ABSTRACT
A novel mesoporous Al-SBA-15 modified by N,N'-(1,2-phenylene)bis(2-aminobenzamide) dichloro cobalt has been prepared and applied as a reusable catalyst in the 3-cinnamoyl coumarins synthesis via three-component reaction between benzaldehydes, salicylaldehydes and ethyl acetoacetate by the assist of ultrasonic irradiation. By using of the nanocatalyst and also ultrasound irradiation, the easiness and velocity of the abovementioned reaction were enhanced and an environment friendly condition was provided to synthesis of various 3-cinnamoyl coumarin compounds. The properties and structure of nanocatalyst have been specified by methods including powder X-ray diffraction (XRD), energy dispersive spectroscopy (EDS), Fourier transform infrared spectroscopy (FT-IR), transmission electron microscopy (TEM), nitrogen adsorption–desorption analysis and scanning electronic microscopy (SEM). Superiority of this novel and viable method is due to mild reaction condition, short reaction times, high yields of 3-cinnamoyl coumarins, environmentally benign, recoverability of the CoCl2N,N'-(1,2-phenylene)bis(2-aminobenzamide)/Al-SBA-15 catalyst and reusability with important preservation in its catalytic activity.
GRAPHICAL ABSTRACT
1. Introduction
Ordered structures of mesoporous silica containing SBA-n, MSU-X and M41S as significant category of compounds have been raised for different applications inclusive separation and catalysis (Citation1,Citation2). SBA-15 has attracted attention of many researchers because of its great specific surface area, prominent mechanical and thermal stability, high pore dimensions, particular surface acidity and monotonous size of pores (Citation3–8). The elements like zirconium, titanium and aluminum can be inserted into hexagonal silica structure to create substances with extensive applications in ion exchange and catalysis processes. Between these metal-incorporated mesoporous materials, aluminum substituted-silica have revealed prominent potential as acidic catalyst in numerous reactions (Citation9–11). Various studies have incorporated organic–inorganic multifunctional groups into heterogeneous catalysts, which can applied to catalyze chemical processes (Citation12,Citation13). A lot of active sites in the functional organic–inorganic hybrid catalysts seriously enhanced the rate of organic reactions (Citation14). These materials represent imperative applications in biomedicine, catalysis, biotechnology and drug delivery (Citation15–17). Mesoporous silica functionalized by organic–inorganic hybrid structures has been used to catalyze some organic reactions by creating environmentally mild condition, excellent surface area, effective recovery from reactants and reusability (Citation18,Citation19).
In the recent years, sonochemistry has attracted many attention as an effective method which was preponderantly employed in the numerous fields including catalyzed reactions (e.g. heterogeneous catalysis, enzymatic reactions, using ion exchange resin, phase transfer catalysis and biphasic reactions) (Citation20–24). The application of ultrasonic technique in organic synthesis has plentiful significant advantages such as milder reaction condition, lower reaction length, higher efficiencies of products, nontoxic and environmental friendly solvent, minimization of side products, saving money and energy in comparison with the conventional heating methods (Citation25–29). In fact, ultrasound irradiation accelerates numerous reactions by means of the making, development and implosive bubbles collapse in liquid phase (Citation30). Cavitational collapse generates high pressures, extreme heating and too small lifetimes. Cavitation as a condensing agent cooperates to dispersion the ultrasound energy (Citation31,Citation32).
Coumarins have some pharmacological and biological activities for example anticoagulant, antimicrobial, antibacterial, pesticidal and fungicidal (Citation33–37). Moreover, different derivatives of coumarin find in numerous pharmacologically active structures, components of some natural products (Citation38), brighting compounds, dyes of laser devices (Citation39) and a categories of pigments in dye solar cells (Citation40–42). Chalcones constitute remarkable component of many natural compounds such as tea, soy-based foodstuffs, fruits and vegetables (Citation43,Citation44). The chalcones represent biological activities containing anti-inflammatory (Citation45), antioxidant (Citation46), antifungal (Citation47), antimalarial (Citation48), analgesic (Citation49), antituberculosis (Citation50), antitumor (Citation51) and anti-HIV (Citation52) functions. In addition, other significant properties of chalcones are monitoring cholesterol amount, blood purification, intensification of immune system, controlling blood pressure, improving metabolism, preventing of thrombus formation and suppressing acid secretion (Citation53). Synthesis of coumarin-chalcone compounds is generally performed by Claisen–Schmidt aldol reaction between aromatic aldehydes and 3-acetylcoumarins using basic or acidic catalyst such as piperidine (Citation54–56) and heterogeneous systems (e.g. Bi(OTf)3) (Citation57). Disadvantages of the above procedures are light harvests of products, toxic reaction condition, nonrecoverability and nonreusability of catalyst. Also in these methods, an additional step for the synthesis of substrate 3-acetylcoumarin is needed to perform.
Thus, to avoid these problems, the development of an alternative method with convenient and environment friendly procedure by a catalyst with high catalytic performance for 3-cinnamoyl coumarins synthesis is still favored. The target of ongoing investigation is minimalization of aforementioned limitations for the 3-cinnamoyl coumarins preparation by a three-component, one-pot procedure using functionalized mesoporous Al-SBA-15 and also with the assist of ultrasound irradiation. Therefore, the novel hybrid CoCl2 N,N'-(1,2-phenylene) bis(2-aminobenzamide)/Al-SBA-15 (CoCl2NN'PhBIA/Al-SBA-15) was prepared, identified and used in the sonochemically synthesis of 3-cinnamoyl coumarin compounds by three-component reaction of salicylaldehyde, ethyl acetoacetate and benzaldehydes (Scheme 1). This method makes many superiorities such as providing sustainable and green condition, using simple one-pot way to prepare 3-cinnamoyl coumarins, benign reaction condition, recoverable and reusable hybrid nano-catalyst.
2. Results and discussions
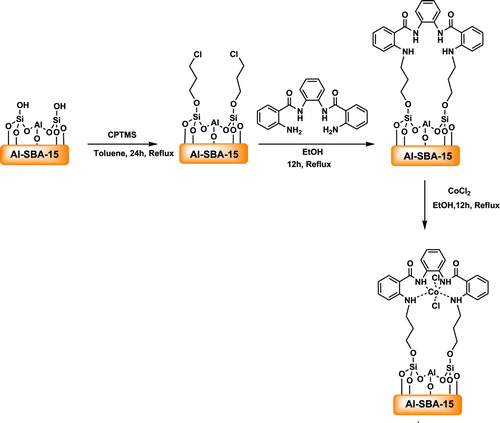
2.1. Preparation and identification of mesoporous CoCl2NN'PhBIA/Al-SBA-15
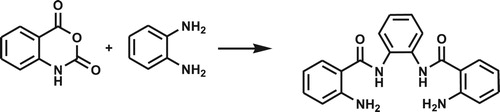
N,N'-(1,2-phenylene)bis(2-aminobenzamide) (NN'PhBIA) was made via the condensation reaction of isatoic anhydride and 1,2-phenylene diamine by molar ratio 2:1 (Scheme 2).
A pathway for the synthesis of CoCl2NN'PhBIA/Al-SBA-15 is represented in Scheme 3. The process was started from the functionalization of Al-SBA-15 surface by chloropropyl linkers through the reaction with (3-Chloropropyl) trimethoxysilane (CPTMS). Next the ligand (NN'PhBIA) was immobilized on the Al-SBA-15 by the replacement of amin (in the ligand) with chloro on the CPTMS/Al-SBA-15 surface. At the final step, dichloro cobalt was attached to the NN'PhBIA/Al-SBA-15 complex to create CoCl2NN'PhBIA/Al-SBA-15 catalyst was created. Structure properties and functional groups of the nanocatalyst was specified using methods containing XRD, FT-IR, TEM, SEM, EDS and N2 adsorption–desorption.
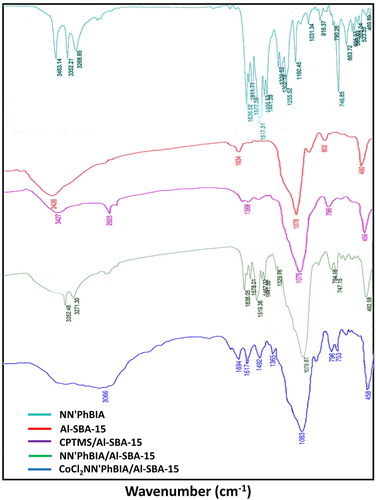
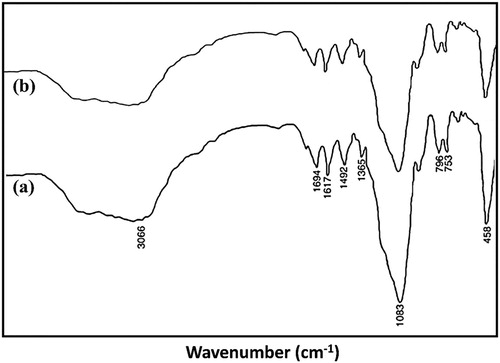
FT-IR spectrums of NN'PhBIA ligand, mesoporous Al-SBA-15 and modified Al-SBA-15 materials (CPTMS/Al-SBA-15, NN'PhBIA/Al-SBA-15 and CoCl2 NN'PhBIA/Al-SBA-15) is illustrated in . The detected bands in the range of 600–1200 cm−1 is recognized as Si–O vibrations in the hexagonal framework of silica. The specific peaks of compounds involving Al-SBA-15 observed at 460, 802 and 1078 cm−1 are charactrized as the O–Si bending vibration, stretching and anti-stretching vibrations of the Si–O–Si group, correspondingly. In the ligand FT-IR spectra, absorption peaks at frequencies 3268, 3352 and 3463cm−1 are associated with –NH2 stretching vibration of the amide and amine substitutions in ligand structure. The specified peaks of carbonyl in the aminobenzamid is appeared at 1636 cm−1 and also the bands in 1577 and 1611 cm−1 frequencies are detected due to stretching vibration of aromatic rings. Stretching vibration peaks of N-H for amine and amide in the NN'PhBIA attached to Al-SBA-15 spectra are revealed at 3271 and 3352 cm−1 frequencies with less peak severity which confirms linkage of ligand to CPTMS/Al-SBA-15. By loading cobalt on NN'PhBIA/Al-SBA-15 complex, transference the characteristic bands at 1636–1694 cm−1 approves the creation of connection between cobalt and NN'PhBIA. These consequences are evidence for the immobilization of the ligand on the Al-SBA-15 surface and anchoring to Co and moreover the molecular structure of the functional parts is absolutely preserved in the CoCl2NN'PhBIA/Al-SBA-15.
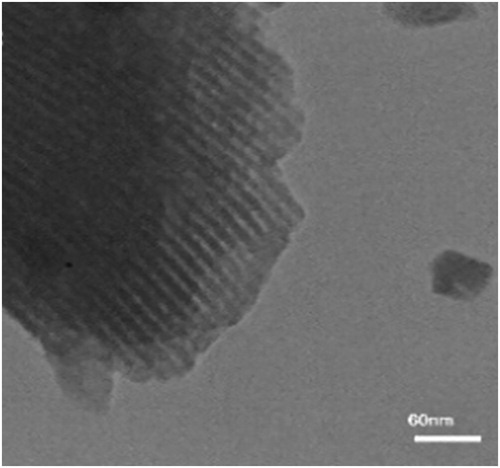
represents the TEM picture of the CoCl2NN'PhBIA immobilized on Al-SBA-15 which proves regularly distribution of hexagonal structure in the pores channels of complex. The Co aggregation is not shown in the pores, which demonstrated incorporation of Co2+ ions to nitrogen atoms in the NN'PhBIA.
The SEM micrograph of Al-SBA-15 at (a) reveals comparatively uniform sizes of bagel-shaped units. (b) depicts SEM picture of CoCl2NN'PhBIA/Al-SBA-15 which demonstrates no remarkable change in Al-SBA-15 surface morphology.
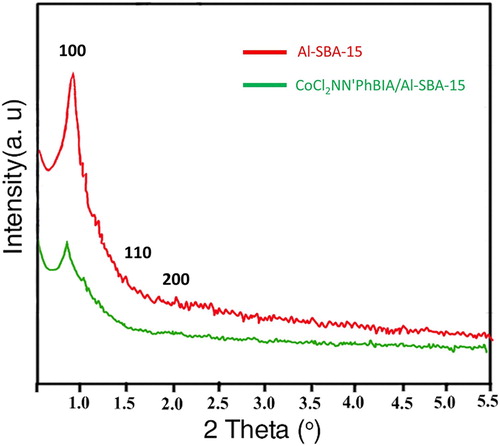
shows the XRD patterns of bare Al-SBA-15 and organic–inorganic hybrid CoCl2NN'PhBIA/Al-SBA-15. The observed Bragg peaks in 2θ = 0.8–2 related to the two-dimensional arrangement of the hexagonal SBA-15 compounds. After immobilization of the ligand and functionalization with Co, the XRD pattern exhibits retention of the Al-SBA-15 framework. Diffraction peaks for the cobalt atoms are not detected, and the Al-SBA-15 mesoporous structure retains unchanged. With these outcomes, immobilization of Co into the Al-SBA-15 hexagonal channels as atom distribution and no existence of crystal cobalt in the catalyst are resulted. The weakend violence of peaks in the CoCl2NN'PhBIA/Al-SBA-15 pattern is because of functionalization which can partially block the channels and also diminish the mesoscopic ordering (Citation58).
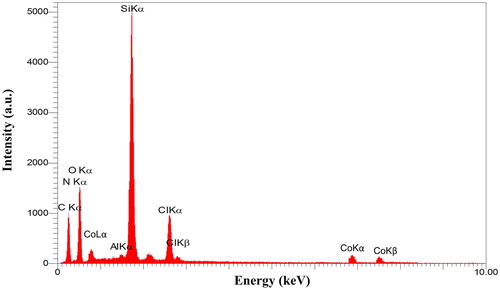
Elemental analysis of the CoCl2NN'PhBIA/Al-SBA-15 nanocatalyst attained by Energy Dispersive Spectroscopy (EDS) confirms the existence of nitrogen, carbon, oxygen, silicon, aluminum, chlorine and cobalt with the weight percentage 7.71, 32.05, 33.91, 13.29, 0.29, 7.31 and 5.44 (wt %), respectively ().
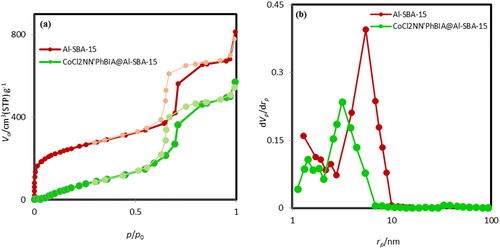
Distinguished surface area of mesoporous Al-SBA-15 and Al-SBA-15 modified by CoCl2NN'PhBIA were determined via nitrogen adsorption–desorption analysis as indicated in (a). The curves of N2 adsorption–desorption designate a typical isotherm with a H1 hysteresis node (commencing from P/P0 = 0.6) of type IV. These results are in well consistency with mesostructure compounds. The nanocatalyst CoCl2NN'PhBIA/Al-SBA-15 surface area was measured at 582 m2/g which was fewer than Al-SBA-15 specific surface area (815 m2/g) owing to insertion of NH2, phenyl and cobalt into the Al-SBA-15 hexagonal channels. The pore dimension distribution diagrams for modified and bare Al-SBA-15 (depicted in (b)) represent the bounded pore size dispensation for them placed on 3.17 and 5.52 nm, respectively. Decrement in CoCl2NN'PhBIA/Al-SBA-15 pore dimension is an approval for modification of Al-SBA-15 surface by the ligand and Co atom.
2.2. Preparation of 3-cinnamoyl coumarins
In the course of our efforts to introduce a green and sustainable route for the synthesis of 3-cinnamoyl coumarin compounds, we here in report three-component reaction of ethyl acetoacetate, salicylaldehyde and different substituted benzaldehydes at presence the CoCl2NN'PhBIA/Al-SBA-15 nano-catalyst under ultrasonic irradiation.
In order to optimization the reaction condition, the preparation of 4e was designated as a model reaction. The influence of various catalysts, solvents, amount of catalyst and different reaction condition on the model reaction were investigated (). The entries 1–6 of this table are related to the investigation of different solvent on this reaction which shows the best result is provided by ethanol as solvent under ultrasound irradiation (entry 6 of ). Study the effect of various catalysts containing P2O5, CuO, CoCl2, ZnO, mesoporous Al-SBA-15 and organic–inorganic hybrid CoCl2NN'PhBIA/Al-SBA-15 (, entries 6-12) demonstrated that the model reaction effectively proceeded using CoCl2NN'PhBIA/Al-SBA-15 as catalyst. Also the maximum yield for 4e was afforded by 3 mg of it (entry 14 of ). According to the results of this table, the ultrasonic irradiation provided higher yields of 4e at shorter reaction times in comparison with the heating process. Ultrasound condition caused to increasing the number and dimension of active cavitation bubbles resulted in upper maximum collapse temperature, thus the preparation of 3-cinnamoyl coumarin derivatives was accelerated under ultrasonic irradiation. Also, sonication technique is more environmental friendly owing to its fundamental green chemistry conception.
Table 1. Investigation the reaction condition of the model reactiona. 
To find the best power of ultrasonic irradiation for the three-component reaction of salicylaldehydes, benzaldehydes and ethyl acetoacetate this reaction was performed under various powers of ultrasonic irradiation (). The resultants prove the reaction is impressively improved by 3 mg of CoCl2NN'PhBIA/AL-SBA-15 under power 55 W of the ultrasound irradiation.
Table 2. Examination of the ultrasonic power effect on the 4e synthesisa.
Sonochemically synthesis of 3-cinnamoyl coumarin derivatives from three-component reaction of different salicylaldehydes, ethyl acetoacetate and varied benzaldehydes was performed by CoCl2NN'PhBIA/AL-SBA-15 under similar condition. Great yields of 3-cinnamoyl coumarin were gained by applying different functional groups on the salicylaldehyde and aromatic aldehydes ().
Table 3. Sonosynthesis of 3-cinnamoyl coumarin derivatives by the CoCl2NN'PhBIA/Al-SBA-15a.
2.3. Proposed mechanism
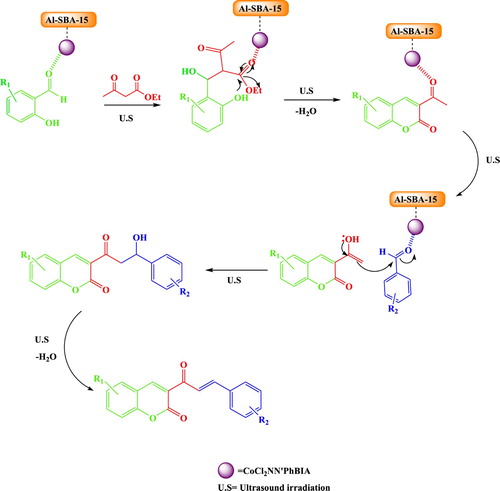
Scheme 4 demonstrates a conceivable pathway for the three-component synthesis of 3-cinnamoyl coumarins using the organic–inorganic hybrid catalyst. It is presumed that cobalt from the hybrid catalyst as a Lewis acid activates carbonyl groups of the substrates by coordination to the oxygen (from carbonyl groups). At first, the O from the carbonyl group of salicylaldehyde created a complex with Co from CoCl2NN'PhBIA/AL-SBA-15 which then reacts with active methylene group of ethyl acetoacetate. 3-acetylcoumarin is produced by transesterification reaction in this intermediate and following dehydration. Afterward, oxygen from carbonyl groups of 3-acetyl coumarin coordinates to Co from CoCl2NN'PhBIA/AL-SBA-15 and enol is generated. Next, the aldol condensation takes place between aromatic aldehyde and the enol form. Nitrogen atoms in NN'PhBIA ligand forms hydrogen bonding with the hydrogens of enol hydroxyl and accelerates this reaction. At the end of process, dehydration reaction yields 3-cinnamoyl coumarins. The CoCl2NN'PhBIA/Al-SBA-15 improves the reaction condition due to its high surface area. Moreover, the coordination of carbonyls to cobalt atoms accelerates the conjugation and the nucleophile addition to the reactants. Also, the N atoms of NN'PhBIA ligand can activate the aldol condensation and dehydration through hydrogen bonding. These routes is significantly proceed by the cavitation effect of ultrasonic irradiation. Under ultrasonic irradiation, the electronic currents in the conjugated systems of reactants and intermediates are improved and reaction is quickly proceeded to formation of product. Generally, faster electronic resonances in reactants lead to faster nucleophile attacks resulted in increasing the rate of various steps in the proposed mechanism specially transesterification and aldol condensation. In addition, better electronic resonances provide more effective adsorption of reactants on the catalyst surface. Moreover, the electronic resonance in NN'PhBIA of catalyst is increased by ultrasound irradiation and ability of hydrogen bonding formation in N atoms of NN'PhBIA is raised. Also, the bonding energy is created by shock wave and small hot bubbles generated through the cavitation effect of ultrasonic irradiations in liquid phase. These occurrences enhance the adsorption of reactants on the CoCl2NN'PhBIA/Al-SBA-15 surface through enhancing mass transportation of substrates from the liquid phase to the catalyst surface.
2.4. Recyclability of CoCl2NN'PhBIA/Al-SBA-15 nano-catalyst
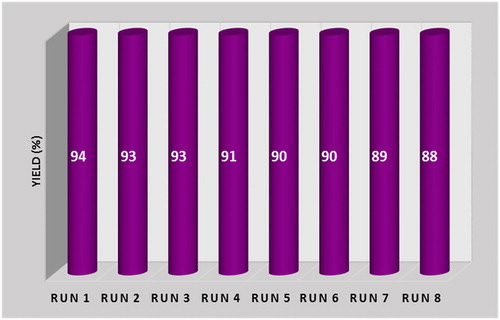
The CoCl2NN'PhBIA/Al-SBA-15 organic–inorganic hybrid is recoverable and reusable with remarkable preservation in its catalytic performance. After ending of the corresponding reaction, the catalyst was recycled from the reaction blend by centrifuging and washed several times with hot ethanol to separate the remaining product. The CoCl2NN'PhBIA/Al-SBA-15 was applied in the other three-component reaction of ethyl acetoacetate, salicylaldehyde and benzaldehydes for eight times. Negligible loss in catalytic activity was afforded and the 3-cinnamoyl coumarins were formed by great yields ().
Figure 7. Recovery chart of CoCl2NN'PhBIA/Al-SBA-15 organic–inorganic hybrid for the model reaction. Required reaction time for every run: run1 = 10 min, run2 = 10 min, run3 = 12 min, run 4 = 15 min, run5 = 15 min, run6 = 15 min, run7 = 20 min, run8 = 20 min.
The structure of mesoporous CoCl2NN'PhBIA/Al-SBA-15 nanocatalyst was investigated using FT-IR spectra before apply in the reaction and after recovery of eight cycles (). As seen in this figure, the corresponding peaks are equal for CoCl2NN'PhBIA/Al-SBA-15 organic–inorganic hybrid before and after the corresponding reaction. These results approve the excellent stability of the mesoporous nanocatalyst in reaction.
3. Conclusion
In the ongoing investigation a convenient, advanced and eco-friendly procedure was presented to prepare 3-cinnamoyl coumarin compounds through tree component condensation of ethyl acetoacetate, salicylaldehyde and different benzaldehydes using mesoporous CoCl2NN'PhBIA/Al-SBA-15 organic–inorganic hybrid as a recoverable and very effectual catalyst under sonication condition. Green technique, the catalyst recyclability and facile reusability for eight times, moderate condition, providing supreme efficiencies of 3-cinnamoyl coumarin compounds at low times are several significant superiorities of the introduced method.
4. Experimental
4.1. Chemicals and devices
The chemicals were bought from Sigma-Aldrich and Merck corporations and were consumed in experiments without extra purification methods.
The ultrasound energy was employed in reactions using a multi-wave ultrasound producer (Sonicator 3200; Bandelin, MS 73, made in Germany), involving a transformer and Ti vibrator (horn) (diameter = 12.5 mm), working at 20 kHz and eldest output power is equal to 200 W. The ultrasound producer advice spontaneously regulates the power balance. Products melting points were determined by using an Electro thermal 9200 device. A Magna 550 device was employed for gaining the FT-IR peaks of products with KBr plates. Mass spectrometer (Finnigan-MAT-8430) was used to obtain electron ionization mass spectral (EIMS) by 70 eV in m/z. The NMR spectrums were achieved by assist of a spectrometer (Bruker Avance-400 MHz) at present tetramethylsilane (TMS) internal standard. Nanomaterials morphologies were imagined by SEM (MIRA 3 TESCAN). Structure and particle size of the organic–inorganic CoCl2NN'PhBIA/Al-SBA-15 hybrid were imagined with a Philips CM10 transition electron microscope (30 kV). The nanocatalysts XRD pattern were recorded on a PHILIPS PW 1510 (made in Netherland) X-ray diffractometer by Cu-Kα light (λ = 0.154056 nm, 2θ = 0.8°–10°). By using a Sigma ZEISS (Oxford Instruments Field Emission) EDS of the nanocatalyst were visualized. The nitrogen adsorption/desorption analyses of bare mesoporous Al-SBA-15 and CoCl2NN'PhBIA/Al-SBA-15 hybrid catalyst were carried out by an automatical gas adsorption device (BEL SORP mini II) at 120°C.
4.2. Synthesis of 2-Amino-N-{2-[(2-aminobenzoyl) amino] phenyl} benzamide
Isatoic anhydride (2 mmol, 0.326 gr) was poured to a mixture containing 1 mmol of 1,2-Phenylenediamine (0.108 g) in 10 ml of hot water. This mixture was mixed on magnetic stirrer at 60°C during 3 h. Heating to the solution was performed until the bubbliness of carbon dioxide was discontinued. The blend was stayed for overnight and afterward NN'PhBIA ligand was purified by recrystallization in methanol. The afforded white powder was specified by NMR and IR methods as: FT-IR (KBr): 3463, 3352, 3268, 1636, 1611, 1577, 1517, 1441, 1255, 1160, 1031, 746 cm−1. 1HNMR (400 MHz, DMSO-d6), δ (ppm) = 9.73 (2H, s, NH), 7.59 (4H, m, Ar–H), 7.26 (2H, m, Ar–H), 7.18 (2H, t, J = 7.6 Hz, Ar–H), 6.74 (2H, d, J = 8.4 Hz, Ar–H), 6.53 (2H, t, J = 7.6 Hz, Ar–H), 6.44 (4H, s, NH2).
4.3. Synthesis of mesoporous Al-SBA-15
Solution A was made by dissolving of pluronic acid (P123 = 2 g) in hydrochloric acid (75 ml, pH = 1.5) and mixing on magnetic stirrer for 6 h at 40°C. Solution B was prepared via mixing tetraethylorthosilicate (TEOS) (3.2 ml), HCl solution (5 ml, pH = 1.5) and aluminum triisopropoxide (0.22 g). The solution B was intensely mixed in a restricted flask during 1.5 h up to being transparent. Subsequently solution B was poured in the solution A and strongly mixed at 40°C in 20 h. The attaining transparent solution was heated to 100°C for 20 h in an autoclave (Teflon-covered stainless steel). The resulting white powder was separated by filtration, cleaned 3 times with water and 2 times by EtOH and then was dried at 100°C during 12 h. The pluronic acid was eliminated through calcination (temperature = 550°C, heating ramp = 1°C /min) for 10 h.
4.4. Immobilization of CPTMS on the Al-SBA-15
1 mL of CPTMS (5.5 mmol) solution was slowly added to an Al-SBA-15 (1 g) suspension in 30 mL of anhydrous toluene and then was refluxed at ambient atmosphere during 24 h. The gained sediment was filtrated and cleaned several times by CH2Cl2 to delete the remained substrates and dried under vacancy for 8 h at 120°C to result CPTMS/Al-SBA-15 powder.
4.5. NN'PhBIA/Al-SBA-15 synthesis
2 mmol of NN'PhBIA (0.7 g,) was poured in an ethanolic mixture of 2 g CPTMS/Al-SBA-15. This mixture was refluxed during 24 h, afterward the sediment was separated by centrifuging, cleaned by cold ethanol and dried at 25°C by means of infrared radiation to attain pure NN'PhBIA/Al-SBA-15 (white solid).
4.6. Synthesis of CoCl2NN'PhBIA/Al-SBA-15
A suspension involving 12.9 mg CoCl2, 0.2 g of NN'PhBIA/Al-SBA-15 and 30 ml of absolute ethanol was mixed by stirrer for 24 h at 25°C. The resultant solid was centrifuged, frequently cleaned by absolute EtOH and subsequently dried using infrared radiation at 25°C to yield CoCl2NN'PhBIA/Al-SBA-15 organic–inorganic hybrid.
4.7. Preparation of 3-cinnamoyl coumarins
A blend of corresponding salicylaldehyde (1 mmol), aromatic aldehyde (1 mmol), ethyl acetoacetate (1.2 mmol) and CoCl2NN'PhBIA/Al-SBA-15 (3 mg) nanocatalyst in ethanol (5 ml) was ultrasonicated at 55 W power for adequate times. After the reaction was terminated (examined by TLC), the blend (with temperature = 65°C) was cooled to 25°C. Then it was poured in acetone and the precipitated catalyst was isolated by centrifuging, washed several times with hot EtOH to separate the residue product and dried for reuse. Also, the residual 3-cinnamoyl coumarin was purified through recrystallization in ethanol.
4.8. Characterization of 3-cinnamoyl coumarin derivatives
1-(3-Coumarinyl)-3-phenyl-2-propen-1-one (4a)
M.p. 179–181°C; FT-IR (KBr): 3032, 2965, 1712, 1680, 1612 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.69 (s, 1H, =CH), 7.88 (d, 1H, J = 11.6, =CH), 7.65–7.51 (m, 4H, Ar–H), 7.40–7.28 (m, 5H, Ar–H), 6.72 (d, 1H, J = 11.6, =CH). 13C NMR (100 MHz, CDCl3), δ (ppm) = 194.25, 162.33, 154.61, 145.63, 139.86, 135.32, 131.98, 130.22, 129.98 (2C, Ar–C), 129.04, 127.85, 126.51 (2C, Ar–C), 125.47, 123.91, 121.57, 113.92. EIMS (70 eV): m/z = 276 (M+).
1-(3-Coumarinyl)-3-(para bromophenyl)-2-propen-1-one (4b)
M.p. 190–191°C; FT-IR (KBr): 3035, 1714, 1676, 1611 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.67 (s, 1H, =CH), 7.88 (d, 1H, J = 12.8, =CH), 7.69–7.51 (m, 4H, Ar–H), 7.36–7.28 (m, 4H, Ar–H), 6.69 (d, 1H, J = 12.8, =CH). 13C NMR (100 MHz, CDCl3), δ (ppm) = 188.95, 159.85, 155.72, 146.74, 137.84, 136.55, 135.16, 131.72, 130.08 (2C, Ar–C), 129.47, 128.70, 126.45, 125.01 (2C, Ar–C), 123.58, 121.29, 115.06. EIMS (70 eV): m/z = 354 (M+).
1-(3-Coumarinyl)-3-(para methoxyphenyl)-2-propen-1-one (4c)
M.p. 141–143°C; FT-IR (KBr): 3041, 1710, 1680, 1611, 1606 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.64 (s, 1H, =CH), 7.78 (d, 1H, J = 13.6, =CH), 7.63-7.54 (m, 4H, Ar–H), 7.41–7.28 (m, 3H, Ar–H), 6.87 (d, 2H, J = 7.2, Ar–H), 3.81 (s, 3H, OCH3). 13C NMR (100 MHz, CDCl3), δ (ppm) = 191.01, 165.52, 162.64, 156.97, 147.87, 139.21, 132.67, 131.52, 130.97 (2C, Ar–C), 129.31, 128.42, 126.69, 125.07 (2C, Ar–C), 124.38, 121.59, 113.97, 56.47. EIMS (70 eV): m/z = 306 (M+).
1-(3-Coumarinyl)-3-(meta nitrophenyl)-2-propen-1-one (4d)
M.p. 204–206°C; FT-IR (KBr): 3038, 1714, 1680, 1609 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.75 (s, 1H, =CH), 8.23 (t, 1H, J = 2.8, Ar–H), 8.092-8.057 (dt, 1H, J1 = 8.4, J2 = 2.8, Ar–H), 7.78–7.69 (m, 2H, Ar–H), 7.47 (t, 1H, J = 8.4, Ar–H), 7.29–7.27 (m, 2H, Ar–H), 7.12-7.01 (m, 3H, Ar–H). 13C NMR (100 MHz, CDCl3), δ (ppm) = 189.12, 165.48, 155.23, 150.13, 146.88, 140.51, 138.39, 136.06, 134.97, 131.24, 130.83, 129.55, 128.74, 126.21, 124.04, 123.62, 120.10, 114.37. EIMS (70 eV): m/z = 321 (M+).
1-(3-Coumarinyl)-3-(para nitrophenyl)-2-propen-1-one (4e)
M.p. 155–157°C; FT-IR (KBr): 3039, 1712, 1680, 1610 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.68 (s, 1H, =CH), 8.15 (d, 2H, J = 6.8, Ar–H), 7.80 (d, 1H, J = 14.4, =CH), 7.56 (d, 2H, J = 6.8, Ar–H), 7.32–7.27 (m, 2H, Ar–H), 7.10–6.99 (m, 3H, Ar–H). 13C NMR (100 MHz, CDCl3), δ (ppm) = 190.11, 164.59, 156.72, 148.73, 139.83, 136.86, 135.03, 131.54, 130.12, 129.85 129.01, 126.46 (2C, Ar–C), 125.57, 123.60 (2C, Ar–C), 120.97, 114.05. EIMS (70 eV): m/z = 321 (M+).
1-(3-Coumarinyl)-3-(para hydroxyphenyl)-2-propen-1-one (4f)
M.p. 175–177°C; FT-IR (KBr): 3370, 3034, 1705, 1683, 1610 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.69 (s, 1H,=CH), 7.89 (d,1H, J = 12.8, =CH), 7.75–7.61 (m, 4H, Ar–H), 7.48–7.31 (m, 3H, Ar–H), 6.88 (d, 2H, J = 12.8, Ar–H), 5.01 (s, 1H, OH). 13C NMR (100 MHz, CDCl3), δ (ppm) = 189.98, 164.21, 162.47, 155.92, 148.23, 139.03, 132.45, 131.30, 129.71, 128.64 (2C, Ar–C), 127.91, 126.79, 125.13, 123.51, 117.14 (2C, Ar–C), 115.26. EIMS (70 eV): m/z = 292 (M+).
1-(3-Coumarinyl)-3-(ortho hydroxyphenyl)-2-propen-1-one (4g)
M.p. 79–81°C; FT-IR (KBr): 3402, 3039, 1712, 1683, 1611 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.72 (s, 1H, =CH), 7.81 (d, 1H, J = 11.6, =CH), 7.18-7.16 (dd, 1H, J1 = 6.4, J2 = 2.8, Ar–H), 7.07 (td, 1H, J1 = 8.4, J2 = 2.8, Ar–H), 6.99-6.88 (m, 4H, Ar–H), 6.78 (d, 1H, J = 11.6, =CH), 6.68 (td, 1H, J1 = 8.8, J2 = 2.4, Ar–H), 6.59 (dd, 1H, J1 = 8.4, J2 = 2.4, Ar–H), 5.08 (s, 1H, OH). 13C NMR (100 MHz, CDCl3), δ (ppm) = 190.99, 163.01, 155.97, 144.78, 139.14, 135.47, 134.73, 131.08, 130.91, 129.47, 128.95, 128.04, 127.75, 126.84, 125.13, 124.73, 123.62, 115.06. EIMS (70 eV): m/z = 292 (M+).
1-(3-Coumarinyl)-3-(para fluorophenyl)-2-propen-1-one (4h)
M.p. 172–173°C; FT-IR (KBr): 3035, 1715, 1667, 1613 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.61 (s, 1H, =CH), 7.81 (d, J = 12.8, 1H, =CH), 7.38–7.29 (m, 4H, Ar–H), 7.16–6.93 (m, 5H, Ar–H). 13C NMR (100 MHz, CDCl3), δ (ppm) = 191.46, 164.27, 156.47, 147.38, 139.76, 136.01, 135.43, 131.28, 130.12, 129.57, 128.12 (2C, Ar–C), 127.79, 125.60, 124.27, 121.37 (2C, Ar–C), 114.81. EIMS (70 eV): m/z = 294 (M+).
1-(3-Coumarinyl)-3-(ortho chlorophenyl)-2-propen-1-one (4i)
M.p. 157–159°C; FT-IR (KBr): 3032, 1715, 1681, 1612 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.61 (s, 1H, =CH), 7.93 (d, 1H, J = 13.6, =CH), 7.33–7.27 (m, 4H, Ar–H), 7.19–7.04 (m, 4H, Ar–H), 6.91 (d, 1H, J = 13.6, =CH). 13C NMR (100 MHz, CDCl3), δ (ppm) = 188.24, 161.68, 159.22, 148.28, 146.97, 140.66, 137.95, 135.08, 133.78, 132.93, 130.55, 129.34, 129.01, 126.49, 125.27, 124.59, 122.37, 114.28. EIMS (70 eV): m/z = 310 (M+).
1-(3-Coumarinyl)-3-(meta chlorophenyl)-2-propen-1-one (4j)
M.p. 173–175°C; FT-IR (KBr): 3033, 1713, 1680, 1610 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.58 (s, 1H, =CH), 7.89 (d, 1H, J = 12.8, =CH), 7.36–7.29 (m, 2H, Ar–H), 7.19–7.10 (m, 4H, Ar–H), 7.08-7.02 (m, 3H, Ar–H). 13C NMR (100 MHz, CDCl3), δ (ppm) = 193.62, 166.41, 155.64, 148.71, 139.28, 138.24, 136.45, 135.37, 131.87, 130.08, 129.81, 127.95, 127.54, 125.19, 123.73,122.68, 121.87, 114.58. EIMS (70 eV): m/z = 310 (M+).
1-(3-(7-Methoxy Coumarinyl))-3-phenyl-2-propen-1-one (4k)
M.p. 181–182°C; FT-IR (KBr): 3043, 1711, 1685, 1612, 1601, 1240cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.65 (s, 1H, =CH), 7.84 (d, 1H, J = 12.4, =CH), 7.48–7.46 (m, 2H, Ar–H), 7.38–7.29 (m, 4H, Ar–H), 7.18 (d, 1H, J = 12.4, =CH), 6.71- 6.68 (m, 2H, Ar–H), 3.35 (S, 3H, OCH3). 13C NMR (100 MHz, CDCl3), δ (ppm) = 183.31, 161.82, 158.64, 152.39, 150.56, 147.38, 138.71, 134.24, 130.45 (2C, Ar–C), 129.11, 126.89 (2C, Ar–C), 123.30, 118.77, 116.91, 114.63, 58.16. EIMS (70 eV): m/z = 306 (M+).
1-(3-(6-Methoxy Coumarinyl))-3-phenyl-2-propen-1-one (4l)
M.p. 164–166°C; FT-IR (KBr): 3048, 1709, 1686, 1618, 1603, 1236 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.58 (s, 1H, =CH), 7.72 (d, 1H, J = 13.6, =CH), 7.38–7.35 (m, 2H, Ar–H), 7.24–7.17 (m, 3H, Ar–H), 7.06 (d, 1H, J = 13.6, =CH), 6.96 (d, 1H, J = 8.8, Ar–H), 6.83 (d, 1H, J = 2.8, Ar–H), 6.67 (dd, 1H, J1 = 8.8, J2 = 2.8, Ar–H), 3.58 (S, 3H, OCH3). 13C NMR (100 MHz, CDCl3), δ (ppm) = 181.46, 157.61, 155.23, 150.17, 143.74, 140.58, 135.67, 131.82, 127.19 (2C, Ar–C), 125.29, 123.08 (2C, Ar–C), 121.99, 120.18, 119.03, 117.75, 115.83, 55.47. EIMS (70 eV): m/z = 306 (M+).
1-(3-(6-Bromo Coumarinyl))-3-phenyl-2-propen-1-one (4m)
M.p. 148–150°C; FT-IR (KBr): 3038, 1716, 1673, 1616, 971 cm−1. 1HNMR (400 MHz, CDCl3), δ (ppm) = 8.68 (s, 1H, =CH), 7.79 (d, 1H, J = 12.8, =CH), 7.54.7.41 (m, 2H, Ar–H), 7.21–7.07 (m, 5H, Ar–H), 6.99 (d, 1H, J = 12.8, =CH), 6.86 (d, 1H, J = 6.4, Ar–H). 13C NMR (100 MHz, CDCl3), δ (ppm) = 186.93, 161.72, 154.28, 149.34, 147.68, 138.91, 136.52, 133.56, 131.44, 130.76 (2C, Ar–C), 128.19 (2C, Ar–C), 127.01, 125.86, 124.67, 121.43, 115.81. EIMS (70 eV): m/z = 354 (M+).
Supplemental Material file
Download MS Word (2 MB)Acknowledgements
The authors of this article are appreciative of Kashan University to support this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes on contributors
Zeinab Akbarzadeh obtained her MS degree in Organic Chemistry from the University of Kashan, Iran, in 2013, on the study of synthesis of some dyes based on triarylamine used in nanostructure solar cell. She has also received his PhD degree under the supervision of Professor Javad Safaei-Ghomi at the same university.
Javad Safaei-Ghomi received a BSc degree in Chemistry from the University of Kashan, Iran, in 1985, and MS degree in Organic Chemistry from the University of Mazandaran, Babolsar, Iran, in 1988, and PhD degree in Organic Chemistry from the University of Wollongong, Australia, in 1995. He is a full Professor at the Department of Organic Chemistry at the University of Kashan, Iran. His research interests include asymmetric synthesis of amino acids, antioxidant and antibacterial activity of herbal extracts, using nanoparticles in multicomponent reactions and new methods for functionalization of fullerene.
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Nature 1992, 359, 710–712. doi: 10.1038/359710a0
- Beck, J.; Vartuli, J.; Roth, W.J.; Leonowicz, M.; Kresge, C.; Schmitt, K.; Chu, C.; Olson, D.H.; Sheppard, E.; McCullen, S. J. Am. Chem. Soc. 1992, 114, 10834–10843. doi: 10.1021/ja00053a020
- Garcia, H. Pure Appl. Chem. 2003, 75, 1085–1090. doi: 10.1351/pac200375081085
- Saikia, L.; Srinivas, D.; Ratnasamy, P. Micro. Meso. Mater. 2007, 104, 225–235. doi: 10.1016/j.micromeso.2007.02.026
- Bordoloi, A.; Hwang, Y.; Hwang, J.S.; Halligudi, S. Catal. Commun. 2009, 10, 1398–1403. doi: 10.1016/j.catcom.2009.03.005
- Garcia, R.A.; Van Grieken, R.; Iglesias, J.; Morales, V.; Villajos, N. J. Catal. 2010, 274, 221–227. doi: 10.1016/j.jcat.2010.07.003
- Kumari, S.; Malvi, B.; Ganai, A.K.; Pillai, V.K.; Sen Gupta, S. J. Phys. Chem. C. 2011, 115, 17774–17781. doi: 10.1021/jp2056442
- Alavi, S.; Hosseini-Monfared, H.; Siczek, M. J. Mol. Catal. A: Chem. 2013, 377, 16–28. doi: 10.1016/j.molcata.2013.04.013
- Gracia, M.D.; Balu, A.M.; Campelo, J.M.; Luque, R.; Marinas, J.M.; Romero, A.A. Appl. Catal. A 2009, 371, 85–91. doi: 10.1016/j.apcata.2009.09.033
- Melero, J.A.; Arsuaga, J.M.; Frutos, P.; Iglesias, J.; Sainz, J.; Blazquez, S. Microporous Mesoporous Mater. 2005, 86, 364–373. doi: 10.1016/j.micromeso.2005.07.040
- Gedeon, A.; Lassoued, A.; Bonardet, J.; Fraissard, J. Microporous Mesoporous Mater. 2001, 44, 801–806. doi: 10.1016/S1387-1811(01)00263-3
- Voit, B. Angew. Chem. Int. Ed. 2006, 45, 4238–4240. doi: 10.1002/anie.200504353
- Perozo-Rondon, E.; Martin-Aranda, R.M.; Casal, B.; Duran-Valle, C.J.; Lau, W.N.; Zhang, X.; Yeung, K.L. Catal. Today 2006, 114, 183–187. doi: 10.1016/j.cattod.2006.01.003
- Wang, L.; Qi, T.; Zhang, Y.; Chu, J. Microporous Mesoporous Mater. 2006, 91, 156–160. doi: 10.1016/j.micromeso.2005.11.042
- Phan, N.T.S.; Gill, C.S.; Nguyen, J.V.; Zhang, Z.J.; Jones, C.W. Angew. Chem. Int. Ed. 2006, 45, 2209. doi: 10.1002/anie.200503445
- Srinivas, P.R.D. Microporous Mesoporous Mater. 2007, 105, 170–180. doi: 10.1016/j.micromeso.2007.05.024
- Shi, J.; Jiang, Y.; Wang, X.; Wu, H.; Yang, D.; Pan, F.; Suad, Y.; Jiang, Z. Chem. Soc. Rev. 2014, 43, 5192–5210. doi: 10.1039/C4CS00108G
- Tayebee, R.; Amini, M.M.; Pouyamanesha, S.; Aliakbarib, A. Dalton Trans. 2015, 44, 5888–5897. doi: 10.1039/C4DT03504F
- Veisi, H.; Kordestani, D.; Faraji, A. J. Porous Mater. 2014, 21, 141–148. doi: 10.1007/s10934-013-9758-3
- Gheorghita, N.Z.; Zbancioc, A.M.V.; Mantu, D.; Miron, A.; Tanase, C.; Mangalagiu, I.I. Rev. Roum. Chim. 2010, 55, 983–987.
- Rajendran, V.; Harikumar, K. Ultrason. Sonochem. 2014, 21, 208–215. doi: 10.1016/j.ultsonch.2013.07.016
- Leonelli, C.; Mason, T.J. Chem. Eng. Process. 2010, 49, 885–900. doi: 10.1016/j.cep.2010.05.006
- Luo, J.; Fang, Z.; Smith, R.L. Prog. Energy Combust. Sci. 2014, 41, 56–93. doi: 10.1016/j.pecs.2013.11.001
- Cravotto, G.; Borretto, E.; Oliverio, M.; Procopio, A.; Penoni, A. Catal. Commun. 2015, 63, 2–9. doi: 10.1016/j.catcom.2014.12.014
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Chem. Soc. Rev. 2013, 42, 2555–2567. doi: 10.1039/C2CS35282F
- Cravotto, G.; Cintas, P. Chem. Soc. Rev. 2006, 35, 180–196. doi: 10.1039/B503848K
- Ramazani, A.; Rouhani, M.; Joo, S.W. Ultrason. Sonochem. 2016, 28, 393–399. doi: 10.1016/j.ultsonch.2015.08.019
- Safaei-Ghomi, J.; Akbarzadeh, Z. Ultrason. Sonochem. 2015, 22, 365–370. doi: 10.1016/j.ultsonch.2014.05.016
- Safaei-Ghomi, J.; Akbarzadeh, Z. Ultrason. Sonochem. 2018, 40, 78–83. doi: 10.1016/j.ultsonch.2017.06.022
- Ghaffari Khaligh, N.; Shirini, F. Ultrason. Sonochem. 2015, 22, 397–403. doi: 10.1016/j.ultsonch.2014.06.020
- Suslick, K.S. Sci. Am. 1989, 260, 80–86. doi: 10.1038/scientificamerican0289-80
- Bang, J.H.; Suslick, K.S. Adv. Mater. 2010, 22, 1039–1059. doi: 10.1002/adma.200904093
- Modranka, J.N.; Nawrot, E.; Graczyk, J. Eur. J. Med. Chem. 2006, 41, 1301–1309. doi: 10.1016/j.ejmech.2006.06.004
- Rosselli, S.; Maggio, A.; Bellone, G.; Formisano, C.; Basile, A.; Cicala, C.; Alfieri, A.; Mascolo, N.; Bruno, M. Planta Med. 2007, 72, 116–120. doi: 10.1055/s-2006-951772
- Deng, Y.; Nicholson, R.A. Pestic. Biochem. Physiol. 2005, 81, 39–50. doi: 10.1016/j.pestbp.2004.09.001
- Patel, H.S.; Patel, S.R. J. Macromol. Sci. Part A: Pure Appl. Chem. 1984, 21, 343–352. doi: 10.1080/00222338408069468
- Yagodinets, P.I.; Skripskaya, O.V.; Chernyuk, I.N.; Bezverkhni, V.D.; Vasikand, L.I.; Sinchenko, V.G. Pharm. Chem. J. 1996, 30, 335–336. doi: 10.1007/BF02333976
- Hoult, J.R.S.; Paya, M. Gen. Pharmacol. Vasc. S 1996, 27, 713–722. doi: 10.1016/0306-3623(95)02112-4
- Sekar, N. Colourage 2003, 50, 55–56.
- Hara, K.; Sato, T.; Katoh, R.; Furube, A.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. J. Phys. Chem. B 2003, 107, 597–606. doi: 10.1021/jp026963x
- Hara, K.; Kurashige, M.; Danoh, Y.; Kasada, C.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. New J. Chem. 2003, 27, 783–785. doi: 10.1039/b300694h
- Hara, K.; Tachibana, Y.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Sol. Energy Mater. Sol. Cells 2003, 77, 89–103. doi: 10.1016/S0927-0248(02)00460-9
- Ballesteros, J.F.; Sanz, M.J.; Ubeda, A.; Miranda, M.A.; Iborra, S.; Paya, M.; Alcaraz, M. J. Med. Chem. 1995, 38, 2794–2797. doi: 10.1021/jm00014a032
- Go, M.L.; Wu, X.; Liu, X.L. Curr. Med. Chem. 2005, 12, 483–499. doi: 10.2174/0929867053363153
- Mukherjee, S.; Kumar, V.; Prasad, A.K.; Raj, H.G.; Bracke, M.E.; Olsen, C.E.; Jain, S.C.; Parmar, V.S. Bioorg. Med. Chem. 2001, 9, 337–345. doi: 10.1016/S0968-0896(00)00249-2
- Sivakumar, P.M.; Babu, S.K.G.; Mukesh, D. Chem. Pharm. Bull. 2007, 55, 44–49. doi: 10.1248/cpb.55.44
- Liu, M.; Wilairat, P.; Croft, S.L.; Tan, A.L.G.; Go, M.L. Bioorg. Med. Chem. 2003, 11, 2729–2738. doi: 10.1016/S0968-0896(03)00233-5
- Viana, G.S.B.; Bandeira, M.A.M.; Mantos, F.J.A. Phytomedicine 2003, 10, 189–195. doi: 10.1078/094471103321659924
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Bioorg. Med. Chem. Lett. 1998, 8, 1051–1056. doi: 10.1016/S0960-894X(98)00162-0
- Xia, Y.; Yang, Z.Y.; Xia, P.; Bastow, K.F.; Nakanishi, Y.; Lee, K.H. Bioorg. Med. Chem. Lett. 2000, 10, 699–701. doi: 10.1016/S0960-894X(00)00072-X
- Dorlars, A.; Schellhammer, C.W.; Schroeder, J. Angew. Chem. Int. Ed. 1975, 14, 665–679. doi: 10.1002/anie.197506651
- Wagner, B.D. Molecules 2009, 14, 210–237. doi: 10.3390/molecules14010210
- Nguyen, A.; Vessires, A.; Hillard, E.A.; Top, S.; Pigeon, P.; Joouren, G. Chimia 2007, 61, 716–724. doi: 10.2533/chimia.2007.716
- Gupta, J.K.; Sharma, P.K.; Dudhe, R.; Chaudhary, A.; Singh, A.; Verma, P.K.; Mondal, S.C.; Yadav, R.K.; Kashyap, S. Med. Chem. Res. 2012, 21, 1625–1632. doi: 10.1007/s00044-011-9675-4
- Zhen, M.; Peng, Y. Org. Biomol. Chem. 2016, 14, 3443–3449. doi: 10.1039/C6OB00189K
- Patel, J.C.; Dholariya, H.R.; Patel, K.S.; Patel, K.D. Appl. Organometal. Chem. 2012, 26, 604–613. doi: 10.1002/aoc.2907
- El Aleem, M.A.; El-Remaily, A.A. Chinese J. Catal. 2015, 36, 1124–1130. doi: 10.1016/S1872-2067(14)60308-9
- Gu, Y.H.J.; Elangovan, S.P.; Li, W.Z.Y.; Toshio, I.; Yamazaki, Y.; Shi, J. J. Phys. Chem. C 2011, 115, 21211–21217. doi: 10.1021/jp206132a