?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This review expands for the first time the development of curcumin as reducing and stabilizing agent. Curcumin is widely used in food industries, cosmetic domain and the biomedical field. The combination of green synthesis and curcumin to produce nanoparticles has been effective as the amount of toxic waste is highly reduced. Starting with a general introduction about nanoparticles synthesis and curcumin, the use of curcumin as a principal reducing agent and stabilizing compounds in the production of nanoparticles is developed. Consecutively, the preparation of different nanoparticles as gold nanoparticles (Au NPs), silver nanoparticles (Ag NPs), copper nanoparticles (Cu NPs), iron nanoparticles (Fe NPs), and manganese and manganese oxide nanoparticles (Mn NPs, MnO NPs) using curcumin is investigated and elaborated. The mechanism of interaction of curcumin with the different metal has also been developed. A concluding section summarizes the advantages of using curcumin as reducing/stabilizing agent in nanoparticles formation and their analytical applications.
GRAPHICAL ABSTRACT
1. Introduction
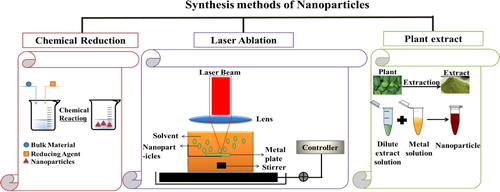
In the old days, synthesis of nanoparticles was made based on three essential categories: starting with the chemical routes, moving to the physical methods and ending with the biological approaches. Hence, whatever the method used to prepare nanoparticles, various shapes are formed (spheres, rods, flower, sheets, wires, etc.). Among all the techniques found in the literature chemical reduction reaction (Citation1–3), laser ablation (Citation4–6) and the use of plants extract (Citation7–9) are widely used and developed (see ).
Despite the advantages of the different methods to produce nanoparticles that include the formation of the nanoparticles in different shapes and sizes, time, etc.; sometimes, the use of toxic and harmful reagents is necessary to produce highly stable and recyclable nanoparticles. In fact, the majority of methods reported to date using reducing agents such as hydrazine, sodium borohydride (NaBH4), and dimethyl formamide (DMF). Thus, these reagents are highly reactive chemicals that can produce several hazardous wastes such as by-products and pose potential environmental and biological risks (Citation10,Citation11).
For this purpose, the development of a new method was necessary to prepare nanoparticles with various shapes, high thermal stability, and using the minimum amount of toxic compounds. Therefore, scientists have started to develop green nanotechnology based on green synthesis to achieve their target (Citation12).
Recently, the green synthesis route is being widely investigated in the production of nanoparticles. Green synthesis is an eco-friendly technique, recyclable and considered a biocompatible process (Citation13). The synthesis of nanoparticles using green synthesis is examined to be an essential tool to reduce the harmful and destructive effects associated with the traditional methods of preparation usually manifested in laboratory and industry (Citation14). In addition, green approaches are significantly attractive since they reduce the toxicity potential of the nanoparticles (Citation15).
The two main criteria of nanoparticle synthesis that should be taken into consideration are the choice of the reducing agent and capping agent (Citation16). In general, the synthesis of nanoparticles through the green chemical reduction method involves two essential steps (Citation17):
The use of reducing agent: in this step, the electrons in the province from curcumin provide the reduction of the metal from Mx+ to M0, meaning that the transformation of the metallic species from its bulk composition to its electric state.
The use of stabilizing agent: in this step, the formed nanoparticles are stabilized and protected from arbitrary aggregation. This involves the presence of a repulsive force that controls the size and the shape of the nanoparticles.
Based on different studies done, researchers have found that chemicals that are generated from plant extract are the greater chemical component used as a reducing agent (Citation18,Citation19).
Indeed, curcumin is one of the greatest nontoxic components, extracted from turmeric Curcuma longa that is developed nowadays to be used as a reducing agent (Citation20,Citation21). It was isolated first by Vogel in1842. Initially, curcumin was isolated from turmeric by solvent extraction, followed by column chromatography (Citation22). Among solvent extraction, soxhlet, ultrasonic, and microwave extraction were useful in the purpose of extracting curcumin (Citation23).
Later on, it was characterized by Milobedeska in 1910, where he found that curcumin is a diferuloylmethane compound, where its IUPAC name is (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-hepadiene-3,5-dione). Finally, it was synthesized and proved by Lamp in 1913 (Citation24,Citation25). The synthesis of curcumin involves five consecutive steps. The raw materials are carbomethoxyferuloyl chloride and ethyl acetoacetate.
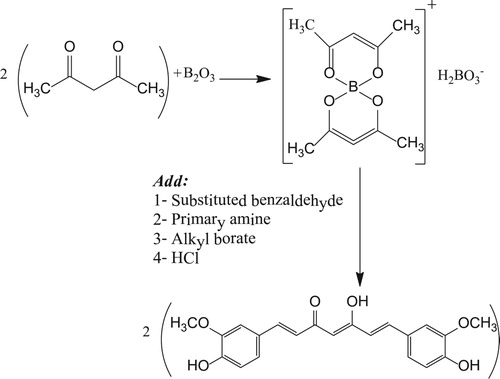
Pabon et al. have developed a new method to prepare curcumin using acetylacetone in the presence of boron trioxide, with different substituted aromatic aldehydes, trialkyl borate, and n-butylamine (Citation26) (see ).
Curcumin was introduced in the 14th century to the western world, and till now is still being used (Citation27). Curcumin in its powdered and roasted form has been used continuously, either consumed with hot milk or administered as anti-dysenteric agent, respectively (Citation28). The importance of curcumin is very clear since more than 400 000 publications were found when searching for the term ‘Curcumin.’
The crystal structure of curcumin has shown some differences in the electron delocalization and intramolecular hydrogen bonding in the fragment –CO–HC=COH–. In addition, an electron delocalization has found also in intermolecular hydrogen bonding. However, it was established that no remarkable differences in the lengths of the C–C and C–O bonds present in the enol form (Citation29). Moreover, it was given that the stability of curcumin is pH dependent. This fact was proven by the change of curcumin color while varying the pH. Hence, in acid media, curcumin has a medium solubility with a yellow color, although, in neutral media, curcumin stands in suspension where it is not completely soluble, with a pale yellow solution’s color. However, at alkaline conditions, curcumin presents high solubility at pH > 8, where the solution color turns dark orange (Citation30,Citation31).
Curcumin has been used worldwide in different ways, regarding its principal health benefits. In India, curcumin has been used in curries. In Japan and Korea, it is mixed with tea and other drinks. In Thailand, it is added as an essential component to cosmetics products. Moreover, in China, it is used as a colorant. In Lebanon, it is used in sweets and cakes. In Malaysia and Pakistan, it founds its benefits as an antiseptic and as an anti-inflammatory reagent, respectively. In addition, in the United States, curcumin is considered a primary ingredient in mustard sauce, it is used as a preservative for various compounds (Citation32,Citation33).
Mainly, the effect of curcumin as anti-cancer (Citation34–36), anti-oxidant (Citation37–39), anti-inflammatory (Citation40–42), anti-bacterial (Citation43–45), etc., agent in the biomedical field has been extremely developed and established (Citation46–48). Therefore, a large number of reviews have adopted this topic and elaborated. However, curcumin shows very poor bioavailability where several studies have showed undetectable concentrations in blood and extra-intestinal tissue. These limitations are initially due to its low absorption, fast metabolism, chemical instability, and very high systemic elimination (Citation49,Citation50). Therefore, to overcome these drawbacks, many methods have been tested to surge its bioavailability. Some of these techniques include the use of curcumin with nanoparticles, the use of adjuvants such as piperine, formulating liposomal curcumin, etc (Citation51). Consequently, curcumin in nanoparticle formation has been introduced (Citation52).
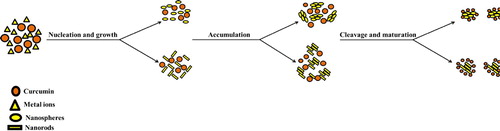
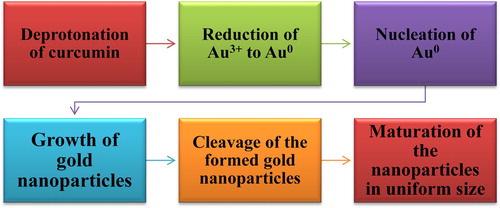
Indeed, a new role of curcumin is being investigated. This role resumes the efficiency of curcumin as a reducing agent. In this area, the use of curcumin in the production of metallic nanoparticles has been developed as a new eco-friendly reducing agent. Generally, the establishment of curcumin functionalized metallic nanoparticles comprises six consecutive steps. The reduction potential starts with the deprotonation of the metal ion, followed by its reduction, moving to the nucleation and growth, afterward cleavage occurs, ending up with the maturation of the nanoparticles (see ) (Citation53,Citation54).
Thus, in this review, we will highlight the role of curcumin as a reducing agent for different nanoparticle preparation by assembling the work presented in the literature. To the best of our knowledge, till now no review has focused on the application of curcumin as a reducing agent though many of the reviews focus on the application of curcumin in biology and medicine.
2. Synthesis of metallic nanoparticles
Nanoparticles including, gold, silver, and iron oxide have gained too much attention, due to their extreme importance and usage in medicine and the biomedical field (Citation55,Citation56). Therefore, it was necessary to develop green synthesis to minimize the toxic waste from the chemical reduction reaction. Lately, researchers have started to replace toxic reducing agents like sodium borohydride and hydrazine by using curcumin as a starting up material to reduce bulk material in the formation of the nanoparticle.
2.1. Gold nanoparticles preparation
In 2013, Sreelakshmi et al. and Singh et al. were the first to elaborate on the role of curcumin as a reducing agent to prepare gold nanoparticles. To start with, Sreelakshmi et al. were able to synthesize gold nanoparticles using curcumin as a reducing and stabilizing agent in basic media (pH = 11) (Citation57). In this work, the authors have established the effect of curcumin’s concentration on the formation of the nanoparticles during one hour. The authors have found that when increasing the concentration of curcumin, the color solution change from light violet to brownish red. This color change explains the formation of smaller size which is in agreement with Mie’s theory. Sreelakshmi et al. have confirmed the formation of Au NPs after 15 min of adding curcumin when the color changes from yellow to light violet. The change of color was verified by a UV-visible spectrophotometer, where after 15 min a surface plasmon resonance (SPR) absorption band was obtained at 530 nm. Hence, after 70 min, an SPR band was obtained with a remarkable increase in the intensity, meaning that the reduction of Au3+ to Au0 was achieved.
Similarly, Singh et al. were able also to produce gold nanoparticles using curcumin only (Citation58). For this purpose, the authors have mixed 4 mg of curcumin with 15 mM of gold chloride (HAuCl4). As curcumin cannot react alone in neutral media, the experiment was carried out at 90°C. The authors have proved the efficiency of curcumin as a reducing agent to produce Au NPs using curcumin only at 90°C. At high temperatures, Singh et al. have enhanced the activity and the solubility of curcumin in the solution. They have determined the effect of HAuCl4 concentration on the synthesis of AuNPs and shown that the solution’s color change from purple to dark brownish when increasing the concentration of curcumin; thus, the change in color solution could also be attributed to the increase on HAuCl4 concentration, where the color changes from brownish to purplish-red color. Here, the change in the color verifies the formation of different sizes. Thereby changing the concentration of HAuCl4, different SPR peaks were obtained situated at λ equal to 528, 527, 552, 546, and 543 nm.
In addition, Sindhu et al. (Citation59) were able to produce Au NPs using curcumin only at pH 9.2. The only main difference between Sindhu et al., Singh et al., and Sreelakshmi et al. is that Sindhu and his colleagues have carried out the synthesis for three consecutive days to make sure that all curcumin has been reacted and to get the maximum reduction. Moreover, Sindhu et al. have dissolved the precipitate of Au NPs after centrifuging in Milli Q water. This step has increased the stability of Au NPs for more than 6 months. In consequence, the Au NPs prepared with Sindhu et al. were efficacy to be used in cell culture study.
The same procedure was adopted by Shaabani et al. (Citation60) using curcumin as stabilizing and reducing agent to produce gold nanoparticles. However, Shaabani et al. have investigated the synthesis of Au NPs using curcumin or citrate as a reducing agent. It was found that the amount of Au3+ reduced is higher when using curcumin. Therefore, it was necessary to add sodium borohydride to the citrate, in order to induce the reduction of Au3+ to Au0. Curcumin-coated Au NPs were smaller in size and have shown better antioxidant activity than the citrate-coated NPs.
Furthermore, Abdelwahab et al. have monitored the synthesis of Au NPs using curcumin as a reducing agent and by adding HAuCl4 dropwise at pH equal to 9.3 (Citation61). In this case, when adding HAuCl4 drop by drop, the solution color starts to change from yellow to colorless, then black, and finally burgundy red. These color changes are attributed to the reaction of curcumin with HAuCL4 in order to form Au NPs. When the color changes from yellow to transparent, it means that all curcumin has been reacted by reducing Au3+ to Au0. Later on, the change in the color from colorless to black verifies the beginning of the formation of Au NPs. Finally, the variation in the color from black to burgundy red confirms the synthesis of stable Au NPs.
Yet, it was found that the stability of the Au NPs increases, and the inhibition of aggregation is achieved where surfactants are added. For this reason, several researchers tend to prepare Au NPs using curcumin as a reducing agent and specific surfactant as a stabilizing agent.
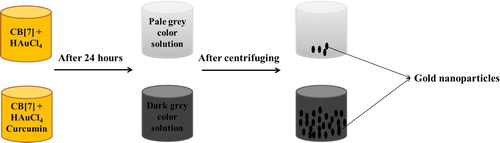
El Kurdi et al. were able to prepare gold nanoparticles with different shapes and sizes using curcumin as a reducing agent and several surfactants, under different reaction parameters. First of all, El Kurdi et al. (Citation62,Citation63) have prepared gold nanoparticles solution using curcumin and cucurbit[7]uril as a stabilizing agent in neutral media. They were able to form Au NPs solution containing two different shapes: spheres and rods in the size range of 10–20 nm.
The effect of curcumin as a reducing agent was obvious when preparing Au NPs without curcumin, where in the absence of curcumin the solution color changed from orange to pale gray after 24 h. However, in the presence of curcumin after 24 h, the color solution changed to dark gray. In the absence of curcumin, after centrifugation, the precipitation yield was almost 10% indicating that Au3+ was totally reduced to Au0. Yet, in the presence of curcumin, the yield of the reaction was increased remarkably, where after centrifuging the precipitate was obtained in high yield (see ). Cucurbit[7]uril in this case acted as a host molecule to protect the formed Au NPs from aggregation.
Later on, El Kurdi et al. (Citation54,Citation64) have established new gold nanoparticles using curcumin and poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) as stabilizing agent (F-108) in neutral media. El Kurdi et al. in their work have summarized the preparation of gold nanoparticles in six steps (see ):
The authors have demonstrated the role of curcumin as a reducing agent to reduce Au3+ to Au0 in this way. The reduction began with the formation of Cur3- when the hydrogen atom from the hydroxyl group of the enolic curcumin is dissociated. When Cur3- is formed, the electron on the O- ion enhanced the reduction of Au3+ to Au0. Therefore, Au0 atoms will form clusters that undergo cleavage and form small fragments protected with F108 polymer. Moreover, the authors were interested in studying the effect of curcumin concentration during Au NPs synthesis. According to their analysis, the results showed that the size of the nanoparticles depends strongly on the amount of curcumin added during the synthesis. For example, when adding 1 and 5 mM of curcumin, Au NPs were obtained in different shapes with diameters between 120–140 nm and 100–120 nm, respectively. In addition, at 50 mM of curcumin, Au NPs in spheres shape were formed with a size range between 30 and 40 nm. However, when adding 10 mM of curcumin, smaller spheres were obtained with a size range between 10 and 20 nm. Hence, in all cases, curcumin has reduced Au3+ to Au0. Thus, different sizes were obtained. This difference is due to the fact that at low concentration, the density of the functional groups of curcumin molecule is low; therefore, the clusters formed did not undergo the cleavage phase, and thereby, the particles remain big. Although at a high concentration of curcumin, the solubility of curcumin decreased, this leads to the precipitation of curcumin in the solution, inhibiting the formation of smaller nanoparticles. Thus, a medium concentration of curcumin (10 mM) induced the formation of small and stable Au NPs.
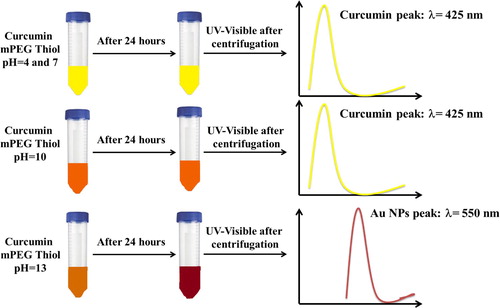
Another type of polymer was used by Al Shehab et al. in order to produce Au NPs in the presence of curcumin (Citation21). Al Shehab et al. have used poly(ethylene glycol) methyl ether thiol polymer (mPEG thiol). As mentioned before, curcumin in solution depends strongly on the pH of the media. Meaning that, in acidic and neutral media, curcumin is present in bis-keto form. Therefore, curcumin is considered a proton donor. Yet, in basic media, curcumin acts as an electron donor since it exists as an enolate form where the heptadienone chains predominate (Citation38). For this reason, the authors were interested in studying the effect of pH on the Au NPs synthesis using curcumin as a reducing agent. Therefore, four different pHs (4, 7, 10, and 13) were studied by Al Shehab et al. to investigate their effect on the synthesis of Au NPs. The effect of the pH on the synthesis was established by a UV-visible spectrophotometer. The authors have found that at high pH, curcumin reacted totally since the absorption peak of curcumin was absent and a peak around 550 nm, relative to the Au NPs, was obtained. Nevertheless, at pH 4, 7, and 10, the absorption peak of curcumin at 425 nm was remarkable as obtained in the UV-visible spectra. This study proved the role of curcumin at high pH as an electron donor, inducing the reduction of Au3+ to Au0. Meaning that, at low pH, the curcumin is kept unreacted and inhibits the formation of Au NPs ().
Nonetheless, El Kurdi et al. have confirmed the role of curcumin as a reducing agent in acidic, neutral, and basic media in the presence of cetyltrimethylammonium bromide (CTAB) (Citation65). The authors have tested the ability of curcumin to reduce Au3+ to Au0 at pH 4, 7, 8, 9, 11, and 13. In contrast to the results obtained with Al by Shehab et al., Au NPs were obtained at all pHs. However, different shapes and sizes were obtained. Using curcumin as a reducing agent and cetyltrimethylammonium bromide as a stabilizing agent has enhanced the production of Au NPs at different pHs. The authors have verified the production of gold nanoparticles through the color change of the solution. In fact, after 24 h and at 45°C, the solution turned from orange to brown at pH 4, 7, 8, 9, and 11. Hence, the big difference was occurred at pH 13, when the solution color turned from orange to dark red. This difference in the color was attributed to the different shapes obtained, where at pH 13 nanoparticles in nanowires shape were obtained, and for the other pH, spherical nanoparticles were formed.
This difference in the results between Al Shehab et al. and El Kurdi et al. could be attributed to the type of surfactant present in the solution. Therefore, the study established by El Kurdi et al. enhances the activity of curcumin as a reducing agent in acidic, neutral, and basic media.
Moreover, additional work was done by Moussawi et al. aims to produce Au NPs using CTAB and curcumin at pH equal to 9.2 (Citation66). In this work, the authors have proved the role of curcumin as a secondary reducing agent to improve the gold nanoparticles formation in a rod shape. It was found that when adding curcumin and silver nitrate to CTAB capped Au NPs seed, nanorods in high yield were formed. According to Moussawi et al., the above procedure was simple, decreased the susceptibility of impurities, and enhances the reproducibility of the product distribution. However, nanorods were obtained in moderate yield when adding curcumin alone but with the presence of some nanospheres. Thus, the addition of silver nitrate has enhanced the activity of curcumin and 100% nanorods were obtained.
Thus, Moussawi et al. were able to form Au nanorods at pH 9.2, where El Kurdi et al. have obtained spherical shapes. These different results emphasize the role of curcumin as a reducing agent in the production of different shapes of gold nanoparticles at the same pH. Meaning that, upon the reaction conditions, curcumin acts in a different way to produce several shape and size of gold nanoparticles.
Finally, a study was published by Patra et al. using curcumin and potassium carbonate (Citation67). The preparation of Au NPs was occurred using curcumin as a reducing agent, where Au3+ were easily reduced to Au0. Patra et al. have established the kinetic reaction of the Au NPs synthesis in order to follow up the reduction process. In fact, when adding curcumin, an amount of Au3+ was reduced to Au0. This was confirmed by the UV-Visible spectrophotometer where a minor peak was obtained at 425 nm (curcumin peak) and a major peak was obtained at 525 nm (Au NPs). However, within the time, the absorbance at 425 nm decreased dramatically and the absorbance at 525 nm increased gradually after 4 h. This change in the absorbance is due to the fact that curcumin is being reacted in the solution and thereby achieving the reduction of Au3+ to Au0.
To end up, based on the formed Au NPs, several sensing nanoprobes were produced in order to detect different analytes. For example, El-Kurdi et al. were able to detect uric acid (Citation64), adenosine triphosphate (Citation62,Citation63), glucose (Citation54), and alpha-tocopherol (Citation65,Citation68). Al Shehab et al. (Citation21) and Patra et al. (Citation67) were able to detect melamine and nucleic acid, respectively. However, each type of Au NPs prepared was selective for specific analytes, in different ranges of concentration. Besides that, Sreelakshmi et al. (Citation57) have evaluated the cytotoxicity of the prepared Au NPs against three different cell lines, where Au NPs have shown an effective role in the inhibition of the proliferation. Furthermore, Sindhu et al. (Citation59) have verified the biocompatibility of the Au NPs, where the studies have shown that the prepared Au NPs are biocompatible when treating with normal human blood cells. Finally, Singh et al. (Citation58) and Shaabani et al (Citation60) have established the role of Au NPs as an antioxidant. Hence, whatever is the procedure followed to prepare the nanoparticles, the use of curcumin enhances the activity of the Au NPs, where they have shown promising features based on their biomedical applications.
2.2. Synthesis of silver nanoparticles
Kundu et al. were the first to establish the role of curcumin as a reducing and stabilizing agent for silver nanoparticle synthesis. Ag+ ions were immediately reduced to Au0 under continuous stirring at 80°C. According to Kundu et al. (Citation69) when heating the curcumin in an aqueous solution at high temperature, the orange color started to disappear. After almost 20 min, the color changes from orange to faint light yellow. This change in color is due to the nucleation of Ag particles. Later on, after one hour, the color changes to dark yellow/yellowish green. This color indicates the maturation of Ag clusters and thereby the formation of Ag NPs. The mechanism of Ag NPs can be explained as below (see ).
Another study by Verma et al. (Citation70) was conducted using curcumin as a reducing agent in the presence of NaOH. Verma et al. have verified the use of basic media as an effective condition to accelerate the reaction of curcumin. Curcumin in basic media is easily deprotonated. Thus, the reduction of Ag+ to Ag0 easily occurs. In this case, the color changes from yellowish red to greenish yellow in five minutes under ultrasonication.
To evaluate the difference between Ag NPs prepared using curcumin and other reducing agents, Selvan et al. have prepared silver nanoparticles using garlic, tea extract, and curcumin (Citation71). In the three cases, the reducing agent was dissolved in NaOH, added in a second step to Ag NO3 solution, and kept under stirring overnight. Despite that curcumin reacted immediately in basic media, the solution was kept for overnight to have the same condition for all reducing agents. The reduction of Ag+ to Ag0, according to Selvan et al., is due to the flavonoids present in the garlic, tea extract, and curcumin. However, these flavonoids consist of hydroxyl groups that have a strong ability to bind with Ag+ ions. Hence, once the interaction occurred, Ag+ is reduced to Ag0 and therefore induces the formation of Ag NPs. However, Ag NPs prepared using curcumin were smaller in size compared to the ones prepared using garlic and tea extract. Hence, no major difference was observed during the preparation and characterization analysis. Thus, based on Selvan et al.’s results, the most relevant difference was that the Ag NPs prepared using curcumin had the most biological effect.
In addition, Alsammarraie et al. (Citation72) and Sathishkumar et al. (Citation73) were in agreement with the statement proposed by Selvan et al. Hence, when the curcumin solution changes in color, this is mainly due to the surface excitation of the plasmon resonance phenomenon of silver metal. Yet, curcumin contains a high level of terpenoids which make it an excellent reducing agent for the synthesis of metallic nanoparticles. Therefore, the enolic tautomer form of curcumin can interact easily with the acceptor orbital and be bound to the core of the Ag+ ions. Curcumin in its enolic form will act as a potent reducing agent where Ag+ will be reduced to Ag0, leading to the formation of Ag nuclei and finally to saturated Ag NPs.
Another explanation was adopted by Khan et al. (Citation74) to evaluate the role of curcumin as a reducing agent to produce Ag NPs. Briefly, Khan et al. have dissolved a specific amount of curcumin in NaOH. According to the authors when adding NaOH to curcumin, curcumin oxide is formed. Hence, in the first place when adding curcumin to Ag NO3, a complex is formed (equation 1). This complex reacted with curcumin oxide in order to produce Ag0 (equation 2).
(1)
(1)
(2)
(2)
Similarly, Shameli et al. have adopted the same concept (Citation75,Citation76). According to Shameli et al., [Ag(Cur)]+ reacted with an aldehyde group presented in the methanolic group of curcumin (curcumin oxide) to form curcumin reduced and stabilized Ag NPs. The reduction of Ag+ is in the first case due to the oxidation of the aldehyde group into the carboxylic acid group.
Al Namil et al. (Citation77,Citation78) were able to synthesize Ag NPs through green solid-state reaction, using curcumin and silver nitrate. In this case, the authors have mixed Ag NO3 plus curcumin and ground them for about one hour until homogeneous powder is obtained. The color changed from yellow to dark orange. Then, a specific volume of ethanol was added and the growth of the Ag NPs was studied for 16 h. Despite the reduction of Ag+ to Ag0, the method involved by Al Namil et al. has increased its role as a stabilizing agent. After complete reduction, the surface charge of curcumin plays a major role as a stabilizing agent, inducing the stabilization and the formation of uniform Ag NPs. Hence, this is due to the C=O group in the enol form of curcumin and/or to the biding of silver nanoparticles with the hydroxyl group in curcumin. El Khoury et al. (Citation79) in their experiment have also verified the interaction of Ag with the C=O group in curcumin to assure a complete reduction. Hence, to increase the stability of the formed Ag NPs, the authors have added glycerol to the mixture during synthesis. In this case, monodisperse Ag NPs were obtained. The addition of glycerol did not alter the formation of Ag NPs in its absence. Yet, glycerol has been disposed on the curcumin’s surface, inducing the stability enhancement of the produced Ag NPs.
Abdulwahab et al. (Citation61) have compared the preparation of Ag NPs using curcumin or citrate. It was found that using curcumin as a reducing agent smaller particles were formed. Hence, Abdulwahab et al. have confirmed the preparation of Ag NPs under continuous stirring without adjustment of pH. According to the authors and under continuous stirring, curcumin can act as a reducing agent without changing pH, since under basic media, curcumin can be degraded. Another study was done by Yang et al. (Citation80) to verify the difference between the use of curcumin and citric acid as a reducing agent. According to Yang et al., the curcumin-stabilized AgNPs were more stable in a physiological environment. Hence, they possess higher and better antiviral properties.
To finish up, Ag NPs can be applied in different ways and different fields. Hence, Al Namil et al. (Citation77) and Verma et al.(Citation70) have used their Ag NPs as catalysis agents to enhance the catalytic reduction reaction of 4-nitrophenol. Moreover, Al Namil et al. (Citation78) have investigated the role of Ag NPs as nanoprobe to detect human serum albumin, in addition to El Khoury et al. (Citation79) that were also able to detect nucleic acid using Ag NPs synthesized in their way. Furthermore, Alsammarraie et al. (Citation72), Sathishkumar et al. (Citation73), and El Khoury et al. (Citation79) have elaborated the role of Ag NPs as an antibacterial against different microorganisms. In addition, the antioxidant and anticancer activity was also established by Selvan et al. (Citation71) and Yang et al. (Citation80), respectively.
2.3. Synthesis of mixed nanoparticles (Au/Ag)
Abdelghani et al. were the first to synthesize mixed metallic nanoparticles using curcumin as a reducing and stabilizing agent (Citation81). In this case, curcumin was mixed with HAuCl4 and AgNO3 aqueous solutions and kept under stirring for 24 h. In this case, curcumin reduced consecutively gold and silver ions. However, the mechanism of curcumin as a reducing agent when preparing mixed nanoparticles does not differ from the mechanism when preparing Au NPs or Ag NPs. Hence, in an aqueous solution, the enol form of curcumin induces the reduction of Au3+ and Ag+ to Au0 and Ag0. However, mixed nanoparticles can be effective to be used as anticancer, antioxidant, and antimicrobial agents.
2.4. Synthesis of copper and iron nanoparticles
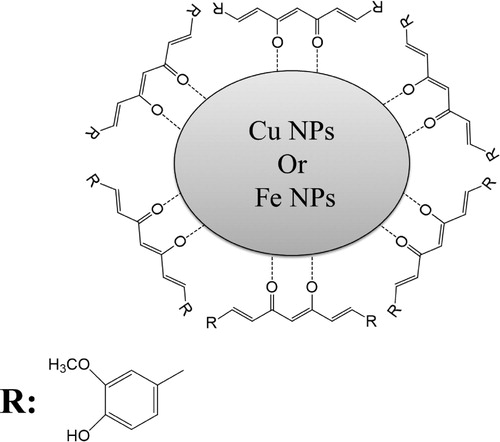
In 2016, Sankar et al. (Citation82) were the first to synthesis copper nanoparticles (Cu NPs) and iron nanoparticles (Fe NPs) using curcumin as a reducing agent and stabilizing agent at the same time. Sankar et al. have evaluated the reaction of curcumin in deionized water without adjusting the pH. However, a relative amount of curcumin was dissolved in deionized water and stirred for 5 consecutive hours. Later on, a specific amount of cupric sulfate and iron chloride hexahydrate was added. The solution mixture was kept under stirring for 24 h and then centrifuged at 12000 rpm for 20 min. The authors were able to verify the formation of Cu NPs and Fe NPs by color change. The color was changed from yellow to dark brown. The change in color is due to the excitation of the surface plasmon vibrations during nanoparticle formation. The use of curcumin as a reducing agent and stabilizing agent induced in the first place the reduction of the metals ions and then stabilized the formed NPs on their surface by the enol form (see ).
Hence, Sankar et al. have investigated the effective role of Fe NPs and Cu NPs in treating A549 lung cancer cells. Few years later in 2019, Jayarambabu et al. (Citation83) were able to synthesize Cu NPs using curcumin as a reducing agent and stabilizing agent to produce Cu NPs. Hence, to reduce the experiment time, the authors have used microwave irradiation. In this case, the reaction time was estimated to be 180 s. Thus, the only difference in this case is that the particles obtained were more agglomerated. In addition, this method enhanced the purity of the Cu NPs formed since no alternative peaks of CuO and CuO2 were obtained in the XRD diffractogram and the amount of oxygen atom was almost absent in the EDX analysis. The Cu NPs prepared by Jayarambabu et al. have shown an important antibacterial activity with both Gram-positive and Gram-negative microorganisms. Furthermore, these NPs can be applied in the industrial field as textile coatings, antiseptic creams, cosmetic applications, and disinfectants.
2.5. Synthesis of manganese nanoparticles
In 2015, Jayandran et al. have synthesized manganese nanoparticles (Citation84) using both lemon extract as a main reducing agent and curcumin as a second reducing agent and stabilizing agent. Gold, silver, and iron can be reduced easily using curcumin. Hence, for manganese bulk material, it is necessary to use two different reducing agents in order to increase its reduction reaction. The color changes from pale green to reddish-brown color. The final color confirms the stability of Mn NPs. Mainly, Mn NPs are used as antimicrobial products. Yet, Jayandaran et al. have established the antimicrobial activity of the prepared nanoparticles toward different funguses, Gram-positive and Gram-negative microorganisms. summarizes the reagent used for nanoparticle preparation and their application.
Table 1. Reagent used for the different metallic nanoparticles and their applications.
3. Synthesis of metallic oxide nanoparticles
As drug carriers, inorganic nanomaterials such as metal oxides present numerous advantages over their organic counterparts. Particularly, some metal oxides like zinc oxide (ZnO), copper oxide (CuO), and iron oxide (Fe2O3) have confirmed quite high stability and less toxicity. Metal oxide nanoparticles are fairly low-cost supplies and have the potential to balance the therapeutic effect of curcumin as they have shown therapeutic effects by themselves (Citation85).
3.1. Synthesis of zinc curcumin oxide nanoparticles
Zinc oxide nanoparticles present excellent ultraviolet absorbability and visible light transparency. Hence, it has been reported that ZnO NPs with particle sizes greater than 100 nm are considered to be relatively biocompatible (Citation86). Recently, curcumin-conjugated zinc oxide nanoparticles have been widely investigated, since the combination of both materials enhances their biomedical effect.
In 2019, Varaprasad et al. have synthesized core–shell curcumin–ZnO nanocomposite, based on the precipitation of curcumin under ultrasonication. The synthesized nanostructures have shown complete water dispersibility. Interestingly, the core–shell curcumin–ZnO nanocomposite confirmed a superior antibacterial performance against Staphylococcus pneumoniae (S. pneumoniae) and E. coli than commercial antibiotic amoxicillin (Citation87).
Additionally, Arab et al. and Nasrallah et al. have produced spherical zinc curcumin oxide nanoparticles using zinc nitrate hexahydrate and potassium hydroxide. Remarkably, Arab et al. have shown that these nanoparticles were found to be a great nanoprobe for the detection of ascorbic acid (Citation88) and a quite good adsorbent for the adsorption of Congo red dyes (Citation89). Likely, Nasrallah et al. have evaluated the effect of zinc curcumin as an antioxidant agent (Citation90).
3.2. Synthesis of curcumin conjugated copper oxide nanoparticles
Copper oxide nanoparticles are a narrow bandgap p-type semiconductor. CuO NPs own different properties like good electrochemical activities, proper redox potential, and outstanding stability in solutions (Citation52).
In 2018, Senosy et al. have produced curcumin–CuO complex, where commercial CuO and curcumin were mixed and ground together. The formed NPs were reported for biomedical applications, where it was found curcumin-CuO nanocomplex has emerged an anti-diabetic effect in vivo on streptozotocin (STZ) diabetic mice (Citation91).
Besides, Qassem et al. have produced curcumin-conjugated copper oxide nanoparticles, by mixing cupric sulfate and curcumin in basic media under reflux. Qassem et al. have established the usage of curcumin-conjugated copper oxide nanoparticles in the biomedical field as nanoprobe. Hence, they have found that these nanoparticles can be used as nanoprobe in the detection of cations as mercury (Citation92) ions and anions as persulfate (Citation93). Additionally, the detection of aminoacids as cystine (Citation94) and dopamine (Citation95) was also achieved with high selectivity using curcumin-conjugated CuO NPs. As well, curcumin-conjugated CuO NPs were found to be effective in the environmental field as a catalyst for the reduction of methylene blue (Citation20).
3.3. Synthesis of iron oxide nanoparticles
Iron oxide nanoparticles are receiving attention in the biomedical domains, such as contrasting agents in MRI imaging, cell separation and detections, drug delivery.
In 2016 Bhandari et al. reported a simple coating of Fe3O4 NPs using curcumin. The formed nanoparticles were found to be useful as an antioxidant agent. The resulting curcumin-coated iron oxide nanoparticles have confirmed lower toxicity than uncoated Fe3O4 or pure curcumin to human umbilical vein endothelial cells (HUVEC) (Citation96).
Also, Khan et al. have successfully synthesized curcumin-capped iron oxide nanoparticles. It was found that these nanoparticles were being an excellent prospect for effective pancreatic cancer treatment (Citation97).
4. Future challenges
It is clear that green synthesis facilitates the production of nanoparticles. However, this simple method can also be developed to produce stable and very uniform nanoparticles. In addition, since nanoparticles could be synthesized in different shapes, additional studies could be handled in order to control precisely the size and the shape. Moreover, as nanoparticles are being recently emerged in the biological application, various separation and purification techniques could be elaborated to increase their activity and decrease the toxicity.
5. Conclusion
As discussed earlier, curcumin has been recently used to act as a cheap reducing and stabilizing agent. However, it was found that curcumin can be easily added during the preparation of nanoparticles without any further modifications. Moreover, in most of the preparation, the ability of curcumin to reduce and stabilize nanoparticles eliminates the necessity of using several reagents that acts specifically as reducing and stabilizing agent. Therefore, despite the different procedures adopted by the researchers, the activity of curcumin as a reducing agent was not altered. Finally, the nanoparticles formed using curcumin can be used in different fields and act as nanoprobes to detect different analytes, anticancer, antioxidant, and antimicrobial compounds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A Chemical Reduction Approach to the Synthesis of Copper Nanoparticles. Int. Nano Lett. 2016, 6 (1), 21–26.
- Manikam, V.R.; Cheong, K.Y.; Razak, K.A. Chemical Reduction Methods for Synthesizing Ag and Al Nanoparticles and Their Respective Nanoalloys. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176 (3), 187–203.
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of Silver Nanoparticles by Chemical Reduction Method. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 256 (3), 111–115.
- Crivellaro, S.; Guadagnini, A.; Arboleda, D.M.; Schinca, D.; Amendola, V. A System for the Synthesis of Nanoparticles by Laser Ablation in Liquid That is Remotely Controlled with PC or Smartphone. Rev. Sci. Instrum. 2019, 90 (3), 1–7.
- Correard, F.; Maximova, K.; Estève, M.A.; Villard, C.; Roy, M.; Al-Kattan, A.; Sentis, M.; Gingras, M.; Kabashin, A.V.; Braguer, D. Gold Nanoparticles Prepared by Laser Ablation in Aqueous Biocompatible Solutions: Assessment of Safety and Biological Identity for Nanomedicine Applications. Int. J. Nanomedicine 2014, 9 (1), 5415–5430.
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 2017 (34), 80–90.
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31 (2), 346–356.
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of Silver Nanoparticles Using Aqueous Extract of Medicinal Plants’ (Impatiens Balsamina and Lantana Camara) Fresh Leaves and Analysis of Antimicrobial Activity. Int. J. Microbiol. 2019, 2019, 1–9.
- Ahmed, S.; Ikram, S. Synthesis of Gold Nanoparticles Using Plant Extract: An Overview. Nano Res. Appl. 2015, 1 (15), 1–6.
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely ‘Green’ Synthesis and Stabilization of Metal Nanoparticles. JACS 2003, 125 (46), 13940–13941.
- Sharma, G.; Pandey, S.; Ghatak, S.; Watal, G.; Rai, P.K. Potential of Spectroscopic Techniques in the Characterization of ‘Green Nanomaterials’. In Nanomaterials in Plants, Algae, and Microorganisms, vol 1, Elsevier Inc.: Amsterdam, 2018; pp 59–77.
- Shanker, U.; Jassal, V.; Rani, M.; Kaith, B.S. Towards Green Synthesis of Nanoparticles: From bio-Assisted Sources to Benign Solvents. A Review. Int. J. Environ. Anal. Chem. 2016, 96 (9), 801–835.
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7 (1), 17–28.
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnology 2018, 16 (1), 1–24.
- Gour, A.; Jain, N.K. Advances in Green Synthesis of Nanoparticles. Artif. Cells, Nanomedicine Biotechnol 2019, 47 (1), 844–851.
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review. Adv. Mater. Res. 2014, 832, 350–355.
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the Methodologies Used in the Synthesis Gold Nanoparticles by Chemical Reduction. J. Alloys Compd. 2019, 798, 714–740.
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 1–18.
- Kharissova, O.V.; Kharisov, B.I.; González, C.M.O.; Méndez, Y.P.; López, I. Greener Synthesis of Chemical Compounds and Materials. R. Soc. Open. Sci. 2019, 6 (11), 1–41.
- Qasem, M.; El Kurdi, R.; Patra, D. Green Synthesis of Curcumin Conjugated CuO Nanoparticles for Catalytic Reduction of Methylene Blue. ChemistrySelect 2020, 5 (5), 1694–1704.
- Al Shehab, S.; El Kurdi, R.; Patra, D. Curcumin Mediated PEG Thiol Acid Conjugated Gold Nanoparticles for the Determination of Melamine. Microchem. J. 2020, 153, 1–26.
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112.
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of Pulsed Ultrasonic and Microwave-Assisted Extraction for Curcuminoids by Response Surface Methodology and Kinetic Study. Food Chem. 2014, 165, 29–34.
- Noorafshan, A.; Ashkani-Esfahani, S. A Review of Therapeutic Effects of Curcumin. Curr. Pharm. Des. 2013, 19 (11), 2032–2046.
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and Diabetes: A Systematic Review. Evidence-based Complement. Altern. Med. 2013, 2013, 1–16.
- Pabon, H.J.J. A Synthesis of Curcumin and Related Compounds. Recueil 1964, 83 (4), 379–386.
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin : The Indian Solid Gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S., Eds.; Springer-Verlag New York Inc.: New York, 2015; pp 1–75.
- Pandeya, N.K. Old Wives’ Tales : Modern Miracles — Turmeric as Traditional Medicine in India. Trees Life J. 2005, 1, 1–3.
- Tønnesen, H.H. Chemistry of Curcumin and Curcuminoids. In In Phenolic Compounds in Food and Their Effects on Health; American Chemical Society: Washington, DC, 1992; pp 143–153.
- Prado-Audelo, D.; María, L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9 (2), 1–28.
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology, vol 39; Elsevier: Amsterdam, 2014; pp 113–204.
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15 (1), 195–218.
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6 (10), 1–11.
- Pongrakhananon, V.; Rojanasakul, Y. Anticancer Properties of Curcumin, no. May. 2011.
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20 (5), 1–26.
- Wang, X.; Hang, Y.; Liu, J.; Hou, Y.; Wang, N.; Wang, M. Anticancer Effect of Curcumin Inhibits Cell Growth Through miR-21/PTEN/Akt Pathway in Breast Cancer Cell. Oncol. Lett. 2017, 13 (6), 4825–4831.
- Tanvir, E.M.; Hossen, M.; Hossain, M.; Afroz, R.; Gan, S.H.; Khalil, M.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma Longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 1–9.
- Palash, O.F.; Butea, P.; Lam, M.; Kumar, A.; Dwivedi, K.N.; Ram, B. Degradation Studies of Curcumin. Pharm. Rev. Res. 2015, 5 (3), 286–292.
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an Antioxidant and Anti-Inflammatory Agent, Induces Heme Oxygenase-1 and Protects Endothelial Cells Against Oxidative Stress. Free Radic. Biol. Med. 2000, 28 (8), 1303–1312.
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory Action of Curcumin and its use in the Treatment of Lifestyle-Related Diseases. Eur. Cardiol. Rev. 2019, 14 (2), 117–122.
- Jurenka, J.S.; Ascp, M.T. Anti-inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa : A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14 (2), 141–153.
- Boroumand, N.; Samarghandian, S.; Hashemy, S.I. Immunomodulatory, Anti-Inflammatory, and Antioxidant Effects of Curcumin. J. HerbMed Pharmacol. 2018, 7 (4), 211–219.
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.B.; Peh, S.C. Antibacterial Action of Curcumin Against Staphylococcus Aureus: A Brief Review. J. Trop. Med. 2016, 2016, 1–11.
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I is Associated with Damaging of Bacterial Membrane. PLoS One 2015, 10 (3), 1–15.
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial Effects of Curcumin: An in Vitro Minimum Inhibitory Concentration Study. Toxicol. Ind. Health 2016, 32 (2), 246–250.
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied Curcumin for Different Diseases Therapy. Biomed Res. Int. 2014, 2014, 1–24.
- Sou, K. Curcumin Towards Nanomedicine. Recent Patents Nanomedicinee 2012, 2 (2), 133–145.
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17 (6), 1341–1356.
- Lopresti, A.L. The Problem of Curcumin and its Bioavailability: Could its Gastrointestinal Influence Contribute to its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9 (1), 41–50.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60 (5), 1620–1637.
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a low Bioavailability. J. Herb. Med. 2015, 5 (2), 57–70.
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin Nanoformulations with Metal Oxide Nanomaterials for Biomedical Applications. Nanomaterials 2021, 11 (2), 1–25.
- Vinayagam, R.; Pai, S.; Varadavenkatesan, T.; Narasimhan, M.K.; Narayanasamy, S.; Selvaraj, R. Structural Characterization of Green Synthesized α-Fe2O3 Nanoparticles Using the Leaf Extract of Spondias Dulcis. Surfaces and Interfaces 2020, 20 (July), 100618.
- El Kurdi, R.; Patra, D. Tuning the Surface of Au Nanoparticles Using Poly(Ethylene Glycol)- Block -Poly(Propylene Glycol)- Block -Poly(Ethylene Glycol): Enzyme Free and Label Free Sugar Sensing in Serum Samples Using Resonance Rayleigh Scattering Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20 (14), 9616–9629.
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8 (11), 1–23.
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to Metallic Nanoparticles. J. Pharm. Bioallied Sci. 2010, 2 (4), 282–289.
- Sreelakshmi, C.; Goel, N.; Datta, K.K.R.; Addlagatta, A.; Ummanni, R.; Reddy, B.V.S. Green Synthesis of Curcumin Capped Gold Nanoparticles and Evaluation of Their Cytotoxicity. Nanosci. Nanotechnol. Lett. 2013, 5 (12), 1258–1265.
- Singh, D.K.; Jagannathan, R.; Khandelwal, P.; Abraham, P.M.; Poddar, P. In Situ Synthesis and Surface Functionalization of Gold Nanoparticles with Curcumin and Their Antioxidant Properties: An Experimental and Density Functional Theory Investigation. Nanoscale. 2013, 5 (5), 1882–1893.
- Sindhu, K.; Rajaram, A.; Sreeram, K.J.; Rajaram, R. Curcumin Conjugated Gold Nanoparticle Synthesis and its Biocompatibility. RSC Adv. 2014, 4 (4), 1–11.
- Shaabani, E.; Amini, S.M.; Kharrazi, S.; Tajerian, R. Curcumin Coated Gold Nanoparticles: Synthesis, Characterization, Cytotoxicity, Antioxidant Activity and its Comparison with Citrate Coated Gold Nanoparticles. Nanomedicine 2017, 4 (2), 115–125.
- Abdulwahab, F.; Henari, F.Z.; Cassidy, S.; Winser, K. Synthesis of Au, Ag, Curcumin Au/Ag, and Au-Ag Nanoparticles and Their Nonlinear Refractive Index Properties. J. Nanomater. 2016, 2016, 1–8.
- El Kurdi, R.; Patra, D. Capping of Supramolecular Curcubit[7]Uril Facilitates Formation of Au Nanorods During pre-Reduction by Curcumin. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 553, 97–104.
- El Kurdi, R.; Patra, D. Nanosensing of ATP by Fluorescence Recovery After Surface Energy Transfer Between Rhodamine B and Curcubit[7]Uril-Capped Gold nanoparticles. Microchim. Acta 2018, 185 (7), 1–8.
- El Kurdi, R.; Patra, D. Gold Nanoparticles Functionalized with Pluronic are Viable Optical Probes for the Determination of Uric Acid. Microchim. Acta 2018, 2, 1–7.
- El Kurdi, R.; Patra, D. The Role of OH − in the Formation of Highly Selective Gold Nanowires at Extreme pH: Multi-Fold Enhancement in the Rate of the Catalytic Reduction Reaction by Gold Nanowires. Phys. Chem. Chem. Phys. 2017, 19 (7), 5077–5090.
- Moussawi, R.N.; Patra, D. Synthesis of Au Nanorods Through Prereduction with Curcumin: Preferential Enhancement of Au Nanorod Formation Prepared from CTAB-Capped Over Citrate-Capped Au Seeds. J. Phys. Chem. C 2015, 119 (33), 19458–19468.
- Patra, D.; Moussawi, R.N. Curcumin Conjugated Gold Nanoparticles for Nucleic Acid Sensing. IEEE Int. Conf. Nanotechnol. 2016, 401–404.
- El Kurdi, R.; Patra, D. Amplification of Resonance Rayleigh Scattering of Gold Nanoparticles by Tweaking Into Nanowires: Bio-Sensing of ?-Tocopherol by Enhanced Resonance Rayleigh Scattering of Curcumin Capped Gold Nanowires Through non-Covalent Interaction. Talanta 2017, 168, 82–90.
- Kundu, S.; Nithiyanantham, U. In Situ Formation of Curcumin Stabilized Shape-Selective Ag Nanostructures in Aqueous Solution and Their Pronounced SERS Activity. RSC Adv. 2013, 3 (47), 25278–25290.
- Verma, A.D.; Jain, N.; Singha, S.K.; Quraishi, M.A.; Sinha, I. Green Synthesis and Catalytic Application of Curcumin Stabilized Silver Nanoparticles. J. Chem. Sci. 2016, 128 (12), 1871–1878.
- Selvan, A.D.; Mahendiran, D.; Senthil Kumar, R.; Kalilur Rahiman, A. Garlic, Green tea and Turmeric Extracts-Mediated Green Synthesis of Silver Nanoparticles: Phytochemical, Antioxidant and in Vitro Cytotoxicity Studies. J. Photochem. Photobiol. B Biol. 2018, 180, 243–252.
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green Synthesis of Silver Nanoparticles Using Turmeric Extracts and Investigation of Their Antibacterial Activities. Colloids Surfaces B Biointerfaces 2018, 171, 398–405.
- Sathishkumar, M.; Sneha, K.; Yun, Y. Immobilization of Silver Nanoparticles Synthesized Using Curcuma Longa Tuber Powder and Extract on Cotton Cloth for Bactericidal Activity. Bioresour. Technol. 2010, 101 (20), 7958–7965.
- Khan, M.J.; Shameli, K.; Sazili, A.Q.; Selamat, J.; Kumari, S. Rapid Green Synthesis and Characterization of Silver Nanoparticles Arbitrated by Curcumin in an Alkaline Medium. Molecules 2019, 24 (4), 1–12.
- Shameli, K.; Ahmad, M.B.; Zamanian, A.; Sangpour, P.; Shabanzadeh, P.; Abdollahi, Y.; Zargar, M. Green Biosynthesis of Silver Nanoparticles Using Curcuma Longa Tuber Powder. Int. J. Nanomedicine 2012, 2012, 5603–5610.
- Shameli, K.; Ahmad, M.B.; Shabanzadeh, P.; Al-Mulla, E.A.J.; Zamanian, A.; Abdollahi, Y.; Jazayeri, S.D.; Eili, M.; Jalilian, F.A.; Haroun, R.Z. Effect of Curcuma Longa Tuber Powder Extract on Size of Silver Nanoparticles Prepared by Green Method. Res. Chem. Intermed. 2014, 40 (3), 1313–1325.
- Al-Namil, D.S.; El Khoury, E.; Patra, D. Solid-State Green Synthesis of Ag NPs: Higher Temperature Harvests Larger Ag NPs but Smaller Size Has Better Catalytic Reduction Reaction. Sci. Rep. 2019, 9 (1), 1–9.
- Al-Namil, D.; Patra, D. Green Solid-State Based Curcumin Mediated Rhamnolipids Stabilized Silver Nanoparticles: Interaction of Silver Nanoparticles with Cystine and Albumins Towards Fluorescence Sensing. Colloids Surfaces B Biointerfaces 2019, 173, 647–653.
- El Khoury, E.; Abiad, M.; Kassaify, Z.G.; Patra, D. Green Synthesis of Curcumin Conjugated Nanosilver for the Applications in Nucleic Acid Sensing and Anti-Bacterial Activity. Colloids Surfaces B Biointerfaces 2015, 127, 274–280.
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin Modified Silver Nanoparticles for Highly Efficient Inhibition of Respiratory Syncytial Virus Infection Xiao. Nanoscales 2016, 3, 1–9.
- Abdelghany, A.M.; Oraby, A.H.; Hindi, A.A.; El-Nagar, D.M.; Alhakami, F.S. Green Synthesis of Mixed Metallic Nanoparticles Using Room Temperature Self-Assembly. J. Adv. Phys. 2017, 13 (2), 4671–4677.
- Sankar, R.; Rahman, P.K.; Varunkumar, K.; Anusha, C.; Kalaiarasi, A.; Shivashangari, K.S.; Ravikumar, V. Facile Synthesis of Curcuma Longa Tuber Powder Engineered Metal Nanoparticles for Bioimaging Applications. J. Mol. Struct. 2017, 1129, 8–16.
- Jayarambabu, N.; Akshaykranth, A.; Venkatappa Rao, T.; Venkateswara Rao, K.; Rakesh Kumar, R. Green Synthesis of Cu Nanoparticles Using Curcuma Longa Extract and Their Application in Antimicrobial Activity. Mater. Lett. 2020, 259, 1–11.
- Jayandran, M.; Muhamed Haneefa, M.; Balasubramanian, V. Green Synthesis and Characterization of Manganese Nanoparticles Using Natural Plant Extracts and its Evaluation of Antimicrobial Activity. J. Appl. Pharm. Sci. 2015, 5 (12), 105–110.
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics. 2020, 5 (27), 1–47.
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc Oxide Nanoparticles: a Promising Nanomaterial for Biomedical Applications. Drug Discov. Today 2017, 22 (12), 1825–1834.
- Varaprasad, K.; Yallapu, M.M.; Jayaramudu, T.; Karthikeyan, C. Generation of Engineered Core–Shell Antibiotic Nanoparticles. RSC Adv. 2019, 9, 8326–8332.
- Arab, C.; El Kurdi, R.; Patra, D. Chitosan Coated Zinc Curcumin Oxide Nanoparticles for the Determination of Ascorbic Acid. J. Mol. Liq. 2021, 328, 1–7.
- Arab, C.; El Kurdi, R.; Patra, D. Efficient Removal of Congo red Using Curcumin Conjugated Zinc Oxide Nanoparticles as new Adsorbent Complex. Chemosphere 2021, 276, 130158.
- Nasrallah, O.; El Kurdi, R.; Mouslmani, M.; Patra, D. Doping of ZnO Nanoparticles with Curcumin: pH Dependent Release and DPPH Scavenging Activity of Curcumin in the Nanocomposites. Curr. Nanomater. 2018, 3 (3), 147–152.
- Al-Senosy, Y.; Prince, A.E.-B.; Abd El Zaher, A. Biochemical Study on the Potential Therapeutic use of Novel Curcumin Copper Oxide Nanocomposite in Diabetic Complications in Rats. Benha Vet. Med. J. 2018, 34 (2), 182–190.
- Qasem, M.; El Kurdi, R.; Patra, D. F108 Stabilized CuO Nanoparticles for Highly Selective and Sensitive Determination of Mercury Using Resonance Rayleigh Scattering Spectroscopy. Anal. Methods 2020, 12 (12), 1631–1638.
- Qasem, M.; El Kurdi, R.; Patra, D. Selective Resonance Rayleigh Scattering Spectroscopic Determination of Persulfate Using Cetyl Trimethylammonium Bromide Capped cuo Nanograins. Microchem. J. 2020, 155, 1–5.
- Qasem, M.; El Kurdi, R.; Patra, D. Glutathione-capped CuO Nanoparticles for the Determination of Cystine Using Resonance Rayleigh Scattering Spectroscopy. Microchim. Acta 2020, 187 (364), 1–8.
- Qasem, M.; El Kurdi, R.; Patra, D. Preparation of Curcubit [ 6 ] Uril Functionalized CuO Nanoparticles : A New Nanosensing Scheme Based on Fluorescence Recovery After FRET for the Label Free Determination of Dopamine. ChemistrySelect 2020, 5, 4642–4649.
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single Step Synthesis, Characterization and Applications of Curcumin Functionalized Iron Oxide Magnetic Nanoparticles. Mater. Sci. Eng. C 2016, 2016, 1–21.
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Hafeez, B.B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; Ganju, A. Biomaterials Superparamagnetic Iron Oxide Nanoparticles of Curcumin Enhance Gemcitabine Therapeutic Response in Pancreatic Cancer. Biomaterials 2019, 208, 83–97.