?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nickel oxide nanoparticles (NiO NPs) were synthesized via the facile sol–gel method. The synthesized NiO NPs were characterized using X-ray diffraction (XRD), Field-Emission Scanning Electron Microscopy (FESEM), Transmission Electron Microscopy (TEM), Raman and Fourier Transform Infrared (FT-IR) techniques. The effect of particle size was analyzed on structural alterations, electrochemical behaviors, and cytotoxic effect of synthesized NiO NPs. According to TEM results, the particle sizes of synthesized NiO NPs were 8.2, 15.4, and 21.7 nm at 300, 400, and 500 °C, respectively. Electrochemical behaviors of synthesized NiO NPs were assessed through the cyclic voltammetry (CV) technique. The results showed that the magnitude of the current density was decreased in treated samples exposed to higher temperature values. The cytotoxic activity of synthesized nanoparticles was investigated against human liver cancer cell (Hep-G2), breast cancer cell (MCF-7), and colon cancer cell (HT-29) lines using the MTT assay. The results demonstrated that synthesized NiO NPs had higher cytotoxicity at 300 °C than at 400 and 500 °C, because of their small particle size. Thus, synthesized NiO NPs exhibit acceptable cytotoxic effects against Hep-G2, MCF-7, and HT-29 cancer cell lines so that they could be a good choice for cancer treatment.
GRAPHICAL ABSTRACT

1. Introduction
Nowadays, the incredible impact of nanotechnology on the research and development process has made nanotechnology one of the most significant research priorities in many countries (Citation1). Nanotechnology is currently used in broad research areas, such as medicine, food, health, disease treatment, environment, energy, electronics, computers, information, materials, manufacturing, aerospace, biotechnology, agriculture, national security, and defense (Citation2). The application of nanotechnology is not restricted to just a few domains or activities. In fact, it is an empowering group of technologies entering all parts of industries or scientific fields. In other words, nanotechnology has the ability to make fundamental changes in plenty of biological or medical methods and instruments in order to make them more economical, individualized, transportable, secure, and manageable (Citation3–8).
Nanomaterials have recently attracted much attention because of their remarkable properties, effective surface area, and high reactivity. On the other hand, with rapid advancement in nanotechnology, nanomaterials are produced in a wide variety of shapes and sizes and used in the manufacture of various industrial and medical products (Citation1,Citation9). Nanoparticles are employed in different medical fields, including drug delivery and imaging of organs and cells, and can be potentially used for cancer diagnosis and treatment (Citation10). Nanoparticles in nanometer size can bind to nano-sized pharmaceuticals and be specifically absorbed by cancer cells. In this way, normal cells would not be exposed to anticancer drugs, and the side effects are diminished (Citation11).
Metal oxide NPs (MONPs), among all the substances which should be produced in nanoscale diameters, have indicated fascinating features because they have a wide range of biological tasks according to their special electronic, biomedical, and optical features(Citation12,Citation13). It has also been suggested that metal-based nanomedicines enhance the antibacterial, antifungal, and anticancer features of antimicrobial factors against bacteria persistence (Citation14,Citation15).
Nickel 0xide nanoparticles (NiO NPs), among metal oxides, are P-type semiconductors possessing an extensive band gap in the range of 3.6 eV to 4.0 eV (Citation16). Nanocrystalline NiO has fantastic magnetic features which are relevant to size and surface impacts (Citation17,Citation18). Because of the anticipated and amazing features for their broad usage in various disciplines like high density recording media, spin valves, magnetic resonance imaging, ferrofluid technology, and magnetocaloric refrigeration, nanomaterials have turned into an interesting subject in nanoscience (Citation19,Citation20). One of the novel semiconductors is diluted magnetic semiconductor (DMS) that is prepared by utilizing magnetic transition metal ions or scarce earth metal ions to accidently substitute the non-magnetic cations in semiconductors and to cause the semiconductor to show magnetic features (Citation21). Semiconductors like it can be possibly applied in spin monitored tools. Overall, NiO NPs are possessing particular structures and properties that are used in photovoltaic fields, catalysts, sensors, ceramics, gas sensors, and optical filters (Citation22–24). The increasing use of NiO NPs needs a better understanding of their potential impact on the environment and human health. Therefore, the toxic effects of NiO NPs were investigated in different human cell lines, namely lung, breast, and colon cancer cells.
The precise mechanism of nanoparticles on cancer cells has not been fully elucidated, but the most controversial procedure of nanoparticles toxicity regarding biological systems is their potential to arouse the formation of reactive oxygen species (ROS), resulting in the destruction of the cell because of the oxidative strain. It is well established that superoxide anions and hydrogen peroxide are extremely reactive oxygen types, which are able to form oxidative strain in the cell system (Citation25–27). When nanomaterials are activated by cancer cells, the cellular defense mechanism is stimulated to minimize damages. However, if the stimulation of ROS production by the nanoparticles exceeds the antioxidant defense capacity of the cells, cells undergo apoptosis (Citation28).
According to the reports already done, ZnO, TiO2, and Ag nanoparticles can ruin the biological constructions by the oxidative strain procedure because of ROS formation (Citation29–31). Sasidharan et al. suggested an analogous procedure in their research which indicated that ZnO nanocrystals in the acidic cancer microenvironment led to oxidative strain which was because of increased ROS strain, mitochondrial superoxide generation, and depolarization of the mitochondrial membrane, which resulted in apoptosis (Citation32). Based on the research already done, autophagy is highly relevant to cellular ROS and NPs can trigger ROS and oxidative strain (Citation25,Citation33). To comprehend the underlying procedures of NiO NPs-mediated autophagy, Cho et al. measured the intracellular ROS levels in cells subjected to NiO NPs. Their outcomes proposed that the autophagy induction triggered by NiO NPs associates with oxidative strain. They also realized that mitochondria can be one of the potential sources of ROS formation (Citation34). According to Ibrahim et al., NiO NPs were formed using green protocol from medicinal plant Terminalia chebula fruit extract as a chemical covering and decreasing factor. They examined the cytotoxicity of NiO by investigating cell feasibility and a strong contrary correlation between the production of ROS and decrease in the cellular feasibility on breast cancerous cells. The potential procedures were also assessed by ROS and mitochondrial membrane potential (MMP) activity on breast cancerous cells. Green formed NiO NPs indicated toxicity to breast cancerous cells in a dose-dependent manner from 0 to 100 μg/mL which indicates high cell feasibility, ROS action, and releasing MMP (Citation26).
There are various methods for the synthesis of NiO NPs, such as thermal decomposition (22), hydrothermal (23), co-precipitation (24), sol–gel (25), and micro-emulsion (26). The majority of these procedures are very costly and boring protocols, very complex tools, and poisonous and non-eco-friendly ways for producing NPs. In order to solve the challenge of poisonous wastes, energy disparity, and for the sake of biocompatibility, an eco-friendly and greener procedure for the development of nanoparticles has been proposed (Citation35–42). Biodegradable materials such as microorganisms (MOs), fungi, and plants can be utilized to form NPs, among which the extracts of plants have more and more become prevalent nowadays (Citation27, Citation43). The sol–gel method is well known for obtaining highly pure and homogeneous products from simple and inexpensive methods (27). To confirm why the sol–gel method has earned such high enthusiasm and to calculate the opportunities for future uses, one should regard the merits and demerits of the process. It would and will be applied only when it is economical, or its profit ratio is acceptable. The basic negative point includes the higher expenditures for the starting substances. Alkoxides are certainly much more-costly than oxides. Another negative point is perhaps the processing time from the beginning to the end of the product (Citation44). However, there are some positive points which are itemized to ease comprehension: (i) the potential to monitor the construction and combination at a molecular level, (ii) the capability to present some parts in one step, (iii) the potential to force kinetic restrictions on a system and fix meta-stable steps, and (iv) the potential to adjust the activation treatment of a sample and detect the outset of active breeds. Eventually, with either single-piece or multi-piece systems, sol–gel formation permits various product shapes to be formed (Citation45). A fundamental development that acts well to typify this fact is utilizing sol–gel science for biomedical uses. It has also been announced that the sol–gel procedure is an effective way for creating ceramic oxides with varied combinations and possessing significant industrial uses (practically altered flat glass, fibers, aeorogels, and so on.) (Citation46). One thing that is sure is the fact that attempts along this direction can be improved by a better cooperation between academic and industrial scholars. Academic researchers should know about the obstructions of new technologies. Are sol–gel substances able to present novel chemistry or tackle the restrictions of the present substances formed by other procedures? Simultaneously, industrial scholars should understand the chances offered by sol–gel substances by either accepting this preparative method or moving novel concepts to the cases on which they are working.
We used gelatin instead of chemicals in this method as a reducing agent. Gelatin is a biocompatible, non-toxic, inexpensive, and available substance. Therefore, in this study, NiO NPs were synthesized using the sol–gel method, and their cytotoxic effects were analyzed against Hep-G2, MCF-7, and HT-29 cancer cells.
2. Experimental procedures
2.1. Materials and methods
The synthesis of NiO NPs was carried out according to the methods previously described (28,29). Nickel nitrate was used as a precursor, gelatin as a polymerization agent, and distilled water as a solvent. Nickel nitrate (Ni(NO3)2.6H2O) was purchased from the Acros Organic Company (UK) at a purity of 99%. Gelatin (Type B derived from bovine skin) was procured from Sigma-Aldrich and used without further purifications. The isolation of pure products was performed via preparative thin-layer chromatography (Silica Gel 60 GF254; Merck). Solvents and organic/inorganic compounds were obtained from Merck and used without further purifications.
In brief, for the synthesis of NiO NPs, 5 g of Ni(NO3)2.6H2O was dissolved in 10 ml of distilled water. Then, 2.5 g of gelatin was dissolved in 40 ml of distilled water and stirred for 60 min at 50 °C. Next, the resulting solution was gently added to the salt solution. In the following step, the obtained mixture was stirred in a water bath at 80 °C for 12 min to obtain the dark green gel. The obtained gel was dried in an oven for 24 h, and the dried powder was calcined in the furnace at 300, 400, and 500 °C for 2 h to obtain NiO NPs.
2.2. Electrochemical measurements
NiO NP electrodes were prepared according to the methods previously described (26). NiO NPs were chosen to take the position of working electrodes, while graphite and Hg/HgO were utilized as the counter and reference electrodes, respectively. At different scan rates, cyclic voltammetry (CV) was conducted using a potentiostat (Versa STAT 3, AMETEK). All of the experiments were performed utilizing the freshly prepared KOH solution (1M) to act as an electrolyte at room temperature.
2.3. Cytotoxicity assessment
The in-vitro cytotoxicity of the synthesized compounds was examined against three types of human cancer cells, namely Hep-G2, MCF-7, and HT-29, using the MTT colorimetric assay. In this assay, culture medium and cell lines served as negative and positive controls, respectively. Briefly, a certain number of cells (104) were seeded onto a 96-well microplate and incubated in a humidified atmosphere of 5% CO2 and 95% air at 37 °C to reach 70-90% confluence. Afterward, 150 μl of each of the synthesized nanoparticles (previously incubated at 37 °C in serum-containing media for 24 h) was added to each one well. After 24 h of incubation, the medium was removed from each well, were washed twice for 2–3 min with 150 μl of the phosphate-buffered saline (PBS). Then, 25 μl of the MTT (3-(4,5 Dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium, Sigma-Aldrich, USA) stock solution was transferred into each one well and incubated in a humidified atmosphere of 5% CO2 and 95% air for 4 h at 37 °C. In this stage, the tetrazolium ring is created by selective cleavage of mitochondrial dehydrogenases in viable cells, producing blue/purple formazan crystals. In order to dissolve the formazan crystals, 100 μl of DMSO was added to each one well. The optical absorption (OD) of the solution was read at a wavelength of 570 nm by an Elisa plate reader (Model 50, Bio-Rad Corp, Hercules, and CA). All experiments were performed in triplicate. The cell viability was considered as the percentage of the mean optical density values of each compound compared with the optical density values of the positive control by the below following formula:
2.4. Characterization of synthesized NPs
The phase purity and crystallite size of NiO NPs were characterized using XRD (Philips, X’pert, and CuKα) with a radiation wavelength of λ=1.54056 Ǻ. Transmission electron microscopy (TEM) was carried out on a Hitachi-7100 transmission electron microscope to calculate the average particle size of NPs. The morphology of samples was assessed under a JSM-7600F FESEM operated at 5–20 KV. The infrared spectra were recorded on a Shimadzu FTIR-8400S spectrometer.
3. Results and discussion
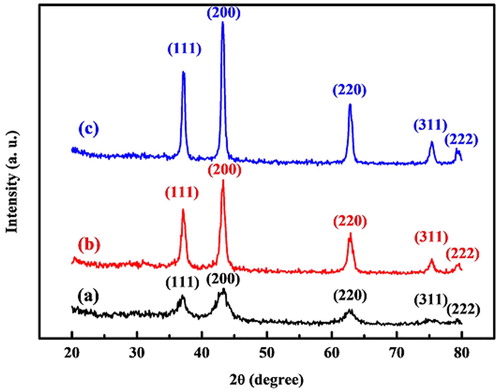
shows the XRD patterns of NiO NPs at different temperature values. All of the patterns clearly exhibit the crystal planes of (111), (200), (220), (311), and (222), corresponding to the face-centered cubic (FCC) structure (PDF card No. 01-078-0423). It indicated that synthesized NiO NPs contained high purity without showing any impurity peaks. It is perceived that a higher annealing temperature can increase the intensity of diffraction peaks. It implies that the crystallite size of NiO NPs increases in parallel with an increase in the values of annealing temperature (28). The crystalline size of synthesized nanoparticles was calculated using the Scherrer equation (EquationEq. 1(1)
(1) ):
(1)
(1) where D is the grain size, K is the constant of the Scherrer formula related to the index of hkl and the shape of nanoparticles, λ is the X-ray wavelength, θ is the XRD diffraction angle, and β is the full width at half maximum (FWHM) of the diffraction peak measured in radians. According to this equation, the crystalline size was estimated to be 10.8, 18.2, and 23.4 nm for synthesized NiO NPs at 300, 400, and 500 °C, respectively.
The morphology and structure of synthesized NiO NPs in various annealing temperature values are demonstrated by FESEM and TEM images (). A flake-like structure of NiO NPs is displayed in the FESEM images (a-c). Moreover, it indicates that the porosity structure grows rapidly as the annealing temperature increases. The mentioned observations seem to be in agreement with previous reports (30). The TEM images of NiO NPs at different temperature values in d-f exhibit a similar structure for all synthesized samples. g-i show the histograms of synthesized NiO NPs at different temperature values. According to the size distribution histogram, the average particle sizes were 8.2, 15.4, and 21.7 nm for NiO NPs at 300, 400, and 500 °C, respectively. a represents the structure of NiO NPs at 300 °C, demonstrating nanoparticles with an average size of 8.2 nm and porosity space that has been generated by the evaporation of water molecules and decomposition of chemically bound groups (29). As the annealing temperature increases, NiO NPs are aggregated and create larger particles with more porosity space, as shown in b and c.
Figure 2. FE-SEM images, TEM images, and size distribution graphs of synthesized NiO NPs at 300, 400, and 500 °C.
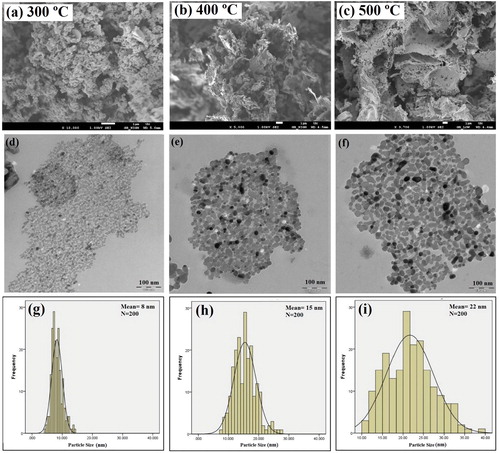
As specified in FESEM and TEM images, all of the synthesized samples exhibit the flake-like and porosity structures, and these structures differ only in size (29,30). The formation mechanism of the flake-like structure includes nucleation, growth, and oriented attachment steps. Nucleation appears to be strongly dependent on the degree of supersaturation, which is very high in this stage. The particles tend to aggregate since the electrostatic repulsive barriers are quite low. In the second step, the aggregation of primary NiO NPs (formed at low temperature) has a crucial role in the growth of NiO NPs that are annealed at higher temperatures. Due to higher annealing temperatures, the coalescence of primary particles in the aggregates leads to the elimination of primary particles, having a size lower than 10 nm. The small primary particles tend to be connected to each other and produce larger particles along with rapidly minimized interfacial free energy, which is proportional to 2γ/R, where R is the radius of spherical particles, while γ stands for the specific interfacial free energy (31). It is well known that the surface energy reduction is considered a principle rule for crystal growth in the attachment step, and by minimizing the high surface energy; this morphology progression is promoted by a greater reduction in surface energy (32). It is possible that the tendency towards minimizing the surface energy is the reason for the formation of NiO NPs that are attached along a particular direction to create flake-like structures.
Particle growth was greater than crystal growth, denoting that the aggregation of primary nanoparticles (formed at low temperature) plays a vital role in the growth of nanoparticles that are annealed at higher temperatures. NiO NPs annealed at 400 and 500 °C had larger particle sizes with a wider size distribution, compared with NiO NPs annealed at 300 °C. Because of higher annealing temperature, the coalescence of primary particles in the aggregates led to the elimination of primary particles possessing a size smaller than 10 nm. The small primary particles have the tendency to be connected to each other, resulting in the formation of larger particles along with rapidly minimized interfacial free energy, which is proportional to 2γ/R (31).
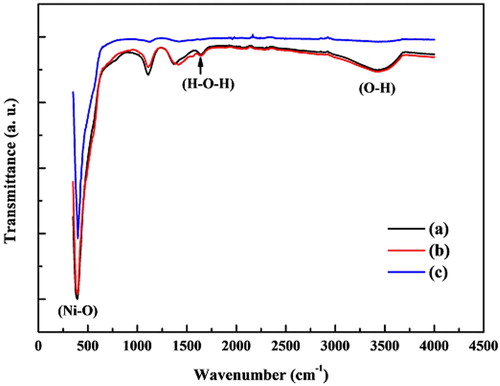
The FT-IR spectra of NiO NPs were recorded between 350 and 4000 cm−1 (). The intense peak at 400 cm−1 is assigned to the stretching vibration modes of the Ni–O molecule and is in agreement with previous studies conducted by Nadeem et al. (33) and El-Kemary et al. (34). Besides the Ni–O vibration, a broad absorption band at 3426 cm−1 corresponds to the O–H stretching vibration, while a weak band at 1645 cm−1 is assigned to H–O–H bending vibrations mode due to the adsorption of water in the air (34). The intensity of peaks corresponding to bending and stretching vibration of OH group decreases by an increase in the annealing temperature as a result of the evaporation of water.
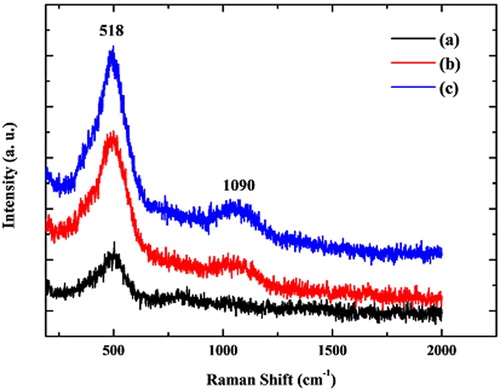
In order to confirm the effect of the annealing temperature on NiO NPs, the Raman analysis was carried out. The Raman spectra of samples were recorded and demonstrated in . According to Mironova-Ulmane report (35), the Raman spectrum exhibits several bands for bulk NiO at room temperature, as follows; a one-magnon (1M) band at ∼34 cm−1; five vibrational bands that include one-phonon (1P) TO at 440 cm−1 and LO at 560 cm−1 modes, two-phonon (2P) 2TO at 740 cm−1, TO + LO at 925 cm−1 and 2LO at 1100 cm−1 modes; a two-magnon (2M) band at ∼1500 cm−1. shows two Raman peaks that indicate the intensity of absorption peaks are proportional to the annealing temperature and size distribution. The peaks of NiO NPs observed at 518 and 1090 cm−1 are assigned to the longitudinal optical (LO and 2LO) phonon modes, associated with the oscillation of Ni-O (36-38). However, when NiO NPs were compared with each other, it was observed that the intensity of peaks is increased parallel with the increase in the particle size; therefore, Raman results also indicate the effect of annealing temperature on NiO NPs (38-40).
The cyclic voltammetry (CV) technique was utilized to analyze the electrochemical properties of synthesized NiO NPs by means of the sol–gel method in the presence of KOH (1M). The oxidation peak appears by the conversion of NiO into NiOOH, while the reduction peak is generated by the reverse reaction (EquationEq. 2(2)
(2) ) (28).
(2)
(2)
The results of TEM images showed that the size of NiO NPs is proportional to the annealing temperature. Besides, FESEM images revealed that the morphology of annealed NPs at 300 °C was changed from nano-flake-like to macro flake-like at 500 °C. The change in the structure and morphology of NiO NPs is correlated with different capacitive properties of NiO samples. By comparison of the CV curves of NiO NPs as electrodes () prepared at different annealing temperature values in the same electrochemical condition (scan rates 30 mV/S & 1M KOH), the magnitude of the current density decreased for samples treated at higher temperatures.
Figure 5. The cyclic voltammograms of synthesized NiO NPs at (a) 300, (b) 400, and (c) 500 °C temperatures.
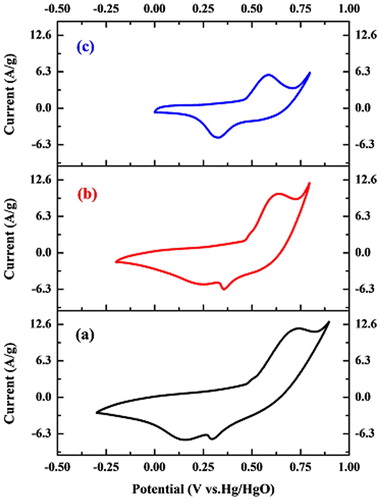
The intensity of the current density depends on the number of OH- ions that diffuse within electroactive materials in the redox reactions. Two outer-pore and inner-pore structures can be considered for the electroactive material layer (41). The suitable pore size of electroactive material plays an essential role in the penetration and diffusion of OH- ions into the inner-pore structure.
The results showed that synthesized NiO NPs at 300 °C possess a higher current density in comparison with those prepared at two temperature values, which might be attributed to the morphology of NiO electrodes with smaller particles, resulting in higher surface area and porous structures (). This feature allows a great number of ions to penetrate through the NiO electrode (28). The results demonstrated that the surface reaction of electrodes decreases when the annealing temperature is elevated (42). In other words, the surface reaction of NiO NPs electrodes decreases when the size of NiO NPs increases with higher annealing temperature.
The cytotoxic effect of synthesized NiO NPs (300, 400, and 500 °C) was investigated on Hep-G2, MCF-7, and HT-29 cancer cell lines using the MTT assay, which is established by the colorimetric assay. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, as an indicator of cell viability and cytotoxicity, measures the cellular metabolic activity in mitochondria of cells treated with different compounds (43,44).
Various concentrations of nanoparticles (0-200 µg/mL) were exposed to cancer cell lines for 24 h. Our findings indicated that the cytotoxic effect of NiO NPs was dependent on the size and concentration of nanoparticles. As shown in , the cytotoxicity of NPs increased in line with an increase in the concentration and a decrease in the size of the nanoparticles. The cell viability values were reported to be 19.28%, 30.17%, and 50.59% at a concentration of 50 μg/mL against MCF-7 cells; 29.82%, 37.83% and 48.00% at a concentration of 100 μg/mL against Hep-G2 cells, and 14.77%, 41.21% and 52.03% at a concentration of 200 μg/mL against HT-29 cells when synthesized nanoparticles were used at 300, 400 and 500 °C, respectively (). So, synthesized NiO NPs at 300 °C exhibited higher cytotoxicity compared with those prepared at 400 and 500 °C, as a result of their small particle size. In presents several studies to investigate the toxicity effects of nickel oxide nanoparticles.
Figure 6. The cell viability of synthesized NiO NPs against (a) MCF-7, (b) Hep-G2, and (c) HT-29 cancer cell lines after 24 h of incubation.
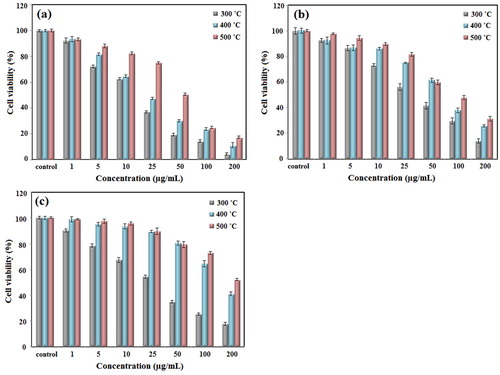
Table 1. The investigation the toxicity effects of nickel oxide nanoparticles in different studies.
Ezhilarasi et al. showed that the cytotoxicity of NiO NPs depends on the particle size, specific surface area, and the release rate of Ni+2 ions. They showed that biosynthesized NiO NPs showed a high degree of cytotoxicity against the A549 cell line due to the production of ROS and oxidative stress by nanoparticles (52). In another study, Ezhilarasi et al. found similar results for biosynthesized NiO NPs against the HT-29 cell lines (53). Siddiqui et al. analyzed the cytotoxic effects of biosynthesized NiO NPs on human airway epithelial (HEp-2) and human breast cancer (MCF-7) cells. They suggested that the mechanism of nanoparticle cytotoxicity is mediated by ROS generation and oxidative stress (53). In the present study, synthesized NiO NPs showed an acceptable degree of cytotoxicity against Hep-G2, MCF-7, and HT-29 cancer cell lines; so, they could be a useful choice for the treatment of different types of cancer.
Conclusion
Nickel oxide nanoparticles were synthesized through a simple, rapid, and inexpensive method using gelatin. The results demonstrated that the size of the synthesized nanoparticles was about 8-22 nm, and it was observed that in parallel with an increase in calcination temperature, the particle size markedly decreases. Our findings showed that particle size influences the electrochemical behavior and cytotoxic activity. Hence, larger particles can reduce the effectiveness of these properties. The results indicated that the surface reaction of NiO NP electrodes is diminished in line with an increment in the particle size. Also, the results of cytotoxicity showed that synthesized nanoparticles had higher cytotoxic effects at 300 °C than at other temperatures. Hence, synthesized NiO NPs have acceptable physiochemical properties, and they can be employed in medical and industrial fields.
Ethical approval
The research was performed on synthesis and cytotoxic activity of nickel oxide nanoparticles (NiO NPs) on human liver cancer cell (Hep-G2), breast cancer cell (MCF-7) and colon cancer cell (HT-29) lines. The cytotoxic activity of nanoparticles was performed using MTT assay. The Hep-G2, MCF-7 and HT-29 cell lines were prepared from Pasteur Institute of Iran. Therefore, this research does not require the approval of Animal Experimentation Committee.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nath, D.; Banerjee, P. Green Nanotechnology–a new Hope for Medical Biology. Environ. Toxicol. Pharmacol. 2013, 36 (3), 997–1014.
- Kharissova, O.V.; Dias, H.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The Greener Synthesis of Nanoparticles. Trends Biotechnol. 2013, 31 (4), 240–248.
- Buazar, F. Impact of Biocompatible Nanosilica on Green Stabilization of Subgrade Soil. Sci. Rep. 2019, 9 (1), 1–9.
- Buazar, F.; Baghlani-Nejazd, M.H.; Badri, M.; Kashisaz, M.; Khaledi-Nasab, A.; Kroushawi, F. Facile one-pot Phytosynthesis of Magnetic Nanoparticles Using Potato Extract and Their Catalytic Activity. Starch-Stärke 2016, 68 (7-8), 796–804.
- Buazar, F.; Bavi, M.; Kroushawi, F.; Halvani, M.; Khaledi-Nasab, A.; Hossieni, S. Potato Extract as Reducing Agent and Stabiliser in a Facile Green one-Step Synthesis of ZnO Nanoparticles. J. Exp. Nanosci. 2016, 11 (3), 175–184.
- Koopi, H.; Buazar, F. A Novel one-pot Biosynthesis of Pure Alpha Aluminum Oxide Nanoparticles Using the Macroalgae Sargassum Ilicifolium: A Green Marine Approach. Ceram. Int. 2018, 44 (8), 8940–8945.
- Buazar, F.; Sweidi, S.; Badri, M.; Kroushawi, F. Biofabrication of Highly Pure Copper Oxide Nanoparticles Using Wheat Seed Extract and Their Catalytic Activity: a Mechanistic Approach. Green Processing and Synthesis 2019, 8 (1), 691–702.
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9 (1), 1–10.
- Nasirmoghadas, P., et al. Nanoparticles in Cancer Immunotherapies: An Innovative Strategy. Biotechnol. Prog. 2020, 37 (2), e3070.
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanodrug Delivery Systems: a Promising Technology for Detection, Diagnosis, and Treatment of Cancer. AAPS PharmSciTech. 2014, 15 (3), 709–721.
- Ghaz-Jahanian, M.A.; Abbaspour-Aghdam, F.; Anarjan, N.; Berenjian, A.; Jafarizadeh-Malmiri, H. Application of Chitosan-Based Nanocarriers in Tumor-Targeted Drug Delivery. Mol. Biotechnol. 2015, 57 (3), 201–218.
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Mashhadi Malekzadeh, A. Biosynthesis of Metallic Nanoparticles Using Plant Extracts and Evaluation of Their Antibacterial Properties. Nanochemistry Research 2018, 3 (1), 1–16.
- Behzad, F.; Naghib, S.M.; Tabatabaei, S.N.; Zare, Y.; Rhee, K.Y. An Overview of the Plant-Mediated Green Synthesis of Noble Metal Nanoparticles for Antibacterial Applications. J. Ind. Eng. Chem. 2021, 94, 92–104.
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic Effects of Combinatorial Chitosan and Polyphenol Biomolecules on Enhanced Antibacterial Activity of Biofunctionalaized Silver Nanoparticles. Sci. Rep. 2020, 10 (1), 1–13.
- Gholizadeh, B.S.; Buazar, F.; Hosseini, S.M.; Mousavi, S.M. Enhanced Antibacterial Activity, Mechanical and Physical Properties of Alginate/Hydroxyapatite Bionanocomposite Film. Int. J. Biol. Macromol. 2018, 116, 786–792.
- Wang, Y.; Zhu, J.; Yang, X.; Lu, L.; Wang, X. Preparation of NiO Nanoparticles and Their Catalytic Activity in the Thermal Decoration of Ammonium Perchlorate. Science Direct, Thermochimica Acta 2005, 437, 106–9.
- Kodama, R.H.; Makhlouf, S.A.; Berkowitz, A.E. Finite Size Effects in Antiferromagnetic NiO Nanoparticles. Phys. Rev. Lett. 1997, 79 (7), 1393.
- Winkler, E.; Zysler, R.; Mansilla, M.V.; Fiorani, D. Surface Anisotropy Effects in NiO Nanoparticles. Physical Review B 2005, 72 (13), 132409.
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Acta Mater. 2000, 48 (1), 1–29.
- McHenry, M.; Laughlin, D. Nano-scale Materials Development for Future Magnetic Applications. Acta Mater. 2000, 48 (1), 223–238.
- Ohno, H.; Munekata, H.; Penney, T.; Von Molnar, S.; Chang, L. Magnetotransport Properties of p-Type (In, Mn) As Diluted Magnetic III-V Semiconductors. Phys. Rev. Lett. 1992, 68 (17), 2664.
- Zhang, F.; Wang, X.; Zhang, X.; Turxun, M.; Yu, H.; Zhao, J. The Catalytic Activity of NiO for N2O Decomposition Doubly Promoted by Barium and Cerium. Chem. Eng. J. 2014, 256, 365–371.
- Boukhachem, A., et al. Study of Substrate Temperature Effects on Structural, Optical, Mechanical and Opto-Thermal Properties of NiO Sprayed Semiconductor Thin Films. Mat. Sci. Eng.: B 2014, 188, 72–77.
- Dalvand, H.; Khayati, G.R.; Darezereshki, E.; Irannejad, A. A Facile Fabrication of NiO Nanoparticles from Spent Ni–Cd Batteries. Mater. Lett. 2014, 130, 54–56.
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: the Clash Between Damage and Metabolic Needs. Cell Death Differ. 2015, 22 (3), 377–388.
- Ibraheem, F.; Aziz, M.H.; Fatima, M.; Shaheen, F.; Ali, S.M.; Huang, Q. In Vitro Cytotoxicity, MMP and ROS Activity of Green Synthesized Nickel Oxide Nanoparticles Using Extract of Terminalia Chebula Against MCF-7 Cells. Mater. Lett. 2019, 234, 129–133.
- Buazar, F.; Moavi, J.; Sayahi, M.H. Algal Magnetic Nickel Oxide Nanocatalyst in Accelerated Synthesis of Pyridopyrimidine Derivatives. Sci. Repor. 2021, 11, 6296.
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Delivery 2010, 7 (9), 1063–1077.
- Hu, Z., et al. Visible Light Driven Photodynamic Anticancer Activity of Graphene Oxide/TiO2 Hybrid. Carbon. N. Y. 2012, 50 (3), 994–1004.
- Sriram, M.I.; Kanth, S.B.M.; Kalishwaralal, K.; Gurunathan, S. Antitumor Activity of Silver Nanoparticles in Dalton’s Lymphoma Ascites Tumor Model. Int. J. Nanomed. 2010, 5, 753–762.
- Krishnamoorthy, K.; Moon, J.Y.; Hyun, H.B.; Cho, S.K.; Kim, S.-J. Mechanistic Investigation on the Toxicity of MgO Nanoparticles Toward Cancer Cells. J. Mater. Chem. 2012, 22 (47), 24610–24617.
- Sasidharan, A.; Chandran, P.; Menon, D.; Raman, S.; Nair, S.; Koyakutty, M. Rapid Dissolution of ZnO Nanocrystals in Acidic Cancer Microenvironment Leading to Preferential Apoptosis. Nanoscale. 2011, 3 (9), 3657–3669.
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxid. Redox Signaling 2009, 11 (4), 777–790.
- Cho, Y.-L., et al. Dual Role of Oxidative Stress-JNK Activation in Autophagy and Apoptosis Induced by Nickel Oxide Nanoparticles in Human Cancer Cells. Free Radical Biol. Med. 2020, 153, 173–186.
- Moradnia, F.; Fardood, S.T.; Ramazani, A.; Min, B.-K.; Joo, S.W.; Varma, R.S. Magnetic Mg0. 5Zn0. 5FeMnO4 Nanoparticles: Green sol-gel Synthesis, Characterization, and Photocatalytic Applications. J. Cleaner Prod. 2021, 288, 125632.
- Azar, B.E.; Ramazani, A.; Fardood, S.T.; Morsali, A. Green Synthesis and Characterization of ZnAl2O4@ ZnO Nanocomposite and its Environmental Applications in Rapid dye Degradation. Optik. (Stuttg) 2020, 208, 164129.
- Yeganeh, M.S.; Kazemizadeh, A.R.; Ramazani, A.; Eskandari, P.; Angourani, H.R. Plant-mediated Synthesis of Cu0. 5Zn0. 5Fe2O4 Nanoparticles Using Minidium Leavigatum and Their Applications as an Adsorbent for Removal of Reactive Blue 222 dye. Mater. Res. Express 2020, 6 (12), 1250f4.
- Moradnia, F.; Fardood, S.T.; Ramazani, A.; Gupta, V.K. Green Synthesis of Recyclable MgFeCrO4 Spinel Nanoparticles for Rapid Photodegradation of Direct Black 122 dye. J. Photochem. Photobiol., A 2020, 392, 112433.
- Fardood, S.T.; Forootan, R.; Moradnia, F.; Afshari, Z.; Ramazani, A. Green Synthesis, Characterization, and Photocatalytic Activity of Cobalt Chromite Spinel Nanoparticles. Mater. Res. Express 2020, 7 (1), 015086.
- Mehr, E.S.; Sorbiun, M.; Ramazani, A.; Fardood, S.T. Plant-mediated Synthesis of Zinc Oxide and Copper Oxide Nanoparticles by Using Ferulago Angulata (Schlecht) Boiss Extract and Comparison of Their Photocatalytic Degradation of Rhodamine B (RhB) Under Visible Light Irradiation. J. Mater. Sci.: Mater. Electron. 2018, 29 (2), 1333–1340.
- Behzad, F.; Jafarirad, S.; Samadi, A.; Barzegar, A. A Systematic Investigation on Spectroscopic, Conformational, and Interactional Properties of Polypeptide/Nanomaterial Complex: Effects of bio-Based Synthesized Maghemite Nanocomposites on Human Serum Albumin. Soft. Mater. 2020, 18 (4), 471–486.
- Hafez Ghoran, S., et al. Biosynthesis of Zinc Ferrite Nanoparticles Using Polyphenol-Rich Extract of Citrus Aurantium Flowers. Nanomedicine Res. J 2020, 5 (1), 20–28.
- Moavi, J.; Buazar, F.; Sayahi, M.H. Algal Magnetic Nickel Oxide Nanocatalyst in Accelerated Synthesis of Pyridopyrimidine Derivatives. Sci. Rep. 2021, 11 (1), 1–14.
- Dislich, H. Sol-gel: Science, Processes and Products. J. Non-Cryst. Solids 1986, 80 (1-3), 115–121.
- Ibraheem, F.; Aziz, M.H.; Fatima, M.; Shaheen, F.; Ali, S.M. Huang. In Vitro Cytotoxicity, MMP and ROS Activity of Green Synthesized Nickel Oxide Nanoparticles Using Extract of Terminalia Chebula Against MCF-7 Cells. Mater. Lett. 2019, 1 (234), 129–133.
- Kumar, P.V.; Ahamed, A.J.; Karthikeyan, M. Synthesis and Characterization of NiO Nanoparticles by Chemical as Well as Green Routes and Their Comparisons with Respect to Cytotoxic Effect and Toxicity Studies in Microbial and MCF-7 Cancer Cell Models. SN Applied Sciences 2019, 1 (9), 1–5.