?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Polycyclic aromatic hydrocarbons (PAHs), a class of toxic organic molecules containing multiple fused aromatic rings, are found in air, soil and water as a by-product from the incomplete combustion of organic matter. We report on the modification of silica gel using lipophilic molecules containing carboxylic acids, for anchoring to the surface via hydrogen bonds. The lipophilic component captures aqueous PAH, specifically phenanthrene, through the agency of π–π and π–sp3 interactions. The structure–sorption-relationships suggest optimum phenanthrene adsorption is achieved with unsaturated bonds in linear chains or two phenyl rings. Examples include linoleic acid or 2,3-diphenylpropionic acid with removal values of 281 and 283 ng PAH per gram of silica gel, respectively. Saturation adsorption is achieved within four hours. Proposed modes of binding of the new reverse phase silica gels with phenanthrene are described. Recycling of the silica gel is accomplished by washing with organic solvents to remove PAH.
GRAPHICAL ABSTRACT
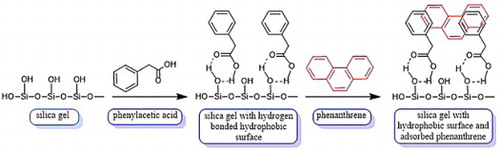
Introduction
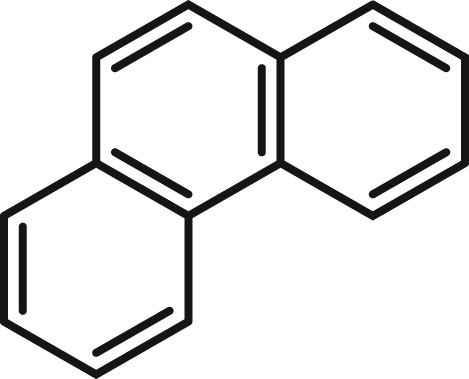
Polycyclic aromatic hydrocarbons (PAHs) are a class of organic molecules containing multiple fused aromatic rings. PAHs are uncharged, non-polar molecules found naturally in coal and tar deposits and produced by the incomplete combustion of organic molecules, specifically in engines, incinerators or forest fires. PAHs are found in air, water and soil samples, often with adversely high levels ( Citation1–3). PAHs can induce a number of adverse effects such as immunotoxicity, genotoxicity, mutagenicity, and carcinogenicity ( Citation4) Due to high toxicity and ability to bioaccumulate, these contaminants are a significant threat to the health of living organisms ( Citation5). Examples of naturally occurring PAH include naphthalene, phenanthrene, chrysene, benzo[a]pyrene and benzo[c]fluorene ( Citation6). Of these, phenanthrene is chosen as a prototypical PAH, the structure of which is shown in .
The conventional methods of decomposition or removal of PAHs from source water are classified into following major groups: (i) biodegradation ( Citation7), (ii) chemical ( Citation8), and (iii) physical methods ( Citation9) and combinations of above methods ( Citation9). Biodegradation of PAHs using microorganisms cannot always guarantee satisfactory results, especially in industrial wastewater treatment, since many of the organic and inorganic pollutants and water pH are lethal for bacteria and other biological sources. Chemical-based strategies for PAH removal include catalytic oxidation and photo degradation while physical methods such as coagulation, membrane filtrations, sorption are employed. Various other types of sorbents, such as activated carbons, resins, electrospun nanofibrous membranes (ENFMs), carbon nanomaterials, bentonite, coconut shell, and chemically modified cellulose have been described ( Citation10–12).
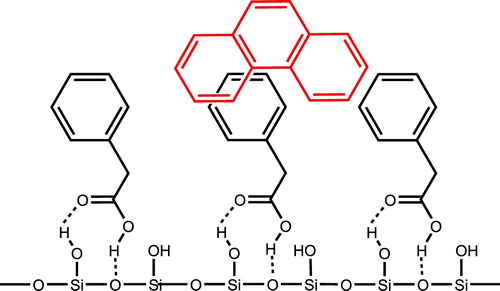
We report on a modification of the hydrophilic surfaces of silica gel that render them lipophilic for efficient PAH capture. Specifically, silica gel is coated with a series of hydrophobic compounds containing a carboxylic acid whose role is to secure the molecules to the surface through hydrogen bonding and, perhaps, dipole–dipole interactions with the Si–OH functional groups. The hydrophobic groups on the surface facilitate the capture of phenanthrene with lipophilic π–π and π–sp3 interactions between the modified surface of silica gel and phenanthrene. Phenyl rings and alkyl chains for the hydrophobic components of the organic molecules are chosen to facilitate maximum van-der Waal and π–π interactions ( Citation13, Citation14, Citation15) with the aromatic rings of PAH. shows the proposed hydrogen bonding interactions between phenylacetic acid and the surface of silica gel along with the hydrophobic-hydrophobic interactions between phenanthrene (red) and phenylacetic acid. The structure-sorption-relationships and kinetics of the removal of phenanthrene with modified silica gel are described. Of the materials examined, silica gel treated with linoleic acid or 2,3-diphenylpropionic acid provide the most effective binding domains for phenanthrene adsorption with removal values of 281 and 283 ng PAH per gram of silica gel, respectively. Saturation adsorption is achieved within four hours. Recycling of the adsorbent for additional PAH capture trials is affected by rinsing with ethanol, which removes PAH and regenerates the hydrophobic surface, without significant loss of adsorption capacity.
Materials and methods
Reagents
Cyclohexylacetic acid, linoleic acid, diphenylacetic acid and 3,3-diphenylpropionic acid were purchased from Acros Organics. Phenylacetic acid was purchased from Sigma Aldrich. 3-Phenylpropionic acid and cyclopentyl(phenyl)acetic acid were purchased from Alfa Aesar. 1-Naphthaleneacetic acid was purchased from Fisher Scientific. 2,3-Diphenylpropionic acid was purchased from TCI Americas. All chemicals were used without further purification. Silica gel 60 Å, 70–230 mesh was obtained from Dynamic Absorbents Inc. (Norcross, GA). Deionized water is utilized throughout.
Loading lipophilic acids onto silica gel
In a typical procedure, silica gel was dried at 120°C for 2 h under vacuum and cooled to room temperature in a desiccator. To a solution of 0.20 g of 3,3-diphenylpropionic acid in 10 mL of methyl t-butyl ether (MTBE) in a capped Erlenmeyer flask was added 1.0 g of silica gel. The mixture was stirred with a stir bar overnight at room temperature. An aliquot was taken from the mixture to determine the extent of adsorption compared to the original 3,3-diphenylpropionic acid solution by UV spectrum with an Agilent 8453 UV/visible photodiode array spectrophotometer. The liquid was decanted. The silica gel was rinsed with 10 mL MTBE. The treated silica gel was dried at room temperature overnight. For non-UV active carboxylic acids, the starting and final weights of the silica gels were subtracted from each other. In addition, a blank silica gel sample was treated in the same manner as the impregnated sample (i.e. soaking in MTBE without a carboxylic acid, filtering, drying). The average percent weight gained due to moisture and solvent adsorption was calculated and subtracted from the non-UV active carboxylic acid samples. As such, the molar amounts of absorbed acids for each carboxylic acid were calculated. An FTIR spectrum of the silica gel after exposure to the lipophilic carboxylic acids revealed a new peak at 1703 cm−1 indicative of the carbon–oxygen double bond stretch of the carboxylic acid bound to the silica gel surface.
lists the measured maximum absorption wavelength, λmax, and molar extinction coefficient, ε, for some aromatic-containing lipophilic carboxylic acids. shows the UV absorption of 3,3-diphenylpropionic acid before and after reacting with silica gel. The moles of lipophilic acids loaded on the silica gel were calculated from the difference of the UV absorbance of the initial concentration of the carboxylic acid compared to the absorbance of the final concentration.
Figure 3. UV/vis absorption spectra of 3,3-diphenylpropionic acid in MTBE before (orange) and after (blue) binding to silica gel.
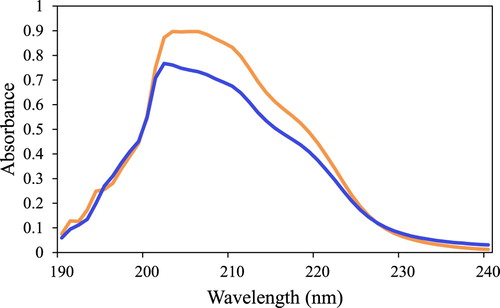
Table 1. Spectroscopic data of selected lipophilic carboxylic acids.
Measuring the capacity of PAH adsorption by treated silica gels
Fluorescence calibration curve of phenanthrene
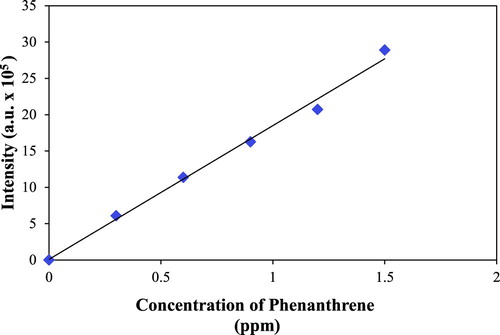
Phenanthrene was chosen as a prototypical PAH compound for removal. A 1.50 ppm aqueous stock solution of phenanthrene was prepared, and serial dilutions performed with deionized water. The fluorescence intensities of the dilute standards were measured at 365 nm with a Photon Technology International (PTI) spectrofluorometer equipped with a 1.0 cm quartz cell. The exaction wavelength was set to 251 nm, and the emission spectra were taken in the range of 275–400 nm. The slit widths of the exaction and emission monochromators were set to 0.5 mm. A calibration curve was constructed as the fluorescence intensity vs. the concentration (ppm) of phenanthrene as shown in .
Adsorption of phenanthrene by designer reverse phase silica gels
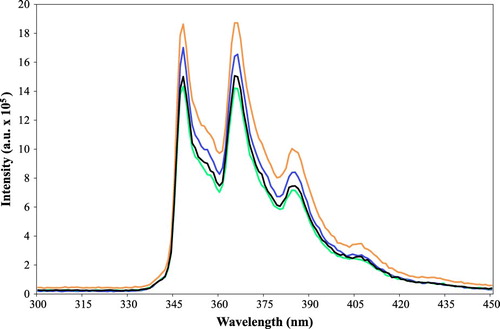
In a typical procedure at neutral pH, a mixture of 0.10 g of a modified silica gel in 50 mL phenanthrene solution (1.5 ppm) in a capped brown bottle is stirred with a magnetic stirring bar. The fluorescence intensity of phenanthrene in the solution is measured at different times () with a 1-cm cuvette, and after each measurement the solution returned from the cuvette to the soaking bottle to maintain the total volume constant (50 mL). After the adsorption is complete the decreasing of the fluorescence intensity at 365 nm is used to calculate the mass (ng) of PAH adsorbed by modified silica gel using the fluorescence calibration curve (). For each type of lipophilic carboxylic acid-treated silica gel, the PAH adsorption capacity (ng PAH/g silica gel) was measured in triplicate. Ambient room temperature is used throughout. For experiments performed at low or high pH values, the solutions were adjusted with 0.1 N NaOH or 0.1 N HCl.
Figure 5. Fluorescence spectra of an aqueous solution (1.5 ppm) of phenanthrene (orange) and after exposure to silica gel treated with 3,3-diphenylpropionic acid at 1 h (blue), 2 h (black) and 3 h (green).
Using the calibration curve the change of fluorescence intensity is converted to the change of PAH concentration by adsorbent silica gel. The capacity of PAH adsorption, i.e. ng PAH adsorbed on one gram of silica gel, can be calculated as:where ΔC is the change of the ppm concentration (mg/L) of phenanthrene, V is the volume of the solution (L), and 178.23 is the molar mass of phenanthrene.
Recycling
To the PAH-saturated silica gel (0.5 g) was added ethanol (50 mL). The mixture was stirred overnight, filtered and washed with ethanol. The recycled silica gel was dried. Exposure of 0.10 g of a modified silica gel in 50 mL phenanthrene solution (1.5 ppm) in a capped brown bottle is stirred with a magnetic stirring bar. The fluorescence intensity of phenanthrene in the solution is measured at similar time points to ascertain the level of PAH capture.
Molecular modeling
Calculations of the binding of phenanthrene with each target hydrophobic-containing carboxylic acid were performed at the semi-empirical level of theory. Specifically, the quantum chemical calculations of each reactant molecule and the product aggregate were executed using the PM3 semi-empirical Hamiltonian set of parameters as a resident in the Spartan ‘16 software suite.
Results and discussion
Determining the amount of lipophilic carboxylic acids loaded on the silica gel surface
All unsaturated lipophilic carboxylic acids tested have a strong UV absorption in the MTBE solution. Since binding to the silica gel ( Citation16, Citation17) reduces the concentration and absorbance of the lipophilic acid in solution, the amount of the acid loaded is calculated from the change of UV absorbance from the initial concentration. A report converting the silanol hydrogen (Si–OH) on the surface of silica gel to dimethylsilyl (Si–O–SiH(CH3)2 ) showed the concentration of silanol groups on silica gel (60 Å) to be 2.54 ± 0.08 mmol/g or 3.3 per 100 Å2 ( Citation18, Citation19). shows that the mmol amount of the carboxylic acids binding to silica gel is 20-180-fold lower than the calculated amount of free silanol groups. Increasing the loading time or the quantity of acid did not significantly change the amount of loading. The molecular volumes of the carboxylic acids in are much larger compared to dimethylsilyl (data not shown). Steric congestion coupled with the small pore diameter of silica gel (60 Ǻ) suggests that the large PAH-capturing carboxylic acids cannot completely penetrate inside the mesopore structure. For example, the adsorption of mono-substituted carboxylic acid chains, entries 3 and 4, provide higher percent loading of 7–9% than di-substituted chains, entries 7, 8 and 9 with of 2–3% loading. The molecular environment surrounding the hydrogen bond network of silanols has a significant influence on carboxylic acids loading capacity. The weight % of acid loaded is calculated by dividing the gram of acid loaded by the initial gram of acid used.
Table 2. PAH adsorption capacities by modified silica gels.
The capacity of PAH adsorption by various reverse phase silica gels
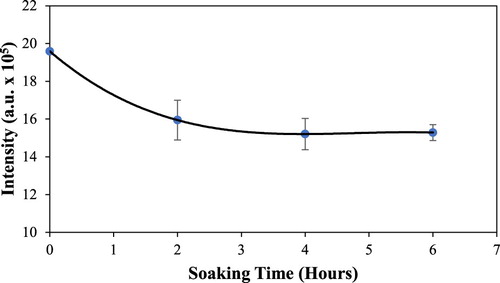
Phenanthrene is used as a prototypical compound for the removal of PAH molecules from aqueous solutions. It has strong fluorescence in the UV region (λex = 251 nm and λem = 365 nm), therefore, its aqueous concentration is monitored by fluorescence intensity. When exposed to the reverse phase silica gels in a stirring environment the fluorescence intensity of phenanthrene rapidly decreases indicating the adsorption of phenanthrene onto the lipophilic domains of the silica gel. As shown in the silica gel coated with cyclopentyl(phenyl)acetic acid reaches saturation between 3 and 4 h. This is generally within the time frames of rapid adsorbing materials, which include waste ion exchange resin ( Citation20), periodic mesporous organosilica ( Citation21) and granulated activated carbon ( Citation22, Citation23), and slower adsorbing substances such as activated carbon ( Citation24)
Figure 6. Changes of fluorescence intensity at 365 nm of a 1.5 ppm aqueous phenanthrene solution (50 mL) as it is adsorbed onto 0.10 g of silica gel coated with cyclopentyl(phenyl)acetic acid (entry 6) in a stirring environment over the course of 6 h.
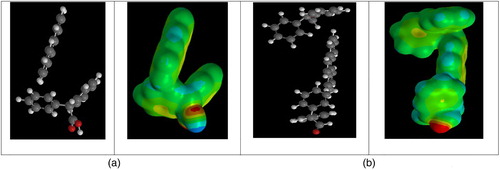
The phenanthrene adsorption capacities (ng PAH/mg silica) of the lipophilic carboxylic acids bound to silica gel (entries 1–9) under neutral pH conditions are shown in . As a comparison, the sorption capacity by untreated silica gel (entry 10) is also reported. All modified silica gels have much higher adsorption capacities than untreated silica gel ( Citation16) confirming that the lipophilic component of the carboxylic acids provides effective phenanthrene-binding domains ( Citation17). Linoleic acid (entry 2), with cis-double bonds at C-9 and C-12, assumes a favorable topology for PAH capture (281 ng PAH). (a) shows the molecular model of binding interactions of linoleic acid with phenanthrene using a combination of π−π and π–sp3 interactions. Introducing phenyl rings on the carboxylic acid scaffold is shown in entries 3–9. Phenylacetic acid (entry 3) absorbs comparably to cylcohexylacetic acid (entry 1), as well as 1-naphthalene acetic acid (entry 5). When judged with cyclopentyl(phenyl)acetic acid (entry 6), diphenylacetic acid (entry 7) is comparable. These results suggest a situation wherein phenanthrene binding does not discriminate between π−π interactions and π–sp3. Entries 8 and 9 represent different spacial arrangements of two aromatic rings on a propionic acid scaffold. The improved performance of entry 9 ((b)) may reflect a somewhat more suitable binding platform with the two aromatic rings separated by an sp3 carbon for a PAH edge-to-face interaction ( Citation25, Citation26).
Figure 7. Binding model of linoleic acid (entry 2). (a) with phenanthrene; (b) with 2,3-diphenylpropionic acid (entry 9). The red atoms represent the oxygen atoms in the carboxylic acids.
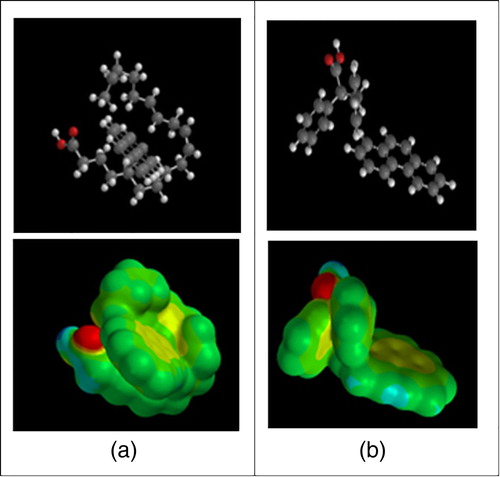
From this data, we conclude that the sorption ability of these modified silica gels can be separated into three categories. The first adsorbing group, entries 2 and 9, are likely to have the best surface topology for binding to phenanthrene by maximizing Van der Waal and π−π interactions ( Citation14) which impacts the magnitude of de-solvation and directly affects Gibbs free energy values for increased sorption capacity. Therefore, the molecular recognition between adsorbent and PAH is the predominant factor. As a group, the second collection, entries 6, 7, and 8, use two rings as platforms for phenanthrene binding but are not as effective adsorbents. The third group of adsorbents, with the smaller surface areas, as shown as entries 3, 4, and 5, are not as successful in binding to the larger surface area of phenanthrene. We calculate that to remove a liter of 1.5 ppm of aqueous phenanthrene would require 5.3 g of the linoleic acid (entry 2) or 2,3-diphenylpropionic acid (entry 8) modified silica gel.
The binding ratio between lipophilic acid and phenanthrene
In addition to measuring the binding affinity of lipophilic acids to phenanthrene, the molar ratio between the two was calculated. Using UV absorption spectroscopy the moles of lipophilic carboxylic acids loaded onto the silica gel surface could be determined. Subsequently, these treated silica gels were used to measure the moles of PAH adsorbed on the surface using fluorescence spectroscopy. The results from comparing the molar ratio of the lipophilic acid to phenanthrene in the binding reaction are summarized in . We envision phenanthrene binding to the hydrophobic binding domains on the surface of the modified silica gel could occur through a combination of contacts, especially π−π or π–sp3 alignment. That is, a single lipophilic group and a phenanthrene molecule could combine in a 1:1 molar ratio or with near neighbor group cooperativity (2:1). For lipophilic acids with molar ratios between 1 and 2, we suggest a combination of 1:1 and 2:1 associations. Molar ratio values much greater than 2 imply a larger number of molecules must supportively contribute to binding to phenanthrene. Alternatively, because of the irregular surface topology of silica gel, some lipophilic domains may remain inaccessible to phenanthrene resulting in a high molar ratio.
Table 3. Binding ratio of selected lipophilic carboxylic acids bound to silica gel with phenanthrene.
(a,b) illustrate potential binding modes of the lipophilic domains of substituted carboxylic acids and phenanthrene in different ratios. For a 1:1 molar ratio, we propose structure (a) of diphenylacetic acid (entry 7) and phenanthrene with an edge-to-π binding or T-shaped alignment and parallel displaced orientation of the π−π orbitals. An example of a 2:1 molar ratio is shown in (b) with diphenylacetic acid (entry 7) bound to phenanthrene employing additional T-shaped interactions. Binding models of the other lipophilic carboxylic acids listed in with phenanthrene are located in Supplementary Material.
Recycling
To establish the feasibility of recycling the modified silica gels after saturation with phenanthrene a series of solid–liquid extraction experiments were performed. The program goal was the identification of a solvent that would preferentially dissolve adsorbed phenantherene and not the lipophilic component of the silica gel surface. It was hoped the energy diffence between phenanthrene’s binding affinity with the lipohphilic silica gel surface compared to dissolution with solvent would be sufficient to undertake recycling. Several of the materials that had reached saturation of phenanthrene after 4 h were treated with organic solvents, such as MTBE, toluene and ethanol. Of these, ethanol proved superior in removing phenanthrene from the surface of the reverse phase silica gel. summarizes the results of the recycling experiements. The ability to effeciently adsorb PAH again is seen for entries 8 and 9 after the first recycling with percent recoveries of 90–97% compared to the initial adsorption capacities. The small erosion of adsorption capacity after second recycling may reflect fractional removal of the lipophilic acid from the silica gel surface with a polar solvent.
Table 4. Recycling ability of modified silica gels.
Conclusion
We have described a strategy for changing the surface composition of silica gel from a hydrophilic environment to a hydrophobic one with the addition of carboxylic acids containing a high carbon content that adhere to silica gel via hydrogen bonds. The resulting modified silica gels, containing hydrophobic binding domains that are suitable for interactions with phenanthrene (PAH) using π−π and π–sp3 interactions, are superior to silica gel for the removal of PAH from water. The structure-sorption-relationships suggest the introduction of unsaturated bonds (entry 2) in linear chains is a good strategy for the adsorption of PAH compared to all saturated carbon atoms (data not shown). Incorporating phenyl rings into the carboxylic acid scaffold was also an effective approach. Surfaces with two phenyl rings (entries 7 and 8) were superior compared to single aromatic ring analogs (entries 3, 4, 6). Of the diphenyl analogs, the capture of aqueous PAH is best achieved with 2,3-diphenylpropionic acid (entry 9) coated silica gels. No significant changes to adsorption are observed with solutions ranging from pH 2 to pH 12. Unlike most other adsorbents, recycling of the modified silica gels is possible by washing the materials with ethanol with no considerable loss in PAH adsorption. To remove a liter of 1.5 ppm of aqueous phenanthrene would require 5.3 g of the linoleic acid (entry 2) or 2,3-diphenylpropionic acid (entry 9) modified silica gel.
Acknowledgements
All authors certify and assert that there are no conflicts of interest, and that no animals or humans were used to obtain results reported in this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr. John Regan is an associate professor of Chemistry at Manhattan College.
Jessi Dolores graduates from the Department of Chemistry & Biochemistry of Manhattan College.
Megan Lepore graduates from the Department of Chemistry & Biochemistry of Manhattan College.
Michael Gualano graduates from the Department of Chemistry & Biochemistry of Manhattan College.
Jeovanna Badson graduates from the Department of Chemistry & Biochemistry of Manhattan College.
Amanda Zimnoch graduates from the Department of Chemistry & Biochemistry of Manhattan College.
Dr. Joseph F. Capitani is a professor of Chemistry at Manhattan College.
Dr. Jianwei Fan is a professor of Chemistry at Manhattan College.
ORCID
John Regan http://orcid.org/0000-0002-3594-3071
Additional information
Funding
References
- Achten, C.; Andersson, J.T. Overview of Polycyclic Aromatic Compounds (PAC). Polycycl. Aromat. Compd. 2015, 35, 177–186. doi: 10.1080/10406638.2014.994071
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. doi: 10.1016/j.ejpe.2015.03.011
- Mastral, A.M.; Callén, M.S.; Garcia, T. Toxic Organic Emissions From Coal Combustion. Fuel Process. Technol. 2000, 67, 1–10. doi: 10.1016/S0378-3820(00)00088-6
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to Polycyclic Aromatic Hydrocarbons with Special Focus on Cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. doi: 10.1016/S2221-1691(15)30003-4
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. doi: 10.1080/10406638.2014.892886
- Andersson, J.T.; Achten, C. A Critical Look at the 16 EPA PAHs. Polycycl. Aromat. Compd. 2015, 35 (2–4), 143–146. doi: 10.1080/10406638.2015.1005241
- Haritash, A.K.; Kaushik, C.P. Biodegradation Aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. J. Hazard. Mater. 2009, 169 (1–3), 1–15. doi: 10.1016/j.jhazmat.2009.03.137
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic Aromatic Hydrocarbons (PAHs) Removal by Sorption: A Review. Chemosphere 2016, 148, 336–353. doi: 10.1016/j.chemosphere.2016.01.036
- Smol, M.; Włodarczyk-Makuła, M. The Effectiveness in the Removal of PAHs From Aqueous Solutions in Physical and Chemical Processes: A Review. Polycycl. Aromat. Compd. 2017, 37 (4), 292–313. doi: 10.1080/10406638.2015.1105828
- Bhadra, B.N.; Song, J.Y.; Lee, S.K.; Hwang, Y.K.; Jhung, S.H. Adsorptive Removal of Aromatic Hydrocarbons From Water Over Metal Azolate Framework-6-Derived Carbons. J. Hazard. Mater. 2018, 344, 1069–1077. doi: 10.1016/j.jhazmat.2017.11.057
- Valderrama, C.; Cortina, J.L.; Farran, A.; Gamisans, X.; Lao, C. Kinetics of Sorption of Polyaromatic Hydrocarbons Onto Granular Activated Carbon and Macronet Hyper-Cross-Linked Polymers (MN200). J. Colloid Interface Sci. 2007, 310, 35–46. doi: 10.1016/j.jcis.2007.01.039
- Stien, D.; Gastaldi, S. Design of Polyaromatic Hydrocarbon-Supported Tin Reagents: A New Family of Tin Reagents Easily Removable From Reaction Mixtures. J. Org. Chem. 2004, 69, 4464–4470. doi: 10.1021/jo049868o
- McGaughey, G.B.; Gagne, M.; Rappe, A.K. Pi-Stacking Interactions. J. Biol. Chem 1998, 273, 15458–15463. doi: 10.1074/jbc.273.25.15458
- Hunter, C.A.; Sanders, J.K.M. The Nature of.Pi.-.Pi. Interactions. J. Am. Chem. Soc. 1990, 112 (14), 5525–5534. doi: 10.1021/ja00170a016
- Martinez, C.R.; Iverson, B.L. Rethinking the Term “Pi-Stacking.” Chem. Sci. 2012, 3, 2191–2201. doi: 10.1039/c2sc20045g
- Curthoys, G.; Davydov, V.Y.; Kiselev, A.V.; Kiselev, S.A.; Kuznetsov, B.V. Hydrogen Bonding in Adsorption on Silica. J. Colloid Interface Sci. 1974, 48, 58–72. doi: 10.1016/0021-9797(74)90328-2
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of Organic Molecules on Silica Surface. Adv. Colloid Interface Sci. 2006, 121 (1-3), 77–110. doi: 10.1016/j.cis.2006.05.028
- Yoshinaga, K.; Yoshida, H.; Yamamoto, Y.; Takakura, K.; Komatsu, M. A Convenient Determination of Surface Hydroxyl Group on Silica Gel by Conversion of Silanol Hydrogen to Dimethylsilyl Group with Diffuse Reflectance FTIR Spectroscopy. J. Colloid Interface Sci. 1992, 153 (1), 207–211. doi: 10.1016/0021-9797(92)90312-A
- Bush, S.G.; Jorgenson, J.W.; Miller, M.L.; Linton, R. Transmission Near-Infrared Technique for Evaluation and Relative Quantification of Surface Groups on Silica. J. Chromatogr. A 1983, 260, 1–12. doi: 10.1016/0021-9673(83)80001-6
- Long, C.; Lu, J.; Li, A.; Hu, D.; Liu, F.; Zhang, Q. Adsorption of Naphthalene Onto the Carbon Adsorbent From Waste Ion Exchange Resin: Equilibrium and Kinetic Characteristics. J. Hazard. Mater. 2008, 150 (3), 656–661. doi: 10.1016/j.jhazmat.2007.05.015
- Vidal, C.B.; Barros, A.L.; Moura, C.P.; de Lima, A.C.A.; Dias, F.S.; Vasconcellos, L.C.G.; Fechine, P.B.A.; Nascimento, R.F. Adsorption of Polycyclic Aromatic Hydrocarbons From Aqueous Solutions by Modified Periodic Mesoporous Organosilica. J. Colloid Interface Sci. 2011, 357 (2), 466–473. doi: 10.1016/j.jcis.2011.02.013
- Valderrama, C.; Gamisans, X.; de las Heras, X.; Farrán, A.; Cortina, J.L. Sorption Kinetics of Polycyclic Aromatic Hydrocarbons Removal Using Granular Activated Carbon: Intraparticle Diffusion Coefficients. J. Hazard. Mater. 2008, 157 (2-3), 386–396. doi: 10.1016/j.jhazmat.2007.12.119
- Eeshwarasinghe, D.; Loganathan, P.; Kalaruban, M.; Sounthararajah, D.P.; Kandasamy, J.; Vigneswaran, S. Removing Polycyclic Aromatic Hydrocarbons From Water Using Granular Activated Carbon: Kinetic and Equilibrium Adsorption Studies. Environ. Sci. Pollut. Res. 2018, 25 (14), 13511–13524. doi: 10.1007/s11356-018-1518-0
- Haro, M.; Cabal, B.; Parra, J.B.B.J.B.; Ania, C.O. On the Adsorption Kinetics and Equilibrium of Polyaromatic Hydrocarbons From Aqueous Solution. Adsorpt. Sci. Technol. 2011, 29 (5), 467–478. doi: 10.1260/0263-6174.29.5.467
- Nishio, M. The CH/π Hydrogen Bond: Implication in Chemistry. J. Mol. Struct. 2012, 1018, 2–7. doi: 10.1016/j.molstruc.2012.03.012
- Brandl, M.; Weiss, M.S.; Jabs, A.; Sühnel, J.; Hilgenfeld, R.C.-H. π-Interactions in Proteins. J. Mol. Biol. 2001, 307 (1), 357–377. doi: 10.1006/jmbi.2000.4473