?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In 1 M HCl pickling media, the paprika extract was used as an ecofriendly anti-corrosive material for carbon steel. Many strategies were utilized to estimate the mitigation efficacy such as potentiodynamic polarization (PP), frequency modulation (EFM), electrochemical impedance spectroscopy (EIS), and mass loss. Surface analyses were accomplished by scanning microscopy (SEM) and dispersion X-ray (EDX) techniques. The mitigation efficacy ameliorates with the concentration of extract and temperature. The inhibition is ascribed to the building of a protective coating at the surface of carbon steel that reduces its dissolution. The adsorption of Paprika extract on steel metal satisfied Langmuir, s model. The adsorption belongs to the physical and chemical types. The adsorption of the extract affected both anodic and cathodic reactions. Tafel curves demonstrated that the Paprika is a mixed inhibitor. The Nyquist curves confirmed that Paprika extract prohibits the disintegration of steel in acid media without changing the dissolution reaction mechanism. The adsorption of the Paprika extract on the carbon steel metal was confirmed by ATR - FTIR analysis and SEM examination. Using 300 ppm of Paprika extract, a maximum inhibitory efficacy of 95% was observed at 313 K.
GRAPHICAL ABSTRACT
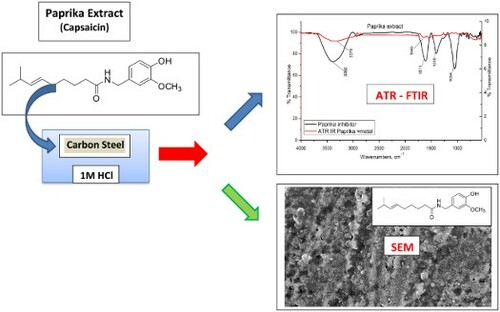
1. Introduction
Using carbon steel as a structural material in industrial sectors has been a major challenge for chemical engineers and researchers in recent years. The majority of the acidic industry applications like pickling, industrial spotless, refining crude oil, descaling, and petrochemical processes utilize carbon steel as an essential material. Also, alloys of carbon steel are cheap and have good characteristics over the other metal alloys. In the industrial sector, HCl is widely applied for scrubbing and descaling processes. The main problem concerned with this process is the fact that carbon steel rusts when exposed to the acid environment. As a result, the use of inhibitors to prevent metal disintegration would be unavoidable if steel was to be memorized in acid environments.
The majority of organic compounds contain heteroatoms and exhibit anti-corrosive properties; however although, the preparation of these compounds is costly and venomous to humans and the environment. Because eco-friendly compounds are substantially less expensive than classic compounds, scientists have focused on their utilization. One of these categories of compounds is plant extracts. The plant extracts are biodegradable, easily obtainable, and renewable resources of materials. Besides, they are green inhibitors and free from heavy metals or other venomous compounds [Citation1–3].
The plant extract contains a variety of heteroatoms like O, S, P, and N. These atoms attach with the metal through free electrons and prevent metal dissolution via the figuration of a preventive film at the metal surface. The naturalistic materials as disintegration inhibitors for a variety of metals in diverse solutions have been reported by plentiful researchers [Citation4–16]. For the dissolution of steel alloys in acid solutions, numerous plants extractors have been used effectively and efficiently [Citation17–29]. Chen et al. [Citation23] utilize Magnolia grandflora leaves extract to prevent Q235 steel corrosion in 1 M HCl. Dehgani and coworkers [Citation24] use Aloysia citrodora leaves extract to retard the corrosion of mild steel in acidic solution. Asfia and others [Citation25] prevent the corrosion of AISI 304 stainless steel in HCl medium by using Garlic extract. Anh et al [Citation26] inhibit steel corrosion in HCl medium by Ficus racemosa leaf extract.
Paprika extracts are generally utilized as natural flavoring and coloring agents in many foods. Also, it is used dried or fresh in different pharmacological preparations. It has long been used as a source of biologically effective compounds, like flavonoids, carotenoids, phenols, capsaicinoids, and vitamins. Capsicum fruits contain coloring pigments, resins, protein, cellulose, mineral elements, and quite small volatile oil, whilst the seeds contain fixed (non-volatile) oil. Triglycerides make up the majority of the fixed oil (approximately 60%), with linoleic acid and other non-saturated fatty acids dominating.
The active ingredient in Paprika is capsaicin, a homo-vanillic acid derivative (8-methyl-N-vanillyl-6-nonenamide, ). The quantity of the capsaicin in the Paprika is about 0.025%, and in the hot pepper attains 0.25% [Citation30]. It is a many-sided agent, and it is utilized in a diversity of scopes like pharmacology, nourishment, chemical arms, and shark fend off [Citation31]. Capsaicin has a high molecular mass (305.418 g/mol) and various groups such as NH, OH, and C = O that can coordinate with different metals via free electrons. As a result, we hypothesized that Capsaicin, which is present in Paprika extract, could serve as a useful inhibitor for reducing carbon steel dissolution in pickling HCl solution.
The goal of this research is to examine how Paprika extract reduces carbon steel disintegration in a 1M HCl pickling solution. The adsorption performance of eco-friendly environment Paprika extract on carbon steel was investigated. The study also comprises the impact of temperature on inhibition efficacy. In addition, the surface of carbon steel samples devoid of and with Paprika extract was examined.
2. Materials and methods
2.1. Steel coupons
Carbon steel with the following composition (wt. %): 0.19 C, 0.0026 Si, 0.024 P, 0.34 Mn, and 99.4 Fe was used for the measurements. Carbon steel sheets with dimensions 2 × 2 × 0.2 cm3 were polished with various grades of silicon carbide emery boards, degreased with petroleum ether, and scrubbed with bi-distilled water. Eventually, the specimens were desiccated at room temperature and weighed by analytical balance [Citation14]. The working electrode utilized in PP, EIS & EFM measurements having an exposed surface area of 1 cm2.
2.2. Preparation of pickling solution
1 M HCl is used as a corrosive medium for carbon steel specimens. It was prepared from analytical grade condensed 32% HCl, and doubly distilled water.
2.3. Making the Paprika extract
The Paprika plant was dried and milled to a fine powder using an electrical mill at room temperature. 200 mg of Paprika powder was soaked in 800 mL of methanol (100%). Under vacuum, the extract was removed and dried by a rotary evaporator. The extract was liquefied in absolute ethanol (1 g/L) and kept in the refrigerator [Citation32].
2.4. Experimental procedures
2.4.1. Mass loss measurements
The weighed steel coupons were submerged in 100 mL of 1 M hydrochloric acid devoid of, and with different quantities of Paprika extract for 180 min. At different time intervals (30 min), the specimens were taken away from a solution, washed, dried, and finally weighed precisely. To ensure reproducibility, each experiment was repeated at least three times. The corrosion rate (CR) was determined by the subsequent equation:
(1)
(1) Where CR is the rate of corrosion (mg/cm2/min), W1 is the mass of coupon before submersion, and W2 is the mass after. S is the area of the coupon, & t is the exposition time.
The mitigation efficacy (η%) can be calculated from the calculated CR values through the given equation [Citation33]:
(2)
(2) Where CR1, and CR2 are the corrosion rate values devoid of, and with distinct quantities of Paprika extractor, respectively.
2.4.2. Electrochemistry studies
The electrochemistry studies were accomplished by using Potentiostat / Galvanostat / ZRA. A standard glass cell with a Pt-counter electrode (CE), and a saturated Hg2Cl2 electrode (SCE) was utilized. The working electrode (WE), carbon steel rod, was pressed into a poly tetra fluoro ethylene (PTFE) holder, exposing 1 cm2 of surface area to the corrosive media. Before any experiment, the WE was immersed in corrosive acid for 30 min to obtain a stationary-open potential. The polarization curves were swept at a rate of 1 mV s−1 from a starting potential of −500 mV to a final potential of +500 mV vs. SCE [Citation34]. EIS tests were established in a wide scope of frequency 100 kHz – 10 MHz using a sine wave with a magnitude of 10 mV [Citation35–40]. Frequency modulation (EFM) is a promising technique with high sensitivity and accuracy due to the measurement of corrosion parameters at harmonics and intermodulation of input frequencies. Two different frequencies were used for electrochemical modulation: 2, and 5 Hz. The undermost frequency was 0.1 Hz that the waveform renews every one second. The spectrum includes current responses, ascribed to harmonically, and inter-modification current peaks. The larger humps were accustomed to estimate the current of corrosion (i), the slopes of Tafel, and also the causality factors [Citation41].
2.4.3. Attenuated total refraction infrared (ATR-FTIR) analysis
ATR-FTIR spectrum of the Paprika extractor and the coating created at the steel surface was investigated using Thermo Fisher Scientific (Nicoleti S10 model) within a wavenumber scope of 400–4000 cm −1.
2.4.4. Surface examination
SEM analysis was carried out to gain insight into the changes at the carbon steel surface before and after the addition of Paprika extract. The coupons were polished, washed, and immersed in 1M HCl for 24 h in the absence and presence of 300 ppm Paprika extract at 298 K. A JEOL JSM-5500 scanning electron microscope was used.
3. Results and discussion
3.1. Mass loss tests
The mass loss of steel coupons versus time was studied in the pickling solution, 1M HCl, utilizing various quantities of Paprika extract for 120 min. According to , the mass loss of steel coupons decreases with increasing the extract concentration. Along the same line, the mitigating efficacy also increases with the concentration of extract [Citation42]. Raising the temperature between 298 and 313 K improves the inhibition efficacy. The improvement of mitigating efficacy with increasing temperature is chiefly assigned to the chemical adsorption of Paprika extract from the steel surface at elevated temperatures.
Table 1. Variation of the corrosion rate, and inhibition efficiency, with the concentration of the Paprika extract at different temperatures.
3.2. Type of adsorption and chemical thermodynamics approach
The inhibition steps and the thermodynamic variables of the disintegration process can be elucidated and determined by adsorption isotherms. Different types of isotherms were studied (Langmuir, Frumkin, Freundlich & Temkin models). The results are shown in . The Langmuir isotherm only fitted experimental data. Langmuir isotherm can be described by the subsequent relation:
(3)
(3) Where θ is the coverage of the surface; determined from the mass loss experiments, Cinh is the inhibitor concentration and Kads is the adsorption equilibrium constant. The latter is computed from the intercept of the straight line on the (Cinh/θ) versus Cinh plot ().
Figure 2. Langmuir adsorption isotherms for the Paprika extract on carbon steel different temperatures.
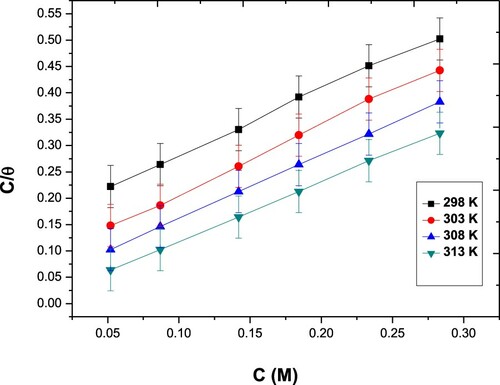
Figure 3. Frumkin adsorption isotherms for the Paprika extract on carbon steel different temperatures.
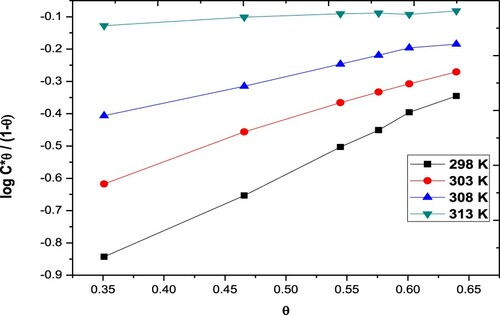
Figure 4. Freundlich adsorption isotherms for the Paprika extract on carbon steel different temperatures.
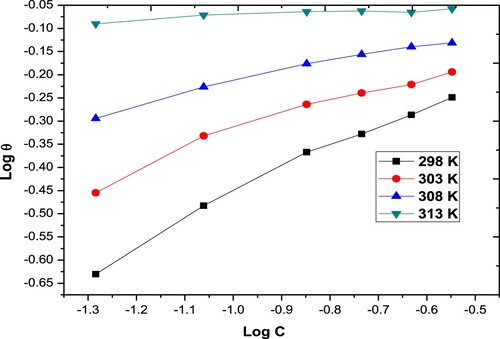
Figure 5. Temkin adsorption isotherms for the Paprika extract on carbon steel different temperatures.
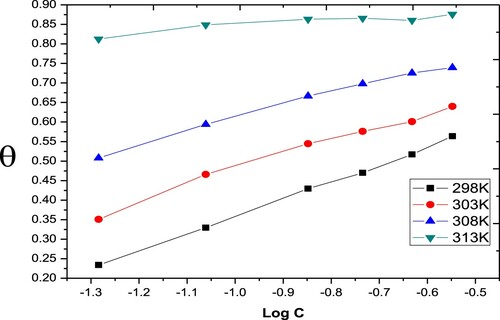
Kads estimated from the inverse of the intercept of isotherm lines are listed in . The Paprika extract is mightily adsorbed on carbon steel at different temperatures. The standard Gibbs energy change for the adsorption process, ΔGoads, is concerned with adsorption equilibrium constant (Kads) via the subsequent equation:
(4)
(4) Where R is the gas constant, T is the absolute temperature and 55.5 is the water concentration in the molarity unit. ΔGoads magnitudes for the extract are negative, referring that the adsorption process proceeds from its own accord and the steadiness of the adsorbed layer. The magnitudes of ΔGoads are smaller than - 20 kJ mol−1 when the temperature is 308 K or less, indicating that the adsorption is physisorption. On the other hand, as the temperature attains 313 K, ΔGoads turns into - 24.39 kJ mol−1, indicating that the adsorption is changed to chemisorption. As a result, the adsorption of Paprika extract on the surface of carbon steel can be classified as both physical and chemical adsorption. The adsorption heat, ΔHoads, can be predestined in the light of the Van’t Hoff equation:
(5)
(5) ΔHoads magnitude is estimated from the slope of the linkage between ln Kads vs. 1/T, which is equal to –ΔHoads/R. The magnitude of ΔHoads is negative, glimpsing that the adsorption of the extract is accompanied by an evolution of heat. Entropy change of the inhibitor adsorption (ΔSoads) was estimated utilizing the subsequent equation:
(6)
(6) The acquired ΔSoads values are scheduled in . As the temperature rises from 298 to 313 K, ΔSoads increase from −25.1–7.64 J/K. This result indicates that the disordering increases with temperature as a result of the replacement of high numbers of water molecules by Paprika extract [Citation43–49].
Table 2. Adsorption parameters of the Paprika extract on carbon steel in 1M HCl at different temperatures.
3.2.1. Inhibition mechanism
Adsorption of the inhibitor onto the metal surface is the first step in any corrosion inhibition reaction. The adsorption can occur via electron transfer to the metal surface (chemisorption) or via charge transfer from the charged inhibitor to the charged metal surface (physisorption).
Several studies on the adsorption of a corrosion inhibitor onto a metal's surface have found that the presence of hetero atoms (N, S, O, or P) in an aromatic system or long carbon chain, as well as π-electrons, will aid the adsorption of the inhibitor onto the metal surface. The adsorption of Paprika extract's components onto the steel metal surface may explain its inhibitory effect on corrosion. Because the adsorbed layer acts as a barrier between the metal surface and the acidic solution, the corrosion rate is reduced. According to the chemical characteristics of Paprika extract, its constituents include chemical organic compounds with hetero atoms and carbonyl groups rich in electrons, which serve as an excellent adsorption site on metal. The adsorption of these components resulted in a reduction in the reactivity between steel metal and acid media (HCl), and hence a reduction in the rate of corrosion.
3.3. Electrochemical measurements
3.3.1. Potentiodynamic tests
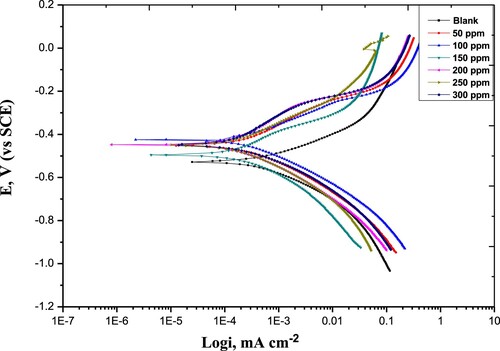
The impact of Paprika extract on the V- I plots of carbon steel in 1M HCl is depicted in . The Paprika extract inhibits the cathodic and anodic reactions. The corrosion current diminishes greatly with extract concentration. The extract effectively inhibits the disintegration of carbon steel metal in the pickling HCl solution. The cathodic polarization curves in show an analogous trend, referring that the adsorption of Paprika extract at the metal surface did not alter the reaction mechanism of the cathode. Meanwhile, the slopes of the anodic curves are varied; pointing out that the mechanism of the anodic reaction has altered. This alludes to that the adsorption of Paprika extract at the metal surface can mightily prohibit the dissolution process.
Figure 6. Potentiodynamic Polarization curves for carbon steel in 1 M HCl without and with various concentrations of the Paprika extract at 298 K.
In the absence of Paprika extract, Cl- ions played an important role in iron disintegration, as shown below:
(7)
(7)
(8)
(8)
(9)
(9) Because the experiments were carried out in aerated acid solution, the cathodic reactions comprise both oxygen reduction and hydrogen evolution.
(10)
(10)
(11)
(11) Even though, it has been reported [Citation50] that the impact of dissolved O2 reduction on iron dissolution is only noteworthy at pH values above 4. As a result, the cathodic H2 evolution reaction was the main one in this work.
shows the magnitudes of the disintegration current (i), disintegration potential (Ecorr), cathodic, and anodic Tafel slopes (βc), (βa), respectively. The presence of the Paprika extract in the pickling media reduces the disintegration current of steel metal. The prepared extract can be classified as a mixed-type [Citation51, Citation52]. Because the anodic and cathodic polarization curves are a symmetrical about Ecorr, The sum of βc and βa does not equal 1.
Table 3. Potentiodynamic polarization parameters of carbon steel in 1M HCl without and with various concentrations of the investigated extract at 25°C.
The mitigating efficacy can be estimated in step with the equation:
(12)
(12) Where, i (free) and i (inh) are the disintegration current densities devoid of and with extract, respectively. In step with , the mitigating efficacy ameliorates with the concentration of extract [Citation53, Citation54]. These results are in good consent with the results of the mass loss technique, which has been argued previously.
3.3.2. Impedance study
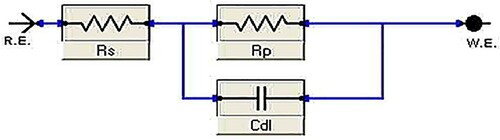
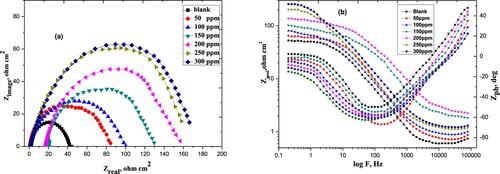
The purpose of EIS tests was to study the mechanism of the dissolution process. Nyquist & Bode-phase angle plots of carbon steel in 1M HCl devoid of, and with distinct quantities of Paprika extract is given in . The Nyquist curves found a consistent trend, confirming that Paprika extract hinders steel disintegration in acidic media without modifying the dissolution reaction mechanism, (a). The high-frequency loops are not ideal semicircles as anticipated from the hypothesis of EIS. The discrepancy can be expounded by the roughness and heterogeneity of the electrode surface [Citation55]. It may be noticed in (b), the Bode plot, that there is one peak only, appears within the phase angle, proving that the extract produces a time constant when adsorbed to the surface carbon steel. The equivalent Randles circuit is demonstrated in . The circuit comprises the charge transfer resistance Rct that is connected in parallel to the double-layer capacitance Cdl [Citation56, Citation57]. The output of both is attached to the solution resistance Rs. Our experimental findings are completely consistent with this model.
Figure 7. The Nyquist (a) and Bode-phase angle (b) plots of carbon steel in 1 M HCl in the absence and presence of various concentrations of the Paprika extract at 298 K.
The mitigation efficacy was estimated via Rct as follow:
(13)
(13) Where, Rct and Roct are the charge transfer resistance devoid of and with Paprika extract, respectively. The impedance variables are illustrated in . The magnitude of Rct raises however, Cdl values drop with increment the extract concentration. This is mainly due to the progressive exchange of H2O molecules by the extract molecules at the steel surface. This will minify the dissolution reaction. As the Rct values become higher the disintegration of the steel gets slower. The decrease in Cdl values is assigned to the decreasing of the dielectric constant and rising of the electrical double-layer thickness. This result indicates that the extract molecules work by adsorption on the metal/solution boundary. The adsorption occurs via the interaction of the Paprika extract with adsorbed Cl- anions. Also, the interaction may be between the π-electrons or unpaired electron pairs of Capsaicin molecules and the empty orbitals of the metal [Citation58, Citation59].
Table 4. EIS parameters for carbon steel corrosion in the absence and presence of various concentrations of the Paprika extract at 25°C.
The difference in the magnitudes of mitigation efficacy obtained from PP & EIS techniques is concerned with the variation in the surface state of the working electrode in the two techniques.
3.3.3. Frequency modulation tests
The modulation spectra that got from EFM tests for the disintegration of carbon steel in 1 M HCl devoid of and with distinct concentrations of Paprika extract is depicted in .
Figure 9. EFM data for carbon steel in 1 M HCl in the absence and presence of different concentrations of the Paprika extract at 298 K.
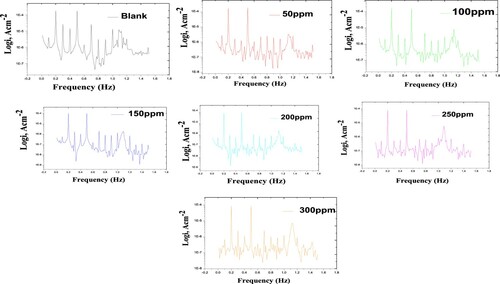
The input frequencies and frequency constituents are included in the current response. The latter is the product of the multiples, differences, and sum of the two input frequencies. There are two major peaks at 0.2, and 0.5 Hz, which are the response to the excitation frequencies. The major peaks are used to compute the corrosion current (i), the Tafel slopes, and also the causality factors (CF-2&CF-3). displayed the inhibition efficacy, Tafel constants, and the causality factors for distinct concentrations of the Paprika extract at 298 K. The corrosion current density decreases as the concentration of Paprika extract in the corrosive medium increases. This is due to the formation of a protective coating on the surface of carbon steel, which reduces dissolution. The interaction between the Paprika extract and the adsorbed Cl- anions causes the adsorption [Citation60, Citation61]. The values of the causality factors directly correlate to the theoretical ones 2 and 3. This indicates that the results obtained are of high quality.
Table 5. EFM parameters for carbon steel in 1 M HCl in the absence and presence of different concentrations of the Paprika extract.
3.3.4. ATR-FTIR analysis
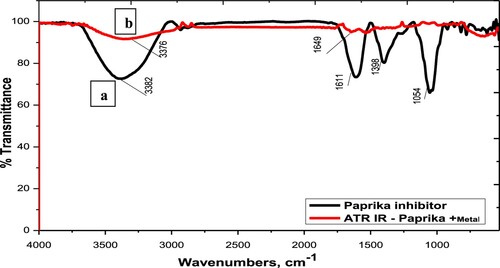
ATR-FTIR spectroscopy is a rapid, nondestructive, noninvasive, reagent-free, cheap, sensitive, and highly reproducible physicochemical tool for characterization molecules. This technique is used to identify the adsorbed functional groups on the metal surface. illustrates the ATR-FTIR spectra of Paprika extract and the surface of carbon steel metal after soaking for 6 hrs in 1 M HCl in the presence of 300 ppm Paprika extract. The spectrum of Paprika extract includes the stretching -OH frequency at 3382 cm−1, The stretching – C = O frequency at 1649 cm−1, the bending SP3 -C–H frequency at 1398 cm−1, and stretching -C–O frequency at 1054 cm−1. The spectrum of steel metal after immersion in 1 M HCl + 300 ppm Paprika extract, on the other hand, is free of the previous functional groups. This suggests that the active functional groups in Paprika extract (primarily Capsaicin) bind to the surface of the carbon steel metal and prevent it from dissolving.
3.3.5. Scanning electron microscope (SEM) examination
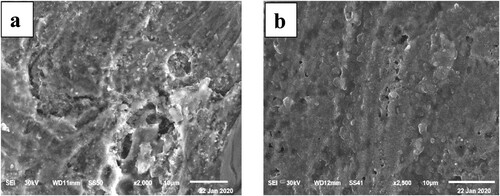
This investigation is carried out on the metal surface before and after immersion in a 1M HCl solution devoid of and containing 300 ppm of Paprika extract. (a) depicts the morphology of a carbon steel metal surface after 6 h of exposure to a 1 M HCl solution. It is possible to see that the entire steel surface is severely corroded due to the pitting action of chloride ions. The morphology of the steel surface treated with the 300 ppm Paprika extract improved significantly in (b). The Paprika extract is responsible for the formation of a shield layer on the steel surface that prevents it from corrosion.
3.4. A comparison of the inhibition efficiency of Paprika extract and other plant extracts in the literature for steel corrosion.
summarizes the comparison of the investigated Paprika extract and other plant extracts reported in the literature for corrosion protection under the same experimental conditions. This comparison shows that Paprika extract is more effective than other extracts as a corrosion inhibitor.
Table 6. Comparison between the tested Paprika extract and other plant extracts reported in the literature.
4. Conclusions
In 1 M HCl, the paprika extract is an effective carbon steel disintegration inhibitor. The concentration of extract and temperature affect the effectiveness of inhibition. As the Paprika extract concentration and temperature are increased, the inhibitory efficacy increases. Using 300 ppm of Paprika extract at 313 K, the maximum inhibitory efficacy of 95% was attained. The Langmuir model describes the adsorption of the extract on the carbon steel surface. Chemical and physical types of adsorption exist. The Paprika extract is strongly adsorbed on the carbon steel surface, as indicated by the negative sign of standard free energy change. Paprika is a mixed inhibitor. The adsorption of the Paprika extract on the carbon steel metal was confirmed by ATR - FTIR analysis and SEM examination. Other plant extracts are less effective as corrosion inhibitors than paprika extract.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdallah, M.; Asghar, B.H.; Zaafarany, I.; Fouda, A.S. The Inhibition of Carbon Steel Corrosion in Hydrochloric Acid Solution Using Some Phenolic Compounds. Int. J. Electrochem. Sci 2012, 7, 282–304.
- Singh, A.; Ebenso, E.E.; Qurashi, M.A. Corrosion Inhibition of Carbon Steel in HCl Solution by Some Plant Extracts. Int. J. Corros 2012, 2012, 1–20.
- Soltani, N.; Tavakkoli, N.; Ghasemi, M. Corrosion Inhibition of low Carbon Steel by Strychnos nux-Vomica Extract as Green Corrosion Inhibitor in Hydrochloric Acid Solution. Int. J. Electrochem. Sci 2016, 11, 8827–8847.
- Yaro, A.S.; Khadom, A.A.; Ibraheem, H.F. Peach Juice as an Anti-Corrosion Inhibitor of Mild Steel. Anti-Corros. Methods Mater 2011, 5, 116–124.
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot Juice as Green Corrosion Inhibitor of Mild Steel in Phosphoric Acid. Alexandria Eng. J 2013, 52, 129–135.
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Garlic Powder as a Safe Environment Green Corrosion Inhibitor for Mild Steel in Acidic Media; Adsorption and Quantum Chemical Studies. J. Chin. Chem. Soc 2014, 61, 615–623.
- Hassan, K.H.; Khadom, A.A.; Kurshed, N.H. Citrus Aurantium Leaves Extracts as a Sustainable Corrosion Inhibitor of Mild Steel in Sulfuric Acid. South Afr. J. Chem. Eng 2016, 22, 1–5.
- Nathiya, R.; Raj, V.J. Evaluation of Dryopteris Cochleata Leaf Extracts as Green Inhibitor for Corrosion of Aluminium in 1 M H2SO4. Egypt. J. Petroleum 2017, 26, 313–323.
- Okafor, P.; Uwah, I.; Ekerenam, O.; Ekpe, U.J. Combretum Bracteosum Extracts as eco-Friendly Corrosion Inhibitor for Mild Steel in Acidic Medium. Pigment Resin Technol. 2009, 38 (4), 236–241.
- Okafor, P.; Ikpi, M.E.; Uwah, I.; Ebenso, E.; Ekpe, U.; Umoren, S.A. Inhibitory Action of Phyllanthus Amarus Extracts on the Corrosion of Mild Steel in Acidic Media. Corros. Sci 2008, 50, 2310–2317.
- Okafor, P.C.; Ebenso, E.E.; Ekpe, U.J. Azadirachta Indica Extracts as Corrosion Inhibitor for Mild Steel in Acid Medium. Int. J. Electrochem. Sci 2010, 5, 978–993.
- Eddy, N.; Ebenso, E.E. Inhibitive and Adsorption Properties of Musa Sapientum for the Corrosion of Mild Steel in Acid Medium. Afr. J. Pure Appl. Chem 2008, 2, 46–54.
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Xanthium Strumarium Leaves Extracts as a Friendly Corrosion Inhibitor of low Carbon Steel in Hydrochloric Acid: Kinetics and Mathematical Studies. S. Afr. J. Chem. Eng 2018, 25, 13–21.
- Fouda, A.S.; Mosallam, H.; El-Khateeb, A.; Fakih, M. Cinnamomum Zeylanicum Extract as a Green Corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Solutions. Prog. Chem. Biochem. Res 2019, 2 (3), 120–133.
- Senthil, D.; Saratha, R.; Vasantha, R. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Medium Using Plant Extracts. Int. J. of Sci 2016, 5 (12), 3324–3340.
- Fouda, A.A.; Rashwan, S.M.; Abdelfatah, M. Corrosion Inhibition of Stainless Steel 304 in Hydrochloric Acid Solution Using Clindamycin Antibiotic as eco-Friendly Inhibitor. Zastita Materijala 2019, 60, 3–17.
- Krishnaveni, K.; Ravichandran, J.; Selvaraj, A. Effect of Morinda Tinctoria Leaves Extract on the Corrosion Inhibition of Mild Steel in Acid Medium. Acta Metall. Sin 2013, 26, 321–327.
- Hsissou, R. Review on Epoxy Polymers and its Composites as Potential Anticorrosive Coatings for Carbon Steel in 3.5% NaCl Solution: Computational Approaches. J. Mol. Liq 2021, 336, 116307.
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel Derivative Epoxy Resin TGETET as a Corrosion Inhibition of E24 Carbon Steel in 1.0 M HCl Solution. Experimental and Computational (DFT and MD Simulations) Methods. J. Mol. Liq 2019, 284, 182–192.
- Hsissou, R.; Benzidia, B.; Rehioui, M.; Berradi, M.; Berisha, A.; Assouag, M.; Hajjaji, N.; Elharf, A. Anticorrosive Property of Hexafunctional Epoxy Polymer HGTMDAE for E24 Carbon Steel Corrosion in 1.0 M HCl: Gravimetric, Electrochemical, Surface Morphology and Molecular Dynamic Simulations. Polym. Bull 2020, 77, 3577–3601.
- Hsissou, R.; Abbout, S.; Berisha, A.; Berradi, M.; Assouag, M.; Hajjaji, N.; Elharf, A. Experimental, DFT and Molecular Dynamics Simulation on the Inhibition Performance of the DGDCBA Epoxy Polymer Against the Corrosion of the E24 Carbon Steel in 1.0 M HCl Solution. J. Mol. Struct 2019, 1182, 340–351.
- Echihi, S.; Hsissou, R.; Benzbiria, N.; Afrokh, M.; Boudalia, M.; Bellaouchou, A.; Guenbour, A.; Azzi, M.; Tabyaoui, M. Performance of Methanolic Extract of Artemisia Herba Alba as a Potential Green Inhibitor on Corrosion Behavior of Mild Steel in Hydrochloric Acid Solution. Biointerface Res. Appl. Chem 2021, 11 (6), 14751–14763.
- Chen, S.; Chen, S.; Zhu, B.; Huang, C.; Li, W. Magnolia Grandiflora Leaves Extract as a Novel Environmentally Friendly Inhibitor for Q235 Steel Corrosion in 1 M HCl: Combining Experimental and Theoretical Researches. J. Mol. Liq 2020, 2020 (311), 113312.
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Aloysia Citrodora Leaves Extract Corrosion Retardation Effect on Mild-Steel in Acidic Solution: Molecular/Atomic Scales and Electrochemical Explorations. J. Mol. Liq 2020, 310, 113221.
- Asfia, M.; Rezaei, M.; Bahlakeh, G. Corrosion Prevention of AISI 304 Stainless Steel in Hydrochloric Acid Medium Using Garlic Extract as a Green Corrosion Inhibitor: Electrochemical and Theoretical Studies. J. Mol. Liq 2020, 315, 113679.
- Anh, H.; Vu, N.; Huyen, L.; Tran, N.; Thu, H.; Bach, L.; Nam, N. Ficus Racemosa Leaf Extract for Inhibiting Steel Corrosion in a Hydrochloric Acid Medium. Alex. Eng. J 2020, 59 (6), 4449–4462.
- Ahmed, Z.; Ali, E.; Radhi, I. Corrosion Protection of Carbon Steel in Saline Solution Using Plant Extracts. Int. J. Pharm. Res 2020, 1, 2078–2086.
- Haldhar, R.; Prasad, D.; Mandal, N.; Benhiba, F.; Bahadur, I.; Dagdag, O. Anticorrosive Properties of a Green and Sustainable Inhibitor from Leaves Extract of Cannabis Sativaplant: Experimental and Theoretical Approach. Colloids Surf. A: Physicochem. Eng. Asp 2021, 614, 126211.
- Tan, B.; He, J.; Zhang, S.; Xu, C.; Chen, S.; Liu, H.; Li, W. Insight Into Anticorrosion Nature of Betel Leaves Water Extracts as the Novel and eco-Friendly Inhibitors. J. Colloid Interface Sci 2021, 2021 (585), 287–301.
- Lazić, B.; Povrtarstvo. Paprika (Capsicum annuum L.), m Poljoprivredni fakultet, Univerzitet u Novom Sadu, Jugoslavija, 1995 (in Serbian).
- Holzer, P. Capsaicin: Cellular Targets, Mechanisms of Action, and Selectivity for Thin Sensory Neurons. Pharmacol. Rev 1991, 43, 143–201.
- Richard, C. Paprika extract chemical and technical assessment, Ph.D. dissertation, for the 69th JECFA, 2008,1, 1–11.
- Verma, C.; Olasunkanmi, L.; Obot, I.; Ebenso, E.E.; Quraishi, M. 5-Arylpyrimido-[4, 5-b] Quinoline-Diones as new and Sustainable Corrosion Inhibitors for Mild Steel in 1 M HCl: a Combined Experimental and Theoretical Approach. RSC Adv. 2016, 6, 15639–15654.
- Khaled, K.; Amin, M.A. Computational and Electrochemical Investigation for Corrosion Inhibition of Nickel in Molar Nitric Acid by Piperidines. J. Appl. Electrochem 2008, 38, 1609–1621.
- Fouda, A.; Megahed, H.; Fouad, N.; Elbahrawi, N.M. Corrosion Inhibition of Carbon Steel in 1 M Hydrochloric Acid Solution by Aqueous Extract of Thevetia Peruviana. J. Bio- Tribo-Corros 2016, 2, 1–13.
- Hsissou, R.; Benhiba, F.; Dagdag, O.; El Bouchti, M.; Nouneh, K.; Assouag, M.; Briche, S.; Zarrouk, A.; Elharfi, A. Development and Potential Performance of Prepolymer in Corrosion Inhibition for Carbon Steel in 1.0 M HCl: Outlooks from Experimental and Computational Investigations. J. Colloid Interface Sci 2020, 574, 43–60.
- Hsissou, R.; Abbout, S.; Benhiba, F.; Seghiri, R.; Safi, Z.; Kaya, S.; Briche, S.; Serdaroğlu, G.; Erramli, H.; Elbachiri, A.; Zarrouk, A.; El Harfi, A. Insight Into the Corrosion Inhibition of Novel Macromolecular Epoxy Resin as Highly Efficient Inhibitor for Carbon Steel in Acidic Mediums: Synthesis, Characterization, Electrochemical Techniques, AFM/UV–Visible and Computational Investigations. J. Mol. Liq 2021, 337, 16492.
- Hsissou, R.; Benhiba, F.; Abbout, S.; Dagdag, O.; Benkhaya, S.; Berisha, A.; Erramli, H.; Elharfi, A. Trifunctional Epoxy Polymer as Corrosion Inhibition Material for Carbon Steel in 1.0 M HCl: MD Simulations, DFT and Complexation Computations. Inorg. Chem. Commun 2020, 115, 107858.
- Hsissou, R.; Abbout, S.; Safi, Z.; Benhiba, F.; Wazzan, N.; Guo, L.; Nouneh, K.; Briche, S.; Erramli, H.; Ebn Tuhami, M.; Assouag, M.; Elharfi, A. Synthesis and Anticorrosive Properties of Epoxy Polymer for CS in [1 M] HCl Solution: Electrochemical, AFM, DFT and MD Simulations. Constr. Build. Mater 2021, 270, 121454.
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of Corrosion Inhibition Performance of Phosphorus Polymer for Carbon Steel in [1 M] HCl: Computational Studies (DFT. MC and MD Simulations). J. Mater. Res. Technol 2020, 9 (3), 2691–2703.
- Kalla, A.; Benahmed, M.; Djeddi, N.; Akkal, S.; Laouer, H. Corrosion Inhibition of Carbon Steel in 1 M H2SO4 Solution by Thapsia Villosa Extracts. Int. J. Ind. Chem 2016, 7, 419–429.
- Guo, W.; Umar, A.; Zhao, Q.; Alsaiari, M.A.; Al-Hadeethi, Y.; Wang, L.; Pei, M. Corrosion Inhibition of Carbon Steel by Three Kinds of Expired Cephalosporins in 0.1 M H2SO4. J. Mol. Liq 2020, 320, 114295.
- Soltani, N.; Tavakkoli, N.; Attaran, A.; Karimi, B.; Khayatkashani, M. Inhibitory Effect of Pistacia Khinjuk Aerial Part Extract for Carbon Steel Corrosion in Sulfuric Acid and Hydrochloric Acid Solutions. Chem. Pap 2020, 74 (6), 1799–1815.
- Fouda, A.S.; Azeem, M.A.; Mohamed, S.A.; El-Hossiany, A.; El-Desouk, A.E. Corrosion Inhibition and Adsorption Behavior of Nerium Oleander Extract on Carbon Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci 2019, 14, 3932–3948.
- Megahed, H.E.; Sobhi, M.; Nour, S. Celery (Apium Graveolens) Extract as Corrosion Inhibitor for Carbon Steel in 1 M HCl. J. Basic Environ. Sci 2017, 4, 170–177.
- Bourazmi, H.; Tabyaoui, M.; Hattabi, L.; El Aoufir, Y.; Taleb, M. Methanolic Extract of Salvia Officinalis Plant as a Green Inhibitor for the Corrosion of Carbon Steel in 1 M HCl. J. Mater. Environ. Sci 2018, 9, 928–938.
- El-Etre, A.Y. Inhibition of Acid Corrosion of Carbon Steel Using Aqueous Extract of Olive Leaves. J. Colloid Interface Sci 2007, 314, 578–583.
- Hamdy, A.; El-Gendy, N.S. Thermodynamic, Adsorption and Electrochemical Studies for Corrosion Inhibition of Carbon Steel by Henna Extract in Acid Medium. Egyptian Journal of Petroleum 2013, 22 (1), 17–25.
- Benabbouha, T.; Siniti, M.; El Attari, H.; Chefira, K.; Chibi, F.; Nmila, R.; Rchid, H. Red Algae Halopitys Incurvus Extract as a Green Corrosion Inhibitor of Carbon Steel in Hydrochloric Acid. J. Bio- Tribo-Corros 2018, 4, 1–9.
- Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E. Adsorption and Corrosion Inhibition Properties of N-{n-[1-R-5-(Quinoxalin-6-yl)-4, 5-Dihydropyrazol-3-yl] Phenyl} Methanesulfonamides on Mild Steel in 1 M HCl: Experimental and Theoretical Studies. RSC Adv. 2016, 6, 86782–86797.
- Fouda, A.; El-Awady, G.; El Behairy, W. Prosopis Juliflora Plant Extract as Potential Corrosion Inhibitor for low-Carbon Steel in 1 M HCl Solution. J. Bio- Tribo-Corros 2018, 4 (1), 1–12.
- Ali, I.H.; Suleiman, M.H. Effects of Acid Extract of Leaves of Juniperus Procera on Corrosion Inhibition of Carbon Steel in HCl Solution. Int. J. Electrochem. Sci 2018, 13, 3910–3922.
- Abboud, Y.; Tanane, O.; Bouari, A.E.; Salghi, R.; Hammouti, B.; Chetouani, A.; Jodeh, S. Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution Using Pomegranate Leave Extracts. Corros. Eng. Sci. Technol 2016, 51 (8), 557–565.
- Verma, C.; Quraishi, M.; Ebenso, E.E.; Bahadur, I. A Green and Sustainable Approach for Mild Steel Acidic Corrosion Inhibition Using Leaves Extract: Experimental and DFT Studies. J. Bio- Tribo-Corros 2018, 4 (33), 1–12.
- Fouda, A.; Etaiw, S.H.; Elnggar, W. Punica Plant Extract as Green Corrosion Inhibitor for C-Steel in Hydrochloric Acid Solutions. Int. J. Electrochem. Sci 2014, 9, 4866–4883.
- Mobin, M.; Basik, M.; Aslam, J. Pineapple Stem Extract (Bromelain) as an Environmental Friendly Novel Corrosion Inhibitor for low Carbon Steel in 1 M HCl. Meas. J. Int. Meas. Confed 2019, 134, 595–605.
- Liu, Y.; Song, Z.; Wang, W.; Jiang, L.; Zhang, Y.; Guo, M.; Song, F.; Xu, N. Effect of Ginger Extract as Green Inhibitor on Chloride-Induced Corrosion of Carbon Steel in Simulated Concrete Pore Solutions. J. Clean. Prod 2019, 214, 298–307.
- Lebrini, M.; Lagrenee, M.; Traisnel, M.; Gengembre, L.; Vezin, H.; Bentiss, F. Enhanced Corrosion Resistance of Mild Steel in Normal Sulfuric Acid Medium by 2,5-bis(n-Thienyl)-1,3,4-Thiadiazoles: Electrochemical, x-ray Photoelectron Spectroscopy and Theoretical Studies. Appl. Surf. Sci 2007, 253, 9267–9276.
- Babić-Samardžija, K.; Lupu, C.; Hackerman, N.; Barron, A.R.; Luttge, A. Inhibitive Properties and Surface Morphology of a Group of Heterocyclic Diazoles as Inhibitors for Acidic Iron Corrosion. Langmuir 2005, 21, 12187–12196.
- Fouda, A.; Nazeer, A.A.; Elbehairy, W.T. Assessment of Begonia Extract as new eco-Friendly Inhibitor for low-Carbon-Steel Corrosion in Acidic Environment. J. Bio- Tribo-Corros 2018, 4 (7), 1–13.
- Belarbi, N.; Dergal, F.; Chikhi, I.; Merah, S.; Lerari, D.; Bachari, K. Study of Anti-Corrosion Activity of Algerian L. Stoechas oil on C38 Carbon Steel in 1 M HCl Medium. Int. J. Ind. Chem 2018, 9 (2), 115–125.
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa Canina Fruit Extract as a Green Corrosion Inhibitor for Mild Steel in 1 M HCl Solution: A Complementary Experimental, Molecular Dynamics and Quantum Mechanics Investigation. J. Ind. Eng. Chem 2019, 69, 18–31.
- Hussin, M.H.; Kassim, M.J.; Razali, N.; Dahon, N.; Nasshorudin, D. The Effect of Tinospora Crispa Extracts as a Natural Mild Steel Corrosion Inhibitor in 1 M HCl Solution. Arab. J. Chem 2016, 9, S616–S624.
- Alibakhshi, E.; Ramezanzadeh, M.; Haddadi, S.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. Persian Liquorice Extract as a Highly Efficient Sustainable Corrosion Inhibitor for Mild Steel in Sodium Chloride Solution. J. Clean. Prod 2019, 210, 660–672.
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and Adsorption Study of the Corrosion Inhibition of Mild Steel by Euphorbia Heterophylla L. Extract in 1.5 M HCl. Results Mater 2020, 5, 100074.
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Green Eucalyptus Leaf Extract: a Potent Source of bio-Active Corrosion Inhibitors for Mild Steel. Bioelectrochemistry 2019, 130, 107339.
- Emori, W.; Zhang, R.H.; Okafor, P.C.; Zheng, X.W.; He, T.; Wei, K.; Lin, X.Z.; Cheng, C.R. Adsorption and Corrosion Inhibition Performance of Multi-Phytoconstituents from Dioscorea Septemloba on Carbon Steel in Acidic Media: Characterization, Experimental and Theoretical Studies. Colloids Surf. Physicochem. Eng. Asp 2020, 590, 124534.
- Faiz, M.; Zahari, A.; Awang, K.; Hussin, H. Corrosion Inhibition on Mild Steel in 1 M HCl Solution by Cryptocarya Nigra Extracts and Three of its Constituents (Alkaloids). RSC Adv. 2020, 10, 6547–6562.
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential Role of a Novel Green eco-Friendly Inhibitor in Corrosion Inhibition of Mild Steel in HCl Solution: Detailed Macro/Micro-Scale Experimental and Computational Explorations. Constr. Build. Mater 2020, 245, 118464.
- Ahanotu, C.C.; Onyeachu, I.B.; Solomon, M.M.; Chikwe, I.S.; Chikwe, O.B.; Eziukwu, C.A. Pterocarpus Santalinoides Leaves Extract as a Sustainable and Potent Inhibitor for low Carbon Steel in a Simulated Pickling Medium. Sustain. Chem. Pharm 2020, 15, 100196.