?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effect of Olive leaf as a corrosion inhibitor for C-steel in 10% Sulfamic acid (NH2SO3H) solution was tested utilizing different techniques. The effect of temperature (298–328 K) was determined and activation and adsorption thermodynamic parameters were counted and discussed. The surface morphology was analyzed utilizing a scanning electron microscope (SEM). Potentiodynamic polarization procedures evidenced that Olive leaf acts as mixed-type inhibitor for corrosion of C-steel in 10% NH2SO3H solution. The addition of Olive leaf produces an increase in the charge transfer resistance (Rct) and reduction in capacitance of the double layer (Cdl). The inhibition mechanism involves physisorption. The adsorption of this extract on C-steel surface follows Langmuir isotherm. All the studied techniques gave similar results.
GRAPHICAL ABSTRACT
1. Introduction
C-steel is a metal applied in several industrial objectives, such as industrialization of equipment for oil, fertilizers, and others. It is important to prevent the corrosion of C-steel by utilizing inhibitors. Organic compounds can be used for this purpose as it contains functional groups of O, N, P, S atoms, but it is considered danger and not safe environmentally (Citation1). Plant extracts are “Green corrosion inhibitors” which are biodegradable, do not contain heavy metals or other toxic elements, renewable nature extracted source and have the perfect action of C-steel inhibition in corrosive media. This is reported by many research groups (Citation2). Several authors tried to use plant extracts as green corrosion inhibitors (Citation3–9).
The present research will examine the inhibitory performance of Olive leaf extract for C-steel in 10% NH2SO3H as a corrosive medium, that is because Olive leaf is considered as a sustainable source, biodegradable, and less expensive than other green inhibitors. Sulfamic acid is chosen as a corrosive medium for C-steel because it is widely used in the industry.
1.1. Plant extract preparation
Olive leaf was dried at room temperature away from the sun and greatly milled utilizing an electrical mill. 10 gm of the powder was dissolved by b-idistilled water and put on a hot plate, then after cooling, the solution was filtrated. In order to determine the concentration of the dissolved part, 10 ml filtrate with 5 ml ethyl alcohol was put in a flask of evaporating purpose. After evaporating the solvent, the remainder substance weights are the same as the equivalent weight dissolved 10 ml filtrate. By this way, the desired concentration of the extract solution was obtained (Citation10).
2. Experimental
2.1. Solutions
The aggressive solution utilized is Sulfamic acid (10% NH2SO3H) which was obtained from analytical reagent degree Sulfamic acid 99.8% by dilution with b-idistilled water. The needed concentrations were obtained from the stock solution by dilution.
2.3. Weight difference test
This test was carried out utilizing six identical C-steel pieces with dimensions of (2 × 2 × 0.2) cm, which were perfectly scraped with various degrees of emery papers. Acetone and bi-distilled water were utilized for cleaning and washing of the pieces, thereafter their weights were determined utilizing a sensitive balance. The pieces were suspended in solutions of 100 ml of 10% NH2SO3H in the absence and existence of various extract concentrations. Through a period of 30 min, the pieces were taken out, washed, dried, and weighted again through 3 h, at different temperatures (298–328 K) (Citation11,Citation12). Surface coverage (θ) and inhibition competence (IE %) can be accounted utilizing the following equation:(1)
(1) where W is the weight difference in the existence of extract and W° is the weight difference in the absence of extract. Corrosion rates (k) were calculated utilizing the following equation:
(2)
(2)
where t is the time, min and A is the species area, cm2 (Citation13).
2.4. Electrochemical techniques
Electrochemical estimations were done utilizing the cell of (a) working electrode consists of C-steel solder with Cu-wire to conduct electricity and mounted into a glass tube of suitable diameter to make the contact area of C-steel electrode 1 cm2. The working electrode is scraped as explained before. (b) Saturated calomel electrode, SCE (reference electrode). All potential values were registered vs. SCE. (c) Platinum foil (1 cm2) is the auxiliary electrode. The measurements were done utilizing Gamry instrument Potentiostat /Galvanostat, ZRA(PCI4-G750) which consists of DC105, EIS300, and EFM140, connecting with a computer to collect and analyze the data utilizing Echem analyst V.6.03.
Initially, C-steel electrode potential was measured against time for 1 h to obtain open circuit potential (OCP) and the steady state.
Potentiodynamic polarization tests were conducted by applying a potential range (−1200 mV to + 1200 mV) to obtain corrosion current densities (icorr). Values of corrosion current densities (icorr) and corrosion potential (Ecorr.) were attained by extrapolating of two Tafel area. IE % and θ from potentiodynamic polarization technique were accounted utilizing the following equation:(3)
(3) where icorr is the corrosion current density in the existence of extract and i°corr is the corrosion current densities in the absence of extract.
Tests of electrochemical impedance spectroscopy (EIS) were done utilizing Ac signals through the frequency extent (0.1 Hz–100 kHz) and 5 mV peak to peak capacity. Analyzing and explaining of outcomes from EIS depend on the utilized equivalent circuit. (θ) and (IE %) were attained by the following equation.(4)
(4) where Rct is the charge transfer resistance in the existence of extract and R°ct is the charge transfer resistance in the absence of extract (Citation14).
Tests of electrochemical frequency modulation (EFM) were achieved utilizing 2 and 5 Hz frequencies. The waveform was found to repeat every 1 s because the main frequency was 0.1 Hz. The spectra include current responses connected with harmonically and intermodulation current peak. Values of Tafel slopes (βc and βa), (icorr.), and the causality factors (CF-2 and CF-3) were acquired utilizing the large peaks (Citation15,Citation16).
2.5. Surface check
The metal surface was analyzed, after 24 h in 10% NH2SO3H with and without the extract, utilizing a scanning electron microscope (SEM, JSM-T20, Japan).
3. Outcomes and debate
3.1. Weight difference (ΔW) test
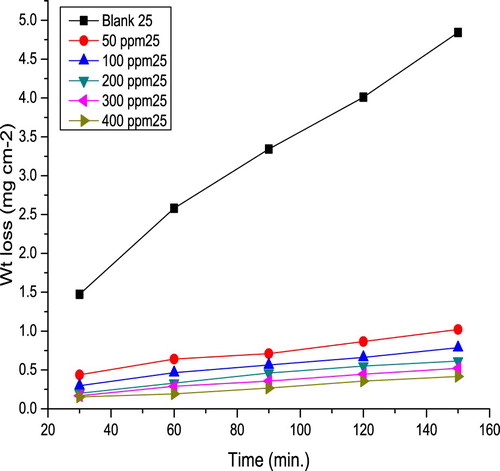
displays ΔW against time for C-steel in 10% NH2SO3H solutions in the absence and existence of various extract concentrations at 298 K. It is clear that the curves with the extract are significantly below than that without the extract. This indicated that the extract molecules are adsorbed on C-steel surface forming a film which reduces the correction rate.
3.2. Temperature influence
The temperature influence on the metal corrosion inhibition in acid media is very complex (Citation17), due to the surface changes, for example, desorption or decomposition of the inhibitor molecules. Impact of temperature on IE% was examined utilizing weight loss test at (298–328 K). shows corrosion rate (k) against temperature in the absence and existence of various extract concentrations. It is evident that k is inversely proportional to the extract concentration while directly proportional to temperature. Values of k and IE% are shown in . Results showed that k values were lower in the presence of the extract compared to the blank solution. Also, IE% increases with an increase in the extract concentration until it reaches 89.6. This result might be due to the fact that the amount of adsorption and the metal surface coverage increase with an increase in the inhibitor concentration (Citation18). IE% decreased with an increasing temperature, indicating that at higher temperatures, dissolution of the metal predominated on extract adsorption (Citation19,Citation20).
Figure 2. Corrosion rate against temperature in the absence and existence of various extract concentrations.
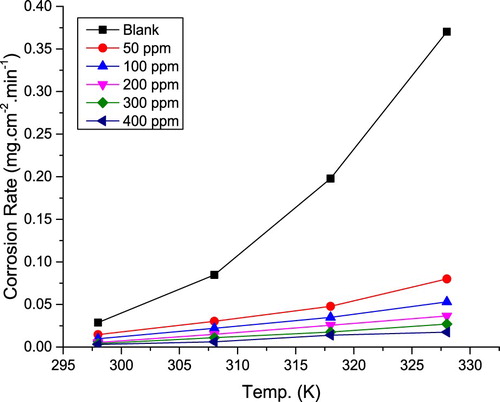
Table 2. Calculated value of k, IE% for C-steel in 10%NH2SO3H without and with Olive leaf extract at (298–328 K).
The relationship of k with T can be shown in the following equation (Citation21):(5)
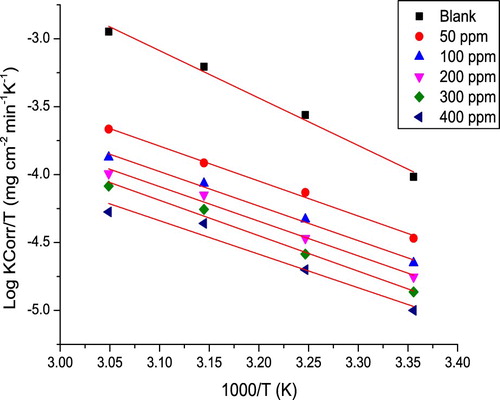
(5) where E*a is the activation energy, T is the absolute temperature (K), R is the universal gas constant (joule/k.mole), and A is the frequency factor. Plots of log k of C-steel in 10% NH2SO3H against 1/T without and with various extract concentrations are shown in .
Figure 3. Plots of log k against 1000/T for C-steel in 10% NH2SO3H in the absence and existence of various extract concentrations.
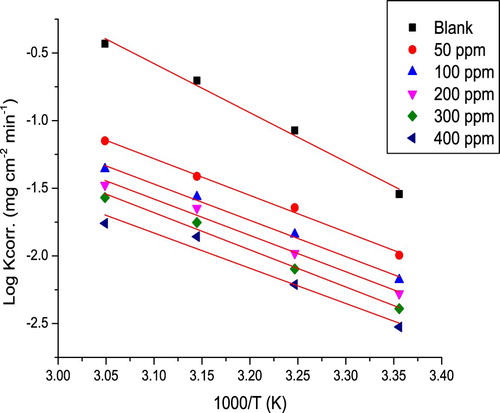
It is obvious from E*a values (in ) that as the extract concentration increases, the value of E*a increases, which points that at larger extract concentration, the corrosion reaction will be more pushed to the surface sites with higher E*a values (Citation22). Meaning that a barrier layer is formed through the extract adsorption on the surface of C-steel, this layer impedes the metal corrosion reactions (Citation23), explaining that the increase in E*a is due to the reduction in the adsorption of the extract as the temperature increases. Lower adsorption means more desorption, thus large surface area of C-steel connects with the acid medium, prompting the rise of corrosion rates. But this trouble not present at high extract concentration due to the reduction in surface coverage that occurs when close to saturation (Citation24). Higher E*a values in the existence of the extract show physical adsorption (electrostatic interaction) (Citation25), actually extract molecule with heteroatom forms cation in acid solution, thus the electrostatic adsorption of the cation gives perfect protective characteristics of the extract.
Table 3. Activation parameters for C-steel in 10% NH2SO3H in the absence and existence of various extract concentrations.
The enthalpy of activation change (ΔH*) and entropy of activation change (ΔS*) were calculated by plotting log k/T versus 1/T () according to the following equation (Citation26):(6)
(6) where h is Planck’s constant and N is Avogadro’s number. This relationship results in straight lines, where ΔH* is obtained from slopes and ΔS* from the intercepts. ΔH* and ΔS* values are displayed in . ΔH* has a positive sign, which points that C-steel dissolution is endothermic and slow (Citation27). ΔS* has a negative sign, which reveals that the activated complex performs an association instead of dissociation, signals that from reactants to the activated complex the decrease in disorder occurs (Citation28).
3.3. Adsorption isotherms
Adsorption is considered the first step in inhibition process. The adsorption relies on inhibitor structure, absolute temperature and electrochemical potential at the interface of metal/solution. So the adsorption procedure can be considered as the aquasi-exchange between organic compound in solution [Org (Sol.)] and adsorbed water particle [H2O(ads.)] (Citation29).(7)
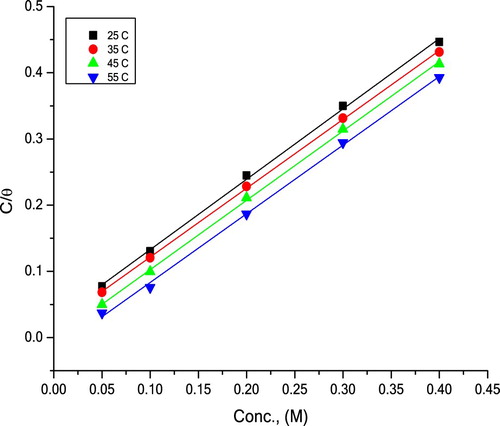
(7) where x is the number of replaced water molecule. The interaction between extract and the metal surface can be demonstrated utilizing adsorption isotherms. Among different isotherms such as Langmuir, Temkin, Frumkin and Flory-Huggins, the ideal fit was Langmuir. This isotherm was utilized for other inhibitors (Citation30,Citation31). Langmuir isotherm condition is as per the following:
(8)
(8) where Kads is the equilibrium constant. displays plots of C/θ versus C at various temperatures, from which linear relationships are acquired with slopes almost unity, which indicate that Langmuir isotherm is good obeyed in our study.
The values of Gibbs free energy (ΔG°) were accounted utilizing the following equation:(9)
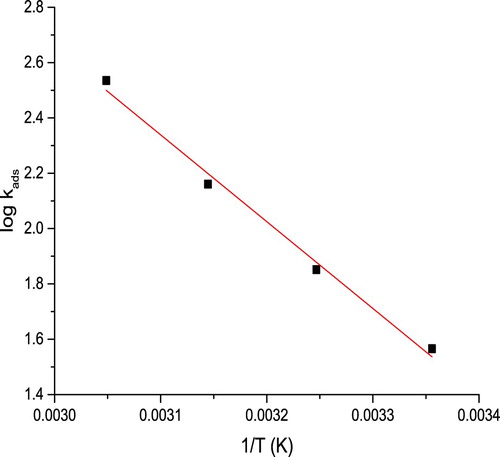
(9) where 55.5 is the water molarity in the solution. Straight line is acquired from plotting log Kads versus 1/T (), as the slope equals enthalpy of adsorption (ΔH°ads) according to the following equation:
(10)
(10)
Adsorption parameters for C-steel in 10% NH2SO3H with the extract are grouped in . It is spotted that estimations of Kads are high, which indicates stronger extract adsorption on the metal surface because of the existence of heteroatom and π-electrons in the various molecules present in the extract. The inhibition process occurs by these various molecules at different contents, i.e. mixture of inhibitors from major to minor components, higher inhibition efficiency (Citation32) is due to the intermolecular synergistic effect of these various molecules in the extract.
Table 4. Langmuir adsorption isotherm results for C-steel in 10% NH2SO3H with the extract at various temperatures.
ΔG°ads have negative values that signalize spontaneous adsorption process. In general, the estimations of ΔG°ads until −20 kJ mol−1, demonstrate physisorption process, while those about −40 kJ mol−1 or more, show chemisorption process as an outcome of sharing or transmit of electrons from molecule on metal surface (Citation33). As evident here, ΔG°ads estimations were in extent of −19 and −27 kJmol−1, suggesting that the adsorption mechanism collected physisorption and chemisorption, together (Citation34), anyway, the primary contributor to the adsorption mechanism was physisorption whereas chemisorption just a little contributed. The positive sign of ΔH°ads indicates endothermic adsorption of extract molecule on C-steel.
Entropy of adsorption (ΔS°ads) was deduced from the following equation (Citation35):(11)
(11) Estimations of ΔS°ads (in ) were positive, indicating that the entropy increases through the adsorption process, due to the desorbed solvent molecules from the C-steel surface. So the disorder of the system increases.
3.4. Open circuit potential (OCP)
shows impact of various Olive leaf extract concentrations on OCP of C-steel in aerated non-stirred 10% NH2SO3H solution at 298 K. OCP was discovered to be high negative than it at t = 0, indicating that the oxide film from air that formed before immersion must dissolve before the steady state is attained (Citation36). This potential (Ecorr.), attained through nearly 10 min, indicates the free bare metal corrosion (Citation37). Obviously (Ecorr.) becomes more negatively without variation of the public feature of E/t curve.
Figure 7. OCP for C-steel in 10% NH2SO3H solutions in the absence and existence of various extract concentrations.
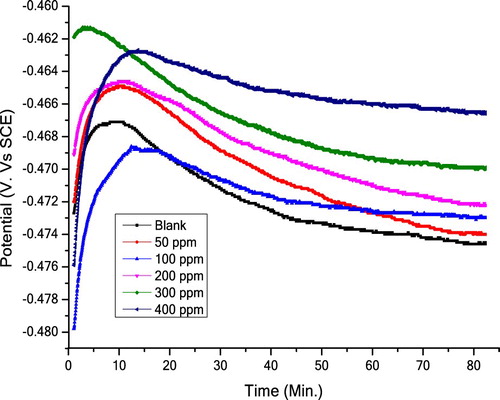
Furthermore, increasing of extract concentration shifts the OCP, initially to low negative values (because of corrosion inhibition) reaching a maximum, then at definite time, depends on the extract concentration, the potential decreases until a steady value (because of metal dissolution). This will move the OCP towards more negative values. These results revealed that Olive leaves inhibit C-steel corrosion in 10% NH2SO3H solutions efficiently.
3.5. Potentiodynamic polarization tests
includes plots from potentiodynamic polarization tests of C-steel in 10% NH2SO3H in the absence and existence of various extract concentrations. IE% values from polarization tests were counted utilizing Equation (3). Electrochemical parameters related with polarization tests (icorr, Ecorr, Tafel slopes (βa, βc) and IE %) are shown in .
Figure 8. Curves from potentiodynamic polarization tests for C-steel in 10% NH2SO3H solutions in the absence and existence of various extract concentrations.
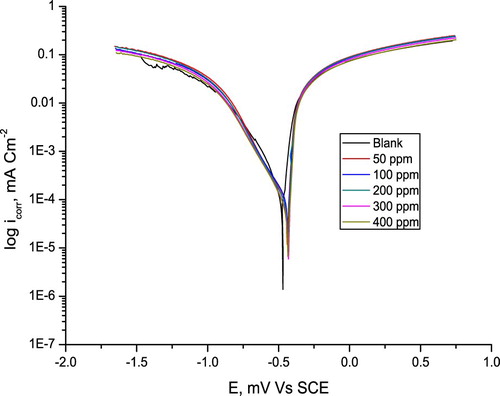
Table 5. Electrochemical parameters that related with polarization tests (icorr, Ecorr, βa, βc and IE %) for C-steel corrosion in 10% NH2SO3H solutions in the absence and existence of various extract concentrations.
It is known that iron dissolution in acid occurs spontaneously according to the following equation:(12)
(12)
Joined by the following cathodic equation:(13)
(13)
Anodic and cathodic Tafel sections indicate a bend over the entire utilized potential range. The anode bend may be because of a non-passive film produced from the products from corrosion or the deposited impurities on C-steel (for example, Fe3C). For cathodic section, stationary solution makes the diffusion of H+ slow, and concentration polarization works to diminish the area of cathodic linear.
Since acidic solution is aerated 10% NH2SO3H solution, the solution will contain two cathodic reactions (evolution of hydrogen and reduction of dissolved oxygen) with the anodic reaction (metal dissolution). The complicated cathodic part may show no linearity or show short Tafel area as shown here (Citation38). So, we depend on the software to obtain the Tafel slopes and icorr values although we do not trust it much. Like polarization, EIS cannot be used to determine icorr values. Unlike polarization, EFM is a perfect method to obtain the values of Tafel slopes and icorr.
It is clear that, as the extract concentration increases, (Ecorr) becomes more positive and (icorr.) decreases. The IE % increases with the increasing extract concentrations, until reaching 77.2. This shows that the extract is a perfect corrosion inhibitor. The inhibiting influence of the extract may be concerning on adsorption on the steel surface to form a barrier layer. Based on the results, it is found that the extract limits the disintegration of C-steel, also delays hydrogen evolution reaction. This referred that the extract molecules absorb upon both anodic and cathodic sites of the metal surface, thus the extract could be classified as a mixed type inhibitor (Citation39).
Values of Tafel slopes are slightly changed at the addition of extract, this result reveals that Olive leaf is efficacy mixed type green corrosion inhibitor. The process of inhibition does not change the mechanism of corrosion, only deactivates a fraction of the surface in the destructive solution (Citation40). IE% values were found to increase by increasing the extract concentration.
3.6. EIS tests
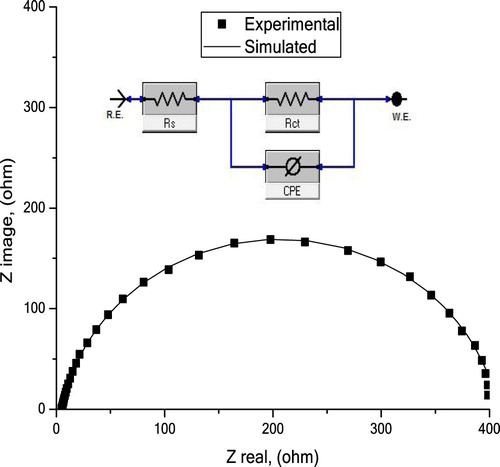
represents (CPE) an equivalent circuit was suggested for corrosion of C-steel electrode in 10% NH2SO3H solutions. Where Rs is the resistance of solution, Rct is the resistance of charge transfer, and CPE is the constant phase element, utilized to give further exact fit and determine (Cdl) the capacitance double layer (Citation41). The semicircle appears due to the stationary time of charge-transfer and double layer capacitance (Citation42). This semicircle produces an angle about 68.5° with an actual axis and affords 4.8 Ω cm2 through its intersection for the resistance of the solution (Rs) found between reference and working electrodes.
Figure 9. Impedance curve together with the equivalent circuit utilizing for stimulation the registered EIS data for C-steel in 10% NH2SO3H solution at 298 K.
It is obvious from Nyquist plots () with and without Olive leaf extract, the shapes of the plots do not change indicating that, as obtained from polarization tests, the existence of extract does not change the mechanism of the corrosion reaction. The same results were obtained in past studies (Citation43). (Rs + Rct) values were obtained from the intersection point between semicircle and actual axis. The greatest frequency (fmax) of the maximal imaginary ingredient of the impedance was applied in the following equation to obtain Cdl.(14)
(14) IE% values from EIS tests were attained by Rct values utilizing Equation (4). Through the C-steel corrosion in 10% NH2SO3H solutions, the surface roughness increases. The obtained EIS parameters for C-steel corrosion in 10% NH2SO3H solutions in the absence and existence of various extract concentrations at 298 K are given in .
Figure 10. Plots from EIS tests for C-steel corrosion in 10% NH2SO3H solutions in the absence and existence of various extract concentrations at 298°K.
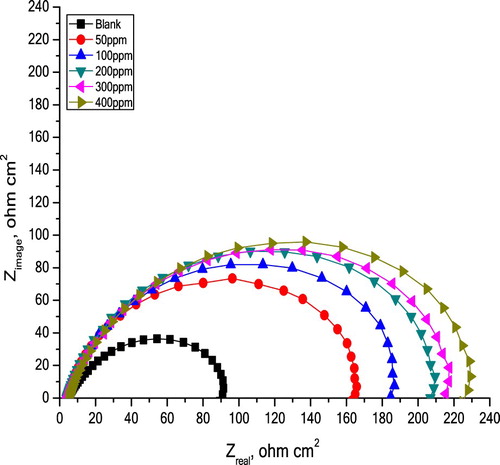
Table 6. Counted values of impedance parameter (Rs, Rct, Cdl and IE%) related to impedance measurements for corrosion of C-steel in 10% NH2SO3H in the absence and existence of various extract concentrations.
The Rct values are observed to increase as the extract concentration increases, while Cdl values diminish. In reality, capacitance is oppositely proportional to the double layer thickness (Citation44). Capacitance might decrease by supplantation of water particles at the electrode interface by extract molecules. As the extract concentration increases, the capacitance decreases by increasing the double layer thickness, since more protonated molecules will be electrostatically adsorbed on the negative electrode surface, which produce a salient reduction in Cdl, this is in accordance with Helmholtz model, given by the following equation:(15)
(15) where d is the double layer thickness, ε is the dielectric constant, ε° is the vacuum permittivity and A is the effective surface area.
Results in illustrate that the inhibition efficiency increases as the extract concentration increases. This outcome assures the results obtained from the previous measurements and confirms that Olive leaf extract is efficacy green corrosion inhibitor.
3.7. EFM tests
EFM is a small signal ac method that utilizes two sine waves (at various frequencies) with one another to cell. Without period information of Tafel constant, EFM immediately gives estimations of the corrosion current.
In EFM tests, the current response to potential is non-linear. The current response includes two enter frequencies, and its ingredients (total, difference, and multiples). The two enter frequencies must be small, integer multiples of an main frequency which is responsible for the experiment length. shows the current response as a function of frequency, as acquired from EFM tests. IE% was calculated from Equation (3). The parameters gained from the EFM method (IE %, icorr, βa, βc and causality factors (CF-2 and CF-3)) at various concentrations of the extract, are seen in .
Figure 11. Current response with the frequency for C-steel in 10% NH2SO3H in the absence and existence of various extract concentrations.
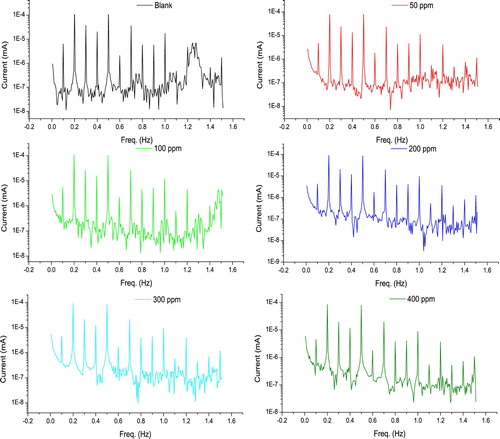
Table 7. The outcomes gained from EFM method (IE %, icorr, βa, βc, CF-2 and CF-3) for C-steel electrode in 10% NH2SO3H in the absence and existence of various extract concentrations.
The large quality of EFM comes from causality factor that utilizes as an inward test on the rightness of the EFM tests (Citation45). As illustrated in , causality factors are compatible with theoretical values, that is 2.0 and 3.0 for CF-2 and CF-3, respectively, which confirms the rightness of Tafel slopes and icorr. It is apparent that the outcomes acquired from all utilized tests were in large agreement with each other.
3.8. SEM analysis
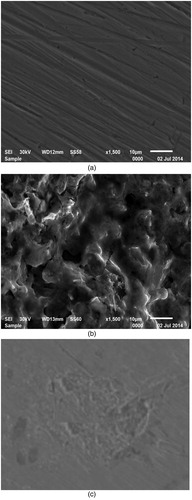
(a–c) shows SEM micrographs for surface of C-steel prior and after its putting in aerated 10% NH2SO3H solution without and with the addition of 400 ppm of Olive leaf. As expected, (a) indicates metallic surface is evident, whereas in (b), without extract, the metallic surface is deteriorated by aerated 10% NH2SO3H solution, while in (c), with extract, the metallic surface is not affected by acid corrosion.
Figure 12 . SEM micrographs of C-steel (a) before exposure to aerated 10% NH2SO3H solution (b) after 24 h. immersion in aerated 10% NH2SO3H solution (c) after 24 h. of immersion in aerated 10% NH2SO3H + 400 ppm of Olive leaf. All at 298 K.
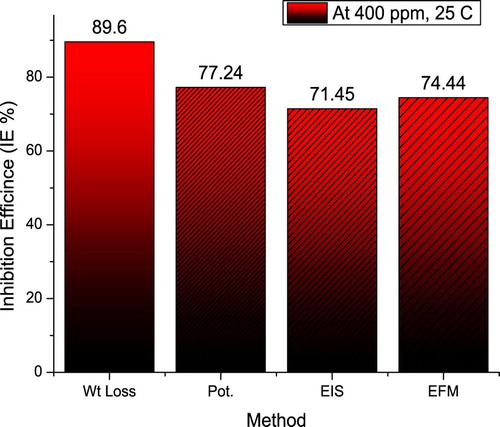
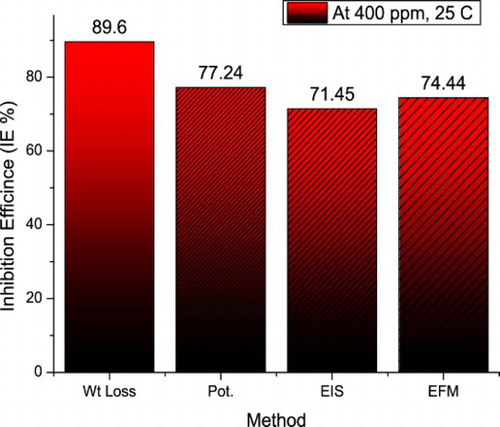
shows a comparison of the inhibition efficiencies derived from different investigated corrosion estimations methods. As shown in the figure, the different methods gave approximately similar results. This confirms the valid obtained results.
3.9. Inhibition mechanism
From experiment results, we notice that there is a logical inhibition mechanism for corrosion operation of C-steel in 10%NH2SO3H solutions by Olive leaf as a green corrosion inhibitor, may be delineated on the adsorption foundation. Inhibitor structure, charged metal surface, and charge dispersion along the inhibitor molecule, all affected the adsorption operation. The adsorption can be depicted through two essential kinds of reaction: physisorption and chemisorption. As a rule, physisorption procedure needs the presence of charged metal surface (steel in acid medium is positively charged (Citation46)) and species with charge in the bulk of solution (protonation of some extract components). The species of anionic acid first adsorbed on steel surface form negatively charged surface, and after that, the protonated species get adsorbed on it. Chemisorption procedure includes sharing or transferring of charge from extract molecule to the surface of the metal, to form a coordinate bond. The existence of transition metal owns electron orbital that is unoccupied and has low energy, and extract molecules contain free electrons or heteroatoms (such as N, O, S) own a lone pair of electrons that is essential (Citation47).
4. Conclusions
Weight loss, OCP, Tafel, EIS, EFM, and SEM, were utilized to check the inhibitory action of Olive leaf extract on corrosion of C-steel in 10% NH2SO3H solutions. The principal conclusions were
IE% values increase as the Olive leaf extract concentration increases.
OCP becomes more positive as the Olive leaf extract concentration increases.
Increasing in Olive leaf concentration leads to decease in icorr.
Rct values increase with increasing of Olive leaf concentration, while Cdl decreases.
Physisorption is suggested as a corrosion mechanism.
IE% acquired from chemical and all electrochemical tests were concurrent with each other.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
H.M. Elabbasy currently works as lecturer of chemistry in essential sciences department at Misr higher Institute of Engineering and Technology, Mansoura, Egypt. Main research interests are in corrosion inhibition of metals and alloys using some synthesis organic compounds and green corrosion inhibitors.
A. S. Fouda currently works at Mansoura University, Mansoura, Egypt. He is professor of physical chemistry at chemistry department (College of Science). Their current project is ‘synthesis and characterization of some organic compounds as corrosion inhibitors’.
ORCID
A. S. Fouda http://orcid.org/0000-0002-3239-4417
References
- Beenakumari, K.S. Inhibitory Effects of Murraya Koenigii (Curry Leaf) Leaf Extract on the Corrosion of Mild Steel in 1 M HCl. Green Chem. Lett. Rev. 2011, 4 (2), 117–120.
- Sharma, S.K.; Mudhoo, A.; Jain, G.; Sharma, J. Inhibitory Effects of Ocimum Tenuiflorum (Tulsi) on the Corrosion of Zinc in Sulphuric Acid: A Green Approach. Rasayan J. Chem. 2009, 2 (2), 332–339.
- Fouda, A.S.; Shalabi, K.; Nofal, A.M.; El-zekred, M.A. Methanol Extract of Rumex Vesicarius L.as Eco-Friendly Corrosion Inhibitor for Carbon Steel in Sulfuric Acid Solution. Chem. Sci. Trans. 2018, 7 (1), 101–111.
- Fouda, A.S.; Abdel Haleemb, E. Berry Leaves Extract as Green Effective Corrosion Inhibitor for Cu in Nitric Acid Solutions. Surf. Eng. Appl. Electrochem. 2018, 54 (5), 498–507.
- Fouda, A.S.; Rashwanb, S.M.; Darwishb, M.M.K.; Armana, N.M. Corrosion Inhibition of Zn in a 0.5 M HCl Solution by Ailanthus Altissima Extract, Portugaliae. Electrochim. Acta 2018, 36 (5), 309–323.
- El-Aziz, A.; Fouda, S.; El-Dossoki, F.I.; El-Nadr, H.A.; El-Hussein1, A. Moringa Oleifera Plant Extract as a Copper Corrosion Inhibitor in Binary Acid Mixture (HNO3 + H3PO4). ZASTITA MATERIJALA 2018, 59 (3), 422–435.
- Fouda, A.S.; El-Ewady, G.Y.; El-Abbasy, H.M.; Zidan, S.M. Vitex Agnus Castus Plant Extract as Safe Corrosion Inhibitor for Carbon Steel 1018 in 1M Hydrochloric Acid. JCBPSC 2018, 8 (3), 469–491.
- Fouda, A.S.; Abou Shahba, R.M.; El-Shenawy, A.E.; Seyam, T.J.A. Adsorption and Corrosion Inhibition of Cassia Angustifolia (Senna) Fruit Extract on Mild Steel in Hydrochloric Acid Solution. Chem. Sci. Trans. 2018, 7 (2), 163–180.
- El-Aziz S, A.; Fouda, R.M.; Shahba, A.; El-Shenawy, A.E.; Seyam, T.J.A. Evaluation of Cleome Droserifolia (Samwah) as Green Corrosion Inhibitor for Mild Steel in 1 M HCl Solution. Int. J. Electrochem. Sci. 2018, 13, 7057–7075.
- Ramananda Singh, M. A Green Approach: A Corrosion Inhibition of Mild Steel by Adhatoda Vasica Plant Extract in 0.5 M H2SO4. J. Mater. Environ. Sci. 2013, 4 (1), 119–126.
- Fouda, A.S.; Elmorsi, M.A.; Fayed, T.; El said, I.A. Oxazole Derivatives as Corrosion Inhibitors for 316 L Stainless Steel in Sulfamic Acid Solutions. Desalin. Water Treat. 2014, 57 (10), 4371–4385.
- Obi-Egbedi, N.O.; Obot, I.B. Xanthione: A New and Effective Corrosion Inhibitor for Mild Steel in Sulphuric Acid Solution, Arab. J. Chem. 2010, 6 (2), 211–223.
- Bentiss, F.; Traisnel, M.; Gengebre, L.; Lagrenee, M. A New Triazole Derivative as Inhibitor of the Acid Corrosion of Mild Steel: Electrochemical Studies, Weight Loss Determination, SEM and XPS. Appl. Surf. Sci. 1999, 152, 237–249.
- Amin, A.M.; Khaled, K.F. Copper Corrosion Inhibition in O2-Saturated H2SO4 Solutions. Corros. Sci. 2010, 52, 1194–1204.
- Bosch, R.W.; Hubrecht, J.; Bogaerts, W.F.; Syrett, B.C. Electrochemical Frequency Modulation: A New Electrochemical Technique for Online Corrosion Monitoring. Corrosion 2001, 57 (1), 60–70.
- Abdel-Rehim, S.S.; Khaled, K.F.; Abd- Elshafi, N.S. Electrochemical Frequency Modulation as a New Technique for Monitoring Corrosion Inhibition of Iron in Acid Media by New Thiourea Derivative. Electrochim. Acta 2006, 51, 3269–3277.
- Bentiss, F.; Lebrini, M.; Lagrenee, M. Thermodynamic Characterization of Metal Dissolution and Inhibitor Adsorption Processes in Mild Steel/2,5-Bis(n-Thienyl)-1,3,4- Thiadiazoles/Hydrochloric Acid System. Corros. Sci. 2005, 47, 2915–2931.
- Bouknana, D.; Hammouti, B.; Serghini caid, H.; Jodeh, S.; Bouyanzer, A.; Aouniti, A.; Warad, I. Aqueous Extracts of Olive Roots, Stems, and Leaves as Eco-Friendly Corrosion Inhibitor for Steel in 1 MHCl Medium. Int. J. Ind. Chem. 2015, 6 (4), 233–245.
- Bouknana, D.; Hammouti, B.; Messali, M.; Aouniti, A.; Sbaa, M. Phenolic and Non-Phenolic Fractions of the Olive Oil Mill Wastewaters as Corrosion Inhibitor for Steel in HCl Medium, Port. Electrochim. Acta 2014, 32 (1), 1–19.
- Bouknana, D.; Hammouti, B.; Messali, M.; Aouniti, A.; Sbaa, M. Olive Pomace Extract (OPE) as Corrosion Inhibitor for Steel in HCl Medium Olive Pomace Extract (OPE) as Corrosion Inhibitor for Steel in HCl Medium, Asian Pac. J. Trop. Dis. 2014, 4 (2), S963–S974.
- Behpour, M.; Ghoreishi, S.M.; Gandomi-Niasar, A.; Soltani, N.; Salavati-Niasari, M. The Inhibition of Mild Steel Corrosion in Hydrochloric Acid Media by two Schiff Base Compounds. J. Mater. Sci. 2009, 44, 2444–2453.
- Mansfeld, F. Corrosion Mechanism; Marcel Dakkar: NewYork, NY, 1987, p. 119.
- Umoren, S.A.; Obot, I.B.; Obi-Egbedi, N.O. Quantum Chemical Studies on the Corrosion Inhibition of Mild Steel by Piperidin-4-One Derivatives in 1 M H3PO4. J. Mater. Sci. 2009, 44, 863–877.
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption Properties and Inhibition of Mild Steel Corrosion in Hydrochloric Solution by Some Newly Synthesized Diamine Derivatives: Experimental and Theoretical Investigations. Corros. Sci. 2010, 52 (9), 3042–3051.
- Solmaz, R.; Kardas, G.; Culha, M.; Yazici, B.; Erbil, M. Investigation of Adsorption and Inhibitive Effect of 2-Mercaptothiazoline on Corrosion of Mild Steel in Hydrochloric Acid Media. Electrochim. Acta 2008, 53, 5941–5952.
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry, vol2; Plenum Press: New York, 1977, p. 1267.
- Guan, N.M.; Xueming, I.; Fei, I. Synergistic Inhibition Between o-Phenanthroline and Chloride ion on Cold Rolled Steel Corrosion in Phosphoric Acid. Mater. Chem. Phys. 2004, 86, 59–68.
- Obot, I.B.; Obi-Egbedi, N.O. The Synergistic Inhibitive Effect and Some Quantum Chemical Parameters of 2,3-Diaminonaphthalene and Iodide Ions on the Hydrochloric Acid Corrosion of Aluminum. Corros. Sci. 2009, 52, 276–282.
- Sein, L.T.; Wei, Y.; Jansen, S.A. The Role of Adsorption of Aniline Trimers on the Corrosion Inhibition Process: A ZINDO/1 Study. Comput. Theor. Polym. Sci. 2001, 11, 83–88.
- Kissi, M.; Bouklah, M.; Hammouti, B.; Benkaddour, M. Establishment of Equivalent Circuits From Electrochemical Impedance Spectroscopy Study of Corrosion Inhibition of Steel by Pyrazine in Sulphuric Acidic Solution. Appl. Surf. Sci. 2006, 252, 4190–4097.
- Machnikova, E.; Whitmire, K.H.; Hackerman, N. Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Furan Derivatives. Electrochim. Acta 2008, 53, 6024–6032.
- Villamil, R.F.V.; Corio, P.; Rubim, J.C.; Agostinho, S.M.L. Effect of Sodium Dodecyl Sulfate on Copper Corrosion in Sulfuric Acid Media in the Absence and Presence of Benzotriazole. J. Electroanal. Chem. 1999, 472 (2), 112–119.
- Lagrenee, M.; Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Study of the Mechanism and Inhibiting Efficiency of 3, 5-bis (4-Methylthiophenyl)-4H-1, 2, 4-Triazole on Mild Steel Corrosion in Acidic Media. Corros. Sci. 2002, 44, 573–588.
- Donahue, F.M.; Nobe, K. Theory of Organic Corrosion Inhibitors Adsorption and Linear Free Energy Relationships. J. Electrochem. Soc. 1965, 112, 886–891.
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Electrochemical and Quantum Chemical Studies of 5-Substituted Tetrazoles as Corrosion Inhibitors for Copper in Aerated 0.5 M H2SO4 Solution. Corros. Sci. 2010, 52, 1472–1481.
- Bouklah, M.; Hammouti, B.; Lagrenee, M.; Bentiss, F. Thermodynamic Properties of 2,5-bis(4-Methoxyphenyl)-1,3,4-Oxadiazole as Corrosion Inhibitor for Mild Steel in Normal Sulfuric Acid Medium. Corros. Sci. 2006, 48, 2831–2842.
- Li, X.; Deng, S.; Fu, H. Adsorption and Inhibition Effect of Vanillin on Cold Rolled Steel in 3.0 M H3PO4. Prog. Org. Coat. 2010, 67, 420–426.
- Reshetnikov, S.M. Inhibitors of Metal Acid Corrosion; Chimica: Leningrad, 1986.
- Bouknana, D.; Hammouti, B.; Jodeh, S.; Sbaa, M.; Lgaz, H. Extracts of Olive Inflorescence Flower Pre-Anthesis, at Anthesis and Grain Pollen as Eco-Friendly Corrosion Inhibitor for Steel in 1M HCl Medium. Anal. Bioanal. Electrochem. 2018, 10 (6), 751–777.
- Jones, D.A. Principles and Prevention of Corrosion; MaCmillan: New York, 1992.
- Rosborg, B.; Pan, J.; Leygraf, C. Tafel Slopes Used in Monitoring of Copper Corrosion in a Bentonite/Ground Water Environment. Corros. Sci. 2005, 47, 3267–3279.
- Flitt, H.J.; Schweinsberg, D.P. A Guide to Polarisation Curve Interpretation: Deconstruction of Experimental Curves Typical of the Fe/H2O/H+/O2 Corrosion System. Corros. Sci. 2005, 47, 2125–2156.
- Labjar, N.; Lebrini, M.; Bentiss, F.; Chihib, N.E.; El Hajjaji, S.; Jama, C. Corrosion Inhibition of Carbon Steel and Antibacterial Properties of Aminotris-(Methylnephosnic) Acid. Mater. Chem. Phys. 2010, 119 (1–2), 330–336.
- Abdel Rehim, S.S.; Hassan, H.H.; Amin, M.A. Corrosion and Corrosion Inhibition of Al and Some Alloys in Sulphate Solutions Containing Halide Ions Investigated by an Impedance Technique. Appl. Surf. Sci. 2002, 187, 279–290.
- Li, P.; Lin, J.Y.; Tan, K.L.; Lee, J.Y. Electrochemical Impedance and X-ray Photoelectron Spectroscopic Studies of the Inhibition of Mild Steel Corrosion in Acids by Cyclohexylamine. Electrochim. Acta 1997, 42, 605–615.
- Damaskin, B.B.; Petrii, O.A.; Batraktov, B. Adsorption of Organic Compounds on Electrodes; Plenum Press: New York, 1971.
- Fouda, A.S.; Ali, A.H. Egy- Dronate Drug as Promising Corrosion Inhibitor of C-Steel in Aqueous Medium. ZASTITA MATERIJALA 2018, 59 (1), 126–140.