ABSTRACT
Mechanochemistry is a powerful tool to develop environmentally benign syntheses of active pharmaceutical ingredients. In this context, we report here the synthesis of the anti-epileptic agent rufinamide through a one-pot sequential multi-component approach, which was fully carried out applying a ball milling method. This solid-state synthesis of rufinamide has proved to be more efficient in terms of sustainability than the equivalent conventional synthetic process in solution. The mechanochemical method presents several advantages such as shorter reaction times, pot economy, the absence of external heating and solvent use. The ball milling-based synthesis of rufinamide has shown excellent green metrics and it is in agreement with several principles of Green Chemistry.
GRAPHICAL ABSTRACT
Introduction
The sustainability concept and the development of environmentally and economically efficient synthetic strategies and processes are crucial research topics for different areas of chemistry (Citation1). In this respect, organic synthetic processes have been traditionally conceived by the assembly of structural fragments of the target molecule in consecutive transformations, involving work-up, purification and isolation processes after each step, leading to so-called stop-and-go processes. Thus, conventional multi-step synthetic routes produce high amounts of waste (especially hazardous organic solvents) and display low environmental efficiency values. Furthermore, the purification and isolation processes have a significant impact on the cost of the finished product from the pharmaceutical and fine chemical industries, in which solvents represent around 85% of the total mass of waste produced (Citation2). The development of new synthetic processes that minimize the undesired use of solvents and manufacturing costs is a challenge for synthetic chemists. This goal has been explored from different approaches: (a) Development of more efficient organic reactions that display good atom economy and chemical yield, especially those that are able to generate several bonds in a single operation such as multi-component (Citation3), one-pot (Citation4) and click reactions (Citation5). (b) Development of new processes that reduce environmental costs, such as continuous and sequential processes (Citation6). (c) Development and application of new green technologies for organic synthesis, such as microwave heating, ball milling and flow chemistry (Citation7–9), which have emerged as powerful tools in the quest for eco-friendly synthetic processes (Citation10, Citation11).
Mechanochemistry has emerged in recent times as a versatile approach for the synthesis of organic compounds, offering benefits such as the reduction or avoidance of solvents as reaction media and short reaction times. While reactive milling of solids is a highly promising technology that will play a relevant role in the synthesis of small-molecule active pharmaceutical ingredients (APIs) for the pharmaceutical industry, few examples of drug synthesis carried out completely in a ball mill have been reported (Citation12, Citation13). In comparison with classical solution-based methods, mechanochemistry provides better opportunities for ‘benign by design' chemistry by allowing the design of overall sustainable processes while simplifying the experimental set-up and procedures.
Epilepsy is a chronic neurological disorder characterized by recurrent seizures, which affects up to 50 million patients worldwide, according to the World Health Organization (WHO) (Citation14). The therapeutic arsenal against epilepsy is formed by 24 anti-epileptic drugs, including rufinamide, which was approved by the FDA in 2008 for the treatment of the Lennox-Gasteaut syndrome, a rare form of epilepsy. Rufinamide is a triazole derivative structurally different from any currently marketed anti-epileptic drugs (Citation15), with a proposed mechanism of action that involves prolongation of the inactive state of voltage-gated sodium channels, resulting in a membrane stabilizing effect. Several stop-and-go synthetic routes to rufinamide in solution have been described, which need long reaction times and are carried out under solvent reflux conditions. Furthermore, the 1,2,3-triazole moiety is assembled by cycloaddition of 2,6-difluorobenzyl azide, with alkynes or enol ethers as dipolarophiles, using hazardous solvents such as toluene and dimethylsulfoxide (DMSO) and expensive Cu(I) catalysts (Citation16).
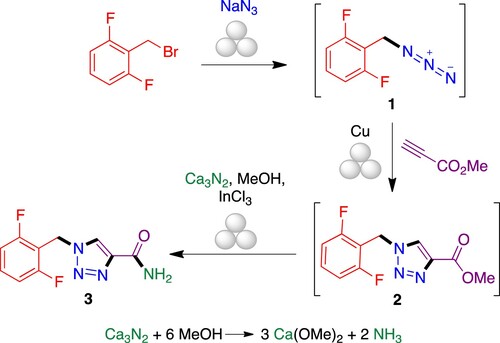
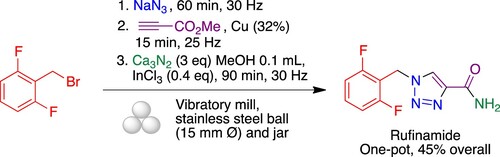
As part of our ongoing project on the sustainable synthesis of bioactive heterocycles (Citation17), we describe here a rapid and environmentally friendly synthesis of rufinamide, which involved the azidation step of low-cost 2-(bromomethyl)-1,3-difluorobenzene, followed by the Huisgen 1,3-dipolar cycloaddition of the formed azide 1 and methyl propiolate catalyzed by copper powder, to obtain the heterocyclic system 2, and finally the amidation step of the methyl ester group employing calcium nitride (Ca3N2) in the presence of methanol as a source of ammonia and indium trichloride as catalyst (Citation18). The synthetic process is performed as a one-pot sequence of three steps under ball milling and solvent-free conditions that generates four bonds (). Although low molecular weight azides are known to be shock-sensitive, azides have often been employed under ball milling conditions without observing any explosion or increased exothermicity. In fact, because the reactions are conducted in a sealed steel container, the authors that pioneered this chemistry describe it as safer than when carried out in glassware (Citation19). Indeed, mechanochemical reactions with azides have even been proposed as suitable experiments for the synthetic undergraduate lab (Citation20).
Results and discussion
We initially focused on the optimization of each individual step, starting with the preparation of 2-(azidomethyl)-1,3-difluorobenzene 1. Organic azides are important intermediates in organic synthesis and have been widely used as precursors of 1,2,3-triazoles (Citation21–23). Alkyl azides are synthesized by nucleophilic substitution of the corresponding alkyl halides with sodium azide, usually in solution under heating, but some examples of this transformation have been reported under microwave heating and by a mechanochemical approach. In reactions performed under ball milling, the energy input can be generated and transferred to the reactants by stress pressure, impact or friction forces, which have a high influence on the reaction time and yield of the final product. Thus, we decided to compare the ball milling reaction on a planetary mill (where the friction forces are predominant) and vibration mill (where impacts are the major contribution to energy input).
Optimization of the synthesis of azido compound 1
We started our work by determining the optimal reaction conditions of the first individual step to obtain azide 1 from 2-(bromomethyl)-1,3-difluorobenzene. To explore the effects of the grinding parameters such as mass (number and size) of balls and rotational or oscillation speed, we first employed a planetary mill using a steel jar containing a powdered mixture of the benzyl halide derivate and sodium azide under several conditions (, entries 1–3), but we recovered exclusively starting material in all cases, even in the presence of sodium chloride as an abrasive grinding additive (, entry 4). Due to these results, we moved to explore vibratory milling and the same mixture of benzyl halide/azide was set to 25 Hz, over 30 min as standard parameters. We first studied the effect of the number of balls and the model reaction was assayed in a steel jar containing 1, 3, 5, 6, 7, or 9 steel balls 5 mm in diameter; the number and size of balls have effect on the number of effective impacts over the grinding time. In this case, NMR analysis of the crude reaction mixtures showed the formation of azide 1 and we found the best result when the reaction was performed with 6 balls, to obtain a 30% conversion (, entries 5-10; for an example of the NMR study, see figure S1). We next increased the reaction time to 60 min under optimal reaction conditions previously found, to give a conversion of 89% (, entry 11). Stimulated by these results, we decided to explore the vibration frequently parameter, which was increased up to 30 Hz, to give an excellent 97% of conversion (, entry 12). When we scaled up the reaction to 0.5 g of benzyl bromide, under the same conditions, conversion decreased slightly to 88% (, entry 13). Ball number optimization showed 6 balls of 5 mm diameter as optimal, while 7 and 9 balls were less effective. On the other hand, when the reaction was carried out with a single stainless steel ball 15 mm in diameter, complete conversion was observed both at 30 and 25 Hz (, entries 14 and 15).
Table 1. Optimizing the mechanochemical reaction conditions to obtain azide 1.Table Footnotea
Optimization of the one-pot azidation-Huisgen cycloaddition sequential process
There is a considerable difference between stop-and-go processes, where the side products formed at each step are eliminated before the next one, and one-pot sequential processes, which must consider the effect of the unreacted starting materials and byproducts of the previous transformation. In this context, the most critical step in the planned one-pot synthesis of rufinamide is the Huisgen cycloaddition between azide 1 and methyl propiolate, since the presence of residues from the previous step could inhibit the catalytic process and consequently decrease the regioselectivity and the chemical yield of the cycloaddition. With the first set of parameters in hand, we studied the mechanochemical reaction conditions to obtain the triazole 2 in a one-pot sequential process (), using as guidance previous work on mechanochemical Huisgen reactions (Citation19, Citation24).
Figure 2. One-pot mechanochemical synthesis of methyl 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxylate 2.
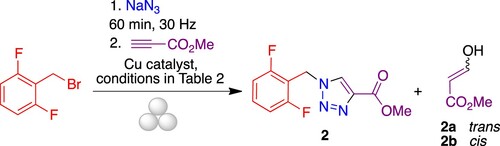
Copper and other metals have been widely described as catalysts of the thermal Huisgen reaction (Citation25) to improve the formation of the 1,4-disubstituted 1,2,3-triazole regioisomer. Metal-free conditions have also been described for the Huisgen reaction (Citation26) and 1,3-dipolar cycloadditions (Citation27). Although copper(I) salts are the favorite choice to perform the alkyne/azide cycloaddition (CuAAc), the active copper I catalyst can also be generated from copper (II) salts using sodium ascorbate as a reducing agent or by in situ oxidation of metallic copper to generate copper (I). We initially studied the formation of triazole in the absence of catalyst, which led to a poor conversion of 40% and the formation of 1,4- and 1,5-disubstituted 1,2,3-triazole regioisomers, after 60 min of reaction. Then, we focused our attention on the use of copper catalysts in different oxidation states. Thus, azide 1 was obtained from a 1:2.4 stoichiometric mixture of 2-(bromomethyl)-1,3-difluorobenzene and sodium azide in a stainless steel jar containing a single ball of the same material (15 mm diameter) for 60 min. Then, we added 1.2 equivalents of methyl propiolate and copper (II) sulfate in a catalytic amount, together with sodium ascorbate as a reducing agent, according to the conditions described by the Sharpless group, and the reaction was milled for an additional 60 min at 30 Hz to yield 1,2,3-triazole 2 in a good conversion and excellent regioselectivity. The most common side reaction in the Huisgen dipolar cycloaddition, i.e. the competing homocoupling of the alkyne starting material (Glaser reaction), was not observed. However, NMR analysis of the crude reaction product showed the formation of side products 2a and 2b, arising from the Michael addition of water to methyl propiolate. To improve the yield and decrease the formation of this side product, we studied other Cu(I) catalysts such as the tetrakis(acetonitrile)copper(I)hexafluorophosphate and the (phenanthroline)bis (triphenylphosphine)copper(I) nitrate dichloromethane adduct, and also copper powder, under the reaction conditions described above, to obtain 95%, 90% and 87% conversions, respectively (, entries 3, 4 and 5; for an example of the NMR study, see Figure S2). Then, we examined the effect of reaction time using as catalysts tetrakis(acetonitrile)copper(I) hexafluorophosphate, copper sulfate/sodium ascorbate and copper powder and reaction times of 30 or 15 min for the cycloaddition step (, entries 6–10). A decrease in conversion was observed upon reduction in reaction time when the copper (II) system was used as catalyst (, entries 2 and 7) and copper (I), not lead to full conversion of azide 2 under any of the conditions (, entry 6 and 9). Copper powder led to full conversion in all cases and gave the best ratio between the target compound and side products (, entries 8 and 10). As an additional advantage, when using metal copper the catalyst waste can be removed with simple work-up techniques. Then, we performed two additional experiments where the catalyst load was reduced to 32% and 16% respectively. The reaction using a 32% load (10 mg of catalyst/100 mg of starting material) gave full conversion of azide 1 to the expected triazole, while a 16% load afforded a lower conversion (, entries 10–12, Figure S2).
Table 2. Optimization of the one-pot synthesis of compound 2 via azidation-Huisgen cycloaddition from 1 (0.5 mmol) under mechanochemical conditions.Table Footnotea
One-pot, sequential four-component synthesis of rufinamide
Following azidation/CuAAC optimization, we studied the full three-step azidation/CuAAC/amidation sequence in a one-pot process. We started the study by the screening of ammonia sources from ammonium formate, ammonium chloride, ammonium acetate, magnesium nitride and calcium nitride. Thus, the first two steps were carried out under the conditions previously optimized and stoichiometric amounts of the different ammonium salts were used in the last step, leading only to triazole 2 in all cases, together with unidentified side products. However, when the reaction was performed with the Mg3N2/MeOH or Ca3N2/MeOH systems in the presence of indium trichloride as a catalyst, rufinamide was obtained with an excellent conversion after 90-min milling in the final step. While both reactions gave similar results, the work-up was simpler for the case of Ca3N2, since the formation of emulsions due to the presence of magnesium salts was prevented. Under these conditions, the reaction mixture was easily purified by simple extraction followed by recrystallization from ethanol–water, without the need for purification by column chromatography, giving rufinamide in 40% overall yield. It is interesting to note that in previous work the mechanochemical amidation of esters with nitride salts had required the presence of indium trichloride as a Lewis acid catalyst (Citation18), whose role is presumably played here by a copper species generated in the second reaction. In an effort to improve the outcome of the process, we added indium trichloride at the last step and obtained a slight increase in yield to 45% (). Nevertheless, bearing in mind that the overall process comprises three reactions that generate four new bonds, an overall yield of 45% corresponds to about 77% for each individual step. Moreover, some loss of material probably took place during the final isolation step, since crystallization is usually less efficient (although greener) than chromatography.
One final issue to be considered is the possibility of the incorporation to the final product of traces of metals resulting from the mechanical process, and for this reason, we determined the content of iron and manganese in our final product using the ICP-MS technique. Although not arising directly from the mechanochemical process, we also considered relevant the determination of copper and indium, which had been used as catalysts along the process, in the final product. The results obtained are collected in compared with the permitted daily exposure (PDE) values for each element found in the Guideline for elemental impurities issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (Citation27) and in the USP Pharmacopoeia (Citation28). The contents of each element are well below the PDE values, bearing in mind that the starting dose of rufinamide in adult patients is 400 mg/day and that the maximum recommended dose is 3200 mg/day.
Table 3. Content of selected metals of the mechanochemically-obtained rufinamide.
Green metrics
In recent decades, several environmental factors have been adopted to evaluate the eco-efficiency of manufacturing processes, which is not represented by traditional chemical yield. Some relevant metrics for green chemistry (Citation29, Citation30) are the environmental factor (E factor) and process mass intensity (PMI), which evaluate the global efficiency of the process and it is calculated as the ratio of the mass of waste per mass of product. To measure the efficiency of our one-pot mechanochemical process, we have calculated the value of these parameters and we have compared them with two of the most efficient solution synthesis described for rufinamide (Citation31). Thus, our approach to the synthesis of rufinamide exhibited a calculated E factor of 18 for the overall 3-step process, which is significantly less than the pharmaceutical industry standard (25–100) (Citation29) and improves the values of synthetic routes that use the stop and go approach, working in solution. In addition, process mass intensity (PMI) is a key mass-based metric for evaluating the green character of an individual or a sequence of reactions and is based on the evaluation of the amount of material used to make a molecule. Our process exhibited an excellent PMI score of 10 (), which supports the postulated environmental benefits of one-pot, solid-phase processes for the synthesis of APIs. It is worth noting that the mechanochemical method does not require any chromatographic separation, since the final product is isolated simply by washing the crude with water followed by recrystallization from ethanol–water.
Table 4. Compared quantitative metrics of classical and mechanochemical approaches to the synthesis of rufinamideTable Footnotea.
Regarding other economic and environmental metric as external heating and reaction times, in the selected patents the azide 1 was formed in aqueous solution, by heating at 70°C for a period of 15 and 30 h respectively, while our mechanochemical strategy requires only 1 h and the reaction was carried in the absence of solvent and external heating. The patent WO2010/043849 described the triazole ring formation by reaction with propiolic acid to give a carboxylic acid intermediate, which on subsequent reaction with methanol in the presence of sulfuric acid gives the corresponding triazole 2 (Citation33). Later, in the order to increase chemical yield, the cycloaddition reaction was performed with an activated carboxylic acid, which increased the synthetic sequence in an additional step.
Conclusion
We report an eco-friendly synthesis of anti-epileptic drug rufinamide, using a one-pot sequential multi-component approach, which is performed in a vibratory ball mill. This method offers several advantages such as operational simplicity, good overall yield, solvent-free reaction conditions, and avoids the use hazardous solvents and expensive catalyst. In the process, four carbon–nitrogen bonds are generated and sodium bromide and magnesium methoxide, are the only waste generated, besides the reagents in excess. The process is carried out without chromatography and purifying by recrystallization only at the last step and shows better green chemistry metrics than known methods performed in solution.
Experimental
General experimental information
Mechanochemical reactions were carried out in a Retsch MM400 mill operating at a frequency of 25 or 30 Hz using a 25 mL stainless steel milling jar and balls of 5 mm or a single ball of 15 mm in diameter, made of the same material. For planetary ball milling, a PM100 planetary ball mill operating at rotation between 200 and 400 rpm was employed, using a 25 mL stainless steel milling jar and 5 mm balls of the same material. All reagents and solvents were of commercial quality and were used as received. Reactions were monitored by thin layer chromatography on aluminum plates coated with silica gel and fluorescent indicator. Infrared spectra were recorded with a ATR spectrophotometer equipped with a diamond accessory; wavenumbers are given in cm−1. NMR spectroscopic data were recorded using a spectrometer maintained by the Resonancia Magnética Unit, UCM, operating at 250 or 500 MHz for 1H NMR, and 63 or 126 MHz for 13C NMR; chemical shifts are given in (δ) parts per million and coupling constants (J) in Hertz.
Warning! The application of magnesium nitride in the conversion of esters to amides in alcoholic solution at 80°C has generated occasional explosions, especially when working at a relatively large scale involving the use of 1.3 g of magnesium nitride and 6 mL of methanol (Citation34). In the conditions described here (calcium nitride below 400 mg, 0.2 mL ethanol, ball milling in grinding flasks with screw caps), we never observed any sign of exothermicity nor explosions. The same remark holds for the use sodium azide.
Optimization of the synthesis of compound 1
The reactions were carried out from 100 mg (0.5 mmol) of 2-(bromomethyl)-1,3-difluorobenzene and 80 mg (1.2 mmol, 2.4 eq) of sodium azide, using a stainless steel jar and balls.
Optimization of the one-pot synthesis of compound 2 via azidation-huisgen cycloaddition from 1
A mixture of 100 mg (0.5 mmol) of 2-(bromomethyl)-1,3-difluorobenzene and 80 mg (1.2 mmol, 2.4 eq) of sodium azide, was subjected to ball milling at 25 Hz using a single stainless steel individual ball (size 15 mm) for 60 min. Then 0.5 mL (0.6 mmol, 1.2 eq) of methyl propiolate and the corresponding catalyst were added to the mixture, which was submitted to additional milling under the appropriate parameters.
Mechanochemical one-pot reaction
A mixture of 0.5 mmol (100 mg) of 2-(bromomethyl)-1,3-difluorobenzene and 1.2 mmol (80 mg, 2.4 eq) of sodium azide, was subjected to ball milling at 25 Hz using a single stainless steel ball (15 mm diameter) for 60 min. Then 0.6 mmol (0.5 ml, 1.2 eq) of methyl propiolate and 0.08 mmol (10 mg, 0.16 eq) of powdered copper were added to the crude mixture and subsequently submitted to ball milling for 15 min at a frequency of 25 Hz. Finally, 1.5 mmol (107 mg) of calcium nitride, 0.2 mmol (44 mg, 0.4 eq) of indium trichloride and 0.1 mL of methanol were added and the reaction was milled at a frequency of 30 Hz for an additional 90 min. The product was purified by washing with distilled water and dried via vacuum filtration. The resulting solid was finally crystallized in ethanol/H2O to give rufinamide as a white solid in 45% overall yield (535 mg). IR νmax (film): 3391 (NH2), 3135, 2307, 1652 (CONH2), 1624, 1470, 1233, 1031, 793. 1H NMR (500 MHz, DMSO-d6) δ 8.56 (s, 1H), 7.87 (s, 1H), 7.61–7.46 (m, 2H), 7.20 (t, J = 8.1 Hz, 2H), 5.73 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ, 161.34, 160.8 (dd, J1 = 249.9 and 8.1 Hz), 142.83, 131.9 (t, J = 10.3 Hz), 126.84, 112.0 (d, J = 19.6 Hz), 111.08 (t, J = 20.1 Hz), 41.2 (t, J = 4.4 Hz) (Figure S3).
Supplemental Material
Download MS Word (1.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- (a) Becker, J.; Manske, C.; Randl, S. Curr. Opin. Green Sust. Chem. 2022, 33, 100562. (b) Erythropel, H.C.; Zimerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.Z.; Tu, Q.; Pincus, L.N.; Falinski, M.M.; Shi, W.; Coish, P.; Plata, D.L.; Anastas, P.T. Green Chem. 2018, 20, 1929. (c) Lange, J.-P. ChemSusChem 2009, 2, 587–592.
- (a) González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.-S.; am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; Yee, S.; Reintjens, R.; Wells, A.; Massonneau, V.; Manley, J. Org. Process Res. Devel. 2011, 15, 900–911. (b) Rogers, L.; Jensen, K.F. Green Chem. 2019, 21, 3481–3498.
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Green Chem. 2014, 16, 2958–2975.
- Hayashi, Y. Chem. Sci. 2016, 7, 866–880.
- Arévalo, M. J., López, Ó., Gil, M. V. Green Chemical Synthesis and Click Reactions. In Click Reactions in Organic Synthesis: Chandrasekaran, S., Ed, Wiley-VCH, 2016; Chapter 2.
- Rogers, L.; Jensen, K.F. Green Chem. 2019, 21, 3481–3498.
- Trojanowicz, M. Molecules 2020, 25, 1434.
- Friščić, T.; Mottillo, C.; Titi, H.M. Angew. Chem. Int. Ed. 2020, 59, 1018–1029.
- Leonardi, M.; Villacampa, M.; Menéndez, J.C. Chem. Sci. 2018, 9, 2042–2064.
- Kappe, C.O.; Dallinger, D. Nature Rev. Drug Discov. 2006, 5, 51–63.
- (a) Egorov, I.N.; Santra, S.; Kopchuk, D.S.; Kovalev, I.S.; Zyryanov, G.V.; Majee; Ranu, B.C.; Rusinov, V.L.; Chupakhin, O.N. Green Chem. 2020, 22, 302–315. (b) Ardila-Fierro, K.J.; Hernández, J.G. ChemSusChem 2021, 14, 2145–2162.
- (a) Tan, D.; Loots, L.; Friščić, T. Chem. Commun. 2016, 52, 7760–7781. (b) Sović, I.; Lukin, S.; Meštrović, E.; Halasz, I.; Porcheddu, A.; Delogu, F.; Ricci, P.C.; Caron, F.; Perilli, T.; Dogan, A.; Colacino, E. ACS Omega 2020, 5, 28663–28672.
- (a) Venegas, M.P.; Juaristi, E. ACS Sust. Chem. Eng. 2020, 8, 8881–8893. (b) Ying, P.; Yu, J.; Su, W. Adv. Synth. Catal. 2021, 363, 1246–1271. (c) Bento, O.; Luttringer, F.; El Dine, T.M.; Pétry, N.; Bantreil, X.; Lamaty, F. Eur. J. Org. Chem. 2022, e202101516.
- “Epilepsy: A Public Health Imperative”. World Health Organization. https://www.who.int/publications/i/item/epilepsy-a-public-health-imperative.
- Hakimian, S.; Cheng-Hakimian, A.; Anderson, G.D.; Miller, J.W. Expert Opin. Pharmacother. 2007, 8, 1931–1940.
- Padmaja, R.D.; Chanda, K. Org. Process Res. Devel. 2018, 22, 457–466.
- (a) Rocchi, D.; González, J.F.; Menéndez, J.C. Green Chem. 2013, 15, 511–517. (b) Estévez, V.; Villacampa, M.; Menéndez, J.C. Org. Chem. Front. 2014, 1, 458–463. (c) Piquero, M.; Font, C.; Gullón, N.; López-Alvarado, P.; Menéndez, J.C. ChemSusChem 2021, 14, 4764–4775.
- Gómez-Carpintero, J.; Sánchez, J.D.; González, J.F.; Menéndez, J.C. J. Org. Chem. 2021, 86, 14232–14237.
- Cook, T.L.; Walker, J.A.; Mack, J. Green Chem. 2013, 15, 617–619.
- E. Colacino, A. Porcheddu. Introducing Practical Organic Mechanochemistry Into Undergraduate Curricula. In Mechanochemistry. A Practical Introduction From Soft to Hard Materials: Colacino, E., Ennas, G., Halasz, I., Porcheddu, A., Scano, A., Eds., 2021, Walter de Gruyter GmbH; Chapter 4.
- Li, J.-J. Synlett. 2007, 505–506.
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem. Int. Ed. 2005, 44, 5188–5240.
- Jun, Y.; Xiaoyue, J.; Shugui, H.; Jing, W. Chinese J. Org. Chem. 2018, 38, 791–801.
- Mukherjee, N.; Ahammed, S.; Bhadra, S.; Ranu, B.C. Green Chem. 2013, 15, 389–397.
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. Macromol. Rapid Commun. 2020, 41, 1900359.
- Rodríguez-Rodríguez, M.; Gras, E.; Pericàs, M.E.; Gómez, M. Chem. Eur. J. 2015, 21, 18706–18710.
- Das, S.; Chanda, K. RSC Adv. 2021, 11, 32680–32705.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Guideline for Elemental Impurities, QE3(R2); 2022.
- Elemental impurities-limits United States Pharmacopeia-National Formulary USP39-NF34, Chapter 232.
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Green Chem. 2002, 4, 521–527.
- Sheldon, R.A. ACS Sust. Chem. Eng. 2018, 6, 32–48.
- The ACS Green Chemistry research tools. https://www.acs.org/content/acs/en/greenchemistry/research-innovation/tools-for-green-chemistry.html
- Bodhuri, P.; Green Stuart, P.; Karadeolian, A.; Cammisa, E.G. WO2014121383A1; 2014. https://patents.google.com/patent/WO2014121383A1/en.
- (a) Crane, S. Chem. Eng. News 2009, 87 (15), 2–4. (b) Ley, S.V. Chem. Eng. News 2009, 87 (23), 4–5. (c) Buske, G. Chem. Eng. News 2009, 87 (28), 2–4.