ABSTRACT
The sorption of cationic dyes was studied using sorbents derived from pulverized papaya seeds and pomelo rind. A convenient fixed sorbent envelope protocol with methods for quality check on the readiness of sorbent material for subsequent sorption was developed. Sorbents based on papaya seeds had higher affinity for methylene blue and brilliant green dyes than pomelo rind. Amongst Langmuir, Freundlich and Temkin isotherms, the sorption of both cationic dyes fitted the Langmuir isotherm best. The multi-step linearity behavior on the intra-particle diffusion model with a positive intercept indicated that intra-particle diffusion was not the sole sorption rate-determining factor. The utility of the envelope protocol as a convenient evaluation method for sorbent characteristics and effectiveness was also demonstrated. Magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy was explored and found to differentiate between the two fruit wastes and had the potential for elucidating sorbent–sorbate interactions.
GRAPHICAL ABSTRACT
Introduction
Sorption with sorbents derived from agricultural waste generated growing interest amongst researchers as evidenced by several reviews in literature (Citation1–9). Various methods have been used to treat these types of sorbent materials before sorption studies, as highlighted in a review by Patel (Citation8). However, detailed sorbent preparation methods were seldom given together with sorption capacity for target pollutants. This may be due to investigators using sorbent materials as they are or with minimal preparation to maintain their cost-effectiveness. An attempt was made here to use chemical oxygen demand (COD) and conductivity contributions of the agro-waste in the water used for cleaning to define the state of the biomaterial before use as sorbents. Depending on the subsequent remediation stage, a certain level of COD could be desirable and a COD determination of the sorbent cleaning effluent could provide an estimate of the contribution of organic content by the sorbent material into the water. In addition, conductivity measurement would give an idea of the inorganic contribution by the agro-waste under sorption conditions.
As landfill options were un-attractive for solid wastes like fruit wastes from plantations and juice makers, the utilization of such wastes for water remediation was also investigated. Carica papaya seeds were reported to form up to 15–22% of the papaya fruit (Citation10–12). The reported sorption capacity for methylene blue (MB) dye were 555.557 mg/g (Citation13) for C. papaya seeds, 769 mg/g for non-defatted papaya seeds and 1250 mg/g for defatted papaya seeds (Citation14), and 344.83 mg/g (Citation15) and 133 mg/g (Citation16) for Citrus grandis rind. C. grandis rind were found to contain up to 30% pectin and to constitute close to 40–50% of the fruit (Citation17). The potentially high % solid wastes available from C. papaya and C. grandis fruit, juice and confectionary production, together with the reported high MB dye sorption capacity make it worthwhile to investigate their potential as sorbent materials. Therefore, a comparative study of pomelo peel and papaya seeds in sorption using a simplified envelope protocol was carried out.
For the harmonization of a protocol for sorbent performance evaluation (Citation18), several factors had been highlighted in a study of fungal by-products for removal of metallic pollutants including, preparation of sorbent, sorbate concentration ranges, sorbent-to-sorbate ratio, and conditions of monitoring methods, just to name a few. Here the sorption of C. grandis rind and C. papaya seeds for MB and brilliant green (BG) dyes were compared using both the standard protocol reported in literature and a simplified envelope protocol developed in this study. The Langmuir, Freundlich and Temkin isotherm fits, together with pseudo-second order and intra-particle diffusion plots were used to validate the proposed envelope protocol for sorbent performance evaluation. In addition, magic angle spinning nuclear magnetic resonance (MAS NMR) and Fourier Transform Infrared (FTIR) spectroscopies were used to confirm the presence of sorbent surface sites responsible for sorbent–sorbate interactions, and to obtain new insights for determining the quality of the sorbents and characterizing the sorbent–sorbate interactions.
Experimental
Sorbates and chemicals
MB and BG dyes were selected for this study as they can be model cationic dyes. MB dye had been a popular choice as sorbate for sorption studies (Citation13–16) as evidenced by a dedicated review on the sorption of MB dye (Citation6). MB and BG dyes are structurally different from each other with MB dye having a relatively flat 3-D structure. Cationic sorbates are expected to have high sorption onto largely anionic surfaces of fruit waste biomass. These dyes had high solubility in water and MB dye sorption had been widely reported. The intent of this paper is to assess and compare different aspects of sorbent behavior for bulk removal of dyes from water using a laboratory protocol.
Sorbent type and preparation
C. papaya (papaya) seeds and C. grandis (pomelo) rind were selected here due to their reported advantages such as minimal pre-treatment needed and high affinities for MB dye. As the aim was to show the utility of the envelope protocol, both papaya seeds and pomelo rind were used in the optimization and comparison studies. These fruits were purchased from a local supermarket. The papaya fruit was from Malaysia and the pomelo fruit was from Thailand. Upon separating from the edible portion, the papaya seeds and pomelo rind were sun-dried for five to seven days after which they were pulverized with a coffee grinder, sieved to required size and stored in desiccators before use. They were cleaned with water either in a column set-up or in envelopes prior to sorption experiments. Cleaning of sorbent by the column method involved packing the material in a glass column and passing water through it till the spent water was colorless and conductivity values were less than 5 μScm−1. No further preparation was performed on the sorbent material other than cleaning with water. Conductivity measurements were made on a HACH Q40D Multi-versatile pH-Oxygen-Conductivity meter and pH determinations were performed with Metrohm 827 pH Lab meter. Upon cleaning or chemical treatment, the sorbent were dried in a 70°C oven. The solid sorbent before and after sorption were characterized using BET (Brunauer, Emmet and Teller) surface analyses and CHNS measurements, FTIR and MAS NMR. BET surface analyses were performed using a Micrometrics ASAP 2020 Surface Analysis and Porosity Analyzer, using N2 at 77 K. CHNS measurements were made on an Elementar Vario Micro Cube Analyzer. FTIR analyses on KBr disc were performed on a Bio-Rad Excalibur Series Infrared spectrometer with Varian Resolution 4.0 software. MAS NMR scans were obtained using Bruker 400 with Xwinnmr 2.6 software and signal processing was performed with TopSpin 1.3 software.
Sorption and desorption
Free and fixed sorbent processes
Free sorbent process had been most widely employed in current sorption research which involved weighing a fixed amount of sorbent material and allowed it to come into direct contact with the dye sorbate solutions in stoppered flasks or bottles (Citation19). A further step to separate the sorbent with dye pollutant from the sorbate solution was required to determine the concentration of the dye in solution. Another common process used was the column protocol (Citation20, Citation21) where the sorbent was packed into columns or discs and the sorbate solution was allowed to pass through the column for sorption to occur. Here, a fixed sorbent envelope protocol i.e. a modified “tea-bag” procedure (Citation22, Citation23) was studied. This involved packing the sorbent material into envelope form prior to suspending it in sorbate solutions for sorption experiments. A comparison of the advantages and disadvantages of these protocols are summarized in .
Table 1. A comparison of enveloped and free sorbent systems.
Equilibrium studies
Equilibrium studies at several different concentrations were conducted. 0.05–0.06 g of sorbent powder was allowed to contact with 20 mL MB or BG dye solutions in water of varying concentrations (1, 10, 20, 50, 100, 200 mg/L) in glass culture tubes with screw-cap or 100 mL conical flasks with stopper, allowed to equilibrate overnight before determination of dye concentration in solution with UV spectroscopy using the free sorbent process reported widely in literature (Citation13–16). The wavelengths for maximum absorbance were 664 nm for MB dye and 623 nm for BG dye.
Kinetics and thermodynamics studies
For kinetics study, 0.5–1.0 g of sorbent powder was allowed to contact with 200 or 250 mL of dye solution with 30 mg/L using both free sorbent and fixed sorbent envelope technique. For kinetic and thermodynamic studies, larger amounts of sorbent then that for the equilibrium studies were used to allow sorbate solutions to be drawn over different time intervals and at different temperatures. This also permitted sufficient dye-laden sorbent to be collected for subsequent characterization experiments. At regular time intervals of 15–60 min within the first 3 h and subsequently on hourly basis thereafter for up to 24 h, samples of the sorbate solutions were drawn for UV analysis. For thermodynamics study, 0.5 g of sorbent powder was allowed to equilibrate at 25°C (298 K), 40°C (313 K) and 50°C (323 K) overnight before the concentration of the dye in each of the sorbate solution was determined by UV analysis.
Desorption
The sorbent powder system was allowed to be saturated with the BG dye by allowing 1 g of sorbent to equilibrate overnight with a low concentration of 5 mg/L of dye sorbate solution followed by a second equilibration with a higher concentration of 150 mg/L of dye sorbate solution till concentration of dye sorbate solution remained constant based on UV analysis. It was noted that the sorbent could swell during first stage equilibrium and dye molecules could get into interaction sites readily at the second stage equilibrium. The dye-laden sorbent powder was allowed to undergo desorption in water–ethanol mixtures. Concentration of dyes in the aqueous mixtures were measured with UV analysis over time for desorption studies.
Results and discussion
Sorbent characterization
Surface area, elemental analysis, pH at point zero charge determination
The BET surface area and porosity data in show that papaya seed (PAP) sorbent exhibits larger pore size, surface area and pore volume than pomelo rind (OP) sorbents. From CHNS elemental analysis data shown in , papaya seed cleaned with water using column set-up (PAw) had lower P content, slightly higher C/N ratio than pristine papaya seed (Pau) (13.3 vs. 11.1 resp.). Pristine papaya seed had a lower C/N ratio than pristine pomelo rind (POu) (11.1 vs. 38.0). pH measurements at point zero charge (pHpzc) were made using the immersion technique (Citation24). The values were found to be 3.54–3.97 for pristine pomelo rind, 6.95 for pre-boiled papaya seed and close to 6 for pristine papaya seed, as shown in . The pHpzc for defatted papaya seed was reported to be around 6 (Citation14). A value of 3.5 had been reported for orange rind (Citation25) which was part of the Citrus family that pomelo belonged to. Although pHpzc was found to be not useful for directly elucidating sorption mechanism, it gave an idea of the overall surface charge of the sorbent under study and thus the sorbent natural affinity for a specific class of sorbates or target pollutants. With a lower than neutral surface charge, the higher affinity for cationic targets by a negatively charged surface was expected.
Table 2. BET surface area and pore size analysis.
Table 3. CHNS and elemental analysis data of pomelo rind and papaya seeds fruit waste sorbents (wt %).
Table 4. pH at point zero charge (pHpzc) using immersion technique.
Infrared spectroscopic analysis
Band assignments of FTIR spectra with respect to possible function groups present on the rind are tabulated in . and show the magnitude and directions of the band shifts observed.
Table 5. FTIR based on KBr discs or pellets of C. grandis rind samples.
Table 6. Shift in FTIR band positions (cm−1) of C. grandis rind before (OP) and after BG dye sorption (OP + BG).
Table 7. Shift in FTIR band positions (cm−1) of C. grandis rind and C. papaya seeds before and after MB dye sorption.
Large shifts post MB dye sorption observed for the –OH stretch around 3407–3420 cm−1. Bands for non-ionic carboxylate at 1727–1744 cm−1 and ionic carboxylate vibration at 1624–1639 cm−1 showed that these functional groups present in fresh pomelo rind (OP) participated in the sorption process. It was also shown that the –OH stretch and ionic carboxylate vibration participated in the adherence of cationic MB dye to the rind surface. These findings were consistent with MB dye sorption by Brazilian pine-fruit (Citation25), divalent cadmium sorption by orange rind (Citation26), and divalent copper, nickel, cadmium, lead and zinc sorption by orange, lemon and banana peels (Citation27). For the sorption of divalent cadmium by orange rind (Citation26), it was noted that frequency shifts in the polysaccharide fingerprint IR regions were difficult to assign unambiguously. Hence, MAS NMR was performed to further confirm that the polysaccharide anionic functionalities participated in the sorption process for the cationic dyes under study.
Solid-state nuclear magnetic resonance characterization
Cross Polarisation Magic Angle Spinning Nuclear Magnetic Resonance (13C CP-MAS NMR) measurements of fresh pristine C. grandis rind were made using different spin rates. It was found that 8 kHz spin rate was suitable for obtaining the characteristic NMR trace of the rind sample (see Figures S1 and S2 in Supplementary Information).
Hence, further 13C CP-MAS NMR on the rind sample before and after sorption of MB dye were performed at 8 kHz spin rate and the NMR spectra are illustrated in Figure S1 where OP = fresh pristine pomelo rind and OPMB = fresh pristine rind post MB dye sorption.
The High Resolution Magic Angle Spinning Nuclear Magnetic Resonance (1H HR-MAS NMR) spectrum of fresh pomelo rind is shown in Figure S2(a). Compared to the spectrum for fresh pomelo rind sorbed with MB dye in Figure S2(b), it can be seen that there is a change in chemical shifts (δ) in the region of around 3.0–4.5 ppm.
When the 1H HR-MAS NMR spectra for the fresh pomelo rind and decayed pomelo rind were compared, a similar change was also found in the same shift region. The 1H HR-MAS NMR results were found to be more discerning between fresh and decayed rind, pristine rind and rind sorbed with MB dye compared with 13C CP-MAS NMR. The peak assignments for 13C CP-MAS NMR and 1H HR-MAS NMR of C. grandis rinds and C. papaya seeds are compiled together with that obtained from wood chars (Citation27) in and .
Table 8. Function group assignments of 13C CP-MAS NMR.
Table 9. Function group assignments of 1H HR-MAS NMR.
From the NMR shifts of 13C CP-MAS NMR of pomelo rind and papaya seed in , it was found that carbonylic C was non-participating in the dye sorption process. There was, however, an indication that alkylic C in papaya seed was accessible to the dyes investigated, but not pomelo rind. The results obtained in this study showed that MAS NMR is a powerful tool to elucidate chemical groups participating in the sorption. In addition, 1H HR-MAS NMR could be a useful identification tool as it was able to distinguish between pomelo rind and papaya seed. Furthermore 1H HR-MAS NMR can potentially be used as a tool to ascertain the quality of agro-waste for applications as sorbents.
Column and envelope protocol
Cleaning of sorbents
It was found that when conductivity of the wash effluent reached below 5 µScm−1, up to 80% reduction in COD contribution of the C. grandis rind could be achieved. For C. papaya seeds, close to 98% reduction in COD contribution was achievable. Thus conductivity measurement of the wash effluent could potentially serve as a quick and useful quality control parameter for sorbent preparation in the field.
Equilibrium, kinetics and thermodynamics of sorption
Several studies had reported and demonstrated the adsorption isotherms and kinetics fits of the sorption of cationic dyes and metallic ions (Citation30–34). The findings in terms of linear correlation factors (R2) for the Langmuir, Freundlich and Temkin isotherm fits are summarized in –. The pseudo-second-order kinetics fits are shown in .
Table 10. Comparison of linear isotherm fit of pomelo rind (OP), vs. papaya seeds for MB cationic dye sorption at 25°C.
Table 11. Linear isotherm fits (R2) for hot water washed (HOP) and MeOH washed (MOP) pomelo rinds vs. papaya seeds (PAP) for cationic dye sorption at 25°C.
Table 12. Linear Langmuir isotherm fits (R2) for hot water washed (HOP) and MeOH (MOP) washed pomelo rind vs. papaya seeds (PAP) for cationic dye sorption at different temperatures.
Table 13. Pseudo-second-order kinetics linear fit for pristine and blended fruit waste for sorption of MB dye.
For both pomelo rind and papaya seed sorbent, the linear isotherm fits were the best for Langmuir, followed by Freundlich and Temkin. The worst fit was found with Temkin isotherm. The Temkin isotherm also did not describe divalent nickel sorption by Sargassum wightii (Citation35) (a dark brown seaweed) well. This could implied that the assumption of the Temkin isotherm, i.e. linear reduction of heat of adsorption with increase sorbent surface coverage, might not hold true for the sorption of MB and BG dyes by pulverized pomelo rind and papaya seed. The linear isotherm fit pattern with Langmuir being the best and Temkin being the worst was also found with the sorbents pre-treated in a column using different washing solvents like hot water and methanol as shown in and 11. Sorption of both MB and BG dyes by C. grandis rind cleaned with MeOH till effluent was colorless or with hot water till effluent achieved COD < 50 mgL−1 and conductivity values < 5 µScm−1 also demonstrated good fit with the Langmuir isotherm. This could be a reason why researchers studying pristine sorbents did not report exactly how “clean” their sorbent materials were. All agricultural biomass may leach color into water streams and hence some researchers employed activated charcoal or other carbonized forms of sorbents derived from biomass sources as alternative low-cost sorbents (Citation32, Citation33). The methods based on mild treatment with water or alcohol of C. grandis rind at ambient conditions as used in this study, and Soxhlet extracted C. papaya seeds or defatted papaya seeds were also found to exhibit excellent linear fits to the Langmuir isotherm, suggesting that the bulk sorption behavior should be predictable across cellulosic-based materials. The Langmiur and Freundlich isotherms were identified by most dye sorption studies in literature to best fit in their linear forms at ambient. Since the reported correlation coefficients were generally quite close for those isotherms, for instance 0.98 for Freundlich and 0.99 for Langmuir or vice versa, there had been no conclusion to-date that the Freundlich isotherm did not describe satisfactorily the processes that best fit Langmuir isotherm in its linear form. In our opinion this is suggestive of multi-modal adsorption mechanisms, consistent with the FTIR and MAS NMR spectroscopic data presented here that varied functional groups present in the natural polymers were responsible for the sorption affinity. The underlying assumptions of the Langmuir isotherm were that all sorption sites were identical and energetically equivalent. Consequently, once the dye sorbate occupies a site, no further adsorption would occur. On the other hand, the key assumption of the Freundlich isotherm was that reversible adsorption could occur and was not limited to formation of a monolayer. High correlation to Freundlich isotherm implied that more than one mechanism and some degree of heterogeneity could be possible for the ionic sorbates in solution and the sorbent surface. Simple sorbent preparation like washing with water or alcoholic solutions conducted in this study did not significantly change the linear modeling fits of the equilibrium isotherms. It could be seen that the envelope protocol did not produce significantly different linear kinetics fits of the sorption system under study.
Pseudo-second-order kinetics
Slope of the pseudo-second-order rate law linear fit gave the inverse of the sorption capacity. Sorption of pomelo rind and papaya seeds was investigated individually in enveloped form. This protocol did not significantly alter the linear fit of pseudo-second-order kinetics described in literature (Citation36). The data are presented in . Sorption of divalent lead by citrus pectins (Citation37) also followed well the pseudo-second-order kinetics rate law. Similar findings with MB sorption by sugar beet pulp had been reported (Citation38).
Intra-particle diffusion
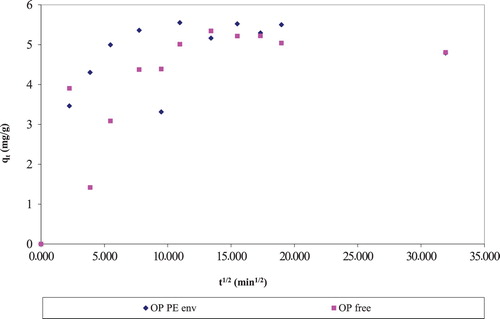
shows the intra-particle diffusion plots of enveloped and free pomelo rinds and it illustrated that the fixed envelope protocol showed similar trend and profile with the widely employed free sorbent protocol. The sorption of dyes by fruit waste blends also showed similar profiles.
Desorption
Desorption kinetics of methanol-washed sorbents
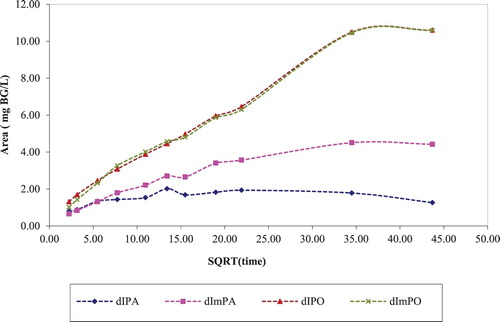
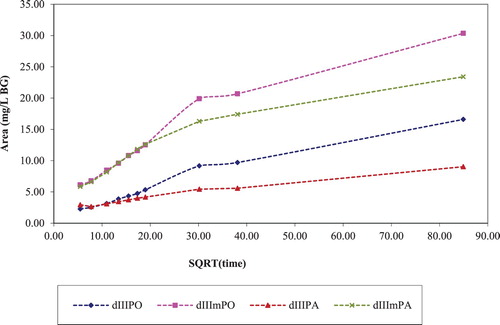
and show desorption kinetics plots of BG dye in different washout solvents. The increase in the concentration of BG dye was plotted against the square root of time. In these figures the label d1 referred to desorption cycle 1 and the letters that follow, i.e. PO referred to pomelo rind and mPO referred to pomelo rind washed with methanol.
It is shown in that the BG dye desorption from pomelo rind samples (dIPO and dImPO) was faster and a higher final equilibrium concentration was achieved relative to papaya seed samples (dIPA and dImPA). It was found that for subsequent third desorption in an ethanol (EtOH): water mixture, the methanol-washed pomelo rind (dIIImPO) had higher initial desorption rate than pristine pomelo rind (dIPO) and the methanol-washed papaya seed (dIIImPA) also had higher desorption rate than pristine papaya seed. These results implied that waste-stream containing residual alcohol from synthetic processes could potentially be used to prepared fruit waste sorbents for color removal and subsequent regeneration.
Discussion
Envelope protocol
The envelope protocol used does not produce results that deviate from previous results reported in literature in terms of linear equilibrium isotherms and kinetics expression fits (Citation19, Citation39–Citation42). Thus the protocol can potentially be used for convenient screening and evaluation of sorbents both on a small laboratory scale and larger field trials. It may offer advantages of ease of handling and transportation in larger scale sorption evaluation. The column set-up is attractive due to its efficient used of material and influent. However for the use of fruit rinds containing thickening or gelling polysaccharides like C. grandis rinds, there would be practical problems in the scaling up of the sorption process. The use of the envelope system partially alleviates these problems whilst at the same time facilitating large scale handling and transportation. Shaker-bath based free sorbent sorption processes would be difficult to scale-up and hence lack practical utility. Either the column or the envelope system is expected to have better scale-up potential. The envelope system would be more manageable in distributed rural areas of developing countries. It is also useful for reutilization of fruit wastes generated in the household, dispersed fruit stalls and juice manufacturing.
Sorbent characterization
As agro-waste like C. grandis rinds and C. papaya seeds were susceptible to decay, methods to determine quality or state of sorbents would be useful. To our knowledge, this is the first time MAS NMR for pomelo rind and papaya seeds were presented. Traditional techniques of solid sorbent characterization included elemental analysis, particle size and surface area analysis and FTIR spectroscopy. To our knowledge, solid-state NMR had been less extensively employed in sorption studies. 13C-MAS NMR was reported for metallic sorption in a previous study (Citation39). It was shown that 1H HR-MAS NMR could differentiate the two sorbents under study more distinctively than FTIR techniques and was able to provide useful information on chemical groups participating in sorption. Key benefits of using MAS NMR techniques are that dried biomass samples can be probed directly and less than 500 mg sample are required (Citation41).
The results of higher MB sorption can be explained as follows. Since MB has three N-sites and is relatively planar compared to BG dye with two N-sites and trigonal about the central C to which the three aromatic rings are directly attached. MB dye could potentially adhere onto surfaces like a plaster. Furthermore both MB and BG dyes are cationic dyes and are expected to have affinity for negatively charged surfaces or surfaces possessing electron-donating groups or function of Lewis base character.
Equilibrium and kinetics modeling
Langmuir and Freundlich isotherms had been widely reported in sorption studies to fit systems involving fruit and vegetable wastes to remove cationic dyes and metals in aqueous systems. Pseudo-second-order kinetics and intra-particle diffusion models had also been successfully applied to such systems. As modeling results obtained for the proposed envelope protocol also suggested similar fits, it is considered that future research efforts should be focussed on practical means to effectively transfer sorption techniques from the bench-top to the field. One such practical approach would be cleaning and preparation of sorbent material in columns with subsequent packing in thins envelopes for sorption. Pre-packed sorbent could be conveniently used in recovery operations, storage for further bioconversion, and eventually for disposal. Sorbate or sorbent recovery could be performed both in columns and envelope systems. Comparison of inorganic and organic wash liquors in desorption cycles, sorption of single and multi-case envelope systems using anionic dye model and laboratory-constituted textile dye effluent are potential aspects to be studied. Although linear correlation fit data suggested Langmuir isotherm as the best fit, other isotherm conditions could also apply. For example, good but not the best linear correlation for the Freundlich isotherm may suggest multi-modal adsorption mechanisms. This observation is consistent with FTIR and NMR spectroscopic data. The presence of various function groups in natural polymers could be responsible for the sorption affinities. A practical scheme has been developed in this study for both laboratory evaluation of sorbents and field study protocols for colored wastewater remediation with simple and readily available apparatus and instruments.
Conclusions
Sorbents derived from papaya seeds showed better sorption capacity towards cationic dyes than pomelo rind both in this study. pH and COD determinations of the wash effluents used for sorbent cleaning served as good indicators of sorbent quality. pHpzc measurements could be used to characterize different classes of sorbent like citrus vs. non-citrus based sorbents.
A practical framework to utilize abundant solid bio-wastes like rinds and seeds in the envelope systems was developed. This scheme can be used to prepare sorbents for bulk removal of dyes from dye-house wastewater with the convenience of handling in the field. A suitable laboratory protocol which can be used to rapidly evaluate diverse types of biomaterials for use as sorbents is proposed. Since waste materials may not be available in the right quantity and quality at any time, the simple and expeditious laboratory protocol develop in this study would be useful in such situations to determine the performance of various materials and blends. Low-cost sorbents based on fruit rinds and seeds can be employed as initial stage bulk removal of dye pollutants thereby extending the service life-time of membrane filters or activated carbon with additional benefits of solid waste re-utilization.
The envelope protocol shortened sorption experiments by saving a separation step in determining sorbate concentration in solutions and facilitate desorption studies immediately after sorption in a similar way as column set-ups. This would allow ease of comparative studies of the wide array of waste sorbents for a targeted wastewater stream. Schweier and co-workers (Citation26) studied extensively the use of orange rind for sorption of divalent metal ions, including desorption of Cd (II) from orange rind. It may not be economically attractive to recover inexpensive dyes compared to more expensive metals like platinum or gold-based catalysts. Nevertheless, desorption studies could shed light on the reversibility of the sorption process.
MAS NMR was found to be useful to probe more deeply into the molecular interactions involved in sorption. This tool could be further harnessed to profile the vast varieties of agricultural wastes for environmental remediation through sorption. This tool had not only been utilized for sorption of humic substances by soil particles (Citation29) but also assessment of compost maturity (Citation43). These studies use 13C NMR for characterization of sorption of organic compounds by plant materials (Citation44,Citation45). Thus the potential of its applicability in sorption or “sorption-omics” to develop predictive sorbent–sorbate models can be envisaged. The results obtained in this study also show that more sophisticated instruments like solid-state NMR could be useful for sorption research.
Supplemental Material
Download MS Word (194.8 KB)Acknowledgements
The authors thank Dr Ji’En Wu and Mdm Yan Hui Han for the MAS NMR scans.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
M. Q. Ho appreciates her mother’s support in the harvest of fruit waste samples for her research project.May Quan Ho has been a part-time graduate research student at the Department of Chemistry, National University of Singapore. She is now working in product stewardship and regulatory affairs at a chemical multinational company.
S. F. Y. Li is a faculty member at the Department of Chemistry, National University of Singapore. He received his B.Sc., Ph.D. and DSc degrees from Imperial College, UK. His research interests include biosensors, capillary electrophoresis, metabolomics, environmental science and technology, photocatalysis, microbial fuel cells and nanomaterials. He has authored/co-authored 359 publications in international peer review journals. He serves/served on editorial advisory boards of several international scientific journals, including Electrophoresis, Analytical Sciences and Journal of Chromatographic Science.
ORCID
S. F. Y. Li http://orcid.org/0000-0002-2092-9226
Additional information
Funding
References
- Zümriye, A. Process Biochem. 2005, 40, 997–1026.
- Mahwish, A. Water Air Soil Pollut. 2012, 223, 2417–2435.
- Bhatnagr, A.; Silanaä, M. Chem. Eng. J. 2010, 157, 277–296.
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Desalination 2011, 280, 1–13.
- Mahmound, D.K.; Salleh, M.A.M.; Karim, W.A.W.A. J. Purity Util React Environ. 2012, 1, 170–199.
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. J. Hazard. Mater. 2010, 177 (1–3), 70–80.
- Sanghi, R.; Verma, P. Color. Technol. 2013, 129, 1–24.
- Patel, S. Rev. Environ. Sci. Bio/Tech. 2012, 11, 365–380.
- Sharma, P.; Kaur, H.; Sharma, M.; Sahore, V. Environ. Monit. Assess. 2011, 183, 151–195.
- Chan, T.; Heu, A. J. Food Sci. 1978, 43, 255–256.
- Passera, C.; Spettoli, P. Qual. Plant. Plant Foods Hum. Nutr. 1981, 3, 77–83.
- Marfo, E.K.; Oke, O.L.; Afolabi, O.A. Food Chem. 1986, 22, 259–266.
- Hameed, B.H. J. Hazard. Mater. 2009, 162 (2–3), 939–944.
- Unuabonah, E.I.; Adie, G.U.; Onah, L.O.; Adeyemi, O.G. Chem. Eng. J. 2009, 155 (3), 567–579.
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Coll. Surf. A Physicochem. Eng. Asp. 2008, 316 (1), 78–84.
- Zeng, J.; Zhu, H.; Kong, J. Adv. Mat. Res. 2013, 634–638, 178–181.
- Pichaiyongvongdee, S.; Haruenkit, R. Kasetsart J. Nat. Sci. 2009, 43 (1), 28–36.
- Roux, J.-C.; Fourest, E.; Sahut, C. In Biotechnology for Waste Management and Site Restoration; Ronneau, C., Bitchaeva, O., Eds.; Kluwer Academic Publishers: Dordrecht, 1997; pp 141–146.
- Balaria, A.; Schiewer, S. Sep. Purif. Technol. 2008, 63 (3), 577–581.
- Naja, G.; Volesky, B. Coll. Surf. A Physicochem. Eng. Asp. 2008, 281 (1–3), 194–201.
- Tamez Uddin, M.; Rukanuzzaman, M.; Maksudaur Rahman Khan, M.; Akhtarul Islam, M. J. Environ. Manag. 2009, 90 (11), 3443–3450.
- Volesky, B. Sorption and Biosorption, BV Sorbex: Montreal, 2004; pp 103–116.
- Chong, K.H.; Volesky, B. Biotechnol. Bioeng. 1996, 1996 (49), 629–638.
- Fiol, N.; Villaescusa, I. Environ. Chem. Lett. 2009, 7 (1), 79–84.
- Contreras, E.; Sepúlveda, L.; Palma, C. Int. J. Chem. Eng. 2012, 2012. DOI: 10.1155/2012/679352.
- Schiewer, S.; Iqbal, M. J. Hazard. Mater. 2010, 177 (1–3), 899–907.
- Munusamy, K.; Somani, R.S.; Bajaj, H.C. BioResources 2011, 6 (1), 537–551.
- Cao, X.; Pignatello, J.; Li, Y.; Lattao, C.; Campbell, M.A.; Chen, N.; Miller, L.F.; Mao, J. Energ. Fuels 2012, 26, 5983–5991.
- Francioso, O.; Ferrari, E.; Saladini, M.; Montecchio, D.; Gioacchini, P.; Ciavatta, C. J. Hazard. Mater. 2007, 149, 408–417.
- Ho, Y.S.; McKay, G. Can. J. Chem. Eng. 1998, 76 (4), 822–827.
- Ho, Y.S.; McKay, G. Chem. Eng. J. 1998, 70, 115–124.
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Vaghetti, J.C.P.; Simon, N.M.; Calvete, T.; Veses, R.C. J. Hazard. Mater. 2009, 164 (2–3), 1213–1222.
- Pavan, F.A.; Gushikem, Y.; Mazzocato, A.C.; Dias, S.L.P.; Lima, E.C. Dyes Pigm. 2007, 72 (2), 256–266.
- Pavan, F.A.; Lima, E.C.; Dias, S.L.P.; Mazzocato, A.C. J. Hazard. Mater. 2008, 150 (3), 703–712.
- Asgher, M.; Bhatti, H.N. Ecol. Eng. 2012, 38 (1), 79–85.
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I.; Kumar, R. Clean Soil Air Water 2010, 38 (5–6), 437–445.
- Mahmoud, D.K.; Salleh, M.A.; Karim, W.A.; Idris, A.; Abidin, Z.Z. Chem. Eng. J. 2012, 181, 449–457.
- Vijayaraghavan, K.; Planivelu, K.; Velan, M. Bioresour. Technol. 2006, 97 (2), 1411–1419.
- Ho, Y.S.; and McKay, G. Trans. IChemE. 1998, 76, 313–318.
- Ho, Y.S.; McKay, G. Process Saf. Environ. Prot. 1998, 76 (2), 183–191.
- Ho, Y.S.; McKay, G. Process Biochem. 1999, 34 (5), 451–465.
- Vučurović, V.M.; Razmovski, R.N.; Tekić, M.N. J. Taiwan Inst. Chem. Eng. 2012, 43 (1), 108–111.
- Kumar, D.S.; Kumar, P.S.; Rajendran, N.M.; Anbuganapathi, G. J. Agric. Food Chem. 2013, 61, 11326–11331.
- Chen, B.; Johnson, E.J.; Chefetz, B.; Zhu, L.; Xing, B. Environ. Sci. Technol. 2005, 39 (16), 6138–6146.
- Shechter, M.; Xing, B.; Kopinke, F.D.; Chefetz, B. J. Agric. Food Chem. 2006, 54 (20), 7761–7768.