ABSTRACT
In this article, we applied ultrasonic irradiation as an ecofriendly, green, efficient approach for the synthesis of a new 1,3-thiazoles and 1,3,4-thiadiazines under solvent-free conditions. Thus, thiocarbohydrazide condenses with N-(thiazol-2-yl)acetamide (2) in different molar ratios under ultrasonic irradiation afforded mono and bis thiocarbohydrazons 3a,b, respectively. Thiocarbohydrazones were reacted with α-haloketone, α-haloester as well as hydrazonoyl halides under ultrasonic conditions afforded 1,3-thiazole 4, 1,3-thiazolinone 5 and 1,3,4-thiadiazines 6 and 11a–c, respectively. The reaction of monohydrazone 3a with phthalic anhydride derivatives afforded phthalazines 7a,b in a good yield. Ultrasonic irradiation has benefits such as greater purity, lower cost, high yields, and simple workups compared to the conventional methods. All the new compounds were screened for antimicrobial activity against different tested microbes. Most of the newly synthesized compounds exhibited potent to moderate activities toward the tested microbes. Compounds that exhibited potent antimicrobial activities were selected to measure their MIC and MBC values. The results revealed that compounds 3a, 3b, 8, 10, and 11a showed lower MIC against Staphylococcus aureus with the values of 39.06, 78.13, 9.77, 39.06 and 39.06 µg/ml, respectively. Meanwhile, the MBC values were found to be acceptable for 3a and 10 of 156 and 312, respectively.
GRAPHICAL ABSTRACT
1. Introduction
Green chemistry is the process that minimizes the use of hazardous substances. In recent decades, the application of green methodologies that are environmentally friendly and can minimize chemical disposal and energy consumption became the goal of organic chemists. One of the green chemistry techniques is ultrasound irradiation (sonochemistry). Ultrasound irradiation is widely utilized as an alternative source of energy to enhance the yield of the chemical reactions (Citation1–3) in high yields, short reaction times, economically attractive and pure products compared to traditional methods (Citation4–7). Ultrasound technology has recently been used to accelerate a large number of organic reactions (Citation8,Citation9). Thiazoles displayed diverse biological activities such as anticancer (Citation10–16), anti-inflammatory (Citation17,Citation18), antimicrobial (Citation19–26), anti-diabetic (Citation27,Citation28), antioxidant (Citation29,Citation30), and anticonvulsant (Citation31). Thiazoles are found in many potent bioactive synthetic drugs such as Sulphathiazole and Meloxicam. Moreover, many natural products have thiazole scaffolds such as thiamin pyrophosphate (TPP) and thiamin (vitamin B1). Cystothiazole A (antibiotic) was isolated in 1998 from the myxobacterium culture (). In addition, 1,3,4-thiadiazines possess pharmacological activities including antibacterial and antifungal (Citation32–34) and anticancer (Citation35,Citation36) activities. Based on the information provided and as an extension of our green synthesis (Citation37–39), our efforts have directed to a green, efficient and rapid procedure for the synthesis of new series of 1,3-thiazole and 1,3,4-thiadiazine by utilizing ultrasound irradiation under solvent-free condition and evaluate their antimicrobial activity.
2. Results and discussion
2.1. Chemistry
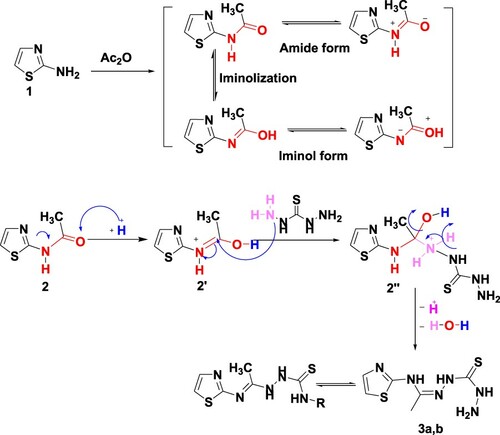
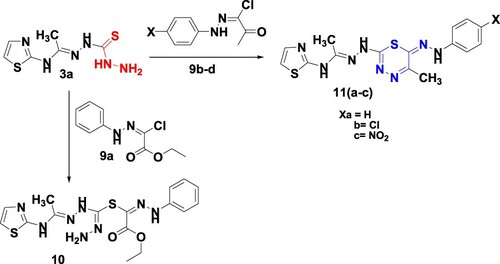
The synthetic pathways for the target compounds are illustrated in Schemes 1–3. Ultrasonic irradiation is a simple method for preparing organic compounds with high yield, pure products, low costs, less energy consumption for chemical transformations and less release of hazardous chemicals to air, as it is solvent-free reactions. Firstly before we move on to the ultrasound-assisted green synthesis of 1,3-thiazoles and 1,3,4-thiadiazines, we would like to refer that, in 2015 Abdel-Aziem reported the synthesis of new derivatives of 1,3-thiazoles and 1,3,4-thiadiazines linked to the coumarin moiety under thermal conditions (traditional method) (Citation32). By applying the ultrasound technique, the reaction is completed in a short time in addition, high yields and pure products are obtained compared to the traditional method. Thus, acetylation of 2-aminothiazole (1) with acetic anhydride by irradiation for 20 min at 70°C under ultrasound irradiation afforded N-(thiazol-2-yl)acetamide (2). The formation of acetyl derivative 2 was confirmed by the 1H NMR spectrum which displayed new signals at 2.14 and 12.06 ppm for CH3 and NH function, besides two doublet signals at 7.17 and 7.44 ppm assigned for H2 and H3 of thiazole, respectively. The reaction of 2 with thiocarbohydrazide (Citation40) in 1:1 and 2:1 ratio under ultrasonic irradiation for 20 min at 70°C furnished the desired mono and bishydrazones 3a,b, respectively, in a good yield (Scheme 1).
The amide nitrogen is poor nucleophile (they do not undergo nucleophilic addition on carbonyl carbon) due to the mesomeric effect (Scheme 1). An iminol nitrogen is much more nucleophilic than the corresponding amide nitrogen (Citation41). Since amides are stable to nucleophilic substitution, they must be activated before the reaction. The addition of acid protonates the amide oxygen converting it to the iminol form. As a result, it is reasonable to suppose that compounds 3a and 3b are produced by protonation of the carbonyl oxygen followed by nucleophilic addition of the thiocarbohydrazide molecule to the positively charged carbonyl carbon. The final step is the formation of the final product via the elimination of water molecules.
Based on spectroscopic data, the structures of the synthesized compounds were verified. The IR spectrum of hydrazones 3a,b demonstrated peaks in the region 3400, 3350, 3270 cm−1 assignable to NH2, and N–H function, additionally to 1260–1282 cm−1 for C═S function. Furthermore, 1H NMR (CDCl3) of 3a showed signals at δ 2.45, 5.84, 7.38, 10.91 and 11.39 related to CH3, NH2 and 3NH protons, correspondingly, besides two doublet signals accounted for thiazole ring. Its 13C NMR spectrum (CDCl3) exhibited signals at 17.13 (CH3), 118, 138, 148, 159 (Ar-C), 171.74 (C=S). On the other side, in the 1H NMR spectrum of bishydrazone 3b, a singlet signal was seen at δ 2.11 ppm for 2CH3 besides two doublet signals at 7.13 and 7.40 ppm for H2 and H3 of the thiazole moiety as well as two singlet signals at 8.35 and 12.02 ppm for 4NH.
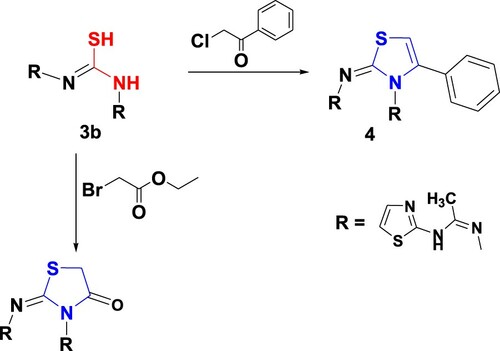
Moreover, the treatment of bishydrazones 3b with phenacyl chloride and/or ethyl bromoacetate in anhydrous sodium acetate under ultrasonic irradiation for 60 min at 60°C afforded 1,3-thiazole 4 and 1,3-thiazolinone 5, respectively (Scheme 2). The spectroscopic data of compounds 4 and 5 supported their structures. In the infrared spectrum of 5 absorption peaks at 3250, 3190, 1710 and 1605 cm−1 were observed, which correspond to 2NH, C=O and C=N, respectively. The 1H NMR of 5 demonstrated a singlet signal at δ 2.11 and 4.33 ppm for two methyl and methylene protons, 7. 13 and 7.40 ppm, two doublet signals for H2 and H3 thiazole and singlet signals at 12.02 for the 2NH function.
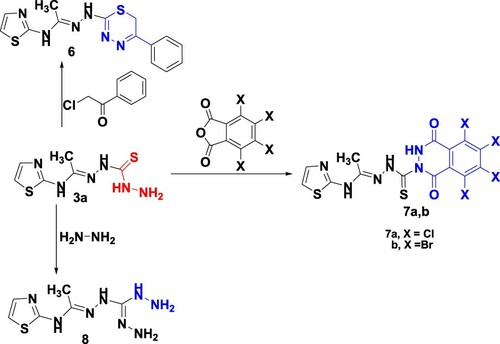
Similarly, monohydrazones 3a was reacted with phenacyl chloride under the previous ultrasonic conditions afforded 1,3,4-thiadiazines 6 based on spectral data. The 1H NMR spectrum of 6 recoded singlet signals at δ 2.14 and 4.99 ppm corresponds to methyl and methylene groups. In addition, two doublet signals at 7.14 and 7.44 ppm are attributed to H2 and H3 of the thiazole moiety besides multiplet at δ 7.56–8.04 ppm for the phenyl group and NH protons, as well as a singlet signal at 12.06 ppm for NH. Also, the reaction of 3a with tetrachloro or tetrabromophthalic anhydrides gave the corresponding phthalazines 7a,b, respectively (Scheme 3). The infrared spectrum of 7a,b displayed characteristic absorption peaks at 1750 and 1760 cm−1 for two carbonyl groups, while the 1H NMR spectrum of 7b recorded a singlet signal at δ 2.11 ppm corresponding to the CH3 group. In addition, two doublet signals at δ 7.14 and 7.44 ppm are attributed to H2 and H3 of thiazole, besides singlet signals at δ 9.60 and 12.02 ppm for 2NH and NH protons. The hydrazinyl hydrazine 8 was prepared from the reaction of monohydrazone 3a with hydrazine hydrate via loss of hydrogen sulphide.
Finally, thiocarbohydrazones 3a was reacted with C-ethoxycarbonyl-N-phenylhydrazonoyl chloride 9a under ultrasound irradiation in triethylamine yielded the condensation product 10 based on its spectral data. The 1H NMR spectrum demonstrated triplet and quartet signals for the ester group at δ 1.18 and 4.16 ppm which proved the open structure of compound 10. In contrast, the ultrasound irradiation of 3a with hydrazonoyl halides 9(b–d) (Citation42–44) furnished one isolable product assigned as 1,3,4-thiadiazines 11(a–c) as outlined in Scheme 4. For instance, the 1H NMR spectrum of 11a revealed signals at δ 2.11, 3.75 and 5.20 ppm for 2CH3 and NH groups, respectively, as well as at triplet signal at δ 6.63 ppm, 7.19–7.42 (m, 4H, ArH's + NH), 7.67 (d,1H, J = 8 Hz, H2-thiazole) and 12.08 ppm (s, NH).
2.2. Antimicrobial activity and (SAR)
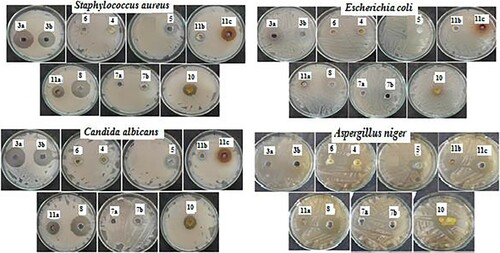
All the newly prepared compounds have been screened for the antimicrobial activity against different tested microbes including Staphylococcus aureus ATCC 6538 (G+ve), Escherichia coli ATCC 25922 (G−ve), Candida albicans ATCC 10231 (yeast), and Aspergillus niger NRRL A-326 (fungus), using the cup diffusion method (Citation45) at a single dose of 250 µg. The antibiotic neomycin and the antifungal cyclohexamide were used as positive controls. The results are shown in and as a growth inhibition zone (mm).
Table 1. In vitro antimicrobial activities of the newly synthesized compounds against pathogenic bacteria and fungi.
2.3. SAR investigation
According to the screening results, monohydrazone 3a demonstrated the most potent activity against all tested microbes, with growth inhibition zones of 33, 31, 35, and 38 mm. Also, compound 8 bearing the hydrazinyl moiety showed substantial action against S. aureus with a 32 mm inhibitory zone, whereas both bishydrazone 3b and 1,3,4-thiadiazine 11c had moderate activity (29 and 25 mm). Thiazole 4, thiazoloinone 5, phthalazine derivative 7b, and 1,3,4-thiadiazine 11a and 11b bearing phenyl or p-chlorophenyl group showed low activities toward S. aureus with inhibition values of 15, 22, 16, 22 and 18 mm, respectively. But, thiadiazine 6 and phthalazine derivative 7a did not have antibacterial activities against S. aureus. Furthermore, thiazole 4 showed potent activity toward E. coli with a growth inhibition zone of 32 mm which was higher than the 30 mm of reference neomycin, but compound 3b was moderately active with growth inhibition zones of 27 mm. Compounds 5, 8, 10, and 11c revealed low activities (14, 23, 22, and 21 mm).
On the other hand, bishydrazone 3b, thiazole 4, hydrazinyl 8, phenyl hydrazo 10, and 1,3,4-thiadiazine bearing p-nitrophenyl group 11c showed significant growth inhibition zones of 36, 35, 30, 35, and 35 mm, respectively, against A. niger compared to the reference drug cyclohexamide (39 mm). Additionally, thiazolinone 5, thiadiazine 6, and 1,3,4-thiadiazine carrying phenyl group 11a were moderately active against A. niger with inhibition zones of 25, 28, and 25 mm, respectively. With respect to C. albicans, bishydrazone 3b and hydrazinyl 8 revealed significant activities with growth inhibition zones of 32 and 31 mm, respectively, whitest, thiazolinone 5 and thiadiazine 11c were moderately active (24 and 24 mm). It was observed that thiadiazine 6 and phthalazine 7a were inactive against all tested microbes except with A. niger compound 6 was moderately active (28 mm).
The following are the findings of the SAR investigation:
It has been noticed that the antimicrobial activity is significantly influenced by different groups attached to thiazolyl moiety. The most active compounds were mono and bishydrazons 3a,b, thiazol 4, hydrazinyl 8, and 1,3,4-thiadiazine bearing p-nitrophenyl group 11c.
The following is the order of reactivity in the case of G+ve bacteria (S. aureus): 3a (33 mm) > 8 (32 mm) > 3b (29 mm) > 11c (25 mm).
In the case of G-ve bacteria (E. coli), the order of reactivity is as follows: 4 (32 mm) > 3a (31 mm) > 3b (27 mm) > 8 (23 mm) > 10 (22 mm) > 11c (21 mm).
With regard to C. albicans, the order of reactivity is: 3a (35 mm) > 3b (32 mm) > 8 (23 mm) > 5, 11c (24 mm) > 11a (22 mm).
For yeast A. niger, the reactivity order is: 3a (38 mm) > 3b (36 mm) > 4, 10, 11c (35 mm) > 6 (28 mm) > 5, 11a (25 mm).
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the promised compounds (Citation46).
The minimum inhibitory concentrations (MIC) and the minimum bactericidal concentrations (MBC) of the promised compounds 3a, 3b, 4, 5, 8, 10, 11a, and 11c were evaluated from the cup diffusion method (, ). The results revealed that compounds 3a, 3b, 8, 10, and 11a showed lower MIC against S. aureus with the values of 39.06, 78.13, 9.77, 39.06, and 39.06 µg/ml, respectively. Meanwhile, compounds 4 and 5 exhibited higher MIC values of 312 µg/ml. For E. coli, most compounds showed higher MIC values compared to S. aureus. Moderate MIC values were recorded for compounds 3a, 3b, and 10 (156 µg/ml), while slightly higher MIC values were reported for compounds 4, 5, and 11c (312 µg/ml). Higher MIC values were found for compounds 10 and 11a (625 µg/ml). Regarding MBC results, it has been found that 3a, 5, and 10 (156, 321 and 312 µg/ml, respectively) exhibited moderate MBC values.
Figure 2. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of promised compounds from the cup diffusion method 3a, 3b, 4, 5, 8, 10, 11a, and 11c against S. aureus (G+ve bacteria) and E. coli (G−ve bacteria).
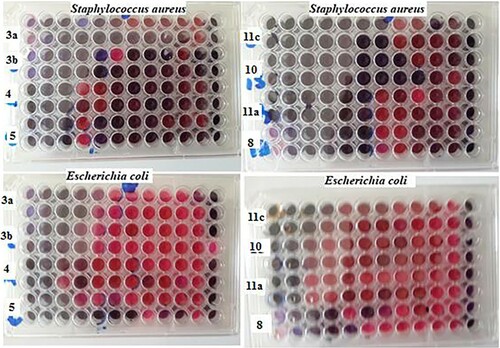
Table 2. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the promised compounds against S. aureus (G+ve bacteria) and E. coli (G−ve bacteria).
From MIC and MBC it is clear that thiazole 4 and thiazolinone 5 demonstrated higher MIC and MBC values against the two tested microbes.
3. Experimental section
All reagents and solvents were of analytical grade. All melting points of the synthesized compounds were measured on a digital Gallen Kamp MFB-595 instrument and maybe uncorrected. A Shimadzu 440 spectrophotometer was utilized for recording the infrared spectra. 1H and 13C NMR spectra of the new derivatives were measured on a Bruker spectrometer at 400 and 100 MHz, respectively, and TMS is the internal standard. The symbols (s) singlet, (d) doublet, (t) triplet, (q) quartet, and (m) multiplet were expressed for hydrogen coupling patterns and chemical shifts (δ) were defined as parts per million (ppm) relative to the solvent peak.
3.1. Synthesis of N-(thiazol-2-yl)acetamide (2)
An equimolar amount of 2-aminothiazole and acetic anhydride was transferred to Pyrex tubes. The mixture was irradiated in a water bath under ultrasonic irradiation at 60°C for 20 min. The reaction progress was monitored through this period using TLC. The formed solid was recrystallized from ethanol affording 2. Pink powder; yield (85%), mp.: 115–16°C; 1H NMR: δ 2.14 (s, 3H, CH3), 7.17 (d,1H, J = 8 Hz, H2-thiazole), 7.44 (d,1H, J = 8.2 Hz, H3-thiazole), 12.06 (s, 1H, NH). Anal. calcd. for C5H6N2OS (142.18): C, 42.24; H, 4.25; N, 19.70; S, 22.55%; found: C, 42.35; H, 4.36; N, 19.61; S, 22.62%
3.2. Synthesis of thiocarbohydrazones 3a,b
A mixture of N-(thiazol-2-yl)acetamide (3) and thiocarbohydrazide (in molar ratio 1:1 or 2:1) were mixed together with a few drops of acetic acid and then transferred to Pyrex tubes. The reaction mass was irradiated in a water bath under ultrasonic irradiation at 60°C for 20 min. The reaction progress was monitored through this period using TLC. The produced solid recrystallized from ethanol furnished 3a, b, respectively.
3.3. 1-(1-(Thiazol-2-ylamino)ethylidene)thiocarbonohydrazide (3a).
Orange powder; yield (77%), mp.: 131–32 °C; IR (KBr): 3303, 3272, 3183 (NH, NH2), 3074, 2983 (CH), 1607(C=N) and 1282 cm−1 (C=S); 1H NMR: δ 2.45 (s, 3H, CH3), 5.84 (s, 2H, NH2), 7.38 (s, 2H, 2NH), 7.47 (d,1H, J = 8.3 Hz, H2-thiazole), 7.82 (d,1H, J = 8.2 Hz, H3-thiazole), 10.92 (s, 1H, NH);13C NMR: δ 17.13 (CH3), 118, 138, 148, 159 (Ar-C), 171.74 (C = S). Anal. calcd. for C6H10N6S2 (230.31): C, 31.29; H, 4.38; N, 36.49; S, 27.84%; found: C, 31.20; H, 4.46; N, 36.40; S, 27.78%.
3.4. 1,5-Bis(1-(thiazol-2-ylamino)ethylidene)thiocarbonohydrazide (3b)
Orange powder; yield (81%); mp.: 168-69°C; IR (KBr): 3340, 3325, 3290 (4NH), 3015, 2990 (CH), 1600 (C=N) and 1265 cm−1 (C=S), 1H NMR: δ 2.11 (s, 6H, 7. 13 (d, 2H, J = 8.3 Hz, H2-thiazole), 7.40 (d, 2H, J = 8.2 Hz, H3-thiazole), 8.35 (s, 2H), 12.02 (s, 2H); 13C NMR: δ 12.99 (2CH3), 118.42, 138.59, 148.28, 159.06 (Ar-C), 173.55 (C=S). Anal. calcd. for C11H14N8S3 (354.48): C, 37.27; H, 3.98; N, 31.61; S, 27.14%; found: C, 37.30; H, 3.99; N, 31.59; S, 27.12%.
3.5. General method for synthesis of 1,3-thiazole 4 and 1,3-thiazolinone 5
An equimolar ratio of 3b, phencyl bromide and/or ethyl bromoacetate (0.01 mol each), and (0.004 mol) anhydrous sodium acetate were mixed together then transferred to Pyrex tubes. After the addition of 3–4 drops of ethanol, the reaction mass was irradiated in a water bath under ultrasonic irradiation at 60°C for 60 min. The reaction progress was monitored through this period using TLC until it is completed. The obtained products 4 and 5 were recrystallized using ethanol.
3.6. N'-(E)-4-phenyl-2-(-(1-(thiazol-2-ylamino)ethylidene)hydrazono)thiazol-3(2H)-yl-N-(thiazol-2-yl)acetimidamide (4)
Orange powder, yield (80%); mp.:226–28°C; IR (KBr): 3275, 3180 (2NH), 3070, 2980 (CH), 1607 cm−1 (C=N); 1H NMR: δ 2.11 (s, 3H), 2.19 (s, 3H), 5.38 (s, 1H), 7.16-7.89 (m, 10H), 11.53 (s, 1H); 13C NMR: δ 12.59, 14.51 (2CH3), 103.26-152.51 (Ar-C). Anal. calcd. for C19H18N8S3 (454.49): C, 50.20; H, 3.99; N, 24.65; S, 21.16%; found: C, 50.23; H, 4.06; N, 24.58; S, 21.22%.
3.7. N'-((E)4-oxo-2-(-(1-(thiazol-2-ylamino)ethylidene)hydrazono)thiazolidin-3-yl)-N-(thiazol-2-yl)acetimidamide (5)
Orange powder, yield (88%); mp.: 252–53°C; IR (KBr): 3410 broad (2NH), 3020, 2990 (CH), 1710 (C=O), 1605 cm−1 (C=N); 1H NMR: δ 2.11 (s, 6H), 3.19 (s, 2H), 7. 13 (d, 2H, J = 8.3 Hz, H2-thiazole), 7.40 (d, 2H, J = 8.2 Hz, H3-thiazole), 12.02 (s, 2H); 13C NMR: δ 13.51, 14.07 (2CH3), 43.49 (CH2), 103.26–152.51 (Ar-C), 171.73 (C=O). Anal. calcd. for C13H14N8OS3 (394.05): C, 39.58; H, 3.58; N, 28.40; S, 24.38%; found: C, 39.69; H, 3.46; N, 28.50; S, 24.49%.
3.8. Synthesis of N'-(5-phenyl-6H-1,3,4-thiadiazin-2-yl)-N-(thiazol-2-yl) acetohydrazonamide (6)
An equimolar ratio of 3a, phencyl bromide (0.01 mol), and (0.004 mol) of anhydrous sodium acetate were mixed together then transferred to Pyrex tubes. After the addition of 3–4 drops of ethanol, the reaction mass was irradiated in a water bath under ultrasonic irradiation at 60°C for 60 min. The reaction progress was monitored through this period using TLC. The orange solid was recrystallized using ethanol afforded 6.
Orange powder; yield (78%); mp.:163–65°C; IR (KBr): 3280, 3190 (2NH), 2990, 2940 (CH), and 1609 cm−1 (C=N); 1H NMR: δ 2.14 (s, 3H), 5.01 (s, 2H), 7.14 (d,1H, J = 8.3 Hz, H2-thiazole), 7.44 (d,1H, J = 8.1 Hz, H3-thiazole), 7.56–7.71 (m, 5H), 8.04 (s, 1H), 12.06 (s, 1H); 13C NMR: δ 16.79 (CH3), 21.96 (CH2), 102.77, 105.80, 117.96, 121.96, 127.60, 128.60, 128.54, 130.55, 143.53, 147.55, 164.73 (Ar-C). Anal. calcd. for C14H14N6S2(330.43): C, 50.89; H, 4.27; N, 25.43; S, 19.41%; found: C, 50.98; H, 4.32; N, 25.54; S, 19.49%.
3.9. General method for synthesis of phthalazines 7a,b
An equimolar ratio of 3a (0.01 mol), tetrachlorophthalic anhydride, or tetrabromophthalic anhydride (0.01 mol) and a catalytic amount of triethylamine were mixed together, then transferred to Pyrex tubes. The reaction mass was irradiated in a water bath under ultrasonic irradiation at 60°C for 60 min. The reaction progress was monitored through this period using TLC. The solid produced was recrystallized from ethanol to give pure products of 7a,b, respectively.
3.10. N'-(5,6,7,8-Tetrachloro-1,4-dioxo-1,2,3,4-tetrahydrophthalazine-2-carbonothioyl)-N-(thiazol-2-yl)acetohydrazonamide (7a)
White powder; yield 65%, mp.: 226–28°C; IR (KBr): 3300, 3250, 3190 (3NH), 3010, 2890 (CH), 1750, 1760 (2C=O), 1609 (C=N), 1250 (C=S), 740 cm−1 (C-Cl); 1H NMR: δ 2.11 (s, 3H), 8.09 (d,1H, J = 8 Hz, H2-thiazole), 8.16 (d,1H, J = 8 Hz, H3-thiazole), 8.28 (s, 1H), 8.42 (s, 1H), 11.73 (s, 1H). Anal. calcd. for C14H8Cl4N6O2S2 (498.19): C, 33.75; H, 1.62; Cl, 28.47; N, 16.87; S, 12.87%; found: C, 33.84; H, 1.73; Cl, 28.56; N, 16.78; S, 12.79%.
3.11. N'-(5,6,7,8-Tetrabromo-1,4-dioxo-1,2,3,4-tetrahydrophthalazine-2-carbonothioyl) -N-(thiazol-2-yl)acetohydrazonamide (7b)
Yellowish white powder; yield 70%, mp.: 287–89°C; IR (KBr): 3300, 3200, 3180 (3NH), 3005, 2990 (CH), 1750, 1760 (2C=O), 1600 (C=N), 1260 (C=S),740 cm−1 (C–Br); 1H NMR: δ 2.11 (s, 3H,), 7.14 (d,1H, J = 8 Hz, H2-thiazole), 7.44 (d,1H, J = 8 Hz, H3-thiazole), 8.50 (s, 1H), 9.60 (s, 1H), 12.03 (s, 1H). Anal. calcd. for C14H8Br4N6O2S2(676.69): C, 24.87; H, 1.19; Br, 47.28; N, 12.43; S, 9.49%; found: C, 24.95; H, 1.24; Br, 47.36; N, 12.50; S, 9.59%.
3.12. Synthesis of hydrazine derivative 8
A mixture of 3a (0.01 mol) and (0. 1 mol) of hydrazine hydrate was mixed together then transferred to Pyrex tubes. After the addition of 3–4 drops of ethanol, the reaction mass was irradiated in a water bath under ultrasonic irradiation at 60 °C for 60 min. The reaction progress was monitored through this period using TLC. The formed solid was recrystallized from ethanol to give 8.
Yellowish white powder; yield (72%), mp.: 168–69°C; IR (KBr): 3300, 3200, 3180 (3NH), 3005, 2990 (CH), 1600 (C=N), 1260 cm−1 (C = S); 1H NMR: δ 2.11 (s, 3H), 5.36 (s, 1H), 7.16 (d,1H, J = 6.10 Hz, H2-thiazole), 7.20-7.42 (m, 4H, H3-thiazole + NH-NH2), 7.75 (s, 2H), 7.87 (s, 1H). Anal. calcd. For C6H12N8S(228.28): C, 31.57; H, 5.30; N, 49.09; S, 14.05%. found: C, 31.53; H, 5.35; N, 49.04; S, 14.01%.
3.13. Synthesis of compounds 10 and 11 (a–c)
An equimolar ratio of 3a (0.01 mol), the appropriate hydrazonoyl halides 9a–d (0,01 mol) and three drops of triethylamine were mixed together and then transferred to Pyrex tubes. The reaction mass was irradiated in a water bath under ultrasonic irradiation at 60°C for 60 min. The reaction progress was monitored through this period using TLC. The product was recrystallized from ethanol to give pure products of 10, 11 (a–c), respectively.
3.14. 2-Ethoxy-2-oxo-N'-phenylacetohydrazonic-2-(1-(thiazol-2-ylamino)ethylidene) hydrazinecarbohydrazonic thioanhydride (10)
Orange powder; yield (75%), mp.: 176–77°C; IR (KBr): 3420, 3350, 3220, 3200, 3150 (3NH + NH2), 3010, 2990 (CH), 1740 (C=O ester),1605 cm−1 (C = N); 1H NMR: δ 1.18 (t, 3H, J = 7 Hz), 2.14 (s, 3H), 4.16 (q, 2H, J = 7 Hz), 7.0–7.04 (m, 3H), 7.14 (d,1H, J = 8.2 Hz, H2-thiazole), 7.33–7.39 (m, 4H + NH2), 7.44 (d,1H, J = 8.1 Hz, H3-thiazole), 9.80 (s, 1H), 10.82 (s, 1H), 11.38 (s, 1H). Anal. calcd. for C16H20N8O2S2(420.51):C, 45.70; H, 4.79; N, 26.65; S, 15.25%; found: 45.83; H, 4.68; N, 26.52; S, 15.36%.
3.15. N'-(5-methyl-6-(2-phenylhydrazono)-6H-1,3,4-thiadiazin-2-yl)-N-(thiazol-2-yl)acetohydrazonamide (11a)
Orange powder; yield (75%), mp.: 182–84°C; IR (KBr): 3220, 3190 (3NH), 2990, 2890 (CH), 1590 cm−1 (C=N); 1H NMR: δ 2.11 (s, 3H), 3.75 (s, 3H), 5.20 (s, 1H), 6.63 (t, 1H, J = 7.6 Hz), 6.75 (d,1H, J = 8.2 Hz), 7.19–7.42 (m, 4H + NH), 7.67 (d,1H, J = 8.2 Hz, H2-thiazole), 12.08 (s, 1H); 13C NMR: δ 13.45, 29.73 (2CH3), 102.40–159.84 (Ar-C). Anal. calcd. for C15H16N8S2 (372.42): C, 48.37; H, 4.33; N, 30.08; S, 17.22%; found: C, 48.40; H, 4.33; N, 30.07; S, 17.20%.
3.16. N'-(6-(2-(4-chlorophenyl)hydrazono)-5-methyl-6H-1,3,4-thiadiazin-2-yl)-N-(thiazol-2-yl)acetohydrazonamide (11b)
Orange powder; yield (70%), mp.: 172–74°C; IR (KBr): 3400 broad (3NH), 2980 (CH), 1608 cm−1 (C=N); 1H NMR: δ 2.10 (s, 3H), 3.69 (s, 3H), 5.37 (s, 1H), 7.17 (t, 1H, J = 8.1 Hz), 7.38–7.43 (m, 2H + NH), 7.67–7.71 (m, 2H), 7.78 (d,1H, J = 8.3 Hz, H2-thiazole), 11.61 (s, 1H, NH-thiazole); 13C NMR: δ 14.17, 29.73 (2CH3), 121.18–161.70 (Ar-C). Anal. calcd. for C15H15N8ClS2 (406.92): C, 44.27; H, 3.72; Cl, 8.71; N, 27.54; S, 15.76%; found: C, 44.25; H, 3.75; Cl, 8.70; N, 27.55; S, 15.77%.
3.17. N'-(E)-5-methyl-6-(2-(4-nitrophenyl)hydrazono)-6H-1,3,4-thiadiazin-2-yl-N-(thiazol-2-yl)acetohydrazonamide (11c)
Orange powder; yield (75%), mp.: 159–60°C; IR (KBr): 3300, 3220 (3NH), 2990, 2850 (CH), 1600 cm−1 (C=N); 1H NMR: δ 1.97 (s, 3H), 2.10 (s, 3H), 7.67 (t, 1H, J = 7.4 Hz), 7.24–7.44 (m, 4H + NH), 7.52 (s,1H), 7.93 (d, 1H, J = 8.1 Hz), 8.10 (d, 1H, J = 8.3 Hz), 11.54 (s, 1H). Anal. calcd. for C15H15N9O2S2 (417.47): C, 43.16; H, 3.62; N, 30.20; S, 15.36%; found: C, 43.20; H, 3.61; N, 30.19; S, 15.35%.
Conclusions
Considering the ultrasound irridiation as a green, environmentally friendly and as an efficient organic chemical methodology, we reported herein the synthesis of some new 1,3-thiazole and 1,3,4-thiadiazine derivatives by utilizing the ultrasound irradiation technique. All the new compounds have been screened for antimicrobial activity against different tested microbes. Most of the newly synthesized compounds exhibited potent to moderate activities toward the tested microbes. The MIC and MBC values were found to be acceptable for the most synthesized compounds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cravotto, G.; Cintas, P. Power Ultrasound in Organic Synthesis: Moving Cavitational Chemistry from Academia to Innovative and Large-Scale Applications. J. Chem. Soc. 2006, 35 (2), 180–196.
- Muravyova, E.A.; Desenko, S.M.; Musatov, V.I.; Knyazeva, I.V.; Shishkina, S.V.; Shishkin, O.V.; Chebanov, V.A. Ultrasonic-Promoted Three-Component Synthesis of Some Biologically Active 1,2,5,6-Tetrahydropyrimidines. J. Comb. Chem. 2007, 9, 797.
- Li, J.T.; Yin, Y.; Li, L.; Sun, M.X. A Convenient and Efficient Protocol for the Synthesis of 5-Aryl-1,3-Diphenylpyrazole Catalyzed by Hydrochloric Acid Under Ultrasound Irradiation. Ultrason. Sonochem. 2010, 17, 11.
- Banaei, A.; Salmanpour, H.; Karimi, S. Ultrasound Irradiation Promoted Synthesis of Bispyrimidine and Bispyrazolines as Selective Adsorbents for the Selective Removal of Ag(I) and Pb(II) from Aqueous Solutions. Monatsh Chem. 2017, 148, 683.
- Lu, C.W.; Wang, J.J.; Li, F.; Yu, S.J.Y. Efficient Synthesis of 2-Amino-3-Cyano-4H-Pyran Derivatives via a non-Catalytic one-pot Three-Component Reaction. Res. Chem. Intermed. 2018, 44, 1035.
- Safari, J.; Tavakoli, M.; Ghasemzadeh, M.A. Ultrasound-promoted an Efficient Method for the One-pot Synthesis of Indeno Fused Pyrido[2,3-d]Pyrimidines Catalyzed by H3PW12O40 Functionalized Chitosan@Co3O4 as a Novel and Green Catalyst. J. Organomet. Chem. 2019, 880, 75.
- Maddila, S.N.; Maddila, S.; Khumalo, M.; Bhaskaruni, S.V.; Jonnalagadda, S.B. An Eco-Friendly Approach for Synthesis of Novel Substituted 4H-Chromenes in Aqueous Ethanol Under Ultra-Sonication with 94% Atom Economy. J. Mol. Struct. 2019, 5, 357.
- Martins, M.A.P.; Pereira, C.M.P.; Cunico, W.; Moura, S.; Rosa, F.A.; Peres, R.L.; Machado, P.; Zanatta, N.; Bonacorso, H.G. Ultrasound Promoted Synthesis of 5-Hydroxy-5-Trihalomethyl-4,5-Dihydroisoxazoles and β-Enamino Trihalomethyl Ketones in Water. Ultrason. Sonochem. 2006, 13, 364.
- Rouhani, M.; Ramazani, A.; Joo, S.W. Novel, Fast and Efficient one-pot Sonochemical Synthesis of 2-Aryl-1,3,4-Oxadiazoles. Ultrason. Sonochem. 2014, 21, 262.
- Sayed, A.R.; Gomha, S.M.; Taher, E.A.; Muhammad, Z.A.; El-Seedi, H.R.; Gaber, H.M.; Ahmed, M.M. One-Pot Synthesis of Novel Thiazoles as Potential Anti-Cancer Agents. Drug. Des. Devel. Ther. 2020, 14, 1363.
- Gomha, S.M.; Abdelhamid, A.O.; Kandil, O.M.; Kandeel, S.M.; Abdelrehem, N.A. Synthesis and Molecular Docking of Some Novel Thiazoles and Thiadiazoles Incorporating Pyranochromene Moiety as Potent Anticancer Agents. Mini Rev. Med. Chem. 2018, 18, 1670.
- Gomha, S.M.; Abdel-Aziz, H.M.; Badrey, M.G.; Abdulla, M.M. Efficient Synthesis of Some New 1,3,4-Thiadiazoles and 1,2,4-Triazoles Linked to Pyrazolylcoumarin Ring System as Potent 5α-Reductase Inhibitors. J. Heterocycl. Chem. 2019, 56, 1275.
- Gomha, S.M.; Abdelhady, H.A.; Hassain, D.Z.H.; Abdelmonsef, A.H.; El-Naggar, M.; Elaasser, M.M.; Mahmoud, H.K. Thiazole-Based Thiosemicarbazones: Synthesis, Cytotoxicity Evaluation and Molecular Docking Study. Drug Des, Devel Ther 2021, 15, 659.
- Łączkowski, K.Z.; Anusiak, J.; Świtalska, M.; Dzitko, K.; Cytarska, J.; Baranowska-Łączkowska, A.; Plech, T.; Paneth, A.; Wietrzyk, J.; Białczyk, J. Synthesis, Molecular Docking, ctDNA Interaction, DFT Calculation and Evaluation of Antiproliferative and Anti-Toxoplasma Gondii Activities of 2,4-Diaminotriazine-Thiazole Derivatives. Med. Chem. Res. 2018, 27, 1131.
- Prashanth, T.; Avin, B.R.V.; Thirusangu, P.; Ranganatha, V.L.; Prabhakar, B.T.; Chandra, J.N.N.S.; Khanum, S.A. Synthesis of Coumarin Analogs Appended with Quinoline and Thiazole Moiety and Their Apoptogenic Role Against Murine Ascitic Carcinoma. Biomed. Pharmacother. 2019, 112, 108707.
- Anuradha, P.S.; Patle, R.; Parameswaran, P.; Jain, A.; Shard, A. Design, Computational Studies, Synthesis and Biological Evaluation of Thiazole-Based Molecules as Anticancer Agents. Eur. J. Pharm. Sci. 2019, 134, 20.
- Siddiqui, N.; Arya, S.K.; Ahsan, W.; Azad, B. International Journal of Drug Development & Research. October-December 2011. Vol. 3. Issue 4. ISSN 0975-9344| Available Online Http://www. Ijddr. in Covered in Official Product of Elsevier, The Netherlands© 2010 IJDDR. Int J Drug Dev Res 2011, 3 (4), 55.
- Mohareb, R.M.; Zaki, M.Y.; Abbas, N.S. Synthesis, Anti-Inflammatory and Anti-Ulcer Evaluations of Thiazole, Thiophene, Pyridine and Pyran Derivatives Derived from Androstenedione. Steroids 2015, 98, 80.
- Eryılmaz, S.; Çelikoğlu, E.T.; İdil, O.; İnkaya, E.; Kozak, Z.; Mısır, E.; Gül, M. Derivatives of Pyridine and Thiazole Hybrid: Synthesis, DFT, Biological Evaluation via Antimicrobial and DNA Cleavage Activity. Bioorg. Chem. 2020, 95, 103476.
- Meghdadi, S.; Amirnasr, M.; Yavari, E.; Mereiter, K.; Bagheri, M. Synthesis, Characterization, and X-ray Crystal Structures of Copper(I) Halide and Pseudohalide Complexes with 2-(2-Quinolyl)Benzothiazole. Diverse Coordination Geometries and Electrochemical Properties. C. R. Chim 2017, 20 (7), 730.
- Biernasiuk, A.; Kawczyńska, M.; Berecka-Rycerz, A.; Rosada, B.; Gumieniczek, A.; Malm, A.; Dzitko, K.; Łączkowski, K.Z. Synthesis, Antimicrobial Activity, and Determination of the Lipophilicity of ((Cyclohex-3-Enylmethylene)Hydrazinyl)Thiazole Derivatives. Med. Chem. Res. 2019, 28, 2023.
- Ansari, A.; Ali, A.; Asif, M.; Rauf, M.A.; Shamsuzzaman, O.M. Facile One-Pot Multicomponent Synthesis and Molecular Docking Studies of Steroidal Oxazole/Thiazole Derivatives with Effective Antimicrobial, Antibiofilm and Hemolytic Properties. Steroids 2018, 134, 22.
- Ouf, S.A.; Gomha, S.M.; Ewies, M.M.; Sharawy, I.A.A. Synthesis, Characterization, and Antifungal Activity Evaluation of Some Novel Arylazothiazoles. J. Heterocycl. Chem. 2018, 55 (1), 258.
- Biernasiuk, A.; Kawczyńska, M.; Berecka-Rycerz, A.; Rosada, B.; Gumieniczek, A.; Malm, A.; Dzitko, K.; Łączkowski, K.Z. Synthesis, Antimicrobial Activity, and Determination of the Lipophilicity of ((Cyclohex-3-Enylmethylene)Hydrazinyl)Thiazole Derivatives. Med. Chem. Res. 2019, 28, 2023.
- Ansari, A.; Ali, A.; Asif, M.; Rauf, M.A.; Shamsuzzaman, O.M. Facile One-Pot Multicomponent Synthesis and Molecular Docking Studies of Steroidal Oxazole/Thiazole Derivatives with Effective Antimicrobial, Antibiofilm and Hemolytic Properties. Steroids 2018, 134, 22.
- Pricopie, A.-I.; Ionut, I.; Marc, G.; Arseniu, A.-M.; Vlase, L.; Grozav, A.; Gaina, L.I.; Vodnar, D.C.; Pîrnau, A.; Tiperciuc, B.; Oniga, O. Design and Synthesis of Novel 1,3-Thiazole and 2-Hydrazinyl-1,3-Thiazole Derivatives as Anti-Candida Agents: In Vitro Antifungal Screening, Molecular Docking Study, and Spectroscopic Investigation of Their Binding Interaction with Bovine Serum Albumin. Molecules 2019, 24, 3435.
- Asif, M.; Ali, A.; Zafar, A.; Farhan, M.; Khanam, H.; Hadi, S.M. Microwave-assisted One Pot Synthesis, Characterization, Biological Evaluation and Molecular Docking Studies of Steroidal Thiazoles. J. Photobiol. B Biol. 2017, 166, 104.
- Abumelha, H.M.A. Synthesis and Antioxidant Assay of New Nicotinonitrile Analogues Clubbed Thiazole, Pyrazole and/or Pyridine Ring Systems. J. Heterocycl. Chem. 2020, 57, 1011.
- Iqbal, A.M.; Khan, A.Y.; Kalashetti, M.B.; Belavagi, N.S.; Gong, Y.D.; Khazi, I.A.M. Synthesis, Hypoglycemic and Hypolipidemic Activities of Novel Thiazolidinedione Derivatives Containing Thiazole/Triazole/Oxadiazole Ring. Eur. J. Med. Chem. 2012, 53, 308.
- Paudel, Y.N.; Ali, M.R.; Shah, S.; Adil, M.; Akhtar, M.S.; Wadhwa, R.; Bawa, S.; Sharma, M. 2-[(4-Chlorobenzyl) Amino]-4-Methyl-1,3-Thiazole-5-Carboxylic Acid Exhibits Antidiabetic Potential and Raises Insulin Sensitivity via Amelioration of Oxidative Enzymes and Inflammatory Cytokines in Streptozotocin–induced Diabetic Rats. Biomed. Pharmacother. 2017, 89, 651.
- Siddiqui, N.; Ahsan, W. Triazole Incorporated Thiazoles as a New Class of Anticonvulsants: Design, Synthesis and in Vivo Screening. Eur. J. Med. Chem. 2010, 45 (4), 1536.
- Abdel-Aziem, A. Synthesis and Antimicrobial Activity of Some Novel Thiazoles, 1,3,4-Thiadiazines, 1,3,4-Thiadiazoles Incorporating Coumarin Moiety. J. Heterocycl. Chem. 2015, 52, 251.
- Shubakar, K.; Umesha, K.B.; Srikantamurthy, N. Synthesis and Antimicrobial Evaluation of Novel Derivatives of 1, 3, 4-Thiadiazine Incorporated with Pyrazole-4-Carboxylic Acid Moiety. J. Chethan. Bulgarian Chem. Comm. 2013, 45 (3), 274.
- Puthiyapurayil, P.; Poojary, B.; Chikkanna, C.; Buridipad, S.K. Synthesis, Spectral Characterization and Biological Evaluation of a Novel Series of 6-Arylsubstituted-3-[2-(4-Substitutedphenyl)Propan-2-yl]-7H-[1,2,4]Triazolo[3,4-b][1,3,4]Thiadiazines. Eur. J. Med. Chem. 2012, 57, 407.
- Abdel-Aziem, A. Utility of 2-Acetyl Benzofuran for the Synthesis of New Heterocycles as Potential Anticancer Agents. J. Heterocycl. Chem. 2017, 54, 2985.
- Khan, I.; Zaib, S.; Ibrar, A.; Rama, N.H.; Simpson, J.; Iqbal, J. Synthesis, Crystal Structure and Biological Evaluation of Some Novel 1,2,4-Triazolo[3,4-b]-1,3,4-Thiadiazoles and 1,2,4-Triazolo[3,4-b]-1,3,4-Thiadiazines. Eur. J. Med. Chem. 2014, 78, 167.
- Abdel-Aziem, A.; Rashdan, H.R.M.; Mohamed Ahmed, E.; Shabaan, S.N. Synthesis and Cytotoxic Activity of Some Novel Benzocoumarin Derivatives Under Solvent Free Conditions. Green Chem. Lett. Rev. 2019, 12 (1), 9.
- Abdel Aziem, A.N.H.A.R. An Efficient and Simple Synthesis of 2, 3-Dihydro-1, 3, 4-Thiadiazoles, Pyrazoles and Coumarins Containing Benzofuran Moiety Using Both Conventional and Grinding Methods. Int. J. Pharm. Sci. 2015, 7 (1 ), 61–68.
- Abdel-Aziem, A.; Abdelhamid, A.O. Int. J. Adv. Res. 2013, 1 (9), 717.
- Zhou, J.; Wu, D.; Gou, D.J. Optimization of the Production of Thiocarbohydrazide Using the Taguchi Method. Chem. Technol. Biotechnol. 2010, 85 (10), 1402.
- Takahashi, O.; Kirikoshi, R. Intramolecular Cyclization of Aspartic Acid Residues Assisted by Three Water Molecules: A Density Functional Theory Study. Comput. Sci. Discovery 2014, 7, 015005.
- Asiri, A.M.; Al-Youbi, A.O.; Zayed, M.E.M.; Ng, S.W. 1-Chloro-1-[(4-chlorophenyl)hydrazinylidene]propan-2-one. Acta. Cryst. Sec. E Struct. Rep. 2011, 67 (Pt 8), o1962.
- Eweiss, N.F.; Osman, A. Synthesis of Heterocycles. Part II. New Routes to Acetylthiadiazolines and Alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713.
- Abdelhamid, A.O.; El-Shiatey, F.H.H. Reactions with Hydrazidoyl Halides ii [1]: Synthesis and Reactions of 2-Bromothienylglyoxal-2-Phenylhydrazone. Phosphorus Sulfur Silicon Relat. Elem. 1988, 39, 45.
- Maigali, S.S.; Abd-El-Maksoud, M.A.; El-Hussieny, M.; Soliman, F.M.; Abdel-Aziz, M.S.; Shalaby, E.S.M. Chemistry of Phosphorus Ylides: Part 41 Synthesis of Antimicrobial Agents from the Reaction of Aminoantipyrine, Coumarin- and Quinoline-Carbaldehyde with Phosphacumulene and Phosphaallene Ylides. J. Chem. Res. 2014, 38 (12), 754.
- Barry, A.L. The Antimicrobial Susceptibility Test, Principle and Practices, 4th ed.; ELBS: London, 1976; p. 180.