ABSTRACT
While porphyrin-related syntheses have become classic experiments in the undergraduate curriculum, traditional syntheses of the free-base meso-tetraphenylporphyrin (H2TPP) and its metallated derivatives can be energy-intensive, use hazardous solvents, and generate appreciable amounts of waste. In an attempt to make the synthesis of H2TPP and its metallated derivatives (MTPP where M = metal) better aligned with the principles of green chemistry, we optimized a microwave-assisted, microscale synthesis of H2TPP and alternative routes toward the metallation of H2TPP including solvent-substituted reflux and mechanochemical syntheses for the undergraduate teaching laboratory. The greenness of these syntheses were evaluated using various green metrics and qualitative comparison to previously reported syntheses.
GRAPHICAL ABSTRACT
Introduction
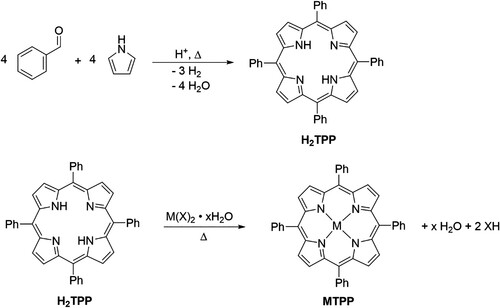
The syntheses and reactions of porphyrins, such as meso-5,10,15,20-tetraphenyl-21H,23H-porphine (H2TPP, commonly referred to as tetraphenylporphyrin) shown in Scheme 1, are prevalent in undergraduate chemistry teaching laboratories worldwide (Citation1–3). The H2TPP molecule is most widely used in this setting arguably due to its relative ease of synthesis and favorable physical properties for isolation and purification. The metallation of H2TPP to MTPP (M = metal ion, Scheme 1) is especially useful in inorganic teaching laboratories for discussing changes in electronic structure, coordination chemistry, bioinorganic chemistry, catalysis, and more (Citation4–11). The synthesis of these highly colored compounds offers an instructive opportunity to expand lecture content within the laboratory, such as ring currents in aromatic systems, and electronic transitions through the use of 1H-NMR and UV-vis spectroscopies, respectively. Likewise, the importance of porphyrins and their derivatives in dye-sensitized solar cells, as photocatalysts, and in biology offers additional context for these compounds as timely and relevant advanced topics (Citation12–14).
Unfortunately, traditional syntheses of H2TPP and MTPP are oftentimes energy intensive, use hazardous or environmentally-unfriendly solvents, and generate excessive waste due to dilute solutions and/or the use of column chromatography (Citation1–11). For example, the ‘simplified’ Adler-Longo method toward H2TPP uses excessive solvent in relatively harsh reactions conditions (Citation15), whereas the Lindsey method uses environmentally-unfriendly reagents such as trifluoroacetic acid (TFA) or BF3•Et2O (Citation16, Citation17). Efforts to make the H2TPP synthesis more environmentally friendly in the undergraduate teaching laboratory have been reported through reducing waste and using alternative heating methods. Initial efforts began with a shift to microscale syntheses as originally reported by Marsh and colleagues (Citation18, Citation19). The microwave-assisted synthesis of H2TPP and its derivatives were also accomplished using domestic microwave ovens as early as 1992 (Citation20, Citation21). The microwave-assisted synthesis of H2TPP as a greener alternative was introduced to the undergraduate lab by Warner and Hutchison (Citation22), but still relied on purification by column chromatography which reduced its effective ‘greenness’ by generating relatively substantial amounts of organic waste. Other microwave-assisted approaches in the teaching lab include the addition of nitrobenzene as an oxidant/co-solvent; however this compound is toxic and a suspected carcinogen (Citation23).
With a free-base porphyrin in hand (e.g. H2TPP), many metals and semimetals can be inserted thermally via a metathesis reaction with an appropriate metal salt to form the metalloporphyrin complex (e.g. MTPP). Some challenges in this synthesis center around finding a solvent which solubilizes both the metal salt and the porphyrinogen, as well as conditions that force the reaction toward the product while overcoming the slow kinetics of metallation (Citation24). The most common approach to metallation involves refluxing in a high-boiling solvent such as toxic dimethylformamide (DMF) or environmentally undesirable mixtures with chlorinated solvents (e.g. CHCl3/MeOH) (Citation18, Citation23, Citation24). A similar evolution of ‘greener’ metallation reactions in undergraduate teaching akin to that of the porphyrin ligands began with an initial shift to microscale reactions (Citation25), and the use of microwave-assisted syntheses (Citation6, Citation26). More recently, a number of research publications have also reported the mechanochemical metallation of porphyrins using a ball mill (Citation27–29), which can circumvent some of the health/environmental hazards and associated energy costs related to the handling, heating, and removal of solvents. However, to the authors knowledge the latter approach had yet to be implemented in a teaching laboratory prior to this work.
While the sequential synthesis of a porphyrin followed by its metallation is useful from an educational viewpoint, it is worth mentioning that one-pot syntheses can be performed, where the porphyrin is formed in-situ and a metal subsequently inserted to give the metalloporphyrin in one step (Citation30).
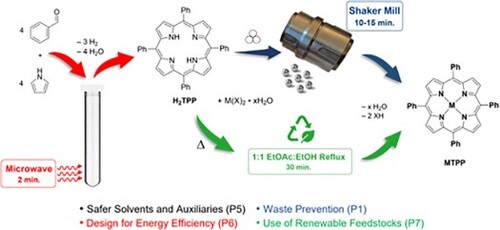
With these previous studies in mind, the microwave-assisted synthesis of H2TPP using a research-grade microwave synthesizer and simplified workup was reinvestigated, and alternative routes toward metallation using a solvent-substituted reflux or mechanochemistry have been developed for the undergraduate teaching laboratory. The results of these investigations and their implementation with students are reported herein.
Experimental section
General considerations
All chemicals were purchased from commercial vendors (e.g. Fisher Scientific, Aldrich) and used as received, unless otherwise specified. Fresh pyrrole could be used without further purification; otherwise it should be distilled under an inert atmosphere prior to use and stored in the freezer (−20°C) for up to six months without significant degradation. Microwave-assisted syntheses of H2TPP were carried out using a CEM DiscoverTM or Discover 2.0TM. Reaction vessels and silicone caps were also purchased from CEM. TeflonTM-coated stir bars (10 mm × 3 mm) for the 10 mL reaction vessels were purchased through Fisher Scientific. A Retsch Mixer Mill 400TM using 10 mm mixer balls and 50 mL stainless steels jars were used for all mechanochemical reactions.
Hazards
Proper protective equipment (e.g. goggles, lab coat, nitrile gloves, etc.) should be worn at all times and work performed in a fume hood where possible. Ethyl acetate (EtOAc), ethanol (EtOH), methanol (MeOH), and propionic acid are highly flammable. Refluxing solvent and hotplates can cause burns. Metal salts are generally toxic and environmentally hazardous, and the cobalt and nickel acetate salts are suspected carcinogens. Propionic acid is corrosive and can cause severe skin and eye irritation or damage.
Microwave Synthesis of H2TPP
To a 10 mL CEM microwave reaction vessel, a stir bar, 2.0 mL (26.8 mmol) of propionic acid, 0.2 mL (11 mmol) deionized H2O, 1.0 mL (9.8 mmol) benzaldehyde, and 0.68 mL (9.8 mmol) pyrrole were added. The reaction vessel was placed immediately in the CEM DiscoverTM reaction chamber. The synthesis of H2TPP was carried out in Dynamic or Fixed-power mode depending on preference. Dynamic mode. Pre-stirring: ON, 60 s; T = 200°C; time at set point = 2:00 min; Power: 200 W; Pressure: 200 psi; Stirring: high; PowerMAX: ON. Fixed-power mode. Pre-stirring: OFF; Tmax = 250°C; time = 2:00 min; Power: 300 W; Pressure: 200 psi; Stirring: high; PowerMAX: OFF.
After cooling, 1 mL of cold MeOH was added to the dark reaction mixture and the vessel was placed on ice for 10 min. The reaction mixture was then vacuum filtered using a small Hirsch funnel. The resulting crystalline, purple precipitate was washed with 0.5 mL cold MeOH (3x), followed by 0.5 mL boiling H2O (3x) and dried on the Hirsch funnel for 10 min. Yield ∼ 30 mg (15%).
Metallation by reflux in EtOH:EtOAc (1:1 v/v)
Working inside a fume hood, a small Erlenmeyer flask with a ground glass joint and stir bar was charged with 15 mg of H2TPP and ten molar equivalents of the corresponding metal(II) acetate hydrate salt (Zn, 0.054 g; Cu, 0.049 g). Next 10 mL of EtOH:EtOAc solvent mixture (1:1 v/v) was added before attaching a reflux condenser (with a small amount of silicon grease and connected to circulating water). The solution was heated to a gentle reflux for at least 30 min before allowing the reaction mixture to cool to room temperature. The UV-vis spectrum was obtained using a few drops of the reaction mixture which was further diluted with EtOAc.
Metallation by mechanochemistry
To two stainless steel 50 mL jars containing seven 10-mm mixer balls, 15 mg of H2TPP and ten molar equivalents of the desired metal(II) acetate hydrate salt (Zn, 0.054 g; Cu, 0.049 g; Ni, 0.061 g; Co, 0.061 g; Ag 0.042 g as AgNO3) were weighed and added. The reactants were shaken at 5-min intervals (15 min total) at 25 Hz. Samples were scraped from the jar and a small amount of product dissolved in EtOAc for UV-vis analysis.
Results and discussion
A recent survey suggests that the vast majority of post-secondary inorganic chemistry teaching laboratories minimally focus on green chemistry principles (Citation31). In part, this served as a call-to-action for the development of this report of a microwave-assisted, microscale synthesis of meso-5,10,15,20-tetraphenyl-21H,23H-porphine (H2TPP), and the solvent-substituted reflux and mechanochemical metallation routes to form MTPP (M = metal).
Microwave synthesis of H2TPP
A traditional microscale synthesis of H2TPP for the chemistry teaching laboratory has previously been described (Citation19). In this reaction, equimolar amounts of pyrrole and benzaldehyde are refluxed in an excess of propionic acid. Upon cooling, the H2TPP crystallizes out of solution and is isolated by filtration and washed repeatedly with water and methanol. Other ‘greener’ alternatives to the synthesis of H2TPP have also been described in the chemical literature, and these approaches either relied on conventional microwave ovens, column chromatography, or were not repeatable (Citation22, Citation32–35). For instance, Henriques et al. (Citation33) reported the synthesis of H2TPP using a research-grade microwave synthesizer (CEM DiscoverTM) with similar molar amounts of pyrrole, benzaldehyde and water to produce H2TPP in 27% yield (Dynamic mode). The authors claimed that the microwave parameters (temperature, time and power), along with the water concentration, had significant effects on the product yield. However contrary to the report, multiple attempts to repeat these experiments by the authors (M.A.C./M.A.N, and J.R.D) and others (personal communication) were unsuccessful and only yielded an intractable black glass that renders this synthetic approach inappropriate for an undergraduate lab. Another microwave-assisted synthesis of H2TPP originally reported by Pereira et al. (Citation25, Citation35) uses nitrobenzene as an oxidant and co-solvent which reportedly increased the yield substantially; however, this compound is highly toxic and a suspected carcinogen that the authors' would prefer to eliminate as a hazard for students (Citation10).
In an attempt to understand where the previously reported microwave-assisted synthesis of H2TPP in aqueous media failed (Citation33), the microwave experiment parameters and water concentration were reinvestigated at the microscale using a significantly reduced amount of propionic acid (Table S1). It should be noted that with 0.5 mL H2O an intractable black glass was formed that led to stir bar and/or vessel damage, whereas above 0.1 mL H2O diminished product yield (<10%) was noted. clearly shows that H2TPP yield (in Dynamic mode) strongly depends only on the reaction temperature, as opposed to microwave power and time. When running the reaction in Dynamic mode on the CEM DiscoverTM microwave synthesizer, a reaction hold time of 5 min translated to a 10–15 min total reaction time due to the ramp time to reach the specified temperature, 5-min hold time and the cooling time to reach 50°C. While this is less than half of the reaction time reported in Ref. (Citation19), it is not a realistic reaction time for a teaching laboratory if only a single microwave synthesizer is available (assuming a class size of 20 students and 2–3 h meeting time). This is in part due to the bottleneck of running reactions back-to-back, compounded by the additional time to precipitate, filter, wash, and dry the H2TPP product.
Figure 1. Plots of the percent yield of H2TPP as a function of reaction time (top), microwave power (middle), and reaction temperature maintained (bottom). General reaction conditions using Dynamic mode: Pre-stirring 1.0 min, stirring = high, reaction time = 5.0 min, power setting = 300 W, and temperature = 200°C (when time, power and temperature are not varied) using 0.133 mL benzaldehyde, 0.090 mL pyrrole, 0.100 mL water, 2.00 mL propionic acid. Cooled using house air.
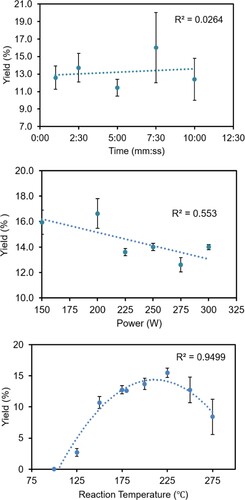
In an effort to streamline the synthesis of H2TPP to better accommodate the teaching laboratory under the aforementioned criteria, the best reaction conditions in Dynamic mode was then translated to Fixed-Power mode on the CEM DiscoverTM. Here, a shift to higher power (300 W vs. 200 W) for a shorter duration (2 min vs. 5 min) led to a lower percent yield (15% vs. 23%) that was still acceptable for characterization by FT-IR, UV-vis and 1H-NMR spectroscopies in the undergraduate teaching lab. By reducing the hold time from 5 to 2 min and using the Fixed-Power mode, there was no longer a need for a ramp cycle to meet a desired temperature. Furthermore, reducing the reaction time aligns with the ‘Design for Energy Efficiency’ approach within the 12 Principles of Green Chemistry framework developed by Anastas and Warner (Citation36), albeit at the cost of some product yield and therefore possible ‘Waste Prevention’.
With the optimized Dynamic and Fixed-power methodologies in hand, scale-up of the microwave-assisted H2TPP synthesis was also investigated. Using a 30 mL reaction vessel, the reaction was readily scaled by a factor of 10 without significant loss in yield. However, it should be noted that when scaling the reaction, the vessel size and related parameters must be considered to avoid vessel over-pressurization which results in venting or vessel failure (see implementation discussion below). Thus, a reduction in Tmax and adjustment of the reaction time may be appropriate.
To determine if there was an improvement with regards to the 12 Principles of Green Chemistry, the original synthesis of Adler and Longo (Citation15), the microscale reflux of Marsh et al. (Citation19), a microwave-assisted reaction published in an undergraduate laboratory manual (Citation22), and this work were compared using the following green metrics: E-factor (Citation37, Citation38), atom economy (Citation39), relative mass efficiency (Citation40), mass intensity (Citation40), scale risk index (Citation41), green star area index (GSAI, 42), and Eco-scale (Citation43) ( and Figure S1). A single metric was not chosen to show the variability in the analysis. In all cases, the atom economy is the same as H2TPP is produced from the same stoichiometry of benzaldehyde to pyrrole. The microwave-assisted, microscale reaction reported herein performs best with respect to four of the seven selected metrics (E-factor, mass intensity, scale risk index, and GSAI) due to decreased mass of the reactants and waste reduction. However, the microscale reflux reaction performed best with respect to the Eco-scale and relative mass efficiency metrics. Neither the traditional bulk-scale reaction of Adler and Longo, nor the ‘green’ microwave-assisted reaction of Warner et al. scored well due to scale and/or the use of column chromatography. While this comparison is not exhaustive with respect to the number of green metrics available it aids in the validation of our approach.
Table 1. Comparison of green metrics for traditional vs. MW-assisted H2TPP syntheses.Table Footnotea
Metallation of H2TPP
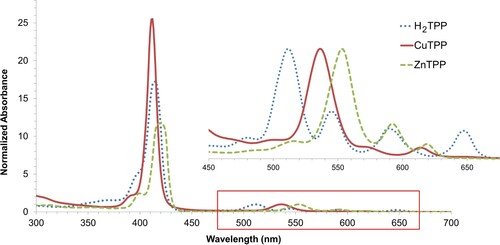
Two different microscale approaches were explored as greener routes to the metalloporphyrins: a solvatothermal method and a mechanochemical method. The reflux method of metallation was developed using the alternative solvent mixture of EtOH:EtOAc (1:1 v/v), which was selected based on previous work by Zovinka et al. (Citation44). Both of these solvents are recommended on available solvent selection guides and can be renewably sourced from biomass feedstocks (Citation45). This substitution aligns with the use of ‘Safer Solvents and Auxiliaries’ and ‘Use of Renewable Feedstocks’ within the 12 Principles of Green Chemistry framework (Citation36). Using an excess of metal acetate salt, this refluxing solvent combination (azeotrope b.p. of 71.8°C) readily inserted both Zn2+ and Cu2+ into H2TPP after 30 min, which was essentially quantitative by UV-vis (). Attempts to insert Ni2+ or Co2+ using either the acetate or nitrate salts were unsuccessful. Interestingly, this is in contrast to the reported microwave-assisted synthesis using this solvent mixture, where both metals were successfully inserted in <15 min (Citation44). This demonstrates the advantages of microwave methods where the solvent mixture can be rapidly superheated past its boiling point (i.e. to 110°C or 150oC in Ref. (Citation44)) which presumably helps overcome the kinetics of metallation. These results follow the general order for rates of formation, Cu > Zn > Co > Fe > Ni, (Citation46–49) from which one would expect Zn and Cu to be more readily incorporated at lower temperatures, while other metals such as Ni require the higher temperatures achieved by microwave-methods or higher boiling solvents such as DMF (b.p. = 153oC) or much longer reaction times such as in refluxing CHCl3/MeOH (∼60°C for five days) (Citation50). This likely reflects Adler et al.’s original report where they commented that a number of solvents were investigated (including methanol, ethanol, propanol, butanol) for the metallation but refluxing DMF ‘proved to be a very useful and good reaction medium’ for most metals (Citation51), and as such has widely been adopted as the solvent of choice.
Figure 2. Representative UV-vis spectra in EtOAc of the hydrated acetate salts of Cu2+ (solid red line) and Zn2+ (dashed green line) from EtOH:EtOAc (1:1 v/v) reflux. The MW-synthesized H2TPP (dotted blue line) is included for comparison. Red boxed area containing the Q-band region has been enlarged for clarity. The main peaks of H2TPP (480, 511, 545, 590, and 648 nm), CuTPP (537 and 615 nm), and ZnTPP (517, 554, 593, and 619 nm) are in good agreement with those reported in the literature (Citation44). Spectra were normalized on the highest intensity Q-band.
Mechanochemical methods present the opportunity to complete a synthetic reaction without using solvents (except for spectroscopic identification). This aligns with ‘Waste Prevention’ and ‘Safer Solvents and Auxiliaries’ within the 12 Principles of Green Chemistry framework (Citation36). Previous research efforts demonstrate the promise of mechanochemical metal insertion (Citation27–29), reporting success in metallating H2TPP with metals such as Zn2+ and Cu2+, but difficulty in inserting metals such as Fe3+, Ag+ and Co2+ as might be expected based on the discussion above.
To develop the mechanochemical synthesis of MTPP for the undergraduate teaching laboratory, the number of grinding balls and mixer frequency were investigated. During this study, it was found the number of 10 mm stainless-steel grinding balls in a 50 mL stainless steel reaction chamber had a significant impact on the reaction progress (Table S2). Metallation reactions attempting less than five grinding balls required either very long reaction times (≥45 min) or produced no reaction, depending on the metal. As the number of grinding balls increased, the reaction time decreased plateauing around seven grinding balls. Thus, reaction progress was optimized with respect to mixer frequency with seven 10 mm grinding balls.
At a frequency of 13.3 Hz, Cu2+ and Zn2+ were readily inserted into H2TPP in 15 min using a 50-mL stainless steel jar with seven grinding balls requiring as little as a 1:1 mol ratio of metal salt to ligand (). At 13.3 Hz, both the Ni2+ and Co2+ insertions (using the metal acetate salts) were unsuccessful over a 45-min reaction time; however, increasing the mixing frequency to 25 Hz yielded NiTPP and CoTPP quantitatively by UV-vis in as little as 10 min. Using AgNO3 and a frequency of 25 Hz led to Ag+ insertion within ∼15 min. Thus, the amount of metal salt could be reduced by increasing the frequency to 25 Hz while maintaining the same number of grinding balls.
Table 2. Summary of attempted metallation reaction conditions using seven 10-mm grinding balls as quantified by UV-vis.Table Footnotea
Three previous papers studying metal insertion into porphyrins provided extensive background and examined different routes to metalloporphyrins. Atoyebi and Brückner (Citation28) reported an extensive study of metal insertion reactions using a planetary ball mill, while Gomes et al. (Citation29) and Ralphs et al. (Citation27) used a commercial shaker mill (Retsch MM400TM) for the metal insertion processes. Atoyebi and Brückner used five 10-mm grinding balls in their 50-mL agate reaction chamber, Ralphs et al. used a 25-mL stainless steel chamber with only one 10-mm grinding ball, and Gomes et al. used a 10-mL stainless steel jar with two 7-mm grinding balls. A direct comparison of the work reported above can be made to that by Gomes et al. and Ralphs et al. as they completed their study using the same shaker mill (Retsch MM400TM) and a comparable reaction frequency. The difference in results is presumably due to the reactor volume, and number and size of grinding balls noted across the three studies. Ralphs et al. were unable to insert Co2+ into H2TPP at 25 Hz over a 60-min period using one grinding ball in the reaction jar, whereas Gomes et al. attained a 61% yield at 30 Hz over a 250-min time period using two grinding balls. In this study, CoTPP is attained quantitatively at 25 Hz after 10 min using seven grinding balls (). The results are similar for Ag + . The use of one grinding ball or two grinding balls does not insert Ag+ into the ligand, however, here the use of seven grinding balls allows Ag+ insertion (from AgNO3) in 15 min at 25 Hz. In the case of Ni2+, two grinding balls (Citation29) did not provide enough energy in a 10-mL stainless steel jar for Ni2+ insertion, yet one grinding ball in a 25-mL stainless steel jar (Citation27) enabled Ni2+ insertion after 40 min. In this study using seven grinding balls in a 50-mL jar reduced the Ni2+ insertion reaction to 15 min. From these results, the general reaction conditions suggested in the experimental section are recommended to ensure a successful reaction independent of the metal salt selected, although more optimized conditions can be used to further improve the ‘greenness’ of the reaction for certain salts. Attempts to metallate using a mortar and pestle or using liquid-assisted grinding (LAG) methods were unsuccessful, providing further evidence that a minimum mechanochemical force threshold is needed for metallation.
As briefly mentioned above, one of the authors (E.P.Z.) also developed a microwave-assisted route toward metallating porphyrins for the undergraduate teaching laboratory which could be implemented in combination with the work reported herein (Citation44). This greener route allowed for incorporation of a variety of metal species (Zn2+, Cu2+, Ni2+, ClCo2+, ClFe2+, ClMn2+) in short reaction times (<15 min.) using a microwave reactor with the same EtOH:EtOAc solvent mixture (1:1 v/v) used in our reflux approach.
Implementation with undergraduate students
The learning objectives (LOs) for students completing this undergraduate teaching experiment are to:
gain familiarity with green chemistry principles, specifically;
o P1 – Prevent Waste
o P5 – Safer Solvents and Auxiliaries
o P6 – Design for Energy Efficiency
o P7 – Use of Renewable Feedstocks
use less-conventional reaction methods of microwave and mechanochemistry
use and interpret UV-vis spectroscopy for product identification
It is worth noting that in the absence of a microwave reactor, the previous micro-scale reflux route of Ref. (Citation19) toward synthesizing H2TPP can be employed followed by metallation routes discussed within this work. Alternatively, H2TPP is also commercially available. With respect to the metallation reactions discussed herein, the reflux route is generally accessible to educators as this technique is widely utilized and is a variation of the traditional approach toward metallation. The mechanochemical route relies on the use of shaker mills which are more specialized equipment that all institutions may not have access to. Additionally, only a limited number of reactions can be simultaneously run on the shaker mills (two in the case of the instrument used for this work), although the speed of the reactions (<15 min.) should allow for numerous reactions to be completed in a typical 3–4 h undergraduate teaching laboratory period. In comparison, the microwave-assisted route previously reported by Zovinka et al. requires at least 10–15 min. per reaction, which on many microwave reactor models is limited to a single reaction at a time (Citation44). For both metallation methods, no further product work-up or isolation were performed, with the UV-vis obtained directly from the reaction mixtures to confirm formation of the products. This reduces additional time and solvent waste required for purification steps to remove the excess metal salts which are not detectable in the spectra anyways.
The microwave-assisted synthesis of H2TPP and greener reflux metallation combination was carried out in a second-year undergraduate inorganic course at the University of Toronto with approx. 80 students (∼20 students per 4 h lab session). The Fixed-Power method was performed at twice (2x) the scale detailed in the experimental section with the Tmax = 230°C to avoid over-pressurization due to reduced headspace in the 10 mL vessels. The average reported student percent yield of H2TPP was 12.5 (±4.9) %. The same students also used their microwave-synthesized H2TPP for the reflux metallation in EtOH:EtOAc solvent mixture (1:1 v/v) with one of either Zn2+, Cu2+, or Ni2+ acetate hydrate salts (Note: as discussed vide supra Ni2+ does not successfully incorporate by this route). The reflux was allowed to go longer (∼1 h total) than necessary to provide time for training and collecting UV-vis of the porphyrin. The students then shared their metallation reaction UV-vis spectra with one another in order to obtain spectral data for reactions of H2TPP with all three metal salts. Nearly all students using the Zn2+ or Cu2+ salts were able to successfully obtain a UV-vis showing formation of the metalloporphyrin; the Ni2+ salt was included as a ‘red herring’ so that students critically interpreted their data to recognize that the metalloporphyrin was not formed in this case. Anecdotally, students enjoyed the laboratory activity, including applying green chemistry principles, using a microwave reactor, and the ability to select the metal salt investigated for the metallation reaction.
In a Jr./Sr.-level (3rd/4th-year) inorganic course at Saint Francis University, the mechanochemical metallation was completed by 20 undergraduates (across two semesters) using the Retsch Mixer Mill 400TM. The students were provided commercially available H2TPP and metal salts to insert Cu2+ and Zn2+. The students tracked the reaction by removing a small sample from the jar every 5 min until the H2TPP Q-bands were no longer present. As the mill has two reaction jars, two groups of students could complete their reaction simultaneously. Since the jars are completely isolated systems, student groups either duplicated reactions of the other group or chose to complete the other metallation reaction. While the mixer mill was in use, other student groups attempted to metallate using liquid-assisted grinding (LAG) of metal salts with the porphyrin ligand in agate mortars. The students could then compare their results from hand grinding (unsuccessful) to the machine-assisted mixing (successful!) and consider the importance of force in obtaining a metallation.
An example student laboratory manual combining the implementations is provided for instructors in the Supporting Information. Other modifications of the outlined activity are possible, for example, a variation has also been carried out at Saint Francis University were students explored the impact of the number of grinding balls on product yield for the mechanochemical metallation. This undergraduate teaching experiment offers several opportunities to introduce and infuse green chemistry principles, including explicit discussions within the introduction as well as pre-/post-lab questions regarding the green chemistry principles associated with the modifications or approaches in our syntheses compared to traditional routes as discussed vide supra. For example, students can be asked to calculate and compare green chemistry metrics, such as the Mass Intensity (MI) between the original H2TPP ‘Adler-Longo’ synthesis vs. the microwave-assisted synthesis they performed in the lab.
Conclusions
In summary, the well-known tetraphenylporphyrin (H2TPP) synthesis and metallation teaching experiment was redeveloped with an emphasis on green chemistry principles. A robust microwave-assisted, microscale synthesis of H2TPP was optimized and assessed using green chemistry metrics. New routes toward metallation in the teaching space were also developed by reflux in a greener EtOH:EtOAc solvent mixture (1:1 v/v) (M = Zn2+ or Cu2+) or by solventless mechanochemical grinding using a shaker mill (M = Zn2+, Cu2+, Ni2+, Co2+, or Ag+). The experiments were successfully implemented in undergraduate inorganic chemistry courses and provided an opportunity to introduce and discuss several green chemistry concepts.
Supporting information
A Summary of microwave parameter ranges for the microwave-assisted, microwave synthesis of H2TPP, mechanochemical reaction parameters and results, UV-vis absorption spectra for mechanochemical experiments, and an example student laboratory manual are provided.
Supplemental Material
Download MS Word (2.5 MB)Acknowledgements
This research was supported, in part, by Colorado State University Pueblo (CSUP) Department of Chemistry (M.A.C), SFU Chemistry Department (E.P.Z.), and the University of Toronto Department of Chemistry (J.R.D.). This research was also support by a CSUP Communities to Build Active STEM Education (CBASE) fellowship (M.A.N.) and the Saint Francis University Office of Student Research (E.P.Z.). The authors wish to thank Prof. Kyle A. Grice (DePaul University) for testing the optimized microwave-assisted porphyrin synthesis. We also thank Beyond Benign, with support from Millipore Sigma, for bringing the authors together within the green chemistry community and for additional support including a Faculty Fellowship (J.R.D.).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beckmann, B.A.; Buchman, A.; Pasternack, R.F.; Reinprecht, J.T.; Vogel, G.C. An Advanced Laboratory Experiment in Bioinorganic Chemistry. J. Chem. Educ. 1976, 53 (6), 387–389. doi:10.1021/ed053p387.
- Bozak, R.E.; Hill, C.L. Synthesis of Tetraphenylporphin: A Convenient Undergraduate Organic Laboratory Experiment. J. Chem. Educ. 1982, 59 (1), 36. doi:10.1021/ed059p36.
- Beeston, R.F.; Stitzel, S.E.; Rhea, M.A. Investigation of Atropisomerism in Ortho-Substituted Tetraphenylporphyrins: An Experimental Module Involving Synthesis, Chromatography, and NMR Spectroscopy. J. Chem. Educ. 1997, 74 (12), 1468–1471. doi:10.1021/ed074p1468.
- Brisbin, D.A.; Asgill, J.O. The Micro-Determination of Porphyrins: An Integrated Laboratory Experiment. J. Chem. Educ. 1974, 51 (3), 211–213. doi:10.1021/ed051p211.
- Maitland, M.S.; Langley, R.H. Metalloporphyrins. J. Chem. Educ. 1984, 61, 566. doi:10.1021/ed061p566.1.
- Arnold, D.P. Aromatic Ring Currents Illustrated-NMR Spectra of Tin(IV) Porphyrin Complexes: An Advanced Undergraduate Experiment. J. Chem. Educ. 1988, 65 (12), 1111–1112. doi:10.1021/ed065p1111.
- Geiger, D.K. Axial Ligand Effects on the Spin State and Electrochemistry of Iron Porphyrins: An Advanced Laboratory Experiment. J. Chem. Educ. 1991, 68 (4), 340–342. doi:10.1021/ed068p340.
- Geiger, D.K.; Paviak, E.J.; Kass, L.T. The Determination of Axial Ligand Binding Constants for Iron Porphyrins by Cyclic Voltammetry. J. Chem. Educ. 1991, 68 (4), 337–339. doi:10.1021/ed068p337.
- Girolami, G.S.; Rauchfuss, T.B.; Angelici, R.J. Synthesis and Technique in Inorganic Chemistry, 3rd ed.; University Science Books: Melville, NY, 1999.
- Megehee, E.G.; Russo, R.J.; Hyslop, A.G. Synthesis, Characterization and Investigation of Trends in Metallated Porphyrin Complexes: An Interlaboratory Approach. Chem. Educ. 2005, 10 (2), 120–125.
- Megehee, E. G.; Hyslop, A.; Rosso, R. J. Interlaboratory Collaborations in the Undergraduate Setting. J. Chem. Educ.. 2005, 82 (9), 1345–1348. doi:10.1021/ed082p1345.
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent Advances in Eco-Friendly and Cost-Effective Materials Towards Sustainable Dye-Sensitized Solar Cells. Green Chem. 2020, 22 (21), 7168–7218. doi:10.1039/D0GC01148G.
- Gopalakrishnan, V.N.; Becerra, J.; Pena, E.F.; Sakar, M.; Béland, F.; Do, T.-O. Porphyrin and Single Atom Featured Reticular Materials: Recent Advances and Future Perspective of Solar-Driven CO2Reduction. Green Chem. 2021, 23 (21), 8332–8360. doi:10.1039/D1GC02439F.
- Suslick, K.S.; Reinert, T.J. The Synthetic Analogs of O2-Binding Heme Proteins. J. Chem. Educ. 1985, 62 (11), 974–983. doi:10.1021/ed062p974.
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A Simplified Synthesis for Meso-Tetraphenylporphine. J. Org. Chem. 1967, 32 (2), 476–476. doi:10.1021/jo01288a053.
- Lindsey, J.S.; Hsu, H.C.; Schreiman, I.C. Synthesis of Tetraphenylporphyrins Under Very Mild Conditions. Tetrahedron Lett. 1986, 27, 4969–4970. doi:10.1016/S0040-4039(00)85109-6.
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo Reactions Revisited: Synthesis of Tetraphenylporphyrins Under Equilibrium Conditions. J. Org. Chem. 1987, 52 (5), 827–836. doi:10.1021/jo00381a022.
- Marsh, D.; Mink, L. Microscale Synthesis and Electronic Absorption Spectroscopy of Tetraphenylporphyrin H2(TPP) and Metalloporphyrins ZnII(TPP) and NiII(TPP). J. Chem. Educ. 1996, 73 (12), 1188–1190. doi:10.1021/ed073p1188.
- Marsh, D.F.; Falvo, R.E.; Mink, L.M. Microscale Synthesis and 1H NMR Analysis of Tetraphenylporphyrins. J. Chem. Educ. 1999, 76 (2), 237–239. doi:10.1021/ed076p237.
- Petit, A.; Loupy, A.; Maiuardb, P.; Momenteaub, M. Microwave Irradiation in Dry Media: A New and Easy Method for Synthesis of Tetrapyrrolic Compounds. Synth. Commun. 1992, 22 (8), 1137–1142. doi:10.1080/00397919208021097.
- Chauhan, S.M.S.; Sahoo, B.B.; Srinivas, K.A. Microwave-Assisted Synthesis of 5,10,15,20-Tetraaryl Porphyrins. Synth. Commun. 2001, 31 (1), 33–37. doi:10.1081/SCC-100000176.
- Warner, M.; Succaw, G.; Doxsee, K.; Hutchison, J. Microwave Synthesis of Tetraphenylporphyrin. In Greener Approaches to Undergraduate Chemistry Experiments, Kirchhoff, M., Ryan, M. A., Eds.; American Chemical Society: Washington, DC, 2002; pp 27–31.
- Neyadi, S.S.A.; Alzamly, A.; Al-Hemyari, A.; Tahir, I.M.; Al-Meqbali, S.; Ahmad, M.A.A.; Bufaroosha, M. An Undergraduate Experiment Using Microwave-Assisted Synthesis of Metalloporphyrins: Characterization and Spectroscopic Investigations. World J. Chem. Educ. 2019, 7 (1), 26–32. doi:10.12691/wjce-7-1-4.
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the Preparation of Metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. doi:10.1016/0022-1902(70)80535-8.
- Buchler, J. W. 10 – Synthesis and Properties of Metalloporphyrins. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, 1978; pp 389–483.
- Gonsalves, A.M.d.R.; Varejão, J.M.T.B.; Pereira, M.M. Some New Aspects Related to the Synthesis of Meso-Substituted Porphyrins. J. Heterocycl. Chem. 1991, 28 (3), 635–640. doi:10.1002/jhet.5570280317.
- Bufaroosha, M.; Neyadi, S.S.A.; Alnaqbi, M.A.R.; Marzouk, S.A.M.; Al-Hemyari, A.; Abuhattab, B.Y.; Adi, D.A. An Undergraduate Experiment Using Microwave-Assisted Synthesis of First Raw Metalloporphyrins: Characterizations and Spectroscopic Study. World J. Chem. Educ. 2019, 7 (3), 225–231. doi:10.12691/wjce-7-3-6.
- Ralphs, K.; Zhang, C.; James, S.L. Solventless Mechanochemical Metallation of Porphyrins. Green Chem. 2017, 19 (1), 102–105. doi:10.1039/C6GC02420C.
- Atoyebi, A.O.; Brückner, C. Observations on the Mechanochemical Insertion of Zinc(II), Copper(II), Magnesium(II), and Select Other Metal(II) Ions into Porphyrins. Inorg. Chem. 2019, 58 (15), 9631–9642. doi:10.1021/acs.inorgchem.9b00052.
- Gomes, C.; Peixoto, M.; Pineiro, M. Modern Methods for the Sustainable Synthesis of Metalloporphyrins. Molecules 2021, 26 (21), 6652. doi:10.3390/molecules26216652.
- Sharma, R.K.; Ahuja, G.; Sidhwani, I.T. A New One Pot and Solvent-Free Synthesis of Nickel Porphyrin Complex. Green Chem. Lett. Rev. 2009, 2 (2), 101–105. doi:10.1080/17518250903117463.
- Connor, M.C.; Pratt, J.M.; Raker, J.R. Goals for the Undergraduate Instructional Inorganic Chemistry Laboratory When Course-Based Undergraduate Research Experiences Are Implemented: A National Survey. J. Chem. Educ. 2022, 99, 4068–4078. doi:10.1021/acs.jchemed.2c00267.
- Nascimento, B.F.O.; Pineiro, M.; Rocha Gonsalves, A.M.d.; Ramos Silva, M.; Beja, M.; Paixão, A.; A, J. Microwave-Assisted Synthesis of Porphyrins and Metalloporphyrins: A Rapid and Efficient Synthetic Method. J. Porphyr. Phthalocyanines 2007, 11 (2), 77–84. doi:10.1142/S1088424607000102.
- Henriques, C.A.; Pinto, S.M.A.; Aquino, G.L.B.; Pineiro, M.; Calvete, M.J.F.; Pereira, M.M. Ecofriendly Porphyrin Synthesis by Using Water Under Microwave Irradiation. ChemSusChem 2014, 7 (10), 2821–2824. doi:10.1002/cssc.201402464.
- Pineiro, M. Microwave-Assisted Synthesis and Reactivity of Porphyrins. Curr. Org. Synth. 2014, 11 (1), 89–109. doi:10.2174/15701794113106660088.
- Pinto, S.M.A.; Vinagreiro, C.S.; Tomé, V.A.; Piccirillo, G.; Damas, L.; Pereira, M.M. Nitrobenzene Method: A Keystone in Meso-Substituted Halogenated Porphyrin Synthesis and Applications. J. Porphyr. Phthalocyanines 2019, 23 (04n05), 329–346. doi:10.1142/S1088424619300039.
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, New York, 2000.
- Sheldon, R.A. Organic Synthesis – Past, Present and Future. Chem. Ind. 1992, 23, 903–906. doi:10.1021/acssuschemeng.7b03505.
- Sheldon, R.A. Consider the Environmental Quotient. CHEMTECH 1994, 24 (3), 38–47.
- Trost, B.M. The Atom Economy – A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. doi:10.1126/science.1962206.
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘Green’ Chemistry—Which Are the Best? Green Chem. 2002, 4 (6), 521–527. doi:10.1039/B206169B.
- Duarte, R.C.C.; Ribeiro, M.G.T.C.; Machado, A.A.S.C. Reaction Scale and Green Chemistry: Microscale or Macroscale, Which Is Greener? J. Chem. Educ. 2017, 94 (9), 1255–1264. doi:10.1021/acs.jchemed.7b00056.
- Ribeiro, M.G.T.C.; Costa, D.A.; Machado, A.A.S.C. “Green Star”: A Holistic Green Chemistry Metric for Evaluation of Teaching Laboratory Experiments. Green Chem. Lett. Rev. 2010, 3 (2), 149–159. doi:10.1080/17518251003623376.
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a Semi-Quantitative Tool to Select an Organic Preparation Based on Economical and Ecological Parameters. Beilstein J. Org. Chem. 2006, 2(3). doi:10.1186/1860-5397-2-3.
- Arnold, A.M.; Kwak, D.J.; Lofgren, E.; Walters, B.M.; Wilt, A.L.; Woldemeskel, S.A.; Zovinka, E.P. Microwaving Metals: Inserting Metals Into Porphyrin Ligands Using Microwave Methods. J. Chem. Educ. 2014, 19, 296–298.
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process 2016, 4 (1), 7. doi:10.1186/s40508-016-0051-z.
- Kingham, D.J.; Brisbin, D.A. Kinetics of Metalloporphyrin Formation in Glacial Acetic Acid. Inorg. Chem. 1970, 9 (9), 2034–2037. doi:10.1021/ic50091a016.
- Longo, F.R.; Brown, E.M.; Quimby, D.J.; Adler, A.D.; Moet-Ner, M. Kinetic Studies on Metal Chelation by Porphyrins. Ann. N. Y. Acad. Sci. 1973, 206 (1), 420–442. doi:10.1111/j.1749-6632.1973.tb43227.x.
- Hambright, P. The Coordination Chemistry of Metalloporphyrins. Coord. Chem. Rev. 1971, 6 (2), 247–268. doi:10.1016/S0010-8545(00)80041-7.
- Schneider, W. Kinetics and Mechanism of Metalloporphyrin Formation. In Biochemistry; Structure and Bonding; Springer: Berlin, Heidelberg, 1975; Vol. 23, pp 123–166.
- Strohmeier, M.; Orendt, A.M.; Facelli, J.C.; Solum, M.S.; Pugmire, R.J.; Parry, R.W.; Grant, D.M. Solid State 15N and 13C NMR Study of Several Metal 5,10,15,20-Tetraphenylporphyrin Complexes. J. Am. Chem. Soc. 1997, 119 (30), 7114–7120. doi:10.1021/ja970447g.