ABSTRACT
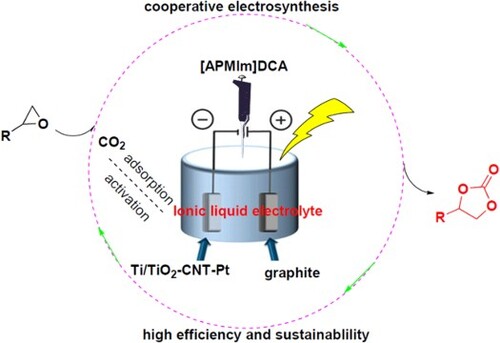
A novel and highly efficient electro-catalytic protocol for the synthesis of cyclic carbonates from epoxides and CO2 using 1-aminopropyl-3-methylimidazolium dicyanamide ([APMIm]DCA) as the supporting electrolyte, graphite as the anode, and Ti/TiO2-CNT-Pt as the cathode has been developed. Based on the cooperative effect between active Ti/TiO2-CNT-Pt cathode and active sites of supporting ionic liquid electrolyte [APMIm]DCA, CO2 and epoxides could be effectively converted into the corresponding cyclic carbonates in high to excellent yields under mild conditions. Moreover, the electro-catalytic system could be easily recovered and reused for six successive cycles without a considerable decrease in catalytic activity. This work provides a sustainable and efficient cooperative strategy for the chemical fixation of carbon dioxide into valuable cyclic carbonates.
GRAPHICAL ABSTRACT
Introduction
Chemical fixation of carbon dioxide (CO2) into value-added products such as cyclic carbonates was one of the promising strategies for CO2 utilization as CO2 as an inexpensive, nontoxic, inflammable, and abundant C1 feedstock (Citation1,Citation2). As a promising way for CO2 chemical utilization, the cycloaddition reaction of CO2 with epoxides is promising due to both the view of 100% atomic economy and the wide applications of cyclic carbonates products in fine chemistry and industry (Citation3–5). However, due to the thermodynamic stability and kinetic inertness of CO2, considerable efforts have been devoted to develop efficient catalytic systems for CO2 sustainable transformation. Recently, a wide range of catalytic systems has been successfully developed for the synthesis of cyclic carbonates, including K, B-CN-4/Bu4NBr (Citation6), Beta zeolite N-660 (Citation7), organocatalysts (Citation8–11), metal-organic frameworks (Citation12–14), ionic liquids (Citation15–19), metal complexes (Citation20–23), and others (Citation24–31). Despite these advances, the development of a green and sustainable strategy for the chemical fixation of CO2 into cyclic carbonates via the cycloaddition with epoxides remains a challenge to synthetic chemistry.
Electrochemical synthesis has attracted considerable interest as an environmentally compatible process in areas of fine chemicals and sustainable synthesis, owing to avoiding the use of conventional unstable or hazardous reagents by the replacement with electric current or ‘clean’ electrons, reducing energy consumption, affording less waste production and often fewer reaction steps than conventional processes (Citation32–35). Recently, the electrochemical fixation of CO2 into cyclic carbonates has been reported and showed its advantages and environmental-friendly characteristics, in which CO2 could be effective activation and electrochemical cycloaddition of CO2 into valuable corresponding carbonates products (Citation36–38). However, some of these approaches suffered from disadvantages such as low yields, long reaction time, and laborious separation of products. In electrochemical synthesis, the suitable choice of electrodes and electrolyte conditions is a critical parameter in controlling the conversion and selectivity of electrochemical reactions. Therefore, developing new and efficient electrodes and electrolytes for the selective electrochemical transformation CO2 into cyclic carbonates are still in demand.
Ionic liquids (ILs), as a type of environmental-friendly materials, have attracted considerable attention in synthetic chemistry and have played important roles in a range of CO2 chemical utilization areas including the cycloaddition reaction of CO2 with epoxides for the synthesis of cyclic carbonates (Citation17–20, 39). In addition, ionic liquids are an important electrochemical electrolyte media in electrocatalytic synthesis due to their fascinating properties such as high thermal and chemical stability, low liquid vapor pressure, non-flammablity, good ionic conductivity, wide electrochemical windows, etc. (Citation40–45). Nanocrystalline TiO2 integrates a broad spectrum of unique properties including as high specific surface areas, well-aligned nanostructures, good adhesion to the Ti substrate, good hydrothermal stability, and excellent materials compatibility. Immobilization of functionalized active material onto the surface of TiO2 for the formation of environmental-friendly and efficient composite electrodes has captured considerable attention in electrochemical applications (Citation46–54). Bearing these in mind, we wish to report a green and efficient protocol for the electrochemical fixation of CO2 with epoxides for the synthesis of cyclic carbonates in the presence of ionic liquid electrolyte and Ti/TiO2-based composite electrode without any additional co-catalysts under mild conditions (Scheme 1). Furthermore, the effects of synthesis conditions and possible electro-catalytic cycle mechanisms were studied. This new electro-catalytic system offers significant improvements on the efficiency and sustainable utilization of CO2 via cycloaddition with epoxides.
Experimental
Apparatus and reagents
The chemicals, reagents, and ionic liquids were obtained from commercial suppliers and used directly without further purification. Composite electrode Ti/TiO2-CNT-Pt was prepared according to the same procedures described in literatures (Citation45,Citation46), and other electrode materials were supplied from Baoji Qixin Titanium Industry Co., Ltd. and Jiangsu Yianteng special electrodes Co., Ltd. (China). Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) were recorded with a JSM-7500F electron microscope. Powder X-ray diffraction patterns were recorded on a Ultima IV diffractometer using Cu Kα radiation (λ = 1.5405 Å), with a scan speed of 4°/min. 1H NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer in CDCl3 with tetramethylsilane (TMS) as an internal reference. Elemental analysis was obtained on a Vario Micro cube Elemental Analyzer. Metal loadings of cathodes were determined on an Avio 200 ICP Optical Emission Spectrometer.
Electro-catalytic cycloaddition of CO2 and epoxides to cyclic carbonates
The electro-catalytic cycloaddition reactions of CO2 and epoxides were conducted in a single-compartment cell with a magnetic stirring bar inside. Typically, the galvanostatic electrolysis at a current density of 3.4 mA cm−2 was carried out in a solution of acetonitrile (15 mL), supporting ionic liquid electrolyte [APMIm]DCA (10 mL) under an N2 atmosphere in a single compartment cell equipped with a graphite anode and Ti/TiO2-CNT-Pt cathode (1 cm × 2 cm). Prior to every experiment, the solution was bubbled with N2 for 20 min. After the consumption of an electric charge of 2 F mol−1, the epoxide (10 mmol) was added to the solution. The reaction mixture was stirred at a constant current at 50°C for a desired time under an atmosphere of CO2 (balloon). The reaction progress was monitored by HPLC. After completion of the reaction, the solvent was evaporated and the residue was then extracted with CH2Cl2 (3 × 10 mL), followed by evaporation of the solvent of the combined organic phase under vacuum to afford the corresponding products. For recycling experiments, the recovered supporting ionic liquid electrolyte was firstly dried under vacuum at 40°C for 1 h, then fresh epoxide, acetonitrile, and CO2 (balloon) were added and recycled under identical reaction conditions. All target products are known and commercial, thus were verified by comparison with those of standard compounds or by 1H NMR and Elemental analysis.
Spectroscopic data for the cyclic carbonates products
1,3-Dioxolan-2-one (, entry 1): 1H NMR (400 MHz, CDCl3), δ/ppm: 4.52 (s, CH2, 2H). Elemental analysis for C3H4O3: C, 40.86; H, 4.55; O, 54.46. Found: C, 40.92; H, 4.58; O, 54.50.
Propylene carbonate (, entry 2): 1H NMR (400 MHz, CDCl3), δ/ppm: 1.46 (dd, CH3, 3H), 3.96 (t, CH, 1H), 4.52 (t, CH, 1H), 4.81 (m, CH, 1H); Elemental analysis for C4H6O3: C, 47.03; H, 5.89; O, 46.98. Found C, 47.06; H, 5.92; O, 47.01.
(Chloromethyl)ethylene carbonate (, entry 3): 1H NMR (400 MHz, CDCl3), δ/ppm: 3.76 (dd, CH2, 2H), 4.33 (t, CH2, 1H), 4.58 (t, CH2, 1H), 4.92 (m, CH, 1H); Elemental analysis for C4H5ClO3: C, 35.15; Cl, 25.94; O, 35.12. Found C, 35.19; Cl, 25.96; O, 35.15.
4-(Hydroxymethyl)-1,3-dioxolan-2-one (, entry 4): 1H NMR (400 MHz, CDCl3), δ/ppm: 3.57 (t, CH2, 2H), 4.27–4.51 (dd, CH2, 2H), 4.77 (m, CH, 1H), 5.23 (t, OH, 1H). Elemental analysis for C4H6O4: C, 40.66; H, 5.09; O, 54.16. Found: C, 40.68; H, 5.12; O, 54.19.
1,2-Butylene glycol carbonate (, entry 5): 1H NMR (400 MHz, CDCl3), δ/ppm: 0.92 (t, CH3, 3H), 1.56–1.62 (m, CH2, 2H), 4.05 (t, CH2, 2H); 4.43 (d, CH2, 1H); 4.62 (m, CH, 1H); Elemental analysis for C5H8O3: C, 51.70; H, 6.91; O, 41.31. Found: C, 51.72; H, 6.94; O, 41.34.
Hexahydrobenzo[d][1,3]dioxol-2-one (, entry 6): 1H NMR (400 MHz, CDCl3), δ/ppm: 1.36–1.44 (m, CH2CH2, 4H), 1.73–1.81 (m, 2CH2, 4H), 4.62 (t, 2CH, 2H); Elemental analysis for C7H10O3: C, 59.13; H, 7.06; O, 33.72. Found C, 59.15; H, 7.09; O, 33.76.
Styrene carbonate (, entry 7): 1H NMR (400 MHz, CDCl3), δ/ppm: 4.31 (t, CH2, 1H), 4.74 (t, CH2, 1H), 5.68 (t, CH2, 1H), 7.27–7.39 (m, Ar-H, 5H); Elemental analysis for C9H8O3: C, 65.81; H, 4.88; O, 29.22. Found C, 65.85; H, 4.91; O, 29.24.
Results and discussion
Initially, propylene oxide was chosen as the benchmark substrate, and the electro-catalytic synthesis of propylene carbonate via the cycloaddition of propylene oxide and CO2 was carried out in the presence of a series of supporting ionic liquid electrolytes (, entries 1–14). It was observed that the type of supporting ionic liquid electrolytes had an important effect on the cycloaddition. The results showed that the supporting ionic liquid electrolyte [APMIm]DCA showed the highest efficiency affording 94% product yield in short time of 2 h with high TOF values of 1285 h−1 (, entry 6), whilst with the [BMIm]Cl or [PrSO3HMIm]Cl as the electrolyte, the yield was only 58% or 65% after 10 h with the low TOF of 173 or 206 h−1 (, entries 1 and 3), and. As control experiment, when in the absence of ionic liquid, much low product yield of 27% after 24 h was obtained (, entry 15). The results meant that [APMIm]DCA was the most suitable supporting ionic liquid electrolyte for the cycloaddition, may be due to the proper active sites (such as amino groups, dicyanamide anion, etc.) of the ionic liquid, which could promote the activation of epoxide and the CO2 adsorption capacity. The influence of the amount of ionic liquid electrolyte [APMIm]DCA on the cycloaddition was also studied. When the amount of ionic liquid was increased from 3 to 10 mL, the product yield was markedly increased from 52% to 94%, and the TOF increased from 471 to 1285 h−1 (, entries 16–19 and 6). No significant enhancement in the yield was observed with further increasing the ionic liquid amount to 12 mL (, entry 19). The charge passed during the electro-catalytic synthesis strongly influenced on the cycloaddition. It can be seen that the yield increased with increasing charge from 0.5 to 2 F mol−1 (, entries 20–22 and 6). However, the yield did not increase with further increasing in the charge to 2.5 F mol−1 (, entry 23). The results of the study suggested that 10 mL of ionic liquid electrolyte [APMIm]DCA with charge 2 F mol−1 was the optimum amount for further investigation.
Table 1. Screening of supporting ionic liquid electrolytes for the cycloaddition of propylene oxide with CO2 to produce propylene carbonate.Table Footnotea
Next, the effects of electrode materials, constant current, reaction temperature, and reaction time on the electrocatalytic efficiency in the cycloaddition of propylene oxide with CO2 to produce propylene carbonate were investigated (). The influence of the electrode materials was proved to be a key factor on the cycloaddition, different types of electrode materials such as Ti/TiO2, Ti/TiO2-Pt, Ti/TiO2-CNT, Ti/TiO2-CNT-Pt, Pt/TiO2-ZrO2, Ti, Pt, CNT, TiO2, and Cu were investigated (a). The results indicated that Ti, Pt, CNT, TiO2, Cu, and Ti/TiO2 achieved bad performance with low yields from 24% to 57%, while the electrode materials of Ti/TiO2-Pt, Ti/TiO2-CNT, Ti/TiO2-CNT-Pt, and Pt/TiO2-ZrO2 were valid for the cycloaddition, the product yield increased in order of Ti/TiO2-Pt < Pt/TiO2-ZrO2 < Ti/TiO2-CNT < Ti/TiO2-CNT-Pt under the same electro-catalytic reaction conditions. Furthermore, the surface morphology and elemental composition of the valid electrodes of Ti/TiO2-Pt, Pt/TiO2-ZrO2, and Ti/TiO2-CNT were determined by SEM-EDX (Figures S1 and S2) and ICP-OES (Table S1). It was observed the three electrodes did not maintain their chemical environment during the electro-catalytic cycloaddition. The observed surface morphology and elemental composition changes on the three electrodes before and after the reaction lead to a limitation in their catalytic activities, resulting in low product yields comparing to that of Ti/TiO2-CNT-Pt. The results indicated that Ti/TiO2-CNT-Pt electrode was found to be the most effective. As expected, the cycloaddition was feeble in the case of the absence of electrode material. Therefore, Ti/TiO2-CNT-Pt was the best electrode cathode material for further studies. The influence of current density on the cycloaddition was investigated ((b)). It can be seen that the yield increased with increasing current density up to 3.4 mA cm−2. When the current density was 3.4 mA cm−2, the yield reached 94%. However, with further increasing in the current density beyond 3.4 mA cm−2, the yield decreased, which might be attributed to the formation of byproducts CO or oxalate dianions during the electrocatalysis at higher current densities. Therefore, the optimum current density is 3.4 mA cm−2. The influence of reaction temperature was also investigated ((c)). It can be seen that the yield was increased in the reaction temperature up to 50°C. Nevertheless, the use of higher temperature beyond 50°C would lead to a decreased yield. Accordingly, the appropriate temperature is 50 °C. The influence of reaction time was also investigated under identical reaction conditions ((d)). The product yield increased sharply with an increase in reaction time to 2 h. However, the yield almost no change when the reaction time increasing longer than 2 h. Thus, the appropriate time would be 2 h.
Figure 1. Effect of reaction parameters on the electrocatalytic cycloaddition (a) Effect of electrode material on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, current density 3.4 mA cm−2, 50°C, 2 h), (b) effect of current density on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, 50°C, 2 h), (c) effect of reaction temperature on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 2 h), and (d) effect of reaction time on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 50°C).
![Figure 1. Effect of reaction parameters on the electrocatalytic cycloaddition (a) Effect of electrode material on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, current density 3.4 mA cm−2, 50°C, 2 h), (b) effect of current density on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, 50°C, 2 h), (c) effect of reaction temperature on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 2 h), and (d) effect of reaction time on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 50°C).](/cms/asset/cd9dcd94-3e0b-46b7-ab96-8f973a0e9853/tgcl_a_2163192_f0001_oc.jpg)
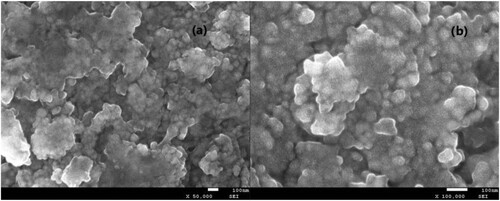
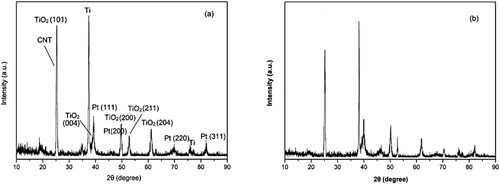
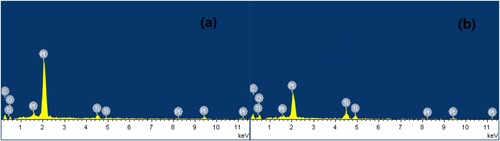
Furthermore, the reusability of the electro-catalytic system was conducted under optimal conditions, and the results are shown in . After completion of the cycloaddition, the electro-catalytic system could be easily separated and recovered (supporting ionic liquid electrolyte [APMIm]DCA by facile extraction with CH2Cl2, graphite anode, and Ti/TiO2-CNT-Pt cathode by facile washing with acetonitrile) and subjected to the next cycle. The electro-catalytic system could be easily reused for up to six consecutive cycles without obvious loss of activity (90∼94% yields), indicating its high stability and excellent recyclability. Scanning electron microscopy (SEM) analysis was carried out to obtain the surface morphology of the used and fresh of the prepared Ti/TiO2-CNT-Pt cathode. As shown in , both the fresh and used cathodes exhibited the nano-porous network morphology with uniform and well-distributed nanoparticles around 30 nm, which was helpful for the electron transfer and had a promoting effect on the improvement of the electro-catalytic activity. Over the cycles of use, the surface morphology of cathode did not change, suggesting that the cathode is relatively stable meanwhile maintaining its catalytic activity during the electro-catalytic process. The structural stability of the active Ti/TiO2-CNT-Pt cathode was also investigated by wide angle X-ray powder diffraction analysis (). The diffraction peaks at 2θ values of 25.3°, 38.6°, 48.2°, 53.7°, and 62.2° were belonged to the (111), (004), (200), (201), (200), (211), and (204) diffractions of the TiO2 nanoparticles, and the diffraction peak 25.3° was also belonged to the typical peak for CNT according to JCPDS78-1621. The diffraction peaks observed at 36.8°, and 75.9° were belonged to the typical peak for Ti according to JCPDS21-1276. The diffraction peaks at 2θ values of 39.5°, 48.1°, 68.7°, and 82.1°were belonged to the (111), (200), (220), and (311) diffractions of Pt nanoparticles. It is clear that in the diffraction peaks of the fresh and six cycles recovered cathode there is no significant changes on the typical peaks. Besides, compared with the fresh cathode, the EDX of the recovered cathode after six cycles is similar to that of the fresh one (), which revealed the presence of the corresponding elemental signals including Ti, O, C, and Pt. Additionally, metal loadings of the fresh and reused Ti/TiO2-CNT-Pt cathode was determined by ICP-OES. The metal loadings analysis data shown in Table S1 confirmed that the cathode is highly stable and durable for the reaction, suggesting that the leaching is negligible during the electro-catalytic process. These above results evidenced that the chemical environment of the active Ti/TiO2-CNT-Pt cathode was well remained during the recycling process.
Figure 2. Recycling stability of the electro-catalytic system (propylene oxide (10 mmol), CO2 (balloon), acetonitrile (15 mL), recovered supporting ionic liquid electrolyte [APMIm]DCA, used graphite anode and Ti/TiO2-CNT-Pt cathode in a single compartment cell with current density 3.4 mA cm−2 at 50°C for 2 h).
![Figure 2. Recycling stability of the electro-catalytic system (propylene oxide (10 mmol), CO2 (balloon), acetonitrile (15 mL), recovered supporting ionic liquid electrolyte [APMIm]DCA, used graphite anode and Ti/TiO2-CNT-Pt cathode in a single compartment cell with current density 3.4 mA cm−2 at 50°C for 2 h).](/cms/asset/a349372e-0692-4c32-aed2-89b21ff3dc14/tgcl_a_2163192_f0002_oc.jpg)
To demonstrate the generality of this approach for the cycloaddition, a range of epoxides was then investigated, and the results are summarized in . The epoxides containing electron-donating or electron-withdrawing groups could be successfully converted into the corresponding cyclic carbonates in high to excellent yields (86∼95%) with high selectivities (96.1∼98%) and high TOF values (967∼1416 h−1) and under the optimal reaction conditions (, entries 2–7). Interestingly oxiran-2-ylmethanol was the most reactive substrate and converted into 4-(hydroxymethyl)-1,3-dioxolan-2-one within 1 h (, entry 4). Furthermore, the electrocarboxylation of 7-oxabicyclo[4.1.0]heptane required a long time of 5 h to obtain a good yield (, entry 6), which may be due to the high steric hindrance during the reaction. These results indicated that the designed electro-catalytic system could be widely and efficiently used for the synthesis of cyclic carbonates through cycloaddition of electro-catalytic activated CO2 to epoxides.
Table 2. Electro-catalytic synthesis of cyclic carbonates from epoxides and CO2.Table Footnotea
To evaluate the advantages of the catalytic efficiency of the electro-catalytic system, the comparison of this electro-catalytic system with other different catalytic systems is summarized in Table S2. The presented data show that this electro-catalytic system is a type of suitable and efficient system for the chemical fixation of CO2 and epoxides into cyclic carbonates under mild conditions in terms of reaction time, temperature, CO2 pressure, and product yields.
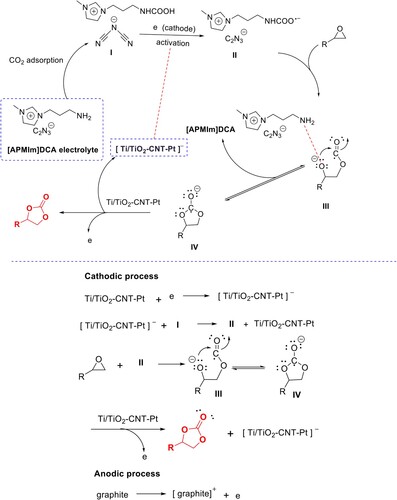
On the basis of experimental findings and previous studies (Citation13–18, 28, 29, 36–38), a possible mechanism for the electro-catalytic cycloaddition of CO2 with epoxides to cyclic carbonates is proposed (Scheme 2). Initially, the ionic liquid electrolyte [APMIm]DCA is helpful for CO2 adsorption, and the adsorbed CO2 could be activated by the NH2 group of [APMIm]DCA for the formation of intermediate I, which can be further activated by the reduction in Ti/TiO2-CNT-Pt cathode via its high electrocatalytic activity (one electron transfer happened from the graphite anode) for the formation of CO2 radical anion -NHCO2•− as the intermediate II. At the same time, the substrate epoxide could be activated via the coordination interaction with amino group of ionic liquid electrolyte [APMIm]DCA and then the intermediate II adds to the less sterically hindered C atom of epoxide via the nucleophilic attack, the intermediate III can be generated via the nucleophilic attack of -NHCO2•−, followed by the intramolecular substitution of anion to form the intermediate IV. Next, intermediate IV could be oxidized by Ti/TiO2-CNT-Pt cathode surface to form the corresponding cyclic carbonate product. The regeneration of ionic liquid electrolyte and Ti/TiO2-CNT-Pt cathode were used for the next cycle. The cooperative effect of ionic liquid electrolyte promoting the activation of epoxide and the CO2 adsorption capacity, the cathode activating CO2, thus this novel and highly efficient electro-catalytic system stimulates the production of high levels of cyclic carbonates under mild conditions.
Conclusions
In conclusion, a novel, convenient, and highly efficient electro-catalytic cycloaddition system equipped with graphite anode, Ti/TiO2-CNT-Pt cathode, and supporting ionic liquid electrolyte [APMIm]DCA was developed, and found to be capable of effectively converting CO2 and epoxides into cyclic carbonates in high to excellent yields without any addition of cocataysts under mild conditions. This superior effectiveness of electro-catalytic system may be ascribed to the cooperative effect between active Ti/TiO2-CNT-Pt cathode and active sites of supporting ionic liquid electrolyte [APMIm]DCA. In addition, the electro-catalytic system could be easily recovered and reused for six successive cycles without a considerable decrease in catalytic activity. The protocol demonstrated advantageous in terms of ease work-up, feasibility, cleaner reaction profile, sustainable and stable recyclability of catalyst. This protocol provides a green and promising strategy for the synthesis of cyclic carbonates toward electro-catalytic activation and cycloaddition of CO2 to epoxides. Further modification of functional groups of ionic liquid and design of new highly active cathodes, and construction of catalytic and sub-stoichiometric amount of functionalized ionic liquid-based electro-catalytic system for the efficient and solvent-free cycloaddition of CO2 to epoxides are ongoing.
Supplemental Material
Download MS Word (9.9 MB)Acknowledgements
The authors acknowledge the support from analysis and testing center of Anshun University and Jinggangshan University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tappe, N.A.; Reich, R.M.; D’Elia, V.; Kühn, F.E. Dalton Trans. 2018, 47, 13281–13313.
- Huang, K.; Zhang, J.Y.; Liu, F.; Dai, S. ACS Catal. 2018, 8, 9079–9102.
- Claver, C.; Yeamin, M.B.; Reguero, M.; Masdeu-Bultó, A.M. Green Chem. 2020, 22, 7665–7706.
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. ACS Catal. 2018, 8, 419–450.
- Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S.W.; Harvey, A. J. Environ. Chem. Eng 2021, 9, 105113.
- Wang, X.; Yang, L.; Fu, G.; Chen, Y.; Yang, C.; Sun, J. J. Colloid Interf. Sci. 2022, 609, 523–534.
- Duan, R.; Hu, C.; Zhou, Y.; Huang, Y.; Sun, Z.; Zhang, H.; Pang, X. Ind. Eng. Chem. Res. 2021, 60, 1210–1218.
- Guo, L.; Lamb, K.J.; North, M. Green Chem. 2021, 23, 77–118.
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kühn, F.E. ChemSusChem. 2015, 8, 2436–2454.
- Whiteoak, C.J.; Nova, A.; Maseras, F.; Kleij, A.W. ChemSusChem. 2012, 5, 2032–2038.
- Sabet-Sarvestani, H.; Izadyar, M.; Eshghi, H.; Norozi-Shad, N. Phys. Chem. Chem. Phys. 2020, 22, 223–237.
- Pal, T.K.; De, D.; Bharadwaj, P.K. Coordin. Chem. Rev. 2020, 408, 213173.
- Xiang, W.; Ren, J.; Chen, S.; Shen, C.; Chen, Y.; Zhang, M.; Liu, C. Appl. Energ. 2020, 277, 115560.
- Liu, E.; Zhu, J.; Yang, W.; Liu, F.; Huang, C.; Yin, S. ACS Appl. Nano Mater. 2020, 3, 3578–3584.
- Yue, S.; Qu, H.; Song, X.; Zang, S.; Deng, G. Catal. Sci. Technol. 2021, 11, 6999–7008.
- Taheri, M.; Ghiaci, M.; Shchukarev, A. New J. Chem. 2018, 42, 587–597.
- Zheng, D.; Ning, P.; Jiang, J.; Liu, F.; Wang, L.; Zhang, J. J. Mol. Liq. 2019, 284, 68–74.
- Martinez, A.S.; Hauzenberger, C.; Sahoo, A.R.; Csendes, Z.; Hoffmann, H.; Bica, K. ACS Sustainable Chem. Eng. 2018, 6, 13131–13139.
- Sodpiban, O.; Phungpanya, C.; Gobbo, S.D.; Arayachukiat, S.; Piromchart, T.; D’Elia, V. Chem. Eng. J. 2021, 422, 129930.
- Hwang, S.; Ryu, J.Y.; Jung, S.H.; Park, H.R.; Lee, J. Polyhedron 2020, 178, 114353.
- Ramírez-Cuellar, K.P.; Salas-Martin, K.P.; Méndez-Ocampo, P.A.; Martínez-dlCruz, L.; García- Márquez, A.; Guerrero-Ríos, I. Catal. Today 2020, 358, 45–50.
- Kiriratnikom, J.; Laiwattanapaisarn, N.; Vongnam, K.; Thavornsin, N.; Sae-ung, P.; Kaeothip, S.; Euapermkiati, A.; Namuangruk, S.; Phomphrai, K. Inorg. Chem. 2021, 60, 6147–6151.
- Meléndez, J.; North, M.; Villuendas, P.; Young, C. Dalton Trans. 2011, 40, 3885–3902.
- Bayer, U.; Liang, Y.; Anwander, R. Inorg. Chem. 2020, 59, 14605–14614.
- Chen, A.; Chen, C.; Xiu, Y.; Liu, X.; Chen, J.; Guo, L.; Zhang, R.; Hou, Z. Green Chem. 2015, 17, 1842–1852.
- Zhang, X.; Qiu, B.; Zou, Y.; Wang, S.; Mai, W.; Cao, Y.; Wang, Y.; Chen, J.; Li, T. Micropor. Mesopor. Mater. 2021, 319, 110758.
- Arayachukiat, S.; Kongtes, C.; Barthel, A.; Vummaleti, S.V.C.; Poater, A.; Wannakao, S.; Cavallo, L.; D’Elia, V. ACS Sustain. Chem. Eng. 2017, 5, 6392–6397.
- Natongchai, W.; Luque-Urrutia, J.A.; Phungpanya, C.; Solà, M.; D’Elia, V.; Poater, A.; Zipse, H. Org. Chem. Front. 2021, 8, 613–627.
- Natongchai, W.; Posada-Pérez, S.; Phungpanya, C.; Luque-Urrutia, J.A.; Solà, M.; D’Elia, V.; Poater, A. J. Org. Chem. 2022, 87, 2873–2886.
- Pourhassan, F.; Khalifeh, R.; Eshghi, H. Fuel 2021, 287, 119567.
- Rajabzadeh, M.; Khalifeh, R.; Eshghi, H.; Hafizi, A. J. Ind. Eng. Chem. 2020, 89, 458–469.
- Jia, G.; Zhang, W.; Jin, Z.; An, W.; Gao, Y.; Zhang, X.; Liu, J. Electrochim. Acta. 2014, 144, 1–6.
- Rasheed, T.; Shafi, S.; Anwar, M.T.; Rizwan, K.; Ahmad, T.; Bilal, M. Appl. Catal. A: Gen. 2021, 623, 118248.
- Kärkäs, M.D. Chem. Soc. Rev. 2018, 47, 5786–5865.
- Chen, N.; Ye, Z.; Zhang, F. Org. Biomol. Chem. 2021, 19, 5501–5520.
- Anastasiadou, D.; Hensen, E.J.M.; Figueiredo, M.C. Chem. Commun. 2020, 56, 13082–13092.
- Gallardo-Fuentes, S.; Contreras, R.; Isaacs, M.; Honores, J.; Quezada, D.; Landaeta, E.; Ormazábal-Toledo, R. J. CO2 Util. 2016, 16, 114–120.
- Zhang, J.J.; Li, S.M.; Shi, Y.; Hu, Q.L.; Wang, H.; Lu, J.X. New J. Chem. 2020, 44, 11817–11823.
- Chen, Y.; Mu, T. Green Chem. 2019, 21, 2544–2574.
- McNeice, P.; Marr, P.C.; Marr, A.C. Catal. Sci. Technol. 2021, 11, 726–741.
- Cuffaro, D.; D’Andrea, F.; Mezzetta, A.; Guazzelli, L.; Chiappe, C.; Nuti, E.; Rossello, A. Green Chem. Lett. Rev. 2020, 13, 295–302.
- Leu, M.; Campbell, P.; Mudring, A.V. Green Chem. Lett. Rev. 2021, 14, 128–136.
- Hullio, A.A.; Afridi, H.I.; Mastoi, G.M. Green Chem. Lett. Rev. 2017, 10, 274–284.
- Koohestani, F.; Sadjadi, S. J. Mol. Liq. 2021, 334, 115754.
- Kathiresan, M.; Velayutham, D. Chem. Commun. 2015, 51, 17499–17516.
- Cakabay, Ö; Achhab, M.E.; Schierbaum, K. J. Phys. Chem. C 2016, 120, 9061–9067.
- Wu, L.; Brennaman, M.K.; Nayak, A.; Eberhart, M.; Miller, A.J.M.; Meyer, T.J. ACS Central Sci. 2019, 5, 506–514.
- Chu, D.; Zhang, X.; He, J.; Hou, Y.; Xiao, Y. Acta Chim. Sinica. 2010, 68, 125–130.
- Zhang, Z.; Li, X.; Zhang, R.; Zhang, Z.; Yu, J. Inorg. Chem. 2019, 58, 7303–7309.
- Chu, D.; Feng, D.; Zhang, J.; Li, X. J. Electrochem. 2005, 11, 219–223.
- Fang, W.Y.; Wang, F.W.; Xu, M.; Zhu, C.G.; Wei, Y.J.; Zhu, Q.Y. Chin. J. Inorg. Chem. 2011, 27, 1155–1159.
- Kong, J.; Qin, Y.H.; Wang, T.L.; Wang, C.W. Int. J. Hydrogen Energ. 2020, 45, 1991–1997.
- An, M.; Li, L.; Tian, Y.; Yu, H.; Zhou, Q. RSC Adv. 2018, 8, 18870–18879.
- Gopi, C.V.V.M.; Singh, S.; Reddy, A.E.; Kim, H.J. ACS Appl. Mater. Interf. 2018, 10, 10036–10042.