ABSTRACT
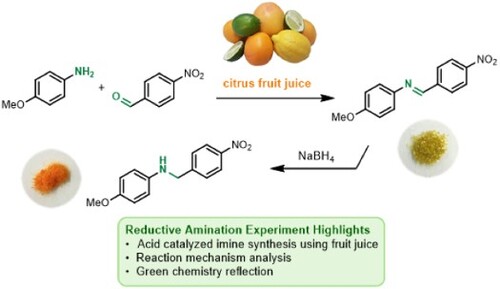
A less hazardous and energy efficient reaction performed using freshly squeezed citrus juice as solvent has been designed and implemented within a sophomore level organic chemistry laboratory. The primary learning objectives are to enable students to (i) identify and reflect upon various green chemistry principles such as waste prevention, atom economy, less hazardous synthesis, use of safer chemicals, catalysis, design for energy efficiency, and inherently safer chemistry for accident prevention; (ii) use proton NMR spectroscopic data to characterize a synthesized Schiff base (imine); and (iii) describe the reaction mechanism for imine formation, including the reasoning for why citrus juice is an excellent reaction medium. Specifically, 4-nitrobenzaldehyde is combined with 4-methoxyaniline at room temperature in the presence of four different fruit juices as reaction media to successfully synthesize an imine that is expensive to procure commercially. This is followed by students undertaking reduction of the imine to form a secondary amine which has a dramatically distinct color due to the disruption in conjugation. In performing this overall reductive amination, students expand their knowledge on acid-catalyzed imine synthesis and its mechanism, strengthen their practical skills in the laboratory, and reflect on green chemistry principles within the context of fundamental organic reactivity.
GRAPHICAL ABSTRACT
Introduction
The degree of wasted food in the United States (both that which reaches the plates of consumers and that which does not) is at a staggering level. A decade-old report by the U.S. Natural Resources Defense Council stated that 40% of food was going uneaten across the country, with this being the largest single component of national municipal solid waste (Citation1). In addition, the cost of transforming food from ‘farm to fork’ was estimated at 10% of the total U.S. energy budget. More recently, the Food and Agriculture Organization of the United Nations disclosed that ‘globally around 14 percent of the world’s food is lost from production before reaching the retail level’ (Citation2), and Target 12.3 of the U.N. Sustainable Development Goals (within ‘Responsible Consumption and Production’) advocates cutting the per capita global food waste in half by 2030 (Citation3). Of relevance to this target is the notion of repurposing so-called ‘ugly food,’ including produce such as fruits and vegetables that are visually unpalatable to the shopper and remain unsold (Citation4). Bioactive compounds (including enzymes, polyphenols, vitamins, and carotenoids) can be extracted from fruit and vegetable waste and potentially utilized elsewhere (Citation5). Methods of fruit waste valorization (making waste valuable) have been described in the chemistry pedagogical literature, including conversion of sugars and lignocellulosic biomass from pineapple waste to biofuel (Citation6), employing pectin from citrus peel to generate shear-thinning gels (Citation7), and promoting Nobel Prize-winning ‘click’ reactions with ascorbic acid derived from fruit peels and juices (Citation8). Fruits and fruit juices have also previously been explored from other teaching perspectives in the undergraduate chemistry laboratory (Citation9–12). From a research perspective, the juice from squeezed citrus fruits has been used as a solvent that provides an acid catalyst for several organic transformations (Citation13–15). It is with these latter publications in mind that we sought to design a synthesis for the introductory organic teaching laboratory to (i) showcase the use of different citrus juices; and (ii) to highlight an approach towards combatting the issue of wasted food.
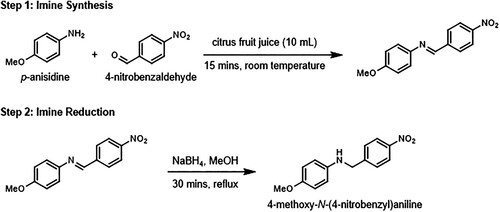
The Schiff base (imine) functional group is routinely discussed in sophomore organic chemistry courses, along with its preparation by reacting an aldehyde or ketone with a primary amine under weakly acidic conditions (Citation16). Many imines display biologically important properties including cytotoxicity and antimicrobial activity (Citation17–19). Unsurprisingly, the synthesis of imines has been a focus of several undergraduate laboratory experiments (Citation20–24) which include preparation of cholesteric liquid crystals (Citation23) and a metal–organic cage capable of trapping benzene (Citation24). ‘Greening’ imine synthesis has recently become an applied research focus with groups reporting use of greener solvents such as water and ethyl lactate (Citation25–27) and alternative catalysts (Citation28, Citation29). Our interest and previous collaboration between the University of Toronto (UT) and University of Pittsburgh Johnstown (UPJ) in designing greener organic experiments (Citation30) led us to develop the preparation of a highly colored yellow/green imine through reaction of 4-nitrobenzaldehyde and 4-methoxyaniline (p-anisidine). This was undertaken by students in the presence of different citrus juices (orange, lemon, lime, and grapefruit) as reaction media (Scheme 1, Step 1), with the weakly acidic pH of the fruit juices being ideal for acid-catalyzed imine synthesis. This experiment highlights various green chemistry principles including waste prevention, atom economy, less hazardous synthesis, use of safer chemicals, catalysis, design for energy efficiency, and inherently safer chemistry for accident prevention. To extend the experiment further, a second reaction step was incorporated where the imine product was reduced to form the corresponding secondary amine (4-methoxy-N-(4-nitrobenzyl)aniline, Scheme 1, Step 2) with the overall two-step transformation representing a reductive amination. Step 2 was primarily developed to showcase a vivid color change to orange due to alteration of the compound conjugation. Other direct reductive amination procedures have been reported in the pedagogical literature (Citation31–33), two of which take place under solvent-free conditions (Citation32, Citation33).
Specific experiment learning objectives
After completing this reaction sequence, successful students will be able to:
Complete a two-step reductive amination involving an acid-catalyzed imine synthesis in a citrus juice solvent.
Perform a variety of fundamental laboratory techniques including heating under reflux, vacuum filtration, and crude product recrystallization.
Characterize the imine and amine products by both proton nuclear magnetic resonance (1H NMR) spectroscopy and melting point determination.
Identify the principles of green chemistry highlighted in the experiment and describe how each are used to help make chemical work safer and more responsible.
Describe the reaction mechanism for the imine synthesis, including the effect of media acidity.
Participants & practical considerations
In this two-part laboratory experiment, students performed an environmentally friendly imine synthesis using citrus fruit juices as the solvent and acid catalyst, followed by reduction to an amine product. The activity was implemented at both UT and UPJ within sophomore organic chemistry courses that contain lecture and laboratory components, geared toward students with an interest in chemistry. Students first earned a single semester credit in introductory organic chemistry (also containing a lecture and laboratory component) as a prerequisite prior to taking each course. At UT, students who performed this activity were enrolled in a course of 65–80 students who completed eleven 4.5-hour experiments over a 12-week semester. Experiments in this course highlight modern laboratory techniques, discovery-based learning opportunities, and green chemistry principles. Students performed this experiment in Week 4 of 12 and had prior experience with introductory organic laboratory techniques including vacuum filtration, recrystallization, spectroscopic analysis (IR and 1H/13C NMR), and melting point determination. Students worked independently at UT to gather results in the laboratory and submitted individual post-laboratory reports. Despite working separately in the laboratory, students in the course belonged to a demonstrator group of 10–14 peers, each guided by a graduate student teaching assistant. The practical curriculum and activities were supervised by a faculty coordinator who was assisted by a laboratory technician. The two-part experiment described here was performed by 131 students over two years at UT. In comparison, the students who performed the activity at UPJ were enrolled in a laboratory course of 7–30 students who completed twelve 4-hour experiments over a 13-week semester. The pedagogical goals for sophomore students at UPJ were comparable to those at UT, and students again had prior experience with introductory organic chemistry laboratory techniques. The laboratory curriculum and activities were supervised by a faculty member teaching the laboratories (UPJ is a small school with no teaching assistants). At UPJ the experiment was performed with 23 students over two semesters.
Experimental overview
In this experiment, students performed the two-step reductive amination transformation in one 4-hour practical session (Scheme 1). The simple and robust methods are easily executed by students using basic equipment and inexpensive reagents, making it a versatile laboratory activity in a variety of teaching environments. Students initially undertook an imine condensation reaction between 4-nitrobenzaldehyde and 4-methoxyaniline using freshly squeezed citrus juice as a greener solvent containing a reaction catalyst (from citric, malic and ascorbic acids (Citation34)). Students obtained their fruit juice by selecting and squeezing a fresh lemon, lime, grapefruit, or orange, where larger fruits often provided enough juice for 2–3 students to use in their reaction. At UT, students performed an experiment using the fruit rind in the week prior to the reductive amination experiment as a method of utilizing the whole fruit in the laboratory. To keep the fruit from spoiling, the rind-free fruits were sealed in airtight containers and stored for one week in a refrigerator. After the experiment was complete, the fruit pulp waste was disposed of through a municipal organics disposal system (i.e., a compost program).
The imine product was isolated and recrystallized from isopropanol solvent prior to the subsequent reduction step. Here, students conducted a sodium borohydride reduction reaction of the imine intermediate to furnish the crude secondary amine product and undertook purification via recrystallization from isopropanol. Students characterized and compared the melting point determination data and 1H NMR spectra of the intermediate imine and final amine product. In addition to the learning objectives outlined above, students also explored concepts related to conjugation including how the number of alternating double bonds in molecules is responsible for the color (or absence of color) of organic molecules. Students recognized this phenomenon in the laboratory after synthesizing the highly conjugated yellow/green imine (absorbs at ∼690 nm) and observed the dramatic color change upon reduction to the less conjugated orange amine product (absorbs at ∼475 nm). The obvious color change observed in the laboratory is a simple and useful tool students can use (as a complement to spectroscopy) as a visual confirmation of product formation at each step.
Representative procedure
Step 1: general procedure for imine synthesis and analysis
In a round bottomed flask, 4-nitrobenzaldehyde (453 mg, 3.0 mmol) was stirred with 10 mL of citrus juice. After one minute, 4-methoxyaniline (394 mg, 3.2 mmol) was added and the reaction mixture was stirred at room temperature for 15 min. The imine product was isolated by vacuum filtration, recrystallized from isopropanol, and characterized by melting point determination and 1H NMR spectroscopy.
Step 2: general procedure for imine reduction and analysis
In a round bottomed flask, the imine obtained in Step 1 was dissolved in 10 mL methanol and heated under reflux. Sodium borohydride (189 mg, 5.0 mmol) was carefully added to the reaction mixture and further refluxed for 30 min. After cooling to room temperature, the solution was neutralized with 1 M HCl. The reaction mixture was placed in ice bath and stirred for an additional 10 min. The amine product was isolated by filtration, recrystallized from isopropanol, and characterized by melting point determination and 1H NMR spectroscopy. A slightly modified imine reduction procedure was used at UPJ. Detailed experimental procedures can be found in the Supporting Information.
After completing the practical component of the experiment, students were assessed through post-laboratory reports that included their results (including a yield calculation for each step), characterization of each product, reaction mechanism analysis, and green chemistry reflection.
Student experimental results
Prior to completing this two-part experiment, students had some understanding of reductive amination reactions, including acid-catalyzed imine synthesis. To facilitate this, students were provided with readings and associated problems to complete prior to performing the laboratory activity (Supporting Information). The first two specific experiment learning objectives were evaluated by assessing the overall student success rate in completing each step of the two-part reductive amination experiment, their ability to execute key laboratory techniques, and their ability to isolate the imine intermediate and final amine product. This assessment of student work was determined from post-laboratory reports and instructor observation during the laboratory sessions. The use of student data in this study was approved by the University of Toronto Research Ethics Board (REB). All participants provided informed consent as required by the University’s REB. In general, instructors observed that students were able to complete the experiment without difficulty and generated consistent results when compared to their peers in other cohorts. At UT, 97% of students successfully isolated some imine intermediate (100% at UPJ) while 90% of them were able to progress through the two-step reductive amination process and isolate amine product after recrystallization (83% at UPJ). It is suspected that students who were unsuccessful in executing one or both steps of the experiment either did not follow the procedure as described or suffered from a laboratory mishap (e.g. product spill, improper measurement of reagents) that is common in the organic teaching laboratory. It is notable that in all cases the experiment was successfully carried out in under 4 h, particularly if provided with ‘time-saving tips’ in the protocol (Supporting Information). Students at UT performed this experiment in Week 4 of the semester, following other activities that required them to conduct many of the introductory laboratory techniques needed to complete the reductive amination protocol (e.g. heating under reflux, vacuum filtration, and recrystallization). As a result, this familiarity strengthened student competence and confidence in executing the reactions and purifications with little difficulty.
In their post-laboratory analyses, students reported the yield and melting point data for each of the imine and amine products. They were able to isolate the imine intermediate in high purity with recrystallized yields that ranged from 41% to 50% at UT depending on the type of citrus juice used (). Interestingly, the highest yields for imine formation were recorded by students who used orange juice (50%) or lime juice (47%) as the solvent during the imine condensation reaction, with grapefruit juice (44%) and lemon juice (41%) furnishing slightly lower yields. Students were able to measure accurate melting point data regardless of the specific fruit juice used in the imine synthesis, with more than 87% of students reporting sharp melting point ranges between 125–133 °C (representative literature values: 125–127 °C (Citation35); 131–132 °C (Citation36); 131.1–133.5 °C (Citation37)), indicating that pure product could be easily isolated.
Table 1. Reaction yields based on citrus juice (UT).
A wider range of yields were obtained with the reduction step, with recrystallized yields for the amine product ranging from 39% to 59%, giving overall yields between 16 and 31% over the two steps. The wider yield range may be attributed to the difficulty associated with working on a smaller scale, particularly when isolating and purifying the final amine product by recrystallization. However, many students were able to isolate pure amine compound based on their melting point determination data. Greater than 88% of students reported a sharp melting point for their amine product, with values obtained between 91 and 98 °C (representative literature values: 91 °C (Citation38); 95–96 °C (Citation39); 97.7–97.9 °C (Citation40)). Student yield and purity results from UPJ were consistent with the data obtained at UT.
Assessment of student written work
Learning objectives 3, 4, and 5 of this experiment were assessed quantitatively through student post-laboratory reports. Firstly, students reported the characterization data for their imine and amine products by 1H NMR spectroscopy and melting point determination. Students obtained their own melting point data, but due to time and facility limitations 1H NMR spectra were provided by the instructor (Supporting Information). Product characterization included signal assignment in the 1H NMR spectra to specific nuclei in each structure. Secondly, students were required to consider the reaction mechanism of the imine condensation reaction as a function of pH, given that lemon, lime, orange, and grapefruit juices are naturally acidic due to the presence of citric, malic and ascorbic acids (pH 1.7–4.9, depending on the fruit (Citation41)). At UT, students were required to propose a reaction mechanism, while at UPJ, students completed a mechanism template containing intermediate structures by drawing mechanism arrows. After completing the reaction mechanism for their report, students at UT explained the following by making specific mention of the mechanistic steps relevant to their answer: (i) why are citrus juices excellent solvents for imine formation?; (ii) what would occur if the reaction medium was at pH = 7.0?; and (iii) what would occur if the reaction medium was at pH = 0.5? Lastly, students were provided with an alternative literature method (Citation42) to synthesize the same imine product which utilized anhydrous magnesium sulfate in dichloromethane solvent and were encouraged to compare the protocol to the one they used in the laboratory by making specific reference to the Twelve Principles of Green Chemistry (Citation43). This reflection was an important method of encouraging students to consider how chemists can responsibly perform their experimental work while taking into consideration the impact on the environment and health and safety concerns.
A total of 139 post-laboratory reports were used to evaluate student performance and learning objectives as shown in (116 from UT and 23 from UPJ). Students performed exceptionally well on their ability to report the correct characterization data for their two products, with an average score of 89%. This was expected given the student's experience performing similar spectral analysis tasks in earlier experiments. Similarly, students performed well when asked to draw the imine reaction mechanism, with an average score of 79%. At UT, students were also required to evaluate the mechanism at various pH values and were able to communicate the role of catalytic acid in the condensation reaction, yet some struggled to identify which mechanistic steps would be attenuated if the reaction media were neutral or too acidic. Lastly, students performed exceptionally well on the green chemistry reflection (average score of 91%) with most students correctly identifying which principles of green chemistry were represented in the imine formation they performed in the laboratory. They were also able to compare the procedure with a less environmentally friendly protocol (Citation42), making specific reference to sustainability and green chemistry principles.
Table 2. Student assessment on post-laboratory reports.
Student feedback
Approximately 92% of UT students participated in a voluntary and anonymous survey after submitting their post-laboratory reports. This feedback, along with anecdotal evidence collected from student-instructor interactions, suggests that individuals greatly enjoyed the experiment with all students reporting the experiment as a valuable part of the course curriculum. In addition, over 80% of respondents reported that the experiment effectively demonstrated principles of green chemistry in a way that was easy to understand, including the role of alternative solvents in organic synthesis. One student commented
I thought it was very interesting and valuable to physically demonstrate the use of alternative solvents. It gives credibility to the principles of green chemistry since we were able to produce a good yield with an entirely environmentally friendly catalyst.
Conclusion
The problem of worldwide food wastage is of serious concern and is embedded into Goal 12 of the U.N. Sustainable Development Goals (Citation3), that works to promote responsible and sustainable production and consumption of resources. This experiment exemplifies to students how wasted fruit that is visibly unappealing and unavailable to the consumer can potentially be valorized in the undergraduate organic laboratory through the application of citrus juice as a reaction solvent. The operational simplicity of the imine formation step (stirring the reactants in juice followed by vacuum filtration and recrystallization) means it could be positioned on its own in a precursor course, without a detailed mechanistic or spectroscopic analysis undertaken. Overall, the two-step reductive amination described in this article has proved successful based upon positive student feedback and more importantly by demonstrating student success in meeting the stated learning objectives.
Supplemental Material
Download MS Word (2 MB)Acknowledgements
We are grateful to the Department of Chemistry at the University of Toronto for their financial support through the Chemistry Teaching Fellowship Program, and to the University of Pittsburgh for a Mascaro Grant. Special thanks to Loise Perruchoud (UT), Bret Tantorno (UPJ) and Azariah Arthur (UPJ) who contributed to the preparation and execution of this laboratory experiment, and to the participating undergraduate students (UT and UPJ) and graduate student teaching assistants (UT) for their invaluable feedback.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wasted: How America is losing up to 40 percent of its food from farm to fork to landfill. https://www.nrdc.org/resources/wasted-how-america-losing-40-percent-its-food-farm-fork-landfill (accessed Nov 27, 2022).
- The State of Food and Agriculture 2019: Moving Forward on Food Loss and Waste Reduction; FAO, Ed.; Food and Agriculture Organization of the United Nations: Rome, 2019.
- Food and Agriculture Organization of the United Nations. Sustainable Development Goals Target 12.3 https://www.fao.org/sustainable-development-goals/indicators/1231/en/ (accessed Nov 27, 2022).
- Berkeley Economic Review. The Good, the Bad, and the Ugly Produce Movement https://econreview.berkeley.edu/the-good-the-bad-and-the-ugly-produce-movement/ (accessed Nov 27, 2022).
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization: Fruit and Vegetable Waste. Compr. Rev. Food Sci. Food Saf. 2018, 17 (3), 512–531.
- McCance, K.R.; Suarez, A.; McAlexander, S.L.; Davis, G.; Blanchard, M.R.; Venditti, R.A. Modeling a Biorefinery: Converting Pineapple Waste to Bioproducts and Biofuel. J. Chem. Educ. 2021, 98 (6), 2047–2054.
- Mackenzie, L.S.; Tyrrell, H.; Thomas, R.; Matharu, A.S.; Clark, J.H.; Hurst, G.A. Valorization of Waste Orange Peel to Produce Shear-Thinning Gels. J. Chem. Educ. 2019, 96 (12), 3025–3029.
- Mendes, D.E.; Schoffstall, A.M. Citrus Peel Additives for One-Pot Triazole Formation by Decarboxylation, Nucleophilic Substitution, and Azide–Alkyne Cycloaddition Reactions. J. Chem. Educ. 2011, 88 (11), 1582–1585.
- Navarro, Y.; Soengas, R.; Iglesias, M.J.; Ortiz, F.L. Use of NMR for the Analysis and Quantification of the Sugar Composition in Fresh and Store-Bought Fruit Juices. J. Chem. Educ. 2020, 97 (3), 831–837.
- Soares, C.; Correia, M.; Delerue-Matos, C.; Barroso, M.F. Investigating the Antioxidant Capacity of Fruits and Fruit Byproducts through an Introductory Food Chemistry Experiment for High School. J. Chem. Educ. 2017, 94 (9), 1291–1295.
- Kurushkin, M.; Tracey, C.; Mikhaylenko, M. BYOL: Bring Your Own Lime Hands-On Laboratory Experience. J. Chem. Educ. 2019, 96 (6), 1283–1286.
- Fahey, J.T.; Dineen, A.E.; Henain, J.M. Microwave-Assisted Aspirin Synthesis from Over-the-Counter Pain Creams Using Naturally Acidic Catalysts: A Green Undergraduate Organic Chemistry Laboratory Experiment. In ACS Symposium Series; Fahey, J. T., Maelia, L. E., Eds.; American Chemical Society: Washington, DC, 2016; pp 93–109.
- Gulati, S.; Singh, R.; Sangwan, S. Fruit Juices Act as Biocatalysts in the Efficient Synthesis of Potentially Bioactive Imidazoles. Green Chem. Lett. Rev. 2022, 15 (1), 3–17.
- Anjani, S.; Suprita, S.; Gulati, S.; Singh, R. Green and Environmentally Benign Organic Synthesis by Using Fruit Juice as Biocatalyst: A Review. Int. Res. J. Pure Appl. Chem. 2018, 16 (1), 1–15. https://doi.org/10.9734/IRJPAC/2018/40536.
- Patil, S.; Jadhav, S.D.; Deshmukh, M.B.; Patil, U.P. Natural Acid Catalyzed Synthesis of Schiff under Solvent-Free Condition: As a Green Approach. Int. J. Org. Chem. 2012, 2, 166–171.
- McMurry, J. Organic Chemistry; Brooks/Cole: Boston, MA, 2016. pp. 619-624
- Iqbal, A.; Siddiqui, H.L.; Ashraf, C.M.; Bukhari, M.H.; Akram, C.M. Synthesis, Spectroscopic and Cytotoxic Studies of Biologically Active New Schiff Bases Derived from p-Nitrobenzaldehyde. Chem. Pharm. Bull. 2007, 55 (7), 1070–1072. https://doi.org/10.1248/cpb.55.1070.
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B. Schiff Bases: A Short Review of Their Antimicrobial Activities. J. Adv. Res. 2011, 2 (1), 1–8.
- Suresh, R.; Kamalakkannan, D.; Ranganathan, K.; Arulkumaran, R.; Sundararajan, R.; Sakthinathan, S.P.; Vijayakumar, S.; Sathiyamoorthi, K.; Mala, V., Vanangamudi, G., et al. Solvent-Free Synthesis, Spectral Correlations and Antimicrobial Activities of Some Aryl Imines. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 239–248.
- Bennett, J.; Meldi, K.; Kimmell, C. Synthesis and Analysis of a Versatile Imine for the Undergraduate Organic Chemistry Laboratory. J. Chem. Educ. 2006, 83 (8), 1221–1224.
- Silverberg, L.J.; Coyle, D.J.; Cannon, K.C.; Mathers, R.T.; Richards, J.A.; Tierney, J. Azeotropic Preparation of a C -Phenyl N -Aryl Imine: An Introductory Undergraduate Organic Chemistry Laboratory Experiment. J. Chem. Educ. 2016, 93 (5), 941–944.
- Mancheño, M.J.; Royuela, S.; de la Peña, A.; Ramos, M.; Zamora, F.; Segura, J.L. Introduction to Covalent Organic Frameworks: An Advanced Organic Chemistry Experiment. J. Chem. Educ. 2019, 96 (8), 1745–1751.
- Popova, M.; Bretz, S.L.; Hartley, C.S. Visualizing Molecular Chirality in the Organic Chemistry Laboratory Using Cholesteric Liquid Crystals. J. Chem. Educ. 2016, 93 (6), 1096–1099.
- Go, E.B.; Srisuknimit, V.; Cheng, S.L.; Vosburg, D.A. Guest Capture, and NMR Spectroscopy of a Metal–Organic Cage in Water. J. Chem. Educ. 2016, 93 (2), 368–371.
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev 2018, 118 (2), 747–800.
- Saggiomo, V.; Lüning, U. On the Formation of Imines in Water – A Comparison. Tetrahedron Lett. 2009, 50 (32), 4663–4665.
- Bennett, J.S.; Charles, K.L.; Miner, M.R.; Heuberger, C.F.; Spina, E.J.; Bartels, M.F.; Foreman, T. Ethyl Lactate as a Tunable Solvent for the Synthesis of Aryl Aldimines. Green Chem. 2009, 11 (2), 166–168.
- Patil, R.D.; Adimurthy, S. Catalytic Methods for Imine Synthesis. Asian J. Org. Chem. 2013, 2 (9), 726–744.
- Chakraborti, A.K.; Bhagat, S.; Rudrawar, S. Magnesium Perchlorate as an Efficient Catalyst for the Synthesis of Imines and Phenylhydrazones. Tetrahedron Lett. 2004, 45 (41), 7641–7644.
- Stacey, J.M.; Dicks, A.P.; Goodwin, A.A.; Rush, B.M.; Nigam, M. Green Carbonyl Condensation Reactions Demonstrating Solvent and Organocatalyst Recyclability. J. Chem. Educ. 2013, 90 (8), 1067–1070.
- Carlson, M.W.; Ciszewski, J.T.; Bhatti, M.M.; Swanson, W.F.; Wilson, A.M. A Simple Secondary Amine Synthesis: Reductive Amination Using Sodium Triacetoxyborohydride. J. Chem. Educ. 2000, 77 (2), 270–271.
- Touchette, K.M. Reductive Amination: A Remarkable Experiment for the Organic Laboratory. J. Chem. Educ. 2006, 83 (6), 929–930.
- Goldstein, S.W.; Cross, A.V. Solvent-Free Reductive Amination: An Organic Chemistry Experiment. J. Chem. Educ. 2015, 92 (7), 1214–1216.
- Sinclair, W.B.; Eny, D.M. The Organic Acids of Lemon Fruits. Botan. Gaz. 1945, 107 (2), 231–242.
- Zarei, M.; Mohamadzadeh, M. 3-Thiolated 2-Azetidinones: Synthesis and in Vitro Antibacterial and Antifungal Activities. Tetrahedron 2011, 67 (32), 5832–5840.
- Galván, A.; de la Cruz, F.N.; Cruz, F.; Martínez, M.; Gomez, C.V.; Alcaraz, Y.; Domínguez, J.M.; Delgado, F.; Vázquez, M.A. Heterogeneous Catalysis with Basic Compounds to Achieve the Synthesis and C–N Cleavage of Azetidin-2-Ones under Microwave Irradiation. Synthesis 2019, 51 (19), 3625–3637.
- Jiang, Q.; Wang, J.-Y.; Guo, C. Iodine (III)-Mediated C–H Alkoxylation of Aniline Derivatives with Alcohols under Metal-Free Conditions. J. Org. Chem. 2014, 79 (18), 8768–8773.
- Ballistreri, F.P.; Maccarone, E.; Mamo, A. Kinetics and Mechanism of Benzylation of Anilines. J. Org. Chem. 1976, 41 (21), 3364–3367.
- Gupta, P.; Kour, M.; Paul, S.; Clark, J.H. Ionic Liquid Coated Sulfonated Carbon/Silica Composites: Novel Heterogeneous Catalysts for Organic Syntheses in Water. RSC Adv. 2014, 4 (15), 7461–7470. https://doi.org/10.1039/c3ra45229h.
- Itoh, T.; Nagata, K.; Miyazaki, M.; Ishikawa, H.; Kurihara, A.; Ohsawa, A. A Selective Reductive Amination of Aldehydes by the Use of Hantzsch Dihydropyridines as Reductant. Tetrahedron 2004, 60 (31), 6649–6655.
- Goldmann, M.E. The pH of Fruit Juices. J. Food Sci. 1949, 14 (4), 275–277.
- Shaghafi, M.B.; Grote, R.E.; Jarvo, E.R. Oxazolidine Synthesis by Complementary Stereospecific and Stereoconvergent Methods. Org. Lett. 2011, 13 (19), 5188–5191.
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, 1998.