?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study reports the synthesis of ZnO nanoparticles using the aqueous solution of broccoli extract. The nanoparticles, represented as broc-ZnO (with broccoli extract) and nb-ZnO (without broccoli extract), were obtained after calcination. The nanoparticles were characterized using X-ray diffraction (XRD), Transmission electron microscopy (TEM), Fourier Transform infra-red (FTIR), Energy dispersive spectroscopy (EDX), UV-visible spectroscopy (UV) and Photoluminescence (PL). Hexagonal phases were identified for both nanoparticles, while average crystallite sizes of 14 and 17 nm for broc-ZnO and nb-ZnO, respectively, were obtained. UV studies indicated the bandgap of the broc-ZnO and nb-ZnO as 4.09 and 3.87 eV, respectively. A plausible mechanism for the synthesis of the nanoparticles was suggested. The photocatalytic efficiency of broc-ZnO, obtained via the broccoli extract, was evaluated by studying its photo-enhanced catalytic activity against methylene blue (MB) and phenol red (PR), under UV light irradiation and 74% and 71% degradation efficiency of the successive dyes were achieved.
GRAPHICAL ABSTRACT
1. Introduction
Nature represents an illimitable source of bio-laboratory with replete of resources therein for biogenic synthesis. A large reserve of biomaterials is available, and they provide formidable and remarkable alternatives to the conventional chemical route for the synthesis of nanomaterials. Green synthesis offers many advantages over chemical methods. Out of these, low cost and environment-friendliness remain significant because the nanoparticles are stabilized by biogenic materials ( Citation1, Citation2). Plant materials contain biogenic molecules capable of acting as modifiers and capping agents for the synthesis of nanoparticles. These biomolecules are vital in the green synthesis of environmental-benign nanoparticles, and are good alternatives to the chemicals used in conventional methods. Over the past few years, the preparation of nanomaterials with divergent morphologies via green methods has given a boost to the application of these materials in catalysis and in the manufacture of commercial products ( Citation3, Citation4).
Water sources have suffered an unprecedented contamination due to the huge volume of organic dyes being discharged from textile industries ( Citation5). These dyes are mostly not biodegradable, toxic to the ecosystem and are carcinogenic. Various methods have been reported for the removal of dyes from solutions such as reverse osmosis, oxidation using chemical action, membrane filtration, coagulation and adsorption techniques ( Citation6, Citation7). The materials used in these methods are either expensive or results in the formation of waste that are non-biodegradable. In an attempt to overcome this snag, there is a need for the synthesis of cheap and nontoxic nanoparticles which have a significant photocatalytic activity for dye removal.
Photocatalytic activity is based on the interaction between a photocatalyst, usually a semiconductor, and light to produce reactive hydroxyl radicals or superoxide species that could interact with dye molecules, resulting in enhanced degradation process of the organic dyes. ZnO nanoparticles have been reported as good photocatalyts in the degradation of organic dyes due to their high stability, electron binding energy and large surface area ( Citation8, Citation9). They exhibit photocatalytic degradation ability similar to TiO2 due to their close band gap energies; thus, an efficient alternative for the degradation of organic dyes and potential environmental pollutant. The nanoparticles have oxygen vacancy-induced bandgap narrowing which enhances its visible light photocatalytic activity ( Citation10).
As a semiconductor, ZnO has a wide band gap of 3.37 eV and a large excitonic binding energy of 60 meV ( Citation11). It is non-toxic, and in the nanoparticulate form the particles possess a high surface to volume ratio. The performance and commercial utilization of ZnO nanoparticles could be ascribed to its unique characteristics, a function of its size and morphology ( Citation12). The nanoparticles have unique electrical, chemical and optical properties and these are responsible for their wide applications in solar cells, light-emitting diodes (LEDs), metal–insulator semiconductor diodes, photocatalytic degradation and drug delivery ( Citation13–17). The nanoparticles have also been used in many devices such as acoustic wave ( Citation18) and optical transparent electrodes ( Citation19).
The synthesis of ZnO in nanoscale dimension is predominantly carried out by physical and chemical routes. Physical methods include chemical vapor deposition ( Citation20), metal–organic chemical deposition ( Citation21), pulse laser deposition ( Citation22) and thermal evaporation ( Citation23), while the chemical methods includes techniques such as sonochemical ( Citation24), solvothermal ( Citation25), spray pyrolysis ( Citation26), hydrothermal ( Citation27), sol gel ( Citation28) and electro deposition process ( Citation29). The use of plants to synthesize metal, metal oxide and metal sulfide nanoparticles, although started in the nineteenth century, only came to limelight a few decades ago ( Citation30). Plants contain some active biomolecules, such as phenols, flavonoid polyphenolics, alkaloids, which could act as reducing and capping agents ( Citation31). Many green plants have been reportedly used as stabilizers and capping agents for the synthesis of nanoparticles ( Citation32). A few reported examples include the synthesis of ZnO nanoparticles using aqueous leave extracts of Aloe vera ( Citation33), Nephelium lappaceum L. ( Citation34), Solanium nigrum ( Citation35), Moringa oleifera ( Citation36), Hisbiscus rosa-sinensis ( Citation37), and Corymbia citriodora ( Citation38). Apart from the leaves, other parts of the plants, such as the stem and roots, have been employed in the green synthesis of ZnO nanoparticles ( Citation39). Facile green syntheses of ZnO nanoparticles using aqueous extract of Chironji and Nyctanthes abortistis have been reported ( Citation40, Citation41). The extract performed the role of a biological agent for the synthesis of zinc oxide. A couple of extensive reviews that are worthy of note have been conducted recently on the biological synthesis of ZnO nanoparticles ( Citation42–44).
The plant-mediated synthesis of nanoparticles could be intra-cellular or extra-cellular. Intra-cellular method entails the synthesis of nanoparticles by growing the plant in metal-rich organic media, while extra-cellular method involves the use of plant extract obtained from heating and pulverizing the plant in solvent or aqueous medium ( Citation45). Brassica oleracea L. var. italica (Broccoli) is a member of Brassicaceae family. Broccoli was discovered in the Mediterranean region and it is well dispersed all over Europe and the United States. It is a rich source of folic acid fibers, phytochemicals such as glucosinolates (GLs) and polyphenols. The chemical compositions of broccoli have been reported in previous studies ( Citation46). Phytochemical constituents of broccoli account for its antioxidant activities. There have been a lot of interest on broccoli due to its health-promoting phytochemicals, the protective ability of its constituent metabolites against damaging free radicals in the body, and in green chemistry for their usage as a capping agent in nanoparticle synthesis ( Citation47). Broccoli contains trace amounts of Se in the form of methyl selenocysteine. The Se constituent of broccoli is chemoprotective against certain cancers ( Citation48).
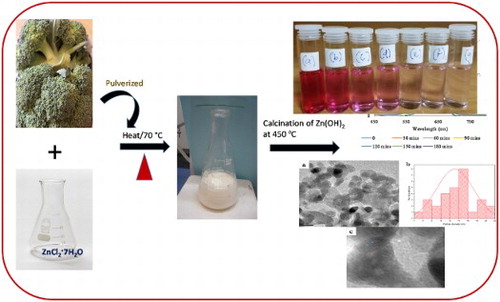
In this study, we report the biosynthesis of ZnO nanoparticles by using the aqueous extract of broccoli and ZnCl2 as Zn precursor. The formation of Zn(OH)2 as an intermediate product occurred during the reaction, which was subsequently calcined to give ZnO nanoparticles. To the best of our knowledge, this is the first report on aqueous broccoli extract-mediated synthesis of ZnO nanoparticles.
2. Experimental
2.1. Materials and methods
Materials: Broccoli plants were purchased at Food Lovers vegetable store in Mafikeng, South Africa and properly identified by a Botanist. Zinc chloride and sodium hydroxide, methylene blue (MB) (C16H18N3SCl, molecular weight 373.90 g-mol−1, absorption wavelength 664 nm) and Phenol red (PR) (C19H14O5S; molecular weight 354.38 g-mol−1; absorption wavelength 556 nm) were purchased from Merck chemicals, South Africa.
2.2. Instrumentation
The X-ray diffraction (XRD) measurement was recorded over a 2θ range of 30–80° at a scanning rate of 0.05 min−1 using a Bruker D8 Advanced, equipped with a proportional counter using Cu Kα radiation (k = 1.5405 Å, nickel filter). Transmission electron microscopy (TEM) images were taken with TECNAI G2(ACI) instrument, operated at an acceleration voltage of 200 KV. Image J software, a computer application for processing image, was employed to measure the average size of nanoparticles from the TEM images. Absorbance studies were recorded on a Cary 30 UV-vis spectrophotometer manufactured by Agilent technologies. Photoluminescence studies were performed using a Perkin Elmer LS 45 fluorescence spectrometer. FTIR spectra of the ZnO and aqueous plant extract were recorded on a Cary 670 FTIR spectrometer from Agilent technologies.
2.3. Preparation of aqueous broccoli extract
Broccoli leaves were cut and air-dried in the laboratory in order to prevent volatile organic component of the broccoli plant from evaporating ( Citation49). After drying, they were pulverized and stored properly for further use. About 8 g of the dried Broccoli florets was weighed out, washed with double de-ionized water to get rid of superficial or apparent impurities. The pulverized broccoli was mixed with 80 mL of de-ionized water and heated at 70 °C for 20 min. The mixture was allowed to cool to room temperature, filtered and the filtrate was stored in the refrigerator for further experiment.
2.4. Synthesis of ZnO nanoparticles using aqueous broccoli extract
ZnO nanoparticles were prepared following a modification of a reported procedure ( Citation11). About 20 mL of the aqueous extract of broccoli and 0.05 M ZnCl2·7H2O were continuously stirred for about 30 min. The solution was then transferred to a 100 mL conical flask and boiled at 70°C for 20 min with a magnetic stirrer and a heater until a brown colored precipitate, which marked the completion of the reaction, was observed. The precipitate was collected while the product was centrifuged at 6000 rpm for 20 min, washed 3 times with ethanol and distilled water to isolate the pure product and subsequently dried in the oven at 80 °C for 6 h given a pale white powder. The product was further calcined at 450°C to afford ZnO nanoparticles, labelled as broc-ZnO. This experiment was repeated using sodium hydroxide to precipitate zinc hydroxide, which was followed by calcination and the product was labelled as nb-ZnO.
2.5. Photocatalytic degradation of MB and PR by broccoli-mediated ZnO nanoparticles
Photocatalytic activity of broc-ZnO nanoparticles was investigated following the modification of a procedure reported in our previous study (
Citation47). Summarily, 20 mg of broc-ZnO was suspended in a beaker containing 25 mL of 5 × 10−5 M of methylene blue dye solution and the mixture was sonicated in the dark to achieve homogenous mixture. In the course of the photocatalytic experiment, the mixture of the broc-ZnO and methylene blue solution was kept in a photoreactor under constant stirring. The photocatalytic degradation of the dye was observed by taking a 2 mL aliquot at reaction intervals: 30, 60, 90, 120, 150 and 180 min (over a period of 3 h) and the aliquots were analyzed using a spectrophotometer. The photodegradation efficiency was monitored by recording the absorption spectra against time at a wavelength of 664 nm. The percentage degradation of the photocatalyst was calculated using the following relation:(1)
(1) where Ao and At are the absorbance of the dye before degradation and final absorbance of dye at different time “t,” respectively. Similarly, the degradation efficiency of broc-ZnO nanoparticles against PR was evaluated sequel to the aforementioned one against MB. The same procedure was followed for the degradation process but instead of methylene blue, phenol red with an absorption wavelength of 560 nm was used.
3. Result and discussion
3.1. p-XRD studies of the nanoparticles
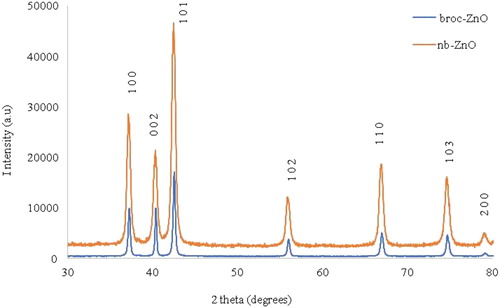
The overlapped p-XRD patterns of both broc-ZnO and nb-ZnO nanoparticles are shown in . broc-ZnO patterns ((a)) showed distinguishable peaks at 2θ values of 37.0°, 40.23°, 42.39°, 55.85°, 66.77°, 74.62° and 78.89° which could be assigned to the (100), (002), (101), (102), (102), (103), (200) planes of hexagonal phase ZnO. The patterns displayed by nb-ZnO showed these planes around 37.22°, 40.38°, 42.54°, 55.88°, 66.93°, 74.70° and 79.14°. The diffraction peaks match well with the powder diffraction standard reported for the hexagonal phase of ZnO nanoparticles (JCPDS No79-2205) (
Citation50). The presence of sharp peaks reveals that the ZnO nanoparticles were highly crystalline, while the average crystallite size of the nanoparticles was calculated using the Debye–Scherer’s Equation (2), (
Citation51)(2)
(2) where D is the crystallite size of ZnO in nm, K is the Scherrer shape factor (0.90),
is the X-ray wavelength used (1.5406 Å),
is the full width at half maximum (FWHM) in radians and, θ is the Bragg diffraction angle in degrees. Average particle sizes of broc-ZnO and nb-ZnO were estimated as 14 and 18.2 nm, respectively. The difference in sizes could be due to the effect of the aqueous broccoli extract on broc-ZnO compared to the bare nb-ZnO. This result is consistent with similar studies in which the uncapped nanomaterial has a larger particle size than the capped one (
Citation52).
3.2. TEM studies of the nanoparticles
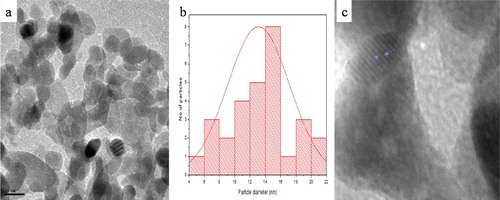
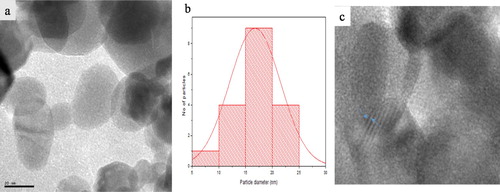
(a) and 3(a) show the TEM micrograph of broc-ZnO and nb-ZnO, respectively. A spherical morphology is observable in both ZnO samples prepared using different routes. The crystallite sizes are in the range 5–25 nm. The average particle size of broc-ZnO is 14 nm, while nb-ZnO has a larger average size of 17 nm. Plausible reason for the variation in the average crystallite sizes of the nanoparticles has earlier been attributed to the effect of the aqueous broccoli extract, which acted as a capping molecule. The broc-ZnO nanoparticles have smaller crystallite size than the ZnO nanoparticles. Particle size distribution histograms for broc-ZnO and nb-ZnO are presented in (b) and 3(b), respectively. The ZnO nanoparticles have partial spherical morphology with slight agglomeration. The image of nb-ZnO presented in (a) showed pronounced agglomeration compared with the TEM image of broc-ZnO and with larger crystallite size. The HRTEM showed the presence of lattice fringes in both nanoparticles presented in (c) and 3(c), an indication of good crystallinity of both broc-ZnO and nb-ZnO. The crystallite size is in close agreement with the size calculated from the XRD.
3.3. UV-vis studies of the nanoparticles
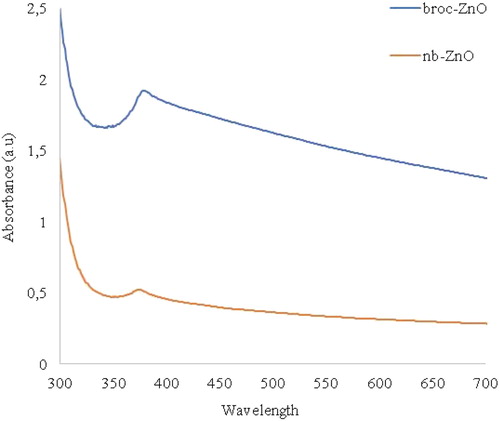
UV-vis spectrophotometer is a technique employed to evaluate the optical property of nanomaterials. Semiconductor nanoparticles experience a change in optical property compared with their bulk counterpart due to quantum confinement. The optical absorption spectra of ZnO nanoparticles were investigated at room temperature in the range 300–700 nm, as shown in . The absorption peaks for broc-ZnO and nb-ZnO were 303 and 320 nm, respectively. A higher degree of blue shift occurred in broc-ZnO than nb-ZnO, relative to the bulk nanoparticles (approx. 367 nm, 3.37 eV). This could be due to the smaller crystallite size of broc-ZnO. Although it appeared like a marginal difference in the two absorption peaks values, the difference in the band gap energy is observable. Optical band gap of the nanoparticles was calculated using Equation (3):(3)
(3) where α is the absorption coefficient, A is a constant, h stands for Plank’s constant, v is the frequency of photon, Eg denotes the optical band gap energy and n is
, which is for a direct band gap semiconductor. A tangent is drawn on the spectra, and an extrapolation of the linear region of the plot of (αhv) on the ordinate axis against the energy of photon (hv) on abscissa axis produces the exact value of the band gap energy of the nanoparticles (
Citation53). Using the relation above, the optical band gaps of broc-ZnO and nb-ZnO were obtained as 4.09 and 3.87 eV, respectively. Quantum size effects in ZnO occur in particles whose crystallite size is less than 7 nm (
Citation54). In this work, the crystallite sizes of the two nanoparticles were more than 7 nm. The smaller the crystallite size of nanoparticles, the higher the band gap energy and this is typical of quantum size effect. The difference in the band gap energy stemmed from the smaller crystallite size of broc-ZnO compared to nb-ZnO.
3.4. Photoluminescence studies
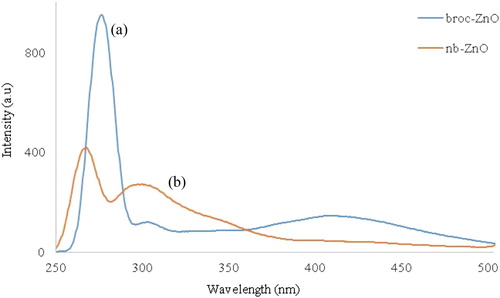
The room temperature photoluminescence spectra of broc-ZnO and nb-ZnO, at an excitation wavelength of 250 nm, are presented in (a,b), respectively. The spectra of broc-ZnO in (a), showed three emission peaks centered at about 268, 298 and 417 nm. The first two peaks are in the UV region, while the third pervades into the visible region typical of ZnO nanoparticles ( Citation55). The emission peaks in the UV region are usually due to the band edge emission or exciton transition ( Citation56), while the third emission peak, which is in the visible region, could be attributed to electronic transition from conduction band tail to valence band tail states. Similar observation has been reported by Wang et al. ( Citation57). Intensity of the emission peak has been reported to decrease with an increase in particle size of nanoparticles ( Citation58). The higher peak intensity of broc-ZnO is consistent with the smaller particle size. Two emission peaks were observed in the spectrum of ZnO, (b), and they occurred in the UV region around 269 and 299 nm. These two peaks emerged from recombination of free exciton and are due to near band edge (NBE) emission. The intensity of the peaks in broc-ZnO is lower compared to that of nb-ZnO, and this is due to the increase in the crystallite size of ZnO nanoparticles.
3.5. Infra-red spectral studies and EDX
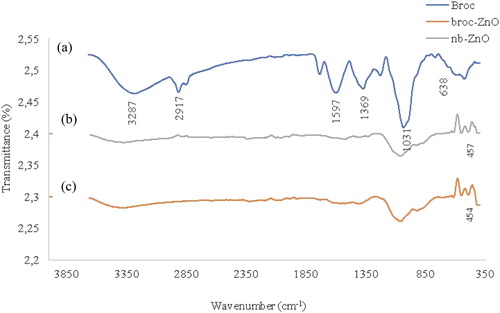
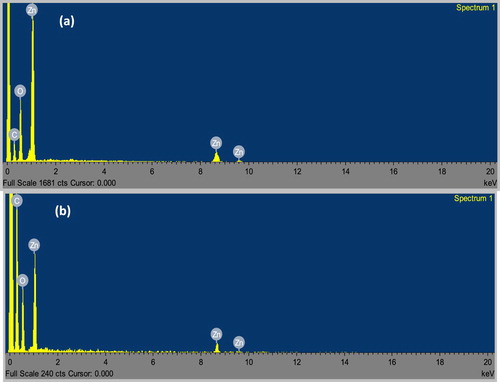
FTIR was employed to investigate the possible functional groups in the broccoli extract and the biosynthesized nanoparticles. presents the FTIR spectra of the broc-ZnO, nb-ZnO and broccoli extract. In the spectrum of the broccoli extract, a broad peak in the range 3200–3600 cm−1 could be assigned to the stretching vibration of the hydroxyl group of phenol, which also overlapped with the N-H of the amines due to different bioactive compounds present in broccoli ( Citation59). Two low intensity peaks around 2916 cm−1 corresponded to the symmetric and asymmetric, C–H of the aliphatic group. A slightly broad peak around 1640 cm−1 is attributed to the bending vibration of the OH group, and might be due to the chemisorbed and/or physiosorbed moisture on the surface of ZnO nanoparticles ( Citation60). The band at 1411 cm−1 is associated with C=C group, possibly as a result of the aromatic conjugates of the biomolecules in the extract. The bands at 1031 and 692 cm−1 depict the presence of C–O stretching vibration of alcohol and C–H vibration of the –CH=CH of the ethylene system, respectively. Comparing these peaks with the spectrum of broc-ZnO reveals a reduction in the peak broadening of broc-ZnO nanoparticles. Prominent peaks around 454 and 357 cm−1 are due to the characteristic Zn-O stretching vibration of the broc-ZnO and nb-ZnO, respectively ( Citation61, Citation62). The biomolecules were responsible for the conversion of ZnCl2 to Zn(OH)2 prior to calcination of the intermediate product to ZnO nanoparticles. Energy dispersive X-ray spectroscopy (EDX) was evaluated to determine the elemental composition of nanomaterials. (a,b) shows the EDX spectra of broc-ZnO and nb-ZnO which revealed that both contain only Zn and O in about 34.13: 65% and 33.4: 66.6% composition, respectively. The observed C peak in the EDX spectra could be ascribed to the carbon tape used as part of the sample holder.
3.6. Mechanism for the formation of biosynthesized ZnO nanoparticles
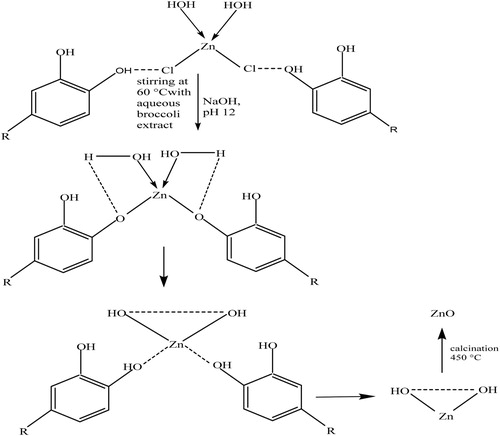
The plausible reaction mechanism for the synthesis of the ZnO nanoparticles is illustrated in Scheme 1. There were two steps to the formation of ZnO nanoparticles, namely, the synthesis of Zn(OH)2 from zinc chloride, and the subsequent calcination of this intermediate product into ZnO nanoparticles at 450°C. Ionic form of metals could be detached from the anionic part and be reduced to the most stable species by chelating to antioxidant phytochemicals, such as polyphenols and flavonoids. The bonding of the OH group of this biomolecule to the metal ions led to the formation of Zn(OH)2 which was accompanied by the formation of a white milky precipitate that marks the completion of the reaction ( Citation63, Citation64). The intermediate product is dried in oven at a temperature of 70°C for 6 h, and subsequently calcined in muffle furnace at 450°C to obtain ZnO nanoparticles. The choice of Quercetin, as a major component of the biomolecules in broccoli in the plausible mechanism, is due to the presence of phenolic group, which is responsible for the formation of hydroxide complex. Quercetin is a bioactive chemical component of green plants that is available in broccoli. It contains phenolic moiety, which is rich in hydroxyl functional group. They are involved in the conversion of zinc chloride to zinc hydroxide. Quercetin reacts with metal chloride to form a complex through weak hydrogen bonds.
3.7. Photocatalytic activity of broc-ZnO nanoparticles against MB and PR dyes
Photocatalytic degradation of the MB dye was conducted using broccoli-mediated synthesized ZnO nanoparticles under UV light. The dye degradation was also visually monitored by the observation of change in color of the MB dye. The color of the dye, MB only, was deep blue and this slightly changed to a lighter blue color after 30 min of UV irradiation. As the time of exposure to UV irradiation progressed from 60 to 180 min, there was a noticeable gradual change in color to different shades of light blue. Ultimately, at 180 min it became colourless and this marked the completion of the degradation ability of the biosynthesized broc-ZnO nanoparticles, as depicted in . In a similar way, the degradation reaction was repeated using an anionic dye, phenol red, and the trend of degradation with time was visually monitored just like the aforementioned scenario, as shown in . The color was observed to have changed from red to faint red at the end of 180 min. The visual observation equally confirmed the degradation of phenol red by broc-ZnO. The choice of broc-ZnO as a photocatalyst over nb-ZnO arose from the smaller particle size of the former. It has been reported that the smaller the nanoparticle size, the better the efficiency of photocatalytic degradation of dyes ( Citation65).
Figure 8. Visual review of color change of the degradation of MB; from blue to colorless revealing the degradation of the dye at different time intervals (a) 0 min, (b) 30 min, (c) 60 min, (d) 90 min, (e) 120 min, (f) 150 min and (g) 180 min.

Figure 9. Visual review of color change of the degradation of PR; from red to colorless revealing the degradation of the dye at different time intervals (a) 0 min, (b) 30 min, (c) 60 min, (d) 90 min, (e) 120 min, (f) 150 min and (g) 180 min.

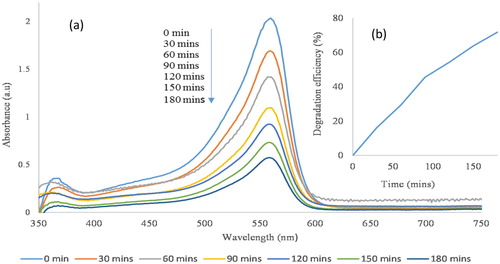
(a) depicts the overlapped absorption spectra of the MB with broc-ZnO, as a catalyst, at different time intervals over a period of 180 min. As shown in the spectra, there is a gradual decrease in the intensity of the diagnostic peak of MB dye located at 664 nm with an increase in time of irradiation from 0 to 180 min. The observed decrease in intensity as a function of exposure to UV light irradiation with time is a reflection of the capability of the broc-ZnO in photocatalytic degradation of the MB solution. The photodegradation efficiency of the catalyst on MB dye was calculated using the relation in Equation (1). The efficiency after 180 min of irradiation with UV light was 74%, as revealed in (b). The percentage degradation was calculated from the initial and final adsorption of the physical change that is associated with the degradation activity of the broc-ZnO nanoparticles was the discoloration of the MB solution. A second dye, an anionic dye, PR was used to study the rate of degradation of broc-ZnO in terms of variation of the absorption maximum at the absorption peak of 560 nm, as shown in (a), and the degradation efficiency of broc-ZnO against PR dye was 71%, as revealed in (b).
Figure 10. (a) Photocatalytic degradation of MB using broc-ZnO as a catalyst and under UV irradiation and inset, (b) Spectra of percentage degradation.
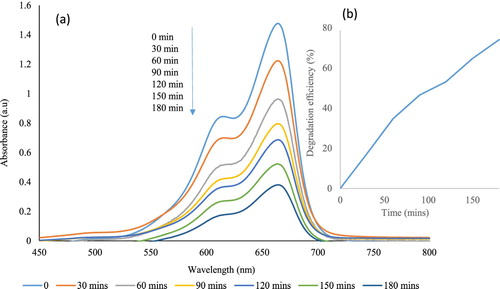
Figure 11. (a) Photocatalytic degradation of PR using broc-ZnO as a catalyst and under UV irradiation and inset and (b) spectra of percentage degradation.
The mechanism of photocatalytic degradation of methylene blue could be illustrated thus: when incident UV light irradiated the broc-ZnO nanoparticles, it created electron–hole pair via oxidation and reduction processes which ultimately generated radicals in the presence of O2 (
Citation66). These photogenerated hydroxyl radicals, superoxide radicals were responsible for the photocatalytic degradation of MB over ZnO nanoparticles. The following equations (4) to (9) illustrate the photocatalytic reactions with carbon dioxide and water as the products of the degradation as reported in different studies (
Citation67, Citation68).
(4)
(4)
(5)
(5)
(6)
(6)
(7)
(7)
(8)
(8)
(9)
(9)
The percentage efficiency of the as-synthesized broc-ZnO in the degradation of MB was around 74%, which is comparable with 97% obtained from the previously reported green synthesized ZnO nanoparticles ( Citation69, Citation70).
3.8. Kinetics study of the photocatalytic degradation
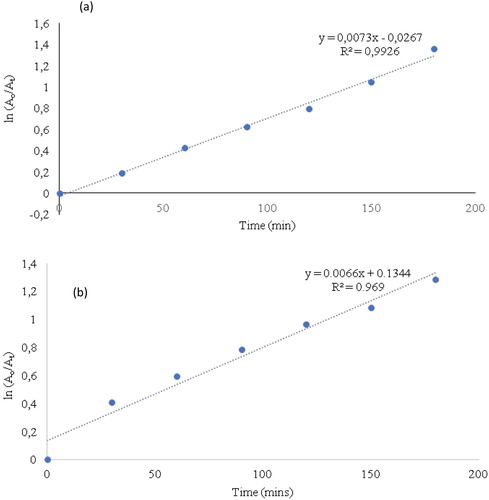
(a) presents a plot of ln(Ao/At) against time and the spectra showed a linear correlation against time. This displayed a pseudo-first-order kinetics of the photodegradation of MB by broc-ZnO via UV irradiation. 0.0073 is the calculated rate constant (k) where the value of the correlation coefficient from the graph is 0.9926. Similarly, (b) presents the plot of ln(Ao/At) as a function of reaction time for the degradation of phenol red by broc-ZnO. A first-order kinetics of photodegradation of PR with calculated rate constant (k) of 0.0066 and a correlation coefficient of 0.969 were obtained. Correlation coefficients in both cases have values close to 1 which revealed the best adsorption rate.
4. Conclusion
ZnO nanoparticles have been prepared using the aqueous extract of broccoli, followed by calcination process at 450°C for 2 h under normal air condition given ZnO nanoparticles. Two approaches were devised and the products were represented as broc-ZnO (with broccoli) and nb-ZnO (without broccoli). The different characterization techniques revealed the nanodimensional nature of the ZnO, with the average crystallite sizes of 14 and 17 nm for broc-ZnO and nb-ZnO, respectively. The difference in particle sizes is probably due to the capping role of the aqueous broccoli extract. XRD indicated that the particles were of hexagonal crystalline phase and optical properties showed that the band gap energies of the ZnO were 4.09 and 3.87 eV for broc-ZnO and nb-ZnO, respectively. FTIR studies showed the presence of capping biomolecules in the prepared ZnO and the Zn-O bond vibration was detected and the EDX confirmed the elemental composition of broc-ZnO and nb-ZnO. A follow-up study was conducted to evaluate the photocatalytic activity of the as-prepared broc-ZnO nanoparticles as catalysts using methylene blue and phenol red as organic dyes. The duration of the UV irradiation was 180 min and 74% and 71% degradation efficiencies were respectively achieved. The study confirmed the role of aqueous broccoli extract in the biogenic synthesis of ZnO.
Acknowledgements
JO appreciates North-West University, South Africa for a Postdoctoral research position and for providing the necessary facilities to carry out this work. The authors gratefully acknowledge Dr Anine Jordaan and Dr Innocent Shuro of the Laboratory of Electron Microscopy (LEM), North-West University, Potchefstroom campus, for Transmission electron microscopy (TEM) and EDX analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Jejenija Osuntokun is presently a Postdoctoral research fellow at North-West University, South Africa. His research interest is on the synthesis of nanomaterials via the chemical and green routes with applications in environmental remediation.
Damian C. Onwudiwe is an Associate professor and the Leader of the Inorganic and Materials Chemistry Research group of the North-West University, South Africa.
Eno E. Ebenso is a Professor of Physical Chemistry, and the Director of Material Science Innovation and Modelling (MaSIM) Research Focus Area, North-West University. He is also the executive Dean of the Faculty of Natural and Agricultural Sciences, North-West University, South Africa.
References
- Sultana, S.; Mohammad, R.; Khan, Z.; Umar, K.; Ahmed, A.S.; Shahadat, M. SnO2-SrO Based Nanocomposites and Their Photocatalytic Activity for the Treatment of Organic Pollutants. J. Mol. Struct. 2015, 1098, 393–399. doi: 10.1016/j.molstruc.2015.06.032
- Suresh, D.; Nethravathi, P.C.; Rajanaika, U.H.; Nagabhushana, H.; Sharma, S.C. Green Synthesis of Multifunctional Zinc Oxide (ZnO) Nanoparticles Using Cassia fistula Plant Extract and Their Photodegradative, Antioxidant and Antibacterial Activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. doi: 10.1016/j.mssp.2014.12.023
- Davar, F.; Majedi, A.; Mirzaei, A. Green Synthesis of ZnO Nanoparticles and Its Application in the Degradation of Some Dyes. J. Am. Ceram. Soc. 2015, 98, 1739–1746. doi: 10.1111/jace.13467
- Gunalana, S.; Sivaraja, R.; Rajendran, V. Green Synthesized ZnO Nanoparticles Against Bacterial and Fungal Pathogens. Prog. Nat. Sci. Mater. Inter. 2012, 22, 693–700. doi: 10.1016/j.pnsc.2012.11.015
- Bhaumik, R.; Upendra, S.; Patel, D. Aqueous Pollutants in Water Bodies can be Photocatalytically Reduced by TiO2 Nano-Particles in the Presence of Natural Organic Matters. Sep. Purif. Technol. 2019, 209, 748–755. doi: 10.1016/j.seppur.2018.09.017
- Bharathi, K.S.; Ramesh, S.T. Removal of Dyes Using Agricultural Waste as Low-Cost Adsorbents: A Review. Appl. Water. Sci. 2013, 3, 773–790. doi: 10.1007/s13201-013-0117-y
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interf. Sci. 2014, 209, 172–184. doi: 10.1016/j.cis.2014.04.002
- Sanna, V.; Pala, N.; Alzari, V.; Nuvoli, D.; Carcelli, M. ZnO Nanoparticles with High Degradation Efficiency of Organic Dyes Under Sunlight Irradiation. Mater. Lett. 2016, 162, 257–260. doi: 10.1016/j.matlet.2015.10.031
- Pavithra, N.S.; Lingaraju, K.; Raghu, G.K.; Nagaraju, G. Citrus maxima (Pomelo) Juice Mediated Eco-Friendly Synthesis of ZnO Nanoparticles: Applications to Photocatalytic, Electrochemical Sensor and Antibacterial Activities. Spectrochim. Acta A. 2017, 185, 11–19. doi: 10.1016/j.saa.2017.05.032
- Sajid, A.A.; Mohammad, M.K.; Shafeer, K.N.; Ambreen, L.; Jintae, H.C. Moo, Oxygen Vacancy Induced Band Gap Narrowing of ZnO Nanostructures by an Electrochemically Active Biofilm. Nanoscale. 2013, 5, 9238–9246. doi: 10.1039/c3nr02678g
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma betulina Natural Extract. Mater. Lett. 2015, 161, 124–127. doi: 10.1016/j.matlet.2015.08.052
- Usui, H. Electrochemical Self-Assembly Synthesis of Zinc Oxide Nanoparticles and Lamellar-Structured Organic/Inorganic Hybrids by Electrodeposition in Surfactant Solution. Electrochim. Acta. 2011, 56, 3934–3940. doi: 10.1016/j.electacta.2011.02.023
- Huang, J.; Yin, Z.; Zheng, Q. Applications of ZnO in Organic and Hybrid Solar Cells. Energy Environ. Sci. 2011, 4, 3861–3877. doi: 10.1039/c1ee01873f
- Pearton, S.J.; Ren, F. Advances in ZnO-Based Materials for Light Emitting Diodes. Curr. Opin. Chem. Eng. 2014, 3, 51–55. doi: 10.1016/j.coche.2013.11.002
- Chen, L.; Hsu, C.; Zhang, X.; Wu, J. Low-Cost ZnO: YAG-Based Metal-Insulator-Semiconductor White Light-Emitting Diodes with Various Insulators. Int. J. Photo Energy. 2014, 959620, 1–4.
- Bomila, R.; Venkatesan, A.; Srinivasan, S. Structural, Luminescence and Photocatalytic Properties of Pure and Octylamine Capped ZnO Nanoparticles. Optik. 2018, 158, 565–573. doi: 10.1016/j.ijleo.2017.12.141
- Huang, X.; Zheng, X.; Xu, Z.; Yi, C. ZnO-Based Nanocarriers for Drug Delivery Application: From Passive to Smart Strategies. Inter. J. Pharm. 2017, 534, 190–194. doi: 10.1016/j.ijpharm.2017.10.008
- Lee, K.J.; Oh, H.; Jo, M.; Lee, K.; Yang, S.S. An Ultraviolet Sensor Using Spin-Coated ZnO Nanoparticles Based on Surface Acoustic Waves. Microelectron, Eng. 2013, 111, 105–109. doi: 10.1016/j.mee.2013.02.025
- Hana, K.; Xie, M.; Zhang, L.; Yan, L.; Wei, J.; Ji, G.; Luo, Q.; Lin, J.; Hao, Y.; Ma, C.-Q. Fully Solution Processed Semi-Transparent Perovskite Solar Cells with Spray Coated Silver Nanowires/ZnO Composite Top Electrode. Sol. Energy Mater. Sol. Cells. 2018, 185, 399–405. doi: 10.1016/j.solmat.2018.05.048
- Chen, S.; Wanga, J.; Zhang, Z.; Briscoe, J.; Warwick, M.E.A.; Li, H.; Hu, P. Aerosol Assisted Chemical Vapour Deposition of Conformal ZnO Compact Layers for Efficient Electron Transport in Perovskite Solar Cells. Mater. Lett. 2018, 217, 251–254. doi: 10.1016/j.matlet.2018.01.090
- Sona, T.; Noha, J.-S.; Park, S. Role of ZnO Thin Film in the Vertically Aligned Growth of ZnO Nanorods by Chemical Bath Deposition Nguyen. Appl. Surf. Sci. 2016, 379, 440–445. doi: 10.1016/j.apsusc.2016.04.107
- Svetlichnyi, V.; Shabalina, A.; Lapin, I.; Goncharova, D.; Nemoykina, A. ZnO Nanoparticles Obtained by Pulsed Laser Ablation and Their Composite with Cotton Fabric: Preparation and Study of Antibacterial Activity. Appl. Surf. Sci. 2016, 372, 20–29. doi: 10.1016/j.apsusc.2016.03.043
- Lv, H.D.D.; Sang, H.D.; Li, X.; Du, B.; Li, D.M.; Zou, G.T. Thermal Evaporation Synthesis and Properties of ZnO Nano/Microstructures Using Carbon Group Elements as the Reducing Agents. Nanoscale Res. Lett. 2010, 5, 620–624. doi: 10.1007/s11671-010-9524-2
- Mohamed, H.H. Sonochemical Synthesis of ZnO Hollow Microstructure/Reduced Graphene Oxide for Enhanced Sunlight Photocatalytic Degradation of Organic Pollutants. J. Photochem. Photobiol. A Chem. 2018, 353, 401–408. doi: 10.1016/j.jphotochem.2017.11.052
- Fenga, W.; Huang, P.; Wang, B.; Wanga, C.; Wang, W.; Wang, T.; Chen, S.; Lv, R.; Qin, Y.; Ma, J. Solvothermal Synthesis of ZnO with Different Morphologies in Dimethylacetamide Media. Ceram. Int. 2016, 42, 2250–2256. doi: 10.1016/j.ceramint.2015.10.018
- Tharsika, T.; Haseeb, A.S.M.A.; Akbar, S.A.; Thanihaichelvan, M. Tailoring ZnO Nanostructures by Spray Pyrolysis and Thermal Annealing. Ceram. Int. 2015, 41, 5205–5211. doi: 10.1016/j.ceramint.2014.12.062
- Kumaresana, N.; Ramamurthi, K.; Babub, R.R.; Sethuramanc, K.; Babu, S.M. Hydrothermally Grown ZnO Nanoparticles for Effective Photocatalytic Activity. Appl. Surf. Sci. 2017, 418, 138–146. doi: 10.1016/j.apsusc.2016.12.231
- Al abdullah, K.; Awad, S.; Zaraket, J.; Salame, C. Synthesis of ZnO Nanopowders by Using Sol-Gel and Studying Their Structural and Electrical Properties at Different Temperature. Energy Procedia. 2017, 119, 565–570. doi: 10.1016/j.egypro.2017.07.080
- Mohammadi, E.; Aliofkhazraei, M.; Sabour, R.A. In-situ Study of Electrophoretic Deposition of Zinc Oxide Nanosheets and Nanorods. Ceram. Inter. 2018, 44, 1471–1482. doi: 10.1016/j.ceramint.2017.10.053
- Ankamwar, B. Biosynthesis of Gold Nanoparticles (Greengold) Using Leaf Extract of Terminalia catappa. Eur. J. Chem. 2010, 7, 1334–9.
- Aromal, S.A.; Philip, D. Green Synthesis of Gold Nanoparticles Using Trigonella foenum-graecum and its Size Dependent Catalytic Activity. Spectrochim. Acta A. 2012, 97, 1–5. doi: 10.1016/j.saa.2012.05.083
- Anamika, M.; Sanjukta, C.; Prashant, M.R.; Geeta, W. Evidence Based Green Synthesis of Nanoparticles. Adv. Mat. Lett. 2012, 3 (6), 519–525. doi: 10.5185/amlett.2012.icnano.353
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe Vera Extract Functionalized Zinc Oxide Nanoparticles as Nanoantibiotics Against Multi-Drug Resistant Clinical Bacterial Isolates. Sol. Energy Mater. Sol Cells. 2018, 185, 399–405. doi: 10.1016/j.solmat.2018.05.048
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO Nanoparticles Using Rambutan (Nephelium lappaceum L.) Peel Extract and their Photocatalytic Activity on Methyl Orange dye. J. Mol. Struct. 2016, 1125, 358–365. doi: 10.1016/j.molstruc.2016.07.029
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green Synthesis of ZnO Nanoparticles Using Solanum nigrum Leaf Extract and their Antibacterial Activity. Spectrochim Acta A. 2015, 136, 864–870. doi: 10.1016/j.saa.2014.09.105
- Sahaa, R.; Karthika, S.; Raju, P.M.; Kumar, S.A.; Suriyaprabhaa, R.; Rajendran, V. Psidium guajava Leaf Extract-Mediated Synthesis of ZnO Nanoparticles Under Different Processing Parameters for Hydrophobic and Antibacterial Finishing Over Cotton Fabrics. Prog. Org. Coat. 2018, 124, 80–91. doi: 10.1016/j.porgcoat.2018.08.004
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M. Studies on the Microwave Assisted and Conventional Combustion Synthesis of Hibiscus rosa-sinensis Plant Extract Based ZnFe2O4 Nanoparticles and Their Optical and Magnetic Properties. Ceram. Int. 2016, 42, 2741–2749. doi: 10.1016/j.ceramint.2015.11.003
- Zheng, Y.; Fu, L.; Han, F.; Wang, A.; Cai, W.; Yu, J.; Peng, F. Green Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Corymbia citriodora Leaf Extract and their Photocatalytic Activity. Green Chem. Lett. Rev. 2015, 8, 59–63. doi: 10.1080/17518253.2015.1075069
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Pavan Kumar, M.A.; RajaNaika, H.; Nagabhushana, H.; Sharma, S.C. Chironji Mediated Facile Green Synthesis of ZnO Nanoparticles and their Photoluminescence, Photodegradative, Antimicrobial and Antioxidant Activities. J. Photochem. Photobiol. B: Biology. 2017, 166, 272–284. doi: 10.1016/j.jphotobiol.2016.12.011
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green Synthesis of Zinc Oxide Nanoparticles Using Flower Extract of Nyctanthes arbor-tristis and Their Antifungal Activity. J. King Saud Univ. Sci. 2018, 30, 168–175. doi: 10.1016/j.jksus.2016.10.002
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G.E.; David, E. Synthesis, Characterization, and Evaluation of the Antimicrobial Efficacy of Boswellia ovalifoliolata Stem Bark-Extract Mediated Zinc Oxide Nanoparticles. Appl. Nanosci. 2016, 6, 581–590. doi: 10.1007/s13204-015-0472-0
- Ahmed, S.; Chaudhry, S.A.; Ikram, S.M. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect Towards Green Chemistry. J. Photoch. Photobio. B: Bio. 2017, 166, 272–284. doi: 10.1016/j.jphotobiol.2016.12.011
- Vishnukumar, P.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K. Recent Advances and Emerging Opportunities in Phytochemical Synthesis of ZnO Nanostructures. Mater. Sci. Semicon. Proc. 2018, 80, 143–161. doi: 10.1016/j.mssp.2018.01.026
- Basnet, P.; Chanu, T.I.; Samanta, D.; Chatterjee, S. A Review on Bio-Synthesized Zinc Oxide Nanoparticles Using Plant Extracts as Reductants and Stabilizing Agents. J. Photoch. Photobio. B: Bio. 2018, 183, 201–221. doi: 10.1016/j.jphotobiol.2018.04.036
- Ankamwar, B. Biosynthesis of Gold Nanoparticles (Green-Gold) Using Leaf Extract of Terminalia catappa. E-J Chem. 2010, 7, 1334–1339. doi: 10.1155/2010/745120
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived By-Products — a Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–392. doi: 10.1111/j.1750-3841.2010.01606.x
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Aqueous Extract of Broccoli Mediated Synthesis of CaO Nanoparticles and Its Application in the Photocatalytic Degradation of Bromocrescol Green. IET Nanobiotech. 2018, 12, 888–894. doi: 10.1049/iet-nbt.2017.0277
- Xu, B.L.; Cao, J.; Chen, W. Structural Characterization of a Broccoli Polysaccharide and Evaluation of Anti-Cancer Cell Proliferation Effects. Carbohyd. Polym. 2015, 126, 179–184. doi: 10.1016/j.carbpol.2015.03.011
- Wang, Y.; Li, X.; Jiang, Q.; Sun H.; Jiang J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. GC-MS Analysis of the Volatile Constituents in the Leaves of 14 Composited Plants. Molecules 2018, 23, 1–12.
- Devi, P.G.; Velu, A.S. Synthesis, Structural and Optical Properties of Pure ZnO and Co Doped ZnO Nanoparticles Prepared by the Co-Precipitation Method. J. Theor. Appl. Phys. 2016, 10, 233–240. doi: 10.1007/s40094-016-0221-0
- Song, D.; Widenborg, P.; Chin, W.; Aberle, A.G. Investigation of Lateral Parameter Variations of Al-Doped Zinc Oxide Films Prepared on Glass Substrates by RF Magnetron Sputtering. Sol Energy Mater. Sol Cells 2002, 73, 1–20. doi: 10.1016/S0927-0248(01)00104-0
- Sahu, N.; Brahme, N.; Sharma, R. Effect of Capping Agent on the Particle Size of CdSe Nanoparticles. Lumin 2016, 31, 1400–1406. doi: 10.1002/bio.3123
- Chaabouni, F.; Abaab, M.; Rezig, B. Effect of the Substrate Temperature on the Properties of ZnO Films Grown by RF Magnetron Sputtering. Mater. Sci. Eng. B. 2004, 109, 236–240. doi: 10.1016/j.mseb.2003.10.105
- Koch, U.; Fojtik, A.; Weller, H.; Henglein, A. Photochemistry of Semiconductor Colloids. Preparation of Extremely Small ZnO Particles, Fluorescence Phenomena and Size Quantization Effects. Chem. Phys. Lett. 1985, 122, 507–510. doi: 10.1016/0009-2614(85)87255-9
- Joshi, R. Facile Photochemical Synthesis of ZnO Nanoparticles in Aqueous Solution Without Capping Agents. Materialia 2018, 2, 104–110. doi: 10.1016/j.mtla.2018.07.001
- Zhong, G.; Li, J.; Su, Q.; Du, G.; Xu, B. Temperature-Dependent Controlled Preparation of ZnO Nanostructures and Their Photoluminescence Properties. Mater. Lett. 2011, 65, 670–673. doi: 10.1016/j.matlet.2010.11.036
- Wang, Q.P.; Zhang, D.H.; Xue, Z.Y.; Hao, X.T. Violet Luminescence Emitted from ZnO Films Deposited on Si Substrate by RF Magnetron Sputtering. Appl. Surf. Sci. 2002, 201, 123–128. doi: 10.1016/S0169-4332(02)00570-6
- Kim, Y.; Kang, S. Effect of Particle Size on Photoluminescence Emission Intensity in ZnO. Acta Mater. 2011, 59, 3024–3031. doi: 10.1016/j.actamat.2011.01.042
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO Nanoparticles via Moringa oleifera Green Synthesis: Physical Properties & Mechanism of Formation. Appl. Surf. Sci. 2017, 406, 339–347. doi: 10.1016/j.apsusc.2017.01.219
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J.; Zhu, Y. Luminescence and Photocatalytic Activity of ZnO Nanocrystals: Correlation Between Structure and Property. Inorg. Chem. 2007, 46, 6675–6682. doi: 10.1021/ic062394m
- Pirhashemi, M.; Habibi-Yangjeh, A. Ultrasonic-assisted Preparation of Novel Ternary ZnO/Ag3VO4/Ag2CrO4 Nanocomposites and Their Enhanced Visible-Light Activities in Degradation of Different Pollutants. Solid State Sci. 2016, 55, 58–68. doi: 10.1016/j.solidstatesciences.2016.02.006
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles Against Gram-Positive and Gram Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003–6009. doi: 10.2147/IJN.S35347
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.W.; Krause, R.W.M. Green Synthesis of Zinc Oxide Nanoparticles Using Solanum torvum (L) Leaf Extract and Evaluation of the Toxicological Profile of the ZnO Nanoparticles-Hydrogel Composite in Wistar Albino Rats. Inter. Nano Lett. 2019, 9, 99–107. doi: 10.1007/s40089-018-0263-1
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green Synthesis and Characterization of Zinc Oxide Nanoparticle Using Insulin Plant (Costus pictus D. Don) and Investigation of its Antimicrobial as Well as Anticancer Activities. Adv. Nat. Sci: Nanosci. Nanotechnol. 2018, 9, 015008–015016.
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic Degradation of Methyl Orange Dye by Pristine Titanium Dioxide, Zinc Oxide, and Graphene Oxide Nanostructures and their Composites Under Visible Light Irradiation. Appl. Nanosci. 2017, 7, 253–259. doi: 10.1007/s13204-017-0565-z
- Kumar, R.; Umar, A.; Kumar, G.; Akhtar, M.S.; Wang, Y.; Kim, S.H. Ce-doped ZnO Nanoparticles for Efficient Photocatalytic Degradation of Direct Red-23 Dye. Ceram. Int. 2015, 41, 7773–7782. doi: 10.1016/j.ceramint.2015.02.110
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Weiliang, W. Highly Efficient Photocatalytic Degradation of Methylene Blue by P2ABSA-Modified TiO2 Nanocomposite Due to the Photosensitization Synergetic Effect of TiO2 and P2ABSA. RSC, Adv. 2017, 7, 23699–23708. doi: 10.1039/C7RA02423A
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-Like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. doi: 10.1021/ie403402q
- Lua, J.; Batjikh, I.; Hurh, J.; Han, Y.; Ali, H.; Mathiyalaganb, R.; Ling, C.; Ahn, C.J.; Yang, D.C. Photocatalytic Degradation of Methylene Blue Using Biosynthesized Zinc Oxide Nanoparticles from Bark Extract of Kalopanax septemlobus. Inter J. Light Electron Optics. 2019, 182, 980–985. doi: 10.1016/j.ijleo.2018.12.016
- Lu, J.; Ali, H.; Hurh, J.; Han, Y.; Batjikh, I.; Rupa, E.J.; Anandapadmanaban, G.; Park, J.K.; Yang, D.-C. The Assessment of Photocatalytic Activity of Zinc Oxide Nanoparticles from the Roots of Codonopsis lanceolata Synthesized by One-Pot Green Synthesis Method. Inter J. Light Electron Optics 2019, 184, 82–89. doi: 10.1016/j.ijleo.2019.03.050