ABSTRACT
Despite various methods for preparation of tetrazoles, they are suffering from some disadvantages such as low yield, long reaction times and harsh reaction conditions, and there are still high demands to improve the common procedures to solve problems. So, the new copper nano-catalyst was prepared by coating of the Fe3O4 magnetic nanoparticles with tetraethyl orthosilicate, functionalization with 3-chloropropyltrimethoxysilane and 3-mercapto-1,2,4-triazole ligands as well as the subsequent complexation with CuCl2. The obtained catalyst was then characterized by different methods (ICP, XRD, EDX, SEM, TEM, TG-DTA and VSM) and applied as a heterogeneous nano-catalyst for the synthesis of diverse tetrazoles from the reaction of various aromatic amines with sodium azide and triethyl orthoformate in solvent-free condition at 100°C in good to high yields.
GRAPHICAL ABSTRACT
Introduction
Tetrazoles are very important class of organic compounds and their derivatives have various medicinal and industrial properties ( Citation1–10). Diverse catalysts were used for the synthesis of various tetrazoles, but the application of the nano-catalysts has received considerable attention due to their unique physical surfaces and catalytic properties ( Citation11–13).
Nanoparticles are more reactive than ordinary particles since they have a greater ratio of the surface area to their volumes. Because the heterogeneous reactions occur on the surface of the catalyst, the nano-catalysts enhance the number of reaction sites for the desired reaction to be occurred.
Since the use of nano-catalysts is not yet well understood and all of its cases have not been fully studied, so caution must be exercised in their use. For example, the application of nanoparticles in medicine is like a double-edged knife. On the one hand, it can penetrate into the human body in places where conventional medicines cannot be administered, and on the other hand, it can be harmful to human health. Therefore, these drugs should be used with extreme caution.
Despite the application of various methods, they suffer from one or more disadvantages, such as the use of toxic reagents, hard reaction conditions, low-efficiency products, difficult preparation of suitable research materials and so on ( Citation14–16). So, the useful catalytic procedures such as the use of ZnCl2 ( Citation17), FeCl3−SiO2 ( Citation18), zeolite ( Citation19), ZAS ( Citation20) and ZnO ( Citation21) were developed for the preparation of various tetrazole derivatives to have all the possible benefits and advantages of the former applied methods without their disadvantages.
Also in this regard, the Fe3O4 magnetic particles were functionalized with 1,10-phenanthroline-5,6-diol and the corresponding Mn complex synthesized as a heterogeneous catalyst to be used for the one-pot three-component synthesis of various tetrazoles, recently ( Citation22, Citation23). In addition, the antioxidant and antibacterial activities of the Mn nano-catalyst and its Phen ligand were in vitro screened with 2,2-diphenyl-1-picrylhydrazyl by free radical scavenging methods. Results showed that the synthesized compounds possess strong antioxidant activity (IC50; 0.172 ± 0.005 mg/ml) as well as a good antibacterial potential in comparison to standards.
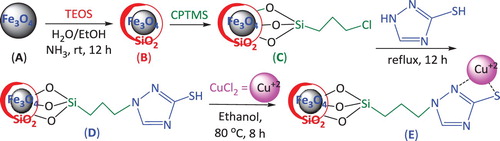
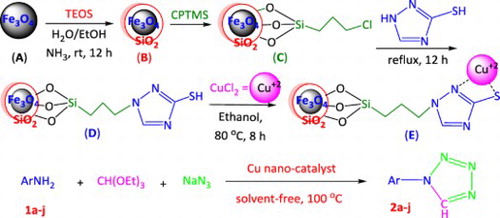
Now, to possess all the benefits of the former methods without their disadvantages, the use of a new Cu nano heterogeneous catalyst is reported which was successively prepared by coating of the Fe3O4 magnetic nanoparticles with tetraethyl orthosilicate (Fe3O4/SiO2), functionalized with 3-chloro-propyltrimethoxysilane (CPTMS) and 3-mercapto-1,2,4-triazole (MT) ligands (Fe3O4/SiO2/CPTMS/MT) and the further complexation with CuCl2 (Fe3O4/SiO2/CPTMS/MT/Cu) (Scheme 1).
The obtained Cu nano-catalyst was then characterized by different methods (ICP, XRD, EDX, SEM, TEM, TG-DTA and VSM) and applied as a heterogeneous nano-catalyst for the synthesis of diverse tetrazoles from the reaction of various aromatic amines with sodium azide and triethyl orthoformate in solvent-free condition at 100°C in good to high yields (Scheme 2).
Results and discussion
Synthesis of the new Cu nano-catalyst
The new copper nano-catalyst was synthesized in successive steps which will comprehensively be explained in the experimental section.
Characterization of the catalyst
Synthesis of the new copper nano-catalyst was confirmed by chemical analysis, inductively coupled plasma (ICP), FT-IR, X-ray diffraction (XRD), dispersive X-ray spectroscopy (EDX), scanning electron microscope (SEM), transmission electron microscope (TEM), thermo-gravimetric-differential thermal analysis (TG-DTA) and vibrating sample magnetometer (VSM).
Characterization of the Cu catalyst by ICP
The ICP analysis of the Cu nano-catalyst showed that the Cu content of the catalyst was about 23.04%.
Characterization of the catalyst by IR spectroscopy
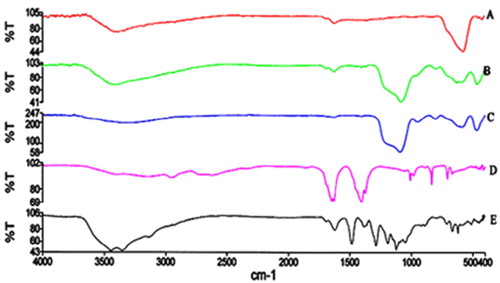
shows the five FT-IR spectra of (A) Fe3O4 magnetic nanoparticles, (B) Fe3O4/SiO2, (C) Fe3O4/SiO2/ CPTMS, (D) Fe3O4/SiO2/CPTMS/MT and (E) Fe3O4/SiO2/CPTMS/MT/Cu. The IR band in 579 cm−1 in the curve A is related to the Fe–O stretching vibrations. Curve B shows a broad band near 1086 cm−1 which indicates the silica-coated magnetite nanoparticles. Curve C shows a new peak at about 583 cm−1 indicating the presence of the C–Cl bond. Curve D shows two new peaks at 1372 and 1632 cm−1 which are attributed to the C–N and C=N bonds, respectively. Curve E shows a shift from 1632 to 1623 cm−1 which is related to the new interaction of the copper with the nitrogen of the C=N bond. As a result, comparison of the IR spectra confirms the successful preparation of the Cu nano-catalyst.
Characterization of the catalyst by the XRD patterns
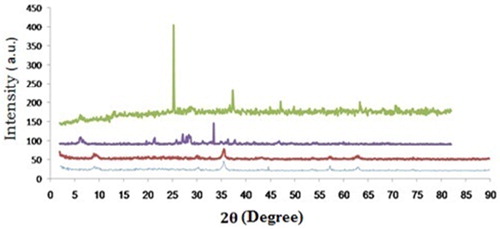
(B, blue) shows the XRD pattern of Fe3O4/SiO2 at about 2θ = 10, 30, 35, 45, 53, 57, 63 and 74, which confirms the structure of the silica-coated magnetite nanoparticles. The appearance of the new peaks at about 2θ = 25, 37, 47, 63 and 71 are attributed to the Cu species (E, green).
Characterization of the catalyst by the EDX analysis
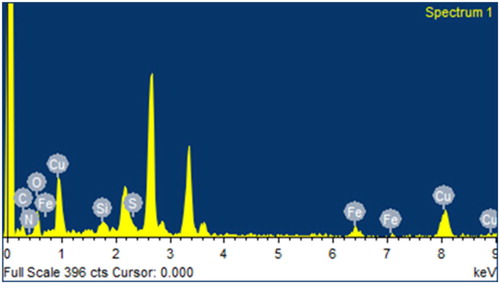
All the predicted elements (C, N, O, S, Si, Fe and Cu) of the new copper nano-catalyst can be seen in the EDX analysis ().
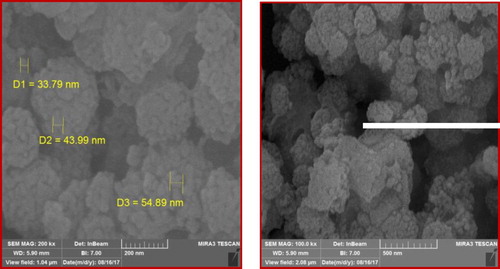
Characterization of the catalyst by the SEM and the TEM images
and show the SEM and TEM particle sizes and morphology images of the new copper nano-catalyst. These images confirmed the nanometer dimensions of the catalyst particles and the sizes of the Cu nano-catalyst particles are in the nanometer ranges (between 33.79 and 54.89 nm).
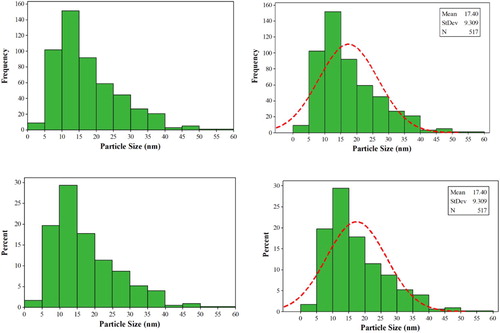
At a closer look, as shown in the particle size distribution histograms (), the sizes of the nanoparticles are between 5 and 40 nm and the average particle size is estimated about 17.4 nm, while 70% of the catalyst particles have an average size of about 5–20 nm.
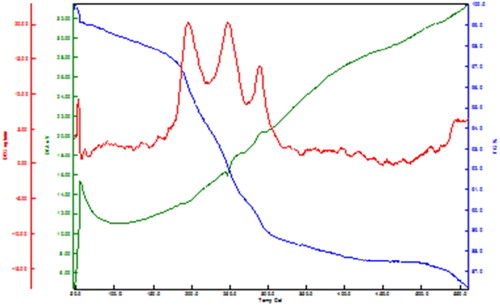
Characterization of the catalyst by the TGA-DTA technique
shows the TGA-DTA curves of the new copper nano-catalyst indicating four weight-loss steps in the temperature ranges of 90°C, 190°C, 230°C, 290°C, respectively. The first weight loss at 90°C is probably due to the residual water, the second step at 190°C is attributed to the thermal decomposition of the complex, the third step at 230°C is probably due to the thermal decomposition of MT ligand, and the fourth step at 290°C is probably due to the thermal decomposition the CPTMS ligand.
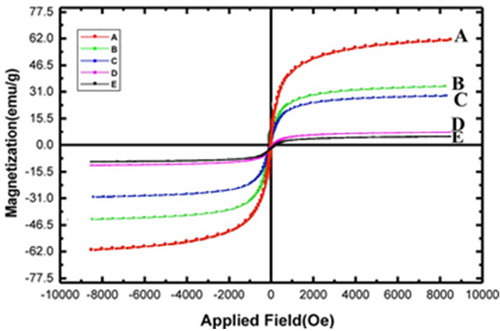
Characterization of the catalyst by the VSM technique
The VSM analyses of the five compounds namely (A) Fe3O4 magnetic nanoparticles, (B) Fe3O4/SiO2, (C) Fe3O4/SiO2/CPTMS, (D) Fe3O4/SiO2/CPTMS/MT and (E) Fe3O4/SiO2/CPTMS/MT/Cu were performed in order to demonstrate and compare their magnetic properties (). As can be seen, all five compounds have magnetic properties; and show a nice decrease from A to E (62, 35, 30, 5.65 and 4.28 emu/g, respectively). This observation can be explained by the reduction of different interactions between nanoparticles before and after various functionalization and complexation which cause the more coating of the Fe3O4 magnetic nanoparticles.
On the basis of these results, modification and the well grafting of ligand groups are verified and indicated that the Cu catalyst has approximately good thermal stability which is probably due to the strong interactions between the SiO2 coating layer, the ligands and the Fe3O4 magnetic nanoparticles.
Optimization of the Cu nano-catalyst
The catalyst activity was optimized in the synthesis of 1-(4-nitrophenyl)-1H-tetrazole from the reaction of 4-nitroaniline with sodium azide and TEOF under different conditions of temperatures, amount of the catalyst and solvents. The best result was obtained with 2 mmole of amine, 2 mmole of sodium azide, 2 mmole of TEOF and 50 mg of the Cu nano-particle catalyst in solvent-free condition at 100°C ().
Table 1. Effect of the amount of the catalyst on the synthesis of 1-(4-nitrophenyl)-1H-tetrazole in solvent-free condition at 100°C.
Table 2. Effect of solvent on the synthesis of 1-(4-nitrophenyl)-1H-tetrazole at 100°C.
Table 3. Effect of temperature on the synthesis of 1-(4-nitrophenyl)-1H-tetrazole in solvent-free condition.
Applying the optimized results from the model reaction (entry 9: synthesis of 1-(4-nitrophenyl)-1H-tetrazole from the reaction of 4-nitroaniline (2.0 mmole) with sodium azide (2.0 mmole) and TEOF (2.0 mmole) in solvent-free condition at 100°C), various tetrazoles were synthesized with good to excellent yields ().
Table 4. Synthesis of various tetrazoles (2a–j).
The structure of all tetrazole products was confirmed by comparison of their spectra with authentic samples which were in agreement with their IR and NMR spectra. In the IR spectra of all products, the sharp NH2 peaks (about 3300–3500 cm−1) were totally disappeared (Supporting Information).
The plausible mechanism
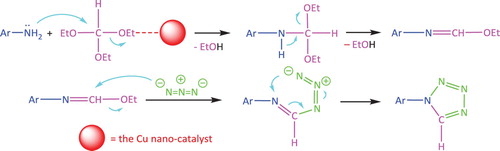
The plausible mechanism for the synthesis of various tetrazoles from the reaction of various amines with sodium azide and TEOF is shown in the presence of the Cu nano-catalyst (Scheme 3). Because of the Lewis acidity, the copper nano-catalyst will attach to the oxygen of the ethoxy group of TEOF to facilitate the nucleophilic attack of the nitrogen atom of the amino group with successive deletion of two molecules of ethanol. Then, the nucleophilic attack of the azide anion will form the intermediate, and with the subsequent cyclization, the final product will be obtained.
Reusability of the catalyst
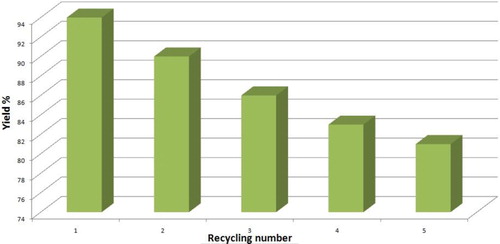
The recyclability of the Cu nano-catalyst was investigated with the model reaction to find the capability of the catalyst. Therefore, the copper nano-catalyst was filtered after the first run, washed with ethanol and dried under vacuum and then reused for the next successive runs under the same conditions (). It was shown that the applied Cu nano-catalyst is capable and reusable even after five runs; and the catalytic activity was almost as same as the fresh one during the preparation of various tetrazoles (94%, 90%, 86%, 83% and 81%, respectively).
Conclusions
In summary, the new Cu nano-catalyst was successfully prepared in four successive stages, characterized with different techniques (ICP, XRD, EDX, SEM, TEM, TG-DTA and VSM) and used for the synthesis of diverse tetrazoles from the reaction of various aromatic amines with sodium azide and tetraethyl orthosilicate in solvent-free conditions at 100°C with good to high yields.
Experimental
The following scientific equipments were used for the characterization of the copper nano-catalyst as well as the tetrazole compounds:
A Perkin–Elmer GX Fourier transforms infrared spectrometer (FT-IR);
A Jeol FT-NMR 90 spectrometer;
A Zeiss-EM10C-80 KV transmission electron microscope;
A Philips XL-30 scanning electron microscope;
A Bruker D8 Advance powder diffractometer (the XRD measurements);
A Zetasizer Nano-ZS-90 (ZEN 3600, MALVERN) instrument (the size distributions measurements);
A Perkin–Elmer ICP/6500 instrument (the ICP measurements), and
A Meghnatis Daghigh Kavir Company Vibrating Sample Magnetometer (VSM).
Chemicals and solvents were purchased from foreign chemical companies and used without further purification.
Synthesis of the novel Cu nano-catalyst (Scheme 1)
The novel Cu nano-catalyst was prepared in the following four successive stages:
Stage 1: general procedure for the preparation of Fe3O4: The solution of FeCl3.6H2O (11.44 g) and FeCl2.4H2O (4.3 g) in water (100 ml) was stirred for 0.5 h in 80°C, and the solution of 37% ammonia added dropwise. The mixture was heated at 70°C with vigorous stirring in pH 10 for 0.5 h, then the black solid filtered, washed with water and dried at 80°C overnight.
Stage 2: Coating of the Fe3O4 magnetic nanoparticles with SiO2 (Fe3O4/SiO2): Fe3O4 MNPs (0.2 g) were dispersed into a solution containing ethanol and distilled water (250 ml, V/V = 4:1) and NH3.H2O (3 ml) under ultrasonication. Then, tetraethyl orthosilicate (2 ml) was slowly added dropwise and the mixture stirred for further 6 h. Fe3O4/SiO2 was obtained by centrifugation, washed with water and EtOH for several times, and dried in vacuo.
Stage 3: Functionalization of the coated Fe3O4 with the CPTMS ligand (Fe3O4/SiO2/CPTMS): The CPTMS ligand (1.0 ml, 5 mmol) dissolved in dry toluene (100 ml) and Fe3O4/SiO2 (1.0 g, prepared in Stage 2) added and stirred for 18 h at 60°C. Then, the obtained product was separated with strong magnet, washed with toluene and dried in vacuo.
Further functionalization with the MT ligand (Fe3O4/SiO2/CPTMS/MT): The MT ligand (0.42 g, 5.0 mmol) and K2CO3 (0.69 g, 5 mmol) in toluene (60 ml) were added to Fe3O4/SiO2/CPTMS (0.1 g) and refluxed for 12 h. Then, the obtained product was separated with strong magnet, washed repeatedly with EtOH and water and dried in vacuo, and
Stage 4: Complexation of Fe3O4/SiO2/CPTMS/MT with CuCl2 (Fe3O4/SiO2/CPTMS/AT/Cu): Fe3O4/SiO2/ CPTMS/MT (0.1 g) was added to a dispersed CuCl2 (0.85 g, 5 mmol) in EtOH and stirred vigorously for 8 h at 80°C. Then, the resulting Cu catalyst was separated with magnet, washed with EtOH and air-dried.
Typical procedure for preparation of the 1-substituted-1H-tetrazoles
A mixture of amine (2.0 mmol), sodium azide (0.13 g, 2.0 mmol), triethyl orthoformate (0.296 g, 2.0 mmol) and the Cu nano-catalyst (50 mg) was stirred at 100°C until the TLC monitoring showed no further progress in the conversion. The reaction mixture cooled to room temperature, the catalyst was separated by strong magnet, the mixture poured over ice, and the organic layer extracted with ethyl acetate (2 × 20 ml). Then, anhydrous MgSO4 was added, the solid filtered and the solvent concentrated and recrystallized from the EtOAc/hexane mixture (1:10).
Spectral data of the products
1-Phenyl-1H-tetrazole (2a): FT-IR (KBr): 3051, 2925, 2854, 1678, 1660, 1585, 1487, 1448, 1316, 1279, 1227, 1209, 1171, 1153, 1076, 986, 899, 806, 765, 754, 694, 619 and 593 cm−1; 1H NMR (90 MHz, CDCl3), δH: 8.27 (s, 1H), 6.98-7.60 (m, 5H).
1-(2,4-Dimethylphenyl)-1H-tetrazole (2b): FT-IR (KBr): 3157, 3015, 2876, 1665, 1496, 1302, 1206, 1036, 815 and 561 cm−1; 1H NMR (90 MHz, CDCl3), δH: 8.39 (s, 1H), 6.96 (m, 3H), 2.29 (s, 6H).
1-(4-Methylphenyl)-1H-tetrazole (2c): FT-IR (Nujol, cm−1): 3027, 2914, 2857, 1672, 1608, 1587, 1521, 1506, 1408, 1313, 1217, 1202, 1174, 1110, 1040, 985, 938, 821, 738, 716, 644, 578; 1H NMR (90 MHz, CDCl3): δH = 8.16 (s, 1H), 7.27-6.92 (m, 4H), 2.30 (s, 3H).
1-(4-Methoxyphenyl)-1H-tetrazole (2d): FT-IR (Nujol): 1667, 1504, 1464, 1243, 1034 and 826 cm−1; 1H NMR (90 MHz, CDCl3), δH: 8.05 (s, 1H), 6.88 (m, 4H), 3.7 (s, 3H).
1-(4-Acetylphenyl)-1H-tetrazole (2e): FT-IR (KBr, cm−1): 3059, 2996, 2962, 1674, 1603, 1589, 1503, 1483, 1411, 1359, 1324, 1303, 1271, 1211, 1186, 1172, 997, 957, 910, 841, 815, 734, 685, 635, 590; 1H NMR (90 MHz, CDCl3): δH = 8.29 (s, 1H), 8.00-7.09 (m, 4H), 2.59 (s, 3H).
1-(4-Bromophenyl)-1H-tetrazole (2f): FT-IR (KBr): 3527, 3151, 3045, 2855, 1660, 1589, 1575, 1483, 1402, 1312, 1291, 1224, 1206, 1073, 1008 and 827 cm−1; 1H NMR (90 MHz, CDCl3), δH: 8.07 (s, 1H), 7.40 (d, J = 8.6 Hz, 2H), 6.90 (d, J = 8.6 Hz, 2H).
1-(2-Chlorophenyl)-1H-tetrazole (2g): FT-IR (KBr): 2998, 2866, 1666, 1583, 1502, 1475, 1454, 1439, 1376, 1309, 1264, 1207, 1132, 1058, 1039, 1000, 935, 825, 752, 702 and 657 cm−1; 1H NMR (90 MHz, CDCl3), δH: 8.07 (s, 1H), 7.44-7.10 (m, 4H).
1-(4-Chlorophenyl)-1H-tetrazole (2h): FT-IR (KBr): 2925, 1663, 1581, 1488, 1405, 1311, 1292, 1226, 1205, 1172, 1090, 1006, 984, 829, 810, 700, 650, 631 and 570 cm−1; 1H NMR (90 MHz, CDCl3), δH: 8.09 (s, 1H), 7.28 (d, J = 6.7 Hz, 2H), 6.96 (d, J = 6.7 Hz, 2H).
1-(4-Nitrophenyl)-1H-tetrazole (2i): FT-IR (Nujol, cm−1): 3105, 2985, 1660, 1599, 1579, 1508, 1396, 1344, 1313, 1301, 1238, 1205, 1173, 1107, 989, 958, 861, 845, 784, 750, 708, 687, 642; 1H NMR (90 MHz, acetone-d6): δH = 8.52 (s, 1H), 8.23 (d, J = 8.8 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H).
1-(3-Trifluoromethylphenyl)-1H-tetrazole (2j): FT-IR (KBr, cm−1): 3275, 3220, 3140, 3005, 2981, 2940, 2840, 1620, 1585; 1H NMR (90 MHz, DMSO-d6): δH = 8.19 (s, 1H), 7.60-7.20 (m, 4H).
Supplemental Material
Download MS Word (2.7 MB)Acknowledgements
The authors are grateful to the Bu-Ali Sina University, Hamedan Iran, for the financial support of this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes on contributors
Dr Davood Habibi, Professor of Chemistry and Head, Organic Chemistry Department, Faculty of Chemistry, Bu Ali Sina University, Hamedan, 6517838683, Iran.
Miss Vahideh Khorramabadi, PhD student, Organic Chemistry Department, Faculty of Chemistry, Bu Ali Sina University, Hamedan, 6517838683, Iran.
Dr Somayyeh Heydari, Researcher at Department of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan, 6517838683 Iran, and Department of research and development of the Faran Shimi, Pharmaceutical Company, Tuyserkan, Iran.
References
- Bulter R.N., In Comprehensive Heterocyclic Chemistry II. Katritzky, A.R.; Rees, C.W.; Scriven, E.F.V. Eds.; Pergamon: New York, 1996.
- Herr, R.J. Bioorg. Med. Chem. 2002, 10, 3379–3393. doi: 10.1016/S0968-0896(02)00239-0
- Mitch, C.H.; Quimby, S.J. Int. Patent WO. 1998, 9851312; Chem. Abstr. 1998, 130, 13997.
- Jursic, B.S.; LeBlanc, B.W. J. Heterocycl. Chem. 1998, 35, 405–408. doi: 10.1002/jhet.5570350224
- Zhao-Xu, C.; Heming, X. Int. J. Quantum Chem. 2000, 79, 350–357. doi: 10.1002/1097-461X(2000)79:6<350::AID-QUA3>3.0.CO;2-T
- Ek, F.; Wistrand, L.-G.; Frejd, T. Tetrahedron 2003, 59, 6759–6769. doi: 10.1016/S0040-4020(03)00818-4
- Rhonnstad, P.; Wensbo, D. Tetrahedron Lett. 2002, 43, 3137–3139. doi: 10.1016/S0040-4039(02)00490-2
- Modarresi-Alam, A.R.; Khamooshi, F.; Rostamizadeh, M.; Kieykha, H.; Nasrollahzadeh, M.; Bijanzadeh, H.R.; Kleinpeter, E. J. Mol. Struct. 2007, 841, 67–72. doi: 10.1016/j.molstruc.2006.11.058
- Jursic, B.S.; Leblanc, B.W. J. Heterocycl. Chem. 1998, 35, 405–408. doi: 10.1002/jhet.5570350224
- Zhao-Xu, C.; Heming, X. Int. J. Quantum Chem. 2000, 79, 350–357. doi: 10.1002/1097-461X(2000)79:6<350::AID-QUA3>3.0.CO;2-T
- Lim, C.W.; Lee, I.S. Nano. Today 2010, 5, 412–434. doi: 10.1016/j.nantod.2010.08.008
- Kusrini, E.; Sakadewa, O.; Pasca, G.; Usman, A.; Yulizar, Y. IOP Conf. Series Mater. Sci. Eng. 2018, 440, 012029. doi: 10.1088/1757-899X/440/1/012029
- Kusrini, E.; Oktavianto, F.; Simanjuntak, A.; Pasca, G.; Usman, A. E3S Web Conf. 2018, 67, 03029. doi: 10.1051/e3sconf/20186703029
- Herr, R.J. Bioorg. Med. Chem. 2002, 10, 3379–3393. doi: 10.1016/S0968-0896(02)00239-0
- Jursic, B.S.; Leblanc, B.W. J. Heterocycl. Chem. 1998, 35, 405–408. doi: 10.1002/jhet.5570350224
- Jin, T.; Kitahara, F.; Kamijo, S.; Yamamoto, Y. Tetrahedron Lett. 2008, 49, 2824–2827. doi: 10.1016/j.tetlet.2008.02.115
- Habibi, D.; Nasrollahzadeh, M.; Faraji, A.; Bayat, Y. Tetrahedron 2010, 66, 3866–3870. doi: 10.1016/j.tet.2010.03.003
- Habibi, D.; Nasrollahzadeh, M. Synth. Commun. 2010, 40, 3159–3167. doi: 10.1080/00397910903370683
- Nasrollahzadeh, M.; Habibi, D.; Shahkarami, Z.; Bayat, Y. Tetrahedron 2009, 65, 10715–10719. doi: 10.1016/j.tet.2009.10.029
- Habibi, D.; Nasrollahzadeh, M. Monatsh. Chem. 2012, 143, 925–930. doi: 10.1007/s00706-011-0670-8
- Habibi, D.; Nasrollahzadeh, M. Synth. Commun. 2012, 42, 2023–2032. doi: 10.1080/00397911.2010.548620
- Habibi, D.; Heydari, S.; Afsharfarnia, M.; Rostami, Z. Appl. Organomet. Chem. 2017, 31, e3826. doi: 10.1002/aoc.3826
- Habibi, D.; Heydari, S.; Gil, A.; Afsharfarnia, M.; Faraji, A.; Karamian, R.; Asadbegy, M. Appl. Organomet. Chem. 2018, 32, e4005. doi: 10.1002/aoc.4005
- Habibi, D.; Nasrollahzadeh, M.; Kamali, T.A. Green Chem. 2011, 13, 3499–3504. doi: 10.1039/c1gc15245a