?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In most African rural communities, ground water is the primary source of drinking water. Fluoride is a harmful substance found in ground water that causes serious health problems. The only answer to the fresh and clean water crisis is water treatment. Sustainable, cost-effective and efficient membrane filtration technologies are becoming progressively vital to solve the scarcity. Owing to their unique tunable physico-chemical properties, carbon nanotubes (CNTs) were used in water treatment. This paper thoroughly reviews the health effects of consuming excess fluoride ions. The recent progress on the synthesis of CNT was also highlighted. A special emphasis was given to green synthetic routes for its preparation. Green catalysts provide defined size and morphology than conventional materials and also prevent metal leaching. The effects of incorporation of CNTs as filler in the matrix have been discussed in detail. The large-scale production of CNTs and their growth mechanisms; for water purification purposes are explored. It has been observed that CNTs have got an excellent filtration performance of fluoride, low biofouling activities and high water flux capacity. Besides, this review attempt to provide a clue for the functionalized CNTs based membranes as potential solutions in water purifications of fluoride ions in the future.
GRAPHICAL ABSTRACT
1. Introduction
Carbon nanotubes (CNTs) have significantly enhanced the momentum of research work in the field of nanoscience and nanotechnology with beneficial applications in various sectors including, catalysis, medicine, energy and environment. CNTs are versatile and have occupied a significant position in nanotechnology applications due to their special physico-chemical properties. The discovery of the tabular carbon structure, CNTs in 1991 was the critical step in the history of this material group. Carbon can form different types of open and closed cages of materials with a honeycomb structure. Graphene is such an open cage honeycomb carbon structure and fullerene molecule is one of the closed carbon crates discovered for the first time. Theoretically, CNTs are obtained by rolling up graphene and are applicable in different research and technology areas. The nanotube consists of multiple rolled layers concentric tube in the form of Russian Doll Model or parchment Model, multiwalled nanotubes with a successive layer of the tube separated by 0.34 nm away from each other. Two years later, single-walled CNTs (SWCNTs) were synthesized (Citation1). Recently, researchers use three common synthetic approaches such as electric arc discharge, laser ablation and chemical vapor deposition (CVD) to fabricate both the multiwalled and single-walled CNTs (MWCNTS and SWCNTs). The manufactured nanotubes are widely characterized by some common electron microscopic and spectroscopic techniques (Citation2) each of which will be discussed thoroughly in this review.
The unique properties of both the MWCNTS and SWCNTs such as antimicrobial activities, higher water flux, tunable physico-chemical, electrical and structural properties, provoke researchers to deeply investigate so that water shortage and pollution problems can be eliminated. Nano adsorption is the most effective technique to purify water (Citation3). These classes of materials have been used in water purification applications as sorbents, catalysts, filters or membranes for the last few decades. Many membrane filtration technologies such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis were used for water treatment activities efficiently. Because of their physical and chemical stabilities, polymer membranes have been in use for water treatment for many years. However, they are hydrophobic in nature and adsorb organic foulants irreversibly. Most of the time the polymer membranes are damaged by membrane fouling (Citation4) mechanisms due to irreversible adsorption of organic, inorganic and biological substances which subsequently decreases the flux of the material. On the other hand, the vertical alignment arrangement of the wall of the CNT favored water molecules' mass transport through the nanotube. It has been observed (Citation5) that the transport rate of water in the CNT membrane is very much greater than the other porous materials of comparable size. Therefore, there has been enhanced interest in using CNTs to develop promising membranes for water purification which renders high flux, selectivity and resistant to fouling (Citation6).
World population is increasing rapidly and the demand for pure drinking water is growing subsequently which resulted in insufficient access to potable water. Ground water is the main source of drinking water supply in most rural communities in Ethiopian rift valley. Even though water has a significantly small microbiological and biological load in general, most of the time, in the rift valley region, water is contaminated with naturally occurring inorganic chemicals. Fluoride ion is one of the most threatening toxicant chemicals in ground water. The World Health Organization (WHO) set the recommended upper limit of fluoride ion concentration in drinking water to be 1.5 mg/l. However, the fluoride ion concentration in some African ground water is beyond that limit by far. The east African regions that extend from Jordan valley down through Sudan, Ethiopia, Uganda, Kenya and Tanzania was reported to have fluoride ion concentration much higher than the recommended value. Particularly a survey of Ethiopian rift valley showed (Citation7) that the fluoride ion concentration exceeded the WHO limit value, 1.5 mg/L by far. Some properties such as high alkalinity and high sodium and bicarbonate in the water samples are the indicators of the fluoridation of drinking water. Water containing a high concentration of fluoride ion is mostly soft water because of the limited contents of calcium and magnesium.
In the recent past, both MWCNTs and SWCNTs were applied for the removal of fluoride from aqueous solutions. Functionalized CNTs have showed remarkable fluoride removal efficiency to an extent beyond 80%. Therefore, in this review work we thoroughly discussed the background concepts on fluorine contamination and de-fluoridation, various methods of synthesis of CNTs with special emphasis on green routes, growth mechanisms, application of CNTs in water purification and surface tunability of carbon families and their advancements in R and D. The Ppzc value (Pristine point of zero charges) of the media at which the filtration capacity of CNT became more effective was also determined. The health effects of consuming excess fluoride ion and the purification strategies done so far and their merit and demerit were also explored in detail. The application of CNTs in environmental protection was highlighted. Finally, the principles of CNT membrane applied for the effective and efficient membrane filtration of fluoride ion from drinking water were determined and future perspectives were proposed.
1.1. Fluoride content, Ethiopian context
Of all water samples taken from different corners of Ethiopian regions, almost all of the samples taken from the rift valley region were found to contain the fluoride ion at a concentration greater than the WHO standard permissible level (Citation8). For instance, the concentration of fluoride ions recorded for rift valley lakes such as lake Shala and lake Abijata were found to be (246 mg/l) and (204 mg/l), respectively (Citation9). On the other hand, high land lakes and springs contain an insignificant level of fluoride ion concentration. Several research works showed that the increase in the concentration of fluoride ion in ground water along the course of the river flow was accountable to the existence of acidic rocks initiated by heat differences generated lengthways across a river flow and the existence of recent volcanic hot eruption.
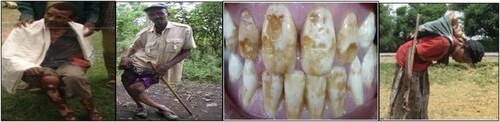
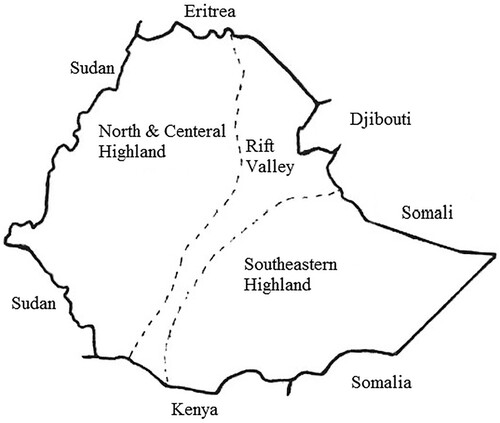
Rift ward flowing groundwater from the plateau may increase the fluoride concentration in the aquifer systems of the rift if the flowing groundwater encounters acidic rocks with heat differences along the flow path. Generally, the landforms of Ethiopian rift valley exist in a variety of terrain that is significantly important to the enrichment of fluoride into groundwater (Citation8). The health effect of fluoride ion on people in the Dugda Bora district, East Shoa Ethiopia is as shown in .
1.2. Fluoride occurrences
A significant amount of fluoride occurs in some classes of igneous rock such as granites and pegmatite. The order of abundance of fluoride in sedimentary rock in decreasing order is shale, carbonate rocks, sandstone respectively but deep sea clay is found to have the richest accumulations of fluorine. The most common source of fluoride in the rift valley region is originated from the volcanic rocks that exist at lower pH such as pumice, obsidian, pyroclastic deposits, ignimbrite and rhyolite. The deposition of volcanic ash and the falling of pumice volcanic rock (Citation10) are the most common potential reservoirs of the fluoride ion enrichment into Ethiopian rift valley groundwater.
There are several factors that control the concentration of fluoride in the rift valley ground water includes lithology, thermal gradient, hydraulic gradient, groundwater water velocity, mean residence time, and depletion of calcium due to evaporation and cation exchange. As shown in , the concentration of fluoride ion in the rift valley region was found to vary from place to place. For example, the fluoride concentration of rift valley lake (Citation11) is about 2–250 mg/L, for spring is about 2–150 mg/L and for thermal groundwater is about 2–68 mg/L and from non-detectable to 6.4 mg/L for cold groundwater.
Figure 2. Fluoride distribution (Citation12).
The ingestion of fluoride is essential for human health provided that the concentration is not beyond the upper level 1.5 mg/L set by WHO in drinking water. The consumption of fluoride ion in food stuff with the limit to the standard value is very important to keep skeletal and dental health. However, the consumption of this mineral above the labeled concentration brings about tremendous health effects. According to the findings (Citation13), the ingestion of fluoride with a concentration between 1.5 and 3 mg/L causes browning and smears of colors of teeth which are commonly called dental fluorosis. This is the onset of the problem which makes the teeth very strong and brittle. Ingestion of fluoride with a concentration between 3.5 and 9 mg/L results in skeletal fluorosis which indicates the worsening of the situation. However, the ingestion of fluoride in the food stuff for a long time with a concentration greater than 10 mg/L brings about crippling fluorosis and this is an irreversible damage to the bone causing immobility as shown in .
It is reported in the literature that excessive ingestion of fluoride ion has many other health effects including muscle fiber degeneration, reduction in the level of hemoglobin, deformation of red blood cells, excessive thirst, headache, skin rashes, depression, gastrointestinal problems, malfunction of urinary tracts, nausea, abdominal pains, tingling sensation in fingers and toes, reduced immunity and neurological manifestations similar to pathological changes that occur in Alzheimer`s disease patients. Nearly all foodstuffs contain at least traces of fluoride and all vegetables contain fluoride which was absorbed from soil and water. However, the ingestion of fluoride from water is much larger than its ingestion from foodstuffs and air. Therefore, great attention should be given to fluoride concentrations in the drinking water supply. According to the WHO recommendation (Citation14), the procedure for the mitigation of fluorosis in endemic area should be prioritized as follows.
It is advisable to search for alternative sources of water for drinking with low fluoride concentration.
Diluting the water containing high fluoride concentration with water containing low/non fluoride concentration until the fluoride concentration is lowered to 1.5 mg/L or below.
Consume a diet containing large amount of calcium, magnesium and vitamin C.
When all the above-listed mitigation strategies are not feasible, remove fluoride ion from water to meet WHO upper limit level 1.5 mg/L.
The ion-exchange reaction between hydroxide and fluoride ions in hydroxyapatite is the center of the fluorosis phenomenon occurrence. The displacement of hydroxide ion from hydroxyapatite by fluoride ion results in the formation of a more acidic resistant structure known as fluoroapatite (Citation15) as presented in Equation (1). There is also an expected decomposition reaction of hydroxyapatite (1) to produce CaF2 and dissolution of calcium and phosphorus into the matrix phase but this is a rare case:
(1)
(1) The early formed fluoroapatite material from the exposure of hydroxyapatite to fluoride ion concentration greater than 1.5 mg/L is more resistant to acidic attack than hydroxyapatite, offers a protective layer to the tooth enamel against acids from the foodstuffs and prevents dental carries. However, the excessive fluoride intake aggravates the reaction beyond the replacement of hydroxide by fluoride so that it results in the decomposition of hydroxyapatite and leads to the formation of another compound called calcium decafluoride as presented in Equation (2) (Citation16):
(2)
(2) This compound is very hard and brittle which is not appropriate for the purposes of skeletal structure where an optimum flexibility is mandatory. There are some de-fluoridation practices as shown in , which have been implemented in Ethiopia so far but the problem of dental fluorosis, skeletal crippling and other complicated health effects continue to persist up-to-date and remain unsolved.
Table 1. Fluoride remediation experience in Ethiopia (Citation12).
Therefore the efficient, effective and low-cost water treatment technology applicable for rural and urban area is a necessity at this time (Citation17). Hence membrane filtration technology is among the advanced water treatment technologies which are being employed in the production of ultrapure water but it is an expensive method. However, the application of CNTs in recent times revealed their greater potentiality towards the water purification process. Even though, CNTs can be synthesized by many physical and chemical methods, Green routes have proved to be eco-friendly, cheaper and simpler methods. This review explored the synthesis of CNT membrane which is from locally abundantly available cheap and easily harvested material as the potential material for the membrane filtration activities. The advantages of green synthetic routes for CNTS over conventional less economical methods were also presented. In addition, the application of CNT membrane filtration to transform the water treatment technology towards access to safe drinking water through consolidation of its unique properties has been discussed.
2. Synthesis of CNTs
CNTs have a proven record of significant applications in the research and development of modern technological applications in various fields. It is the most promising material in the application of nanoadsorbent, catalysis and sensor for environmental protection. It exists in the form of single-walled nanotube (Citation4) and multiwalled nanotube (Citation18). The three aforementioned synthesis techniques have been applied for many years for the fabrication of nanotubes. However, the industrial application of CNTs requires the development of techniques for large-scale and defect-free production where the vapor technique (Citation19) was considered as the promising fabrication technique for large-scale and high purity production of the nanotube. In this following section, the three main production techniques such as electric arc discharge method, laser ablation method and CVD method were described separately.
2.1. Arc discharge method
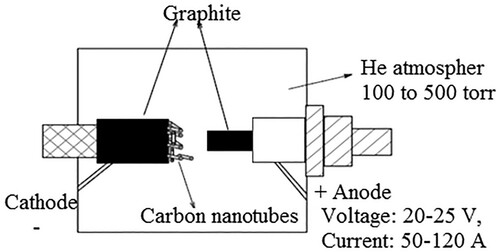
The preparation of a new needle-like CNT was reported for the first time in 1991. The same way fullerene was synthesized, the CNT was also produced by arc discharge evaporation methods. The CNTs, having a diameter ranging from 3 to 29 nm and several micrometers long were developed on the cathode end and the graphite electrode provided for the direct current electric arc discharge evaporation of carbon in an inert gas-filled vessel at 110 Torr (Citation20) as shown in . Carbon black was the first carbonaceous material which was characterized by transmission electron microscopy (TEM), and the image shows that the synthesized nanotube consists of a parallel aligned graphitic sheet numbered from 1 to 51 and it was later called a multiwalled CNT. The arrangement of the carbon atom on each of the tube is in a zigzag fashion about the center axis of the nanotube. The area of the nanotube formed was different from each other and the edge was most of the time covered by pentagon, heptagon and cone-shaped lids.
Therefore, the individual tube was seen to be grown in a helical fashion at the end of the tube and the TEM characterization result of the arc discharge synthesized carbon microtubules showed that its growth morphology varied in shape typically around the tube tips. The most common tube observed by the construction of topological model was pentagons (positives) and heptagons (negatives) at the tube tips. When the carbon atoms are captured by dangling bonds the growth was happened layer by layer and the nanotubes thicken gradually (Citation21), an open-ended growth mechanism is proposed. The change in the growth direction was attained by the nucleation of pentagon and heptagon declinations on open tubes which was resulted in giving different morphologies.
The above technique was used for large-scale production of multiwalled nanotubes. The synthesis of CNTs by using the arch discharge method involves the filling of the bored anode electrode by transition metal catalysts such as Fe, Ni and Co. The potential difference of 20 V and DC current of 200 A was applied between two graphite electrode anode and cathode under high vacuum in the presence of helium atmosphere to generate the arc discharge to synthesis the nanotubes. At a pressure of nearly 500 Torr, a maximum yield of 75% was obtained (Citation22). The three important raw materials for the synthesis of SWNTs are argon, iron and methane. The spherical hat that fits the cylindrically grown CNT was the fullerene molecules.
According to the electron microscopic analysis, the nanotube which was catalyzed by cobalt gave a single-walled layer of uniform diameter ranging from 1 to 0.05 nm. The deposited material observed by SEM was found to comprise a tremendous number of aggregated CNT ropes. The coherent interference of the wave scattered from all atoms in the sample resulted in a diffraction pattern and the X-ray diffraction result (XRD) (Citation23) revealed the alternative arrangement of the tube in the ropes. This characterization result indicates that the growth mechanisms of the nanotubes in arc discharge techniques do not depend on the detail of the experimental condition but depend more on the kinetics of carbon condensation under different rates of reaction condition.
2.2. Laser ablation method
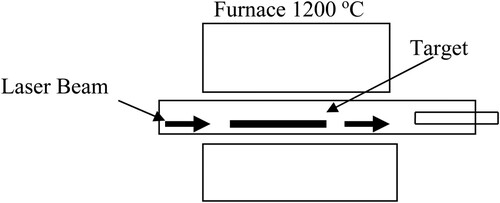
Transition metals such as Ni and Co were used as a catalyst to produce SWCNTs by laser ablation vaporization method at 1200°C as presented in . The result showed that the nanotubes were highly uniform with a diameter range of 3–18 nm and tens to hundreds micrometers long. The lattice constants of the two-dimensional triangular lattice rope formed were about 2 nm through a Van der Waals bonding. It was supposed to be a particular tube of (10, 10) as a dominant component and metallic. The scooter mechanism was used to explain the growth of the nanotube where a transition metal atom Ni or Co was adsorbed chemically onto the active site of the nanotube. The formation of fullerene was inhibited by the metal atom having a sufficient electronegativity and catalyzes the reaction to favor the formation of the nanotube.
The growth of the tube was continued until a large amount of the catalyst atoms aggregates on it. Eventually, the catalysis was poisoned by the large particles when they were either detached or become over coated with sufficient carbon. This way the termination of tube growth takes place with the formation of fullerene-like end or catalyst particles. The advantages of electric arc discharge and laser ablation techniques are the production of about 70% of SWNTs. There are also some disadvantages of these techniques including the use of extremely large temperature around 3000°C to evaporate a carbon atom from a solid target and the entanglement of the nanotube which is difficult to purify. The mixed CNT formation has been persisting a problem for a long time. The separation of SWNTs from MWNTs by using a traditional technique (Citation24) has been a challenging activity since they have many common features. In a laser ablation synthesized nanotubes, it was observed that above 70% endless, tangled ropes of SWNTs and nano scale impurities were present.
The purification method of the grown sample before the cutting of the nanotube was done according to the following procedure. This method was consisting of the refluxing in 2.6 M nitric acid and resuspension of the nanotube in pH 10 water with the surfactant followed by across flow filtration system. By using polytetrafluoroethylene as a filter, a freestanding mat of tangled SWNT ropes called Bucky paper were formed from the resulting purified SWNTs suspension. Of all the cutting techniques available, the prolonged sonification of the nitric acid purified SWNT rope in a mixture of concentrated sulfuric acid at a temperature of 45°C was considered to be the most efficient one.
2.3. CVD method
This technique is based on the thermal decomposition or CVD of hydrocarbon in the presence of the transition metal catalyst which was used to produce carbon filament and fibers since 1960s. This method was also applied to produce multiwalled nanotubes by using the CVD which is easily improved and optimized. The hydrocarbon precursor is thermally decomposed under a temperature of about 600°C–1200°C and hydrocarbon vapor was passed through a tabular reactor in the presence of the catalyst iron, nickel and cobalt. Even if these metal catalysts were used in arc discharge and laser ablation techniques, the large-scale production of the nanotube in both cases was impossible. It has been reported that the interfacial force between the catalyst particles and the substrate influences the nanotube growth by tip or base-growth mechanism from within the catalyst nanoparticles rooted in the perforation.
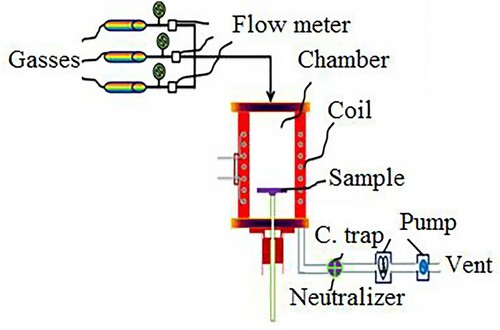
The application of transition metal, iron as a catalyst in the CVD technique resulted in large-scale aligned nanotube production. A mixture of 10% acetylene in nitrogen at a flow rate of 100 cm3/min along with a substrate containing iron nanoparticles rooted in mesoporous silica was placed in the reaction chamber. CNTs were formed on the substrate containing iron nanoparticles catalyst by the deposition of carbon atoms obtained from decomposition of acetylene at 850°C. About 40 shells of multiwalled nanotubes arrays with an outer diameter of 30 nm were formed with a consistent spacing of 100 nm between the pores on the substrate. The SEM result showed the formation of a thin film of nanotube that grew continuously from the bottom to the top with a film length of 50 and 100 mm. The CVD techniques were used to grow consistent arrays of MWCNTs on a perforated silicon wafer substrate. The electrochemical etching of a highly doped n-type Si wafer was used to obtain the porous silicon substrate. The substrate was decorated by Fe films by electron beam evaporation through shallow covered with squared openings having fixed side length and pitch distance. The nanotube with a diameter of 16 nm was formed on the top of the iron catalyst. The CVD process was performed in a tube reactor in the temperature range of 850°C–1000°C from the precursor ethylene under argon atmosphere. This CVD technique was also applied to produce high-quality SWCNTs on a silicon wafer substrate in the presence of transition metal catalyst under noble gas atmosphere. The most commonly used catalyst is Fe/Mo transition metal catalyst on alumina silica composite materials. The characterization of the sample with TEM revealed that a bundle and individual SWCNTs were obtained. The SEM image of the sample also showed that the high quality with a diameter distribution in the range between 0.7 and 5 nm with a peak at 1.7 nm. The increased metal support was the indicator of the large-scale (Citation25) production of the nanotube but the weaker the metal support the larger the agglomeration formation. The section of procedural activity and the schematic diagram of CVD experimental setup for the synthesis of CNT were shown in .
2.4. Green synthesis of CNTs
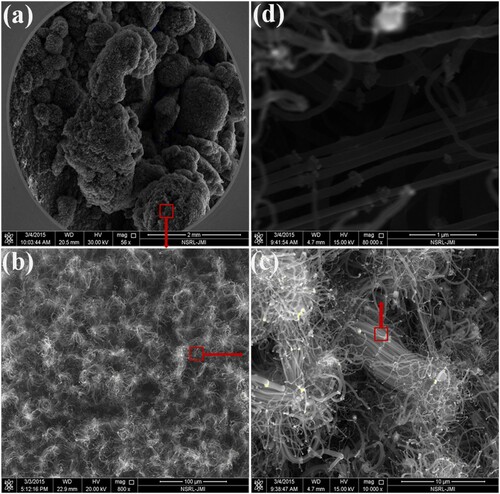
Most of the physico-chemical methods of synthesis of CNTS, discussed in the previous section, pose a serious threat to the environment by introducing unwanted by-products by utilizing toxic chemicals with enhanced energy consumption. In an attempt to develop a clean, cheap, biocompatible and environmentally safe method, researchers ended with a novel process called green synthesis with an intention of exploring biological systems as a source for possible conversion of materials into nanomaterials (Citation26). Green synthetic methods have gained greater significance in the last few decades due to their simple nature, non-toxic and environment-friendly attributes (Citation27). Plant materials are available in plenty in nature and hence their usage for the synthesis of CNTs has been explored by many researchers in the last few decades. Green materials provide better-defined size and morphology for CNTs than conventional materials. The MWCNTs were recently synthesized by the application of green catalyst synthesized from garden grass (Cynodon dactylon), rose (Rosa), neem (Azadirachta indica) and walnut (Juglans regia) plant extracts (Citation28). The microscopic images of MWCNTs grown by walnut extract revealed a tube diameter of 8–15 nm with 3600 µm length. The FESEM images of CNTs demonstrated the formation of ultra-dense network as shown in (a–d) with different magnifications.
Figure 6. The FESEM images of CNTs obtained by green method using walnut extract (Citation28).
In another study, CNT/PANI nanocomposites were prepared by in situ interfacial polymerization using two green solvents. This green route was successfully used to develop other PANI-based composites for multifunctional applications (Citation29). On similar lines, green synthesis of SWCNTs has been demonstrated using olive and coconut oils by pyrolysis technique. The CNT synthesized from olive oil was found to contain single-wall carbon tubes with a diameter of 27 nm. The structural differences in the CNTs obtained from olive and coconut oil were attributed to the amount of unsaturated hydrocarbons (Citation30).
In order to overcome the disadvantages of physical methods, an environmentally friendly and convenient method called one-step water-assisted (quenching) green synthesis method was conducted from graphite flakes, obtained from coconut shell wastes to produce CNTs (Citation31). The TEM images of these CNTs revealed the presence of carbon tubes with irregular morphology and having an average diameter of 123 nm. These CNTS obtained via coconut shell wastes were proved to be better adsorbent for Pb (II) ions. In the case of water treatment, functional CNTs modified with functional groups (chemical groups/polymers) were found to interact with undesirable metal ions. The modification also results in the solubility of the CNTs in respective solutions (Citation32). Functionalized CNTs with silver nanoparticles were synthesized by the application of fresh holly leaf extract, where the phytochemicals of the extract serve the role of reducing agents. The average silver particle size in CNTs/Ag nanomaterials was found to vary from 7 to 11 nm while the CNTs showed diameter in the range of 20 −50 nm (Citation33).
The use of environmentally friendly, renewable green methodologies remains at the core of sustainable development with respect to water, energy and food security (WEF) issues. CNTs synthesized using green routes are believed to solve the problems associated with the WEF-nexus without being harmful to the environment and life (Citation34). Hollow-structured carbon nanomaterials have attracted tremendous research activities due to the availability of interior void spaces in CNTs. Hierarchically porous CNTs (HPCNTs) were synthesized by green method following simple carbonization treatment without any assistance of soft/hard templates and activation procedures. These HPCNTs served as excellent electrode/host materials for high-performance supercapacitors and Li-batteries (Citation35).
3. CNTs growth mechanism
3.1. Experimental growth mechanism
The experimental result showed that the growth mechanism of SWNTs and MWNTs takes place differently because the growth of SWNTs requires the presence of transition metal catalyst and the growth of MWNTs does not need the catalyst. Hence the effects of the growth conditions and parameters on the structural characteristics of the product samples were considered and studied under both experimental and theoretical basis. Firstly, experimental growth mechanism is discussed followed by the theoretical growth mechanism.
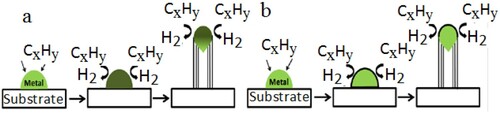
The two different experimental growth mechanisms are vapor–liquid–solid (VLS) and vapor–solid–solid. This classification of growth mechanism was made based on the state of metal catalyst used and the way carbon atom was diffused under high temperature is shown in (a,b). The high temperature was used to vaporize and adsorb the carbon precursor on the surface of low melting point catalyst nanoparticles to form carbon atoms. The freshly evaporated carbon atoms then dissolved into the bulk of the catalyst nanoparticles to form a liquid metal carbide phase. These metal carbides may decompose the catalyst and the carbon atoms are separated away where the carbon atoms are precipitated to form CNTs. As the reaction proceeds a large number of carbon atoms are generated (Citation36) and the growth of nanotubes is continuous. This process is known as the VLS mechanism and the mechanism was studied based on kinetic data and experimental observation.
The other case is the growth mechanism involving vapor–solid–solid by using CVD. This mechanism comprises three successive steps which are shown in Figure 7(b). These steps are, the decomposition of carbon precursor to form carbon atom, these carbon atoms spread over the surface of the catalyst nanoparticles and move toward the interface between catalyst and substrate, and finally, the carbon atom precipitate on the interface in the form of CNT and the growth process was by precipitation-nucleation-crystallization.
The interaction strength between catalyst nanoparticles and substrate involves the growth of nanotubes in two basic models. These are the tip-growth model and the base-growth model as shown in (a,b) respectively. If the interaction strength between catalyst nanoparticles and substrate surface is weak, the carbon precursor decomposes into carbon atoms on the top surface of catalyst particles. These carbon atoms diffuse down through the catalyst and CNTs grow at the bottom of the catalyst and push the whole catalyst away from the substrate.
Figure 8. Interaction strength between catalyst particles and substrate CNTs growth: (a) tip-growth (b) base-growth model.
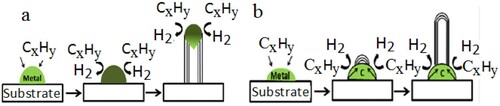
The growth of CNTs become longer and longer if there are still available free surface of the catalysts for more carbon precursor to decomposition. However, the surfaces of the catalyst become deactivated when it is fully enclosed by extra carbon atoms and the growth of CNTs stops. This is a tip-growth model. On the other hand, if the interaction strength between the catalyst nanoparticles and the substrate surface is strong, then the decomposition of carbon precursor and diffusion of carbon atoms takes place at the top of the catalyst. But in this case, the carbon atom forms a hemi spiral dome on the top of the catalyst, which then grows in the form of unified graphitic cylinder to form CNTs. The catalyst always fixes on the base to support the growth of CNTs (Citation37,Citation38), where it is called as base-growth model.
3.2. Theoretical growth mechanism
3.2.1. Multiwalled CNTs
The MWCNTS continues to grow both in thickness and lengths before the closure and this process was observed by experimental manipulation. Even if the presence of the dangling bond at the tip of the nanotubes provokes it to be closed, the nanotubes remain open during its growth. It was suggested that open-ended growth could be explained by a so-called edge-to-edge interaction. It was studied by the tight-binding calculation on MWNTs that the growing edge is stabilized by bridging carbon atoms which extends the life of open-ended structure. To understand the growth process of nanotubes, first principle molecular dynamics simulations on SWNTs and double-walled nanotubes (DWNTs) were performed. Since single-walled nanotube cannot grow in the absence of catalyst, at some experimental temperature the open-end nanotubes closed spontaneously into graphitic dome. The tip closure produced a significant reduction in the localized densities of electronic states on the tips which are close to Fermi energy. This result indicated that the closed nanotubes tips were more stable than the open-ended nanotubes. The possibility of single-walled nanotubes growth by continued incorporation of carbon atoms into the closed tip resulted from the above condition (Citation39).
The chemical bond exists between the edge of respective walls trapped at the end of the DWNTs in a metal were stabilize the energy. The continuous breaking and forming of the bridging bonds accelerate the rapid adsorption of carbon atoms by the nanotubes. By using ab initio and parameterized calculations of the study of morphology and the structural stability of the growing edge of MWNTs, it was found that strong covalent bonds connecting the exposed edges of adjacent walls stabilized nanotubes against dome closure. The role of lip–lip interactions on the growth of DWNTs was investigated by some computer simulations. The lip–lip interaction usually facilitates tube closure by anticipating the transfer of atoms between inner and outer shells and it does not stabilize open-end growth.
3.2.2. Single-walled nanotubes
In the presence of a catalyst, arc discharge and laser ablation techniques were applied for the synthesis of single-walled nanotubes with a narrow diameter distribution. The classical molecular simulation studies on nanotubes revealed that initially open nanotubes continue to grow to maintain the hexagonal structure. Most of the nanotubes was grown in a curved form with a pentagonal structure that eventually goes to the closing of the tube where the diameter of the nanotube is less than the critical diameter 3 nm. The molecular dynamic simulation was applied to study how the open end of a small diameter of SWNTs close spontaneously under the temperature of 3000 K into a graphitic dome where the dangling bonds were totally consumed. Hence, the presence of catalyst helped us to synthesize a nanotube of diameter less than 1 nm.
The catalytic growth of the nanotubes has been studied extensively and much plausible explanation has been made however is not yet clearly understood. The catalyst-assisted tip growth most commonly occurred when the metal atom was placed on the top of precursor cylindrical clusters. The formation of CNT pentagons and the dome closure were prohibited by the metal atoms scoots around the open edge of the cluster. According to the ab initio calculations, Co or Ni atoms were strongly bound to the open edge of the nanotube but yet highly mobile on it. The formation of pentagons which would initiate dome closure was hindered by the metal catalyst however the formation of hexagons and the increasing of the length of the nanotubes were favored by the metal catalyst. With increasing time, the metal atom at the tip of the nanotubes aggregates and the adsorptive energy per metal atom decreases with the increase in the size of the adsorbed metal cluster where the cluster is less reactive and less mobile. The adsorption energy of the cluster decreases such that it peels off the edge when the size of the metal cluster reaches some critical values. The presence of the catalyst assists the prevention of the formation of defects so that initiating the tube closure. This mechanism is consistent with the experimental observation so that no metal particles could be found on the tube grown.
Catalyst-assisted base or root growth of SWNTs is another possible nanotubes growth mechanism. In the presence of transition metal catalysts such as Ni or Co, large productions of the nanotube with different chirality angles were produced using the laser ablation of graphitic powder. The base-growth nanotubes were terminated into nanoparticles consisting of carbon and metal atoms which were produced during the laser ablation process. The nanoparticles could catalyze the growth of SWNTs at the end of the dangling bond, by adding carbon atoms to the tubes. To clearly determine the base-growth mechanisms, molecular dynamics and total energy calculations (Citation40) were carried out for carbon using a realistic three body potential. The mechanism which adopts the concepts of the vapor–liquid–solid model was introduced to explain the growth of silicon whiskers and role of metal catalyst. The theoretical and experimental investigations were necessary in order to anticipate the growth mechanisms in specific experimental conditions and SWNTs with desirable properties.
4. CNTs characterizations
Because of their unique properties, CNTs are the most important carbon nanostructures available for various applications. The overview of nanostructures was determined by SEM and a more accurate examination of defects was revealed by TEM (Citation26). There are also some techniques called small-angle X-ray scattering (SAXS) (Citation2) that have been applied for the qualitative characterization and in-depth investigation of the morphology of the nanotubes. An in situ and real-time study of islands growing on a substrate which gives access to their three dimension is studied well (Citation2).
A SAXS characterization technique is the most important technique when the use of imaging techniques becomes difficult and impossible. It gives structural and morphological information and correlations on CNTs and their mutual orientation. The result obtained from this technique is in agreement with the qualitative ones from SEM and TEM. X-ray absorption near edge structure (XANES) spectroscopy has been a powerful tool (Citation39) which provides information on the local environment around carbon but also investigates the absorption and adsorption of hydrocarbon molecules (Citation41) radicals and atoms with a specific selectivity for the orientation of these compounds. XANES is a local probe, sensitive to chemical impurities, defects, chemical adsorption and curvature induced orbital re-hybridization.
Microscopic techniques are commonly used to observe the position of tip and sidewall, as well as the morphology of CNTs (Citation42). Raman spectroscopy (RS) is one of the most powerful characterization techniques for CNTs. It is routinely employed to evaluate the quality, purity, defects, tube alignment, and assists in the distinction of the presence of MWCNTs relative to other carbon allotropes. The technique has been strikingly successful at describing the structural properties of SWCNTs. Unfortunately; the interpretation of the spectra from an MWCNT is often very complicated and has not yet yielded the same level of output as Raman spectroscopy has achieved for SWCNTs.
For Raman scattering, MWCNTs can be said to be an ensemble of CNTs with diameters ranging from small to very large. Despite this, a number of reports have indicated that Raman spectroscopy can have qualitative and even quantitative characterization power. Because much less theoretical work has been devoted to an understanding of the Raman spectrum of MWCNTs (Citation43), the interpretation of the experimental spectra is usually based on well-established results obtained for SWCNTs. This approach is found to be valuable but has important limitations since the number of effects, absent in SWCNTs, are often found in the spectra of MWCNTs. A Raman spectrum of CNTs shows two main first-order bands including D band and G band. Here the D band is associated with the defects that existed in the CNTs, which is observed at around 1300–1350 cm−1. The G band is related to the degree of graphitization of CNTs, which is approximately located in between 1500 and 1600 cm−1. Hence, the area ratio of the D band and G band (ID/IG) is usually measured to estimate the level of defects in a specific CNTs sample. For example, the lower ID/IG ratio, the lower defects that existed in the CNTs.
The X-ray photoelectron spectroscopy (XPS) and thermogravimetric analysis (TGA) are used to precisely verify the occurrence of functionalization reactions of CNTs for final quality evaluation (Citation44). Therefore, by changing reactants and CVD preparation parameters such as carbon precursor, catalyst, substrate, temperature, pressure, time and gas flow rate assisted with various functionalization and characterization methods, optimized CNTs could be obtained for various practical applications. The summary of characterization techniques used of CNTs has been presented in .
Table 2. The objectives of few characterization techniques of CNTs.
5. Membrane water purification
5.1. Membrane filter
The process of de-fluoridation of water is difficult and expensive in the real-life environment. The large number of natural and synthetic materials have been applied to solve this problem of global concern. The most important methods such as ion-exchange, precipitation, nanofiltration, electrochemical, reverse osmosis and adsorption have been widely applied methods for de-fluoridation of water. Among them, adsorption is a quite effective method because it is easy to operate, needs less space, eco-friendly and cost-effective method. Natural, natural modified and synthetic materials have been widely applied as adsorbents for the removal of fluoride ions from water. The membrane technology which utilizes CNTs as adsorbent, has been widely used and intensively studied for water purification and filtration technology. Membrane surface wettability technology is the means to avoid materials fouling (Citation52). It has been applied for different types of impurities including domestic, commercial and industrial chemicals (Citation53). It has been long since people started to use traditional and conventional methods such as using cloth membranes for the filtration of impure water (Citation54). The cloth materials of different capacities were used for the filtration of drinking water and the separation of various kinds of colloidal solutions. This method of water purification is relatively efficient and has a very low cost which is easily affordable everywhere in the rural areas. However, membrane technologies gradually found their way into industrial applications to serve as viable alternatives to more traditional processes such as distillation, evaporation or extraction (Citation55). Unlike microfiltration, nanofiltration, reverse osmosis and ultrafiltration, CNT-aligned membranes have high flow desalination capacity (Citation56).
In this chapter, the main processes in the technology of membrane separation will be discussed concisely. The need for advanced and efficient membranes increases because of the inefficient accomplishment of the conventional separation techniques. Hence many advanced membrane processes were explored (Citation57). The most commonly known pressure-driven membrane processes are: reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF), and microfiltration (MF) (Citation58). These membrane technologies are the most effective and widely used technologies around the world. The flow charts are given in shows the size boundary and filtration applications.
Figure 9. Flow chart for size boundary, pressure range and types of material separated in filtration technology (Citation59).
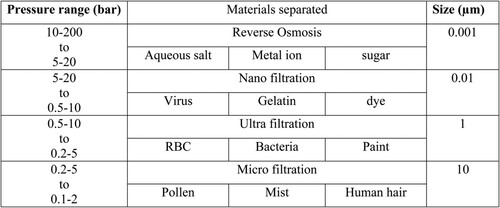
In most membrane separation technologies, the major problem is the cost allocated for the operations with high pressure needed to remove dissolved contaminants (e.g. monovalent ions and small organic molecules). The use of CNT in water purification is totally non-toxins yet desalinate water to the highest degree (Citation60). The dissolved ions and organic solutes are effectively removed by reverse osmosis and nano filtrations. However, high pressures up to 100–1000 psi are required to operate reverse osmosis and nanofiltration membranes. On the other hand, ultrafiltration and microfiltration membranes require much lower pressure up to 5–60 psi. Therefore, those membrane filtrations which use high pressure for their operations such as reverse osmosis and nanofiltration are effectively applied for desalination, water purification, electrodialysis (ED) in chlorine caustic cell and hemodialysis for artificial kidneys. Ultrafiltration is used commonly in the food industry for protein separation from milk and apple juice concentration pervaporation (PV) for dehydration of ethanol, controlled release of drugs, genetic engineering, etc.
The cause of membrane separation process and the types of membrane separation technologies are given in . The commercially available vacuum-driven hollow membranes separation instrument, which is known as Zee Weed membrane is the most intensively used membrane types for industrial applications. The microfilter, consisting of 0.85 µm pores which operates under a small suction created within the hollow fibers by a permeate pump, where the treated water was pumped through for distribution after it is passed through the membrane. The turbulence which was created by the air flow introduced at the bottom of the membrane was used to scrubs and cleans the outside of the membranes allowing them to function at a high flux rate. The air introduced was also used to oxidize iron and other organic compounds generating better quality water than provided by microfiltration alone.
Table 3. Driving forces and their related membrane separation processes.
The access to potable drinking water is the leading question of the world population and will also be a problem in the future if not intervened. Membrane nanotechnology has a great potential in order to deliver efficient, effective and environmentally acceptable solutions for increasing water quality and for accessing the amount of potable water available. Due to their size, nanomaterials have a number of key physico-chemical properties that make them particularly attractive as separation media for water purification. Therefore, they possess a large surface area than their bulk materials. Hence, they are the ideal building block for developing high-capacity sorbent with the ability to be functionalized to enhance their affinity and selectivity. Nanomaterials provide extraordinary opportunities for developing more efficient water purifications catalyst materials and redox-active media due to their large surface areas and their size and shape-dependent optical and electronic properties (Citation22).
5.1.1. Types of filtration
Several membrane technologies have been employed for water treatment activities. The most commonly used membrane filtration technologies are described as follows.
Microfiltration (MF) is a membrane technology which is characterized by a pore size between 0.05 and 2 µm with operation pressure less than or equal to 2 bar. This membrane filtration technique is used for the separation of particles and bacteria from a given solute. Ultrafiltration (UF) is a membrane having a pore size between 2 nm and 0.05 µm and it is operating pressures ranging from 1 to 10 bars. It is applied for the filtration of colloidal such as proteins from small molecules such as sugars and salts.
Nanofiltration (NF) is a sophisticated membrane filtration process characterized by a membrane pore size of 0.5 and 2 nm and work in the pressure range of 5–40 bars. It is used for the separation between sugars and other organic molecules and multivalent salts on one hand from monovalent salts and water on the other hand. NF does not apply to remove dissolved compounds but they are commonly applied for the separation of an aqueous solution of organic solvents to homogenous catalysis, separation of ionic liquids and food processing (Citation6,Citation61).
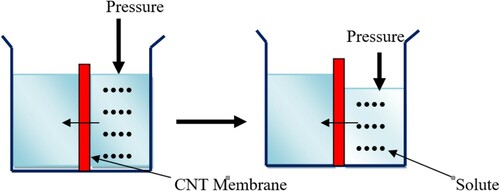
Nanofiltration is broadly classified into organic solvent nanofiltration (OSNF) and solvent resistant nanofiltration (SRNF) where the nanofiltration is usually made from cellulose-based and polyamide composite. Hollow fiber and spiral wounds were typically the basic configuration of the membrane. Their operational conditions and life span vary depending on the type of membrane fabrication techniques and the quality of water obtained from this membrane purification is a function of the pore size and applied pressure. Reverse osmosis (RO) is a physical separation process where the pretreated source of water is delivered as high pressure and filtered by the semi-permeable membrane. In reverse osmosis, the two solutions are separated by a semi-permeable membrane however the pressure is applied to reverse the normal flow of water as shown in and this applied pressure is used to move water from a high concentration compartment to a low concentration compartment. This way the impurities and contaminants residue was excluded and end up on one side of the semi-permeable membrane while the pure water is on the other hand (Citation41). The following set up shows how reverse osmosis basically works.
5.2. Mechanisms of filtration
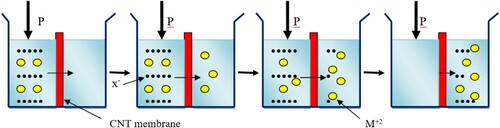
The working principle of membrane filtration at such a small scale size is governed by the Donnan effects of the ion balance in the comparative compartment. According to this principle, the different ion diffuses across a membrane and the speed of diffusion varies from ion to ion based on their size, reactivity and polarity. So in order to keep the charge neutrality of the compartment some ions are sped up and the others are slow down. This phenomenon becomes more pronounced with a very small pore size and the types of charge associated with the membranes. One very important case is when the divalent ions are rejected highly compared to the monovalent ions because of the charge effect. However, to maintain the chemical equilibrium of both sides of the compartment, the exclusion of the monovalent ions will actually decrease as the concentration of the divalent anions is increased in a multicomponent feed solution. The second very important process that takes place in Donnan effect is the occurrence of the negative rejection of the monovalent anion in which case the charge accumulation on the feed end of the membrane leads to concentration polarization. Above all, CNT has a superior separation potential with tunable properties (Citation62).
The exclusion of contaminants using the principle of dielectric rejection mechanism is the most widely used process in electrodialysis rather than ultrafiltration or microfiltration. In this case duo to the charge of the membrane and the polarity of water, the water will show a polarization in the pore. This polarization effect of water results in the decrease of the dielectric constant in the pore, and renders it less favorable for a charged solute to enter. But the transfer of the ion from the bulk to the pore, the change in electrostatic free energy of the ions occurs even in the case when the dielectric constant inside the pore and that of the water is equal which eventually resulted in the ion exclusion. These two mechanisms are not yet clarified in the case of nanofiltration. Most of the works on nanofiltration, the Donnan effect of charge balance, still argue as the Donnan effect is the charge distribution mechanism which is shown in .
There were different approaches to transport the solvent into the membranes which include Kedem and Katchalsky model, Spiegler and Kedem model, Solution-diffusion model, and solution-diffusion imperfection model. The free volume that exists between the segments of the polymer is the core concept of the solvent transport process. Deferent membrane technologies require different amounts of pressure to function. Reverse osmosis is operated in the pressure ranges of 10–100 bar and this technology is used only to pass water through its membrane. RO is currently the widely used technology for the production of municipal drinking water and ultrapure industrial water. In general, above 95% of the municipal water is produced using this technology and the rest 5% is used in important separation process in various applications.
Based on the composition and physico-chemical properties across the cross section of the membranes, they are classified as either isotropic or anisotropic. Anisotropic is the case when the property is depending on the direction of alignment whereas the isotropicity is the uniformity of properties throughout. Anisotropic membranes can be either phase separation membranes or thin-film composite membranes. The phase separation anisotropic membranes are relatively homogenous in chemical composition but not in structure and they consist of a dense layer of polymer on the surface of an increasingly porous layer. However, the thin-film composite membrane ones are heterogeneous in all their chemical and structural constituents. For the fabrication of reverse osmosis, the commonly used materials are cellulose acetate, polyamide, and thin-film composites prepared by interfacial polymerization on the surface of the porous support. However, these materials are distracted easily by the fouling effect therefore carbonaceous materials such as graphene membrane and CNTs are the most preferred materials of the twenty-first century.
5.3. CNT membrane filtration
CNT Filtration membranes are working on the basis of size sieving principles (Citation55). They are able to filter out a wide spectrum of pollutants from aqueous as well as non-aqueous medium (Citation63). In our effort to come up with the noble nature of CNT in membrane filtration, we have performed several laboratory tests and reviewed a large number of literatures and discovered that it has high permeation flux, high pollutant rejection rate and low fouling propensity (Citation46). CNT at lower pH is a promising material for the membrane filtration of highly reactive monovalent anion because it possesses a large surface area, ease of functionalization, has a high aspect ratio, fast water transport even faster than 4–5 order of magnitude than theoretically predicted by Hagen-Poiseuille. It has got a potential to either directly use as a filter or as a filler to improve the performance of the other membranes because of its antifouling nature, strength, disinfection and permeability (Citation64).
According to their fabrication methods, CNTs are categorized into two broad classes: (i) self-supporting CNT membranes (this consists of vertically aligned and Bucky-paper CNT membranes) and (ii) nanocomposite CNT membranes. Unlike the nanocomposite one self-supporting CNT membranes have a high water flow rate and short channel length (Citation62). CNT membranes then have the potential to totally replace microfiltration, nanofiltration, reverse osmosis and ultrafiltration or improves their performances in water treatment. Water crisis remains the greatest challenge of this and the lack of fresh and clean water is the global problem now a day. However, CNT inspires innovative technologies to address the water shortage and the water pollution problems (Citation46). In the latest work, as-prepared carbon/CNT composite (ball-milled sample) demonstrated the effective applicability of the prepared adsorbent for the reduction of elevated fluoride concentration from groundwater to the permissible limit.
5.4. Leaching by CNTs and toxicity
The release of non-biodegradable CNTs into the aqueous system/water bodies will lead to immediate exposure of organisms in all phases. As-prepared CNTs usually contain a significant amount of impurities, including metal catalysts. The leaching of metals from the CNTs, when introduced into natural waters, has been investigated on a short-term basis and no significant toxicity to algae was reported. But at a higher temperature, the exposure risk of aquatic organisms to CNTs was reported to be higher around the point of release. The variation in temperature plays a significant role in decreasing the stability of CNTs. This temperature effect is less significant with time when the media is maintained at comparatively less hot conditions relative to when it is heated up. Significant leaching of CNT metals may happen in the groundwater, seawater, and wastewater. The dynamics of metal catalyst leaching vary widely depending on the nature of CNTs and their respective metals. Almost 6% of CNTs produced and used globally are currently estimated to end up in wastewater treatment plants (Citation65). Moreover, many reports are published on CNT growth using non-metal catalysts. In order to overcome the demerits of conventional metal catalyst CNTs, green catalyst-assisted CNTs’ growth may come as a rescue to overcome the threats (Citation66).
It has been very well established that the addition of CNTs into the water environment, beyond a certain limit will produce certain toxicity to aquatic organisms. In addition, nanosized CNTs can easily penetrate cell walls, cell membranes in a living body, causing lung tumors and cellular inflammation in animals and humans (Citation67). Many researchers in the past, reported that the respiratory system exhibited high susceptibility towards CNTs, which would lead to serious health issues including bronchitis, lung cancer, emphysema and asthma. The use of pulverized CNTs or composites containing fine CNT particles is believed to result in an inhalation exposure (Citation68). Recently conducted research on the inhalation risk has helped in realizing the impact of CNTs on the respiratory tract and to define the exposure limits. The carcinogenicity of CNTs was found to diminish with the decrease in the fiber length (Citation66).
The toxicity study on MWCNTs revealed extremely high phagocytic activity for undifferentiated HL-60 cells and a certain cytotoxicity for already differentiated HL-60 cells (Citation69). In addition, MWCNTs have a certain genotoxicity, which is believed to affect the DNA repair mechanism. The molecular larval studies at aquatic levels showed that MWCNTs affect the transcription of genes involved in apoptosis (Citation70). However, the structure, diameter, and length of CNTs are also significant for their toxicity. The longer the length of the CNTs, the stronger the toxicity. The application prospects of CNTs have been limited by their toxicity. The research on the degradation of CNTs is also gaining momentum so as to reduce its biological toxicity and enhance the biomedical applications of CNTs (Citation67). Therefore, it is very significant to explore the degradation mechanisms for CNTs in the presence of different contaminants and find a highly effective green degradation method.
6. Conclusion
Eastern Shoa zone of Oromia regional state, Ethiopia lies totally in the rift valley and people of this region are at the extreme risk of lack of pure drinking water. Their life is totally relying on underground water, whereas more than 90% of the sample contain fluoride ion above WHO standard level 1.5 mg/l. It was also reported that many villages are totally paralyzed due to fluoride problems. The Oromia regional state government employed some traditional water treatment strategies to rescue the people but it is not yet efficient. The CNTs with remarkable physico-chemical properties exhibit a myriad of applications, including supercapacitors, batteries, catalysts, and adsorption. Thus, in this review, we realized that CNTs membrane filter will be the most effective and efficient nanomaterials to remove fluoride ion from the drinking water by reverse osmosis mechanisms. Even if the conventional membrane filtration mechanisms are expensive, require skilled manpower and time taking, with this review we identified the efficient and effective material, CNT, for the simple, eco-friendly and cheap way of removing fluoride from aqueous media due to the presence of dangling bond-forming capacity and the vertical alignment with the available inner and outer surface of CNT. The toxicity of conventional CNTs can be reduced by the application of green catalysts in the place of metal catalysts. Hence we thoroughly reviewed the green synthesis, growth mechanism and indicated the promising potential applications of CNTs towards the waste water treatment potential in this review article. In addition, exploration of the degradation mechanisms for CNTs in the presence of diverse contaminants is a very challenging and indispensable activity.
Acknowledgments
The authors acknowledge the support provided by Adama Science and Technology University, Ethiopia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hiraoka, T.; Yamada, T.; Hata, K.; Futaba, D.N.; Kurachi, H.; Uemura, S.; Yumura, M.; Iijima, S. Synthesis of Single- and Double-Walled Carbon Nanotube Forests on Conducting Metal Foils. J. Am. Chem. Soc. 2006, 128 (41), 13338–13339. https://doi.org/https://doi.org/10.1021/ja0643772.
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and Structural Characterization of Carbon Nanotube Surfaces. Anal. Bioanal. Chem. 2010, 396 (3), 1003–1014. https://doi.org/https://doi.org/10.1007/s00216-009-3332-5.
- Homaeigohar, S. Water Treatment with New Nanomaterials. Water (Basel). 2020, 12 (1507), 10–13. https://doi.org/https://doi.org/10.3390/w12051507.
- Zhou, O.; Shimoda, H.; Gao, B.O.; Oh, S.; Fleming, L.E.S.; Yue, G. Materials Science of Carbon Nanotubes : Fabrication, Integration, and Properties of Macroscopic Structures of Carbon Nanotubes. Acc. Chem. Res. 2002, 35 (12), 1045–1053. https://doi.org/https://doi.org/10.1021/ar010162f.
- Rashid, H.; Ralph, S.F. Carbon Nanotube Membranes : Synthesis, Properties, and Future Filtration Applications. Am. J. Obs. 2017, 120 (10), 1298–1299. https://doi.org/https://doi.org/10.3390/nano7050099.
- Liu, X.; Zhang, S.; Pan, B. Potential of Carbon Nanotubes in Water Treatment. Recent Prog. Carbon Nanotub. Res. 2012. 1–5. https://doi.org/https://doi.org/10.5772/51332.
- Malago, J.; Makoba, E.; Muzuka, A.N.N. Fluoride Levels in Surface and Groundwater in Africa : A Review 2. Sources of Fluoride in Surface and Groundwater in Africa. Am. J. Water Sci. Eng. 2017, 3 (1), 1–17. https://doi.org/https://doi.org/10.11648/j.ajwse.20170301.11.
- Kebede, S. Groundwater in Ethiopia Features, Numbers and Opportunities, Springer Hydrology: Berlin, 2013. https://doi.org/https://doi.org/10.1007/978-3-642-30391-3.
- Demelash, H.; Beyene, A.; Abebe, Z.; Melese, A. Fluoride Concentration in Ground Water and Prevalence of Dental Fluorosis in Ethiopian Rift Valley : Systematic Review And Syst. Rev. Meta-Analysis. BMC Public Heal. 2019, 19 (1), 1–9. https://doi.org/https://doi.org/10.1186/s12889-019-7646-8.
- Peccerillo, A. Petrogenesis of Silicic Peralkaline Rocks in the Ethiopian Rift : Geochemical Evidence and Volcanological Implications. J. African Earth Sci. 2007, 48, 161–173. https://doi.org/https://doi.org/10.1016/j.jafrearsci.2006.06.010.
- Ansari, M.; Kazemipour, M.; Dehghani, M.; Kazemipour, M. The Defluoridation of Drinking Water Using Multi-Walled Carbon Nanotubes. J. Fluor. Chem. 2011, 132 (8), 516–520. https://doi.org/https://doi.org/10.1016/j.jfluchem.2011.05.008.
- Datturi, S.; Kumsa, A.; Kebede, S.; van S, F.; van B, M. The Right to Smile: Fluoride and Fluorosis in Central Rift Valley (Ethiopia). Groundw. Mag. 2017, (3), 8–26.
- Thole, B. Defluoridation Kinetics of 200 8 C Calcined Bauxite, Gypsum, and Magnesite and Breakthrough Characteristics of Their Composite Filter. J. Fluor. Chem. 2011, 132 (8), 529–535. https://doi.org/https://doi.org/10.1016/j.jfluchem.2011.05.016.
- WHO. Guidelines for Drinking-Water Quality; 2017.
- McCann, H.G. Reactions of Fluoride Ion with Hydroxyapatite. J. Biol. Chem. 1953, 201 (1), 247–259.
- Huber, A.C.; Mosler, H.-J. Determining Behavioral Factors for Interventions to Increase Safe Water Consumption: A Cross-Sectional Field Study in Rural Ethiopia. Int. J. Environ. Health Res. 2013, 23 (2), 96–107. https://doi.org/https://doi.org/10.1080/09603123.2012.699032.
- Collivignarelli, M.C.; Abb, A.; Miino, M.C.; Torretta, V.; Rada, E.C.; Caccamo, F.M.; Sorlini, S. Adsorption of Fluorides in Drinking Water by Palm Residues. Sustainability. 2020, 12 (9), 3786–3712. https://doi.org/https://doi.org/10.3390/su12093786.
- Ando, Y.; Zhao, X.; Sugai, T.; Kumar, M. Growing Carbon Nanotubes. Mater. Today. 2004, 7 (10), 22–29. https://doi.org/https://doi.org/10.1016/S1369-7021(04)00446-8.
- Ma, W.; Zhao Y. Fluoride Removal from Drinking Water by Adsorption Using Bone Char as a Biosorbent Feiqun Ya and Ren Wang Yaqian Zhao. Int. J. Environ. Technol. Manag. 2008, 9 (1), 59–69.
- Ibrahim, K.S. Carbon Nanotubes – Properties and Applications : A Review. Carbon Lett. 2013, 14 (3), 131–144. https://doi.org/https://doi.org/10.5714/CL.2013.14.3.131.
- Popov, V.N. Carbon Nanotubes : Properties and Application. Mater. Sci. Eng. R. 2004, 43 (3), 61–102. https://doi.org/https://doi.org/10.1016/j.mser.2003.10.001.
- Das, R.; Ali, E.; Bee, S.; Hamid, A.; Ramakrishna, S.; Chowdhury Z.Z. Carbon Nanotube Membranes for Water Puri Fi Cation : A Bright Future in Water Desalination. Desalination. 2014, 336, 97–109. https://doi.org/https://doi.org/10.1016/j.desal.2013.12.026.
- Dore, J.; Burian, A.; Tomita, S. Structural Studies of Carbon Nanotubes and Related Materials by Neutron and X-Ray Diffraction. ACTA Phys. Pol. A. 2000, 98 (5), 495–504.
- Bachtold, A.; Hadley, P.; Nakanishi, T.; Dekker, C. Logic Circuits with Carbon Nanotube Transistors. Science. 2001, 294(5545), 1317-1320. https://doi.org/https://doi.org/10.1126/science.1065824.
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes : A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10 (6), 3739–3758. https://doi.org/https://doi.org/10.1166/jnn.2010.2939.
- Murthy, H.C.A.; Desalegn, T.; Kassa, M. Synthesis of Green Copper Nanoparticles Using Medicinal Plant Hagenia Abyssinica (Brace) JF. Gmel. Leaf Extract: Antimicrobial Properties. J. Nanomat. 2020, 2020, 1–12. https://doi.org/https://doi.org/10.1155/2020/3924081.
- Desalegn, T.; Ravikumar, C.R.; Murthy, H.C.A. Eco-friendly Synthesis of Silver Nanostructures Using Medicinal Plant Vernonia Amygdalina Del. Leaf Extract for Multifunctional Applications. Appl. Nanosci. 2020, 11, 535–551. https://doi.org/https://doi.org/10.1007/s13204-020-01620-7.
- Tripathi, N.; Pavelyev, V.; Islam, S.S. Synthesis of Carbon Nanotubes Using Green Plant Extract as Catalyst: Unconventional Concept and its Realization. Appl. Nanosci. (Switzerland). 2017, 7, 557–566. https://doi.org/https://doi.org/10.1007/s13204-017-0598-3.
- Nguyen, V.H.; Shim, J.J. Green Synthesis and Characterization of Carbon Nanotubes/Polyaniline Nanocomposites. J. Spectrosc. 2015, 2015, 1–9. https://doi.org/https://doi.org/10.1155/2015/297804.
- Hamid, Z.A.; Azim, A.A.; Mouez, F.A.; Rehim, S.S.A. Challenges on Synthesis of Carbon Nanotubes from Environmentally Friendly Green oil Using Pyrolysis Technique, Vol. 126, Elsevier B.V., 2017; pp 218–229.
- Hakim, Y.Z.; Yulizar, Y.; Nurcahyo, A. Surya M Green Synthesis of Carbon Nanotubes from Coconut Shell Waste for the Adsorption of Pb(II) Ions. Acta Chim. Asiana. 2018, 1, 6–10. https://doi.org/https://doi.org/10.29303/aca.v1i1.2.
- Araga, R.; Kali, S.; Sharma, C.S. Coconut-Shell-Derived Carbon/Carbon Nanotube Composite for Fluoride Adsorption from Aqueous Solution. Clean - Soil, Air, Water. 2019, 47, 1800286–1800289. https://doi.org/https://doi.org/10.1002/clen.201800286.
- Cao, D.; He, H.Y. Eco-friendly Synthesis and Characterisations of Single-Wall Carbon Nanotubes/Ag Nanoparticle Heterostructures. Mater. Res. Innov. 2020, 25 (2), 76–82. https://doi.org/https://doi.org/10.1080/14328917.2020.1740868.
- Makgabutlane, B.; Nthunya, L.N.; Maubane-Nkadimeng, M.S.; Mhlanga, S.D. Green Synthesis of Carbon Nanotubes to Address the Water-Energy-Food Nexus: A Critical Review. J. Environ. Chem. Eng. 2019, 104736. https://doi.org/https://doi.org/10.1016/j.jece.2020.104736.
- Wang, J.G.; Liu, H.; Zhang, X. Green Synthesis of Hierarchically Porous Carbon Nanotubes as Advanced Materials for High-Efficient Energy Storage. Small 2018, 14, 1–8. https://doi.org/https://doi.org/10.1002/smll.201703950.
- Kumar, M. Carbon Nanotube Synthesis and Growth Mechanism; Applications: India, 1999, https://doi.org/https://doi.org/10.5772/19331.
- Irle, S.; Ohta, Y.; Okamoto, Y.; Page, A.J.; Wang, Y.; Morokuma, K. Milestones in Molecular Dynamics Simulations of Single-Walled Carbon Nanotube Formation : A Brief Critical Review. Nano Res. 2009, 2 (10), 755–767. https://doi.org/https://doi.org/10.1007/s12274-009-9078-8.
- Mandal, S.K.; Hussain, S.; Pal, A.K. Growth Mechanism of Carbon Nanotubes Deposited by Electrochemical Technique. Indian J. Pure Appl. Phys. 2005, 43 (10), 765–771.
- Lehman, J.H.; Terrones, M.; Meunier, V.; Mansfield, E.; Hurst, K.E. Evaluating the Characteristics of Multiwall Carbon. Carbon N. Y. 2011, 49 (8), 2581–2602. https://doi.org/https://doi.org/10.1016/j.carbon.2011.03.028.
- Gavillet, J.; Loiseau, A.; Journet, C.; Willaime, F.; Ducastelle, F.; Charlier, J. Root-Growth Mechanism for Single-Wall Carbon Nanotubes. Phys. Rev. Lett. S. 2001, 87 (27), 2–5. https://doi.org/https://doi.org/10.1103/PhysRevLett.87.275504.
- Ong, Y.T.; Ahmad, A.L.; Hussein, S.; Zein, S.; Tan, S.H. A Review on Carbon Nanotubes in an Environmental Protection and Green Engineering Perspective. Brazilian J. Chem. Eng. 2010, 27 (02), 227–242. https://doi.org/https://doi.org/10.1590/s0104-66322010000200002.
- Ye, Q.; Cassell, A.M.; Liu, H.; Chao, K.; Han, J.; Meyyappan, M.; Field, M.; Nanosystems, I.; Sunny, V. Large-Scale Fabrication of Carbon Nanotube Probe Tips for Atomic Force Microscopy Critical Dimension Imaging Applications. Nano Lett. 2004, 4 (7), 1301–1308. https://doi.org/https://doi.org/10.1021/nl049341r.
- Kardimi, K.; Tsoufis, T.; Tomou, A.; Kooi, B.J.; Prodromidis, M.I.; Gournis, D. Synthesis and Characterization of Carbon Nanotubes Decorated with Pt and PtRu Nanoparticles and Assessment of Their Electrocatalytic Performance. Int. J. Hydrogen Energy. 2012, 37 (2), 1243–1253. https://doi.org/https://doi.org/10.1016/j.ijhydene.2011.09.143.
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and Water Treatment Application of Carbon Nanotubes (CNTs) -Based Composite Membranes : A Review. https://doi.org/https://doi.org/10.3390/membranes7010016.
- Casanova, E.G.O.; Mandujano, A.T.; Rom, M. Microscopy and Spectroscopy Characterization of Carbon Nanotubes Grown at Different Temperatures Using Cyclohexanol as Carbon Source. J. Spectrosc. 2019, 2019, 1–6. https://doi.org/https://doi.org/10.1155/2019/6043523.
- Lee, B.; Baek, Y.; Lee, M.; Jeong, D.H.; Lee, H.H.; Yoon, J.; Kim, Y.H. A Carbon Nanotube Wall Membrane for Water Treatment. Nat. Commun. 2015, 6 (7109), 1–7. https://doi.org/https://doi.org/10.1038/ncomms8109.
- Dikio, E.D. A Comparative Study of Carbon Nanotubes Synthesized from Co / Zn / Al and Fe / Ni / Al Catalyst. E-Journal Chem. 2011, 8 (3), 1014–1021. https://doi.org/https://doi.org/10.1155/2011/252875.
- Costa, S.; Borowiak-Palen, E.; Kruszyńska, M.; Bachmatiuk, A.; Kaleńczuk, R.J. Characterization of Carbon Nanotubes by Raman Spectroscopy. Mater. Sci. 2008, 26 (2), 1–8.
- Cheng, X.; Zhong, J.; Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H.; Xu, H. Characterization of Multiwalled Carbon Nanotubes Dispersing in Water and Association with Biological Effects. J. Nanomater. 2011, 2011, 1–12. https://doi.org/https://doi.org/10.1155/2011/938491.
- Klein, K.L.; Melechko, A.V.; McKnight, T.E.; Retterer, S.T.; Rack, P.D.; Fowlkes, J.D.; Joy, D.C.; Simpson, M.L. ARTICLES. Surface Characterization and Functionalization of Carbon Nanofibers. J. Appl. Phys. 2016, 103 (061301), 1301. https://doi.org/https://doi.org/10.1063/1.2840049.
- Park, M.; Kim, B.; Kim, S.; Han, D.; Kim, G.; Lee, K. Improved Binding Between Copper and Carbon Nanotubes in a Composite Using Oxygen-Containing Functional Groups. Carbon N. Y. 2011, 49 (3), 811–818. https://doi.org/https://doi.org/10.1016/j.carbon.2010.10.019.
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.; Matsuyama, H.; Fane, A.G.; Kyong, H. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination. 2020, 479, 114312. https://doi.org/https://doi.org/10.1016/j.desal.2020.114312.
- Bruggen, B.V.D. The Separation Power of Nanotubes in Membranes : A Review. Nanotechnology. 2012, 2012, 1–17. https://doi.org/https://doi.org/10.5402/2012/693485.
- Zhao, M. World’s Largest Science, Technology & Medicine Open Access Book Publisher. 2017, No. March. https://doi.org/https://doi.org/10.5772/65723.
- Saththasivam, J.; Yiming, W.; Wang, K.; Jin, J.; Liu Z. OPEN A Novel Architecture for Carbon Nanotube Membranes Towards Fast and Efficient Oil / Water Separation. Sci. Rep. 2018, 8 (1), 4–9. https://doi.org/https://doi.org/10.1038/s41598-018-25788-9.
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification Carbon Nanotubes A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4 (135), 1–20. https://doi.org/https://doi.org/10.3390/jcs4030135.
- Biron, S. Ceramic Membranes Applied in Separation Processes, Membrane: Brazil, 2018, https://doi.org/https://doi.org/10.1007/978-3-319-58604-5.
- Bruggen, B.V.D.; Vandecasteele, C.; Volodin, A. How a Microfiltration Pretreatment Affects the Performance in Nanofiltration. Sep. Sci. Technol. 2005, 39 (7), 1443–1459. https://doi.org/https://doi.org/10.1081/SS-120030799.
- Mokhtar, G.; Naoyuki, F. Microfiltration, Nanofiltration and Reverse Osmosis for the Removal of Toxins (LPS Indotoxins) from Wastewater. J. Memb. Sci. Technol. 2012, 2 (3), 1–5. https://doi.org/https://doi.org/10.4172/2155-9589.1000118.
- Pinto, J. Membrane Science & Technology Carbon Nanotubes Membrane for Water Filtration. J. Membr. Sci. Technol. 2020, 10 (204), 1–2. https://doi.org/https://doi.org/10.35248/2155-9589.2020.10.204.
- Shahmansouri A.; Bellona, C. Nanofiltration Technology in Water Treatment and Reuse : Applications and Costs. Water Sci. Technol. 2015, 71 (3), 309–319. https://doi.org/https://doi.org/10.2166/wst.2015.015.
- Wang, R.; Chen, D.; Wang, Q.; Ying, Y.; Gao, W.; Xie, L. Recent Advances in Applications of Carbon Nanotubes for Desalination : A Review. Nanomaterials. 2020, 10 (1203), 1–28. https://doi.org/https://doi.org/10.3390/nano10061203.
- Ihsanullah. Carbon Nanotube Membranes for Water Purification: Developments, Challenges, and Prospects for the Future. Sep. Purif. Technol. 2019, 209, 307–337. https://doi.org/https://doi.org/10.1016/j.seppur.2018.07.043.
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application Potential of Carbon Nanotubes in Water Treatment : A Review. J. Environ. Sci. 2013, 25 (7), 1263–1280. https://doi.org/https://doi.org/10.1016/S1001-0742(12)60161-2.
- Adeleye, A.S.; Keller, A.A. Long-term Colloidal Stability and Metal Leaching of Single Wall Carbon Nanotubes: Effect of Temperature and Extracellular Polymeric Substances. Water Res. 2014, 49, 236–250. https://doi.org/https://doi.org/10.1016/j.watres.2013.11.032.
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 1–12. https://doi.org/https://doi.org/10.3390/jcs4030135.
- Zan, P.; Xiaojuan, L.; Wei, Z.; Zhuotong, Z.; Zhifeng, L.; Chang, Z.; Yang, L.; Binbin, S.; Qinghua, L.; Wangwang, T.; Xingzhong, Y. Advances in the Application, Toxicity and Degradation of Carbon Nanomaterials in Environment: A Review. Environ. Int. 2020, 134, 236–250. https://doi.org/https://doi.org/10.1016/j.envint.2019.105298.
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: a Perspective View. Nanoscale Res. Lett. 2018, 13, 1–9. https://doi.org/https://doi.org/10.1186/s11671-018-2589-z.
- Tabei, Y.; Fukui, H.; Nishioka, A.; Hagiwara, Y.; Sato, K.; Yoneda, T.; Koyama, T.; Horie, M. Effect of Iron Overload from Multi Walled Carbon Nanotubes on Neutrophil-Like Differentiated HL-60 Cells. Sci. Rep. 2019, 9.1, 1–6. https://doi.org/https://doi.org/10.1038/s41598-019-38598-4.
- Martínez-Paz, P.; Negri, V.; Esteban-Arranz, A.; Martínez-Guitarte, J.L.; Ballesteros, P.; Morales, M. Effects at Molecular Level of Multi-Walled Carbon Nanotubes (MWCNT) in Chironomus Riparius (DIPTERA) Aquatic Larvae. Aquat. Toxicol. 2019, 209, 42–48. https://doi.org/https://doi.org/10.1016/j.aquatox.2019.01.017.