ABSTRACT
β-agonists are a class of synthetic phenylethanolamine compounds that were originally designed to reduce asthma and chronic obstructive pulmonary disease. They remain widely used but unfortunately, misuse in animal farming and sports activities due to their huge medical and economic benefits. As a result, they have been found in a variety of matrices and subsequently end up in multiple water bodies in the environment. However, their environmental occurrence and fates are only evaluated on an occasional basis as they happen at a very trace level. The development of appropriate analytical procedures is critical in providing reliable data related to their occurrence and concentrations in various water bodies. Coupling high-performance liquid chromatography and mass spectrometry have been the most extensively used approach for great sensitivity and high selectivity for their determination in the environment. Sample preparation is important for obtaining lower detection limits, and the online solid-phase extraction has been comprehensively used considering the physicochemical properties of β-agonists. This review presented the systematic development of the preceding research on β-agonist detection approaches published between 1985-2020, emphasizing their analysis, resolution in environmental waters, and highlighting the significant scientific approach from the existing analytical techniques suitable for their ultra-trace determination.
GRAPHICAL ABSTRACT
Introduction
β-agonists are made up of phenylethanolamine backbone containing aromatic group, aliphatic nitrogen groups, and beta-hydroxyl group (Citation1). These compounds are referred to as β-adrenergic agonists because they emulate the actions of catecholamines that occur naturally such as dopamine, norepinephrine, and epinephrine (adrenaline). The catecholamines are responsible for regulating physiological (body) functions like breathing rate as well as the heartbeat. They can also activate regulatory proteins (protein kinase) by binding to β-receptors on skeletal muscle cells, resulting in muscle development, increased muscle protein synthesis as well as feed transformation (Citation2–5). Almost all group members have the common -CH-(OH)-CH2-NH side chain bonded to the aromatic ring, but with unique substituent groups on the phenylethanolamine at the amino nitrogen (typically tert.-butyl or isopropyl group) and yet another substituent group at different locations throughout the phenylic ring. (–OH, –Cl, –NH2, –CN, and –CF3) (Citation6,Citation7). They have typical modes of operations such as chemical composition, efficiency, and side effects with various pharmacokinetics which take account of changes in dosage planning and duration of action. The common chemical structure of β-agonists showing the different substituent groups (R1, R2, R3, R4, and R5) is presented in showing the actual structures and other physiochemical parameters of the four β-agonists. They have comparable physical and chemical characteristics, with the majority of the members consisting of white crystalline particles or powders that are odorless and bitter. β-agonists can be dissolved in a wide range of organic polar solvents as well as inorganic solvents with low acidity or alkalinity when they are in their free form. In addition to being readily soluble in alcohol, methanol, and water; they are also somewhat soluble in acetone; however, they are not soluble in ether due to their high electrostatic attraction. Based on the benzene ring structure, β-stimulants are produced as parent molecules that can absorb light in the ultraviolet and visible ranges of wavelengths between 220-310 nm (Citation8). They are synthetic derivatives of naturally produced molecules (catecholamines) which (as the name implies) bind β-receptors to nerve cells to cause instant detectable physiological effects e.g. increase in heart rate (Citation9). Their mode of action resembles that of norepinephrine and epinephrine (endogenous agonists of the β-adrenergic receptor), which, when activated at nerve endings in the bloodstream, constitute an important part of the function of the autonomic (involuntary) nervous system (Citation10–12). The process regulates multiple organs like cardiovascular, gastrointestinal, etc., and metabolic processes (Citation13).
Table 1. Physicochemical properties and some uses of four of the most prominent β-agonists
β-agonists as members of the class of chemicals known as phenyl β-ethanolamine compounds, which includes a variety of functional groups on the aromatic ring as well as on the terminal amino group of the molecule (), they can similarly be classified into three types based on the substituent groups on the aromatic ring: a) the phenolic group, which consists of salbutamol, ractopamine, bamethane, isoxsuprine, and ritodrine; b) the aniline group, which comprises clenbuterol, clenproperol, mabuterol, cimaterol, cimbuterol, brombuterol, and other synthetic analogues and c) the resorcinol group which includes members as terbutaline, fenoterol and metaproterenol (Citation14). There are still reports of unauthorized use of β-agonists in several countries, particularly novel agonists and analogues of agonists, in animal feed, which is particularly in concern because the residue will directly or indirectly be transported to our environmental water. Though established gas or liquid chromatography coupled with mass spectrometry (GC/MS or LC/MS) or immunoassay-based techniques, can identify these compounds to some extent, the novel β-agonists compounds are hard to be identified by these techniques (Citation15). For this reason, rapid screening techniques that involved broad-spectrum detection techniques for different kinds of β-agonists and analogues that can occur in animal feed, urine, blood, and water samples were mostly applied for rapid identification of the new β-agonists compounds. Medical research indicated that people with heart disorder may develop serious diseases such as ischemia or ischemic necrosis of local tissues as a consequence of excessive use of β-agonists, which will promote the activation of β-receptors and result in aberrant changes in the human heart, including heart disease caused by cardiac hypertrophy and fast heartbeat, and even death (Citation8,Citation16). It is the objective of this review to provide a systematic evolution of the previous research on β-agonist investigative techniques conducted and reported between 1985 and 2020, with an emphasis on their analysis, resolution in environmental waters, and highlighting the important scientific approach from the current analytical techniques that may be appropriate for their ultra-trace determination. An explanation of the technique used, year, state (country) for accurate geographical development, and some comparisons of technical advancements in analytical procedures that were investigated and capable of analyzing β-agonists in our natural waters are provided; these descriptions include what such techniques could accomplish as well as the degree of determinations that could be obtained by each approach.
Side effects and misuse of β-agonists
Around three hundred million people of all ages and different ethnicity are believed to be affected by asthma, with the condition posing a significant burden on governments, medical services, households, and patients worldwide, and an estimated two hundred and fifty thousand deaths each year (Citation20,Citation21). The rising prevalence of asthma and the side effects of β-agonists use are of greater importance as additional reports indicated a daily usage of β-agonists for seven days to have a negative effect on the late asthmatic reaction to allergens (Citation22). presented some physicochemical properties and some uses of four of the most prominent β-agonists. In addition to asthma treatment, β-agonists are the most regular medications for treating cardiogenic effect, acute heart failure, bradyarrhythmia disease (heart rate of less than 60 caused by cardiac-controlling electric disorder), tocolysis (birth contraction inhibition), cough suppressant, nasal decongestant, and COPD (Citation23–26). When a β-adrenergic agonist binds to a β-adrenergic receptor, a physiological response is created, and when certain synthetic β-adrenergic receptor agonists are administered orally, they produce a much longer plasma half-life (e.g. clenbuterol which is why it is used as a good doping agent) (Citation27) and induce growth changes with increased skeletal muscular accumulation and reduced fat accumulation. Because of their essential function in nutritional redistribution, these compounds are repeatedly misapplied as growth promoters in dairy, pigs, and other farmstead faunas (food animals) (Citation1,Citation28). These drugs are also exploited to increase physical success in both human and animal athletes (e.g. horses and dogs), administered to stave off and delay exhaustion and injury, ultimately leading to an elevated mood (Citation5,Citation29,Citation30), and also used to boost metabolic rate and stamina. β-agonists have also been listed as counterfeit substances (for their unlawful use by athletes) by the International Olympic Committee's Medical Commission if used in non-therapeutic dosages, as per reports (Citation31,Citation32)) agreeing on the official detection concentration at 0.5 μg m/L using gas chromatography-mass spectrometry (GC–MS) after derivatization (Citation33). Another development was the criterion set up by WADA for the identification of the substance through the coupling of MS with the chromatographic system for separation using liquid chromatography (LC), the analytes’ retention time is expected not to vary above 2% or ± 0.4 min (the lesser is considered) from the reference material. When a single mass full or partial scan is collected for MS detection, all diagnostic ions with an excess relative to above 10% in the reference range must be present in the unknown (Citation34).
Clenbuterol, for instance, is a β-agonist that the European Union (EU) banned as a medication for human use, but it can be used to relieve bronchial congestion in animals. When β-agonists are administered at 5–10 times the required medical doses, these compounds act as growth promoters, increasing the meat-to-fat ratio in cattle and other food animals. These residues, which are concentrated in the liver and meat, are poisonous to people causing many problems including heart complications (Citation9,Citation35,Citation36).
Though the benefits offered by β-agonists were manipulated by administering the medicines in greater measures (Citation5,Citation28) as they were proposed to have tremendous economic gains, long-term use was found to have an illicit degrading physiological aftereffect, resulting in multiple poisonings and impending dangerous consequences (Citation5,Citation24,Citation26,Citation29,Citation37–39). This means that the problem is not avoided by prohibiting the use of β-agonists in humans and allowing the same compounds to be used in animals in multiple folds.
Environmental occurrence of β-agonists and the threat to human health
The data on the occurrence of pharmaceuticals in some water bodies was first reported by Hignite (Citation40), between 1977-1978, and was generally believed that pharmaceutical contaminants occur in the environment at negligible concentrations, but their bioaccumulation typically poses an impending long-term threat to aquatic and land species and are therefore considered emerging environmental contaminants (Citation41). Increased protein synthesis and lipolysis are well-known β-agonists side effects, and when these concentrations are being increased spontaneously, and the current conventional removal/analytical techniques are incapable of removing the residues completely, then the adverse effects of the residue will be devastating (Citation42). As β-agonists are among the residues monitored by one of the procedures used in the investigation of anabolic steroids in the Brazilian National Program for the Control of Residues in Meat (Brazil is a major exporter of food animals), the inclusion of β-agonists in this group of residues could make it possible to analyze a large number of such substances at the same time, reducing the number of β-agonists incidences that would need to be investigated before reaching out to the market, thereby controlling its occurrence in the environment (Citation43). In Ternes (Citation44) research, the β-agonists analyzed were detectable in no less than one urban sewage treatment plant (STP) eluent with higher concentration levels, resulting in pollution of the receiving waters, due to inadequate elimination of the drug residues during the STP passage. Meanwhile, even extremely low doses of pharmaceuticals can have indirect long-term impacts on organisms, the entirety of their ecotoxicological effects on the aquatic environment is imperative to be addressed. Excessive use leading to the presence of β-agonists in our environmental waters degrades the quality of our water supplies due to their sluggish biodegradability and accumulation in aqueous media, resulting in harmful impacts such as endocrine destruction in marine animals and humans, as well as some number of other pronounced side effects (Citation24,Citation26,Citation29,Citation37,Citation38,Citation39). However, there is still a scarcity of data concerning the behavior of drugs after their passage through an STP and the pollution of the aquatic system as a result of the drugs used in medicine (Citation44). It is imperative for trace analysis of β-agonists for quality assurance in wastewaters research and development as well as clinical and pharmaceutical research chemistry (Citation45). The presence of β-agonists in our water does not come without possible human health and environmental consequences as they pass through our water treatment systems making it hard to be easily detected and completely removed. In our water treatment systems, organic pollutants are removed through evaporation to air, sorption to particles and subsequent sedimentation, and biological transformation (Citation46). But removal by evaporation and sedimentations are generally not possible since β-agonists are neither volatile compounds nor solids particles (Citation47).
The presence of β-agonists as a residue in any environmental medium has been identified as a possible threat to human health. These compounds reach water sources by varying means, either by unlawful or by unintended spillage of unmetabolized β-agonists, by stormed rivers from farm fields containing manure of animals administered with β-agonists, or by sewages (Citation9,Citation48,Citation32,Citation49). Either way, it will eventually end up in our water as their polar nature makes it easy for them to be discharged or leak from landfills and into other water storage systems, such as ground, surface, and the wastewaters around our environments (Citation50). Various studies showed that about 70% of these drugs administered and consumed by humans or animals are excreted as a combination of unmetabolized medication and conjugated metabolites with no difference in chemical structure (Citation44,Citation51,Citation52). Salbutamol and clenbuterol are two of the most frequently used medications by asthmatic patients, concerns have been raised about the possibility that β-agonists are contributing to the mortality of asthmatic patients (Citation53). This can be due to the increased use of β-agonist inhalants during asthma attacks, which may induce cardiac arrhythmia. A reported 70% of these medications are known to be excreted unmetabolised into the environment, thereby following through our waterways back to our domestic water bringing in more threat to the environment (Citation44,Citation51).
Current environmental problems are often concealed, which means that long-term risks or intermittent exposure may reorganize ecosystems, frequently resulting in a loss of biodiversity and the loss of critical functions and services. The widespread use of β-agonists in livestock production and other human intakes of either water containing the chemicals (β-agonists) has resulted in harmful consequences. Long-term or high-dose usage has been found to have detrimental physiological side effects, and a sufficiently large single dose may start an immediate toxic reaction characterized by nervousness, muscle tremors, confusion, headache, lightheaded feelings (dizziness), chest distress, heart palpitations, insomnia, itching, limb numbness and, tachycardia on men (Citation5,Citation51,Citation24,Citation26,Citation29,Citation37,Citation38,Citation39,Citation54). The presence of β-agonists in the aquatic environment caused organisms to be faced with greater challenges in altering their homeostasis upkeep, development, behaviors, and reproduction irregularities, thereby changing and lowering fish and other aquatic organisms availability in the area (Citation55,Citation56). Pomati’s group (Citation56) reported destructive effects caused by the presence of salbutamol on exposure with other drugs by preventing the growth of human embryonic stem cells by deregulation the propagation of the cell up to 30%. Endocrine disruption was also observed in some fish classes when salbutamol was induced in fish liver cells (Citation56). These studies (Citation55,Citation56) respectively, contributed to the scientific data showing the possible health threats involved with the existence of β-agonists in the environment. Moreover, negative effects of ractopamine and clenbuterol on more sensitive end-points have also been reported in roundworms (nematodes) (Citation57). Some of these effects include reduced broad size and locomotive behavior, induced intestinal autofluorescence as well as production of reactive oxygen species, and a consequent reduction of lifespans on chronic exposure to 10 μg/L of these compounds (Citation58). Some levels of guinea-pig mortality were also detected (Citation59) on exposure to 12 µg/L concentrations of clenbuterol.
Plants can take in contaminated groundwater containing β-agonists to satisfy the requirements of evapotranspiration and photosynthesis. Bártíková (Citation60) describes the actions of β-agonists in the environment and summarises the details on their toxic effects on plants, while some poisoning cases were individually reported in some parts of the world (Citation37,Citation27).
Regulations on β-agonists
Due to the toxic effects of β-agonist in the environment and their adherent threat to human health, many countries were compelled to enact preventative and stringent protection policies, such as banning the misuse of the compounds and imposing severe penalties on their illegal intake (Citation29,Citation61). Depending on the variety of applications, some national and international regulatory authorities have individually or collectively issued regulation directives prohibiting the use of β-agonists in part or entirely (Citation9). For instance, in 1999, the United States Food and Drugs Administration licensed ractopamine for use as a pig nutrition substitute, and in 2003, the license was extended to livestock feeding diets (Citation62). China, Japan, and the EU have all imposed a full ban on ractopamine in water (Citation9,Citation63,Citation28,Citation39). But it is legalized for poultry and animal production in countries such as Brazil (one of the world's top four food animal producers), Canada, and the United States (the US) (Citation25). The use of clenbuterol for growth-promoting and athletic purposes was also prohibited in countries like China, the EU, and the US due to anabolic effects (Citation1,Citation9,Citation39,Citation61).
Although β-agonist such as clenbuterol (CLB) has been banned for obvious reasons, they are still permitted as the parent drug with maximum residue limits (MRL) recommended by various commissions and international regulatory bodies such as the Codex Alimentarius Commission, which suggested MRL for CLB as 0.2 ng/g in fat and muscle and 0.05 ng/mL for bovine milk, and 0.6 ng/g in liver and kidney while MRL for ractopamine in beef and pork is recommended as 10 µg/kg by the same Codex Alimentarius Commission (Citation24,Citation26). Whereas the European Union proposed an MRL of 0.5 g/kg for CLB in the livers of horses and cattle (Citation24). Similarly, the Chinese National Standard GB/T5009.192–2003 sets the limit of detection (LOD) for CLB detection in animal feeds at 0.5 g/kg. Urine is usually recommended for use as a sample for β-agonist evaluation for the excretion rate of 22–49% in urine after oral therapy (Citation64). The competence of the analytical technique used determines the amount of LOD achieved.
Analysis of β-agonists in environmental waters
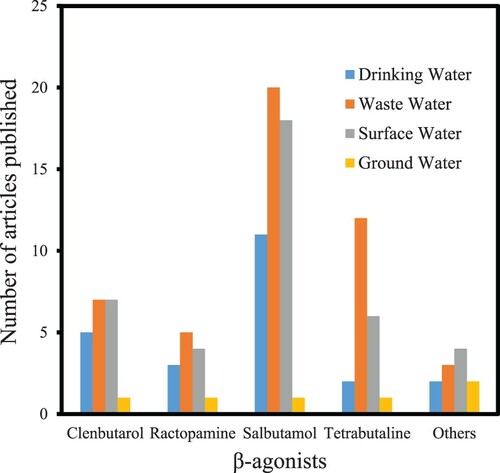
Due to the ubiquitous occurrences and frequent detection of β-agonist in environmental water samples, researchers from various fields of environmental endeavors have carried out investigations for the determination of these toxic compounds in various environmental water matrices. presented the number of articles published on β-agonists determination in water samples for the last 35 years. Despite ample evidence on the transport of medicinal compounds in and around various water sources, only a few experimentations and researches have established the persistence and fate of β-agonists in environmental settings to date (Citation65,Citation66). The presence of β-agonists in drinking water has typically been an alarming factor to the regulatory authorities as well as the public concern, even though their presence were mostly measured at thousands of times lesser than beneficial (therapeutic) doses, this is so because of the bioaccumulation factor of these compounds with time as they are discharged into the environment on a regular basis (Citation66,Citation67). The occurrences of β-agonists in our environment are always measured at lower concentrations commonly under part per million or part per billion or even lower values in an aqueous medium. Their recalcitrant chemical behaviors made their detection very hard, and therefore their detection is always a real analytical challenge. Due to the diversity of their physio-chemical features and continuing intervention of β-agonists in our water will have devastating consequences on both humans and aquatic lives (Citation67–70).
Figure 1. Frequency of publications on β-agonists determination in water samples for the last 35 years
While progress has been made on finding efficient techniques to determine β-agonists at the lowest level possible in various sample mediums, the analytical process is still complex and challenging when dealing with these compounds in water. Their pKa impair greatly their protein binding ability, lipophilicity, solubility, and permeability while having a significant impact on pharmacokinetic characteristics, like absorption, transportation, metabolic, and excretion properties. Reports showed that their concentrations usually exceed 20 mg/L in some treated water for drinking purposes after going through the usual standard water treatment (Citation68,Citation71). Hakk (Citation58) postulated that free ractopamine, (with a reasonable solubility of 31 g/L; log Kow 2.4; pKa 9.4), could be propagated in water (water-borne) from animal waste and is very transportable in the environment.
Many surveys have been run by researchers based on the predicted environmental concentrations (PECs) criteria in calculating the levels of β-agonists in some noticeable areas from different countries. Two surveys were reported by Castiglioni (Citation72) in Italian surface waters and Bound (Citation73) in southeast England using the PECs criteria to measure and compare the levels of salbutamol in their respective area. Both surveys of Castiglioni (Citation72) and Bound (Citation73) found the salbutamol concentrations to have increased. Salem (Citation74) observed and suggested that advanced research and determination of β-agonists in our environmental water is much needed as less than 5% of the more than 3,000 human and veterinary drugs recorded have been evaluated for their existence and involvement in this media. In Linköping, Sweden, Bessel (Citation46) conducted a feasibility study as the foundation for the planning, construction, developmental design, and process of a proposed full-scale oxidation plant to supplement sewage treatment. Terbutaline was successfully detected in a variety of pharmaceuticals after an ozonation stage was undertaken with the primary objective of removing the highest priority compounds from effluent water levels.
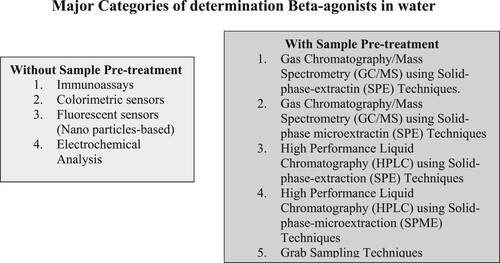
presented the widely used methods for the detection of β-agonists and the methods were currently divided into immunoassay, chromatographic analysis, and electrochemical analysis as reported by Gu (Citation75) and Zhang (Citation9). presented various the literature findings on analytical techniques for determining β-agonist in water ranging from year 1985–2020 using some specific keywords search such as determination of β-agonists in wastewaters, determination of β-agonists in environmental water, techniques for the determination of β-agonists in aqueous solution, determination of β-agonists residues, occurrences and threats of β-agonists as well as individual search which involves a simple and specific search for the β-agonists compounds like determination of terbutaline, salbutamol, or ractopamine, etc. in environmental water in a high-impact database. Many other papers were discovered via the use of mentioned references from published research works and review articles from nations such as Canada, the United Kingdom, Malaysia, the United States, Germany, Spain, Portugal, Sweden, and many countries around the globe. This review focused on our investigation on screening and confirmation methods for the analysis of β-agonist in environmental waters for the past 35 years. The emerging and promising methods were also highlighted.
Determination of β-agonists in environmental waters
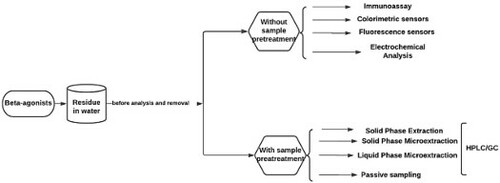
The widespread of β-Agonists in the environmental water sample has been an issue of concern for the past 3 decades. Analytical chemists have proposed various techniques for their determinations in different water matrices using different detection methods, publishing many articles in this regard. The methods employed have been broadly categorized into two:
Those without sample pre-treatments such as immunoassays, electrochemical, colorimetric, and fluorescence methods.
Those involving sample pre-treatment: such as SPE, solid-phase microextraction (SPME), liquid-phase microextraction (LLME), etc.
Determination of β-agonists without sample pre-treatment
Immunoassays
The research using immunoassays has been extensively carried out for more than fifty years now, with the usage of antibodies in numerous methods that have a major impact on the testing and diagnostic fields (Citation76). Antibodies are also applied in quite simple liquid samples and in pregnancy tests that could be directly analysed (Citation77).
This method offers a simple yet very sensitive and selective high-throughput analysis, but an analytical instrumentation technique is needed for conformational purposes when the results are above the recommended limit from the regulatory body or when positive. Many reports using immunoassays on β-agonists have been published in various samples mostly in animal feeds and their products as well as in their biological samples such as urine (Citation9), only a few reports based on the analysis of β-agonist in environmental samples have been established.
Materials with exclusive size-dependent electronics, broad excitation spectra, narrow emission spectra, and electrochemical, optical, and magnetic properties referred to as quantum dots, also known to be highly luminescent semiconductor nanocrystals, have gained significant attention for their potential luminescent applications in conventional aqueous solutions (Citation78). Li (Citation63), showed a sensitive and simultaneous identification of clenbuterol and salbutamol using gold nanoparticles as the coating layer and electron transport accelerator in a quantum-dots-based electro-chemiluminescent immunosensor. A noble time-resolved fluoroimmune experiment (TRFIA), built on a dual-label set-up, was carried out to simultaneously detect clenbuterol and salbutamol in water samples, while the lanthanide ions provide the necessary benefit for time-based (temporal) resolution of signals from the background with the TRFIA detection at a very low detection of 0.007 and 0.014 ng/mL for both clenbuterol and salbutamol, respectively. Comparison of the findings was well compared with those obtained by the HPLC technique, the result showed the capability of the method for the simultaneous identification of β-agonists in the water matrix, with a strong recovery between 72.2–102.2%. The findings were well below the EU’s recommended overall residue limits of 0.05 ng/g for Clenbuterol (Citation79).
Liu group (Citation80) developed an enzyme-linked immunosorbent assay (ELISA) method, aimed at detecting salbutamol in environmental water, with good sensitivity results through the inhibition at half-maximal concentrations (IC50) as the most commonly insightful indicator of a drug’s effectiveness and (limit of detection (LOD (IC50)) values of 0.466 and 0.0021 µg/L, correspondingly. This value indicates that the method managed to detect salbutamol at five times lower than the MRL value. The recoveries of 80.1-115.6% and relative standard deviation of 2.2-8.6% obtained were well within residue detection criteria. Also, the findings correlated well with the results of HPLC with a coefficient of determination of 0.988. This method was recently improved by the same group by adapting a TRFIA previously established for both salbutamol and clenbuterol in swine water (Citation63), their latest attempt was targeting salbutamol in environmental water. The method which utilized lanthanide ions as fluorescent markers have gained a highly sensitive result having a LOD (IC50) value of 0.66 ng/L and it was claimed to have demonstrated a simpler and more stable reproducibility as compared to the immuno-PCR that uses an approach that combines the benefits of ELISA and that of the polymerase chain reaction, employed to ‘visualize’ antigen–antibody interaction (Citation81).
Electrochemical method
β-agonists are typical compounds that have phenolic hydroxyl substituents, oxidation of which dominate their electrochemical actions on the aromatic rings, producing certain free radical sorts that could then form the carbon–carbon single-bond-related dimers that can then adsorb and passivate the electrode surface. Similarly, they also have the amine-substituted group, their electrochemical reaction would include the oxidation of the amine functional group, creating the radical cations that will then submit to the head-to-tail coupling, leading to the development of dimers that can adsorb strongly on the electrodes’ surface (Citation29). Most β-agonists may be oxidized at bare or modified electrodes, making electrochemical techniques one of the ideal methods for the detection of these compounds. Other benefits of electro-chemical techniques are the cheap cost of the instruments and the speed with which they may be analyzed (Citation14). Tang (Citation24) uses the covalent imprinting technique, in which a molecularly imprinted polymer successfully identified and determined clenbuterol in potable water samples, employing clenbuterol as a functional monomer and confirmation analysis with HPLC using an ultra-violet detector (UV-detector). The linear range of the analysis was 5–80 μg/L with the best correlation of 0.9938, a very good recovery of 74.08–107.77%, and a detection limit of 0.12 μg/L. Electrochemical sensors are built by varying electrochemical signals (like current and potential) generated by chemical interactions involving target analytes and electrode-immobilized recognition components. Changes in the output-electrochemical signal are linked to analyte concentrations, that have been extensively utilized for both the quantitative determination and quantitative evaluation of relevant target molecules (Citation64). The voltammetric properties of various β-agonists were assessed by Shen (Citation14) in aqueous samples, and the results were utilized as electrochemical detectors in a liquid chromatography system for the rapid detection of β-agonists utilizing graphite nanosheet (GN) modified glassy carbon (GC) electrodes. Various electrochemical responses were seen on the GN modified electrodes for β-agonists that had different substituent groups on their aromatic ring. The porous structure of the GN film leads to a modified electrode with a larger surface area, which in turn results in an increase in peak current (Citation82) and a detection limit of less than 0.1 µM.
Colorimetric method
Due to their easy interpretation and rapid visual assay through the use of bare eyes, these low-cost and inexpensive devices have been used to detect β-agonists based on the color change produced by chemical reactions (Citation83).
A quick approach to large-scale β-agonists screening in liquid samples with sensitivity comparable to electrochemical methods was demonstrated by He (Citation15) through direct detection of 13 commonly used β-agonists using gold nano-particles (AuNP's) colorimetric absorption process. The test demonstrated the potential by oxidizing the amine group or the group of phenol at the benzene ring of the β-agonist compound, to reduce HAuC14 directly into nuclear gold. The (LOQ) was in the range of 2 × 10−7–4.6 × 10−6 M. The specificity of the established method was however said to be low by Zhang (Citation9) to prove the difficulty in determining the β-agonists in aqueous solutions. Still, the method is facing some significant challenges in reproducibility and imaging, with some difficulties in the determinations of individual components of the mixtures as well as stability/shelf-life (Citation83). A valuation of salbutamol sulfate in pure and various types of pharmaceuticals was recently proposed by Al-rufaie (Citation23). The information (empirical data) for the tactic conformed to the guidelines, having the average percentage recovery of 100.23%, and the LOD down to 0.5510 µg/mL which were considered good.
Fluorescent sensors (nano particles-based) method
Advanced nanomaterials significantly promote the development of sensors in terms of precision, accuracy, and robustness. Zhang (Citation9) and Li (Citation64) explored a large number of research on nanoparticle-based sensors for a long time but were only able to come across two studies from the water medium which were mainly upconversion and quenching methods used for the construction of the fluorescent.
A standard fluorescence quenching of clenbuterol was explored in Tang (Citation84) research using up conversion particles coated on molecularly imprinted polymers, the material was successfully used as a fluorescence probe to selectively detect clenbuterol. The fluorescence quenching was found to have a detection limit of 0.12 µg/L under a linear dynamic range of 5–100 μg/L and a good recovery range of 81.66–102.46% when the analytical performance was assessed with HPLC. While the binding ability of clenbuterol with the MOFs-based (metal–organic framework based) adsorbent (UiO-66) was explored by Yang (Citation85) and discovered to bind to the UiO-66 (MOF), the fluorescence of the MOF is quenched by up to 88% with the linear range of 4.0-40 ng/mL and LOD of 0.17 µM in water.
Determination of β-agonists with sample pre-treatment
Most existing techniques are paired with some type of sample treatment to stimulate the removal of sample matrix interferences as well as analyte enrichment to achieve sufficient method detection limit (MDL) and method quantification limit (MQL) for environmental analysis. With a sufficient and appropriate sample preparation procedure, it would be possible to improve and provide an analytical system with a high degree of sensitivity and efficiency for good quantification and recognition of β-agonists, since it is essential to isolate and pre-concentrate the analytes for precise detection at the lowest possible measure (Citation9,Citation29,Citation86). Several extraction techniques, ranging from liquid–liquid extraction to the discovery of SPE, and to the more recent methods that focused on microextraction as well as passive samplers’ approaches, have steadily been involved in the treatment of β-agonists in water medium over time. However, SPE has been the most common analytical extraction technique used since the end of the century.
Sample preparation coupled with chromatographic techniques
The historical development of chromatography started after the invention of thin-layer chromatography, the field evolved into gas chromatography, liquid chromatography, and eventually high-performance liquid chromatography and capillary column gas chromatography. As a result of persistent development and enhancement of chromatographic detectors, high-sensitivity detection methods such as hydrogen flame ion detection, nitrogen, and phosphorus detection, and mass spectrometry have emerged, allowing the detection limit of trace elements to be significantly increased (Citation8). The target objective of environmental research and analysis is to develop new analytical methods, such as LC coupled with MS or tandem mass spectrometry (LC-MS/MS or HPLC) and GC to be coupled with MS or tandem mass spectrometry (GC MS/MS), that can measure the compounds of interest to the barest minimum MDL (Citation87,Citation88). Due to the sensitivity issue in chromatographic performances that are often limited, some preconcentration and extraction have to be carried out to obtain a maximum yield of the analyte of interest. For a successful identification of β-agonists in a very complex medium like water, some pre-concentration and extraction processes like SPE, SPME (Citation5), and liquid-phase microextraction (LPME) (Citation50,Citation89), were established for separation and pre-concentration of β-agonists before the chromatographic technique.
Many advancements have recently been introduced in the experimental techniques used to measure β-agonists in water (as a complex matrix), from improving the chromatographic resolution, increased specificity, and sensitivity in detection, as well as precision, along with new extraction and clean-up strategies. More importance is being placed on using GC-MS, GC-MS/MS, LC-MS, and LC-MS/MS for β-agonist testing since they can have low detection limits even in complex matrices like wastewater (Citation90,Citation91). The increasing rate of permitted and illegitimate overuse of β-agonists necessitates the need for the accelerated screening and identification of these compounds in anti-doping prevention, toxicological research findings, forensic investigations, and environmental sciences (Citation92). High sensitivity in the MS, provides a more comprehensive scientific framework for the identification of β-agonists in water when coupled with the chromatographic system. LC-MS/MS is usually a very safe choice for trace residue analysis compared to GC-MS (Citation9). The widespread use of automated analytical techniques such as GC-MS, GC-MS/MS, LC-MS, and LC-MS/MS in drug development and research is gaining momentum due to the following benefits: a) You can get compound validation and detailed knowledge on the composition and structure of the compounds you're looking at with them. b) Even though they co-elute, it is possible to successfully isolate and detect molecules of the same molecular mass but different productions.
Solid-Phase extraction techniques with high-performance liquid chromatography
Castiglioni (Citation93), make use of the efficiency in HPLC and reported a way to boost the little existing information about the fates of medicines in STPs and receiving water in Italy. The report describes the determination of salbutamol and analyzed by reverse phase-HPLC–MS/MS in urban wastewaters. Castiglioni uses two SPE extraction steps during the method development and validation obtaining a fair recovery of 65–131% yielding LOD from 0.15–5.2 ng/L, with a LOD of 0.90 ng/L for salbutamol as the MDL of the instrument.
The electrospray ionization (ESI) is a gentle ionization source in which very little residual energy is kept by the analyte, and typically no fragmentation occurs during the ionization process. Furthermore, extremely weak noncovalent interactions were maintained in the gas phase during the ionization process (Citation94). ESI was used as an ion source when using the LC-MS/MS systems because it enables quick, precise, and responsive analysis, as a source of low-energy ionization ESI typically does not induce molecular ion fragmentation and is suggested for thermally labile and polar compounds, especially, β-agonists compounds (Citation95). Salbutamol was detected in the river and waste effluents by Al-Odaini in the year 2010 (Citation95), SPE-LC-ESI-MS/MS was used to obtain good recoveries above 70% in all the target analytes in both the matrices. The LOD of the method was 0.2–281 ng/L and the detection limit for the analyte in both matrices was greater than the method detection limit of 3 ng/L. SPE-LC-ESI-MS/MS method for the positive detections and quantification of salbutamol in wastewaters sampled from 7 Canadian STPs was developed by Lee(Citation6). The developed method was able to detect terbutaline at a limit range of 6-11 ng/L with the recovery on the compounds better than 85%. A minor modification of Lee’s method (Citation6), was successfully applied by Salem (Citation74), in a simultaneous determination of terbutaline in distilled and wastewaters. The optimized and validated method obtained an average recovery between 77.20-97.30% with method detection limits around 0.11–6.74 pg/mL and quantification limits of 0.14–22.88 pg/mL.
High-resolution mass spectrometry combined with SPE pre-treatment technique has been used in the study of β-agonists in the water medium. For example, salbutamol in rivers of Taff in the United Kingdom and Warta Poland were identified by Kasprzyk-Hordern (Citation96) in 2007. An ultra-performance liquid-chromatography–positive-electrospray ionization tandem-mass-spectrometry with SPE for the preconcentration of the analyte (SPE-UPLC–ESI/MS/MS) was used to obtain levels from nanograms to single micrograms per liter. The use of this novel UPLC column with an internal diameter of 1 mm packed with a stationary phase of 1.7 µm particle size allowed to achieve very low flow rates around 0.07 mL/min within very considerable retention periods of 1.3–15.5 min throughout the studied. The limit of quantification (LOQ) and the instrumental detection (MDL) of the analyte (salbutamol) stood at 0.5 and 0.1 μg/L correspondingly, and the process limits range around low nanograms per liter. In the subsequent year, Kasprzyk-Hordern et al. (Citation97) also offered a modified SPE-UPLC–ESI/MS/MS study of salbutamol in surface water and wastewater treatment plant (WWTP) in the Welsh area. The key benefits of these approaches were their high sensitivity, with method LOQ in surface water and wastewater at a considerable level of 0.1 ng/L and 500 μg/L. Kasprzyk-Hordern et al. method (Citation97) was able to achieve very low column equilibration times of less than 4 min and a strong analyte separation accomplished in less than 20 min process time along with averagely higher coefficients of determination above 0.997 for the salbutamol tested using SPE technique.
New developments in environmental mass spectrometric approaches have been on the rise with the attention specifically to hybrid mass spectrometers (Citation98,Citation99). Gros (Citation100) obtained a very low percentage recovery of 33-43% on salbutamol during his analysis via hybrid-triple-quadrupole-linear ion trap mass-spectrometer (QqLIT-MS) coupled in LC using SPE extraction method while tracing salbutamol residues in waters in our environment, the detection limit that ranges from 0.1–55 ng/L is good and acceptable for all the % recovery obtained. UPLC joined with a quadrupole time-of-flight MS (QToF) was also used by Magner (Citation101) by applying two different SPE methods (SPE-Bag and SPE-column) to determine terbutaline as the micro pollutants in wastewater. A detection limit of 40.0 ng/L in effluent and 50.0 ng/L in influent was obtained with a limit of quantification of 120 ng/L in effluent and 150 ng/L in influent, and % recovery between 35.2–80.0%.
Considering increased resolution, sensibility, and reduction of matrix effects, Maria Huerta-Fontel (Citation102), also employs UPLC column coupled with MS/MS (UPLC/QqLIT) using SPE in extracting the analytes to allow for chromatographic separation of salbutamol and terbutaline in less than 9 min with an average study duration of 18 min. Recoveries between 70 and 75%, which were also better than Gros (Citation100) results were obtained with LOQ of 0.02 ng/L in wastewaters from Spain. Utilization of UPLC/MS-MS hybrid triple quadrupole using SPE extraction technique with recoveries over 100% and a detection limit between 0.3–0.8 ng/L both in the effluent and surface water was also presented by Angela Batt (Citation103) designed for the investigation of Albuterol. Full utilization of high-resolution MS in the analysis of β-agonists’ was also reported by Mira Petrovic (Citation104) on the prevalence of salbutamol in different water samples obtained from Serbia. UPLC-MS/MS with hybrid triple quadrupole linear ion-trap (QqLIT) using SPE for sample clean-up and concentration was employed. The pervasive presence of the drug in water was shown in varying concentrations of ng/L to values above 1 μg/L.
To contribute to the LC-MS/MS analytical approach in the study of β-agonists, a selective multi-residue analytical approach for seven β-agonists was successfully developed and validated by Juan (Citation105) in drinking water. The validation of the method was fully done following the steps designated in the European Commission Decisions of 2002/657/EC (Citation106) at a level less than the maximum required performance limit that was projected by the Community Reference Laboratories with required sensitivity for the target analytes. SPE technique was also used for the clean-up and concentration of the solution.
Numerous literatures were found on the determination of β-agonists in different water sources using SPE as a sample pre-treatment and extraction technique. For instance, the presence of clenbuterol and salbutamol in hospital effluent of the Po Valley area of northern Italy was determined using LC-MS/MS and an average detection of 1.4 ng/L and, 1.2 ng/L was attained for clenbuterol and salbutamol respectively (Citation107). Different water sites in Spain were analyzed using LC-Q-TRAP-MS with a detection frequency of over 70% across all the experiments. Valcarcel (Citation108) detected salbutamol at levels between 12 and 29 ng/L in almost all sampling points from the domestic tap water and some selected rivers around the Madrid Region, Spain. Martinez Bueno’s study (Citation109) also, found salbutamol but in lower quantities of 2-4 ng/L in some river waters, while values below the detection and quantification limits were observed by Lopez (Citation110) based on online analysis of albuterol and clenbuterol using turbulent-flow chromatography-tandem mass spectrometry (TFCLC-MS/MS) that is very good in drugs analysis in general. In Germany, Ternes (Citation44), found some β-agonists (salbutamol, fenoterol, clenbuterol, and terbutaline) in urban sewage effluents and rivers at lower concentrations between 0.035–0.061 µg/L using the SPE method as the extracting technique, however, all the β-agonists found were observed to have had an exceptionally low concentration and did not exceed 0.2 µg/L for STP effluent samples. They were only occasionally found in river and stream waters beyond the 0.01 µg/L detection level, with a maximum concentration of 0.061 µg/L.
Terbutaline was shown to display poor method efficiency in all the water matrices tested in Grabic’s (Sweden) (Citation111) trace levels determination in Umea River. However, the method established an acceptable lower detection limit of 1.9 ng/L, 1.6 ng/L, and 0.71 ng/L for terbutaline in all the three different water matrices in Milli-Q water, surface water, and wastewater, respectively. Ractopamine was one of the most regularly found compounds in Taiwanese hospitals’ effluents and its adjacent rivers, with trace quantities ranging from 0.2–12.0 ng/L and detection frequencies ranging from 9 to more than 70% in the river, according to Yu (Citation112) research in 2011. However, some concentrations as low as 38 ng/L were found by Lin (Citation113) using LC-ESI-MS-MS in hospital effluents, pharmaceutical industries, and waste streams around Taiwanese water sites.
Passive sampling techniques with HPLC
Usually, grab sampling techniques were commonly engaged in the determination of drugs in aquatic environments. The effectiveness of samples taken using grab samplers can be restricted when dealing with low concentrations of contaminants, especially when analyzing pollutants that dissolve rapidly or reach the water source in discrete temporal events. Optionally, passive samplers, like polar organic chemical integrative samplers (POCIS) in particular, are used to determine water-soluble substances such as veterinary pharmaceuticals (like β-agonists) where concentrations could be below detection levels in the grab samples collected. POCIS is effective due to the simplicity of implementation and does not usually require several samples of water to be obtained or analysed (Citation48). Dearden (Citation114) used POCIS with HPLC column and identified salbutamol among the highly excreted drugs present in the River Lee, which was North London's sole source of potable water, back in 1985. While Calamari (Citation115) observed salbutamol concentrations to surpassed the trigger value of 10 ng/L using HPLC in river Po, Italy. In line with Ramos’ (Citation116) and other research findings, intensive sampling at a regional WWTP in Portugal successfully determined the daily inconsistency of salbutamol using an HPLC coupled to MS with a reverse-phase column detecting the analyte at 11 ng/L (Citation117).
Ractopamine was detected in an intensive agricultural watershed in Nebraska-Lincoln USA, using POCIS and HPLC-MS/MS, and ESI in positive mode with a reporting limit of 0.02 ng/L and MDL of 0.007 ng/L (Citation48). Consistent with the Jaimes study, dissipation rates of ractopamine in two streams receiving urban wastewater effluents around Nebraska-Lincoln USA were also measured using a passive sampler and analyzed with HPLC-MS/MS with Positive ion mode ESI (Citation118).
Extant of β-agonists were also reported in some Malaysian rivers by different individual researchers at different times. Salbutamol concentration was detected by Najat (Citation95), in the Langat river at a lesser concentration than the maximum detection limit and concentration of 3 ng/L in sewage treatment plants’ effluents using LC-ESI-MS/MS with a run-time of thirty minutes and a recovery of more than 70%. Research focusing on the spatial distribution of some β-agonists (ractopamine, clenbuterol, terbutaline, salbutamol, and other β-agonists) in surface water and recognizing its sources in the Langat River basin as Malaysia's most urbanized watersheds was conducted by Sakai (Citation51). The source sketching indicated the sources of β-agonists from sewage, and quantitative analysis was by LC-MS / MS. The lowest detection limit of the analytes was at 4.3-19.1 ng/L and the catchment areas were visualized using a geographic information system for the sampling points. While in another study by Rodil (Citation68), salbutamol concentration was contemplated to be negligible when a monitoring program on surface, wastewaters, and drinking waters showed it to be characteristically lower than the maximum mandatory level of 10 ng/L using HPLC–ESI–MS/MS.
Spe extraction technique with HPLC identification of chiral β-agonist
Pharmaceutical drugs are among the notable chiral pollutants with numbers exceeding 1500 chiral contaminants around the environment. There is a growing interest in the risk assessment of the environmental outcome of chiral drugs and therefore, enantioselective analyses are considered necessary. Because of the enantioselective biodegradation of chiral pollutants, chiral pollution is a critical concern for human health and the environment believing to have a devastating effect on our society and the scientific community (Citation119,Citation120). As chirality is a key feature of the living characteristic, a variety of guidelines on the processing of chiral chemicals have been issued by regulatory bodies as chiral pollutants currently do not have any regulation or control from the authority (Citation119,Citation121). Residues of chiral β-agonists are difficult to recognize in water matrices due to their comparatively high polarity and the need for a low enantiomer identification (Citation122). Felix(Citation123) presented a set of commercial chiral stationary phases that were best used for the separations of clinical racemic drugs setting the established analytical conditions that deliver the finest enantioselectivity of many β-agonists drugs.
β-agonists do happen as chiral compounds with two different racemic states, both isomers act in a different way causing a conflicting effect. Despite its relevance, the chirality of the β-agonists is frequently overlooked, and little is known about the destiny and consequences of single enantiomers of chiral β-agonists developing in the environment. Because chiral pollutants degrade in an enantioselective manner, they pose a major threat to our health and the environment, causing negative consequences for our culture and science (Citation122). Unfortunately, chiral pollutants are not regulated or controlled, resulting in significant economic losses and at times loss of lives owing to the usage of racemic compounds (Citation119). Macleod (Citation124) performed the first enantiomer fractions (EF) measurement of salbutamol in wastewater and obtained an improved EF measurement with a good average recovery of 78% to 86% and detection limits at 0.1 and 0.4 ng/L for both the effluent and influent respectively. An R2 of more than 0.997 in all the enantiomers using a reverse-phase enantioselective SPE-HPLC-MS-MS system was obtained. Comparable data on salbutamol concentration in wastewaters was also reported in Canada by Lee (Citation6) more than a decade ago.
A multi-residue SPE technique with Chiral-HPLC-MS-MS for the concurrent enantiomeric evaluation of chiral albuterol and clenbuterol and their active metabolites was successfully developed and used in the Guadalquivir River Basin (South Spain) by lopez-serna (Citation125) in 2013. The technique was able to obtain LOD and LOQ levels at low nanograms per liter (< 10 ng/L) precisely, between 3.92 and 4.69 for albuterol and clenbuterol at 1.00–1.07 ng/L with an absolute recovery of more than 50% in all the enantiomers. While a bit higher LOD and LOQ levels were obtained in a Ribeiro study of salbutamol enantiomers, using LC/MS with a triple quadrupole analyzer (QqLIT) on wastewater treatment plant effluents (WWTP) in Portugal (Citation120). The linearity of more than 0.99 was obtained with method identification limits lower than 10 ng/L precisely (R) salbutamol and (S) salbutamol were 5.07 ng/L and 6.29 respectively, and LOQ of 15.4 and 19.1 ng/L for both (R) and (S) enantiomers. The existence of both enantiomers was established utilizing two MS/MS transitions and their ion ratios permitted by the European Commission Decision 2002/657/EC (Citation106).
Direct chiral reverse-phase RP-HPLC with SPE extraction technique was used to analyze salbutamol in natural water where Rosales-Conrado obtained reproducible recoveries between 77 and 98%, with negligible matrix effect and LOD between 0.013–0.018 µg/mL (Citation122). While the full and extensive investigation of salbutamol as a chiral pharmacologically active compound in water and digested-sludge at enantiomeric level addressed the use of Chiral-HPLC-MS-MS with microwave-assisted extraction (MAE-SPE) through a multi-residue approach (Citation98). The overall result for S (+) salbutamol and R (-)salbutamol study gave a relative percentage recovery (% R) of 44.5 ± 5.8 and 51.4 ± 12.5 and detection limit of 2.20 and 2.22 ng/L with the LOQ of 7.32 and 7.41 ng/L for wastewater influent and the %R of 46.9 ± 20.9 and 64.2 ± 13.6 MDL of 0.98 and 1.31 ng/L with LOQ of 3.26 and 4.36 ng/L for wastewater effluent. The %R of 0.8 ± 0.2 and 0.5 ± 0.2 and MDL of 60.03 and 80.03 ng/g with LOQ of 225.1 and 265.1 ng/g for digested sludge (1 kg). The consequence and transportation of ractopamine in the management of wastewaters were also evaluated by Hakk (Citation58) and values around 42.5–51.6% which were much lower than Rosales (Citation122) and, Evans (Citation98) percentage values were obtained.
Solid phase extraction techniques with Gas Chromatography (GC)
Previous research discovered that gas chromatography (GC) combined with incredibly sensitive mass spectroscopy (MS) can provide a quantitative and qualitative analysis of β-agonists, although the analytes must always be derivatized owing to the polar nature of β-agonists, which is possibly attributable to the amines present in the compound (Citation9). The systematic survey conducted proved that there is insufficient documented data on the derivatization and MS analysis of β-agonists (Citation92).
The available researches include the analytical methods for the rapid determination of salbutamol in several aqueous samples that have been developed and presented by Jones (Citation126). Salbutamol with the method detection limit of 1.01 ppb in the selected ion monitoring (SIM) mode and a LOQ of 2.67 ppb was obtained using GC-EI-MS with a derivatized N-methyl-N-trimethylsilyl trifluoroacetamide and recovery from 24 to 51%. GC-MS with SPE method analysis was also utilized to determine the concentration of terbutaline in reclaimed wastewaters and a good method detection limit of 15 and 35 ng/L were obtained from both the effluent and influent (Citation67). As non-volatile metabolites, a derivatization phase is usually needed to increase the number of measurable β-agonists when using GC for its determination to increase the number of detectable compounds. Bisceglia (Citation127) developed a GC method that was able to satisfy the environmental community’s need with an MDL of 2 ng/L and minimum reporting limit of 6 ng/L for the analysis of Albuterol as an important aquatic pollutant by derivatizing with N,O-Bis (trimethylsilyl)trifluoroacetamide (BSTFA) in wastewater. In another development, a method including SPE, silylation derivatization, and GC/MS detection allows detection of β-agonists down to 5 ng/L, with recovery rates exceeding 70% was also presented by Ternes (Citation128). Salbutamol, fenoterol, clenbuterol, and terbutaline were quantified down to 5 ng/L in drinking and river water in Germany, while their concentrations in the STP as well as in the streams and small rivers, were up to 50 ng/L quantified using the SPE technique. The discovery of the contamination of clenbuterol in Portuguese food led to a detailed assessment of the illicit use of clenbuterol in certain cattle farms via some sample sources including drinking water (Citation116). Of all the samples tested, drinking water was found to have the lowest percentage of β-agonists of approximately 14.15% and was found to be positive in concentrations of around 0.03–3.80 mg/L of clenbuterol using the GC-MS method as an analytical instrument for the assessment of the drug, in compliance with the criteria defined by Commission Decision No. 2002/657/ CE (Citation106).
Micro-Extraction techniques in β-agonists detection
Analytical microextraction processes were developed with the notion of scaling down SPE. Those procedures may be described as non-exhaustive sample preparation stages that use a very tiny amount of extraction phase (solid, semi-solid polymeric, or liquid substance) in relation to the sample volume (microliter range or smaller) (Citation129). The micro-extraction provides fewer compound recoveries with a lower volume of organic solvent but significantly improves the final concentration in the organic phase. The amounts of solvent used during the extraction process are also significantly decreased. It is regularly used as a pre-concentration step before the determination stage. Alternative sample pre-treatment such as micro-extraction phases were employed to help detect the analytes in lower concentrations since their concentrations are low (ng/L, μg/L or less) in solutions, and due to the complicated matrices in which they are found, their direct determination tend to be restricted. Analyte enrichment through a good sample preparation that has a pre-concentration effect is needed to help in the successful detection of these ionic compounds that usually happen at trace and ultra-trace concentration levels. Their recalcitrant nature, high polarity, and low volatility made them (β-agonists) persistent specifically in water and therefore bringing the need for some extra analytical techniques to be employed to obtain the compounds found.
Micro extractions with HPLC
Positive achievements have been recorded by researchers’ in terms of minimizing the use of solvents to obtaining the lowest possible concentrations of a β-agonists compound in a water matrix. Determination of salbutamol and terbutaline in water samples around our environment in concentrations around ng/mL with RSD < 10% was made possible by Yamini (Citation89) using HPLC–DAD and HPLC–MS. This was done through 3-phase liquid-phase microextraction (LPME) approach to extract and pre-concentrate the analytes from aqueous solutions with a detection limit of 2.5 and 0.5 ng/mL respectively. Rezazadeh (Citation50), uses the same condition as Yamini (Citation89) for the determination and quantification of salbutamol and terbutaline in wastewaters samples. The separation of the base line at a retention time of 6.5 and 7.6 min was obtained at a total run-time of 25 min using HPLC with a UV detector. Electrically assisted-LPME (EA-LPME) was used to obtain exclusive possible detectability of 10 and 5 ng/mL of extract concentrations for every analyte with high-correlation coefficients (R2) of 0.995 and 0.997 for salbutamol and terbutaline, respectively.
Micro Extractions with GC
The Microextraction technique was also employed for the trace pre-concentrations and extractions of β-agonists using the GC column. Solid-phase microextraction allowed for a very low detection limit of 1.1 and 0.20 ppb and good recovery of 80–90% achievement using on-fiber derivatization by GC coupled with Flame Ionization Detector (GC-FID) and Selected Ion Monitoring Mass Spectrometry (GC-SIMMS) by Engelmann (Citation5) on the quantification of clenbuterol hexamethyldisilazane derivative. With the added advantages of SPME like extraction in a limited amount of solvent or solvent-free extraction, concentration as well as the introduction of complete analytes at a step, the preparation time will be reduced and at the same time with increased sensitivity compared to some extraction approaches.
Conclusion and future recommendations
The achievement of faster, more accurate analytical methods with sufficient sensitivity and selectivity depends on the advancement of all the measures routinely used in analytical methods starting from sampling to data collection and analysis. The improvement of the sample preparation stage, the addition of one or more operations involving the extraction of the target analytes by eliminating any possible interrupting compounds before the final separation, identification, and quantification is often emphasized.
From the reviewed literature, it is obvious that the leading method employed in the determination of β-agonists from water medium is mainly HPLC with UV detection, MS or MS/MS for superior sensitivity. However, before the analytical determination, sample pre-treatment is necessary to remove interfering matrices components and to pre-concentrate the samples for trace analysis of the analytes.
Nowadays, chromatographic and electrophoretic procedures are regarded as suitable for obtaining high selectivity and sensitivity. In some ways, the two procedures are identical, such as in terms of extraction, purification procedures, and detectors.
β-agonists can consequently be easily identified using the electrochemical detection (ED) method since they are electroactive compounds. Capillary electrophoresis with electrochemical detection (CE–ED) devices have been found to give an exceptional separation ability, therefore, more research on these analytes in water matrices is recommended as the previously conducted analysis gave a good detection result. CE has made some progress in the field of speciation, which has been reported from time to time. The hyphenation technology (which combines detection and sample preparation equipment) is a significant step forward in CE, where its sensitivity and selectivity have been improved by linking these units with it. However, no study on this focus has been identified in the literature, these couplings might as well be used to separate β-agonists in water.
Furthermore, with the alarming reports of unauthorized use of novel agonists and analogs of agonists that are directly or indirectly transported to our environmental water, the researchers find difficulties in identifying these compounds employing the common techniques, more rapid screening techniques that comprise broad-spectrum detection techniques for rapid identification of the new β-agonists compounds in water samples are hereby recommended.
Much more new nanomaterials should be created for the development of chemiluminescence sensors for improved sensitivity, accuracy, and stability. Due to its benefits of rapidity, low LOD, large linearity, minimal need for complex devices, and easy operation, chemical-light sensors can extensively be explored and be used in the determination of β-agonists in our environment.
With the idea that SPE is the background of all extraction techniques, the progress of traditional SPE technology has been constrained by a few shortcomings such as limited (sorbents) reusability, low (limited) capacity, restricted physical and chemical stabilities at a low or high pH. Thus, to facilitate the extraction technique of SPE in complicated water matrices, exploration of viable sorbent that can overcome all these shortcomings and even bring out more possibilities of obtaining a very sensitive, fast, and lower-cost analysis is advised.
In line with the above recommendations, the progress in material science has seen the production of new materials like carbon nanotubes, graphene, and the recently discovered metal–organic-frameworks (MOFs). Of all these, MOFs and it’s composite are particularly interesting due to their remarkably high porosity and surface area, lending them as potential adsorbents. MOFs-based composites have excellent physical and chemical properties, allowing them to be used in a wider range of advanced applications most especially in the protection of the environment, and industrial wastewater treatment. The integration of MOFs with functional species or matrix materials not only enhances properties but also extends the variety of applications for MOFs.
Magnetic-SPE (MSPE) constructed on the usage of functionalized magnetic-sorbents is advised to be explored more in this perspective, as the extraction of amine compounds comes with a lot of problems because they have very low solubility (considering their kpa values of above 7) in addition to their charge which normally rendered their attraction very poor; in this case, employing the MSPE will make the extraction possible to get very good extracts as the β-agonists water happens at a very lower level matrices. MOFs were also believed to show a very good adsorption capability on β-agonists via their porous advantages that are tuneable and rich surface chemistry in general.
The quick, simple, cheap, effective, robust, and safe extraction approach (QuEChERS) involves removing analysis with an acetonitrile and salt solution from a homogenized sample, then cleaning the supernatant using a dispersive SPE (dSPE) approach, is another alternative to direct SPE method. This is because the QuEChERS method is a more user-friendly version of standard LLE and SPE.
Developing a data bank on chiral pollutants, as well as rules for regulating the manufacturing, distribution, and use of racemic chemicals and other industrial products is a pressing requirement in the twenty-first century. The governments of the various countries should take the lead in establishing the legislation concerning β-agonists in our waters around our environment. The leading national and international organizations like The US Food and Drug Administration (FDA), the US Environmental Protection Agency (EPA), and the World Health Organization (WHO) are the most important regulatory bodies that should consider β-agonists especially those occurring in water as well as their occurrence as chiral contaminants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Blanca José, M.P.; Morgado, N.; Miguel, Méndez, T.A.; Angela, R.; Hooghuis, H. Determination of Clenbuterol, Ractopamine and Zilpaterol in Liver and Urine by Liquid Chromatography Tandem Mass Spectrometry. Anal. Chim. Acta 2005,, 199–205. doi:https://doi.org/10.1016/j.aca.2004.09.061.
- Lin, Y.P.; Lee, Y.L.; Hung, C.Y.; Huang, W.J.; Lin, S.C. Determination of Multiresidue Analysis of β-Agonists in Muscle and Viscera Using Liquid Chromatograph/Tandem Mass Spectrometry with Quick, Easy, Cheap, Effective, Rugged, and Safe Methodologies. J. Food Drug Anal. 2017, 25 (2), 275–284. doi:https://doi.org/10.1016/j.jfda.2016.06.010.
- Hassan, M.; M. M., R.A.; Kh, S. Effect of Different Cooking Methods on Ractopamine Residues in Beef. Benha Veterinary Medical Journal 2016, 31 (2), 210–212. doi:https://doi.org/10.21608/bvmj.2016.31299.
- Preechakasedkit, P.; Ngamrojanavanich, N.; Khongchareonporn, N.; Chailapakul, O. Novel Ractopamine–Protein Carrier Conjugation and its Application to the Lateral Flow Strip Test for Ractopamine Detection in Animal Feed. Journal of Zhejiang University-SCIENCE B 2019, 20 (2), 193–204. doi:https://doi.org/10.1631/jzus.B1800112.
- Engelmann, M.D.; Hinz, D.; Wenclawiak, B.W. Solid-phase Micro Extraction (SPME) and Headspace Derivatization of Clenbuterol Followed by GC-FID and GC-SIMMS Quantification. Anal. Bioanal. Chem. 2003, 375 (3), 460–464. doi:https://doi.org/10.1007/s00216-002-1688-x.
- Lee, H.; Sarafin, K.; Peart, T.E. Determination of β-Blockers and β2-Agonists in Sewage by Solid-Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr., A 2007, 1148, 158–167. doi:https://doi.org/10.1016/j.chroma.2007.03.024.
- Polettini, A. Bioanalysis of β2-Agonists by Hyphenated Chromatographic and Mass Spectrometric Techniques. J ChromatograpB: Biomed Sci Appl 1996, 687 (1), 27–42. doi:https://doi.org/10.1016/S0378-4347(96)00015-1.
- He, Z.; Fan, H. Research Progress of Electrochemical Detection of β-Agonists: A Mini-Review. Int. J. Electrochem. Sci. 2019, 14 (10), 9449–9458. doi:https://doi.org/10.20964/2019.10.43.
- Zhang, W.; Wang, P.; Su, X. Current Advancement in Analysis of β-Agonists. TrAC, Trends Anal. Chem. 2016, 85, 1–16. doi:https://doi.org/10.1016/j.trac.2016.08.011.
- Slotkin, T.A.; Seidler, F.J. Terbutaline Impairs the Development of Peripheral Noradrenergic Projections: Potential Implications for Autism Spectrum Disorders and Pharmacotherapy of Preterm Labor. Neurotoxicol. Teratol. 2013, 36, 91–96. doi:https://doi.org/10.1016/j.ntt.2012.07.003.
- Hemmati, M.; Rajabi, M.; Asghari, A. Ultrasound-promoted Dispersive Micro Solid-Phase Extraction of Trace Anti-Hypertensive Drugs from Biological Matrices Using a Sonochemically Synthesized Conductive Polymer Nanocomposite. Ultrason. Sonochem. 2017, 39, 12–24. doi:https://doi.org/10.1016/j.ultsonch.2017.03.024.
- Ternes, T.; Joss, A. Human Pharmaceuticals, Hormones and Fragrances - The Challenge of Micropollutants in Urban Water Management. Water Intelligence Online 2006, 5, 1-475. doi:https://doi.org/10.2166/9781780402468.
- Thomas D.; B. B. Sc, Analysis of some Beta-Adrenergic Agonists in Biological Matrices after Evaluation of Various Extraction Methodologies and Determination Procedures.,” 1994.
- Shen, L.; Li, Z.; He, P. Electrochemical Behavior of β2-Agonists at Graphite Nanosheet Modified Electrodes. Electrochem. Commun. 2010, 12 (7), 876–881. doi:https://doi.org/10.1016/j.elecom.2010.04.010.
- He, P.; Shen, L.; Liu, R.; Luo, Z.; Li, Z. Direct Detection of β-Agonists by Use of Gold Nanoparticle-Based Colorimetric Assays. Anal. Chem. 2011, 83 (18), 6988–6995. doi:https://doi.org/10.1021/ac200769f.
- Yu, Q.; Liu, J.; Zhao, G.; Dou, W. A Silica Nanoparticle Based 2-Color Immunochromatographic Assay for Simultaneous Determination of Clenbuterol and Ractopamine. Microchim. Acta 2019, 186 (7), 421. doi:https://doi.org/10.1007/s00604-019-3529-z.
- U. EPA, “US EPA; Estimation Program Interface (EPI),” US EPA; Estimation Program Interface (EPI) Suite. Ver.3.12. Nov 30, 2004. Available from, as of Apr 18, 2006:2006.
- Yalkowsky H. S; He Y.; J. Parijat, “Handbook of Aqueous Solubility Data,” in Handbook of Aqueous Solubility Data: An Extensive Compilation of Aqueous Solubility Data for Organic Compounds Extracted from the AQUASOL Database, Second., Boca Raton, FL: CRC Press LLC, 2019, p. 1620.
- PubChem, “PubChem Terbutaline,” https://www.drugbank.ca/drugs/DB00871
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The Global Burden of Asthma: Executive Summary of the GINA Dissemination Committee Report. Allergy 2004, 59 (5), 469–478. . doi:https://doi.org/10.1111/j.1398-9995.2004.00526.x.
- Jat, K.R.; Khairwa, A. Levalbuterol Versus Albuterol for Acute Asthma: A Systematic Review and Meta-Analysis. Pulm. Pharmacol. Ther. 2013, 26 (2), 239–248. doi:https://doi.org/10.1016/j.pupt.2012.11.003.
- Sears, M.R. Adverse Effects of β-Agonists. J. Allergy Clin. Immunol. 2002, 110 (6), S322–S328. doi:https://doi.org/10.1067/mai.2002.129966.
- Al-rufaie, M.M.; Abdul, A.; Jawad, R.; Sadiq, H.M. Colorimetric Estimation for Salbutamol-Sulphate in Pure Form and in Different Types of Pharmaceutical. iMedPub Journals 2017, 1, 1–7.
- Y. Tang, Determination of Clenbuterol in Pork and Potable Water Samples by Molecularly Imprinted Polymer Through the use of Covalent Imprinting Method. Food Chem. 2016, 190, 952–959. doi:https://doi.org/10.1016/j.foodchem.2015.06.067.
- Li, C.; Wu, Y.L.; Yang, T.; Zhang, Y.; Huang-Fu, W.G. Simultaneous Determination of Clenbuterol, Salbutamol and Ractopamine in Milk by Reversed-Phase Liquid Chromatography Tandem Mass Spectrometry with Isotope Dilution. J. Chromatogr., A 2010, 1217 (50), 7873–7877. doi:https://doi.org/10.1016/j.chroma.2010.10.055.
- Sakai, N.; Sakai, M.; Mohamad Haron, D.E.; Yoneda, M.; Ali Mohd, M. Beta-agonist Residues in Cattle, Chicken and Swine Livers at the wet Market and the Environmental Impacts of Wastewater from Livestock Farms in Selangor State, Malaysia. Chemosphere 2016, 165, 183–190. doi:https://doi.org/10.1016/j.chemosphere.2016.09.022.
- Aboul-Enein, H.Y.; Serignese, V. Quantitative Determination of Clenbuterol Enantiomers in Human Plasma by High-Performance Liquid Chromatography Using the Macrocyclic Antibiotic Chiral Stationary Phase teicoplanin1. Biomed. Chromatogr. 1999, 13 (8), 520–524. doi:https://doi.org/10.1002/(SICI)1099-0801(199912)13:8<520::AID-BMC919>3.0.CO;2-7.
- Zhang, L.Y.; Chang, B.Y.; Dong, T.; He, P.L.; Yang, W.J.; Wang, Z.Y. Simultaneous Determination of Salbutamol, Ractopamine, and Clenbuterol in Animal Feeds by SPE and LC–MS. J. Chromatogr. Sci. 2009, 47 (4), 324–328. doi:https://doi.org/10.1093/chromsci/47.4.324.
- Lomae, A.; Nantaphol, S.; Kondo, T.; Chailapakul, O.; Siangproh, W.; Panchompoo, J. Simultaneous Determination of β-Agonists by UHPLC Coupled with Electrochemical Detection Based on Palladium Nanoparticles Modified BDD Electrode. J. Electroanal. Chem. 2019, 840 (April), 439–448. doi:https://doi.org/10.1016/j.jelechem.2019.04.003.
- Li, Y.; Zhang, W.; Wang, R.G.; Wang, P.L.; Su, X.O. Development of a Efficient and Sensitive Dispersive Liquid-Liquid Microextraction Technique for Extraction and Preconcentration of 10 β2-Agonists in Animal Urine. PLoS One 2015, 10 (9), e0137194–16. doi:https://doi.org/10.1371/journal.pone.0137194.
- Reig, M.; Batlle, N.; Navarro, J.L.; Toldrá, F. Stability of β-Agonist Methyl Boronic Derivatives Before gas Chromatography–Mass Spectrometry Analysis. Anal. Chim. Acta 2005, 529 (1–2), 293–297. doi:https://doi.org/10.1016/j.aca.2004.07.047.
- de de Boer, T.; Bijma, R.; Ensing, K. Modelling of Conditions for the Enantiomeric Separation of β2-Adrenergic Sympathicomimetics by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors in a Polyethylene Glycol gel. J. Pharm. Biomed. Anal. 1999, 19 (3–4), 529–537. doi:https://doi.org/10.1016/S0731-7085(98)00249-0
- Liu, W. Simultaneous Determination of Blockers and Agonists by on-Fiber Derivatization in Self-Made Solid-Phase Microextraction Coating Fiber. Talanta 2015, 132, 915–921. doi:https://doi.org/10.1016/j.talanta.2014.07.064.
- Politi, L.; Groppi, A.; Polettini, A. Applications of Liquid Chromatography—Mass Spectrometry in Doping Control*. J. Anal. Toxicol. 2005, 29 (1), 1–14. doi:https://doi.org/10.1093/jat/29.1.1
- Vulić, A.; Pleadin, J.; Durgo, K.; Scortichini, G.; Stojković, R. Comparison of Clenbuterol and Salbutamol Accumulation in the Liver of two Different Mouse Strains. J. Anal. Toxicol. 2014, 38 (5), 265–271. doi:https://doi.org/10.1093/jat/bku022.
- Beucher, L.; Dervilly-Pinel, G.; Prévost, S.; Monteau, F.; Le Bizec, B. Determination of a Large Set of β-Adrenergic Agonists in Animal Matrices Based on Ion Mobility and Mass Separations. Anal. Chem. 2015, 87 (18), 9234–9242. doi:https://doi.org/10.1021/acs.analchem.5b01831.
- Brambilla, G. Clinical and Pharmacological Profile in a Clenbuterol Epidemic Poisoning of Contaminated Beef Meat in Italy. Toxicol. Lett. 2000, 114 (1–3), 47–53. doi:https://doi.org/10.1016/S0378-4274(99)00270-2
- Yan, K., et al. Rapid Screening of Toxic Salbutamol, Ractopamine, and Clenbuterol in Pork Sample by High-Performance Liquid Chromatography—UV Method. J. Food Drug Anal. 2016, 24 (2), 277–283. doi:https://doi.org/10.1016/j.jfda.2015.12.002.
- Han, S.; Zhou, T.; Yin, B.; He, P. A Sensitive and Semi-Quantitative Method for Determination of Multi-Drug Residues in Animal Body Fluids Using Multiplex Dipstick Immunoassay. Anal. Chim. Acta 2016, 927, 64–71. doi:https://doi.org/10.1016/j.aca.2016.05.004.
- Hignite, C.; Azarnoff, D.L. Drugs and Drug Metabolites as Environmental Contaminants: Chlorophenoxyisobutyrate and Salicylic Acid in Sewage Water Effluent. Life Sci. 1977, 20 (2), 337–341. doi:https://doi.org/10.1016/0024-3205(77)90329-0.
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of Pharmaceutical Pollutants Onto Graphene Nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. doi:https://doi.org/10.1016/j.cej.2014.03.023.
- Leyssens, J.; Driessen, L.; Jacobs, C.; Czech, A.; Raus, J. Determination of β2-Receptor Agonists in Bovine Urine and Liver by gas Chromatography—Tandem Mass Spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications 1991, 564, 515–527. doi:https://doi.org/10.1016/0378-4347(91)80520-M.
- Mastrianni, K.R.; Metavarayuth, K.; Brewer, W.E.; Wang, Q. Analysis of 10 β-Agonists in Pork Meat Using Automated Dispersive Pipette Extraction and LC-MS/MS. J. Chromatogr. B 2018, 1084, 64–68. doi:https://doi.org/10.1016/j.jchromb.2018.03.026.
- Ternes, T.A. Occurrence of Drugs in German Sewage Treatment Plants and rivers1Dedicated to Professor Dr. Klaus Haberer on the Occasion of his 70th Birthday.1. Water Res. 1998, 32 (11), 3245–3260. doi:https://doi.org/10.1016/S0043-1354(98)00099-2.
- Kalambate, P.K.; Rawool, C.R.; Srivastava, A.K. Fabrication of Graphene Nanosheet-Multiwalled Carbon Nanotube-Polyaniline Modified Carbon Paste Electrode for the Simultaneous Electrochemical Determination of Terbutaline Sulphate and Guaifenesin. New J. Chem. 2017, 41 (15), 7061–7072. doi:https://doi.org/10.1039/C7NJ00101K
- Baresel, C.; Malmborg, J.; Ek, M.; Sehlén, R. Removal of Pharmaceutical Residues Using Ozonation as Intermediate Process Step at Linköping WWTP, Sweden. Water Sci. Technol. 2016, 73 (8), 2017–2024. doi:https://doi.org/10.2166/wst.2016.045
- Selkälä, T. Rapid Uptake of Pharmaceutical Salbutamol from Aqueous Solutions with Anionic Cellulose Nanofibrils: The Importance of pH and Colloidal Stability in the Interaction with Ionizable Pollutants. Chem. Eng. J Oct. 2018, 350, 378–385. doi:https://doi.org/10.1016/j.cej.2018.05.163.
- Jaimes-Correa, J.C.; Snow, D.D.; Bartelt-Hunt, S.L. Seasonal Occurrence of Antibiotics and a Beta Agonist in an Agriculturally-Intensive Watershed. Environ. Pollut. 2015, 205, 87–96. doi:https://doi.org/10.1016/j.envpol.2015.05.023.
- O’Neill J. “Antimicrobials in agriculture and the environment: Reducing unnecessary use and waste,” Rev. Antimicrob. Resist., no. December, pp. 1–28, 2015, [Online]. Available: https://amr-review.org/sites/default/files/Antimicrobials in agriculture and the environment - Reducing unnecessary use and waste.pdf.
- Rezazadeh, M.; Yamini, Y.; Seidi, S. Electrically Assisted Liquid-Phase Microextraction for Determination of β2-Receptor Agonist Drugs in Wastewater. J. Sep. Sci. 2012, 35 (4), 571–579. doi:https://doi.org/10.1002/jssc.201100869.
- Sakai, N.; Mohd Yusof, R.; Sapar, M.; Yoneda, M.; Ali Mohd, M. Spatial Analysis and Source Profiling of Beta-Agonists and Sulfonamides in Langat River Basin, Malaysia. Sci. Total Environ. 2016, 548-549, 43–50. doi:https://doi.org/10.1016/j.scitotenv.2016.01.040.
- Dodson, L.G.; Vogt, R.A.; Marks, J.; Reichardt, C.; Crespo-Hernández, C.E. Photophysical and Photochemical Properties of the Pharmaceutical Compound Salbutamol in Aqueous Solutions. Chemosphere 2011, 83 (11), 1513–1523. doi:https://doi.org/10.1016/j.chemosphere.2011.01.048.
- Sirichai, S.; Khanatharana, P. Rapid Analysis of Clenbuterol, Salbutamol, Procaterol, and Fenoterol in Pharmaceuticals and Human Urine by Capillary Electrophoresis. Talanta 2008, 76 (5), 1194–1198. doi:https://doi.org/10.1016/j.talanta.2008.05.026.
- Li, M. Ultrasensitive and Quantitative Detection of a New β-Agonist Phenylethanolamine A by a Novel Immunochromatographic Assay Based on Surface-Enhanced Raman Scattering (SERS). J. Agric. Food Chem. 2014, 62, 10896–10902. doi:https://doi.org/10.1021/jf503599x.
- Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells. Environ. Sci. Technol. 2006, 40 (7), 2442–2447, doi:https://doi.org/10.1021/es051715a.
- Pomati, F.; Cotsapas, C.J.; Castiglioni, S.; Zuccato, E.; Calamari, D. Gene Expression Profiles in Zebrafish (Danio Rerio) Liver Cells Exposed to a Mixture of Pharmaceuticals at Environmentally Relevant Concentrations. Chemosphere 2007, 70 (1), 65–73. doi:https://doi.org/10.1016/j.chemosphere.2007.07.048.
- Zhuang, Z., et al. Adverse Effects from Clenbuterol and Ractopamine on Nematode Caenorhabditis Elegans and the Underlying Mechanism. PLoS One 2014, 9 (1), e85482–11. doi:https://doi.org/10.1371/journal.pone.0085482.
- Hakk, H.; Shelver, W.L.; Casey, F.X.M. Fate and Transport of the β-Adrenergic Agonist Ractopamine Hydrochloride in Soil–Water Systems. J. Environ. Sci. 2016, 45, 40–48.. doi:https://doi.org/10.1016/j.jes.2015.11.026.
- Martin, O.V.; Voulvoulis, N. Sustainable Risk Management of Emerging Contaminants in Municipal Wastewaters. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2009, 367 (1904), 3895–3922. doi:https://doi.org/10.1098/rsta.2009.0115.
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary Drugs in the Environment and Their Toxicity to Plants. Chemosphere 2016, 144, 2290–2301. doi:https://doi.org/10.1016/j.chemosphere.2015.10.137.
- Kuiper, H.A.; Noordam, M.Y.; van Dooren-Flipsen, M.M.; Schilt, R.; Roos, A.H. Illegal use of Beta-Adrenergic Agonists: European Community. J. Anim. Sci. 1998, 76 (1), 195–207. doi:https://doi.org/10.2527/1998.761195x.
- Tang, Y.; Fang, G.; Wang, S.; Li, J. Covalent Imprinted Polymer for Selective and Rapid Enrichment of Ractopamine by a Noncovalent Approach. Anal. Bioanal. Chem. 2011, 401 (7), 2275–2282. doi:https://doi.org/10.1007/s00216-011-5280-0.
- Li, M. Simultaneous Determination of β 2 -Agonists Clenbuterol and Salbutamol in Water and Swine Feed Samples by Dual-Labeled Time-Resolved Fluoroimmunoassay. Food Control 2017, 73, 1039–1044. doi:https://doi.org/10.1016/j.foodcont.2016.10.019.
- Li, G.; Zhang, X.; Zheng, F.; Liu, J.; Wu, D. Emerging Nanosensing Technologies for the Detection of β-Agonists. Food Chem. 2020, 332. doi:https://doi.org/10.1016/j.foodchem.2020.127431.
- WHO, “Pharmaceuticals in drinking-water,” 2012. [Online]. Available: ISBN 978 92 4 150208 5.
- NACWA, “Pharmaceuticals in the Water Environment,” Assoc. Metrop. Water Agencies, p. 38, 2011, [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/44630/9789241502085_eng.pdf?sequence=1.
- Yu, Y.; Wu, L. Comparison of Four Extraction Methods for the Analysis of Pharmaceuticals in Wastewater. J. Chromatogr., A 2011, 1218 (18), 2483–2489. doi:https://doi.org/10.1016/j.chroma.2011.02.050.
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging Pollutants in Sewage, Surface and Drinking Water in Galicia (NW Spain). Chemosphere 2012, 86 (10), 1040–1049. doi:https://doi.org/10.1016/j.chemosphere.2011.11.053.
- Petrovic, M. Analysis and Removal of Emerging Contaminants in Wastewater and Drinking Water. TrAC, Trends Anal. Chem. 2003, 22 (10), 685–696. doi:https://doi.org/10.1016/S0165-9936(03)01105-1.
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107 (6), 907–938. doi:https://doi.org/10.1289/ehp.99107s6907.
- Quintana, J.B.; Rodil, R.; Cela, R. Reaction of β-Blockers and β-Agonist Pharmaceuticals with Aqueous Chlorine. Investigation of Kinetics and by-Products by Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Anal. Bioanal. Chem. 2012, 403 (8), 2385–2395. doi:https://doi.org/10.1007/s00216-011-5707-7.
- Castiglioni, S.; Fanelli, R.; Calamari, D.; Bagnati, R.; Zuccato, E. Methodological Approaches for Studying Pharmaceuticals in the Environment by Comparing Predicted and Measured Concentrations in River Po, Italy. Regul. Toxicol. Pharmacol. 2004, 39 (1), 25–32. doi:https://doi.org/10.1016/j.yrtph.2003.10.002.
- Bound, J.P.; Voulvoulis, N. Predicted and Measured Concentrations for Selected Pharmaceuticals in UK Rivers: Implications for Risk Assessment. Water Res. 2006, 40 (15), 2885–2892. doi:https://doi.org/10.1016/j.watres.2006.05.036.
- Salem, A.A.; Wasfi, I.A.; Al-Nassibi, S.S. Trace Determination of β-Blockers and β2-Agonists in Distilled and Waste-Waters Using Liquid Chromatography-Tandem Mass Spectrometry and Solid-Phase Extraction. J. Chromatogr. B 2012, 908, 27–38. doi:https://doi.org/10.1016/j.jchromb.2012.09.026.
- Gu, C.; Ren, P.; Zhang, F.; Zhao, G.; Shen, J.; Zhao, B. Detection of Six β-Agonists by Three Multiresidue Immunosensors Based on an Anti-Bovine Serum Albumin-Ractopamine-Clenbuterol-Salbutamol Antibody. ACS Omega 2020, 5,, 5548–5555.. doi:https://doi.org/10.1021/acsomega.0c00249.
- Gilbert, J.H.Z.S. Immunoaffinity Column Clean-up Techniques in Food Analysis: A Review. J. Chromatogr. B 2010, 878, 115–132. doi:https://doi.org/10.1016/j.jchromb.2009.05.042.
- Von Urin-schwangerschaftstests, H.Z. Strips of Hope: Accuracy of Home Pregnancy Tests and New Developments. Geburtshilfe. Frauenheilkd. 2014, 74, 661–669. doi:https://doi.org/10.1055/s-0034-1368589
- Jie, G.; Zhang, J.; Wang, D.; Cheng, C.; Chen, H.; Zhu, J. Electrochemiluminescence Immunosensor Based on CdSe Nanocomposites. Anal. Chem. 2008, 80 (11), 4033–4039. doi:https://doi.org/10.1021/ac800052g.
- Cun, L.; Wu, Y.L.; Yang, T.; Zhang, Y.; Huang-Fu, W.G. Simultaneous Determination of Clenbuterol, Salbutamol and Ractopamine in Milk by Reversed-Phase Liquid Chromatography Tandem Mass Spectrometry with Isotope Dilution. J. Chromatogr., A 2010, 1217 (50), 7873–7877. doi:https://doi.org/10.1016/j.chroma.2010.10.055.
- Liu, Z. Highly Efficient Detection of Salbutamol in Environmental Water Samples by an Enzyme Immunoassay. Sci. Total Environ. 2018, 613-614, 861–865. doi:https://doi.org/10.1016/j.scitotenv.2017.08.324.
- Lei, Y.; Yao, X.; Zhang, D.; Zhou, L. Investigation of the Salbutamol Residue Level in Human Urinary Samples by a Sensitive Direct Competitive ELISA. Anal. Methods 2015, 7, 5635–5640. doi:https://doi.org/10.1039/C5AY00544B.
- Keeley, G.P.; Lyons, M.E.G. The Effects of Thin Layer Diffusion at Glassy Carbon Electrodes Modified with Porous Films of Single-Walled Carbon Nanotubes. Int. J. Electrochem. Sci 2009, 4 (6), 794–809.
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit. Rev. Anal. Chem. 2017, 47 (2), 138–153. doi:https://doi.org/10.1080/10408347.2016.1233805.
- Tang, Y. Upconversion Particles Coated with Molecularly Imprinted Polymers as Fluorescence Probe for Detection of Clenbuterol. Biosens. Bioelectron. 2015, 71, 44–50., doi:https://doi.org/10.1016/j.bios.2015.04.005
- Yang, H. Determination and Removal of Clenbuterol with a Stable Fluorescent Zirconium(IV)-Based Metal Organic Framework. Microchim. Acta 2019, 186 (7), 454. doi:https://doi.org/10.1007/s00604-019-3586-3.
- Sarafraz-Yazdi, A.; Amiri, A. Liquid-phase Microextraction. TrAC, Trends Anal. Chem. 2010, 29 (1), 1–14. doi:https://doi.org/10.1016/j.trac.2009.10.003.
- Fatta-kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399 (1), 251–275, doi:https://doi.org/10.1007/s00216-010-4300-9.
- Caban, M.; Migowska, N.; Stepnowski, P.; Kwiatkowski, M.; Kumirska, J. Matrix Effects and Recovery Calculations in Analyses of Pharmaceuticals Based on the Determination of β-Blockers and β-Agonists in Environmental Samples. J. Chromatogr., A 2012, 1258, 117–127. doi:https://doi.org/10.1016/j.chroma.2012.08.029.
- Yamini, Y.; Reimann, C.T.; Vatanara, A.; Jönsson, JÅ. Extraction and Preconcentration of Salbutamol and Terbutaline from Aqueous Samples Using Hollow Fiber Supported Liquid Membrane Containing Anionic Carrier. J. Chromatogr., A 2006, 1124 (1–2), 57–67. doi:https://doi.org/10.1016/j.chroma.2006.05.001.
- Lacorte, S.; Ferna, A.R. Pilot Survey Monitoring Pharmaceuticals and Related Compounds in a Sewage Treatment Plant Located on the Mediterranean Coast. Chemosphere 2007, 66, 993–1002. doi:https://doi.org/10.1016/j.chemosphere.2006.07.051.
- Liu, W. Novel Multifunctional Acceptor Phase Additive of Water-Miscible Ionic Liquid in Hollow-Fiber Protected Liquid Phase Microextraction. Talanta 2012, 88, 43–49. doi:https://doi.org/10.1016/j.talanta.2011.08.017.
- Caban, M.; Stepnowski, P.; Kwiatkowski, M.; Migowska, N.; Kumirska, J. Determination of β-Blockers and β-Agonists Using gas Chromatography and gas Chromatography-Mass Spectrometry - A Comparative Study of the Derivatization Step. J. Chromatogr., A 2011, 1218 (44), 8110–8122. doi:https://doi.org/10.1016/j.chroma.2011.08.093.
- Castiglioni, S.; Bagnati, R.; Calamari, D.; Fanelli, R.; Zuccato, E. A Multiresidue Analytical Method Using Solid-Phase Extraction and High-Pressure Liquid Chromatography Tandem Mass Spectrometry to Measure Pharmaceuticals of Different Therapeutic Classes in Urban Wastewaters. J. Chromatogr., A 2005, 1092, 206–215. doi:https://doi.org/10.1016/j.chroma.2005.07.012.
- Banerjee, S.; Mazumdar, S. Electrospray Ionization Mass Spectrometry: A Technique to Access the Information Beyond the Molecular Weight of the Analyte. Int. J. Anal. Chem. 2012, 2012, 1–40. doi:https://doi.org/10.1155/2012/282574.
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S. Multi-residue Analytical Method for Human Pharmaceuticals and Synthetic Hormones in River Water and Sewage Effluents by Solid-Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr., A 2010, 1217 (44), 6791–6806.doi:https://doi.org/10.1016/j.chroma.2010.08.033.
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue Method for the Determination of Basic/Neutral Pharmaceuticals and Illicit Drugs in Surface Water by Solid-Phase Extraction and Ultra Performance Liquid Chromatography-Positive Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr., A 2007, 1161 (1–2), 132–45. doi:https://doi.org/10.1016/j.chroma.2007.05.074.
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multiresidue Methods for the Analysis of Pharmaceuticals, Personal Care Products and Illicit Drugs in Surface Water and Wastewater by Solid-Phase Extraction and Ultra Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2008, 391 (4), 1293–1308. doi:https://doi.org/10.1007/s00216-008-1854-x.
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of Chiral Pharmaceuticals and Illicit Drugs in Wastewater and Sludge Using Microwave Assisted Extraction, Solid-Phase Extraction and Chiral Liquid Chromatography Coupled with Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 882, 112–126. doi:https://doi.org/10.1016/j.aca.2015.03.039.
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Pharmaceuticals in the Environment. Environ. Chem. Lett. 2012, 10 (3), 239–253. doi:https://doi.org/10.1007/s10311-011-0352-0.
- Gros, M.; Petrovic, M. Tracing Pharmaceutical Residues of Different Therapeutic Classes in Environmental Waters by Using Liquid Chromatography/Quadrupole-Linear Ion Trap Mass Spectrometry and Automated Library Searching. Anal. Chem. 2009, 81 (3), 898–912. doi:https://doi.org/10.1021/ac801358e.
- Magnér, J.A.; Alsberg, T.E.; Broman, D. Bag-SPE—a Convenient Extraction Method for Screening of Pharmaceutical Residues in Influent and Effluent Water from Sewage Treatment Plants. Anal. Bioanal. Chem. 2009, 395 (5), 1481–1489. doi:https://doi.org/10.1007/s00216-009-3099-8.
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Fast Liquid Chromatography-Quadrupole-Linear ion Trap Mass Spectrometry for the Analysis of Pharmaceuticals and Hormones in Water Resources. J. Chromatogr., A 2010, 1217 (25), 4212–4222. doi:https://doi.org/10.1016/j.chroma.2009.11.007.
- Batt, A.L.; Kostich, M.S.; Lazorchak, J.M. Analysis of Ecologically Relevant Pharmaceuticals in Wastewater and Surface Water Using Selective Solid-Phase Extraction and UPLC−MS/MS. Anal. Chem. 2008, 80 (13), 5021–5030. doi:https://doi.org/10.1021/ac800066n.
- Petrović, M.; Škrbić, B.; Živančev, J.; Ferrando-Climent, L.; Barcelo, D. Determination of 81 Pharmaceutical Drugs by High Performance Liquid Chromatography Coupled to Mass Spectrometry with Hybrid Triple Quadrupole-Linear ion Trap in Different Types of Water in Serbia. Sci. Total Environ. 2014, 468-469, 415–428. doi:https://doi.org/10.1016/j.scitotenv.2013.08.079.
- Juan, C.; Igualada, C.; Moragues, F.; León, N.; Mañes, J. Development and Validation of a Liquid Chromatography Tandem Mass Spectrometry Method for the Analysis of β-Agonists in Animal Feed and Drinking Water. J. Chromatogr., A 2010, 1217 (39), 6061–6068. doi:https://doi.org/10.1016/j.chroma.2010.07.034.
- Commission, D.E. “COMMISSION DECISION of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Communities 2002, 657, 8–36.
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital Effluent: Investigation of the Concentrations and Distribution of Pharmaceuticals and Environmental Risk Assessment. Sci. Total Environ. 2012, 430, 109–118. doi:https://doi.org/10.1016/j.scitotenv.2012.04.055.
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of Pharmaceutically Active Compounds in the Rivers and tap Water of the Madrid Region (Spain) and Potential Ecotoxicological Risk. Chemosphere 2011, 84 (10), 1336–1348. doi:https://doi.org/10.1016/j.chemosphere.2011.05.014.
- Bueno, M.J.M. Pilot Survey of Chemical Contaminants from Industrial and Human Activities in River Waters of Spain. Int. J. Environ. Anal. Chem 2010, 90 (3–6), 321–343. doi:https://doi.org/10.1080/03067310903045463.
- López-Serna, R.; Petrović, M.; Barceló, D. Occurrence and Distribution of Multi-Class Pharmaceuticals and Their Active Metabolites and Transformation Products in the Ebro River Basin (NE Spain). Sci. Total Environ. 2012, 440, 280–289. doi:https://doi.org/10.1016/j.scitotenv.2012.06.027.
- Grabic, R.; Fick, J.; Lindberg, R.H.; Fedorova, G.; Tysklind, M. Multi-residue Method for Trace Level Determination of Pharmaceuticals in Environmental Samples Using Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Talanta 2012, 100, 183–195. doi:https://doi.org/10.1016/j.talanta.2012.08.032.
- Yu, T.-H.; Lin, A.Y.-C.; Wang, X.-H.; Lin, C.-F. Occurrence of β-Blockers and β-Agonists in Hospital Effluents and Their Receiving Rivers in Southern Taiwan. Desalin. Water Treat Aug. 2011, 32 (1–3), 49–56. doi:https://doi.org/10.5004/dwt.2011.2677
- Lin, A.Y.-C.; Yu, T.-H.; Lin, C.-F. Pharmaceutical Contamination in Residential, Industrial, and Agricultural Waste Streams: Risk to Aqueous Environments in Taiwan. Chemosphere 2008, 74 (1), 131–141. doi:https://doi.org/10.1016/j.chemosphere.2008.08.027.
- Dearden, J.C.; Nicholson, R.M. QSAR STUDY OF THE FATE OF PHARMACEUTICAL CHEMICALS IN AN AQUATIC ENVIRONMENT. J. Pharm. Pharmacol. 2011, 37 (S12), 71P–71P. doi:https://doi.org/10.1111/j.2042-7158.1985.tb14143.x.
- Calamari, D.; Zuccato, E.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Strategic Survey of Therapeutic Drugs in the Rivers Po and Lambro in Northern Italy. Environ. Sci. Technol. 2003, 37 (7), 1241–1248. doi:https://doi.org/10.1021/es020158e.
- Ramos, F.; Baeta, M.L.; Reis, J.; Silveira, M.I.N. Evaluation of the Illegal use of Clenbuterol in Portuguese Cattle Farms from Drinking Water, Urine, Hair and Feed Samples. Food Additives & Contaminants: Part A 2009, 26 (6), 814–820. doi:https://doi.org/10.1080/02652030902729908.
- Salgado, R. Assessing the Diurnal Variability of Pharmaceutical and Personal Care Products in a Full-Scale Activated Sludge Plant. Environ. Pollut Oct. 2011, 159 (10), 2359–2367. doi:https://doi.org/10.1016/j.envpol.2011.07.004.
- Brown, D.; Snow, D.; Hunt, G.A.; Bartelt-Hunt, S.L. Persistence of Pharmaceuticals in Effluent-Dominated Surface Waters. J. Environ. Qual. 2015, 44 (1), 299–304.. doi:https://doi.org/10.2134/jeq2014.08.0334.
- Basheer, A.A. Chemical Chiral Pollution: Impact on the Society and Science and Need of the Regulations in the 21stCentury. Chirality 2018, 30 (4), 402–406. doi:https://doi.org/10.1002/chir.22808.
- Ribeiro, A.R.; Santos, L.H.M.L.M.; Maia, A.S.; Delerue-Matos, C.; Castro, P.M.L.; Tiritan, M.E. Enantiomeric Fraction Evaluation of Pharmaceuticals in Environmental Matrices by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr., A 2014, 1363, 226–235. doi:https://doi.org/10.1016/j.chroma.2014.06.099.
- West, C. Enantioselective Separations with Supercritical Fluids - Review. Curr. Anal. Chem. 2014, 10 (1), 99–120.
- Rosales-Conrado, N.; Dell’Aica, M.; de León-González, M.E.; Pérez-Arribas, L.V.; Polo-Díez, L.M. Determination of Salbutamol by Direct Chiral Reversed-Phase HPLC Using Teicoplanin as Stationary Phase and its Application to Natural Water Analysis. Biomed. Chromatogr. 2013, 27 (11), 1413–1422. doi:https://doi.org/10.1002/bmc.2937.
- Felix, G.; Berthod, A. Commercial Chiral Stationary Phases for the Separations of Clinical Racemic Drugs. Separation & Purification Reviews 2007, 36 (4), 285–481. doi:https://doi.org/10.1080/15422110701826997.
- MacLeod, S.L.; Sudhir, P.; Wong, C.S. Stereoisomer Analysis of Wastewater-Derived β-Blockers, Selective Serotonin re-Uptake Inhibitors, and Salbutamol by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A Nov. 2007, 1170 (1–2), 23–33. doi:https://doi.org/10.1016/j.chroma.2007.09.010.
- López-Serna, R.; Kasprzyk-Hordern, B.; Petrović, M.; Barceló, D. Multi-residue Enantiomeric Analysis of Pharmaceuticals and Their Active Metabolites in the Guadalquivir River Basin (South Spain) by Chiral Liquid Chromatography Coupled with Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405 (18), 5859–5873. doi:https://doi.org/10.1007/s00216-013-6900-7.
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Analytical Method Development for the Simultaneous Determination of Five Human Pharmaceuticals in Water and Wastewater Samples by Gas Chromatography-Mass Spectrometry. Chromatographia 2003, 58 (7–8), 471–477. doi:https://doi.org/10.1365/s10337-003-0087-6
- Bisceglia, K.J.; Yu, J.T.; Coelhan, M.; Bouwer, E.J.; Roberts, A.L. Trace Determination of Pharmaceuticals and Other Wastewater-Derived Micropollutants by Solid Phase Extraction and gas Chromatography/Mass Spectrometry. J. Chromatogr., A 2010, 1217 (4), 558–564. doi:https://doi.org/10.1016/j.chroma.2009.11.062.
- Ternes, T.A.; Hirsch, R.; Mueller, J.; Haberer, K. Methods for the Determination of Neutral Drugs as Well as Betablockers and β 2 -Sympathomimetics in Aqueous Matrices Using GC/MS and LC/MS/MS. Fresenius. J. Anal. Chem. 1998, 362 (3), 329–340. doi:https://doi.org/10.1007/s002160051083.
- Armenta, S.; Garrigues, S.; de la Guardia, M. The Role of Green Extraction Techniques in Green Analytical Chemistry. TrAC, Trends Anal. Chem. 2015, 71, 2–8. doi:https://doi.org/10.1016/j.trac.2014.12.011.