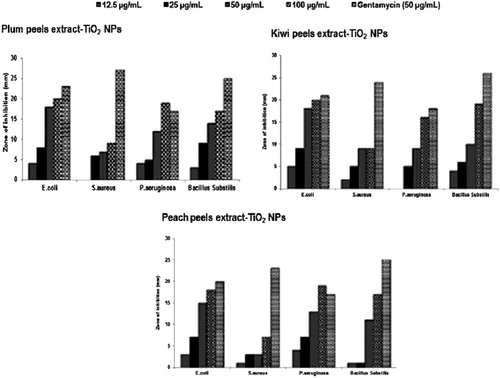
The growth of another gram (+) bacteria, B. substilis, was also inhibited by titanium dioxide nanoparticles with the zone of inhibition of 17–19 mm at 100 µg/mL concentration. Mechanism of titanium dioxide nanoparticles as antibacterial depends on its exposure to sunlight. Lipids, lipopolysaccharides, and peptidoglycans of microorganism are oxidized, when TiO2 NPs gets absorbed on cell wall to formed HO and O2 radicals (Citation42). Due to these features, TiO2 NPs referred to as antiseptic. Under the influence of sunlight, TiO2 disrupt the DNA chains in cells due to its genotoxic nature (Citation43). In many studies, antibacterial effects of TiO2 NPs have highlighted the concentration-dependent activities (Citation44). In the present study also antibacterial activities of TiO2 NPs were found concentration dependent. Moreover, the size and shape of TiO2 NPs also played a role in antibacterial evaluation. TiO2 NPs derived from peels extract of Plum and Kiwi were a somewhat small particle in size as depicted in SEM micrograph (), also found to be better antibacterial activities.
3.3. Antioxidant activities
3.3.1. Evaluation of in-vitro antioxidant activity of fruits peels extract-mediated titanium dioxide nanoparticles
3.3.1.1 DPPH free radical scavenging assay
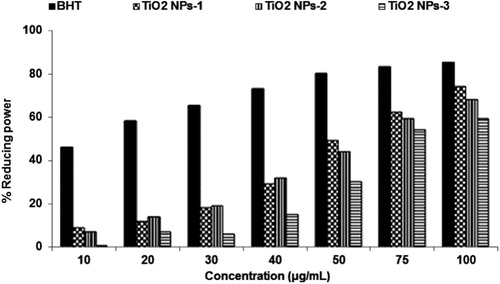
As depicted in , the fruits mediated titanium dioxide nanoparticles showed less free radical scavenging activity at low concentration but it was exhibited very close to standard BHT at higher concentration. The free radical scavenging was 10–79% for Plum (TiO2 NPs-1), 11–69 Kiwi (TiO2 NPs-2) and 8–59% for Peach (TiO2 NPs-3) peel extracts-mediated titanium dioxide nanoparticles as compared to 43–85% effect with standard BHT at 10–100 μg/mL concentrations. Results obtained in this experiment indicated that free radical scavenging was a concentration-dependent activity.
Figure 6. Antioxidant activity of titanium dioxide nanoparticles synthesized using Plum (TiO2 NPs-1), Kiwi (TiO2 NPs-2) and Peach (TiO2 NPs-3) peels extract in DPPH assay.
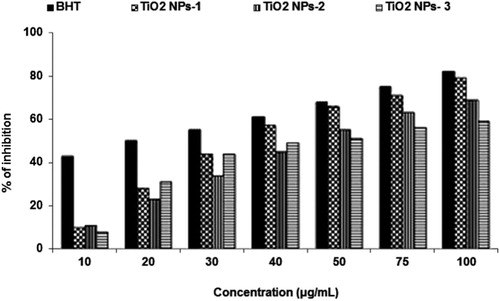
The radical scavenging also depends on the size and morphology of titanium dioxide nanoparticles. Therefore, Plum peels extracts-mediated TiO2 NPs showed the highest radical scavenging at all concentration due to its smallest particle size of 47.1 and 63.21 nm with the overall size of 200 nm. The maximum radical scavenging of peels extracts-mediated TiO2 NPs was found to be 79% at 100 μg/mL concentrations. Similarly, Kiwi peels mediated TiO2 NPs having 69% of radical scavenging at 100 μg/mL concentrations with a particle size of 54.17 and 85.13 nm. The Peach peels mediated TiO2 NPs having a particle size of 200 nm exhibited less radical scavenging ability. Better radical scavenging capacity of metal nanoparticles might be due to the presence of phytoconstituents in the particular extracts (Citation34). It is reported to have a higher amount of phenolic compounds in Plum peels than flesh as compared to Kiwi and Peach (Citation45). This might be the reason that Plum peels-mediated TiO2 NPs acts as better radical scavenging potential than Kiwi and Peach peel synthesized TiO2 NPs.
3.4. Hydrogen peroxide scavenging assay
Similar to DPPH assay, in hydrogen peroxide assay, the titanium dioxide nanoparticles exhibited good scavenging potential and close to standard antioxidant ascorbic acid as especially at the higher concentration shown in .
Figure 7. Antioxidant activity of titanium dioxide nanoparticles synthesized using Plum (TiO NPs-1), Kiwi (TiO2 NPs-2) and Peach (TiO2 NPs-3) peels extract in H2O2 scavenging assay.
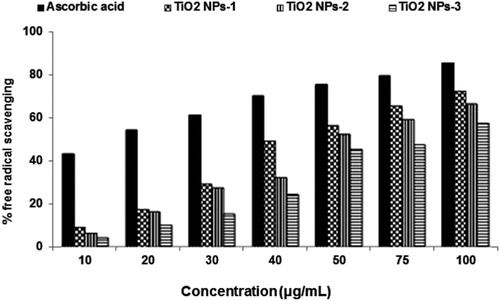
The fruits peel-mediated TiO2 NPs showed 57–72% scavenging at 100 μg/mL concentration, while at 10 μg/mL dose scavenging was 4–9% indicating ineffectiveness at lower concentrations. H2O2 scavenging of the capability of TiO2 NPs synthesized by Plum peels extract was higher in comparison to Kiwi and Peach peels mediated TiO2 NPs. The H2O2 scavenging of three titanium dioxide nanoparticles has been found in the order of TiO2 NPs-1 > TiO2 NPs-2 > TiO2 NPs-3. Moreover, differences in hydrogen peroxide scavenging of these three titanium dioxide nanoparticles were again due to different particle size. TiO2 NPs-1 produced by peels of Plum has the smallest particle size followed by TiO2 NPs-2 which was obtained through peels of Kiwi and TiO2 NPs-3 synthesized by Peach peels extract. Phytoconstituents such as phenols, flavonoids, and proteins also were reported to have a high amount in peels of Plum fruit (Citation45) in comparison to Kiwi and Peach, which acts as capping and reducing agents to form effective titanium nanoparticles to be potential scavenging of hydrogen peroxide.
3.4.1. Nitric oxide scavenging activity
Titanium dioxide nanoparticles were found concentration-dependent scavenging of NO radicals. However, TiO2 NPs showed relatively good NO scavenging activity at 100 µg/mL in comparison to standard ascorbic acid ().
Figure 8. Antioxidant activity of titanium dioxide nanoparticles synthesized using Plum (TiO2 NPs-1), Kiwi (TiO2 NPs-2) and Peach (TiO2 NPs-3) peels extract in NO scavenging assay.
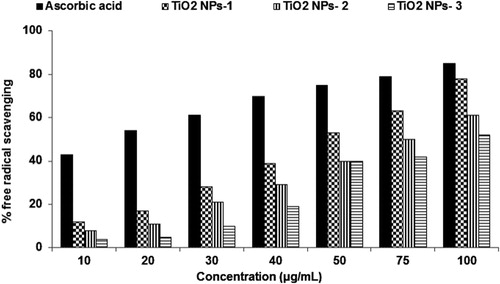
The Plum peels-mediated TiO2 NPs-1 exhibited 12–78% scavenging; Kiwi peels mediated TiO2 NPs-2 exhibited 8–61%, while Peach peels mediated TiO2 NPs-3 showed 4–52% effect indicating size-dependent potency against NO radicals. TiO2 NPs-1 synthesized from peels of Plum has the smallest size produced maximum NO radicals scavenging at all concentrations followed by TiO2 NPs-2 and TiO2 NPs-3.
3.4.1.1. Reducing power assay
Fruit peels extract-mediated titanium dioxide nanoparticles showed good reducing power at higher concentrations but lower than the standard BHT. The reducing was 9–74%, 7–68%, and 1–59%, respectively, with Plum peels-mediated TiO2 NPs-1, Kiwi peels-mediated TiO2 NPs-2 and Peach peels-mediated TiO2 NPs-3 as compared to 46–85% with BHT (). Reducing the power of titanium dioxide nanoparticles has also been found in size-dependent results.
4. Conclusions
Revalorization of agro-waste material such as fruits peels towards the biosynthesis of titanium dioxide nanoparticles is surely a cost-effective eco-friendly approach. In the present study, methanolic extract of P. domestica L. (Plum), A. deliciosa (Kiwi) and P. Persia L. (Peach) fruits peels were employed for the synthesis of three titanium dioxide nanoparticles. The biosynthesized nanoparticles were characterized by XRD, FTIR and SEM. Antibacterial and antioxidant activities of titanium dioxide nanoparticles were performed and found dose- and size-dependent variation. In conclusion, present green chemistry approach for the biosynthesis of titanium dioxide nanoparticles is easy and economically viable and would be a useful biomedical candidate.
Supplemental Material
Download MS Word (1.3 MB)Disclosure statement
There was no any conflict of interest was reported by the authors.
Notes on contributors
Noushin Ajmal has received is Ph.D. award in 2018. She has experienced in the formation of metal nanoparticles using green Chemistry approach.
Keerti Saraswat has expertise in the field of organic and green chemistry for the potential biological interest. Presentally she is working in College of Science and Humanities, Paratap University Jaipur, India as Associate Professor.
Md. Afroz Bakht Received his Ph.D. in pharmaceutical chemistry in 2009. He is currently working as an associate professor in the Department of Chemistry in the College of Science and Humanities Studies, Prince Sattam Bin Abdulaziz University, KSA. His research interest is in the synthesis of potential organic scaffold utilizing new green chemistry technics and studying their pharmacological activities. He has few novel green solvents and catalysts to his name. He has also used ultrasound technology for the synthesis purposes.
Yassine Riadi Received his Ph.D. in organic pharmaceutical chemistry from the University of Orleans in France in 2013. Yassine is an assistant professor and researcher in the College of Pharmacy, Prince Sattam Bin Abdulaziz University. His research focuses on the development of new green catalysts using facile and low-cost processing methods and their use in the synthesis of new drugs and compounds.
Mohammed Jawed Ahsan has completed his Ph.D. in pharmaceutical chemistry from NIMMS university, Jaipur, India in 2012. He has expertise in medicinal and pharmacological activities of compounds/ molecules in the area of antimicrobial, antioxidants and anticancer.
Md. Noushad did his master in nanotechnology. Presentally he is involved in designing, preparation and characterization of nanomaterials in Bottom Up Technologies Corporation [BT Corp], Bangaluru, India.
References
- Narayanan, K.B.; Sakhthivel, N. Adv. Colloid. Interface. Sci. 2010, 156, 1–13.
- Kathiresan, K.; Manivannan, S.; Nabeel, A.M.; Dhivya, B. Colloid. Surf. B: Biointerface. 2009, 71, 133–137.
- Torresday, J.L.G.; Parsons, J.G.; Gomez, E.; Peralta-Video, J.; Troian, H.E.; Santiago, I.P.; Yacaman, M.J. Nano. Lett. 2002, 2, 397–401.
- Bali, R.; Razak, N.; Lumb, A.; Harris, A.T. The Synthesis of Metallic Nanoparticles Inside Live Plants. IEEE Xplore Conference, Brisbane, Australia, July 3-7, 2006, 224–227, 2006.
- Zawrah, M.F.; Abd El-Moez, S.I. Life. Sci. J. 2011, 8, 37–44.
- Azmira, J.; Zaidula, I.S.M.; Rahmana, M.M.; Sharif, K.M.A.; Mohamed, A.; Sahenab, F.; Jahurulb, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. J. Food. Eng. 2013, 117, 426–436.
- Pérez, C.; Cast Gillo, M.L.R.; Gil, C.B.; Flores, G.P. Food. Funct. 2015, 6, 2607–2613.
- Sinha, A.; Khare, S.K. Biores. Tech. 2011, 102, 4281–4284.
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Chem. Rev. 1995, 95, 69–96.
- Fujishima, A.; Rao, T.N.; Truk, D.A. J. Photochem. Photobiol. C: Photochem. 2000, 1, 1–21.
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Biol. Trace. Elem. Res 2011, 141, 3–15.
- Gelis, C.; Girard, S.; Mavon, A.; Delverdier, M.; Pailous, N.; Vicendo, P. Photodermatol. Photoimmunol. Photomed. 2003, 19, 242–253.
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Future. Microbiol. 2011, 6, 933–940.
- Lipovsky, A.; Gedanken, A.; Lubart, R. Photo. Med. Laser. Surg. 2013, 31, 526–530.
- Miller, R.J.; Bennett, S.; Keller, A.A.; Pease, S.; Lenihan, H.S. PLOS. Biol. 2012, 7, 1–7.
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Cancer. Res. 2009, 69, 8784–8789.
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Soil. Biol. Biochem. 2010, 42, 586–591.
- Tahir, M.B.; Sagir, M.; Shahzad, K. J. Hazard. Mater. 2019, 363, 205–213.
- Tahir, M.B.; Nabi, G.; Rafique1, M.; Khalid, N.R. Int. J. Environ. Sci. Technol. 2017, 14, 2519–2542.
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. Res. Effc. Tech. 2017, 3, 406–413.
- Nabi, G.; Aain, Q.; Khalid, N.R.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Hussain, S.; Iqbal, T.; Majid, A. J. Inorg. Organomet. Polym. 2018, 28, 1552–1564.
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. J. Adv. Res. 2016, 7, 17–28.
- Shailajaraj, M.; Roselin, P. Int. J. Pharm. Bio. Sci. 2012, 3, B267–B276.
- Thamima, M.; Karuppuchamy, S. Adv. Sci, Eng. Med. 2014, 6, 1–2.
- Ibrahem, K.H.; Salman, J.A.S. Eur. Sci. J. 2014, 10, 324–338.
- Kirthi, A.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T.; Jayaseelan, C.; Elango, G.; Zahir, A.A.; Kamaraj, C.; Bagavan, A. Mat. Lett. 2011, 65, 2745–2747.
- He, W.; Cui, J.; Yue, Y.; Zhang, X.; Xia, X.; Liu, H.; Lui, S. J. Adv. Colloid. Interface. Sci. 2011, 354, 109–115.
- Rajakumar, G.; Rahuman, A.; Jayaseelan, C.; Santhoshkumar, T.; Marimuthu, S.; Kamaraj, C.; Bagavan, A.; Zahir, A.A.; Vishnu Kirthi, A.; Elango, G.; Arora, P.R.K. Parasitol. Res. 2014, 113, 469–479.
- Velayutham, K.; Rahuman, A.; Rajakumar, G.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; Bagavan, A.; Kirthi, A.; Kamaraj, C.; Zahir, A.; Elango, G. Parasitol. Res. 2012, 111, 2329–2337.
- Malabdi, R.B.; Mulgund, G.S.; Meti, N.T.; Nataraja, K.; Vijayakumar, S. Res. Pharm. 2012, 2, 10–21.
- Nwanya, A.C.; Ugwuoke, P.; Ejikeme, P.M.; Oparaku, E.O. Int. J. Electrochem. Sci. 2012, 7, 11219–11235.
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, V.A.; Kumar, T.S.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M.; et al. Asian. Pac. J. Trop. Med. 2013, 6, 682–688.
- Meruvu, H.; Vangalapatti, M.; Chippada, S.C.; Bammidi, S.R. J. Chem. Sci. 2011, 4, 2017–2022.
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. App. Nano. Sci. 2016, 6, 755–766.
- Rees, D.D.; Palmer, R.M.; Moncada, S. Proc. Natl. Acad. Sci. USA. 1989, 86, 3375–3378.
- Kalyanasundharam, S.; Prakash, M.J. Int. Lett. Chem. Phys. Astron. 2015, 50, 80–95.
- Vijayalakshmi, R.; Rajendran, V. Arch. Appl. Sci. Res. 2012, 4, 1183–1190.
- Ajmal, N.; Saraswat, K. Adv. Bio. Res. 2017, 8, 39–46.
- Shahab, M.; Tabish, T.; Zaman, B.; Tariq, Z.; Kamran, M. Int. J Eng. 2013, 11, 313–316.
- Vetrivel, V.; Rajendran, K.; Kalaiselvi, V. Int. J. Chem. Tech. Res. 2015, 7, 1090–1097.
- Órdenes-Aenishanslins, N.A.; Luis, A.S.; Durán-Toro, V.M.; Juan, P.M. Microb. Cell Fact. 2014, 13, 1–10.
- Kiwi, J.; Nadtochenko, V.A. Langmuir. 2005, 21, 4631–4641.
- Serpone, N.; Salinaro, A.; Emeline, A. Proc. SPIE. 2001, 4258, 86–98.
- Mamonova, I.A.; Babushkina, I.V.; Norkin, I.A.; Gladkova, E.V.; Matasov, M.D.; Puchin’yan, D.M. Nanotech. Russia. 2015, 10, 128–134.
- Walkowiak-Tomczak, D. Pol J. Food. Nutr. Sci. 2008, 58, 401–405.