ABSTRACT
This paper addresses the development and evaluation of a case study designed to introduce both the core chemistry concepts of oxidation and reduction reactions as well as green chemistry within the undergraduate organic chemistry curriculum. The developed case study consisted of two parts that can be used in tandem or independently. Part 1 focused on calculating oxidation states and determining whether the provided bioderived reactions represented oxidation or reduction reactions, and Part 2 focused on evaluating the greenness of oxidation reactions. Students reported positive learning gains for both the core chemistry concepts of oxidation and reduction reactions and green chemistry and reported that they viewed the case study favorably. It is our hope that other educators use this case study module to incorporate green chemistry into their organic courses and use its development as inspiration to create further case studies that integrate green chemistry into the curriculum.
GRAPHICAL ABSTRACT
Introduction
One of the primary concerns faculty have about integrating green chemistry into their undergraduate organic chemistry courses is finding time to incorporate it into an already full curriculum (Citation1, Citation2). Despite these concerns, incorporating green chemistry has been shown to promote student engagement with the curriculum in a meaningful way (Citation3). Thus, using green chemistry and real-world contexts as a platform to teach the curriculum core concepts can be a viable solution for covering both necessary content and green chemistry concepts.
Within the prescribed undergraduate organic curriculum, the oxidation and reduction of organic compounds are outlined as key reactions by the American Chemical Society’s Anchoring Concepts Content Map for organic chemistry (Citation4). Yet, despite their importance, research has shown that many students struggle with these topics (Citation5). Thus, the goal of this study was to develop an instructional module that taught the concepts of oxidation and reduction reactions within the context of green chemistry using case-based learning (CBL).
CBL is a learner-centered active-learning approach that prompts students to apply course content knowledge to real-world situations as presented through case studies. CBL has been shown to increase course relevancy, boost student engagement, promote critical thinking, and improve exam scores (Citation6, Citation7). Within organic chemistry, CBL has been utilized in laboratory experiments (Citation8, Citation9) and as group assignments (Citation10, Citation11). For further details about its use in chemistry education, refer to the systematic review that was recently published by Bernardi and Pazinato (Citation7).
Due to the advantages of CBL, we chose to use a case study focused on the transformations of the biobased compound 5-hydroxymethylfurfural (5-HMF) and its derivatives as a green chemistry platform to teach about oxidation and reduction reactions. This biobased compound has been identified by the Department of Energy as one of the top 14 biomass-derived platform chemicals (Citation12). Despite its importance as a top biomass-derived chemical, to the best of our knowledge 5-HMF is not commonly included in the undergraduate organic chemistry curriculum, with only a couple of reported examples (Citation13, Citation14). This scarcity of 5-HMF in the curriculum is in stark contrast to vanillin, another example of lignocellulosic biomass, which has been the focus of numerous laboratory experiments (Citation15–17). Therefore, we hope this case study promotes the further integration of 5-HMF into the curriculum.
Description of developed case study
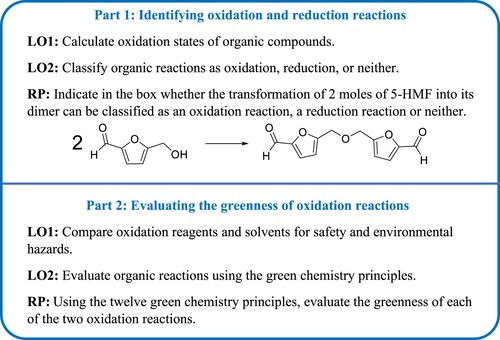
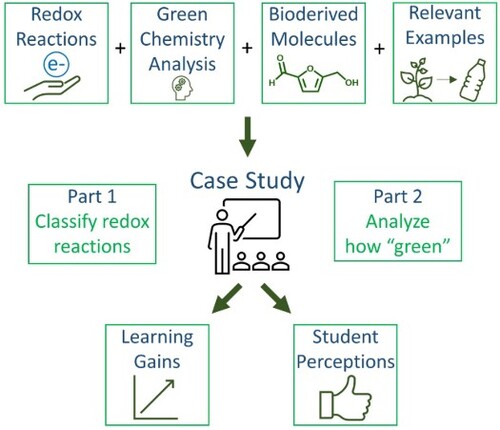
The case study was designed to consist of two parts that can be used either independently or in tandem. As shown in , Part 1 of the case study introduced students to assigning oxidation states and determining whether the specified molecule is oxidized, reduced, or neither. Part 2 of the case study introduced students to the analysis of various oxidation reactions using the green chemistry principles (GCPs).
Both parts of the case study were prefaced with an introduction developed using the Transparency in Learning and Teaching (TILT) framework (Citation18) in which the purpose, task, and criteria for success for the assignment were provided to the students. TILT was chosen because it has been shown to increase student engagement, retention, and academic confidence (Citation18). Further details about its integration into chemistry courses have recently been reported elsewhere (Citation19). However, despite its advantages, the TILT framework has not yet been commonly used in the organic or green chemistry curriculum.
Methods
This project was conducted at a medium-sized midwestern university in the spring and fall semesters of 2022, with the approval of the Institutional Review Board (Protocol # IRB0004053). Study participants were enrolled in one of three courses: Organic Chemistry 1 in Spring 2022 (N = 13), Survey of Organic Chemistry in Fall 2022 (N = 23), and Organic Chemistry 1 in Fall 2022 (N = 125). Students enrolled in Organic Chemistry 1 in Spring 2022 and Survey of Organic Chemistry in Fall 2022 only completed the first part of the case study module. Students enrolled in Organic Chemistry 1 in Fall 2022 completed both parts of the module with 86 students completing Part 1, 89 students completing part 2, and 52 students providing a self-assessment of learning gains after completing both parts.
Development and refinement of the case study
The case study was developed through an iterative process using a participatory research framework, with direct collaboration from students (Citation20). Prior to administering it in a classroom setting, feedback was received from a chemistry professor, graduate students, and an undergraduate student who had taken the course in the prior year. This feedback resulted in modifying the formatting to enhance readability, adding additional problems, and rearranging a few of the problems to ensure they became progressively more difficult as the case study was completed.
To receive further feedback from students, the initial version of the case study was then piloted in an Organic Chemistry 1 course (Spring 2022 [N = 13]), and student feedback about Part 1 of the case study was collected via a survey. The Spring 2022 iteration was used solely to receive student feedback needed for final Fall 2022 revisions. Due to the feedback received, the document was revised to add explanations on how to perform the calculations on the first page of the problem set and to remind students to work in groups at the start of the activity. In addition, in subsequent administrations of this case study, the PowerPoint slides with sample problems were provided to students prior to the class via the course management system.
The revised version was then administered in a Survey of Organic Chemistry course and an Organic Chemistry 1 course in Fall 2022 as one of their in-class activity assignments. Students in the Survey of Organic Chemistry course received nominal bonus points for completing the case study and survey. However, students in the Organic Chemistry 1 course received credit for completing the two parts of the case study since they were assigned as required in-class activities. Prior exposure to redox reactions for both sections was assumed to be in General Chemistry. For the Organic Chemistry 1 course, however, the subsequent course material was connected to the classification of reactions as oxidation, reduction, or neither.
Upon completion of the activity, students in each class provided feedback through a four-question survey that asked students: (i) to provide three learned concepts, (ii) to identify a remaining question, (iii) to list beneficial aspects of the case study, and (iv) to indicate what aspects they would like changed. In addition, students enrolled in the Organic Chemistry 1 course in Fall 2022 completed a post-assessment containing a student assessment of learning gains (SALG). This study focused on the administration of the revised version in Fall 2022 with both parts of the revised case study provided in the supporting information.
Results and discussion
Student feedback for Part 1: identifying oxidation and reduction reactions
Upon finishing the first part of the case study, students completed a survey that explored what they learned and how they perceived the case study. The first question of the survey used a classroom assessment technique that involved asking students to identify three learned concepts from completing the activity. For the Survey of Organic Chemistry course (N = 23), commonly identified learned concepts included the acronym OIL RIG for remembering that oxidation is loss of electrons and reduction is gain of electrons (n = 9, 39%), calculating oxidation states (n = 9, 39%), calculating average oxidation states (n = 7, 30%), and identifying oxidation and reduction reactions (n = 7, 30%). Similarly, for the Organic Chemistry 1 course (N = 86), commonly identified concepts included identifying oxidation and reduction reactions (n = 38, 44%), calculating oxidation states (n = 34, 40%), and calculating average oxidation states (n = 17, 20%).
When asked about what aspects of the case study students found beneficial, the most common responses for students in the Survey of Organic Chemistry course (N = 23) included the PowerPoint slides and introductory lesson (n = 10, 43%), receiving help during class (n = 4, 17%), and working on questions as a group (n = 4, 17%). However, for students in the Organic Chemistry 1 course (N = 86) responses included the case study containing many different practice problems (n = 16, 19%), providing background information about the reaction along with each problem (n = 15, 17%), and including an introduction that used the TILT method to outline the assignment details (n = 12, 14%). Overall, it was exciting to see that 17% of the students reported that providing the background information about the reaction was a beneficial aspect of the case study because they found value in seeing the relevance of the reactions they were learning about. This student appreciation for real-world applications of the chemistry reactions (Citation21) and green chemistry (Citation22) has also been reported in other studies, further supporting its need for inclusion.
Finally, when asked what aspects of the case study students would like changed, the most common responses from the Survey of Organic Chemistry course (N = 23) included shortening the case study (n = 9, 39%), including more instructional time before completing the activity (n = 5, 22%), and that they would not change the activity (n = 3, 13%). Similarly, responses from the Organic Chemistry 1 course (N = 86) included they would not change the activity (n = 20, 23%), would include more information in the introduction section such as examples for determining oxidation and reduction and further explanations of the concepts to be learned (n = 16, 19%), would shorten the background information about the reactions (n = 6, 7%), and would have fewer questions (n = 6, 7%).
Student feedback for Part 2: evaluating the greenness of oxidation reactions
Students enrolled in Organic Chemistry 1 in Fall 2022 completed the second part of the case study (N = 89). Upon completion, students (N = 58) again completed a survey to identify what concepts they learned and how they perceived the second part of the case study. The first question of the survey asked students to identify three concepts they learned by completing the activity. As intended, the most identified learned concepts included evaluating the greenness of reactants, solvents, and reactions (n = 44, 76%), learning about the GCPs and how to apply them (n = 20, 34%), calculating oxidation states (n = 19, 33%), finding and using safety data sheets (n = 17, 29%), and identifying oxidation and reduction reactions (n = 11, 19%).
When asked about what aspects about Part 2 of the case study they found beneficial, the most common responses included providing the Sigma Aldrich website (www.SigmaAldrich.com) and practicing finding and reading safety data sheets (n = 18, 31%), learning about green chemistry (n = 15, 26%), providing the GlaxoSmithKline Solvent Guide (n = 11, 19%), practicing the calculation of oxidation states (n = 10, 17%), and comparing the greenness of reactions (n = 10, 17%), reagents (n = 7, 12%), and solvents (n = 3, 5%). In addition, five students (9%) indicated that they appreciated that the last question required them to put together the concepts learned previously in the case study because it enabled them to see the bigger picture.
Finally, students were asked to provide recommendations for aspects of the case study that they would like changed. The most common responses included do not change it (n = 19, 33%), provide and discuss correct solutions (n = 9, 16%), include more problems on comparing greenness of reactions (n = 5, 9%), and have fewer problems that required consulting safety data sheets (n = 5, 9%).
Student’s self-assessment of learning gains from completing the case study
To assess student learning gains from completing both parts of the case study, students were asked to report a student-assessment of learning gains (SALG) using a Likert-type instrument that was prefaced with the statement ‘As a result of completing the Case Study Modules’ to ensure that their answers were in response to learning from the case study. Only responses from students who completed both parts of the case study were included in this analysis (N = 52). Because the goal of the case study was to teach students about both oxidation and reduction reactions and green chemistry concepts, the prompts for self-assessment were designed to address each of these topics.
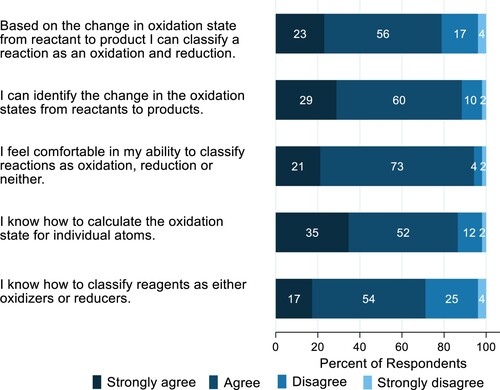
As shown in , most students self-reported that they either strongly agreed or agreed with the statements that they can calculate the oxidation state of an atom and that they can classify a reaction as an oxidation or reduction; thus, providing partial evidence for the effectiveness of the case study for teaching the core chemistry concepts of oxidation and reduction.
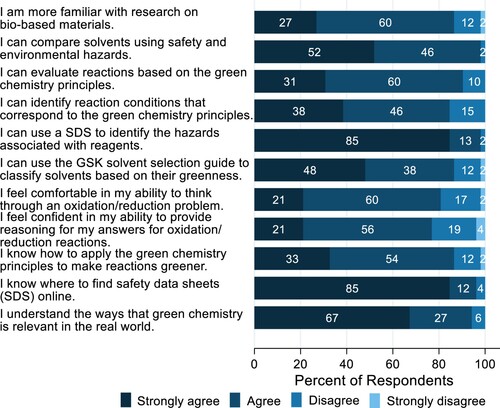
Moreover, as shown in , students indicated that the case study helped them become more familiar with research on biobased materials, learn how to find and use safety data sheets for identifying chemical hazards, and analyze a reaction for its adherance to the GCPs. Furthermore, it was found that the items that most students strongly agreed to corresponded to procedural knowledge, i.e. they learned how to find and use safety data sheets and how to compare solvents for their safety and environmental hazards. Promisingly, 67% of the students strongly agreed with the statement that they understand ways that green chemistry is relevant to the real world. This provided partial evidence that the case study achieved its secondary goal of introducing green chemistry concepts.
Finally, students were asked to respond to the prompt ‘I would like to see similar activities implemented in future courses.’ Of the 52 respondents, most (n = 44, 85%) either strongly agreed or agreed with the statement indicating that they perceived value in the assignment. This perceived value was further elaborated in their open responses to the survey prompts. For example, in response to what aspects of the case study they would like changed, one student wrote ‘I actually really liked this activity. It was easy to complete in a timely manner and made you think outside the box. I wouldn’t change anything about the activity!’ Similarly, in response to beneficial aspects of the case study another student replied, ‘I really liked the real world [sic] applications, which makes what we're learning seem more relevant.’ Responses such as these show that students appreciate when the chemistry content is connected to relevant concepts as it was in this case study.
While these positive student perceptions of the case study and resultant learning gains are encouraging, research has shown that self-reported measures are not always reliable indicators of cognitive learning gains (Citation22–25). Thus, to measure these learning gains, questions about calculating oxidation states, identifying oxidation and reduction reactions, and evaluating the greenness of a reaction were included on the unit exam.
Assessment of student knowledge of oxidation states and redox reactions
To further evaluate student knowledge of oxidation states and redox reactions after completing the case study, the unit exam contained two multiple choice questions for which students had to calculate oxidation states and three multiple choice questions for which students had to identify reactions as either oxidation, reduction, or neither. A total of 125 students completed this exam.
The first question about oxidation states (OS-1) prompted students to calculate the oxidation state of an aldehyde and a ketone. The second prompt on oxidation states (OS-2) asked students to calculate the oxidation state of the secondary carbon attached to the hydroxyl group in 2-propanol that was previously shown in the PowerPoint on oxidation states.
The three questions assessing student ability to classify reactions as oxidation, reduction, or neither featured one of each category. The first of these questions (CR-1) asked students to identify the conversion of glucose to sorbitol as a reduction reaction. The second (CR-2) asked students to identify the conversion of glucose to levoglucosan as neither an oxidation nor a reduction reaction. Finally, the third of these prompts (CR-3) asked students to identify the conversion of glucose to mucic acid as an oxidation reaction.
Psychometric analysis using classical test theory was performed for these items to determine the item difficulty and discrimination index as illustrated in . Item difficulty (p) refers to the proportion of respondents who correctly answered the item; whereas the discrimination index (d) refers to an item’s ability to distinguish between individuals without reference to an external criterion. Items with larger discrimination indexes have a greater percentage of high achieving students than lower achieving students answering the question correctly (Citation26), whereas values close to zero indicate no discrimination (Citation27). A discrimination index less than 0.20 indicates that the item can benefit from revision, between 0.20 and 0.30 indicates that the item is fair, and those between 0.40 and 0.70 are considered good (Citation28). As shown in , the items were found to function well psychometrically.
Table 1. Item difficulties (p) and discrimination values (d) for the calculating oxidation states (OS) and classifying reaction (CR) prompts.
Within the prompts about calculating the oxidation state, it was somewhat surprising to see that students found the 2-propanol prompt (OS-2) more difficult since it was explicitly discussed in class. However, it has been well established in literature that students struggle with interpreting line structures (Citation29) and consider that carbon atoms can have more-than or less-than four bonds (Citation30, Citation31). Thus, it was not surprising that the distractor of +1 was selected by 35% of the respondents indicating that they did not account for the hydrogen that was not explicitly drawn in the line structure.
Within the prompts about classifying the reactions, the conversion of glucose to levoglucosan (CR-2) was found to be the most difficult. This was expected because it required students to calculate the oxidation states to classify the reaction, whereas the other two items could be identified strictly by either remembering the trends in the number of C–H or C–O bonds or trends in functional group transformations.
Assessment of student knowledge of green chemistry
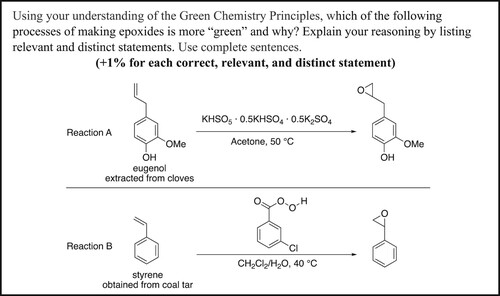
To evaluate student knowledge of green chemistry after completing the case study, the unit exam contained a bonus question asking students to compare the greenness of two reactions as illustrated in . This style of reaction comparison prompts has previously been reported for the evaluation of student knowledge of green chemistry (Citation22, Citation32–34). Expected student responses were that reaction A uses a bioderived molecule (GCP #7) and uses acetone, which is less hazardous than dichloromethane (GCP #3, 5, and 12). The expected student response for reaction B was that it requires a lower temperature and thus would require less energy (GCP #6). While it could be argued that if reaction B required much less dichloromethane than reaction A required acetone, it could make reaction B preferential regarding waste prevention and energy consumption (GCP #1 and 6), and if any of our students had indicated this it would have been counted as a correct response. However, due to the hazards of dichloromethane (DCM) (Citation35) and the EPA working on banning or extremely regulating the use of DCM (Citation36), it would require a very large difference for it to be preferential.
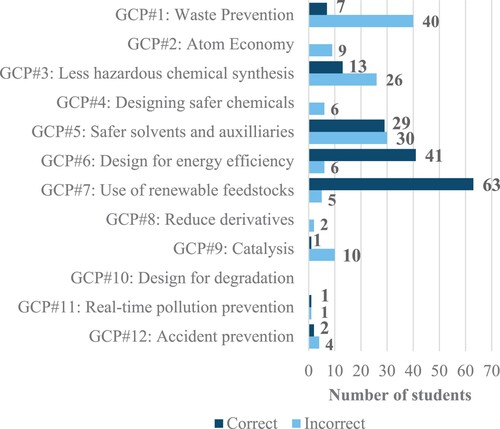
A total of 109 students attempted the green chemistry bonus problem on the exam. It was found that students who submitted Part 2 of the case study correctly applied an average of 1.6 GCPs, whereas students who did not submit Part 2 of the case study correctly applied an average of 1.0 GCPs. Further analysis of student responses to this open-ended prompt indicated that the most common correctly applied GCPs included use of renewable feedstocks (n = 63), design for energy efficiency (n = 41), and safer solvents and auxiliaries (n = 29). Furthermore, the most common incorrectly applied GCPs included waste prevention (n = 40), safer solvents and auxiliaries (n = 30), and less hazardous chemical synthesis (n = 26).
Due to a few students stating more than one incorrect concept for each GCP, the number of responses obtained for each GCP exceeded the number of students shown in . Thus, the following values represent the number of responses obtained. For the principle of waste prevention, a total of 41 incorrect responses were obtained. Of these, the most common incorrect conception was that the number of different reactants used determined the quantity of waste generated in the reaction (n = 12). For the principle of safer solvents and auxiliaries, a total of 31 incorrect responses were obtained. For students who incorrectly applied this principle, the most common incorrect responses were that they only considered the water as the solvent (n = 14) and identified the dichloromethane/water solvent system as safer than acetone (n = 11). Finally, for the principle of less hazardous chemical synthesis, a total of 31 incorrect responses were obtained. Of these, most responses marked incorrect only stated that either reaction A or reaction B had less hazardous chemicals without any further explanation (n = 18). Interestingly, two students indicated the elements making up the reactants determined their overall hazards, and one student indicated the functional groups present on the reactant determined its hazards. Furthermore, an additional two students indicated that a chemical’s hazards are due to the source of the chemical, in this case from cloves versus coal tar.
We were further interested in exploring the effect that completing the case study had on student argumentation levels of their responses. Thus, the use of the structure of observed learning outcome (SOLO) taxonomy was employed to compare the argumentation level of responses from students who submitted Part 2 of the case study to those who did not submit it for points. The use of SOLO taxonomy allows instructors to rank student responses to open-ended prompts based on the structural complexity of their answers (Citation37). This increasing complexity in responses can help measure the progression of student knowledge as they become more familiar about a topic (Citation38).
The five stages of SOLO taxonomy include prestructural, unistructural, multistructural, relational, and extended abstract. Within this study, responses were coded as prestructural if they only indicated which reaction was greener or provided only incorrect reasoning for their answers. Responses were coded as unistructural if they correctly addressed only one or two GCPs in their analysis. Multistructural responses contained at least three correctly applied GCPs, but the analysis did not compare the pros and cons of each reaction. Relational responses not only contained at least three correctly applied GCPs, but also included an analysis of the pros and cons for each reaction. Finally, responses were coded as extended abstract if at least three GCPs and the pros and cons of the reactions were addressed along with at least one recommendation on how to improve the selected greener reaction.
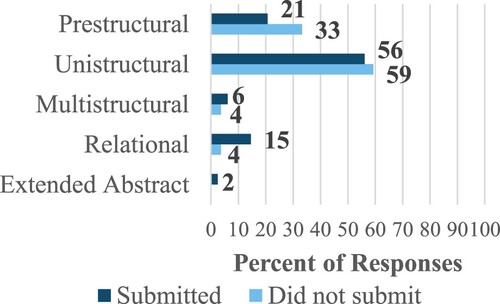
Determining which GCPs were applied in student responses required that the response provided enough information to identify the applicable GCP. Thus, statements referring only to which reaction has a higher temperature without addressing the significance of the temperature difference were not assigned a code. Additionally, statements in which the brevity of details prevented identification of the applicable GCP were also excluded from the analysis. As shown in , students who submitted Part 2 of the case study for credit tended to exhibit a higher level of structural complexity than students who did not submit their case study. In fact, 92% of respondents who did not submit Part 2 scored within the prestructural to unistructural range, whereas only 77% of respondents who submitted it scored within this range. However, one limitation to this analysis is that there may be some students who completed the assignment but simply did not submit it. Although most students remained at the unistructural level, this was their first introduction to the GCPs and so there remains room to progress through the levels in the second semester of organic chemistry and in future courses.
Conclusion
The integration of green chemistry into the curriculum requires that instructors rethink how to teach the material. Instead of teaching the content as it has been for the last century, there is a need to frame it using relevant current examples to increase student interest. In this study, the concepts of oxidation and reduction were tied to green chemistry using the biobased molecule 5-hydroxymethylfurfural. Through focusing on a series of oxidations and reductions of this molecule, students explored the chemical transformations of biomass compounds while learning the prescribed chemistry content. This emphasis was appreciated by our students, who noted it as one of the beneficial aspects of the case study, with one student saying:
I also liked the paragraphs with a little history/summary before the problems because it made the problems more relatable. Organic chemistry seems much more abstract when you can't relate concepts back to your own life. I thought the paragraphs were interesting.
It was also beneficial to have the section ‘tasks of the assignment’ and ‘criteria for success on the assignment’ because it helped me realized [sic] what I should be understanding from this assignment and if I am not then I should be asking questions so that I can understand it.
The case study and corresponding PowerPoint slides have been included in the supporting information to facilitate their use by other faculty. While this case study was designed to be completed as a peer activity within the lecture course, it can be implemented in lecture or laboratory courses when teaching about oxidation and reduction reactions. Implementation in a large enrollment course would benefit from the help of learning assistants or through using the peer-led team learning (PLTL) model (Citation39, Citation40). Furthermore, the two parts of the case study can be used either independently or in tandem. In addition, the case study can be shortened by removing questions or assigning only selected questions for points. In this manner, students who want more practice will have that option; in this study mixed feedback was received with some students wanting more practice and others wanting less. It is our hope that more resources for using green chemistry as a platform to teach organic chemistry are developed and widely disseminated, such as those stemming from initiatives like the Green Chemistry Commitment (Citation41), Green Chemistry Teaching and Learning Community (Citation42), and the Green & Sustainable Chemistry Education Module Development Project (Citation43).
Supplemental Material
Download MS Word (4.3 MB)Acknowledgements
The first author acknowledges the NDSU DBER Education Fellowship. The authors wish to thank the students who participated in this project. We would also like to thank Megan Wolf for her work on developing the Student Assessment of Learning Gains (SALG) questions used in the post-test. We would also like to thank James Nyachwaya, Ariana McDarby, Evan Culver, Spencer Gilman, and Annie Schiro for their feedback on the iterations of the case study. Finally, we would like to thank Kristina Caton from the NDSU Center for Writers for her help with revising the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grieger, K.D.; Hill, B.; Leontyev, A. Exploring Curriculum Adoption of Green and Sustainable Chemistry in Undergraduate Organic Chemistry Courses: Results from a National Survey in the United States. Green Chem. 2022, 24, 8770–8782.
- Mackellar, J.J.; Constable, D.J.C.; Kirchhoff, M.M.; Hutchison, J.E.; Beckman, E. Toward a Green and Sustainable Chemistry Education Road Map. J. Chem. Educ. 2020, 97 (8), 2104–2113.
- Kennedy, S.A.; Chapman, R.M. Green Chemistry as the Inspiration for Impactful and Inclusive Teaching Strategies. In Integrating Green and Sustainable Chemistry Principles into Education; Dicks, A.P., Bastin, L.D., Eds.; Elsevier: Cambridge, MA, 2019; pp 1–30.
- Raker, J.; Holme, T.; Murphy, K. The ACS Exams Institute Undergraduate Chemistry Anchoring Concepts Content Map II: Organic Chemistry. J. Chem. Educ. 2013, 90 (11), 1443–1445.
- Brandriet, A.R.; Bretz, S.L. Measuring Meta-Ignorance Through the Lens of Confidence: Examining Students' Redox Misconceptions About Oxidation Numbers, Charge, and Electron Transfer. Chem. Educ. Res. Pract. 2014, 15 (4), 729–746.
- Hibbard, L. Case Studies for General Chemistry: Teaching with a Newsworthy Story. J. Chem. Educ. 2019, 96 (11), 2528–2531.
- Bernardi, F.M.; Pazinato, M.S. The Case Study Method in Chemistry Teaching: A Systematic Review. J. Chem. Educ. 2022, 99 (3), 1211–1219.
- Schaber, P.M.; Larkin, J.E.; Pines, H.A.; Berchou, K.; Wierchowski, E.; Marconi, A.; Suriani, A. Supercritical Fluid Extraction versus Traditional Solvent Extraction of Caffeine from Tea Leaves: A Laboratory-Based Case Study for an Organic Chemistry Course. J. Chem. Educ. 2012, 89 (10), 1327–1330.
- Saloranta, T.; Lönnqvist, J.E.; Eklund, P.C. Transforming Undergraduate Students into Junior Researchers: Oxidation-Reduction Sequence as a Problem-Based Case Study. J. Chem. Educ. 2016, 93 (5), 841–846.
- Hodges, L.C. From Problem-Based Learning to Interrupted Lecture: Using Case-Based Teaching in Different Class Formats. Biochem. Mol. Biol. Educ. 2005, 33 (2), 101–104.
- Bennett, N.; Cornely, K. Thalidomide Makes a Comeback: A Case Discussion Exercise That Integrates Biochemistry and Organic Chemistry. J. Chem. Educ. 2001, 78 (6), 759–761.
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12 (4), 539–554.
- Simeonov, S.P.; Afonso, C.A.M. Batch and Flow Synthesis of 5-Hydroxymethylfurfural (HMF) from Fructose as a Bioplatform Intermediate: An Experiment for the Organic or Analytical Laboratory. J. Chem. Educ. 2013, 90 (10), 1373–1375.
- Pfab, E.; Filiciotto, L.; Luque, R. The Dark Side of Biomass Valorization: A Laboratory Experiment to Understand Humin Formation, Catalysis, and Green Chemistry. J. Chem. Educ. 2019, 96 (12), 3030–3037.
- Palesch, J.J.; Gilles, B.C.; Chycota, J.; Haj, M.K.; Fahnhorst, G.W.; Wissinger, J.E. Iodination of Vanillin and Subsequent Suzuki-Miyaura Coupling: Two-Step Synthetic Sequence Teaching Green Chemistry Principles. Green Chem. Lett. Rev. 2019, 12 (2), 117–126.
- Winter, R.T.; Van Beek, H.L.; Fraaije, M.W. The Nose Knows: Biotechnological Production of Vanillin. J. Chem. Educ. 2012, 89 (2), 258–261.
- Ruiz, K.A.; López, M.; Suppan, G.; Makowski, K. Crossed Aldol Reactions in Water Using Inexpensive and Easily Available Materials as a Tool for Reaction Optimization Teaching in an Undergraduate Organic Chemistry Laboratory. J. Chem. Educ. 2020, 97 (10), 3806–3809.
- Winkelmes, M.-A.; Bernacki, M.; Butler, J.; Zochowski, M.; Golanics, J.; Weavil, K.H. A Teaching Intervention That Increases Underserved College Students’ Success. Peer Rev. 2016, 18 (1/2), 31–36.
- Medawala, W.; Nugawela, D. Effectiveness of Transparency in Learning and Teaching (TILT) in Chemistry Courses. In Integrating Transparency in Learning and Teaching (TILT): An Effective Tool for Providing Equitable Opportunity in Higher Education; Akella, D., Paudel, L., Wickramage, N., Rogers, M., Gibson, A., Eds.; IGI Global: Hershey, PA,, 2022; pp 113–134.
- Vaughn, L.M.; Jacquez, F. Participatory Research Methods - Choice Points in the Research Process. J. Particip. Res. Methods 2020, 1 (1), 1–13.
- Jones, M.B.; Miller, C.R. Chemistry in the Real World. J. Chem. Educ. 2001, 78 (4), 484–487.
- Armstrong, L.B.; Rivas, M.C.; Zhou, Z.; Irie, L.M.; Kerstiens, G.A.; Robak, M.A.T.; Douskey, M.C.; Baranger, A.M. Developing a Green Chemistry Focused General Chemistry Laboratory Curriculum: What Do Students Understand and Value About Green Chemistry? J. Chem. Educ. 2019, 96 (11), 2410–2419.
- Von Blottnitz, H.; Case, J.M.; Fraser, D.M. Sustainable Development at the Core of Undergraduate Engineering Curriculum Reform: A New Introductory Course in Chemical Engineering. J. Clean. Prod. 2015, 106, 300–307.
- Davis, D.A.; Mazmanian, P.E.; Fordis, M.; Van Harrison, R.; Thorpe, K.E.; Perrier, L. Accuracy of Physician Self-Assessment Compared With Observed Measures of Competence. JAMA 2006, 296 (9), 1094.
- Sitzmann, T.; Ely, K.; Brown, K.G.; Bauer, K.N. Self-Assessment of Knowledge: A Cognitive Learning or Affective Measure? Acad. Manag. Learn. Educ. 2010, 9 (2), 169–191.
- Kline, T.J.B. Classical Test Theory: Assumptions, Equations, Limitations, and Item Analysis. In Psychological Testing: A Practical Approach to Design and Evaluation; Shaw, L.C., Crouppen, M., Hoffman, C.A., Weight, B., Eds.; Sage: Thousand Oaks, CA, 2005; pp 91–106.
- Meyer, J.P. Applied Measurement with jMetrik; Routledge: New York, 2014.
- McGahee, T.W.; Ball, J. How to Read and Really Use an Item Analysis. Nurse Educ. 2009, 34 (4), 166–171.
- Anderson, T.L.; Bodner, G.M. What Can We Do About ‘Parker’? A Case Study of a Good Student Who Didn’t ‘Get’ Organic Chemistry. Chem. Educ. Res. Pract. 2008, 9 (2), 93–101.
- Duis, J.M. Organic Chemistry Educators’ Perspectives on Fundamental Concepts and Misconceptions: An Exploratory Study. J. Chem. Educ. 2011, 88 (3), 346–350.
- Ealy, J. Analysis of Students’ Missed Organic Chemistry Quiz Questions That Stress the Importance of Prior General Chemistry Knowledge. Educ. Sci. 2018, 8 (2), 42.
- Grieger, K.; Leontyev, A. Student-Generated Infographics for Learning Green Chemistry and Developing Professional Skills. J. Chem. Educ. 2021, 98 (9), 2881–2891.
- Grieger, K.; Schiro, A.; Leontyev, A. Development of the Assessment of Student Knowledge of Green Chemistry Principles (ASK-GCP). Chem. Educ. Res. Pract. 2022, 23 (3), 531–544.
- Grieger, K.; Leontyev, A. Teaching Green Chemistry Though Student-Generated Open Educational Resources. J. Coll. Sci. Teach., in press.
- Vidal, S. Safety First: A Recent Case of a Dichloromethane Injection Injury. ACS Cent. Sci. 2020, 6 (2), 83–86.
- Erickson, B.E. EPA Moves to Regulate Methylene Chloride. C&EN Glob. Enterp. 2022, 100 (41), 16–16.
- Hodges, L.C.; Harvey, L.C. Evaluation of Student Learning in Organic Chemistry Using the SOLO Taxonomy. J. Chem. Educ. 2003, 80 (7), 785–787.
- Biggs, J.; Tang, C. Train-the-Trainers: Implementing Outcomes-Based Teaching and Learning in Malaysian Higher Education. Malaysian J. Learn. Instr. 2011, 8, 1–19.
- Wamser, C.C. Peer-Led Team Learning in Organic Chemistry: Effects on Student Performance, Success, and Persistence in the Course. J. Chem. Educ. 2006, 83 (10), 1562–1566.
- Clark, A.; Raker, J.R. Peer-Leaders’ Perceived Roles: An Exploratory Study in a Postsecondary Organic Chemistry Course. Int. J. Teach. Learn. High. Educ. 2020, 32 (2), 180–189.
- Cannon, A.S.; Levy, I.J. The Green Chemistry Commitment: Transforming Chemistry Education in Higher Education. In The Promise of Chemical Education: Addressing Our Students’ Needs: Daus, K., Rigsby, R., Eds.: American Chemical Society: Washington, DC, 2015; pp 115–125.
- Green Chemistry Teaching and Learning Community. https://www.beyondbenign.org/online-community-gctlc/. (accessed Aug 4, 2022).
- Green & Sustainable Chemistry Education Module Development Project. https://www.acs.org/content/acs/en/greenchemistry/students-educators/module-development.html. (accessed Mar 22, 2021).