ABSTRACT
Mica/Fe3O4 nanocomposite was easily synthesized under the mild conditions and completely identified by Fourier transform infrared (FT-IR) spectra, field-emission scanning electron microscopy (FE-SEM) images, X-ray diffraction (XRD) pattern, energy-dispersive X-ray (EDX) analysis, thermogravimetric analysis (TGA) curve and vibrating sample magnetometer (VSM) curve analyses. Also, to environment-friendly, low cost and non-toxic features, this nanocomposite has unique physical and chemical properties such as magnetic separation, thermal stability and uniform size distribution. Then, the activity of mica/Fe3O4 magnetic nanocomposites as an efficient heterogeneous nanocatalyst was investigated in the synthesis of 2-amino-4H-chromene derivatives as an important group of organic pharmaceutical compounds. The catalyst can be separated by an external magnet and reused in numerous cycles without loss of its catalytic activity as well as maintenance of its stability. Green and highly efficient catalyst based on mica and mild reaction conditions is further advantages of the present work.
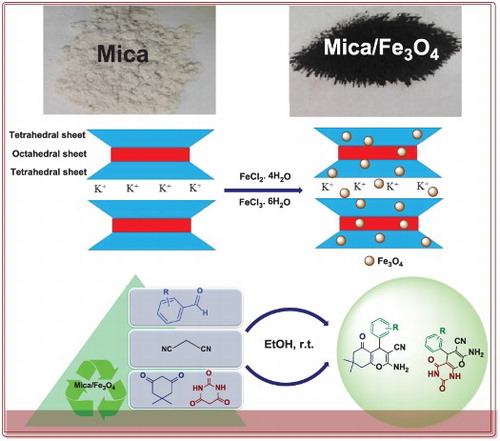
GRAPHICAL ABSTRACT
1. Introduction
Recently, magnetic nanoparticles (MNPs) owing to their considerable properties such as their relatively low cost, suitable reactive sites, high specific surface area and easy separation have been applied in various branches of sciences [Citation1–4]. The surface of MNPs is typically modified or coated with renewable polymers, carbon materials, silica compounds and clay minerals to improve their colloidal stability and reduce their toxicity [Citation5–9]. The obtained magnetic nanocomposites can be used in various fields including medical therapy and diagnosis, adsorption and catalysis [Citation10–14].
Newly, the clay minerals with their environmental compatibility, low cost, high selectivity, reusability and operational simplicity features have received considerable attention in different industries and fundamental researches [Citation15]. Also, they have good potential to be used as a substrate for the preparation of nanocomposite [Citation16]. Clay minerals are divided into different types based on their crystal and structural chemistry. The structure of mica sheets, as a subset of clay mineral, composed of two layers of silica tetrahedrons and one central dioctahedral or trioctahedral layer of alumina [Citation17]. According to mica’s accessibility, eco-friendly character, elasticity, insolubility in acid and alkali solution resistance, it was very much considered for the synthesis of nanocomposites. Also, mica by including large sheets can be modified by different metal oxides such as TiO2, ZrO2, SnO2, Cr2O3, Fe2O3 and Al2O3 with epitaxial growth [Citation18].
Multicomponent reactions (MCRs) are the most effective strategy in the field of green chemistry and are important tools in material, medical and modern synthetic organic chemistry; especially, for the construction of heterocyclic scaffolds [Citation19–21]. 4H–Chromene derivatives with biological activities such as mutagenicity [Citation22], antitumor [Citation23] antimicrobial [Citation24] and antiproliferative [Citation25] are more attractive. Furthermore, the synthesis of 2-amino-4H-chromenes with CN functionality, due to the biomedical properties and usage in the treatment of psoriasis cancer and rheumatoid are considerable importance to chemists [Citation26]. Therefore, according to the importance of these components several homogeneous or heterogeneous catalysts and various methods have been applied to improve the synthesis of 2-amino-4H-chromenes reaction such as Fe3O4@SiO2@propyltriethoxysilane@o-phenylenediamine [Citation27]. Most of these reported methods have undesirable conditions like long catalyst synthesis processes, rigorous catalyst regeneration and long reaction times. In continuation of our research on introducing new recoverable, efficient and eco-friendly nanocatalysts in organic synthesis [Citation28–38], herein, we report the synthesis and characterization of a mica/Fe3O4 nanocomposite as a heterogeneous catalyst and its application in the effective synthesis of 2-amino-4H-chromenes derivatives 5a-j and 6a-d (Scheme 1). To the best of our knowledge, there is no previously reported work on the catalytic activity investigation of magnetized mica.
Among various chromene4H–Chromenes containing CN are of the heterocyclic compounds with unique pharmaceutical and biological activities that has recently attracted great interests by chemists. Due to the importance of 4H–chromene derivatives, various synthetic method with different homogeneous or heterogeneous catalysts such as alumina [Citation30], silica nanoparticles [Citation31] methane sulfonic acid [Citation28], [BMIm]BF4, nano-sized MgO [Citation24], and K3PO4 have been used to promote 2-amino-4H-chromenes [Citation21]. Many of these methods suffer from disadvantages such as toxic catalysts, tedious work-up procedures, long times, low yields and high temperatures.
2. Experimental
2.1. General
Solvents, reagents and chemicals were obtained from Merck, Sigma and Aldrich companies. Melting points were measured on an Electrothermal 9100 apparatus and are uncorrected. Fourier transforms infrared spectroscopy (FT-IR) spectra were recorded by the method of KBr pellet on a Shimadzu IR-470 spectrometer. Thermogravimetric analysis (TGA) was taken by Bahr-STA 504 instrument. Field-emission scanning electron microscopy (FE-SEM) images were achieved by a MIRA 3 TESCAN microscope with an attached camera. Elemental analysis of the nanocatalyst was carried out by energy-dispersive X-ray (EDX) analysis recorded Numerix DXP-X10P. Lakeshore 7407 vibrating sample magnetometers (VSM) was used for examining the magnetic measurement of the solid sample. X-ray diffraction (XRD) measurements was taken on a JEOL JDX–8030 (30 kV, 20 mA). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DRX-500 Avance spectrometer at 500 and 125 MHz, respectively.
2.2. Synthesis of catalyst
2.2.1. Synthesis of mica/Fe3O4 Nanocatalyst
Mica/Fe3O4 nanocomposite was synthesized by analytically pure reagents. Firstly, 50 mL of distilled water was boiled for five minutes to eliminate oxygen. Then, 0.5 g of mica added to boiling water and the suspension vigorously stirred at room temperature with the mechanical stirrer. The mix solution of FeCl3⋅6H2O (1.06 g, 4 mmol) and FeCl2⋅4H2O (0.39 g, 2 mmol) was added to the above solution. Next, sodium hydroxide solution (10 mL, 4 M) was dropped into the mix solution in the atmosphere of nitrogen. The suspension was stirred for 120 min to complete the reaction. Finally, black precipitate was collected using an external magnet and washed several times with ethanol and distilled water. the synthesize nanocomposite mica/Fe3O4 was dried in an air oven at 60°C for 5 h and keep in the furnace at 350°C for 3 h in nitrogen gas flow.
2.2.2. General procedure for the synthesis of 2-amino-4H-chromene derivatives
A mixture of aromatic aldehyde 1 (1 mmol), malononitrile 2 (1 mmol) and dimedone 3 or barbituric acid 4 (1 mmol) in the presence of mica/Fe3O4 nanocatalyst (3.0 mg) was reacted in 3 mL ethanol at room temperature. The completion of the reaction was monitored by thin-layer chromatography (TLC) and all of derivatives were synthesis in less than 10 min. After completion of the reaction, the catalyst was easily separated by an external magnet. The pure products were obtained from the reaction mixture by recrystallization from hot EtOH and no more purification was required. All the products were known compounds which were identified by characterization of their melting points (as indicated in ) and by comparison with those authentic literature samples and also in some cases their FT-IR, 1H and 13C spectral data (See copies of 1H and 13C NMR spectra of the selected products (5a, 5b, 5 h and 6e) in Supplementary Materials file as Figures S1–S7).
2.2.3. Spectral data of the selected products
2-Amino-4-phenyl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (5a)
M.p. 236–238 °C; 1H NMR (500 MHz, DMSO-d6): δH (ppm) = 0.94 (3H, s, CH3), 1.02 (3H, s, CH3), 2.08-2.25, 2.50 (4H, 2 CH2), 4.17 (1H, s, CH), 7.00 (2H, s, NH2) 7.13–7.27 (5H, m, H-Ar). 13C NMR (125 MHz, DMSO-d6): δc (ppm) = 27.4, 29.0, 32.4, 36.2, 40.7, 50.6, 58.9, 113.4, 120.4, 127.2, 127.8, 128.7, 129.0, 145.4, 159.1, 163.1, 196.3.
2-Amino-4-(3-nitrophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (5b)
M.p. 210–212°C; 1H NMR (500 MHz, DMSO-d6): δH (ppm) = 0.93 (3H, s, CH3), 1.01 (3H, s, CH3), 2.08–2.11 (1H, d, J = 16 Hz, CH2), 2.23–2.26 (1H, d, J = 16 Hz, CH2), 2.48 (2H, s, CH2), 4.40 (1H, s, CH), 7.18 (2H, s, NH2), 7.58–7.61 (1H, m, H-Ar), 7.65–7.66 (1H, d, J = 7 Hz, H-Ar), 7.97 (1H, s, H-Ar), 8.05–8.06 (1H, d, J = 7 Hz, H-Ar). 13C NMR (125 MHz, DMSO-d6): δc (ppm) = 27.3, 28.9, 32.4, 36.0, 40.6, 50.5, 57.8, 112.4 120.0, 122.3, 122.4, 130.6, 134.8, 147.6, 148.4, 159.2, 163.8, 196.4.
2-Amino-4-(4-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (5 h)
M.p. 230–232 °C; 1H NMR (500 MHz, DMSO-d6): δH (ppm) = 0.93 (3H, s, CH3), 1.01 (3H, s, CH3), 2.08–2.11 (1H, d, J = 16 Hz, CH2), 2.22–2.25 (1H, d, J = 16 Hz, CH2), 2.48 (2H, s, CH2), 4.28 (1H, s, CH), 7.14 (2H, s, NH2) 7.34– 7.35 (2H, d, J = 8 Hz, H-Ar). 7.74–7.74 (2H, d, J = 8 Hz, H-Ar). 13C NMR (125 MHz, DMSO-d6): δc (ppm) = 27.5, 28.8, 32.4, 36.4, 50.5, 57.7, 60.4, 110.1, 112.4, 119.4, 120.0, 129.0, 133.0, 150.8, 159.2, 163.7, 170.9, 196.3.
7-amino-2,4-dioxo-5-(3,4,5-trimethoxyphenyl)-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carbonitrile (6e)
M.p. 261–262°C; 1H NMR (500 MHz, DMSO-d6): δH (ppm) = 3.77 (3H, s, CH3), 3.84 (6H, s, CH3), 4.49 (1H, s, CH), 6.78 (2H, s, NH2), 6.96 (2H, s, H-Ar), 10.11 (1H, s, NH), 11.05 (1H, s, NH).
3. Results and discussion
In this work, a natural, eco-friendly and magnetic mica/Fe3O4 nanocatalyst was synthesis in the mild condition. Mica/Fe3O4 was fully characterized by FT-IR, FE-SEM, EDX, XRD, TGA, VSM analyzes. All of these analyzes confirmed the synthesis of mica/Fe3O4 with features such as thermal stability, acceptable magnetic property and crystalline nature. Also, the catalytic activity of mica/Fe3O4 as a reusable magnetic catalyst was examined in the synthesis of 2-amino-4H-chromene derivatives without any considerable loss of catalyst activity after 7 cycles.
3.1. Characterization of mica/Fe3O4 nanocatalyst
3.1.1. Fourier transform infrared (FT-IR) spectroscopy
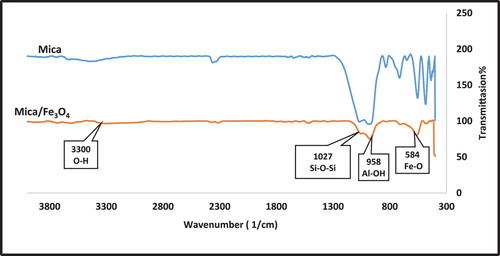
The FT-IR results spectra were used as a common method for investigating the synthesized of mica/Fe3O4 nanocomposite (). As can be seen in the FT-IR spectrum of mica, the bands at 491 and 549 cm−1 were assigned to the bending vibration of Si-O-Si and Al-O-Si, respectively. The peak at 983 cm−1 was related to the bending vibration of Al-OH of mica. Also, the peak at 1043 was attributed to the Si-O-Si bond. All the motion peaks of mica were appeared in the mica/Fe3O4 spectrum, too. The stretching vibrations of hydroxyl groups from iron oxide were shown at 3300 cm−1. The Fe-O adsorption at around 580 cm−1 was overlapped with the bending vibration of Al–O-Si [Citation18].
3.1.2. Scanning electron microscopy (SEM)
The SEM image indicated the morphology and size distribution of the nanocatalyst. As can be seen in , the roughness on the surface of mica can be related to Fe3O4 nanoparticles with its cubic shape. The mica/Fe3O4 nanocatalyst has uniform size distribution with an average size of 20 ± 16 nm.
3.1.3. Energy-dispersive X-ray (EDX)
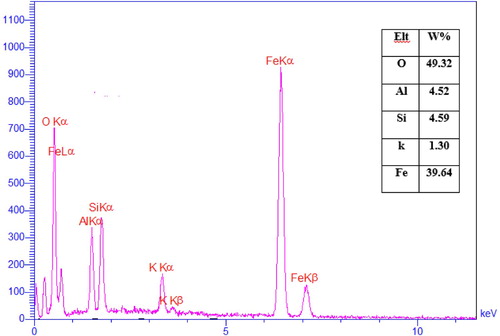
The elemental content of mica/Fe3O4 magnetic nanocomposite was determined through EDX analysis and the result was illustrated in . It confirms the presence of iron (39.6%), aluminum (4.5%), oxygen (49.3%), potassium (1.3%) and silicon (4.5%) elements in the nanocomposite. Furthermore, recycled nanocomposite was characterized by EDX analysis (Figure S8). The existence of aluminum, silicon, iron, oxygen, and potassium elements in the reused nanocomposite confirm the stability of mica/Fe3O4 nanocatalyst.
3.1.4. Vibrating sample magnetometer (VSM)
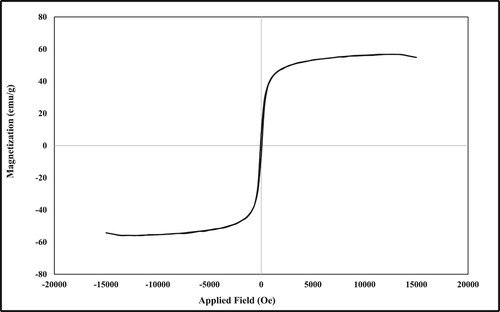
The magnetic properties of mica/Fe3O4 nanocatalyst were measured by VSM curve at room temperature (). According to reported articles, the saturation magnetization value (Ms) of neat Fe3O4 nanoparticles was found around 69 electromagnetic units per gram (emu g−1). While, the mica/Fe3O4 nanocomposite exhibits lower magnetic value around 55.69 emu g−1. This decrease in magnetic property could be attributed to the loading of Fe3O4 MNPs on the surface of mica and confirmed the successful synthesis of mica/Fe3O4 nanocomposite.
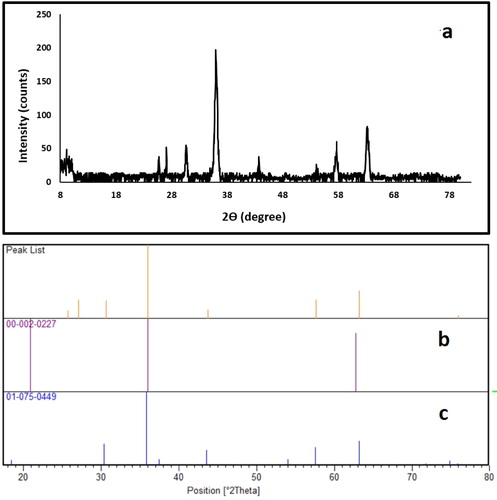
3.1.5. X-ray diffraction (XRD)
The crystal structure of the mica/Fe3O4 nanocomposite was studied by XRD analysis. It can be seen that the diffraction peaks of the nanocomposite appeared at 2θ = 25.70, 27.09, 30.67, 36.00, 43.69, 57.67, 63.17, and 75.79° (a). According to JCPDS card No 00-002-0227 (b), the diffraction peaks at 2θ = 25.70, 30.67 and 63.17° are related to the mica. Moreover, the Fe3O4 cubic magnetic nanoparticles corresponding to the characteristic diffraction angles (2θ) of 30.67, 36.00, 43.69, 57.67, 63.17, and 75.79 are similar to the characteristic data of Fe3O4 (JCPDS card No. 01-075-0449) (c). Therefore, the crystalline nature of nanocomposite and loading of Fe3o4 on mica were confirmed by XRD analysis.
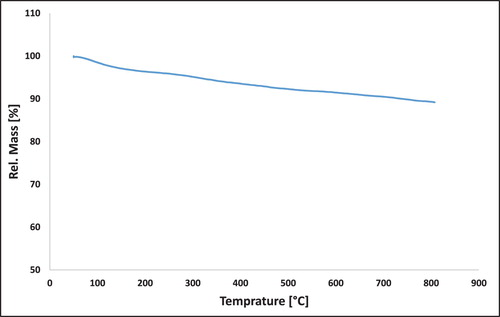
3.1.6. Thermogravimetric analysis (TGA)
The thermal stability of the synthesized nanocomposite was investigated by thermogravimetric analysis (TGA) over the temperature range of 20–800 °C and air atmosphere. As can be seen in the TG curve in , the magnetic nanocomposite is high thermal stability and the degradation is linear. The low percentage losing weight around 20–250°C was related to the evaporation of solvent (water) and other molecules that are physically absorbed on to the surface of mica/Fe3O4 nanocomposite. As a result, the thermal degradation of nanocomposite was very slight and mica/Fe3O4 nanocomposite exhibited remarkable thermal stability.
3.2. Optimization of the mica/Fe3O4 nanocomposite
In order to achieve the highest efficiency of magnetic nanocomposite, multiple amounts of mica and iron salts with the approximate ratio were used to prepare different magnetic nanocomposite. The efficiency of them was investigated in the synthesis of 5a at 10 min. as can be seen in , the performance of the mica/Fe3O4 nanocomposite was decreased by the increase of iron oxide nanoparticles due to agglomeration of iron oxide nanoparticles.
Table 1. Optimization of the mica/Fe3O4 nanocomposite.
3.3. Application of the mica/Fe3O4 nanocomposite for the synthesis of 4H–chromene derivatives
The catalytic activity was investigated in the synthesis of 4H–chromene derivatives. Initially, to optimize the reaction conditions, the three-component reaction of benzaldehyde 1, malononitrile 2 and dimedone 3 was chosen as the model reaction. The effects of the different ratios of mica/Fe3O4 and various solvents on the reaction efficiency were investigated and the results summarized in . The model reaction was studied in solvent-free, H2O and EtOH as green solvents. As can be seen in this table, higher yields of the products were obtained in EtOH rather than other solvents under similar catalyst loading. In the presence of iron nanoparticles and mica the reaction efficiency was low (, entries 3 and 4). Also, Because of the agglomeration of nanoparticles, the catalyst activity of Fe3O4 nanoparticles was lower than the mica. Nevertheless, after using the mica/Fe3O4 nanocomposite the reaction yield increased significantly to 95%. This result confirmed the synergistic effect of mica and Fe3O4. Also, 3.0 mg of catalyst was sufficient to catalyze the reaction and synthesize desired product 5a in high yields by saving both time and energy.
Table 2. Optimization of different parameters in the model reactionTable Footnotea.
Finally, after optimization of the reaction conditions, in order to study the effects of substituents, number of aldehydes such as electron-withdrawing and electron-donating moieties were employed to provide the corresponding 4H–chromene derivatives in high yields (). Also, both of dimedone 3 or barbituric acid 4 have acceptable results to afford the corresponding 5a–j and 6a–d products under similar reaction conditions. Briefly, all desired products were achieved with high yields, short reaction times and mild reaction conditions.
Table 3. Synthesis of 2-amino-4H-chromene derivatives 5a-j in optimal conditionsTable Footnotea.
3.4. Comparison the efficiency of mica/Fe3O4 with previous reported literatures
The effectiveness of mica/Fe3O4 with reported works was compared in the synthesis of 4H–chromene derivatives. As collected in , the reaction condition like Temperature and time of the present work were more reliable than previous ones.
Table 4. Comparison the efficiency of synthesized nanocatalyst with other reported works.
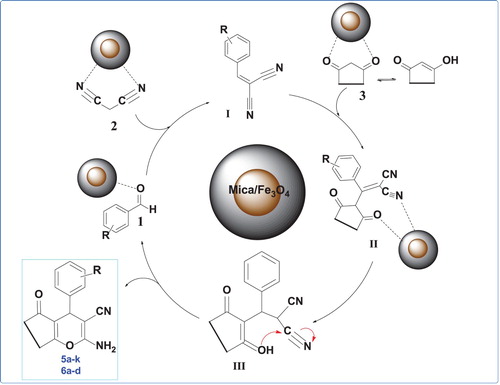
3.5. Proposed mechanism for the synthesis of 4H–chromene derivatives
The plausible mechanism for the synthesis of 4H–chromene derivatives in the presence of nanocomposite is shown in Scheme 2. Firstly, the Knoevenagel condensation between activated aldehyde 1 and malononitrile 2 provide the intermediate I in the existence of mica/Fe3O4. After that, the addition of activated dimedone 3 or barbituric acid 4 to intermediate I produce intermediate II. The tautomerization and dehydration of compound II, gave the compound III. Finally, the cyclization of intermediate III gives the final products 5a–j and 6a–d.
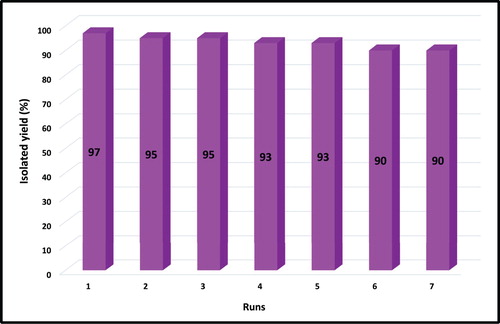
3.5. Reusability of mica/Fe3O4 nanocomposite
The recovery and reusability of the synthesized nanocomposite are one of the most important advantages for industry usage. In this regard, the mica/Fe3O4 nanocatalyst was reused after separation by the external magnet and washing with ethanol in the model reaction. The results confirmed the stability and efficiency of nanocomposite at least 7 times (). Furthermore, the stability of the recycled mica/Fe3O4 nanocatalyst was approved by FT-IR spectra and EDX analysis (Figures S8 and S9).
4. Conclusion
In summary, a green, efficient and magnetic nanocomposite was synthesized in the simple and mild condition. The synthesis of mica/Fe3O4 nanocomposite with special features such as the uniform distribution of the Fe3O4 nanoparticles, magnetic property and the thermal stability was confirmed by FT-IR, EDX, FE-SEM, XRD, VSM and TGA analyses. Then, the catalytic activity of the nanocomposite was investigated in the synthesis of -amino-4H-chromenes derivatives. The products were obtained in high-to-excellent yields at room temperature under mild reaction conditions. The nanocatalyst was easily recovered by an external magnetic field and reused efficiently for several times without a significant decrease in its activity. This is the first report on the performance of mica/Fe3O4 nanocomposite as a heterogeneous catalyst in organic reactions.
Supplemental Material
Download MS Word (3 MB)Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology.
Supplementary materials
Additional supporting information including 1H and 13C NMR of the selected products (5a, 5b, 5h and 6e) and EDX analysis and FT-IR spectrum of the recycled nanocatalyst can be found in the online version of this article at the publisher’s web site.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors

Ali Maleki
Professor Ali Maleki was born in 1980. He received his Ph.D. in Chemistry from Shahid Beheshti University under supervision of Prof. Shaabani in 2009. He started his career as an Assistant Professor in Department of Chemistry, IUST in 2010 where he is currently Full Professor. His research interests focus on design and development of novel multicomponent reactions, organic synthesis, natural and synthetic polymers, heterocycles, pharmaceutical compounds, nanochemistry, magnetic, composite, hybrid, core/shell nanomaterials, catalysts and catalytic reactions, medicinal and green chemistry. Prof. Dr. Maleki has hundreds of ISI-JCR publications, book chapters and executed industrial and applied projects. He has been selected as a distinguished researcher of IUST within 2010-2020. He has awarded IUPAC-CHEMRAWN VII prize for green chemistry in 2016. Dr. Maleki has been ranked as top 1% International Scientists in ESI (Web of Science) in 2018-2020. He had more than 20 highly cited papers in 2010-2020. His H-index is currently 50 including more than 7000 citations.
Zoleikha Hajizadeh
Mrs. Zoleikha Hajizadeh was graduated from Shahid Beheshti University in M.Sc. in organic chemistry. Then, she continued as a Ph.D. student in IUST under supervision of Prof. Ali Maleki on the synthesis of halloysite nanocomposites and evaluation of their catalytic performance in the condensation organic reactions. She has been succeeded to publish several ISI publications. Now, she was graduated from Ph.D. course in 2019.
Kobra Valadi
Mrs. Kobra Valadi was graduated from IUST in M.Sc. in organic chemistry. Then, she continued as a Ph.D. student in IUST under supervision of Prof. Ali Maleki since 2020.
References
- Zhang, W.; Chen, P.; Zhao, Z.; Wang, L.; Wang, S.; Tang, Y.; Wang, B.; Wang, Z.; Zhuang, H. Green Chem. Lett. Rev 2018, 11, 246–253.
- Hajizadeh, Z.; Hassanzadeh-Afruzi, F.; Jelodar, D.F.; Ahghari, M.R.; Maleki, A. RSC Adv. 2020, 10, 26467–26478.
- Maleki, A.; Taheri-Ledari, R.; Soroushnejad, M. ChemistrySelect 2018, 3, 13057–13062.
- Maleki, A.; Hajizadeh, Z. Silicon 2019, 11, 2789–2798.
- Maleki, A.; Firozi-Haji, R.; Hajizadeh, Z. Int. J. Biol. Macromol. 2018, 116, 320–326.
- Bai, Z.Y.; Yang, Q.; Wang, J.L. Int. J. Environ. Sci. Technol. 2016, 13, 483–492.
- Safaei-Ghomi, J.; Eshteghal, F.; Shahbazi-Alavi, H. Green Chem. Lett. Rev. 2018, 11, 345–351.
- Maleki, A.; Ravaghi, P.; Aghaei, M.; Movahed, H. Res. Chem. Intermed. 2017, 43, 5485–5494.
- Maleki, A.; Hajizadeh, Z.; Firozi-Haji, R. Microporous Mesoporous Mater. 2018, 259, 46–53.
- Barabadi, H.; Ovais, M.; Khan Shinwari, Z.; Saravanan, M. Green Chem. Lett. Rev. 2017, 10, 285–314.
- Ruey-Shin, J.; Yao-Chung, Y.; Chien-Shiun, L.; Kuen-Song, L.; His-Chuan, L.; Sea-Fue, W.; An-Cheng, S. J. Taiwan Inst. Chem. Eng. 2018, 90, 51–60.
- Maleki, A.; Azizi, M.; Emdadi, Z. Green Chem. Lett. Rev. 2018, 114, 573–582.
- Hajizadeh, Z.; Maleki, A. Mol. Catal. 2018, 460, 87–93.
- Maleki, A.; Taheri-Ledari, R.; Rahimi, J.; Soroushnejad, M.; Hajizadeh, Z. ACS Omega 2019, 4, 10629–10639.
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. J. Clean. Prod. 2019, 215, 1233–1245.
- Maleki, A.; Panahzadeh, M.; Eivazzadeh-keihan, R. Green Chem. Lett. Rev. 2019, 12, 395–406.
- Murray, H.H. Developments in Clay Science; Tarcher/Putnam: Netherlands, 2006; pp 7–31.
- Xiaojuan, L.; Haiquan, X.; Jing, C.; Juncai, S.; Yuxiang, Y.; Xiangnong, L. Glass Phys. Chem. 2011, 37, 330–342.
- Hajizadeh, Z.; Radinekiyan, F.; Eivazzadeh-Keihan, R.; Maleki, A. Sci. Rep. 2020, 10, 11671.
- Maleki, A.; Hajizadeh, Z.; Salehi, P. Sci. Rep. 2019, 9, 5552.
- Maleki, A.; Hajizadeh, Z.; Abbasi, H. Carbon Lett. 2018, 27, 42–49.
- Hiramoto, K.; Nasuhara, A.; Michikoshi, K.; Kato, T.; Kikugawa, K. Mutat. Res. Genet. Toxicol. Environ. Mutagen 1997, 395, 47–56.
- Gourdeau, H.; Leblond, H.; Hamelin, B.; Desputeau, C.; Dong, K.; Kianicka, I.; Custeau, D.; Boudreau, C.; Geerts, L.; Cai, S.X.; Drewe, J. Mol. Cancer Ther. 2004, 3, 1375–1384.
- Alvey, L.; Prado, S.; Saint-Joanis, B.; Michel, S.; Koch, M.; Cole, S.T.; Tillequin, F.; Janin, Y.L. Eur. J. Med. Chem. 2009, 44, 2497–2505.
- Dell, C.P.; Smith, C.W. Eur. Pat. Appl. EP 537949. Chem. Abstr. 1993, 119, 139102d.
- Gao, Y.; Yang, W.; Du, D.M. Tetrahedron Asym. 2012, 23, 339–344.
- Maleki, A.; Ghalavand, R.; Firozi-Haji, R. Appl. Organomet. Chem. 2018, 32, e3916.
- Maleki, A. Tetrahedron 2012, 68, 7827–7833.
- Maleki, A. Tetrahedron Lett. 2013, 54, 2055–2059.
- Maleki, A. RSC Adv. 2014, 4, 64169–64173.
- Maleki, A. Helv. Chim. Acta. 2014, 97, 587–593.
- Maleki, A. Ultrason. Sonochem. 2018, 40, 460–464.
- Maleki, A. Polycycl. Aromat. Compd. 2018, 38, 402–409.
- Maleki, A.; Varzi, Z.; Hassanzadeh-Afruzi, F. Polyhedron 2019, 171, 193–202.
- Maleki, A.; Hassanzadeh-Afruzi, F.; Varzi, Z.; Esmaeili, M.S. Mater. Sci. Engin. C 2020, 109, 110502.
- Maleki, A.; Kari, T.; Aghaei, M. J. Porous Mater. 2017, 24, 1481–1496.
- Maleki, A.; Aghaei, M. Ultrason. Sonochem. 2017, 38, 585–589.
- Maleki, A.; Movahed, H.; Ravaghi, P.; Kari, T. RSC Adv. 2016, 6, 98777–98787.
- Zeydi, M.M.; Ahmadi, S. Orient. J. Chem. 2016, 32, 2215.
- Gao, S.; Tsai, C.H.; Tseng, C.; Yao, C.F. Tetrahedron 2008, 64, 9143–9149.
- Azizi, N.; Dezfooli, S.; Khajeh, M.; Hashemi, M.M. J. Mol. Liq. 2013, 186, 76.
- Molaei, H.; Sadeghi, B. J. Nanostruc. Chem. 2018, 8, 197–206.
- Mohammadi Ziarani, M.G.; Faramarzi, S.; Asadi, S.; Badiei, A.; Bazi, R.; Amanlou, M. J. Pharm. Sci. 2013, 21, 3–13.