 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
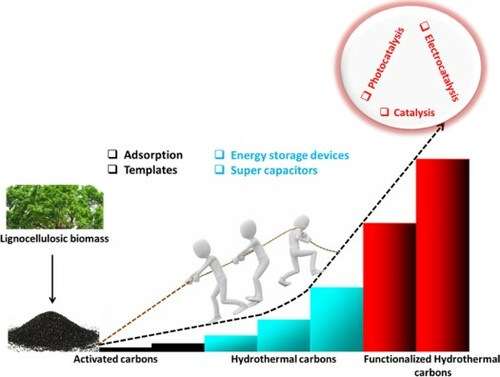
The growing demand for alternative green energy and environmental crises is a significant concern for the world population. Thus, researchers have devoted effort to finding cheap, eco-friendly, and robust functional materials. The bottleneck faced by scientists is the synthesis of a material with high surface area, highly functionalized, and cost-effective. To this end, biomass-based hydrothermal carbons are gaining increasing attention due to the presence of functional groups introduced during hydrothermal carbonization (HTC) and mild/tunable preparation conditions. This review Materials the synthesis, parameters that influence the carbonization process, and mechanisms involved during HTC. Other synthesis routes for enhancing the properties of HTC carbon materials are also discussed. The application of HTC carbon materials, including energy storage devices, catalysis, electrocatalysis, photocatalysis, and adsorption, are reported. Lastly, we present the challenges and possible strategies for improving the HTC process.
GRAPHICAL ABSTRACT
KEYWORDS:
1. Introduction
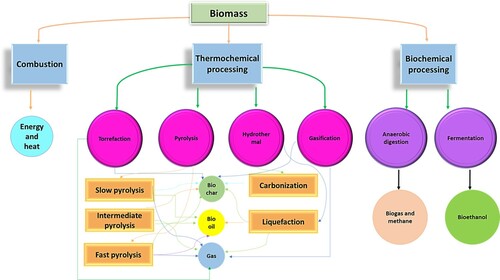
Biomasses are substances formed during photosynthesis in the presence of carbon dioxide (CO2), water, and sunlight (Citation1). There has been tremendous interest in biomass research due to its ability to potentially diminish the effect of carbon dioxide from the energy sector. Contrary to the burning of fossil fuel, which emits CO2, a global warming gas, only the absorbed CO2 in the plant during photosynthesis is released during the combustion of biomass; thus, it is regarded as a carbon-neutral process since no additional CO2 is released into the environment (Citation1). Biomass is of significant benefits and has been demonstrated as a source of renewable raw material and useful in carbon sequestration, energy generation, and improvement of soil fertility (Citation2). However, the most significant drawback to its broad range of applications emanates from a high level of heterogeneity, low mass density, and high moisture content (Citation2, Citation3). Several thermochemical processes, biological processes, and combustion can improve the properties and application of biomass to turn it into products with higher levels of carbon content than the original biomass under anaerobic conditions, as shown in (Citation4). Pyrolysis is one of the oldest thermochemical processes for transforming biomass into usable fuel products, such as oil, gas, and charcoal. It involves the thermal decomposition of biomass in the absence of air at high temperatures of 300–950°C, resulting in unique structures with specific surface areas and high porosity. Pyrolysis is most suitable for biomass possessing a low amount of water, thus giving rise to a high product yield (Citation1, Citation2). However, hydrothermal carbonization (HTC) becomes a method of interest when the biomass contains high moisture.
Figure 1. Several processing strategies for enhancing the properties and application of biomass (Reproduced from reference (Citation4) with permission from The American Chemical Society).
HTC is thermodynamically advantageous as it is performed at a lower temperature range of 150–350°C for the thermochemical decomposition of wet biomasses, such as sewage sludge, municipal organic waste lignocellulosic containing water generating gases, and a solid lignite-like char. The solid lignite-like char is called hydrochar, which serves as fuel and may be processed further to advanced carbon materials (Citation5). The biomass structure is modified by several reactions such as hydrolytic cleavage of ester and ether bonds, dehydration, and decarboxylation. In general, these reactions give rise to the formation of a tangible product that is more brittle than the original material with improved energy density and increased hydrophobicity (Citation6). Because of (i) the economy of synthesis (lower temperature and pressure) and (ii) the propensity to functionalize the end product, these biomass-based hydrothermal carbons have attracted the immense attention of most researchers in many fields and are considered to be promising for a wide array of applications.
This review reports the synthesis route of biomass-based HTC carbons and the reaction mechanisms involved. Some factors that affect the HTC process are discussed. Furthermore, synthesis routes of biomass carbonization and the various strategies for enhancing HTC are presented. The versatility of the biomass-based HTC carbons is revealed through their applications in energy storage devices, photocatalysis, electrocatalysis, and adsorption. Some of the drawbacks of the HTC are also discussed, and possible approaches to improving these are proposed.
2. Synthesis of biomass-based HTC carbons
In the quest for a reliable and green energy source, biomass conversion into forms of high caloric value has been an attractive field of immense research. Several strategies such as pyrolysis, torrefaction, or HTC have been employed for adding value to biomass through chemical and physical transformation (Citation2). The fundamental disparity amongst these methods lies in the medium of reaction used. Torrefaction is pyrolysis carried out under a milder condition at a temperature range of 250–350°C, basically enhancing the caloric value of carbonized biomass by removing moisture and degradation of reactive organic constituents. The torrefaction (drying-up) of fuel-wood is a mild pyrolysis process carried out at 250–350°C (Citation7). This process aims to increase the heat value by removing moisture and decomposing the highly reactive components of organic matter, primarily hemicellulose. Torrefied products are grayish coal with higher caloric values (Citation7).
Furthermore, the torrefied products are most resistant to rot and spontaneous combustion than their source material. While pyrolysis is carried under a high temperature in the absence of water, HTC takes place in the presence of water. In other words, HTC is more suitable for wet biomass. HTC requires pressure at least at a level above the saturated vapor pressure of water to ensure the water in the HTC reactor is maintained in a liquid state (Citation7). Bergius was one of the first to apply hydrothermal carbonization to various organic feedstocks (Citation8). The key advantage of the HTC over other synthesis methods for nanostructured carbonaceous materials is the applicability of cheap and environmentally friendly renewable biomass as carbon source (Citation9, Citation10). HTC is sometimes referred to as cold carbonization. Biomass-containing water is subjected to a temperature range of 180–220°C and a pressure of 25 atm under an anaerobic condition that subsequently gives rise to the formation of hydrocoal (Citation7). During hydrothermal carbonization, biomass, hemicellulose, and cellulose constituents are converted by hydrolysis into oligomers and monomers, while the portion of lignin is unaffected (Citation11). To boost the yield and alter the composition of hydrocoal formed, additives such as acids or bases are sometimes added to catalyze the hydrolysis reaction. The equation for HTC using glucose as a source of carbon can be presented as follows (Citation7):
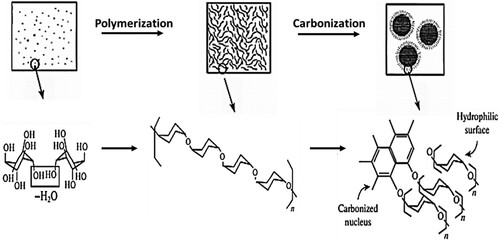
Most researchers have concluded that hydrolysis is the first step of the HTC mechanism; the ether and glycosidic ester bonds in hemicellulose or cellulose react with water to form degraded products, including oligomers (Citation12). The hydrolysis temperature of pristine hemicellulose and pure cellulose are approximately 180 and 230°C, respectively (Citation12, Citation13). Oligomers obtained from the decomposition of cellulose further hydrolyze into glucose and fructose. On the other hand, a fragment of pure lignin at a higher temperature (260°C) degrades and releases phenol and phenol derivatives (Citation7). A scheme representing HTC of glucose as biomass is shown in (Citation14). The remarkable decrease in the oxygen content of biomass is associated with the removal of carboxyl groups, principally from extractive substances, hemicellulose, and cellulose. The hydrolyzed products are further converted into 5-hydroxymethylfurfural (5-HMF), furfurals, aldehydes, and erythrose, with subsequent dehydration and decarboxylation (Citation13).
Figure 2. Diagram of the hydrothermal carbonization of glucose (Reproduced from reference (Citation7) with permission from Springer).
Having highlighted the synthesis and reaction conditions for biomass-based HTC materials, it is essential to discuss the various steps involved for better understanding of the formation of hydrochar, which will enable control of the reaction parameters needed for the formation of desirable products.
3. Mechanisms for biomass-based hydrothermal carbons formation
3.1. Hydrolysis
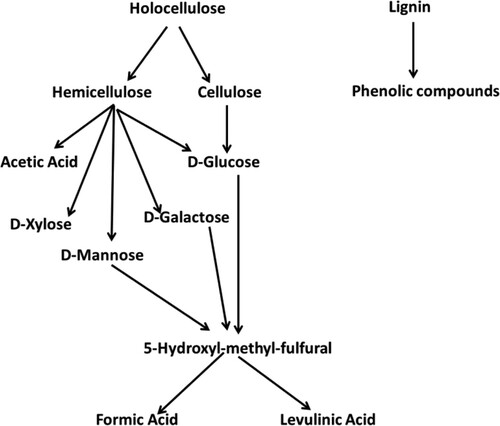
The hydrolytic reaction results in the addition of water molecules across ester and ether bonds, leading to cleavage into a wide variety of cellulosic oligosaccharides and fragments of lignin. For instance, hemicellulose hydrolysis yields acetic acid, D-xilose, D-manose, D-galactose, and D-glucose, as shown in . D-mannose, D-galactose, and D-glucose are transformed into 5-hydroxy-methyl-furfural (5-HMF) and then into formic or levulinic acid (Citation15).
Figure 3. Degradation products and sub-products during hydrolysis of lignocellulosic biomass (Reproduced from reference (Citation15) with permission from Elsevier).
The detailed reaction of the hydrolysis of cellulose subjected to hydrothermal conditions beyond roughly 200°C is reported in the literature (Citation16). Hemicellulose is known to hydrolyze at a lower temperature (180°C); however, a comprehensive understanding of the reaction pathways is still elusive. Contrary, a higher temperature of approximately 200°C is required to degrade lignin due to the predominant presence of ether bonds (Citation17–19).
3.2. Decarboxylation
In decarboxylation reaction, biomass losses part of its carboxyl groups during HTC. Subsequently, the fragmented carboxyl groups and any carbonyl group present quickly degrade above 150°C, forming CO2 and CO, correspondingly (Citation20–25). To date, the effect of water in the reaction and the mechanism are still not fully understood (Citation16). Understanding the formation of CO2 is more rational than a loss of carboxyl group to explain the decarboxylation mechanism. Different possible origins of CO2 include the hydrothermal degradation and decomposition of cellulose, degradation of formic acid, bond cleavages, and condensation reactions (Citation25–29).
3.3. Dehydration
Dehydration of biomass yields products with lower H/C and O/C ratios due to the elimination of water from the biomass matrix without altering its chemical composition (Citation22). Under mild experimental hydrothermal conditions, dehydration may occur without substantial decarboxylation (Citation11, Citation26). The decarboxylation to dehydration ratio is described as between 0.2 for cellulose and 1 for lignite. Under subcritical conditions, it is mostly independent of temperature. Most of the published findings affirm this order of magnitude of ‘r.’ The dehydration rate is known to be faster than decarboxylation during hydrothermal carbonization (Citation11).
3.4. Aromatization
The 13C-NMR is a useful technique to measure the increase in the aromaticity of carbon materials due to hydrothermal carbonization under increasingly reaction conditions. Aromaticity is enhanced under alkaline conditions (Citation30). It is postulated that hydrothermal treatment shows an inverse relationship with the number of aromatic bonds observed in lignin, which possesses a substantial number of aromatic structures, as shown in (Citation11). Regardless of the reaction medium (hydrous or non-hydrous), hemicellulose can be transformed into aromatic structures composed of carbohydrates (Citation11).
Figure 4. Change of carbon content with conversion of biomasses during hydrothermal carbonization (Reproduced from reference (Citation11)).
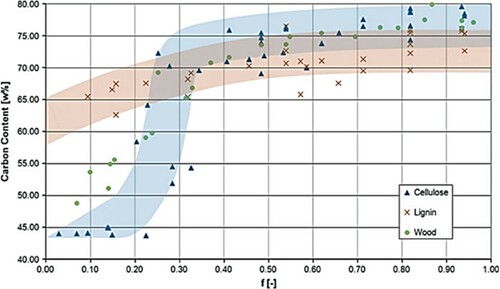
Aromatic structures may be considered the primary backbone of HTC-coal since they exhibit remarkable stability under hydrothermal conditions (Citation31). The close relationship between hydrothermal carbonization and natural coalification may be tied to the cross-linking condensation of aromatic ring structures (Citation31). A good number of literature have reported similarities in chemical structure features between raw coal and HTC-coal (Citation22, Citation32, Citation33).
3.5. Polymerization
The fragments of unsaturated compounds formed from the removal of hydroxyl and carboxyl groups usually undergo polymerization (Citation22). The CO2 may be expelled during condensation reactions. The condensation polymerization, particularly aldol condensation, may be used to characterize the formation of HTC-coal during hydrothermal carbonization reaction (Citation30, Citation34). The low polymerization rate of condensation polymerization may be associated with the step-growth pattern during polymerization (Citation35). Generally, the polymerization of highly reactive species such as the fragments of lignin occurs in minutes beyond 300°C. However, the same reaction may take months to polymerize if carried out under ambient temperature (Citation36, Citation37). A comparatively much slower condensation reaction has been reported for monosaccharides due to the competition between re-condensation to oligosaccharides and cross-linked polymerization (Citation38). The understanding of the elementary steps involved in the polymerization during hydrothermal carbonization progress is principally missing. It is considered an unwanted side reaction that leads to the formation of a solid precipitate. Overall, the HTC process is influenced mainly by the reaction parameters including reaction temperature, phase medium, reaction time, and feedstock type, discussed in the following section.
4. Parameters influencing hydrothermal carbonization of biomasses
4.1. Reaction temperature
Temperature is a critical parameter that affects the nature of the resultant product of the HTC process. For instance, it has been demonstrated that HTC at a temperature higher than 250°C results in hydrochars containing more aromatic and nearly completely depleted carbohydrates having lower ratios of H/C and O/C (Citation39). The decomposition of the lignocellulosic biomass components occurs during HTC due to substantial loss in moisture content. An increase in the fixed carbon content alongside a loss in volatile components occurred due to decomposition reaction during the HTC process (Citation40). On the contrary, the static carbon content rises as temperature increases with the removal of volatiles: the biomass-based samples obtained from the HTC process after 250°C have about six times higher fixed carbon contents than the raw material (Citation40).
4.2. Reaction time
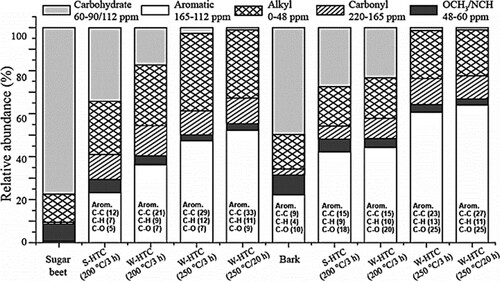
In principle, a longer reaction time gives rise to the complete carbonization of the feedstock material. However, a negligible influence of the reaction time during HTC may be observed if an unduly long treatment is carried out (Citation39). Xiaoyan and coworkers, in their study, observed enrichment of non-protonated aromatic C–C in sugar and beet hydrochars upon increasing reaction residence time from 3 to 20 h at 250°C (), which depicts a shift in their H/C ratios to lesser values (Citation39).
Figure 5. Distribution of major C functional groups in sugar beet, bark, and their hydrochars (Reproduced from reference (Citation39) with permission from American Chemical Society).
There is no universal definition for reaction time; some researchers consider it time following reactor heating, while others include reactor heating (Citation41–46). A large amount of feedstock was solubilized during a short reaction period, with subsequent rises in H/C formation over time until a maximum constant level was reached in accordance with Lu et al. (Citation47) Also, reaction time has been demonstrated to influence the distribution of carbon (Citation41, Citation46–48) and the H/C chemical characteristics and energy content (Citation46, Citation47, Citation49). An increase in reaction time decreases the solid yields (Citation39, Citation46, Citation47, Citation50, Citation51), whereas energy and carbon content increase with reaction time (Citation46, Citation47, Citation49). However, other researchers have taken a counter position from their assessment and on these reports. They opined that reaction time has less effect on the carbonization of product and its properties, which is more likely if carbonization data are taken after the complete conversion of the biomass; as such, the reaction time becomes less significant (Citation15, Citation43, Citation50).
4.3. Feedstock type
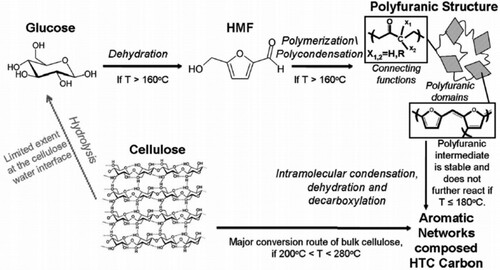
Modifications in feedstock type have been reported to influence the hydrochar and HC properties (Citation42, Citation46, Citation52–56). For instance, model food waste has been reported to yield more considerable energy than food waste from paper or samples from mixed municipal solid waste (MSW) (Citation57). Findings from work carried out by Titirici et al. reveal that all hexose forms of sugar decompose into hydroxymethyl furfural (HMF), yielding solid carbonaceous materials with similar composition chemical features. However, they realized that solids obtained from the carbonization of varieties of pentoses were dissimilar in chemical structures (Citation58). The mechanism exhibited by pentoses differs from hexoses; while the former gives furfural, the latter yields HMF as their dehydration products (Citation59). The hydrochar from pentoses (example; xylose) were in nanometer sizes; conversely, hexoses (examples; glucose and fructose) were spherical with uniform micrometer sizes. The HTC mechanisms for the components of biomass are different from each other. The mechanism of cellulose and lignocellulose is said to be different from that of glucose (Citation60). Polyfuranic intermediate, a unique feature of D-glucose-based hydrochar formed at either at a short reaction time, is absent in the cellulose-based hydrochar formation route, as seen in .
Figure 6. Different hydrothermal carbonization mechanisms for glucose and cellulose under mild processing conditions (180°C < T < 280°C) (Reproduced from reference (Citation59) with permission from Royal Society of Chemistry).
4.4. Reaction phase medium
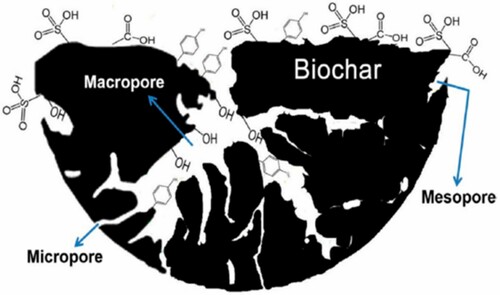
The reaction media significantly affect the nature of the final product. The composition of the HTC material can be controlled by modifying the constituent of the reaction media. One of the major differences between HTC and pyrolysis is the media employed for the reaction. For instance, ionic reactions are more feasible in an aqueous medium compared to pyrolysis. Moreover, biomass-based HTC carbons comprise enormous functional groups, including hydroxyl, carboxyl, and other groups, shown in , reflecting the role of a reaction medium (Citation61). In pyrochars, these functional groups are absent since non-aqueous media are used in pyrolysis.
Figure 7. Schematic of porous biochar containing various functional groups (Reproduced from reference (Citation62) with permission from MDPI).
5. Recent strategies employed for enhancing the performance of HTC
The hydrothermal carbonization (HTC) process for preparing value-added carbon materials has attracted the growing interest of researchers due to its economy and facile approach. However, the carbonized material still falls short of satisfaction; thus, other methods are incorporated during the HTC process to improve its performance (carbonized material). Here, we are taking a look at two significant approaches, namely, co-hydrothermal carbonization (Co-HTC) and catalytic HTC (C-HTC), as described below:
5.1. Co-hydrothermal carbonization (co-HTC)
Scientists have demonstrated co-hydrothermal carbonization (co-HTC) as an alternative strategy for synthesizing hydrochar with improved fuel properties. The co-HTC method involves mixing two solid biomasses as raw materials for the synthesis of a single feedstock with enhanced properties (Citation63). Various mechanisms on integrating two biomasses to improve the properties of hydrochar obtained from hydrothermal treatment have been described in the literature (Citation63, Citation64). For instance, according to Shen et al., the improved fuel properties of hydrochar from lignocellulosic biomass and polyvinyl chloride (PVC) is attributed to their synergistic effect on dechlorination (Citation64). The unbounded –OH in the lignocellulosic biomass improved the substitution of –Cl with –OH, thereby enhancing the dechlorination reaction, which gives rise to the formation of hydrochar with better fuel properties.
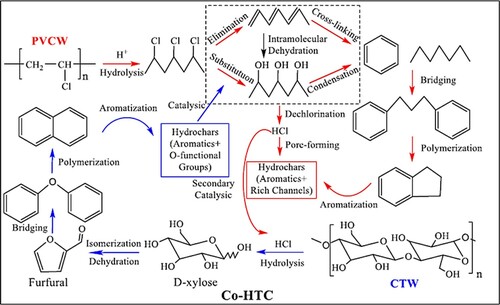
Similarly, Zhihua et al. proposed a mechanism for converting hydrothermal products during the co-HTC process, as shown in (Citation63). The hydrochar obtained through different conversion routes using biomasses of cotton textile waste (CTW) and polyvinyl chloride waste (PVCW) were highly functionalized aromatic materials, which were proposed to be due to the interaction between PVCW and CTW during co-HTC process. In , the PVCW catalyzes the conversion of the CTW. Prior to this catalytic transformation, the CTW undergoes several reactions, including hydrolysis, dehydration, isomerization, polymerization, and aromatization (Citation65). Consequently, the dechlorinated PVCW obtained through hydrolysis underwent cross-linking and condensation and were transformed into aromatic hydrochars by aromatization reaction (Citation63).
Figure 8. Mechanism for the synthesis of hydrochars through co-HTC (Reproduced from reference (Citation63) with permission from Elsevier).
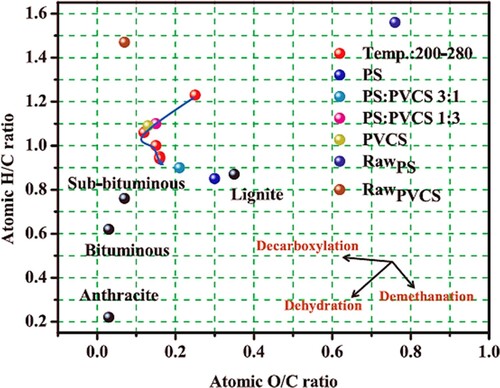
One of the critical benefits of co-HTC is that it is a pretreatment technique that reduces energy demand and improves its quality. Many raw materials have been subjected to the co-HTC method to prepare carbon materials, including biomass, municipal solids and paper, and agricultural wastes (Citation66). The synergistic effect of biomass from pine dust and sewage dust during co-HTC resulted in the creation of hydrochar with a high yield and synergy coefficient (Citation67). Another advantage of co-HTC is that it improves the HVV of the yield; in so doing, the amount of hydrogen and oxygen is decreased. The reduction in the H/C and O/C content of a material is usually illustrated using the Van Krevelen diagram, shown in (Citation68). Under high HTC temperature conditions, a decrease in the mole ratios of H/C and O/C is observed, which may be associated with a rise in unsaturation in the hydrochar. Xu et al. reported that dehydration was the critical response of digested sludge and cow manure biogases during the co-HTC (Citation69). Hydrochar obtained from co-HTC has also been reported to exhibit the remarkable activity of CO2 gasification leading to higher CO quantities. From the high values of gasification efficiency and the LSV of the hydrochar, Ma and colleagues established its outstanding combustion performance (Citation70).
Figure 9. A typical Van Krevelen diagram for hydrochar obtained from co-HTC treatment of pinewood sawdust (PS) and polyvinyl chloride (PVC) (Reproduced from reference (Citation68) with permission from Elsevier).
5.2. Catalytic HTC (c-HTC)
The HTC process may lead to low yield hydrochar formation with poor efficiency, followed by slow deoxidation and denitrification if performed alone without modification. The addition of catalysts to the HTC process helps to subvert these limitations. In various HTC methods, several metal salts, acid, and base were used as catalysts, and the results are presented in (Citation66, Citation71, Citation72).
Table 1. The quality of hydrochar produced by HTC over different catalysts.
These additives are known to regulate the carbonization of sludge and biomass through dehydration, depolymerization, and decarboxylation of their components (Citation71). In the presence of three forms of acids: oxalic, tartaric, and citric acid, a sewage sludge was treated using HTC to compare the removal effect of phosphorus in the prepared hydrochar by Song et al. (Citation72). The acids examined gave varying exciting results. In another example, applying 20 mmol of organic acid, the removal efficiency of P2O5 was improved (Citation73–76). In the presence of oxalic acid, hydrochar displayed an increased phosphorus removal effect, carbon content, and HHV. Elsewhere, other catalyst forms such as acetic acid, zeolite, lithium chloride, and borax were tested in the HTC process. The catalyst helped in speeding up the HTC and hydrolysis reaction. The presence of the ZSM-5 catalyst allegedly affected the carbon content and the degree of sludge carbonization (Citation77). There are different ways in which ZSM-5 affects the product according to Peng et al. (Citation78). Firstly, the addition of the ZSM-5 promoter during the reaction gives rise to the formation of hydrochar with larger aromatic rings relative to the pure carbon aromatic ring. Additionally, it improves the decomposition of various functional groups and carbon into hydrocarbons groups (Citation78). There is a growing interest in the use of biomass-based hydrothermal carbons in several fields including energy, supercapacitors, supports and templates, catalysis, electrocatalysis, photocatalysis, and pollutant remediation. These are highlighted in the following section.
6. Recent advances in the catalytic applications of biomass-based hydrothermal carbons
6.1. Energy storage devices
Materials of interest in energy storage devices, among other properties, must possess high charge density, stability and must be cheap and abundant. Carbon-based and especially hydrothermally synthesized materials meet these requirements and have attracted the increasing attention of researchers. The most common device fabrications in which hydrothermal carbon-based materials are used include Lithium-ion battery, Sodium-ion battery, and Carbon fuel cell.
6.1.1. Li-ion battery
Although the high energy density of Lithium-ion batteries has attracted the interest of electronic gadgets manufacturers, its application in rechargeable batteries is limited by rate capability and longer cycle life. Several nano structural materials such as transition metal oxides, tin-based oxides, and silicon with potentials to meet the requirement of a high-performance anode: high coulombic efficiency and increased capacity to meet have been explored (Citation79–81). However, the two primary setbacks using these promising nano structural materials are low conductivity and considerable volume alteration in the Li insertion and extraction cycle process (Citation79, Citation82–84). Comparatively, the HTC technique has gained the increasing attention f researchers due to its facile approach, cheapness, and potency for commercialization. For instance, Si@SiOx–C nanocomposite synthesized through HTC was reported to exhibit superior lithium-storage capacity, as shown in (Citation79). After electrochemical testing in vinylene carbonate electrolyte, the SiOx–C nanocomposite was recorded to offer a high electronic ability alongside a good cycling electrolyte.
Figure 10. (a) TEM images of the Si@SiOx–C nanocomposite. (b) Cycling and rate performance of pure-Si-nanoparticle and Si@SiOx–C-nanocomposite electrodes cycled in vinylene carbonate (VC)-free and VC-containing 1 M LiPF6 in EC/DMC solutions (solid symbols: charge; empty symbols: discharge) (Reproduced from references (Citation79, Citation85) with permission from German Chemical Society).
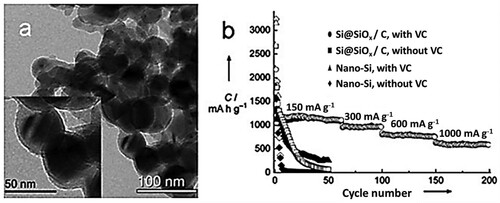
It was Huang et al. who first demonstrated the synthesis and application of HTC carbon as an anode in lithium-ion batteries. The HTC carbons were developed at 1000 C for 5 h using hydrothermally carbonized sugar (Citation86). They developed HTC carbons post-treated at elevated temperature from their reversible lithium insertion/extraction ability to display similar specific capacity ranges as commercial graphite, thus paving the way for HTC carbons content in lithium-ion batteries (Citation79, Citation87).
6.1.2. Sodium-ion battery
Sodium-ion batteries are envisaged as a suitable replacement for Li-ion batteries. Intensive research is ongoing, especially for the synthesis of effective anodes for sodium-ion batteries. Using HTC, several cheap and ubiquitous carbon sources have been transformed hydrothermally for energy storage devices. Studies have shown that hydrothermally treated carbon functions better as an electrode in lithium or sodium-ion batteries than directly pyrolysed biomass (Citation88–96). The presence of micropores and macropores are attributed to the improved performance of hydrothermally prepared carbon electrodes, which are considered desirable for fast ion diffusion (Citation94). There is still no clear understanding of the storage mechanism of sodium.
6.1.3. Carbon fuel cell
Carbon fuel cells are reported to be the most effective route for transforming carbon materials into electrical energy. In a work by Paraknowitsch et al. to investigate the potential of HTC materials using different carbon sources as reference materials for fuel prepared by HTC, observed a significantly enhanced capability of HTC material obtained from D-glucose (Citation97). In addition, they also observed the formation of an indirect carbon fuel with an overall zero-emission balance with respect to greenhouse gas CO2.
6.2. Supercapacitors
Like batteries, supercapacitors are energy storage devices. Supercapacitors can be mainly classified into two: pseudo capacitors and electric double-layer capacitors (EDLCs). Pseudo capacitors store energy via reduction–oxidation reactions at the electrode/electrolyte interface and possess higher energy density over EDLCs rate capacity (Citation98). Supercapacitors are considered more advantageous over Li-ion batteries as they possess a high-power density capacity for electrochemical energy storage with broad operating temperature conditions. The use of capacitors for energy harvesting has been limited due to their low energy density (<10 W.h.kg−1), implying they exhibit few seconds of charge/discharge process. Carbon materials with hierarchical pore structures are important for enhancing the performance of supercapacitors. Carbon-based materials are commonly used as electrodes due to their low cost, good electrical conductivity, varying forms, remarkable physiochemical properties, and tunable porosity (Citation99).
Though HTC materials show low porosity, incorporating an activation process drastically improves the material's porosity. For instance, the use of KOH as an activator was reported to increase the surface area of HTC material processed from many different biomass sources () (Citation100–105). Similarly, the use of ZnCl2 activator reportedly enhanced the mesoporosity of HTC carbon material obtained granulated sugar, which is essential for improving ion diffusion, giving rise to a capacity of ca. 140 F.g−1. Altering the intrinsic structure of carbon material by introducing carbon nanoparticles or doping with heteroatoms such as N, O, S, and Fe, enhance its overall performance (Citation89, Citation98, Citation102, Citation103, Citation106, Citation107). It is noteworthy to point out here that though the introduction of heteroatoms may increase the surface area of HTC carbon materials but does necessarily guarantees a corresponding increase in capacitance. For example, a comparative analysis of HTC glucose and glucosamine reveals that the former showed higher surface area (1766 versus 1238 m2.g−1), but their capacitance was almost similar (280 and 287 F.g−1 respectively, at 0.2 A.g−1). The presence of pyridine and pyridine groups were said to contribute to the pseudocapacitive property of the material, were said to be the basis for the variation in performance of the carbon materials (Citation106).
Table 2. Electrochemical performance of carbon and composite supercapacitor electrodes obtained by HTC.
6.3. Support and templates for catalysts
Unlike activated carbons (ACs), HTC materials are polar and have lower surface areas (less than 1500 m2.g−1). The apolar and hydrophobic nature of ACs is ascribed to their low oxygen content. For this reason, they decrease the reproducibility and performance of catalysts by leaching when employed as support. On the other hand, HTC carbons can display similar properties to ACs by reducing their oxygen content and increasing the carbon level (Citation120). Enhancing the polarity and surface area of HTC carbons support minimizes the leaching of metals and aggregation deposited on it (Citation121). Sometimes, HTC carbons are modified before utilization as catalyst support (Citation107, Citation121–124). It is possible to design more elaborate supports by combining HTC with a porous polymer as a template, as Cheng et al. (Citation125). During the HTC process, a polymer was added in this situation as a prototype that was then extracted in a decreasing atmosphere at 700°C. This thermal treatment greatly increased the BET surface area. The material was then filled with gold nanoparticles and tested with sodium borohydride to hydrogenate 4-nitrophenol to 4-aminophenol, leading to high catalytic activity. HTC can also be achieved with particles of metal oxide (Citation126–128). A magnetically active material can be obtained by using magnetic metal oxide cores. In this way, active catalysts were prepared with palladium and platinum nanoparticles as active sites for the Suzuki–Miyaura cross-coupling reaction (Citation126). In this work, during the carbonization of glucose at 180°C for 4 h, Fe3O4 particles (magnetite) were added. Palladium or platinum nanoparticles were subsequently deposited on the carbon shell, and an additional layer of approximately 31 nm mesoporous silica thickness covered the entire substance. Due to their low density, high surface area, and good permeability, porous hollow spheres have recently attracted growing interest, making these open materials promising candidates for catalysis, sensing, or other applications.
A widely used approach is the precipitation on the template surface of a layer of desired inorganic material (Citation129). Many hollow nanometer-sized photocatalysts have been developed with excellent catalytic efficiency using this method. For example, Yuan and colleagues synthesized hollow spheres of titanium that can decompose methylene blue under UV irradiation with an apparent constant rate of nearly 6 times that of commercial titanium P25 (0.26 and 0.045 min−1, respectively) (Citation129). Liu and colleagues synthesized porous In2O3 hollow nanospheres using glucose-derived carbon spheres as a template that had a satisfactory response to ethanol, methanol, and other organic gases even at very low concentrations (Citation127). The synthesis of carbon template particularly anisotropic nanocarbons can be challenging as a result of scarce anisotropic shapes of hard templates (Citation130). Le et al. demonstrated the role of octadecanol/triblock copolymer F127 surfactant solution in the formation of ring-like shaped templates, without which only nanospheres are formed (Citation130). Additionally, carbonaceous materials synthesized using HTC can be used as templates for encapsulating different metals and the resultant highly porous and shape-selective composites are applicable for the hydrogenation of nitroarenes (Citation131).
6.4. Catalysts for biofuel production
Due to their high stability at elevated temperatures and against harsh reaction conditions, carbon materials can be used as catalyst supports or as catalysts on their own. The benefit of HTC carbons in this application is their polarity and the functional groups on their surface, enabling further modification. A noble metal salt such as Pd0 can be reduced in situ by the carbohydrate aldehyde groups, resulting in HTC carbons loaded with metal nanoparticles. A simultaneous hydrothermal treatment with Ti isopropoxide and glucose was used to build a nanocomposite in HTC process. The high surface area of this carbon-doped titanium dioxide (C@TiO2) can absorb a significant amount of light in the visible range, effectively driving photochemical degradation reactions of organic colorants and pollutants (Citation132).
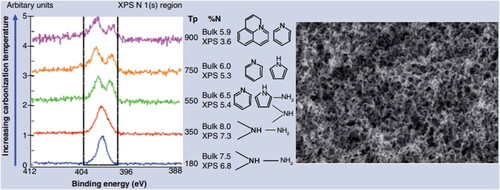
To meet different application requirements, the addition of nitrogen-containing functional groups (N-doping) to HTC carbons generates carbon materials with surface functionality tuned to temperature. For instance, x-ray photoelectron spectroscopy showed that HTC carbons produced hydrothermally at 180°C have amine functionality on the surface. Subjecting these materials to higher temperatures changes their functionality towards pyridinic nitrogen (N incorporated within a graphitic structure), as shown in a. Another benefit is that such materials show a well-defined mesoporosity and high surface areas (b). Selectively and at high conversions, these materials effectively catalyzed different reactions (Citation132).
Figure 11. (a) x-ray photoelectron spectroscopy spectra of the N-doped materials obtained upon hydrothermal treatment of glucose and albumine at different temperatures, showing the change in the surface functionality, (b) Scanning electron micrograph of the hydrothermal carbon aerogel obtained from glucose and albumin (Reproduced from reference (Citation132) with permission from John Wiley).
The addition of sulfonic compounds to HTC synthesized carbon materials gives rise to the introduction of groups of sulfonic acids (), leading to a solid acid catalyst used for catalytic reactions (Citation107). These materials are typically produced at high temperatures by the treatment of porous carbon in concentrated sulphuric acid. In esterification reactions, primarily in the synthesis of biodiesel, several sulfonated hydrothermal carbon catalysts were added (Citation120, Citation133, Citation134).
Figure 12. Schematic view of sulfonation of porous carbon (Adopted from reference (Citation107) with permission from Royal Society of Chemistry).
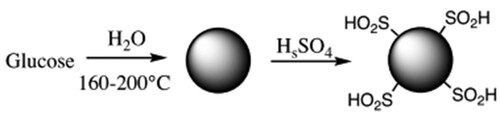
Sulfonated biochars (HTC carbons) have been used as inexpensive heterogeneous catalysts for the production of biodiesel and free fatty acids. These achieved the high yield (88%) of biodiesel products from cooking oil (Citation135). After five cycles, the output of methyl esters decreased from 88% to 80% catalyst reuse, demonstrated by the leaching of functional groups of SO3H (Citation135). The biochar catalysts (HTC carbon catalysts) in terms of ester yield are comparable to non-biochar catalysts (i.e. traditional heterogeneous solid catalysts). However, the stability of HTC carbon catalysts should be improved to avoid post-separation steps for removing S or Ca for the practical use as catalysts for biodiesel production.
6.5. Electrocatalysis
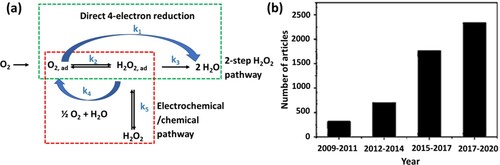
Electrocatalysis is a process that involves the electrochemical conversion of water, CO2, and N2 into added-value products such as hydrogen, oxygenates, and ammonia (Citation136). Oxygen electro-reduction is a very complex mechanism involving a method of multi-electron transfer with many elementary steps. Wroblowa’s widely accepted model presents simplified oxygen reduction pathways through either a ‘direct four-electron’ or a ‘two-step peroxide’ process, as shown in a (Citation137). The sluggish kinetic reactions of electrocatalysis are the main barrier to improving the overall efficiency of fuel cell devices (Citation136). Currently, Pt-group of metals (PGM) commercial catalysts are used as electrocatalysts to enhance the kinetics of oxygen reduction reaction (ORR). These metals demonstrate high catalytic activity towards the ORR; nevertheless, they are scarce and easy to degrade, especially in an acidic electrolyte. To date, ORR transition metal compound-based and heteroatom-doped metal-free carbon-based materials are considered to be promising substitutes for PGM-based electrocatalysts (Citation138). Due to high electronic conductivity, excellent stability, tunable morphology, and simple functionality, various types of carbon materials (carbon nanotube, graphene, carbon nanofiber, etc.) have lately been developed as high-performance ORR electrocatalysts (Citation108). The use of carbon materials as ORR electrocatalysts has attracted increasing attention over the years, as shown in b, displaying the growth in the number of publications related to ORR carbon catalysts (Citation139).
Figure 13. (a) Simplified ORR mechanism through direct four-electron reduction (reaction constant k1) or two-step peroxide pathways (reaction constants k2 and k3); (b) Articles published between 2009 and 2020 according to the results of the search in Network of Science with ‘reduction of carbon-oxygen’ or ‘evolution of carbon-oxygen’ or ‘carbon-hydrogen evolution’ as the keywords (Reproduced from reference (Citation139) with permission from Elsevier).
shows a list of some selected literature on the application of HTC carbon materials as electrocatalysts for ORR and hydrogen reduction reactions (HER) carried out in the past decade. Though these carbon materials are promising ORR electrocatalysts, their commercialization has been limited by low activity and high cost (Citation140). Doping of metal/non-metal is one of the promising strategies for enhancing the performance of HTC electrocatalysts. The metal/non-metal finds its way into the lattice of the carbon atom after doping. These heteroatom dopants induce heteroatom-carbon bonds within the carbon matrix. The polarization of these bonds incorporates active positions on the adjacent carbon matrix, resulting in a decreased energy barrier to electrocatalysis, thus increasing electrical conductivity due to the higher amount of delocalized electrons inside the π system (Citation110, Citation142–144). For the production of electrocatalysts, HTC is an environmentally benign and highly efficient synthesis form.
Table 3. Selected HTC electrocatalysts and applications for the past decade.
Hao and colleagues reported the synthesis of highly porous defective HTC carbon electrocatalysts derivative of seaweed biomass and compared their ORR activity with Pt/C catalyst under acidic and basic medium (Citation148). The ORR catalytic activity of these as-prepared carbon catalysts was assessed through cyclic voltammetry (CV) and linear sweep voltammetry (LSV). They reported a comparative ORR activity of the highly porous defective HTC electrocatalyst to commercial Pt/C catalyst in terms of onset potential (Eonset), a half-wave potential (E1/2), and a limiting current density (JL). For the highly porous defective HTC electrocatalysts ORR activity was recorded to be Eonset = 1.01 V, E1/2 = 0.83 V, and JL = −5.43 mAcm−2, whereas Pt/C catalyst showed Eonset = 1.01 V, E1/2 = 0.84 V, and JL = −5.35 m.Acm−2. Hao and colleagues attributed the high ORR activity to defective carbons formed during the N-removal process. Similarly, recently, LSV and CV were employed by Morais et al. to study the ORR activity of glucose-derived HTC carbon materials (Citation111). They reported two key observations: an increase in onset potential shifts with increased activation time and an upsurge in limiting current density with increased microporosity. HTC is an environmentally benign and highly effective synthesis method for the development of electrocatalysts. However, the structural regulation of a well-defined active site structure is still a challenge because of the dynamics of the process.
6.6. Photocatalysis
In the field of photocatalysis, TiO2 is the most researched photocatalyst due to its remarkable physiochemical properties. Additionally, it is abundant, environmentally friendly, cheap, highly photoactive, and stable (Citation149). Chemical contaminants, air purification, sterilization, and pollutant degradation have been extensively researched using TiO2. Generally, only a small UV fraction of the solar radiation, which accounts for only 3–5% of solar energy, will excite TiO2, with a bandgap of 3.2 eV. Much research effort in the past decade has concentrated on extending the TiO2 photo-response to the visible region. Among these attempts, the most effective approach to enhancing the photocatalytic efficiency of the catalyst was TiO2 doping, either with main group elements or transition metals. Carbon doping is one of the most promising routes for extending the visible absorption of semiconductors. TiO2 doped using carbon from HTC has shown visible light response (Citation132). UV-vis diffuse reflectance spectroscopy showed that C-doped TiO2 material in the 420 to 800 nm range would absorb significantly more light compared to pure TiO2. Therefore, solvothermal prepared C doped TiO2 displayed a robust photocatalytic activity for the degradation of methyl orange under visible light.
Furthermore, the C@TiO2 hybrid was able to generate significant photocurrents under both UV and visible-light irradiation. Other researchers have established higher activity for C-doped TiO2 under visible degradation of acid orange 7 and 2,4-dichlorophenol. The visible-light-induced photocatalysis of C doped TiO2 was ascribed to two forms of sensitization processes; carbon sensitization and dye sensitization (Citation150).
6.7. Sequestration of reducing and greenhouse gases
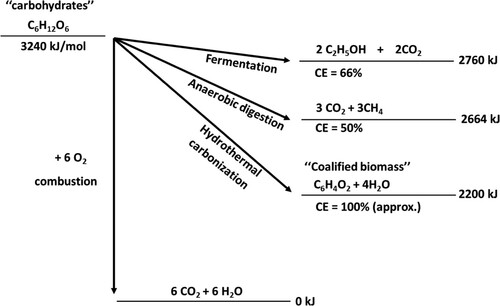
The HTC based products are ‘CO2 – neutral’, which have attracted growing interest in chemistry and technology-based operations. The HTC products can effectively sequester atmospheric CO2 up to a negative point (– CO2) when such a process is carried out over a long period. HTC method to quickly turn biomass into coal may be the most effective tool for CO2 sequestration (Citation32). First of all, the needed acceleration by a factor of 106 to 109 of the coalification of biomass makes it a technically appealing, practical ‘artificial’ instrument for fixing biomass carbon on a large scale. Additionally, it is the most potent carbon fixation technique, with a ‘carbon efficiency’ close to 1 (). Moreover, the HTC route is known to proceed through a milder condition than other conventional carbonization techniques. One of the most attractive features of HTC is that it can be carried in water, as such complicated and high-cost schemes associated with biomass are evaded. Therefore, the HTC method is an appealing technique to deal with a salient part of the CO2 problem (Citation112, Citation113). Recent preliminary studies on CO2 adsorption using nitrogen-containing carbons showed an excellent adsorption property of CO2 adsorption, both in ability and selectivity (Citation32).
Figure 14. Comparison of different ‘renewable energy pathways’ and carbon transfer schemes from carbohydrates. Here, preservation of combustion energy and the ‘carbon efficiency’ of the transformation (CE) are compared. The ‘sum formula’ of the coalified plant material is a schematic simplification (Adopted from reference (Citation32) with permission from The Royal Society of Chemistry).
A comparative analysis of the adsorption properties of activated carbons produced from giant bamboo using HTC and pyrolysis was carried out by Correal et al. (Citation151). The result obtained from the adsorption studies of H2 at −196 and 1 bar reveals the best uptake for carbon material synthesized through HTC. According to the Sevilla et al., in their research on adsorption of H2 using HTC carbons, the average H2 absorption of biomass-derived carbons was between 6.4 and 6.8 percent at −196°C and 20 bar, equivalent to 32–34 m.mol.g−1 (Citation114). A comparative analysis between the Xiao et al. and Correal et al. research reveals that the former had greater H2 adsorption for the HTC content used. Here, the variation in the performance of HTC material similar conditions is attributed to the textural properties such as surface area, pore volume and micropore volume, and synthesis method. While Correa et al. (Citation151) reported (SBET = 2117 m2.g−1; Vtotal = 1.14 cm3.g−1; and Vn = 0.49 cm3.g−1) for the carbon materials, Xiao et al. (Citation115) reported the following (SBET = 980 m2.g−1; Vtotal = 0.78 cm3.g−1; and Vn = 0.46 cm3.g−1).
For the synthesis method, Correa et al. (Citation151) used HTC and chemical activation; Xiao et al. (Citation115) reported HTC-soft templating. Correa et al. (Citation151) measured CH4 adsorption at high pressure and ambient temperature for activated nano carbons provided by microwave-assisted HTC. They found that CH4 uptake increases with pressure, up to a maximum of 8 wt percent at 40 bars. Another fascinating approach to using HTC materials for CO2 capture is their functionalization with amino groups with a high CO2 affinity (Citation87). This material was synthesized in a two-step process: carbonization of hydrothermal glucose in the presence of small amounts of acrylic acid and subsequent treatment of the resulting product with triethylamine, which contains a large number of carboxyl groups. The aminated activated hydrocoal was said to display a high CO2 adsorption potential (at −20°C, up to 4.3 m.mol.g−1). More importantly, at 70°C, these materials showed a very high CO2/N2 ratio (up to 110). The properties of hydrochar can be affected by changing the conditions of its preparation and by introducing specific reagents into the mixture of reactions. The addition of small amounts of vinyl monomers to D-glucose contributes to the creation of carbon materials rich in carboxyl functional groups after hydrothermal carbonization (Citation116).
6.8. Decontamination of pollutants
6.8.1. Inorganic pollutants removal
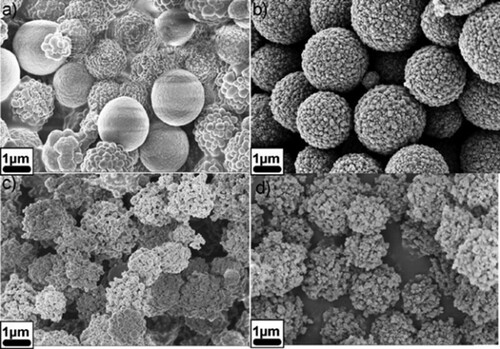
shows functionalized HTC carbon samples used as adsorbents for removing heavy metal ions from water (Citation83). A dramatic increase in adsorption capacity for cadmium and lead was reported with the increasing amount of acrylic acids in the functionalized HTC carbon compared to other samples, which was said to be associated with the development of a pore system and accessibility of binding sites. Furthermore, the adsorption capacity for cadmium 88.8 mg.g−1 (0.79 m.mol.g−1) was higher than that of lead ions 351.4 mg.g−1 (1.7 m.mol.g−1), which is similar to other reports in the literature (Citation117). A comparative analysis reveals the superiority of the adsorption capacity of acrylic acid-functionalized HTC carbons to ion exchange resins and different types of sorption materials (Citation118, Citation119), which underscores their potential for ion binding and ion buffering purposes of these practical hydrothermal carbon products, particularly for environmental applications.
Figure 15. SEM micrographs of hydrothermal carbon dispersions containing (a) 1 wt%, (b) 2 wt%, (c) 5 wt%, (d) 10 wt% of acrylic acid (Adopted from reference (Citation32) with permission from The Royal Society of Chemistry).
HTC-prepared porous carbon materials have also been recognized for their capacity to adsorb and recycle heavy metals from aqueous solutions (Citation152–155). Sun et al. (Citation154) reported the synthesis of KOH ACs for removal of Cd(II) and multiple metals (Pb(II), Cu(II) and Zn(II)) from wastewater, starting from different feedstocks (sawdust, wheat straw, and corn stalk). The characterization results showed that along with N, O, and H, the hydrothermal carbon had a high content of C. As far as porosity is concerned, the powders had surface areas ranges from 4.4 to 9 m2.g−1. The two hydrothermal carbon samples, adjusted and unmodified by KOH, were tested for adsorption of Cd(II). The KOH ACs extracted Cd(II) more readily, around 80%, compared to less than 10% for the pure hydrothermal carbons. Overall, relative to the pure ones, the KOH ACs were much stronger adsorbents for the heavy metals, possibly due to the increased binding sites associated with functional groups containing oxygen. Han et al. (Citation156) also explored the properties of hydrothermal carbon as a Cd(II) sorbent. Adsorbents from swine solids and poultry litter were prepared using HTC, characterized, and used for Cd(II) and Sb (III). For the poultry litter hydrothermal carbon, the maximum adsorption potential for Cd(II) was 19.80 mg.g−1, and for the swine solids, 27.18 mg.g−1. Sb(III) absorption was slightly lower, with a maximum of 3.98 mg g−1 for hydrothermal carbon derived from pig solids, with a maximum of 3.98 mg.g−1. Xiong et al. reported the removal of Cd(II) from aqueous solutions using HTC silicate-carbon composite (Citation157). The maximum Cd(II) uptake for composite materials was 108 mg.g−1, which was higher than that obtained for pure hydrothermal carbon (34.62 mg.g−1) or magnesium silicate (70.42 mg.g−1), the precursor used during synthesis.
6.8.2. Organic pollutants removal
HTC carbons can also be used for the removal of organic pollutants. For water treatment, Roldán et al. (Citation158) used mesoporous carbon doped with N and S, prepared through a single pot of HTC to adsorbed two colorants: methylene blue and rhodamine B. They found that the adsorption ability for methylene blue was more significant than the other dye, which could be primarily due to size-based factors, the second being marginally larger. Both molecules are cationic; as such, the interaction with the support does not differ significantly. The adsorption potential was approximately 106 mg.g−1 for rhodamine B and 123 mg.g−1 for methylene blue, with a surface area of 288 m2.g−1 and a pore volume of 0.56 cm3.g−1 for ZnCl2 activated S-doped porous carbon. Alatalo et al. (Citation159) suggested removing methylene blue from aqueous media using meso-microporous soft templated carbons prepared using a process of HTC-salt templating. During these experiments, when the temperature was increased from 20 to 40°C, the adsorption efficiency increased significantly, possibly due to decreased solution viscosity, leading to an increased diffusion rate of adsorbent molecules through the external boundary layer and the internal pores. At equilibrium (pH 6 at 20°C with a 24 h contact time), the maximum adsorption potential was approximately 96 mg.g−1 for fructose (FruLi) and 64 mg.g−1 for the final carbon network (FruLi + TCA). Correa et al. (Citation151) recorded a very high adsorption potential for methylene blue, approximately 735 mg.g−1, using activated carbon prepared from various chars.
7. Challenges and outlook on hydrothermal carbonization
This review discussed the advantages and applications of hydrothermal carbonization. However, there are limitations to the HTC method which impede its commercialization. The key drawback is the inability to use the post-processing water as liquid fertilizer due to its significant mineral and dissolved organic compounds (Citation160). The liquid effluent obtained during the HTC process is usually treated before further utilization. The cost associated with such an approach constitutes a drawback by increasing the economy of the process (Citation161, Citation162). Although HTC process is a promising method for the transformation of wet material into valuable products with less volume and mass, the nature of the outputs (hydrochars) is widely influenced by the reaction conditions, particularly temperature and residence time (Citation163). Therefore, optimizing temperature and residence time must be considered to synthesize hydrochar with the desired properties, which makes the process tedious, time-consuming, and expensive. Although HTC process requires lesser energy input than other thermochemical processes, its temperature demand in the range 180–250°C is still considered to be expensive from the economy view point (Citation164).
Additionally, HTC exhibits a non-uniform distribution of heat and a long duration for a reaction process (Citation165). Generally, the HTC process employs the traditional method of heating biomass which is usually subjected to elongated residence time, indiscriminate superficial heating, and minimal controllable feature of the heating process. It was also established that HTC yielded more sugar degradation by-products at high temperatures, particularly furan derivatives, which impedes the anaerobic digestion process (Citation166). The hydrophilicity of hydrochar is reportedly lower than its original biomass (Citation167). The decrease in affinity for water in the hydrochar is simply attributed to the loss in oxygen content at high temperature with a resultant increase in the carbon content as established from the van Krevelen diagram in . The selective choice of good filtration process and drying of a solid product constitutes a challenge in HTC process (Citation160). Usually, after the HTC process, the synthesized hydrochar is separated from the liquid content of the reaction mixture using a 0.45 m Whatman filter. The filtration process could be challenging if some particles in the hydrochar pass through the effluent during filtration (for particles smaller than 0.45 m). HTC process is relatively new; the cost of synthesizing hydrochar is considerably higher than coal, which is a significant concern (Citation168, Citation169).
These disadvantages can be overturned or minimized by putting the 7th sustainable development goal into perspective (aimed at achieving affordable and clean energy) and adapting some of the 12 principles of green chemistry which were first established by Paul Anastas and John Warner in 1998 (Citation170). These principles aim at reducing or eliminating life and environmental threatening substances after production. Although it is impossible to achieve all the 12 principles in a particular process at a given time, employing relevant principles including prevention, atom economy, safer solvent and auxiliary, design for energy efficiency, use of renewable feedstock, and catalysis, which would improve the HTC process. The principle of prevention admonishes the avoidance of waste generation, which will save the cost of treating waste produced during the HTC process. The principle of prevention can be achieved by employing the principle of atom economy which lay emphasis on the effective utilization of feedstock to prevent the formation waste products. The principles of less hazardous chemical synthesis advocates the production of chemical products that are eco-friendly. Some researchers have demonstrated the addition of surfactant to HTC reaction media in order to overcome the bulk density, storage, and cost of transporting hydrochar and to enhance its fuel properties (Citation171). In the event that researchers wish to functionalize hydrochar to improve their properties by incorporating chemical reactions including sulfonation, nitration, oxidation, etc., careful selection of precursors must be considered to minimize or eliminate the release of harmful waste into the environment. Although the reaction conditions of HTC is relatively mild, the overlay from the synthesis can be minimized by employing the principle of design for energy efficiency. According to this principle, there are three possible ways the energy efficiency of HTC process can be improved: (i) maintenance and recovery, (ii) choice and conditions of chemical reactions, and (iii) combined heat and power (CHP). The loss of heat and energy can be minimized through the use of insulating systems. Alternatively, the heat generated during the process may be channeled to other purposes such heating up edifices. For the optimal decrease in energy demand in HTC process, this principle suggests the synthesis of hydrochar under ambient temperature and atmospheric pressure involving the use of catalyst. The CHP is the simultaneous generation of electricity and heat energy by a production plant, which may be useful in various processes on site. Recently, there is an increasing attention on co-HTC process which combines two biomass wastes for the synthesis of hydrochar with improved fuel properties (Citation63, Citation64). According to the principle of the use of renewable feedstocks, the chosen biomasses must be biodegradable. For instance, a combination of plastic polymer with lignocellulosic biomass during co-HTC process will lead to the formation a non-biodegradable output, which may be detrimental to the environment. The introduction of catalyst is one of the feasible ways of reducing the energy demand in HTC process; through the reduction in the energy barrier (activation energy) of the reaction. The principle of catalysis in green chemistry encourages the use of biodegradable catalysts in such process, which helps in utilizing less energy, minimizing waste water, and prevent the use of harmful organochlorine in order to protect the environment from toxic chemical substances.
Despite the substantive and potential benefits of catalysts incorporated in the HTC process, some catalysts that have shown potential have not yet been thoroughly explored. Metal-based catalysts have been shown to improve the degree of carbonization when used in HTC (Citation172), and production of microstructures and specific surface areas similar to activated carbons (Citation173). There are, however, only a few studies exploring their application. Many HTC catalyst studies based on biomass only cover HTC temperatures in the range of 180–250°C, likely due to increased pressure and corrosion complications, leaving a lot of space for testing different HTC catalysts based on biomass at higher temperatures. Studies have shown that a better product than an individual catalyst alone will result from mixing multiple catalysts (Citation174), leaving a broad range of catalyst mixing choices to improve product characteristics. Moreover, the analysis of new catalysts may lead to further improvement in performance over what is currently available. The process, which may include testing new activating agents and HTC catalysts with reduced environmental influence and, where possible, the recovering of HTC process water and activation agents, should also consider the environmental impact and cost in addition to performance. An appreciative knowledge of biomass-based HTC catalytic and chemical activation mechanisms, which is still largely vague, will promote the optimization and biomass-based HTC for different applications. Overall, biomass-based HTC can overcome these obstacles and produce activated carbon with specific properties beyond conventional properties of activated carbons without applying a secondary treatment stage even if it is appropriate.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Li, Z.; Liu, M.; Zhang, X.; Chen, Y.; Xue, H.; Ye, E.; Luque, R. Biomass-Derived Carbonaceous Materials: Recent Progress in Synthetic Approaches, Advantages, and Applications. ACS. Sustain. Chem. Eng. 2019, 7, 4564–4585. https://doi.org/https://doi.org/10.1021/acssuschemeng.8b06030
- Titirici, M.; Fühner, C.; Bens, O.; Kern, J.; Emmerich, K. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of wet and dry Pyrolysis. Biofuels. 2011, 2 (1), 71–106.
- Kc, R.; Babu, I.; Alatalo, S.; Föhr, J.; Ranta, T.; Tiihonen, I. Hydrothermal Carbonization of Deciduous Biomass (Alnus Incana) and Pelletization Prospects. J. Sustainable Bioenergy Syst. 2017, 07 (03), 138–148. https://doi.org/https://doi.org/10.4236/jsbs.2017.73010
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. https://doi.org/https://doi.org/10.1021/acs.energyfuels.8b01678
- Jung, D.; Ko, P. Calculating the Reaction Order and Activation Energy for the Hydrothermal Carbonization of Fructose. Chem. Ing. Tech. 2020, 92, 692–700. https://doi.org/https://doi.org/10.1002/cite.201900093
- Hansen, L.J. Impact of Hydrothermal Carbonization on Combustion Properties of Residual Biomass. Biomass Convers. Biorefin 2020.
- Krylova, A.Y.; Zaitchenko, V.M. Hydrothermal Carbonization of Biomass: A Review. Solid Fuel Chem. 2018, 52, 91–103. https://doi.org/https://doi.org/10.3103/S0361521918020076
- Hochschul- und personal- nachrichten, (1914) 260.
- Xie, L.; Wang, H.; Chen, C.; Mao, S.; Chen, Y.; Li, H.; Wang, Y. Cooperative Assembly of Asymmetric Carbonaceous Bivalve-Like Superstructures from Multiple Building Blocks. Research. 2018; Article ID 5807980.
- Feist, F.; Walden, S.L.; Alves, J.; Kunz, S.V.; Micallef, A.S.; Brock, A.J.; Mcmurtrie, J.C.; Weil, T.; Blinco, J.P.; Barner-kowollik, C. Wavelength-Gated Photochemical Synthesis of Phenalene Diimides. Angew. Chem., Int. Ed. 2021, 60, 10402–10408. https://doi.org/https://doi.org/10.1002/anie.202016632
- Funke, A.; Ziegler, F.; Berlin, T.U. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels, Bioprod. Biorefin. 2010, 4 (2), 160–177. https://doi.org/https://doi.org/10.1002/bbb.198
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon N. Y 2009, 47, 2281–2289. https://doi.org/https://doi.org/10.1016/j.carbon.2009.04.026
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction Kinetics of Hydrothermal Carbonization of Loblolly Pine. Bioresour. Technol 2013, 139, 161–169. https://doi.org/https://doi.org/10.1016/j.biortech.2013.04.028
- Sun, X.; Li, Y. Hollow Carbonaceous Capsules from Glucose Solution. J. Colloid Interface Sci 2005, 291, 7–12. https://doi.org/https://doi.org/10.1016/j.jcis.2005.04.101
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal Carbonization as an Effective way of Densifying the Energy Content of Biomass. Fuel Process. Technol 2012, 103, 78–83. https://doi.org/https://doi.org/10.1016/j.fuproc.2011.11.009
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical Biofuel Production in Hydrothermal Media: A Review of sub- and Supercritical Water Technologies. Energy Environ. Sci 2008, 1, 32–65. https://doi.org/https://doi.org/10.1039/b810100k
- Mochidzuki, K.; Sato, N.; Sakoda, A. Production and Characterization of Carbonaceous Adsorbents from Biomass Wastes by Aqueous Phase Carbonization. Adsorption 2005, 11, 669–673. https://doi.org/https://doi.org/10.1007/s10450-005-6004-6
- Hashaikeh, R.; Fang, Z.; Butler, I.S.; Hawari, J.; Kozinski, J.A. Hydrothermal Dissolution of Willow in Hot Compressed Water as a Model for Biomass Conversion. Fuel 2007, 86, 1614–1622. https://doi.org/https://doi.org/10.1016/j.fuel.2006.11.005
- Shoji, D.; Sugimoto, K.; Uchida, H.; Itatani, K.; Fujie, M.; Koda, S. Visualized Kinetic Aspects of Decomposition of a Wood Block in Sub- and Supercritical Water. Ind. Eng. Chem. Res 2005, 44, 2975–2981. https://doi.org/https://doi.org/10.1021/ie040263s
- Murray, J.B.; Evans, D.G. The Brown-Coal/Water System: Part 3. Thermal Dewatering of Brown Coal. Fuel 1972, 51, 290–296. https://doi.org/https://doi.org/10.1016/0016-2361(72)90006-3
- Lau, F.S.; Roberts, M.J.; Rue, D.M.; Punwani, D.V.; Wen, W.W.; Johnson, P.B. Peat Beneficiation by Wet Carbonization. Int. J. Coal Geol 1987, 8, 111–121. https://doi.org/https://doi.org/10.1016/0166-5162(87)90026-7
- Teichmüller, M.; Teichmüller, R. Die stoffliche und strukturelle Metamorphose der Kohle. Geol. Rundschau 1954, 42, 265–296. https://doi.org/https://doi.org/10.1007/BF01773966
- Blazsó, M.; Jakab, E.; Vargha, A.; Székely, T.; Zoebel, H.; Klare, H.; Keil, G. The Effect of Hydrothermal Treatment on a Merseburg Lignite. Fuel 1986, 65, 337–341. https://doi.org/https://doi.org/10.1016/0016-2361(86)90292-9
- Gattung, D. III. Zur Kenntnis der Chromatopien. Helv. Chim. Acta. 1903, 9, 594–599.
- Schafer, H.N.S. Factors Affecting the Equilibrium Moisture Contents of low-Rank Coals. Fuel 1972, 51, 4–9. https://doi.org/https://doi.org/10.1016/0016-2361(72)90029-4
- Berl, E.; Schmidt, A. ober das Verhalten der Cellulose bei der Druckerhitzung mit Wasser. Justus. Liebigs. Ann. Chem. 1928, 461, 192–220.
- Mccollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid Synthesis Under Hydrothermal Conditions by Fischer-Tropsch-Type Reactions. Orig. Life Evol. Biosph. 1999, 29 (2), 153–166. https://doi.org/https://doi.org/10.1023/A:1006592502746
- Yu, J.; Savage, P.E. Decomposition of Formic Acid Under Hydrothermal Conditions. Kinet. Catal. React. Eng. 1998, 5885, 2–10.
- Siskin, M.; Katritzky, A.R. Reactivity of Organic Compounds in hot Water: Geochemical and Technological Implications. Science (80-.) 1991, 254, 231–237. https://doi.org/https://doi.org/10.1126/science.254.5029.231
- Nelson, D.A.; Molton, P.M.; Russell, J.A.; Hallen, R.T. Application of Direct Thermal Liquefaction for the Conversion of Cellulosic Biomass. Ind. Eng. Chem. Prod. Res. Dev 1984, 23, 471–475. https://doi.org/https://doi.org/10.1021/i300015a029
- Inci, U. Lignite and Carbonate Deposition in Middle Lignite Succession of the Soma Formation, Soma Coalfield, Western Turkey. Int. J. Coal Geol 1998, 37, 287–313. https://doi.org/https://doi.org/10.1016/S0166-5162(98)00010-X
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the Black: Hydrothermal Carbonization of Plant Material as an Efficient Chemical Process to Treat the CO2 Problem? New J. Chem 2007, 31, 787–789. https://doi.org/https://doi.org/10.1039/b616045j
- Leibnitz, E.; Könnecke, H.G.; Schröter, M. Zur Kenntnis der Druckinkohlung von Braunkohlen in Gegenwart von Wasser. IV. J. Für Prakt. Chemie 1958, 6, 18–24. https://doi.org/https://doi.org/10.1002/prac.19580060106
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and Fructose Decomposition in Subcritical and Supercritical Water: Detailed Reaction Pathway, Mechanisms, and Kinetics. Ind. Eng. Chem. Res 1999, 38, 2888–2895. https://doi.org/https://doi.org/10.1021/ie9806390
- Smith, R.C.; Howard, H.C. Aromatization of Cellulose by Heat. J. Am. Chem. Soc 1937, 59, 234–236. https://doi.org/https://doi.org/10.1021/ja01281a005
- Masselter, S.; Zemann, A.; Bobleter, O. Analysis of Lignin Degradation Products by Capillary Electrophoresis. Chromatographia 1995, 40, 51–57. https://doi.org/https://doi.org/10.1007/BF02274608
- Aronovsky, S.I.; Gortner, R.A. The Cooking Process I—Role of Water in the Cooking of Wood. Indus. Eng. Chem. 1930, 22, 264–274. https://doi.org/https://doi.org/10.1021/ie50243a017
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on its Manufacture. Starch - Stärke 1990, 42, 314–321. https://doi.org/https://doi.org/10.1002/star.19900420808
- Xiaoyan Cao, J.M.; Ro, K.S.; Libra, J.A.; Kammann, C.I.; Lima, I.; Berge, N.; Li, L.; Li, Y.; Chen, N.; Yang, J.; Deng, B. Effects of Biomass Types and Carbonization Conditions on the Chemical Characteristics of Hydrochars. J. Agric. Food Chem. 2013, 61 (39), 9401–9411.
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal Carbonization of Coniferous Biomass: Effect of Process Parameters on Mass and Energy Yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. https://doi.org/https://doi.org/10.1016/j.jaap.2015.03.012
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. https://doi.org/https://doi.org/10.1021/ef101745n
- Kang, S.; Li, X.; Fan, J.; Chang, J. Solid Fuel Production by Hydrothermal Carbonization of Black Liquor. Bioresour. Technol. 2012, 110, 715–718. https://doi.org/https://doi.org/10.1016/j.biortech.2012.01.093
- Gao, Y.; Wang, X.H.; Yang, H.P.; Chen, H.P. Characterization of Products from Hydrothermal Treatments of Cellulose. Energy 2012, 42, 457–465. https://doi.org/https://doi.org/10.1016/j.energy.2012.03.023
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal Carbonization of Anaerobically Digested Maize Silage. Bioresour. Technol. 2011, 102, 9255–9260. https://doi.org/https://doi.org/10.1016/j.biortech.2011.06.099
- Li, L.; Diederick, R.; Flora, J.R.V.; Berge, N.D. Hydrothermal Carbonization of Food Waste and Associated Packaging Materials for Energy Source Generation. Waste Manag. 2013, 33, 2478–2492. https://doi.org/https://doi.org/10.1016/j.wasman.2013.05.025
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Alvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216–28. https://doi.org/https://doi.org/10.3390/en11010216
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of Reaction Time and Temperature on Product Formation and Characteristics Associated with the Hydrothermal Carbonization of Cellulose. Bioresour. Technol. 2013, 138, 180–190. https://doi.org/https://doi.org/10.1016/j.biortech.2013.03.163
- Knežević, D.; van Swaaij, W.P.M.; Kersten, S.R.A. Hydrothermal Conversion of Biomass: I, Glucose Conversion in Hot Compressed Water. Ind. Eng. Chem. Res. 2009, 48, 4731–4743. https://doi.org/https://doi.org/10.1021/ie801387v
- Jamari, S.S.; Howse, J.R. The Effect of the Hydrothermal Carbonization Process on Palm oil Empty Fruit Bunch. Biomass Bioenergy 2012, 47, 82–90. https://doi.org/https://doi.org/10.1016/j.biombioe.2012.09.061
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of Variables in Energy Densification of Olive Stone by Hydrothermal Carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. https://doi.org/https://doi.org/10.1016/j.jaap.2015.01.031
- Garcia Alba, L.; Torri, C.; Samorì, C.; Van Der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal Treatment (HTT) of Microalgae: Evaluation of the Process as Conversion Method in an Algae Biorefinery Concept. Energy Fuels 2012, 26, 642–657. https://doi.org/https://doi.org/10.1021/ef201415s
- Lu, X.; Berge, N.D. Influence of Feedstock Chemical Composition on Product Formation and Characteristics Derived from the Hydrothermal Carbonization of Mixed Feedstocks. Bioresour. Technol. 2014, 166, 120–131. https://doi.org/https://doi.org/10.1016/j.biortech.2014.05.015
- Sevilla, M.; Fuertes, A.B. Sustainable Porous Carbons with a Superior Performance for CO2 Capture. Energy Environ. Sci. 2011, 4, 1765–1771. https://doi.org/https://doi.org/10.1039/c0ee00784f
- Tremel, A. Entrained Flow Gasification of Biocoal from Hydrothermal Carbonization. Fuel 2012, 102, 396–403. https://doi.org/https://doi.org/10.1016/j.fuel.2012.05.024
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical Modification of Biomass Residues During Hydrothermal Carbonization - What Makes the Difference, Temperature or Feedstock? Org. Geochem. 2013, 54, 91–100. https://doi.org/https://doi.org/10.1016/j.orggeochem.2012.10.006
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2012, 118, 619–623. https://doi.org/https://doi.org/10.1016/j.biortech.2012.05.060
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. https://doi.org/https://doi.org/10.1021/es2004528
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal Carbon from Biomass: A Comparison of the Local Structure from Poly- to Monosaccharides and Pentoses/Hexoses. Green Chem. 2008, 10, 1204–1212. https://doi.org/https://doi.org/10.1039/b807009a
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural and Furfural by Dehydration of Biomass-Derived Mono- and Poly-Saccharides. Green Chem. 2007, 9, 342–35. https://doi.org/https://doi.org/10.1039/b611568c.
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and Structural Differences Between Glucose, Cellulose and Lignocellulosic Biomass Derived Hydrothermal Carbons. Green Chem. 2011, 13, 3273–3281. https://doi.org/https://doi.org/10.1039/c1gc15742f
- Cao, X.; Sun, S.; Sun, R. Application of Biochar-Based Catalysts in Biomass Upgrading: A Review. RSC Adv. 2017, 7, 48793–48805. https://doi.org/https://doi.org/10.1039/c7ra09307a.
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346–35. https://doi.org/https://doi.org/10.3390/catal8090346
- Xu, Z.; Qi, R.; Zhang, D.; Gao, Y.; Xiong, M.; Chen, W. Co-hydrothermal Carbonization of Cotton Textile Waste and Polyvinyl Chloride Waste for the Production of Solid Fuel: Interaction Mechanisms and Combustion Behaviors. J. Clean. Prod. 2021, 316, 128306. https://doi.org/https://doi.org/10.1016/j.jclepro.2021.128306
- Lu, X.; Ma, X.; Chen, X.; Yao, Z.; Zhang, C. Co-hydrothermal Carbonization of Polyvinyl Chloride and Corncob for Clean Solid Fuel Production. Bioresour. Technol. 2020, 301, 122763. https://doi.org/https://doi.org/10.1016/j.biortech.2020.122763
- Huang, N.; Zhao, P.; Ghosh, S.; Fedyukhin, A. Co-hydrothermal Carbonization of Polyvinyl Chloride and Moist Biomass to Remove Chlorine and Inorganics for Clean Fuel Production. Appl. Energy 2019, 240, 882–892. https://doi.org/https://doi.org/10.1016/j.apenergy.2019.02.050
- Zhang, X.; Li, X.; Li, R.; Wu, Y. Hydrothermal Carbonization and Liquefaction of Sludge for Harmless and Resource Purposes: A Review. Energy Fuels 2020, 34 (11), 13268–13290. https://doi.org/https://doi.org/10.1021/acs.energyfuels.0c02467
- Zhang, X.; Zhang, L.; Li, A. Hydrothermal Co-Carbonization of Sewage Sludge and Pinewood Sawdust for Nutrient-Rich Hydrochar Production: Synergistic Effects and Products Characterization. J. Environ. Manage. 2017, 201, 52–62. https://doi.org/https://doi.org/10.1016/j.jenvman.2017.06.018
- Zhang, X.; Zhang, L.; Li, A. Co-hydrothermal Carbonization of Lignocellulosic Biomass and Waste Polyvinyl Chloride for High-Quality Solid Fuel Production: Hydrochar Properties and its Combustion and Pyrolysis Behaviors. Bioresour. Technol. 2019, 294, 122113. https://doi.org/https://doi.org/10.1016/j.biortech.2019.122113
- Xu, Z.; Song, H.; Zhang, S.; Tong, S.; He, Z.; Wang, Q.; Li, B.; Hu, X. Co-hydrothermal Carbonization of Digested Sewage Sludge and cow Dung Biogas Residue: Investigation of the Reaction Characteristics. Energy 2019, 187, 115972. https://doi.org/https://doi.org/10.1016/j.energy.2019.115972
- Ma, J.; Chen, M.; Yang, T.; Liu, Z.; Jiao, W.; Li, D.; Gai, C. Gasification Performance of the Hydrochar Derived from Co-hydrothermal Carbonization of Sewage Sludge and Sawdust. Energy 2019, 173, 732–739. https://doi.org/https://doi.org/10.1016/j.energy.2019.02.103
- Mäkelä, M.; Yoshikawa, K. Simulating Hydrothermal Treatment of Sludge Within a Pulp and Paper Mill. Appl. Energy 2016, 173, 177–183. https://doi.org/https://doi.org/10.1016/j.apenergy.2016.04.017
- Song, E.; Park, S.; Kim, H. Upgrading Hydrothermal Carbonization (HTC) Hydrochar from Sewage Sludge. Energies 2019, 12, 1–9.
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new Method Combining Hydrothermal Carbonization and Mechanical Compression in-Situ for Sewage Sludge Dewatering: Bench-Scale Verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. https://doi.org/https://doi.org/10.1016/j.jaap.2019.02.003
- Liu, T.; Liu, Z.; Zheng, Q.; Lang, Q.; Xia, Y.; Peng, N.; Gai, C. Effect of Hydrothermal Carbonization on Migration and Environmental Risk of Heavy Metals in Sewage Sludge During Pyrolysis. Bioresour. Technol. 2018, 247, 282–290. https://doi.org/https://doi.org/10.1016/j.biortech.2017.09.090
- Li, R.; Teng, W.; Li, Y.; Liu, E. Liquefaction of Sewage Sludge to Produce Bio-oil in Different Organic Solvents with In Situ Hydrogenation. Energy Fuels 2019, 33, 7415–7423. https://doi.org/https://doi.org/10.1021/acs.energyfuels.9b01434
- Biller, P.; Ross, A.B. Potential Yields and Properties of Oil from the Hydrothermal Liquefaction of Microalgae with Different Biochemical Content. Bioresour. Technol. 2011, 102, 215–225. https://doi.org/https://doi.org/10.1016/j.biortech.2010.06.028
- Seiple, T.E.; Skaggs, R.L.; Fillmore, L.; Coleman, A.M. Municipal Wastewater Sludge as a Renewable, Cost-Effective Feedstock for Transportation Biofuels Using Hydrothermal Liquefaction. J. Environ. Manag. 2020, 270, 110852. https://doi.org/https://doi.org/10.1016/j.jenvman.2020.110852
- Escala, M.; Zumbu, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-E ffi Cient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2013, 27 (1), 454–460.
- Hu, Y.S.; Demir-Cakan, R.; Titirici, M.M.; Müller, J.O.; Schlögl, R.; Antonietti, M.; Maier, J. Superior Storage Performance of a Si@SiOx/C Nanocomposite as Anode Material for Lithium-ion Batteries. Angew. Chem., Int. Ed. 2008, 47, 1645–1649. https://doi.org/https://doi.org/10.1002/anie.200704287
- Lou, X.W.; Deng, D.; Lee, J.Y.; Archer, L.A. Preparation of SnO2 / Carbon Composite Hollow Spheres and Their Lithium Storage Properties. Chem. Mater. 2008, 20 (20), 6562–6566. https://doi.org/https://doi.org/10.1021/cm801607e.
- Zakaria, D.; Erragh, F.; Oudahmane, A.; El-Ghozzi, M.; Avignant, D. α-Ba2P2O7. Acta Crystallogr. Sect. E Struct. 2010, 66, i76–i77. https://doi.org/https://doi.org/10.1107/s1600536810043539.
- Zhang, W.M.; Wu, X.L.; Hu, J.S.; Guo, Y.G.; Wan, L.J. Carbon Coated Fe3O4 Nanospindles as a Superior Anode Material for Lithium-Ion Batteries. Adv. Funct. Mater 2008, 18, 3941–3946. https://doi.org/https://doi.org/10.1002/adfm.200801386
- Titirici, M.M.; new Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2010, 39, 103–116. https://doi.org/https://doi.org/10.1039/b819318p.
- Lou, X.W.; Li, C.M.; Archer, L.A. Designed Synthesis of Coaxial SnO2@Carbon Hollow Nanospheres for Highly Reversible Lithium Storage. Adv. Mater. 2009, 21, 2536–2539. https://doi.org/https://doi.org/10.1002/adma.200803439
- Hu, B.B.; Wang, K.; Wu, L.; Yu, S.; Antonietti, M.; Titirici, M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. https://doi.org/https://doi.org/10.1002/adma.200902812
- Wang, Q.; Li, H.; Chen, L.; Huang, X. Monodispersed Hard Carbon Spherules with Uniform Nanopores. Carbon N. Y 2001, 39, 2211–2214. https://doi.org/https://doi.org/10.1016/S0008-6223(01)00040-9
- Titirici, M.; New Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2010, 39, 103–116. https://doi.org/https://doi.org/10.1039/B819318P
- Zheng, P.; Liu, T.; Yuan, X.; Zhang, L.; Liu, Y.; Huang, J.; Guo, S. Ultrastructural Characterization of the Lower Motor System in a Mouse Model of Krabbe Disease. Sci. Rep. 2016, 6, 1–9. https://doi.org/https://doi.org/10.1038/srep26246.
- Hao, E.; Liu, W.; Liu, S.; Zhang, Y.; Wang, H.; Chen, S.; Cheng, F.; Zhao, S.; Yang, H. Rich Sulfur Doped Porous Carbon Materials Derived from Ginkgo Leaves for Multiple Electrochemical Energy Storage Devices. J. Mater. Chem. A. 2017, 5, 2204–2214. https://doi.org/https://doi.org/10.1039/C6TA08169J
- Imtiaz, M.; Zhu, C.; Li, Y.; Pak, M.S.; Zada, I.; Bokhari, S.W.; Chen, Z.; Zhang, D.; Zhu, S. Functionalized Bioinspired Porous Carbon with Graphene Sheets as Anode Materials for Lithium-ion Batteries. J. Alloys Compd. 2017, 724, 296–305. https://doi.org/https://doi.org/10.1016/j.jallcom.2017.07.005
- Zheng, P.; Liu, T.; Zhang, J.; Zhang, L.; Liu, Y.; Huang, J.; Guo, S. Sweet Potato-Derived Carbon Nanoparticles as Anode for Lithium Ion Battery. RSC Adv. 2015, 5, 40737–40741. https://doi.org/https://doi.org/10.1039/c5ra03482e.
- Li, W.; Huang, J.; Feng, L.; Cao, L.; Ren, Y.; Li, R.; Xu, Z.; Li, J.; Yao, C. Controlled Synthesis of Macroscopic Three-Dimensional Hollow Reticulate Hard Carbon as Long-Life Anode Materials for Na-ion Batteries. J. Alloys Compd. 2017, 716, 210–219. https://doi.org/https://doi.org/10.1016/j.jallcom.2017.05.062
- Li, J.; Qi, H.; Wang, Q.; Xu, Z.; Liu, Y.; Li, Q.; Kong, X.; Huang, J. Constructing Graphene-Like Nanosheets on Porous Carbon Framework for Promoted Rate Performance of Li-ion and Na-ion Storage. Electrochim. Acta. 2018, 271, 92–102. https://doi.org/https://doi.org/10.1016/j.electacta.2018.02.147
- Wang, H.; Yu, W.; Shi, J.; Mao, N.; Chen, S.; Liu, W. Biomass Derived Hierarchical Porous Carbons as High-Performance Anodes for Sodium-ion Batteries. Electrochim. Acta. 2016, 188, 103–110. https://doi.org/https://doi.org/10.1016/j.electacta.2015.12.002
- Demir, E.; Aydin, M.; Arie, A.A.; Demir-Cakan, R. Apricot Shell Derived Hard Carbons and Their tin Oxide Composites as Anode Materials for Sodium-ion Batteries. J. Alloys Compd. 2019, 788, 1093–1102. https://doi.org/https://doi.org/10.1016/j.jallcom.2019.02.264
- Ma, L.; Zhao, B.; Wang, X.; Yang, J.; Zhang, X.; Zhou, Y.; Chen, J. MoS2 Nanosheets Vertically Grown on Carbonized Corn Stalks as Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2018, 10, 22067–22073. https://doi.org/https://doi.org/10.1021/acsami.8b04170
- Paraknowitsch, J.; Thomas, A.; Antonietti, M. Carbon Colloids Prepared by Hydrothermal Carbonization as Efficient Fuel for Indirect Carbon Fuel Cells. Chem. Mater. 2009, 21, 1170–1172. http://pubs.acs.org/doi/abs/https://doi.org/10.1021/cm801586c?ai=54q&mi=0&af=R%5Cnpapers2://publication/uuid/CD463170-157B-42CE-82D2-33C207190DAD.
- Lu, H.; Zhao, X.S. Biomass-Derived Carbon Electrode Materials for Supercapacitors. Energy Fuels 2017, 1, 1265–1281.
- Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Boron- and Nitrogen-Doped Carbon Nanotubes and Graphene. Inorganica Chim. Acta. 2010, 363, 4163–4174. https://doi.org/https://doi.org/10.1016/j.ica.2010.07.057
- Salinas-Torres, D.; Lozano-Castelló, D.; Titirici, M.M.; Zhao, L.; Yu, L.; Morallón, E.; Cazorla-Amoros, D. Electrochemical Behaviour of Activated Carbons Obtained via Hydrothermal Carbonization. J. Mater. Chem. A 2015, 3, 15558–15567. https://doi.org/https://doi.org/10.1039/c5ta03574k.
- Wang, Y.; Yang, R.; Li, M.; Zhao, Z. Hydrothermal Preparation of Highly Porous Carbon Spheres from Hemp ( Cannabis Sativa L.) Stem Hemicellulose for use in Energy-Related Applications. Ind. Crops Prod. 2015, 65, 216–226. https://doi.org/https://doi.org/10.1016/j.indcrop.2014.12.008
- Liu, Y.; Huang, B.; Lin, X.; Xie, Z. Biomass-derived Hierarchical Porous Carbons: Boosting the Energy Density of Supercapacitors via an Ionothermal Approach. J. Mater. Chem. A 2017, 5, 13009–13018. https://doi.org/https://doi.org/10.1039/c7ta03639f.
- Wei, T.; Wei, X.; Gao, Y.; Li, H. Large Scale Production of Biomass-Derived Nitrogen-Doped Porous Carbon Materials for Supercapacitors. Electrochim. Acta 2015, 169, 186–194. https://doi.org/https://doi.org/10.1016/j.electacta.2015.04.082
- Zhang, L.; You, T.; Zhou, T.; Zhou, X.; Xu, F. Interconnected Hierarchical Porous Carbon from Lignin-Derived Byproducts of Bioethanol Production for Ultra-High Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 13918–13925. https://doi.org/https://doi.org/10.1021/acsami.6b02774
- Li, C.; He, D.; Huang, Z.-H.; Wang, M.-X. Hierarchical Micro-/Mesoporous Carbon Derived from Rice Husk by Hydrothermal Pre-Treatment for High Performance Supercapacitor. J. Electrochem. Soc. 2018, 165, A3334–A3341. https://doi.org/https://doi.org/10.1149/2.0121814jes
- Zhao, Y.Q.; Lu, M.; Tao, P.Y.; Zhang, Y.J.; Gong, X.T.; Yang, Z.; Zhang, G.Q.; Li, H.L. Hierarchically Porous and Heteroatom Doped Carbon Derived from Tobacco Rods for Supercapacitors. J. Power Sources 2016, 307, 391–400. https://doi.org/https://doi.org/10.1016/j.jpowsour.2016.01.020
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berged, N.; Titirici, M.-M. Recent Advances in Hydrothermal Carbonisation: From Tailored Carbon Materials and Biochemicals to Applications and Bioenergy. Green Chem. 2020, 22, 4747–4800. https://doi.org/https://doi.org/10.1039/D0GC00998A
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2021, 33, 1908232. https://doi.org/https://doi.org/10.1002/adma.201908232
- Wütscher, A.A.; Eckhard, T.; Hiltrop, D.; Lotz, K.; Schuhmann, W.; Andronescu, C. Nitrogen-Doped Metal-Free Carbon Materials Derived from Cellulose as Electrocatalysts for the Oxygen Reduction Reaction. ChemElectroChem n.d., 6, 514–521. https://doi.org/https://doi.org/10.1002/celc.201801217
- Panganoron, H.O.; Pascasio, J.D.A.; Esparcia Jr, E.; Anne, J.; Rosario, D.; Ocon, J.D. Hydrothermally Carbonized Waste Biomass as Reduction Reaction. Catalysts 2020, 10, 1–18.
- Morais, R.G.; Rey-raap, N.; Figueiredo, J.L.; Fernando, M.; Pereira, R. Glucose-derived Carbon Materials with Tailored Properties as Electrocatalysts for the Oxygen Reduction Reaction. Beilstein J. Nanotechnol. 2019, 10, 1089–1102. https://doi.org/https://doi.org/10.3762/bjnano.10.109
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 Capture by Adsorption with Nitrogen Enriched Carbons. Fuel 2007, 86, 2204–2212. https://doi.org/https://doi.org/10.1016/j.fuel.2007.06.001
- Yue, M.B.; Sun, L.B.; Cao, Y.; Wang, Z.J.; Wang, Y.; Yu, Q.; Zhu, J.H. Promoting the CO2 Adsorption in the Amine-Containing SBA-15 by Hydroxyl Group. Microporous Mesoporous Mater. 2008, 114, 74–81. https://doi.org/https://doi.org/10.1016/j.micromeso.2007.12.016
- Sevilla, M.; Sangchoom, W.; Balahmar, N.; Fuertes, A.B.; Mokaya, R. Highly Porous Renewable Carbons for Enhanced Storage of Energy-Related Gases (H2 and CO2) at High Pressures. ACS Sustain. Chem. Eng 2016, 4, 4710–4716. https://doi.org/https://doi.org/10.1021/acssuschemeng.6b00809
- Xiao, P.W.; Guo, D.; Zhao, L.; Han, B.H. Soft Templating Synthesis of Nitrogen-Doped Porous Hydrothermal Carbons and Their Applications in Carbon Dioxide and Hydrogen Adsorption. Microporous Mesoporous Mater. 2016, 220, 129–135. https://doi.org/https://doi.org/10.1016/j.micromeso.2015.08.027
- Demir-Cakan, R.; Makowski, P.; Antonietti, M.; Goettmann, F.; Titirici, M.M. Hydrothermal Synthesis of Imidazole Functionalized Carbon Spheres and Their Application in Catalysis. Catal. Today 2010, 150, 115–118. https://doi.org/https://doi.org/10.1016/j.cattod.2009.05.003
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of Arsenic, Cadmium, and Lead by Chars Produced from Fast Pyrolysis of Wood and Bark During bio-oil Production. J. Colloid Interface Sci. 2007, 310, 57–73. https://doi.org/https://doi.org/10.1016/j.jcis.2007.01.020
- Li, Y.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C. Competitive Adsorption of Pb2+, Cu2+ and Cd2+ Ions from Aqueous Solutions by Multiwalled Carbon Nanotubes. Carbon. N. Y. 2003, 41, 2787–2792. https://doi.org/https://doi.org/10.1016/S0008-6223(03)00392-0
- Kaewsarn, P.; Yu, Q. Cadmium(II) Removal from Aqueous Solutions by pre-Treated Biomass of Marine Alga Padina sp.. Environ. Pollut. 2001, 112, 209–213. https://doi.org/https://doi.org/10.1016/S0269-7491(00)00114-7
- Roldán, L.; Pires, E.; Fraile, J.M.; García-Bordejé, E. Impact of Sulfonated Hydrothermal Carbon Texture and Surface Chemistry on its Catalytic Performance in Esterification Reaction. Catal. Today 2015, 249, 153–160. https://doi.org/https://doi.org/10.1016/j.cattod.2014.10.008
- Dong, W.; Cheng, S.; Feng, C.; Shang, N.; Gao, S.; Wang, C.; Wang, Z. Carbon Nanospheres with Well-Controlled Nano-Morphologies as Support for Palladium-Catalyzed Suzuki Coupling Reaction. Appl. Organomet. Chem. 2017, 31, e3741–6. https://doi.org/https://doi.org/10.1002/aoc.3741
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based Design and Emerging Applications of Nanoporous Carbon Spheres. Nat. Mater. 2015, 14, 763–774. https://doi.org/https://doi.org/10.1038/nmat4317
- Gai, C.; Zhang, F.; Yang, T.; Liu, Z.; Jiao, W.; Peng, N.; Liu, T.; Lang, Q.; Xia, Y. Hydrochar Supported Bimetallic Ni-Fe Nanocatalysts with Tailored Composition, Size and Shape for Improved Biomass Steam Reforming Performance. Green Chem. 2018, 20, 2788–2800. https://doi.org/https://doi.org/10.1039/c8gc00433a.
- Wataniyakul, P.; Boonnoun, P.; Quitain, A.T.; Sasaki, M.; Kida, T.; Laosiripojana, N.; Shotipruk, A. Preparation of Hydrothermal Carbon as Catalyst Support for Conversion of Biomass to 5-Hydroxymethylfurfural. Catal. Commun. 2018, 104, 41–47. https://doi.org/https://doi.org/10.1016/j.catcom.2017.10.014
- Cheng, J.; Wang, Y.; Teng, C.; Shang, Y.; Ren, L.; Jiang, B. Preparation and Characterization of Monodisperse, Micrometer-Sized, Hierarchically Porous Carbon Spheres as Catalyst Support. Chem. Eng. J. 2014, 242, 285–293. https://doi.org/https://doi.org/10.1016/j.cej.2013.12.089
- Sun, Z.; Yang, J.; Wang, J.; Li, W.; Kaliaguine, S.; Hou, X.; Deng, Y.; Zhao, D. A Versatile Designed Synthesis of Magnetically Separable Nano-Catalysts with Well-Defined Core–Shell Nanostructures. J. Mater. Chem. A 2014, 2 (17), 6071–6074. https://doi.org/https://doi.org/10.1039/C3TA14046F
- Yu, J.; Yan, L.; Tu, G.; Xu, C.; Ye, X.; Zhong, Y.; Zhu, W.; Xiao, Q. Magnetically Responsive Core-Shell Pd/Fe3O4@C Composite Catalysts for the Hydrogenation of Cinnamaldehyde. Catal. Letters 2014, 144, 2065–2070. https://doi.org/https://doi.org/10.1007/s10562-014-1328-z
- Zhang, F.; Hu, H.; Zhong, H.; Yan, N.; Chen, Q. Preparation of γ-Fe2O3@C@MoO3 Core/Shell Nanocomposites as Magnetically Recyclable Catalysts for Efficient and Selective Epoxidation of Olefins. Dalt. Trans. 2014, 43, 6041–6049. https://doi.org/https://doi.org/10.1039/c3dt53105h
- Ao, Y.; Xu, J.; Fu, D.; Yuan, C. A Simple Method for the Preparation of Titania Hollow Sphere. Catal. Commun. 2008, 9, 2574–2577. https://doi.org/https://doi.org/10.1016/j.catcom.2008.07.020
- Chen, C.; Wang, Y. The Precise Engineering of Nanostructured Carbon Materials. ACS. Cent. Sci. 2021, 7 (9), 1470–1472. https://doi.org/https://doi.org/10.1021/acscentsci.1c00979
- Liu, J.; Xie, L.; Wang, Z.; Mao, S.; Gong, Y. Biomass-derived Ordered Mesoporous Carbon Nano-Ellipsoid Encapsulated Metal Nanoparticles Inside: Ideal Nanoreactors for Shape-Selective Catalysis. Chem. Commun. 2020, 56 (2), 229–232. https://doi.org/https://doi.org/10.1039/C9CC08066J
- Zhao, L.; Chen, X.; Wang, X.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. One-Step Solvothermal Synthesis of a Carbon@TiO2 Dyade Structure Effectively Promoting Visible-Light Photocatalysis. Adv. Mater. 2010, 22, 3317–3321. https://doi.org/https://doi.org/10.1002/adma.201000660
- Lathiya, D.R.; Bhatt, D.V.; Maheria, K.C. Synthesis of Sulfonated Carbon Catalyst from Waste Orange Peel for Cost Effective Biodiesel Production. Bioresour. Technol. Reports 2018, 2, 69–76. https://doi.org/https://doi.org/10.1016/j.biteb.2018.04.007
- Tang, Z.E.; Lim, S.; Pang, Y.L.; Ong, H.C.; Lee, K.T. Synthesis of Biomass as Heterogeneous Catalyst for Application in Biodiesel Production: State of the Art and Fundamental Review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. https://doi.org/https://doi.org/10.1016/j.rser.2018.04.056
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel Production from Waste Cooking oil Using a Heterogeneous Catalyst from Pyrolyzed Rice Husk. Bioresour. Technol. 2014, 154, 345–348. https://doi.org/https://doi.org/10.1016/j.biortech.2013.12.070
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights Into Materials Design. Science 2017, 355 (6321), 1–13.
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691–27. https://doi.org/https://doi.org/10.1002/adma.201703691
- Wang, W.; Jia, Q.; Mukerjee, S.; Chen, S. Recent Insights Into the Oxygen-Reduction Electrocatalysis of Fe/N/C Materials. ACS Catal. 2019, 9, 10126–10141. https://doi.org/https://doi.org/10.1021/acscatal.9b02583
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based Electrocatalysts for Sustainable Energy Applications. Prog. Mater. Sci. 2021, 116, 100717. https://doi.org/https://doi.org/10.1016/j.pmatsci.2020.100717
- Dai, L. Metal-Free Carbon Electrocatalysts: Recent Advances and Challenges Ahead. Adv. Mater. 2019, 31, 1–2.
- Paper, O. PtRu/Carbon Hybrid Materials Prepared by Hydrothermal Carbonization as Electrocatalysts for Methanol Electrooxidation. Ionics 2012, 18 (1-2), 215–222.
- Links, D.A. Bifunctional Fluorescent Carbon Nanodots: Green Synthesis via soy Milk and Application as Metal-Free Electrocatalysts for Oxygen Reduction. Chem Comm. 2012, 48 (75), 9367–9369.
- Gavrilov, N.; Pas, I.; Mentus, S.; Travas-sejdic, J.; Gordana, C. Superior Capacitive and Electrocatalytic Properties of Carbonized Nanostructured Polyaniline upon a low-Temperature Hydrothermal Treatment. Carbon. N. Y. 2013, 64, 472–486. https://doi.org/https://doi.org/10.1016/j.carbon.2013.07.100
- Meng, F.; Li, L.; Wu, Z.; Zhong, H.; Li, J.; Yan, J. Facile Preparation of N-Doped Carbon Nanofiber Aerogels from Bacterial Cellulose as an Efficient Oxygen Reduction Reaction Electrocatalyst. Chinese J. Catal. 2014, 35, 877–883. https://doi.org/https://doi.org/10.1016/S1872-2067(14)60126-1
- Wang, M.; Lai, Y.; Fang, J.; Li, J.; Qin, F.; Zhang, K.; Lu, H. N-doped Porous Carbon Derived from Biomass as an Advanced Electrocatalyst for Aqueous Aluminium/air Battery. Int. J. Hydrogen Energy 2015, 40, 16230–16237. https://doi.org/https://doi.org/10.1016/j.ijhydene.2015.09.054
- Wei, J.; Liang, Y.; Hu, Y.; Kong, B.; Zhang, J.; Gu, Q.; Tong, Y.; Wang, X.; Jiang, S.P.; Wang, H. Hydrothermal Synthesis of Metal–Polyphenol Coordination Crystals and Their Derived Metal/N-Doped Carbon Composites for Oxygen Electrocatalysis. Angew. Chem. 2016, 128, 12658–12662. https://doi.org/https://doi.org/10.1002/ange.201606327
- Baobing, H.; Lu, P.; Fangfang, Y.; Yuchuan, L.; Zailai, X. Improving ORR Activity of Carbon Nanotubes by Hydrothermal Carbon Deposition Method. J. Energy Chem. 2017, 26 (4), 712–718. https://doi.org/https://doi.org/10.1016/j.jechem.2017.03.016
- Hao, Y.; Zhang, X.; Yang, Q.; Chen, K.; Guo, J.; Zhou, D.; Feng, L.; Slanina, Z. Highly Porous Defective Carbons Derived from Seaweed Biomass as Efficient Electrocatalysts for Oxygen Reduction in Both Alkaline and Acidic Media. Carbon. N. Y. 2018, 137, 93–103. https://doi.org/https://doi.org/10.1016/j.carbon.2018.05.007
- Igenepo, K.; Abdul, A.; Timothy, A.; Pearl, I.; Amune-matthews, C.; Omorogie, M.O. Unravelling the Effect of Crystal Dislocation Density and Microstrain of Titanium Dioxide Nanoparticles on Tetracycline Removal Performance. Chem. Phys. Lett. 2021, 776, 138725. https://doi.org/https://doi.org/10.1016/j.cplett.2021.138725
- Zhong, J.; Chen, F.; Zhang, J. Carbon-Deposited TiO2: Synthesis, Characterization, and Visible Photocatalytic Performance. J. Phys. Chem. C 2010, 114, 933–939. https://doi.org/https://doi.org/10.1021/jp909835 m.
- Correa, C.R.; Ngamying, C.; Klank, D.; Kruse, A. Investigation of the Textural and Adsorption Properties of Activated Carbon from HTC and Pyrolysis Carbonizates. Biomass Convers. Biorefin. 2018, 8, 317–328. https://doi.org/https://doi.org/10.1007/s13399-017-0280-8
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. https://doi.org/https://doi.org/10.1080/10643389.2015.1096880
- Yu, X.F.; Liu, Y.H.; Zhou, Z.W.; Xiong, G.X.; Cao, X.H.; Li, M.; Bin Zhang, Z. Adsorptive Removal of U(VI) from Aqueous Solution by Hydrothermal Carbon Spheres with Phosphate Group. J. Radioanal. Nucl. Chem. 2014, 300, 1235–1244. https://doi.org/https://doi.org/10.1007/s10967-014-3081-6
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of Potassium Hydroxide (KOH) Modified Hydrochars from Different Feedstocks for Enhanced Removal of Heavy Metals from Water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. https://doi.org/https://doi.org/10.1007/s11356-015-4849-0
- Zheng, Z.; Wang, Y.; Zhao, W.; Xiong, G. Adsorptive Removal of Uranyl Ions in Aqueous Solution Using Hydrothermal Carbon Spheres Functionalized with 4-Aminoacetophenone Oxime Group. J. Radioanal. Nucl. Chem. 2017, 312, 187–198. https://doi.org/https://doi.org/10.1007/s10967-017-5209-y
- Han, L.; Sun, H.; Ro, K.S.; Sun, K.; Libra, J.A.; Xing, B. Removal of Antimony (III) and Cadmium (II) from Aqueous Solution Using Animal Manure-Derived Hydrochars and Pyrochars. Bioresour. Technol. 2017, 234, 77–85. https://doi.org/https://doi.org/10.1016/j.biortech.2017.02.130
- Xiong, T.; Yuan, X.; Chen, X.; Wu, Z.; Wang, H.; Leng, L.; Wang, H.; Jiang, L.; Zeng, G. Insight Into Highly Efficient Removal of Cadmium and Methylene Blue by eco-Friendly Magnesium Silicate-Hydrothermal Carbon Composite. Appl. Surf. Sci. 2018, 427, 1107–1117. https://doi.org/https://doi.org/10.1016/j.apsusc.2017.08.115
- Roldán, L.; Marco, Y.; García-bordejé, E. Bio-sourced Mesoporous Carbon Doped with Heteroatoms (N,S) Synthesised Using one-Step Hydrothermal Process for Water Remediation. Microporous Mesoporous Mater. 2016, 222, 55–62. https://doi.org/https://doi.org/10.1016/j.micromeso.2015.10.008
- Alatalo, S.-M.; Mäkilä, E.; Repo, E.; Heinonen, M.; Salonen, J.; Kukk, E.; Sillanpääa, M.; Titirici, M.-M. Meso- and Microporous Soft Templated Hydrothermal Carbons for dye Removal from Water. Green Chem. 2016, 18, 1137–1146. https://doi.org/https://doi.org/10.1039/C5GC01796C
- Magdziarz, A.; Kalemba-rec, I.; Szyma, M. Upgrading of Green Waste Into Carbon-Rich Solid Biofuel by Hydrothermal Carbonization: The Effect of Process Parameters on Hydrochar Derived from Acacia. Energy 2020, 202, 117717. https://doi.org/https://doi.org/10.1016/j.energy.2020.117717
- Rom, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11 (1), 216–28. https://doi.org/https://doi.org/10.3390/en11010216
- Sliz, M. A Comprehensive Investigation of Hydrothermal Carbonization: Energy Potential of Hydrochar Derived from Virginia Mallow. Renewable Energy 2020, 156, 942–950. https://doi.org/https://doi.org/10.1016/j.renene.2020.04.124
- Ismail, H.Y.; Shirazian, S.; Skoretska, I.; Mynko, O.; Ghanim, B.; Leahy, J.J.; Walker, G.M.; Kwapinski, W. ANN-Kriging Hybrid Model for Predicting Carbon and Inorganic Phosphorus Recovery in Hydrothermal Carbonization. Waste Manag. 2019, 85, 242–252. https://doi.org/https://doi.org/10.1016/j.wasman.2018.12.044
- Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M. Enthalpy Change During Hydrothermal Carbonization of Biomass: A Critical Review. J. Therm. Anal. Calorim. 2020, 141, 1251–1262. https://doi.org/https://doi.org/10.1007/s10973-019-09117-4
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Optimization and Characterization of Hydrochar Produced from Microwave Hydrothermal Carbonization of Fish Waste. Waste Manage. 2017, 65, 159–168. https://doi.org/https://doi.org/10.1016/j.wasman.2017.04.016
- Sharma, H.B.; Panigrahi, S.; Sarmah, A.K.; Dubey, B.K. Downstream Augmentation of Hydrothermal Carbonization with Anaerobic Digestion for Integrated Biogas and Hydrochar Production from the Organic Fraction of Municipal Solid Waste: A Circular Economy Concept. Sci. Total Environ. 2020, 706, 135907.
- Basso, D.; Castello, D.; Baratieri, M. Hydrothermal Carbonization of Waste Biomass : Progress Report and Prospect. 21st European Biomass Conf. Exhibition 2013, 3–7.
- Saqib, N. U.; Sharma, H. B.; Baroutian, S.; Dubey, B.; Sarmah, A. K. Valorisation of food waste via hydrothermal carbonisation and techno-economic feasibility assessment. Science of the Total Environment, 2019, 690, 261-276..
- Lama, G.; Pazos, M.; Rosales, E. Production of 3-Hydroxypropionic Acid from Acetate Using Metabolically-Engineered and Glucose-Grown Escherichia Coli. Bioresour. Technol. 2021, 320, 124362. https://doi.org/https://doi.org/10.1016/j.biortech.2020.124362
- Ivanković, A.; Dronjić, A.; Bevanda, A.M.; Talić, S. Review of 12 Principles of Green Chemistry in Practice. Int. J. Sustainable Green Energy 2017, 6, 39. https://doi.org/https://doi.org/10.11648/j.ijrse.20170603.12
- Tu, R.; Sun, Y.; Wu, Y.; Fan, X.; Wang, J.; Cheng, S.; Jia, Z.; Jiang, E. Improvement of Corn Stover Fuel Properties via Hydrothermal Carbonization Combined with Surfactant. Biotechnol. Biofuels 2019, 12, 1–19. https://doi.org/https://doi.org/10.1186/s13068-018-1346-y
- Hamid, S.B.A.; Teh, S.J.; Lim, Y.S. Catalytic Hydrothermal Upgrading of α-Cellulose Using Iron Salts as a Lewis Acid. BioResources 2015, 10, 5974–5986.
- Cui, X.; Antonietti, M.; Yu, S.H. Structural Effects of Iron Oxide Nanoparticles and Iron Ions on the Hydrothermal Carbonization of Starch and Rice Carbohydrates. Small 2006, 2, 756–759. https://doi.org/https://doi.org/10.1002/smll.200600047
- Fechler, N.; Wohlgemuth, S.A.; Jäker, P.; Antonietti, M. Salt and Sugar: Direct Synthesis of High Surface Area Carbon Materials at low Temperatures via Hydrothermal Carbonization of Glucose Under Hypersaline Conditions. J. Mater. Chem. A 2013, 1, 9418–9421. https://doi.org/https://doi.org/10.1039/c3ta10674h
