ABSTRACT
Silver nanoparticles (AgNPs) have been exploited for their broad-spectrum antibacterial effects and their vast applications, which are generating interest of researchers towards green synthesis of AgNPs. In this paper, we describe a novel biosynthesis of AgNPs employing secondary metabolites of Bacillus subtilis (SDUM301120). The mean particle diameter of AgNPs was calculated by the high-resolution transmission electron microscope (HRTEM). HRTEM analysis revealed the particle was spherical and distributed in the range of 2–26 nm. Crystal nature of the nanoparticles in the face-centered cubic structure was confirmed by the peaks in the X-ray diffraction pattern corresponding to (111), (200), (220), and (311) planes. The formation of the reduced AgNPs was monitored by UV–VIS spectrophotometer analysis which displayed a peak in the region of 430–460 nm. We investigated the antibacterial activity of AgNPs against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Vibrio Parahaemolyticus ATCC 17802T, and Acinetobacter baumanni ATCC 19606T. The results show that AgNPs with enhanced antibacterial properties have significant antimicrobial effects compared with pure AgNPs, antibacterial substances (lipopeptide), and the mixture of lipopeptide and pure AgNPs. The bacteriostatic experiments compared with antibiotics showed that the obtained AgNPs had a promising future in the bacterial infections.
GRAPHICAL ABSTRACT
1. Introduction
Nanotechnology, which can be employed in different fields of science, is one of the most promising technologies (Citation1). The application of nanoscale and nanostructure materials is an emerging area of nanoscience and nanotechnology (Citation2). Nanotechnology has shown an unprecedented impact on the pharmaceutical improvement due to the production of many nanomedicines that have the capacity to deliver the drug to the target position (Citation3). Gold and silver metal nanoparticles have attracted much attention among all the high-precision nanomaterials because of their unique physiochemical properties which include antimicrobial activity (Citation4,Citation5), antivirus activity (Citation6,Citation7), antitumor activity (Citation8–10), antifungal activity (Citation11,Citation12), antioxidant activity (Citation13), antiparasitic activity (Citation14), catalyst activity (Citation15), and electronics property (Citation16).
In the global world, multidrug-resistant infections pose a major threat to human health (Citation17). Thus, the development of new antibacterial substances is imminent, especially antibacterial substances that are not prone to bacterial resistance. AgNPs can be considered as the potential candidate to combat multidrug resistance for its wide range of medical potentials, mainly including bactericidal (Citation5), fungicidal (Citation18), larvicidal (Citation19), scolicidal (Citation14), antioxidant (Citation20), anti-inflammatory (Citation20), and anti-cancer agents (Citation21–26).
Physical and chemical methods are common methods for the production of metal nanoparticles. However, these methods are often accompanied by the use of toxic chemicals and excessive consumption of energy. Biological approaches have advantages over aforementioned methods as they are used to prepare AgNPs in a simple, safe, clean, and environmentally friendly manner (Citation27). Researchers prefer biosynthesis (Citation28–35). Different resources such as bacteria (Citation27,Citation36,Citation37), fungi (Citation38,Citation39), plant (Citation4,Citation40,Citation41), algae (Citation42), and even yeast (Citation43) have been reported for the biosynthesis of AgNPs. Arsiya et al. obtained nanoparticles by biomass of Chlorella vulgaris (Citation44); Kalimuthu et al. synthesized AgNPs with a particle size of about 50 nm by mixing the biomass of Bacillus licheniformis with AgNO3 solution for 24 h (Citation36); AbdelRahim et al. employed mycelia extract of the fungi Rhizopus stolonifer to synthetic AgNPs (Citation45); Li et al. synthesized AgNPs with a mean diameter ranging from 1 to 10 nm in the culture supernatants of Aspergillus terreus (Citation46). However, the researchers as mentioned above only synthesized pure AgNPs, they did not pay great attention to the special role of secondary metabolites that played in the synthesis of silver nanoparticle. Up to now, several researchers have begun synthesizing metal nanoparticles with antibacterial effect by employing bioactive ingredients with medical functions found in plants (Citation4,Citation47,Citation48). Parlinska-Wojtan reported the production of AgNPs with antimicrobial effect using camomile extract and clove eugenol (Citation49,Citation50), Kasithevar et al. reported a green process for rapid synthesis of AgNPs against Multi-Drug Resistant Clinical Isolates from Post-Surgical Wound Infections using aqueous leaf extract of Corchorus Capsularis (Citation47). Therefore, we assumed that we could obtain AgNPs with enhanced antibacterial properties by using bioactive substances obtained from antagonistic bacteria Bacillus subtilis. Bacillus subtilis is gram-positive, aerobic, endospore-forming rod commonly found in soil and oceans (Citation51). This bacterium can produce a variety of secondary metabolites with broad spectrum of antimicrobial activity such as peptides, lipopeptides, phospholipids, and polyene (Citation52). In addition, there may be some reducing group in chemically diverse structures of these bioactive components such as amine group or hydroxyl group (Citation52–55), which has the ability to perform functions of reduction of Ag+ to Ag0 (Citation56). Biomolecules were immobilized on the surface of AgNPs while reacting with silver nitrate to form AgNPs.
Considering the antibacterial and reducing ability of secondary metabolites from antagonistic bacteria B. subtilis, the propose of this study is to use the synergistic effect of silver and secondary metabolites to obtain AgNPs with enhanced antibacterial properties. In this paper, we presented an approach for green synthesis of AgNPs with enhanced antibacterial properties. Antibacterial control test compared with antibiotics showed the powerful ability to inhibit bacterial growth of the synthesized AgNPs, which have great application potential in the treatment of bacterial infections and is expected to play an important role in the biomedical field.
2. Materials and methods
2.1. Materials
All reagents used for the AgNPs syntheses were of analytical purity: silver nitrate (AgNO3, purity 99.999%), urea (H2NCONH2, purity 99.0%), sodium dihydrogen phosphate (NaH2PO4·2H2O, purity 99.0%), disodium hydrogen phosphate (Na2HPO4·12H2O, purity 99.0%), sodium dodecylsulfate (C12H25SO4Na, purity 90.0%), and deionized water. All reagents were obtained from Xinyue (Weihai, China). All solutions of reacting materials were prepared using deionized water. Before being used, all glassware was rinsed thoroughly with deionized water. Bacterial pathogens: E. coli ATCC 25922, S. aureus ATCC 29213, V. parahaemolyticus ATCC 17802T, A. baumannii ATCC 19606T and B. subtilis (SDUM301120) were obtained from College of Marine Science, Shandong University of Weihai. B. subtilis (SDUM301120) was isolated from Weihai (37°31′8″N, 122°1′5″E) seabed sediments. All bacterial strains were stored in 15% glycerol containing medium at −80°C.
2.2. Optimization of fermentation conditions for cultivation of Bacillus subtilis (SDUM301120)
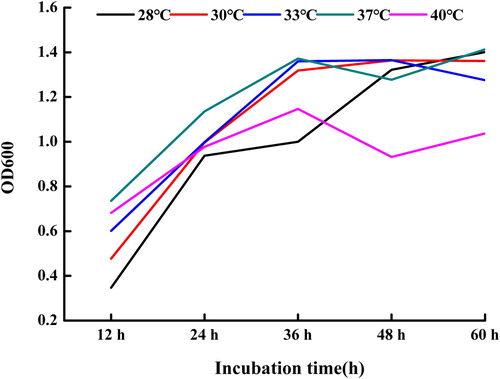
The B. subtilis (SDUM301120) was grown in Landy medium (g L−1) containing glucose 20.0, L – glutamic acid 5.0, KH2PO4 1.0, MgSO4·7 H2O 0.5, KCl 0.5, yeast powder 1.0, MnSO4 · 5 H2O 5.0 × 10−3, CuSO4·5 H2O 0.16 × 10−3, FeSO4· 7 H2O 0.15 × 10−3, L-phenylalanine 2.0 × 10−3, 1 L distilled water. In order to make the fermentation supernatant of Bacillus subtilis (SDUM301120) has the best antibacterial performance, we fluctuated the parameters such as the pH value of medium (6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0), cultural temperature (28°C, 30°C, 33°C, 37°C, 40°C) and incubation time (12, 24, 36, 48, 60 h), and we explored the effect of cultural temperature on the growth of Bacillus subtilis (SDUM301120). The bacterial growth can be demonstrated by measuring the optical density at 600 nm (OD600).
2.3. Isolation of lipopeptide antibacterial substances
The extraction of lipopeptide antibacterial substances was carried out according to the methods reported in the literature (Citation57). Bacillus subtilis (SDUM301120) was incubated in 250 mL flasks with 100 mL seed medium for 18 h with 120 rpm at 37°C, and then 1 mL inoculum was added to a 500 mL flask containing 250 mL fermentation medium. The fermentation was incubated at 37°C under shaking condition (120 rpm) for 36 h. The fermentation broth was centrifuged at 4°C for 15 min (12,000 rpm). The supernatant was collected and placed in a sterile conical flask; the pH of cell-free culture broth was adjusted to 2.0 using 6 M HCl. The bacteria – inhibiting substances (mainly lipopeptides) were precipitated overnight at 4°C. The obtained precipitates were collected by centrifuging at 4°C for 15 min (12,000 rpm), and then extracted twice with deionized water. The antimicrobial substances were maintained at 4°C before being used.
2.4. Optimization and synthesis of AgNPs
The impact of substrate on the production of AgNPs with enhanced antibacterial properties was optimized by varying the substrate concentration (1.00, 1.15, 1.30, 1.43 mM of AgNO3). We obtained optimum concentration by determining the inhibition zone against pathogenic strains (E. coli ATCC 25922) of 50 uL AgNPs (300 µg/mL) with enhanced antibacterial properties. The synthesis of AgNPs with enhanced antibacterial properties was carried in 20 mL solutions of antibacterial substances at pH to 9.0, which was placed on a conical flask. Then, 7 mL 5 mM AgNO3 solution based on the optimization results was added to the conical flask using a pipette. The reaction mixture stirred continuously on a magnetic stirrer at a constant speed (1000 rpm). It was observed that the color of the solution turned from yellow to bright yellow and then to dark brown after 3, 7 and 12 h of the reaction, which indicated the formation of AgNPs with enhanced antibacterial properties.
2.5. Antimicrobial activity of AgNPs with enhanced antibacterial properties
2.5.1. MIC and MBC of AgNPs with enhanced antibacterial properties against pathogenic strains
The determination was carried out according to a previously described method (Citation58). The AgNPs with enhanced antibacterial properties were centrifuged, washed thrice with deionized water, and dried in oven. Dried AgNPs with enhanced antibacterial properties were weighed and dispersed in deionized water to obtain the final concentration of 64 µg/mL. In the sterile 96-well plate, AgNPs with enhanced antibacterial properties were two-fold serially diluted within the range of 1–64 µg/mL. The wells were then inoculated with overnight culture of pathogenic strains in their log phase of growth, which were diluted to give the initial population of approximately 100 CFU/mL, further, incubated at 35°C for 18 h. The bacterial growth could be shown by absorbance increase at 600 nm. MIC was deduced as the lowest concentration of AgNPs with enhanced antibacterial properties at which the growth of tested strain was inhibited completely. The determination of MBC was based on a reduction in the number of viable pathogenic strains.
2.5.2. Antimicrobial activity of AgNPs with enhanced antibacterial properties compared with pure AgNPs and antibacterial substances
In our experiment, we evaluated the antibacterial activity of AgNPs with enhanced antibacterial properties, antibacterial substances (lipopeptide), pure AgNPs and the mixture of lipopeptide, and pure AgNPs. AgNPs with enhanced antibacterial properties were treated according to the methods reported in the literature (Citation59). The AgNPs with enhanced antibacterial properties were washed three times with the pH value of 7.4 phosphate buffer solution (50 mM), treated with the mixture of 8 M urea and 1% sodium dodecyl sulfate, then placed in 75°C water bathes for 15 min to remove antimicrobial substances (lipopeptide) from surfaces of AgNPs with enhanced antibacterial properties to obtain pure AgNPs. The pure AgNPs were washed for three times with deionized water and the pH value of 7.4 phosphate buffer solution (50 mM), respectively. The obtained pure AgNPs were stored in a 4°C refrigerator for later use. The disc diffusion assay was performed as described previously (Citation58,Citation60). On this basis, the method was improved. In short, the overnight log culture of each pathogenic strains (E. coli ATCC 25922, S. aureus ATCC 29213, V. parahaemolyticus ATCC 17802T and A. baumannii ATCC 19606T) with turbidity 0.5 was spread evenly on Luria–Bertani (LB) plate (g L−1) containing peptone 10, yeast extract 5, sodium chloride 10, and agar 20. Then we punched holes (diameter: 7 mm) to add 100 µL sample to be tested and kept for incubation at 35°C for 18 h. After incubation, the zone of inhibition was determined by measuring the diameter of zone around each disk. The experiment was done three times to check the reproducibility.
2.5.3. Antimicrobial activity of AgNPs with enhanced antibacterial properties compared with antibiotics
To determine the strong antibacterial efficiency of the AgNPs with enhanced antibacterial properties, the disk diffusion method was used to assay antibacterial activity of synthesized AgNPs compared with antibiotics (tetracycline, doxycycline, neomycin, minocycline, kanamycin, ceftriaxone) against A. baumannii ATCC 19606T. The standard antibiotics disks were purchased from Xinyue (Weihai, China). In addition, each blank standard paper disk was further impregnated with 30 µL of the freshly prepared AgNPs with enhanced antibacterial properties at a final content of 30 µg/disk. The overnight cultures with turbidity 0.5 were applied to Luria–Bertani (LB) plate along with the standard disks containing the same amounts of AgNPs and antibiotics at 35°C for 18 h. After incubation, the zone of inhibition was evaluated by measuring the diameter of zone around each disk.
2.6. Characterization of AgNPs with enhanced antibacterial properties
2.6.1. UV–visible spectroscopy
UV–visible spectroscopy is one of the most commonly used and the most effective method to identify the nanoparticle formation. To confirm the existence of lipopeptides on the surface of AgNPs, the UV–VIS absorbance spectra of AgNPs with enhanced antibacterial properties, pure lipopeptide, and pure AgNPs were compared by using the UV–Visible spectrophotometer HITACHI-model U-2910. UV–VIS spectrometry showed optical absorption spectra ranged from 220 to 800 nm.
2.6.2. Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) plays a significant role in identifying the surface absorbents present in nanoparticles. FTIR spectrum of the samples was recorded spectrophotometrically using NICOLET 5700 FT-IR Spectrometer (USA) and spectrum was collected in the range of 500–4000 cm−1 at a resolution of 4 cm−1 using the potassium bromide (KBr) pellet technique.
2.6.3. High-resolution transmission electron microscopy
Morphology and particle size were observed by high-resolution transmission electron microscope (HRTEM) (Tecnai G2 F20 transmission electron microscope, USA) operating at 200 kV accelerating voltage. The prepared AgNPs suspensions were diluted with deionized water, then dripped on the carbon-coated copper grid, and dried at ambient temperature before testing.
2.6.4. Energy-dispersive X-ray
Energy-dispersive X-ray (EDX) was carried out using a Field emission electron microscope (Tecnai G2 F20 transmission electron microscope, USA). The EDX spectrum was performed at 200 KV accelerating voltage.
2.6.5. X-ray diffraction analysis
To obtain the XRD pattern of the AgNPs, AgNPs solution was placed on a glass slide and was allowed to dry in a hot air oven at 50°C. The dried samples were analyzed using X-ray diffractometer (Model: UItimaIV, Japan) with a wide selection of Bragg angles 2θ ranging from 30° to 80° at a scanning rate of 5°min−1, operating at a voltage of 40 kV.
2.6.6. Zeta potential
The stability of AgNPs is closely related to Zeta potential. The surface zeta potential of the synthesized AgNPs was measured by Zeta sizer (Model: Zetasizer Nano, Malvern Instruments LtdMalvern, UK).
2.6.7. Surface-enhanced Raman scattering
Raman spectra were recorded by WITec alpha 300R confocal Raman microscope and a 300 grooves/mm diffraction grating was used for the collection of the Raman signals. For all measurements, a 633 nm solid-state laser with an intensity of 4 mW was used as the excitation source, the integration time was 2 s and the number of accumulations was 10. The sample was tableted for stable and repeatable detection of Raman signals.
3. Results and discussion
3.1. Optimization of fermentation conditions
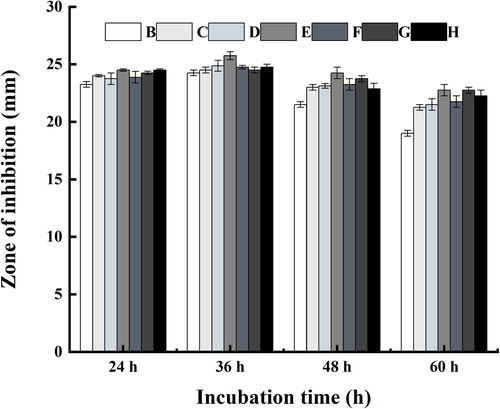
As shown in , the antimicrobial activity of strain (SDUM301120) varied with temperature. In general, the antimicrobial effect improves with the increase of the temperature. The maximum production of antimicrobial substance (lipopeptide) happened at 37°C. It also proved that it was unfavorable for production of antimicrobial substance at 28°C due to the inhibition zone was smaller in size.
Figure 1. Effect of cultural temperature on the antibacterial activity of strain (SDUM301120) fermentation supernatant.
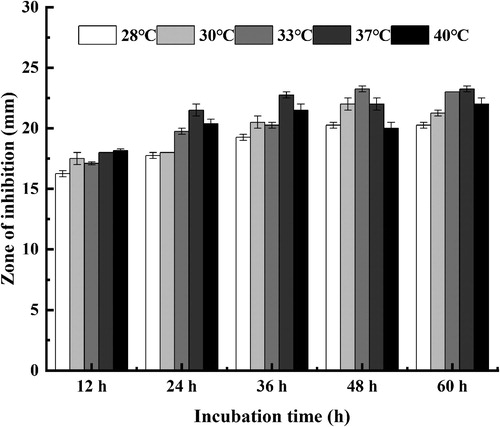
As can be seen from , the OD600 of the fermentation supernatant basically increased with the extension of culture time at different culture temperatures. It was also observed that the OD600 of the fermentation supernatant at 37°C was bigger than those at other temperatures. Therefore, we chose 37°C as the optimal temperature for the growth of strain (SDUM301120).
The impact of the pH of the fermentation supernatant was conducted by using different culture medium adjusted pH to 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0. Then 1 mL fermented broth was taken to determine its antimicrobial property. As shown in , under the same pH condition, the antimicrobial activity of the fermentation supernatant reached a maximum after 36 h of incubation. It was also proved that the optimum pH for maximum production of antimicrobial substance (lipopeptide) was found to be 7.5 compared to other levels, which favored the growth of strain.
3.2. Optimization and synthesis of AgNPs with enhanced antibacterial properties
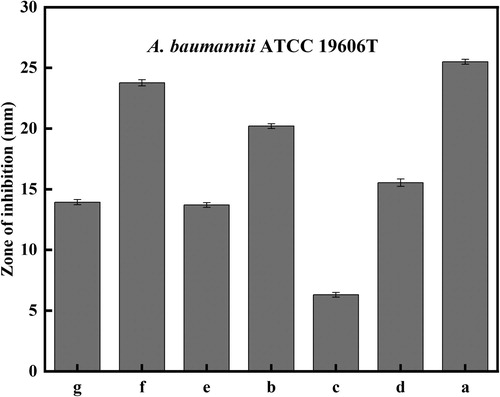
There are many factors affecting antimicrobial properties of AgNPs. The concentration of AgNO3 played a key role in antibacterial activity as well as the yield of AgNPs. We evaluated the correlation between the concentration of AgNO3 and antiseptic properties of AgNPs with enhanced antibacterial properties to determine the optimal concentration. The result showed that the concentration of 1.30 mM AgNO3 caused the strongest bacteriostatic effect of AgNPs (). Then the same regimen was conducted with additions of 1.30 mM AgNO3 to acquire AgNPs with enhanced antibacterial properties. Initially, biosynthesized AgNPs were observed during the reduction reaction mediated by secondary metabolites of Bacillus subtilis (SDUM301120). (A,B) demonstrates the change in color before and after exposure to AgNO3 for 15 h while shaking in the conical flask. The color of the reaction mixture changes gradually from yellowness to dark brown because of the surface plasmon resonance (SPR) of the nanoparticles, indicating that AgNPs with enhanced antibacterial properties were formed. The same type of results could be seen in study that reported that AgNPs were made by Shani Raj (Citation61).
Figure 4. Effect of the AgNO3 concentration on the antibacterial activity of AgNPs with enhanced antibacterial properties.
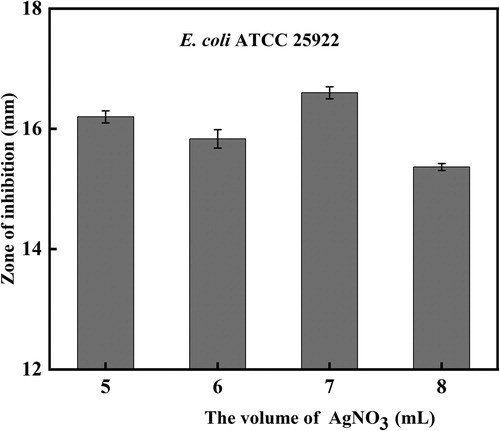
Figure 5. (A) ErlenMeyer flask with Bacillus subtilis (SDUM301120) fermented liquid after exposure to AgNO3 solution (1.30 mM) for a few minutes (no color change); (B) ErlenMeyer flask with Bacillus subtilis (SDUM301120) fermented liquid after exposure to AgNO3 solution (1.30 mM) for 15 h (reddish-brown color).

3.3. Antimicrobial activity of synthesized AgNPs compared with pure AgNPs and antibacterial substances
Inhibition zone was measured by the modified agar diffusion method to identify the inhibitory effect of synthesized AgNPs with enhanced antibacterial properties against different microorganisms. The experiment was done three times and the results were interpreted in terms of mean diameter of inhibition zone. Moreover, pure AgNPs, the mixture (pure AgNPs and lipopeptide) and the antibacterial substances (lipopeptide) were tested as control groups. After the treatment of AgNPs with the solution of 8 M urea and 1% sodium dodecyl sulfate, the treated AgNPs and the antibacterial substance with the same volume and concentration were dispersed by ultrasound to obtain the mixture (pure AgNPs and lipopeptide). AgNPs with enhanced antibacterial properties (b) showed the biggest inhibitory zones when to compare to the control groups in same concentration and loading volume, as shown in and . Particularly, in contrast to the mixture, the AgNPs with enhanced antibacterial properties showed stronger bactericidal effect, which also confirmed that the antimicrobial performance of the AgNPs with enhanced antibacterial properties was not simply additive of pure AgNPs and antibacterial substances, but the synergy action between two substances, which results in stronger antimicrobial ability of the AgNPs with enhanced antibacterial properties. It is also roughly observed that its inhibitory effect against A. baumannii and E. coli were superior to others. Nevertheless, due to the influence of external environment, the diameter of the inhibition zone can only be used as a general reference. In order to obtain an accurate judgment of antimicrobial performance, we determined MIC and MBC of AgNPs with enhanced antibacterial properties. The results indicated that the synthesized AgNPs with enhanced antibacterial properties showed MIC value against pathogenic strains (E. coli ATCC 25922, S. aureus ATCC 29213, A. baumannii ATCC 19606T) in the range of 8–16.2 ug/mL, it again proves that AgNPs with enhanced antibacterial properties have excellent inhibitory performance against pathogenic strains (). The exact mechanism of antibacterial behavior is unclear, but it has been reported that the overproduction of reactive oxygen species (ROS) is regarded as a main mechanism of NPs toxicity and cell damage (Citation62). The high ROS production could destroy the defense capacity of the organisms, resulting in the inactivation of some enzymes, oxidation of proteins, damage of DNA, and other essential biomolecules (Citation63). AgNPs-induced bacterial apoptosis depends on the damage to cell components and the increase of free calcium levels of the bacterial cells caused by ROS (Citation64). The important role of AgNPs in the treatment of diseases that need to maintain the concentration of circulating drug or deliver drugs to specific cells or organs cannot be neglected due to its toxicity in microorganisms (Citation65,Citation66). For example, AgNPs can inactive HIV-1 virus and block the interaction of cells with virus (Citation67). While seeing the benefits of AgNPs to human life, we have to pay attention to the risks behind it. Up to now, the expanding production and usage of commercial AgNPs is inevitably leading to their release to the environment, in this case, the adverse effect of AgNPs on human health needs to be taken into consideration (Citation53). However, little knowledge is available about the potential impacts of AgNPs on human health, which requires further study.
Figure 6. Antimicrobial properties against (A) A. baumannii ATCC19606T; (B) E. coli ATCC 25922; (C) V. parahemolyticus ATCC17802T; (D) S. aureus ATCC 29213. (a) The mixture of lipopeptide and pure AgNPs (150 µg/mL); (b) AgNPs with enhanced antibacterial properties (150 µg/mL); (c) Pure AgNPs (150 µg/mL); (d) Antibacterial substances (lipopeptide) (150 µg/mL).
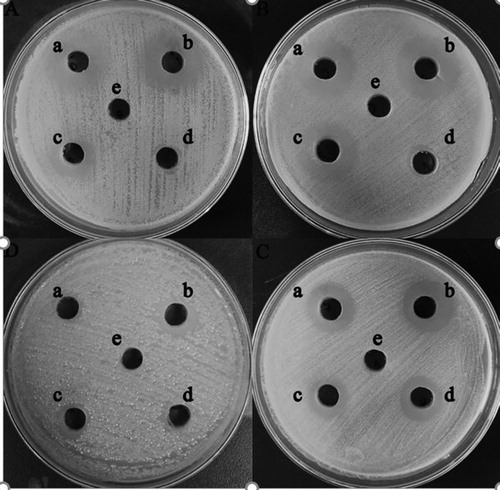
Figure 7. Zone of inhibition against pathogenic strains. (a) The mixture of lipopeptide and pure AgNPs (150 ug/mL); (b) AgNPs with enhanced antibacterial properties (150 ug/mL); (c) Pure AgNPs (150 ug/mL); (d) Antibacterial substances (lipopeptide) (150 ug/mL).
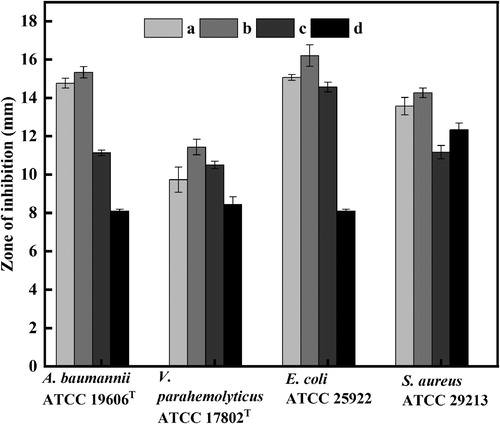
Table 1. MIC and MBC values of AgNPs with enhanced antibacterial properties (µg/mL) against pathogenic microorganisms.
3.4. Antimicrobial activity of AgNPs with enhanced antibacterial properties compared with antibiotics
To demonstrate the potential of AgNPs with enhanced antibacterial properties as an antibiotics substitute, we roughly compared the antibacterial performance of AgNPs with enhanced antibacterial properties with antibiotics via the disk diffusion method. AgNPs with enhanced antibacterial properties (a) owned stronger antimicrobial performance compared with minocycline, kanamycin, ceftriaxone, neomycin, doxycycline and tetracycline in and . To some extent, this result indicated that AgNPs with enhanced antibacterial properties could be seen as a potential candidate for antibiotics in medical fields, such as the treatment of infectious diseases. In fact, the wide application of AgNPs with enhanced antibacterial properties requires to be based on relevant safety tests, which is also the way to continued efforts in the future.
Figure 8. Antimicrobial properties against A. baumannii ATCC19606T: (a) AgNPs with enhanced antibacterial properties (30 µg/disk); (b) minocycline (30 µg/disk); (c) kanamycin (30 µg/disk); (d) ceftriaxone (30 µg/disk), (e) neomycin (30 µg/disk); (f) doxycycline (30 µg/disk); (g) tetracycline (30 µg/disk); (h) Sterile water (control).
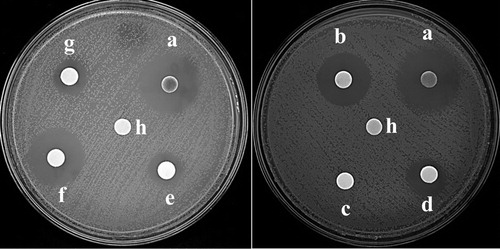
Figure 9. Zone of inhibition against A. baumannii ATCC19606T: (a) AgNPs with enhanced antibacterial properties (30 µg/disk); (b) minocycline (30 µg/disk); (c) kanamycin (30 µg/disk); (d) ceftriaxone (30 µg/disk), (e) neomycin (30 µg/disk); (f) doxycycline (30 µg/disk); (g) tetracycline (30 µg/disk).
3.5. UV–visible spectrum of AgNPs with enhanced antibacterial properties, pure lipopeptide, and pure AgNPs
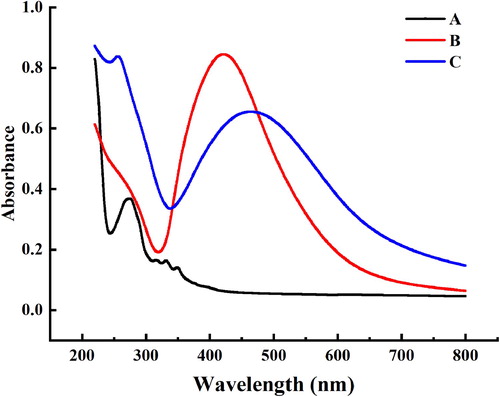
To confirm the existence of lipopeptides on the surface of AgNPs, the UV–VIS absorbance spectra of AgNPs with enhanced antibacterial properties, pure lipopeptide and pure AgNPs were compared. UV–VIS spectrometry showed optical absorption spectra ranged from 220 to 800 nm. Line C () has shown an outstanding absorption peak at 430–460 nm which was specific for the SPR of AgNPs corresponding to the absorption peak of line B. However, the particle size increases with the immobilization of lipopeptide on the surface of nanoparticles, which leads to the red shift of absorption peak (Citation68). It is assumed that the Ag+ were reduced to Ag (0) in the reaction. On top of, there is an absorption peak at 260–280 nm in Line C which is consistent with the absorption peak of Line A characterized as a lipopeptide substance. In summary, the lipopeptides do exist on the surface of AgNPs, so which enhanced antibacterial properties of the synthesized AgNPs.
3.6. HRTEM and EDX analysis of AgNPs with enhanced antibacterial properties
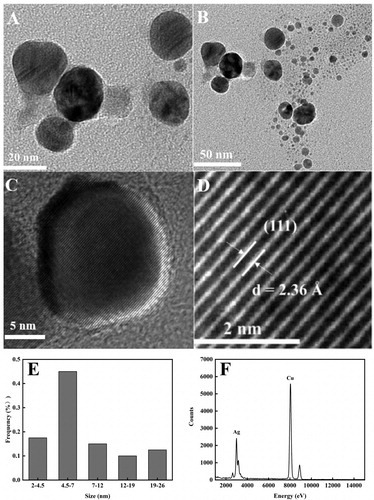
The precise HRTEM analysis is widely used to identify further insight into the shape of the nanoparticles. The prepared AgNPs were dissolved in deionized water and dispersed by ultrasonic. A 5 µL solution was dripped onto the carbon-coated copper grid and dried naturally. The HRTEM images of AgNPs with enhanced antibacterial properties as were shown in (A,B). From the results, it showed that AgNPs with enhanced antibacterial properties exhibited subspherical morphology with higher as well as lower distributions. Higher distribution may be due to little aggregation between particles. Lower distributions may be due to aggregations of larger particles consisting of several small particles (image (B)) (Citation8). The AgNPs with enhanced antibacterial properties have an inter-fringe distance of 0.236 nm, which corresponds to the (111) plane in the face-centered cube (image (C,D)) (Citation69). Size distribution histogram showed average particle sizes ranging from 2 to26 nm, with 17.5% of the nanoparticles between 2 and 4.5 nm and 45% of the nanoparticles between 4.5 and 7 nm, respectively (image (E)). EDX spectroscopic analysis confirmed the formation of elemental silver with the sharp signal band located at 3–4 keV (image (F)) (Citation70).
3.7. XRD analysis of AgNPs with enhanced antibacterial properties
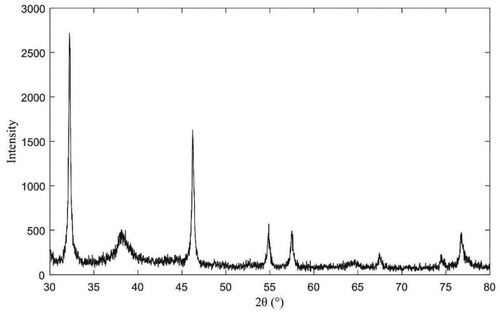
The XRD pattern is shown in which confirmed the crystal nature of AgNPs with enhanced antibacterial properties. The XRD spectrum shows four noticeable peaks were at 2-Theta of 38.16°, 46.24°, 64.70°, and 77.64°, which corresponds to (111), (200), (220), and (311) Bragg’s reflections of the face-centered cubic structure of silver (Citation69,Citation70). The appearance of other diffraction peak (32.24° and 54.88° and 57.5°and 67.44° and 74.58°) may be caused by the binding of antibacterial substances to the surface of AgNPs with enhanced antibacterial properties. Diffraction analysis showed that the antibacterial substances produced by antagonistic bacteria could react with AgNO3 solution to synthesize AgNPs which surface adsorbed some antibacterial substances.
3.8. Zeta potential of AgNPs with enhanced antibacterial properties
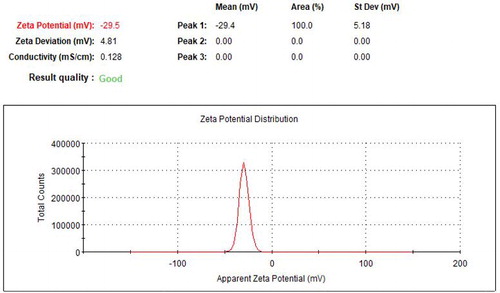
The stability of AgNPs with enhanced antibacterial properties in an aqueous environment could be evaluated by zeta potential. Molecule aggregation has a low probability to come into sight for an element having charge with balance potential of zeta (±30 mV) (Citation42). The value of zeta potential of the AgNPs with enhanced antibacterial properties was −29.5 mV (). This result is consistent with previous research reported by Dutta et al. (Citation71). This result evidently demonstrated that AgNPs with enhanced antibacterial properties were highly stable by electrostatic means ().
3.9. FTIR analysis of AgNPs with enhanced antibacterial properties
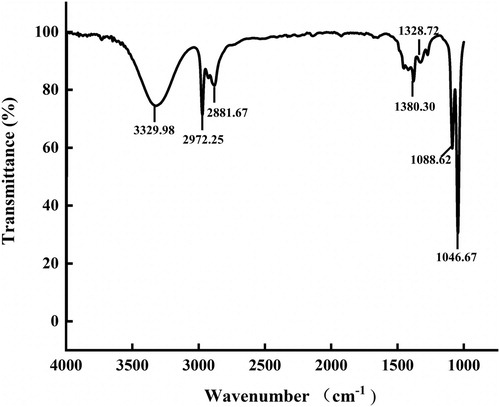
represented the FTIR spectrum of AgNPs with enhanced antibacterial properties that showed O–H stretching vibrations at 3329 cm−1, C–H bond at 2972 and 2881 cm−1, C–N vibration at 1046 cm−1 and C–OH stretching vibration at 1088 cm−1 (Citation4,Citation8,Citation44,Citation48). These functional groups play an important part in the stability of AgNPs as reducing and capping agents. In addition, the hydroxyl groups may participate in the bio-reduction process during AgNPs synthesis.
4. Surface-enhanced Raman scattering
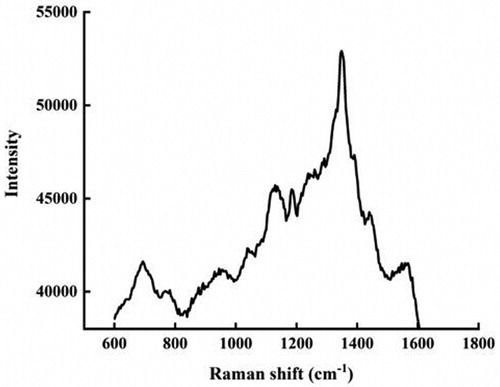
The secondary metabolites of Marine B. subtilis are not a single substance, but a mixture with a common feature: most of which consist of short linear chains or cyclic structures of amino acids that are linked to fatty acids by ester or amide bonds or both (Citation72–75). It can be seen from the Raman spectrum () that the peak value of the characteristic absorption peak is within the range of 1000–2000 cm −1. The most significant absorption peak value is 1300–1400 cm−1. The C–N was observed at 1345, 1284, 1258, and 1141 cm−1 in the Raman experimental spectrum, respectively. The peaks owing to the lipopeptide substances correspond to the C–H in-plane bending (1184 cm−1). These are in agreement with the literature (Citation76,Citation77). All of these have verified from sides that antibacterial active substances are absorbed on the surface of AgNPs with enhanced antibacterial properties.
5. Conclusion
In this article, the antagonistic bacteria B. subtilis (SDUM301120) was employed to acquire AgNPs with enhanced antibacterial properties with stronger antimicrobial potential in an ecofriendly method. Characterizations of this study support the formation of AgNPs with enhanced antibacterial properties. The research on bacteriostatic property also confirms AgNPs with enhanced antibacterial properties have enhanced antibacterial potential. To sum up, we provide a strategy for functionalization of AgNPs and this simple way has several advantages such as cost-effectiveness, compatibility for biomedical and medical applications such as antibacterial, and this material promises to be a substitute for antibiotics.
Acknowledgements
The authors appreciate Shandong University (Weihai) for providing the necessary facilities to carry out this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Patil, M.P.; Kim, G.D. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495.
- Ghorbani, H.R. Biosynthesis of Nanosilver Particles Using Extract of Salmonella typhimurium. Arab. J. Chem. 2017, 10, S1699–S1702.
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent Expansion of Pharmaceutical Nanotechnologies and Targeting Strategies in the Field of Phytopharmaceuticals for the Delivery of Herbal Extracts and Bioactivities. J. Controlled Release 2016, 241, 110–124.
- Balachandar, R.; Gurumoorthy, P.; Karmegam, N.; Barabadi, H.; Subbaiya, R.; Anand, K.; Boomi, P.; Saravanan, M. Plant-Mediated Synthesis, Characterization and Bactericidal Potential of Emerging Silver Nanoparticles Using Stem Extract of Phyllanthus pinnatus: A Recent Advance in Phytonanotechnology. J. Cluster Sci. 2019, 30 (6), 1481–1488.
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid Biosynthesis of Silver Nanoparticles from Bacillus megaterium (NCIM 2326) and Their Antibacterial Activity on Multi Drug Resistant Clinical Pathogens. Colloids Surf. B Biointerfaces 2011, 88 (1), 325–331.
- Saravanan, M.; Asmalash, T.; Gebrekidan, A.; Gebreegziabiher, D.; Araya, T.; Hilekiros, H.; Barabadi, H.; Ramanathan, K. Nano-Medicine as a Newly Emerging Approach to Combat Human Immunodeficiency Virus (HIV). Pharm. Nanotechnol. 2018, 6 (1), 17–27.
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit Peels Waste for the Green Synthesis of Silver Nanoparticles with Antimicrobial Activity Against Foodborne Pathogens. Lwt 2019, 103, 293–300.
- Boomi, P.; Poorani, G.P.; Palanisamy, S.; Selvam, S.; Ramanathan, G.; Ravikumar, S.; Barabadi, H.; Prabu, H.G.; Jeyakanthan, J.; Saravanan, M. Evaluation of Antibacterial and Anticancer Potential of Polyaniline-Bimetal Nanocomposites Synthesized from Chemical Reduction Method. J. Cluster Sci. 2019, 30 (3), 715–726.
- Khatua, A.; Prasad, A.; Priyadarshini, E.; Patel, A.K.; Naik, A.; Saravanan, M.; Barabadi, H.; Ghosh, l.; Paul, B.; Paulraj, R., et al. Emerging Antineoplastic Plant-Based Gold Nanoparticle Synthesis: A Mechanistic Exploration of their Anticancer Activity Toward Cervical Cancer Cells. J. Cluster Sci. 2019, 31, 1329–1340.
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-Cancer Green Bionanomaterials: Present Status and Future Prospects. Green Chem. Lett. Rev. 2017, 10 (4), 285–314.
- Khatua, A.; Priyadarshini, E.; Rajamani, P.; Patel, A.; Kumar, J.; Naik, A.; Saravanan, M.; Barabadi, H.; Prasad, A., Ghosh, l., et al. Phytosynthesis, Characterization and Fungicidal Potential of Emerging Gold Nanoparticles Using Pongamia pinnata Leave Extract: A Novel Approach in Nanoparticle Synthesis. J. Cluster Sci. 2020, 31 (1), 125–131.
- Ocsoy, I.; Demirbas, A.; McLamore, E.S.; Altinsoy, B.; Ildiz, N.; Baldemir, A., Green Synthesis with Incorporated Hydrothermal Approaches for Silver Nanoparticles Formation and Enhanced Antimicrobial Activity Against Bacterial and Fungal Pathogens. J. Mol. Liq. 2017, 238, 263-269.
- Varadharaj, V.; Ramaswamy, A.; Sakthivel, R.; Subbaiya, R.; Barabadi, H.; Chandrasekaran, M.; Saravanan, M. Antidiabetic and Antioxidant Activity of Green Synthesized Starch Nanoparticles: An In Vitro Study. J. Cluster Sci. 2019, 31, 1257–1266.
- Rahimi, M.T.; Ahmadpour, E.; Rahimi Esboei, B.; Spotin, A.; Kohansal Koshki, M.H.; Alizadeh, A.; Honary, S.; Barabadi, H.; Ali Mohammadi, M. Scolicidal Activity of Biosynthesized Silver Nanoparticles Against Echinococcus granulosus protoscolices. Int. J. Surg. 2015, 19, 128–133.
- Wang, N.; Hu, Y.; Zhang, Z. Sustainable Catalytic Properties of Silver Nanoparticles Supported Montmorillonite for Highly Efficient Recyclable Reduction of Methylene Blue. Appl. Clay Sci. 2017, 150, 47–55.
- Nantaphol, S.; Chailapakul, O.; Siangproh, W. Sensitive and Selective Electrochemical Sensor Using Silver Nanoparticles Modified Glassy Carbon Electrode for Determination of Cholesterol in Bovine Serum. Sens. Actuators B 2015, 207, 193–198.
- Saravanan, M.; Ramachandran, B.; Barabadi, H. The Prevalence and Drug Resistance Pattern of Extended Spectrum Beta-Lactamases (ESBLs) Producing Enterobacteriaceae in Africa. Microb. Pathog. 2018, 114, 180–192.
- Wang, S.H.; Chen, C.C.; Lee, C.H.; Chen, X.A.; Chang, T.Y.; Cheng, Y.C.; Young, J.J.; Lu, J.J. Fungicidal and Anti-Biofilm Activities of Trimethylchitosan-Stabilized Silver Nanoparticles Against Candida Species in Zebrafish Embryos. Int. J. Biol. Macromol. 2020, 143, 724–731.
- Barabadi, H.; Alizadeh, Z.; Rahimi, M.T.; Barac, A.; Maraolo, A.E.; Robertson, L.J.; Masjedi, A.; Shahrivar, F.; Ahmadpour, E. Nanobiotechnology as an Emerging Approach to Combat Malaria: A Systematic Review. Nanomedicine 2019, 18, 221–233.
- Lee, G.H.; Lee, S.J.; Jeong, S.W.; Kim, H.C.; Park, G.Y.; Lee, S.G.; Choi, J.H. Antioxidative and Antiinflammatory Activities of Quercetin-Loaded Silica Nanoparticles. Colloids Surf. B Biointerfaces 2016, 143, 511–517.
- Barabadi, H.; Hosseini, O.; Damavandi Kamali, K.; Jazayeri Shoushtari, F.; Rashedi, M.; Haghi-Aminjan, H.; Saravanan, M. Emerging Theranostic Silver Nanomaterials to Combat Lung Cancer: A Systematic Review. J. Cluster Sci. 2020, 31 (1), 1–10.
- Barabadi, H.; Damavandi Kamali, K.; Jazayeri Shoushtari, F.; Tajani, B.; Mahjoub, M.A.; Alizadeh, A.; Saravanan, M. Emerging Theranostic Silver and Gold Nanomaterials to Combat Prostate Cancer: A Systematic Review. J. Cluster Sci. 2019, 30 (6), 1375–1382.
- Barabadi, H.; Mahjoub, M.A.; Tajani, B.; Ahmadi, A.; Junejo, Y.; Saravanan, M. Emerging Theranostic Biogenic Silver Nanomaterials for Breast Cancer: A Systematic Review. J. Cluster Sci. 2019, 30 (2), 259–279.
- Barabadi, H.; Vahidi, H.; Damavandi Kamali, K.; Rashedi, M.; Saravanan, M. Antineoplastic Biogenic Silver Nanomaterials to Combat Cervical Cancer: A Novel Approach in Cancer Therapeutics. J. Cluster Sci. 2020, 31 (4), 659–672.
- Barabadi, H.; Vahidi, H.; Damavandi Kamali, K.; Rashedi, M.; Hosseini, O.; Golnaraghi Ghomi, A.R.; Saravanan, M. Emerging Theranostic Silver Nanomaterials to Combat Colorectal Cancer: A Systematic Review. J. Cluster Sci. 2020, 31 (2), 311–321.
- Barabadi, H.; Alizadeh, A.; Ovais, M.; Ahmadi, A.; Shinwari, Z.K.; Saravanan, M. Efficacy of Green Nanoparticles Against Cancerous and Normal Cell Lines: A Systematic Review and Meta-Analysis. IET Nanobiotechnol. 2018, 12 (4), 377–391.
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Bacillus brevis (NCIM 2533) and Their Antibacterial Activity Against Pathogenic Bacteria. Microb. Pathog. 2018, 116, 221–226.
- Barabadi, H. Nanobiotechnology: A Promising Scope of Gold Biotechnology. Cell Mol Biol (Noisy-le-Grand) 2017, 63 (12), 3–4.
- Barabadi, H.; Honary, S.; Ali Mohammadi, M.; Ahmadpour, E.; Rahimi, M.T.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Green Chemical Synthesis of Gold Nanoparticles by Using Penicillium aculeatum and Their Scolicidal Activity Against Hydatid Cyst Protoscolices of Echinococcus granulosus. Environ. Sci. Pollut. Res. Int. 2017, 24 (6), 5800–5810.
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Mohammadi, M.A.; Alizadeh, A.; Naghibi, F. Microbial Mediated Preparation, Characterization and Optimization of Gold Nanoparticles. Braz. J. Microbiol. 2014, 45 (4), 1493–1501.
- Emmanuel, R.; Saravanan, M.; Ovais, M.; Padmavathy, S.; Shinwari, Z.K.; Prakash, P. Antimicrobial Efficacy of Drug Blended Biosynthesized Colloidal Gold Nanoparticles from Justicia glauca Against Oral Pathogens: A Nanoantibiotic Approach. Microb. Pathog. 2017, 113, 295–302.
- Golnaraghi Ghomi, A.R.; Mohammadi-Khanaposhti, M.; Vahidi, H.; Kobarfard, F.; Ameri Shah Reza, M.; Barabadi, H. Fungus-Mediated Extracellular Biosynthesis and Characterization of Zirconium Nanoparticles Using Standard Penicillium Species and Their Preliminary Bactericidal Potential: A Novel Biological Approach to Nanoparticle Synthesis. Iran. J. Pharm. Res. 2019, 18 (4), 2101–2110.
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Optimization of Myco-Synthesized Silver Nanoparticles by Response Surface Methodology Employing Box-Behnken Design. Inorganic and Nano-Metal Chemistry 2019, 49 (2), 33–43.
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. 2018, 17, 87–97.
- Honary, S.; Barabadi, H.; Ebrahimi, P.; Naghibi, F.; Alizadeh, A. Development and Optimization of Biometal Nanoparticles by Using Mathematical Methodology: A Microbial Approach. Journal of Nano Research 2015, 30, 106–115.
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of Silver Nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65 (1), 150–153.
- Subbaiya, R.; Saravanan, M.; Priya, A.R.; Shankar, K.R.; Selvam, M.; Ovais, M.; Balajee, R.; Barabadi, H. Biomimetic Synthesis of Silver Nanoparticles from Streptomyces atrovirens and Their Potential Anticancer Activity Against Human Breast Cancer Cells. IET Nanobiotechnol. 2017, 11 (8), 965–972.
- Barabadi, H.; Tajani, B.; Moradi, M.; Damavandi Kamali, K.; Meena, R.; Honary, S.; Mahjoub, M.A.; Saravanan, M. Penicillium Family as Emerging Nanofactory for Biosynthesis of Green Nanomaterials: A Journey into the World of Microorganisms. J. Cluster Sci. 2019, 30 (4), 843–856.
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green Synthesis of Silver Nanoparticles Induced by the Fungus Penicillium citrinum. Tropical Journal of Pharmaceutical Research 2013, 12 (1), 7–11.
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of Silver Nanoparticles Using Plants Extract and Analysis of Their Antimicrobial Property. J. Saudi Chem. Soc. 2015, 19 (3), 311–317.
- Saravanan, M.; Barabadi, H.; Ramachandran, B.; Venkatraman, G.; Ponmurugan, K. Emerging Plant-Based Anti-Cancer Green Nanomaterials in Present Scenario. In Engineered Nanomaterials and Phytonanotechnology: Challenges for Plant Sustainability 2019, 87, pp 291–318.
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of Silver Nanoparticles Using Red Algae Portieria hornemannii and its Antibacterial Activity Against Fish Pathogens. Microb. Pathog. 2020, 138, 103780.
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of Silver Nanoparticles Using Yeasts and Evaluation of Their Antifungal Activity Against Phytopathogenic Fungi. Process Biochem. 2016, 51 (9), 1306–1313.
- Arsiya, F.; Sayadi, M.H.; Sobhani, S. Green Synthesis of Palladium Nanoparticles Using Chlorella vulgaris. Mater. Lett. 2017, 186, 113–115.
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.; Husseiny, S.M. Extracellular Biosynthesis of Silver Nanoparticles Using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24 (1), 208–216.
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-Mediated Green Synthesis of Silver Nanoparticles Using Aspergillus terreus. Int. J. Mol. Sci. 2012, 13 (1), 466–476.
- Kasithevar, M.; Periakaruppan, P.; Muthupandian, S.; Mohan, M. Antibacterial Efficacy of Silver Nanoparticles Against Multi-Drug Resistant Clinical Isolates from Post-Surgical Wound Infections. Microb. Pathog. 2017, 107, 327–334.
- Kanagamani, K.; Muthukrishnan, P.; Shankar, K.; Kathiresan, A.; Barabadi, H.; Saravanan, M. Antimicrobial, Cytotoxicity and Photocatalytic Degradation of Norfloxacin Using Kleinia grandiflora Mediated Silver Nanoparticles. J. Cluster Sci. 2019, 30 (6), 1415–1424.
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green Synthesis and Antibacterial Effects of Aqueous Colloidal Solutions of Silver Nanoparticles Using Camomile Terpenoids as a Combined Reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39 (8), 1213–1223.
- Parlinska-Wojtan, M. Green Synthesis and Antibacterial Effects of Aqueous Colloidal Solutions of Silver Nanoparticles Using Clove Eugenol Green Synthesis and Characterization of Antibacterial Effects of silver. Appl. Organomet. Chem. 2018, 32 (4), e4276.
- Wang, T.; Liang, Y.; Wu, M.; Chen, Z.; Lin, J.; Yang, L. Natural Products from Bacillus subtilis with Antimicrobial Properties. Chin. J. Chem. Eng. 2015, 23 (4), 744–754.
- Stein, T. Bacillus Subtilis Antibiotics: Structures, Syntheses and Specific Functions. Mol. Microbiol. 2005, 56 (4), 845–857.
- Manivel, P.; Balamurugan, A.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Novel Synthesis of Silver Nanoparticles Using 2,3,5,6-tetrakis-(morpholinomethyl) Hydroquinone as Reducing Agent. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 95, 305–309.
- Roy, N.; Gaur, A.; Jain, A.; Bhattacharya, S.; Rani, V. Green Synthesis of Silver Nanoparticles: An Approach to Overcome Toxicity. Environ. Toxicol. Pharmacol. 2013, 36 (3), 807–812.
- Chen, W.-C.; Juang, R.-S.; Wei, Y.-H. Applications of a Lipopeptide Biosurfactant, Surfactin, Produced by Microorganisms. Biochem. Eng. J. 2015, 103, 158–169.
- Gurunathan, S. Rapid Biological Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Effects Against Escherichia fergusonii and Streptococcus mutans. Arab. J Chem. 2019, 12 (2), 168–180.
- Chen, H.; Wang, L.; Su, C.X.; Gong, G.H.; Wang, P.; Yu, Z.L. Isolation and Characterization of Lipopeptide Antibiotics Produced by Bacillus subtilis. Lett. Appl. Microbiol. 2008, 47 (3), 180–186.
- Singh, P.; Singh, H.; Kim, Y.J.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Extracellular Synthesis of Silver and Gold Nanoparticles by Sporosarcina koreensis DC4 and Their Biological Applications. Enzyme Microb. Technol. 2016, 86, 75–83.
- Bharde, A.A.; Parikh, R.Y.; Baidakova, M.; Jouen, S.; Hannoyer, B.; Enoki, T.; Prasad, B.L.; Shouche, Y.S.; Ogale, S.; Sastry, M. Bacteria-Mediated Precursor-Dependent Biosynthesis of Superparamagnetic Iron Oxide and Iron Sulfide Nanoparticles. Langmuir 2008, 24 (11), 5787–5794.
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and Effect of Silver Nanoparticles on the Antibacterial Activity of different Antibiotics Against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3 (2), 168–171.
- Raj, S.; Chand Mali, S.; Trivedi, R. Green Synthesis and Characterization of Silver Nanoparticles Using Enicostemma axillare (Lam.) Leaf Extract. Biochem. Biophys. Res. Commun. 2018, 503 (4), 2814–2819.
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the Aquatic Environment: Usage, Properties, Transformation and Toxicity – A Review. Process Saf. Environ. Prot. 2019, 130, 238–249.
- Wang, S.; Gao, M.; Li, Z.; She, Z.; Wu, J.; Zheng, D.; Guo, L.; Zhao, Y.; Gao, F.; Wang, X. Performance Evaluation, Microbial Enzymatic Activity and Microbial Community of a Sequencing Batch Reactor Under Long-Term Exposure to Cerium Dioxide Nanoparticles. Bioresour. Technol. 2016, 220, 262–270.
- Lee, W.; Kim, K.J.; Lee, D.G. A novel Mechanism for the Antibacterial Effect of Silver Nanoparticles on Escherichia coli. Biometals 2014, 27 (6), 1191–1201.
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53 (2), 283–318.
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Delivery Rev. 2003, 55 (3), 329–347.
- Lara, H.H.; Ayala-Nunez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of Antiviral Action of Silver Nanoparticles Against HIV-1. J. Nanobiotechnology. 2010, 8, 1.
- Seoudi, R.; Shabaka, A.; El Sayed, Z.A.; Anis, B. Effect of Stabilizing Agent on the Morphology and Optical Properties of Silver Nanoparticles. Physica E 2011, 44 (2), 440–447.
- Ma, L.; Su, W.; Liu, J.X.; Zeng, X.X.; Huang, Z.; Li, W.; Liu, Z.C.; Tang, J.X. Optimization for Extracellular Biosynthesis of Silver Nanoparticles by Penicillium aculeatum Su1 and Their Antimicrobial Activity and Cytotoxic Effect Compared with Silver Ions. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 77, 963–971.
- El-Baz, A.F.; El-Batal, A.I.; Abomosalam, F.M.; Tayel, A.A.; Shetaia, Y.M.; Yang, S.T. Extracellular Biosynthesis of Anti-Candida Silver Nanoparticles Using Monascus purpureus. J. Basic Microbiol. 2016, 56 (5), 531–540.
- Dutta, P.P.; Bordoloi, M.; Gogoi, K.; Roy, S.; Narzary, B.; Bhattacharyya, D.R.; Mohapatra, P.K.; Mazumder, B. Antimalarial Silver and Gold Nanoparticles: Green Synthesis, Characterization and In Vitro Study. Biomed. Pharmacother. 2017, 91, 567–580.
- Bezza, F.A.; Chirwa, E.M.N. Production and Applications of Lipopeptide Biosurfactant for Bioremediation and Oil recOvery by Bacillus subtilis CN2. Biochem. Eng. J. 2015, 101, 168–178.
- Jha, S.S.; Joshi, S.J.; Geetha, S.J. Lipopeptide Production by Bacillus subtilis R1 and its Possible Applications. Braz. J. Microbiol. 2016, 47 (4), 955–964.
- Vinothkanna, A.; Palanisamy, P.; Sekar, S. Activity of Antibacterial Compounds from Bacillus subtilis Against Cellular Oncoproteins by In Silico Approach. Biocatalysis and Agricultural Biotechnology 2019, 18, 101059.
- Zhou, H.; Cong, B.; Tian, Y.; He, Y.; Yang, H. Characterization of Novel Cyclic Lipopeptides Produced by Bacillus sp. SY27F. Process Biochem. 2019, 83, 206–213.
- Premkumar, R.; Hussain, S.; Mathavan, T.; Anitha, K.; Benial, A.M.F. Surface-Enhanced Raman Scattering and Quantum Chemical Studies of 2-Trifluoroacetylpyrrole Chemisorbed on Colloidal Silver and Gold Nanoparticles: A Comparative Study. J. Mol. Liq. 2019, 290, 111209.
- Saleh, T.A.; Al-Shalalfeh, M.M.; Al-Saadi, A.A. Silver Nanoparticles for Detection of Methimazole by Surface-Enhanced Raman Spectroscopy. Mater. Res. Bull. 2017, 91, 173–178.