ABSTRACT
Complementarity to liquid and gas chromatography, supercritical fluid chromatography (SFC) is becoming a mainstream separation technique. There is a global demand for more SFC users since ‘green’ method development has become a favorable target for research into sustainable technologies. In order to parallel this trend, there is a need to incorporate SFC into the relevant curricula to provide students with this pertinent chromatographic separation knowledge. There is currently limited SFC training in undergraduate modules compared to traditional liquid/gas chromatography and mass spectrometry. Herein, we comment on the evolution of SFC, and the move to this greener chemistry technology with increased industrial applications, to highlight the importance of including this technique in future undergraduate modules.
GRAPHICAL ABSTRACT
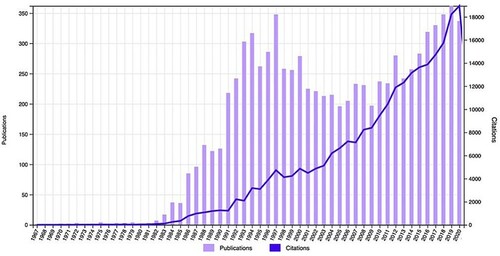
Supercritical fluid chromatography (SFC) is rapidly accelerating as one of the fastest-growing analytical and preparative separation tools (Citation1). However, there is limited exposure to this separation method in universities globally as compared to high-performance liquid chromatography (HPLC) and gas chromatography (GC) (Citation2, Citation3). The topic forms part of a steadily growing area of research, based on the citation report below using the keywords supercritical fluid chromatography as shown in from a Web of Science search.
Figure 1. Times cited and publications of over time using keyword supercritical fluid chromatography (all fields) on Web of Science database (August 2021).
This letter emphasizes the need for SFC teaching at undergraduate levels to broaden the student’s practical and theoretical understanding and provide essential hands-on chromatographic experience. It is aligned to current industry advancements of greener and more sustainable methods in the important field of chromatography. ‘Green’ methodologies is a fast-growing trend in various cutting edge research areas within chemistry, biotechnology, engineering, agriculture, and food science technology. Scientists are aiming to replace traditional methods with more sustainable, energy-efficient alternatives aligning to the 12 Principles of Green Chemistry (Citation4–6). These principles are now increasingly being taught in academic programs in order to train future scientists to examine the environmental impacts of their research (Citation6, Citation7). Much research is also dedicated to revolutionizing past chemical reactions and testing methods to fit within the green chemistry framework (Citation8).
Chromatography is one such field that is striving to incorporate green chemistry principles to instrumentation and analytical techniques i.e. more robust and sustainable chromatographic methods which employ environmentally friendly solvents and produce minimal waste (Citation9–12). Analytical and preparative SFC has the potential to reduce organic solvent waste production and overall consumption as compared to conventional separation techniques such as HPLC (Citation13). The need for more benign manufacturing cannot be overlooked since it significantly benefits pharmaceutical industries in our modern society (Citation11). Therefore, new greener methods such as SFC purification are fast gaining popularity.
SFC has been used for the past four decades, albeit not with industrial applications, until more recently (Citation13, Citation14). In 1958, James E. Lovelock first proposed the concept of SFC at Yale University, thereafter in 1962, Ernst Klesper conducted the first SFC separations (Citation14). This was followed by the development and commercialization of open tubular or capillary SFC in the 1980s, which intended to provide an alternative to GC (Citation14). In 1981, Lee and Novotny introduced capillary SFC columns that employed pressure programming of pure CO2 (Citation15). These developments occurred concurrently with Berger’s group at Hewlett Packard who also developed fused silica capillary columns. Capillary columns for SFC were eventually discontinued due to poor reproducibility, high cost, and inefficient separation (Citation10) Thereafter, Hewlett-Packard converted the 1084 model HPLC into an SFC system using a packed column, and this became the standard type of column moving forward (Citation16). In 1986, the first chiral separation using this technique was demonstrated for enantiomeric resolution on chiral amides and phosphine oxides (Citation10). Interest in packed column SFC was reignited in the early 1990s due to advances involving mixed mobile phases and improved pressure gradient techniques (Citation10). During the mid to late 1990s, the modern SFC was created to purify pharmaceuticals, thereby sparking Hewlett-Packard’s return to the SFC market in 1992 (Citation10). The disadvantages associated with the first generation of SFC’s included inconsistent pump performance, increased detector noise, poor reproducibility, and robustness. Present analytical SFC instruments have, to the most part, successfully overcome these limitations vide infra (Citation10). In 1995 Berger instruments were created introducing preparative scale SFC using packed columns. In 2010, Agilent introduced the Infinity Hybrid SFC/UHPLC and Infinity Analytical SFC while Waters manufactured the Acquity Ultra-Performance Convergence Chromatography systems (Citation14). The aforementioned SFC instruments brought about an expansion and renewed interest to the field of SFC since these instruments could be employed for rapid, robust, and high precision method validation of numerous analytes (Citation10, Citation17–20). Currently there has also been an increase in the number of global SFC manufacturers.
In the past two decades, there have been major advancements with the components and software for SFC. This has triggered its resurgence for increased industrial applications (Citation10). Some significant advances include the use of stacked injections (which allows for higher throughput and the ability to process larger sample volumes) and an electronic back pressure regulator that is used to maintain the critical pressure throughout the SFC system. The instruments are sensitive enough to adjust to gradient flow setups and the added advantage of continuously increasing options of stationary phases (Citation21, Citation22).
The use of supercritical carbon dioxide was initially reserved for the extraction and separation of small polymers, petroleum products, and the decaffeination of tea/coffee. However, modern SFC instruments have expanded its applications to include the purification of fragrances, pharmaceuticals, pesticides, and personal care products (Citation1, Citation10, Citation16, Citation22–Citation25). It has replaced HPLC for applications such as separation, purification, screening, and method development for enantiomers e.g. ketamine (well-known antidepressant) (Citation17–Citation20, Citation22, Citation26, Citation27).
It is an ideal substitute for the separation of hydrocarbons comprising of 80–130 carbon atoms, as GC methods would result in sample cracking at high temperatures (Citation10).
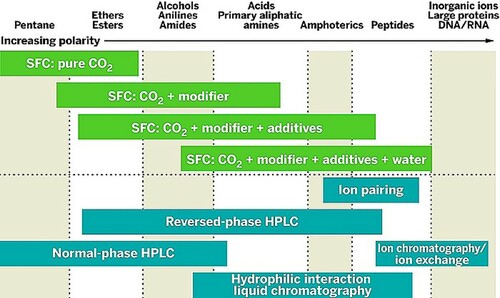
The expansion of usable modifiers for SFC has even allowed for the separation of biologicals such as peptides and proteins (Citation1, Citation23–25). illustrates the variety of modifiers that this technique tolerates, thereby separating a wide range of molecules and broader use.
Figure 2. SFC modifications of the CO2 mobile phase allow for an increased scope of analytes compared to other chromatography methods (Citation14).
The reduction in solvent waste has added health, safety, cost benefits, and reduced environmental impact. It employs carbon dioxide as a mobile phase that can be easily separated from the modifier and recycled (Citation10, Citation16). Other advantages include higher efficiency in separations, shorter analysis times, faster method development, stacked injections, and column equilibrations (Citation17–Citation20, Citation22). Another plus for using an SFC instrument is the ease of recycling an unresolved fraction when overlapping of chromatogram peaks occurs (Citation18).
With a myriad of advantages, SFC has overall increased chromatographic productivities (Citation12, Citation16) and will soon become a mainstream separation technique. Therefore the scientific advances in the field of modern SFC technology together with the increased industrial applications should warrant its incorporation into core undergraduate pharmaceutical sciences and chemistry curricula. Teaching undergraduate students SFC operation and applications could be implemented in a similar manner to that followed by Wang et al. who employed GC-QTOF-MS (Citation2) coupled with green chemistry principles from Cann and Dickneider (Citation7). This technology can be translated in the context of the green chemistry principles for; minimization of waste production, efficient energy methods, proper waste management, sustainable, less toxic, and renewable reagents (Citation5, Citation7, Citation10, Citation12, Citation16).
SFC teaching/training poses one of the key challenges for its implementation amongst trained personal in academic and industry labs (Citation14). According to a 2014 article conducted by Arnaud (Citation14), SFC applications seeks new users, the author interviewed experts in the field of SFC, and they stated that in the past 20 years, academic labs had not implemented the use of SFC since undergraduate and postgraduate students are not trained in this cutting edge technology. As such, a considerable amount of training must be conducted in order for widespread acceptance of SFC as scientists are more accustomed to HPLC (Citation14). SFC is extensively employed in the pharmaceutical industry but is not thoroughly used in academia (Citation10, Citation28). The scarcity of SFC knowledge for graduates may be due to limited academic training in the field and fewer institutions that have access to the equipment based on the historically higher costs than HPLC. The consequences of the failed acceptance, instrument shortages, and limited undergraduate laboratory exposure to this technology create a knowledge gap. Thus graduates without the necessary skills and training will be hampered should they wish to enter industrial sectors already using SFC.
The future of SFC seems to be beaming since it has solved many of the HPLC limitations, it meets successful separation requirements (fast, robust and sensitive), and SFC champions the approach for greener methodologies. We, as educators have a responsibility to accept newer innovative technologies into our curricula that more closely parallel industry trends. It is also imperative that we embrace and teach alternatives to traditional methods that are more sustainable, energy-efficient, and cost-effective leading to a beneficial impact on our environment/health. Hence, the call for higher education institutions to merge SFC topics within undergraduate pharmaceutical sciences/chemistry curricula (under the section of chromatography or green chemistry). Also, established researchers in the field are encouraged to publish ‘simple’ SFC separation introductory experimental procedures to better train students to gain practical knowledge and experience. Finally, it is also recommended for funding agencies to support higher education institutions in purchasing SFC instrumentation to provide graduates with the much-needed enhancement of skills in this fast-growing and promising area.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schiavone, N.M.; Bennett, R.; Hicks, M.B.; Pirrone, G.F.; Regalado, E.L.; Mangion, I.; Makarov, A.A. Evaluation of Global Conformational Changes in Peptides and Proteins Following Purification by Supercritical Fluid Chromatography. J. Chromatogr. B 2019, 1110, 94–100.
- Wang, H.; Li, Z.; Ding, J.; Ren, N. Teaching Graduate Students GC-QTOF-MS Operation and Application via an Analytical Chemistry Experiment. J. Chem. Educ. 2021, 98 (2), 644–650.
- Novaki, L.P.; Chinelatto, A.M.; Silva, V.A.B.B.; El Seoud, O.A. Analysis of Consumer Products: Demonstrating the Power of LC-MS/MS for the Simultaneous Analysis of Caffeine and Other Methylxanthines in Guaraná Fruit Powder Extract. J. Chem. Educ. 2021, 98 (6), 2083–2089.
- Anastas, P.; Warner, J. Green Chemistry; Oxford University Press: Oxford, 1998, 160 pp. ISBN 0-19-850234-6. 1999: p. G21.
- Ivanković, A.; Dronjić, A.; Bevanda, A.M.; Talić, S. Review of 12 Principles of Green Chemistry in Practice. International Journal of Sustainable and Green Energy 2017, 6 (3), 39–48.
- Hjeresen, D.L.; Boese, J.M.; Schutt, D.L. Green Chemistry and Education. J. Chem. Educ. 2000, 77 (12), 1543.
- Cann, M.C.; Dickneider, T.A. Infusing the Chemistry Curriculum with Green Chemistry Using Real-World Examples, Web Modules, and Atom Economy in Organic Chemistry Courses. J. Chem. Educ. 2004, 81 (7), 977.
- Billiard, K.M.; Dershem, A.R.; Gionfriddo, E. Implementing Green Analytical Methodologies Using Solid-Phase Microextraction: A Review. Molecules 2020, 25 (22), 5297.
- Welch, C.J.; Wu, N.; Biba, M.; Hartman, R.; Brkovic, T.; Gong, X.; Helmy, R.; Schafer, W.; Cuff, J.; Pirzada, Z. Greening Analytical Chromatography. Trends Anal. Chem. 2010, 29 (7), 667–680.
- Miller, L.M.; Pinkston, J.D.; Taylor, L.T. Modern Supercritical Fluid Chromatography: Carbon Dioxide Containing Mobile Phases; John Wiley & Sons: USA, 2019.
- Li, J.; Albrecht, J.; Borovika, A.; Eastgate, M.D. Evolving Green Chemistry Metrics Into Predictive Tools for Decision Making and Benchmarking Analytics. ACS. Sustain. Chem. Eng. 2017, 6 (1), 1121–1132.
- Sheldon, R.A. The Greening of Solvents: Towards Sustainable Organic Synthesis. Current Opinion in Green and Sustainable Chemistry 2019, 18, 13–19.
- Ritter, S.K., Growing Interest for Supercritical Fluid Chromatography. In: Asia, in C&En Chemical and Engineering News; American Chemical Society: United States, 2016.
- Arnaud, C.H., Supercritical Fluid Chromatography Seeks New Users. In C&En Chemical and Engineering News; American Chemical Society: United States, 2014.
- Novotny, M.; Springston, S.R.; Peaden, P.A.; Fjeldsted, J.C.; Lee, M.L. Capillary Supercritical Fluid Chromatography. Anal. Chem. 1981, 53 (3), 407–414.
- Berger, T.A. Supercritical Fluid Chromatography; Agilent Technologies: USA, 2015, 186.Primer, publication number 5991-5509EN.
- Miller, L.; Potter, M. Preparative Chromatographic Resolution of Racemates Using HPLC and SFC in a Pharmaceutical Discovery Environment. J. Chromatogr. B 2008, 875 (1), 230–236.
- Speybrouck, D.; Lipka, E. Preparative Supercritical Fluid Chromatography: A Powerful Tool for Chiral Separations. J. Chromatogr., A 2016, 1467, 33–55.
- Miller, L. Evaluation of Non-Traditional Modifiers for Analytical and Preparative Enantioseparations Using Supercritical Fluid Chromatography. J. Chromatogr., A 2012, 1256, 261–266.
- Miller, L. Use of Dichloromethane for Preparative Supercritical Fluid Chromatographic Enantioseparations. J. Chromatogr., A 2014, 1363, 323–330.
- Nogle, L.M.; Mann, C.W.; Watts, W.L., Jr.; Zhang, Y. Preparative Separation and Identification of Derivatized β-Methylphenylalanine Enantiomers by Chiral SFC, HPLC and NMR for Development of New Peptide Ligand Mimetics in Drug Discovery. J. Pharm. Biomed. Anal. 2006, 40 (4), 901–909.
- Płotka, J.M.; Biziuk, M.; Morrison, C.; Namieśnik, J. Pharmaceutical and Forensic Drug Applications of Chiral Supercritical Fluid Chromatography. Trends Anal. Chem. 2014, 56, 74–89.
- Govender, K.; Naicker, T.; Baijnath, S.; Chuturgoon, A.A.; Abdul, N.S.; Docrat, T.; Kruger, H.G.; Govender, T. Sub/Supercritical Fluid Chromatography Employing Water-Rich Modifier Enables the Purification of Biosynthesized Human Insulin. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 2020, 1155, 122126–122126.
- Govender, K.; Naicker, T.; Baijnath, S.; Kruger, H.G.; Govender, T. The Development of a sub/Supercritical Fluid Chromatography Based Purification Method for Peptides. J. Pharm. Biomed. Anal. 2020, 190, 113539.
- Molineau, J.; Hideux, M.; West, C. Chromatographic Analysis of Biomolecules with Pressurized Carbon Dioxide Mobile Phases – A Review. J. Pharm. Biomed. Anal. 2020, 193, 113736.
- Stringham, R.W. Chiral Separation of Amines in Subcritical Fluid Chromatography Using Polysaccharide Stationary Phases and Acidic Additives. J. Chromatogr., A 2005, 1070 (1–2), 163–170.
- Wei, Y.; Chang, L.; Hashimoto, K. A Historical Review of Antidepressant Effects of Ketamine and its Enantiomers. Pharmacol. Biochem. Behav. 2020, 190, 172870.
- Mukhopadhyay, R. SFC: Embraced by Industry But Spurned by Academia. Anal. Chem. 2008, 80 (9), 3091–3094.
