ABSTRACT
A comparative study was carried out between the methods of synthesis of C-tetra(aryl)calix[4]resorcinarenes: by means of reflux, that is to say, carrying out a cyclocondensation reaction catalyzed by hydrochloric acid between resorcinol and different aromatic aldehydes, and by means of the solvent-free method, which is part of the green chemistry methodologies, in which several catalysts were used, viz. p-toluenesulfonic acid, clinoptilolite zeolite, and silica-gel. In the calix[4]resorcinarenes obtained by both methodologies, mainly 2 types of conformers, crown and chair, were observed. The conformers were isolated and characterized via different spectroscopy techniques: FT-IR, HPLC/MS, 1H-NMR, and 13C-NMR. In these solvent-free and reflux reactions, different ratios were obtained for each of the conformers, finding diverse and unexpected ratios for the solvent-free method. On the other hand, for the solvent-free method there was a significant saving of solvent, an important factor in the care of the environment, as well as of energy and time, in addition to minimizing the significant risks involved in the handling of strong mineral acids.
Introduction
One of the current challenges in science is to synthesize organic compounds more efficiently and with a smaller ecological footprint. For this reason, it is essential that synthetic chemists innovate, propose, design, develop, and optimize synthetic methodologies that have a less negative environmental impact. Therefore, the concept of eco-friendly organic synthesis has seen a large boom in recent years. Eco-friendly organic synthesis methods have as a central tenet the care of the environment and human health, and this is possible because they implement several of the principles of green chemistry in their procedures. The fundamental principle is prevention, since it is better to prevent waste than to treat it once it is generated (Citation1).
An example of the way the principle of prevention is implemented is by reducing the generation of residues of organic solvents and hazardous substances. It is currently known that in most cases this type of residue can reach values between 25 and 100 times higher than that of synthesized compounds (Citation2, Citation3).
The situation is worrisome and is partly due to the fact that the production of fine and specialty chemicals often involves a multi-step synthetic procedure, involving large amounts of organic solvents, which in most cases are toxic, as well as the widespread use of reagents in stoichiometric, rather than catalytic amounts (Citation3).
Recently, papers on eco-friendly organic synthesis have been published (Citation4), where concepts such as mechanochemistry (Citation5), microwave (Citation6), ultrasound (Citation7), and solvent-free (Citation8) among the most interesting, have been successfully applied.
It is fascinating to observe the increasing occurrence in recent decades of research related to the development of this type of environmentally friendly methods concerning the synthesis of relatively complex and supramolecular substances, such as macrocyclic compounds (Citation9).
Macrocyclic compounds that structurally contain a cavity that allows the complexation of analytes by molecular insertion, mainly through the host-host mechanism, are of great interest, essentially because of the wide spectrum of applications that these substances have found. These macrocyclic compounds include crown ethers (Citation10), cyclodextrins (Citation11) and resorcin[4]arenes (Citation12–14). Crown ethers are oligomers of ethylene oxide, where its oxygen atoms can coordinate cations within the polyether. Cyclodextrins are oligosaccharides composed of 1,4-D-glucopyranosyl residues that give them a hydrophobic cavity, allowing interaction with nonpolar molecules (Citation10).
Resorcin[4]arenes are oligomeric polyphenols belonging to the group of polyhydroxylated platforms that are very important in supramolecular chemistry. Resorcinarenes have several advantages over other macrocyclics, among them that the synthesis, separation, and purification procedures are relatively simpler and cheaper than those for other macrocyclic compounds. Additionally, they have great versatility of chemical functionalization, because they have active sites for their derivatization. This gives resorcin[4]arenes a high selectivity in molecular affinity processes and therefore numerous applications, such as sensors, catalysis, NMR solvating agents, heavy metal complexation for water purification, chemical separations, etc (Citation15,Citation16). Resorcin[4]arenes are commonly prepared through cyclocondensation in an acid medium between resorcinol and an aliphatic or aromatic aldehyde, using an organic solvent, a mineral acid, and long reaction times (Citation17–19). Continuing with our investigation of the properties and synthesis of resorcin[4]arenes (Citation20–25), this study presents the development of new methodologies for the synthesis of resorcin[4]arenes that eliminate the use of organic solvents and mineral acids with short reaction times. Additionally, the traditional methodology is compared with the new eco-friendly synthesis.
Results and discussion
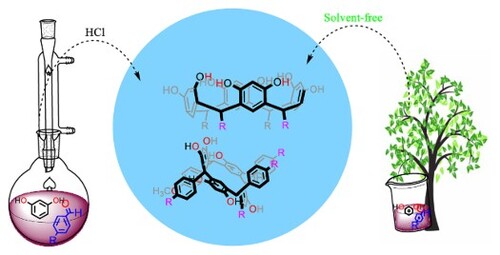
For this comparative study, the first step consisted of carrying out the synthesis of C-tetra(aryl)resorcin[4]arenes by the conventional method, which is described in previous reports (Citation20, Citation21), and the procedure is shown in the experimental section. Reactions between resorcinol and aromatic aldehydes were carried out using a concentrated HCl catalyst, under reflux conditions and using ethanol as a solvent (Scheme 1), where high-percentage yields are usually obtained in a single reaction step but over prolonged periods of time which range between 18 and 24 h.
Initially, the HPLC technique was used for the analysis of the reaction’s mixtures. The chromatographic method was eventually carried out using a gradient with a mobile phase of acetonitrile–water applied at a flow rate of 2 mL/min with a detection wavelength of 210 nm. Under mobile phase conditions, the elution of the samples was completed in less than 15 min. The chromatograms, in all three cases, exhibited two peaks corresponding to the conformational mixture (a) and allowed establishing retention times and the yield of the reaction during the formation of each conformer. For example, the chromatogram for the conformational mixture of the reaction between resorcinol and 4-methoxybenzaldehyde exhibited two peaks, with retention times of 6.00 and 6.98 min, corresponding to chair and crown conformers of C-tetra(p-methoxyphenyl)resorcin[4]arenes (a). Once the formation of the two isomers had been established, the separation of cone and chair conformers was carried out by using the crystallization technique with mixtures of solvents such as water and ethanol, among others, a methodology described in the experimental section. Isolated and purified products were analyzed via HPLC. As shown in b and c for the conformers of C-tetra(p-methoxyphenyl)resorcin[4]arenes, each of the conformers was efficiently separated, finding a longer retention time for the crown conformer (tR = 6.98 min). The isolated and purified products were characterized via FT-IR, 1H NMR, and 13C NMR spectroscopy.
Figure 1 . Chromatograms of the conformational separation via HPLC for C-tetra(p- methoxyphenyl)resorcin[4]arene: a) conformational mixture, b) chair conformer, c) crown conformer.
![Figure 1 . Chromatograms of the conformational separation via HPLC for C-tetra(p- methoxyphenyl)resorcin[4]arene: a) conformational mixture, b) chair conformer, c) crown conformer.](/cms/asset/0622f178-d148-4622-aac9-d0143c6af657/tgcl_a_2290847_f0001_ob.jpg)
The confirmation of the structure of the conformers was carried out mainly via 1H-NMR spectroscopy; in this way, individual assignments of the protons were done based on their positions, multiplicities, integral values, and comparison of spectral data reported in previous papers. As shown in , the 1H-NMR spectra of C-tetra(p-methoxyphenyl)resorcin[4]arene conformers are remarkably different. The conformer with tR = 6.00 min (a) displayed the characteristic signal of a methine bridge at 5.42 ppm, the aromatic hydrogen of the tetrasubstituted resorcinol unit displayed two signals, at 5.52 and 6.07 ppm for the protons in the ortho position, and the signals at 6.25 and 6.26 ppm for meta-protons and the signals at 8.35 and 8.43 ppm were assigned to two types of hydroxyl groups in the resorcinol residue. Finally, signals of the aromatic ring in the lower rim show the characteristic signals for a disubstituted system at 6.40 and 6.47 ppm. This pattern of multiple signals in the 1H-NMR spectrum allowed us to establish that the structure corresponds to C-tetra(p-methoxyphenyl)resorcin[4]arene in the chair conformation.
Figure 2 . 1H NMR of C-Tetra(p-methoxyphenyl)resorcin[4]arene. a) crown conformer, b) chair conformer. The signals at 2.5 and 3.3 ppm corresponds to the solvent DMSO-d6*.
![Figure 2 . 1H NMR of C-Tetra(p-methoxyphenyl)resorcin[4]arene. a) crown conformer, b) chair conformer. The signals at 2.5 and 3.3 ppm corresponds to the solvent DMSO-d6*.](/cms/asset/2422ebb4-d3c2-4755-b3e6-2db7e9bbf410/tgcl_a_2290847_f0002_oc.jpg)
The 1H-NMR spectrum for the product with tR = 6.98 min was obtained with DMSO-d6, displayed a signal for a methine bridge at 5.58 ppm. In the aromatic region, normally the ortho- and meta-protons of resorcin[4]arene moiety attach to a hydroxyl group, producing two separate signals, at 6.12 and 6.49 ppm, respectively. The signals at 6.53 and 6.60 were attributed to the hydrogen in the aromatic ring of the phenyl substituent on the lower rim. Finally, the singlet signal at 8.49 ppm was assigned to hydroxyl groups in the molecule. This pattern of few signals in the 1H-NMR spectrum is characteristic of the crown conformation (b). summarizes the 1H-NMR data for the conformers of tetra(aryl)resorcin[4]arenes 1-3.
Table 1 . 1H-NMR chemical shifts of conformers of tetra(aryl)resorcin[4]arenes 1-3.
Once the structure of the conformers and their retention times were verified, the next step was to do a comparative evaluation of the solvent-free processes, so p-toluenesulfonic acid (TsOH), silica gel, and clinoptilolite-type zeolite were used as catalysts, with a catalytic amount of 5% w/w for all experiments. As described in the experimental section, the general method for the solvent-free process consists of mixing equimolar amounts of resorcinol and the aromatic aldehyde with an acidic catalyst. The reaction mixtures were heated in a sealed vessel in all processes, and the temperature was kept at 80 °C. The rapid consumption of the reactants during the reaction process was shown with TLC analysis, observing in all cases that a period of 5 min was enough to verify the complete reaction. Using this methodology, conformers of tetra(aryl)resorcin[4]arenes 1-3 were extracted and analyzed using the HPLC technique. The relative percentages of both stereoisomers present in the crude product were assessed by integration of the chromatographic peaks in each experiment, and the results obtained for the effects of changing of the acid catalyst were assessed and are summarised in .
Figure 3 . Summary of results, on the y axis, % of the conformer obtained, on the x axis, results for obtaining 1 (bars 1R, 1TA and 1Z), results for obtaining 2 (bars 2R, 2TA and 2S) and results for obtaining 3 (bars 3R, 3TA and 3S).
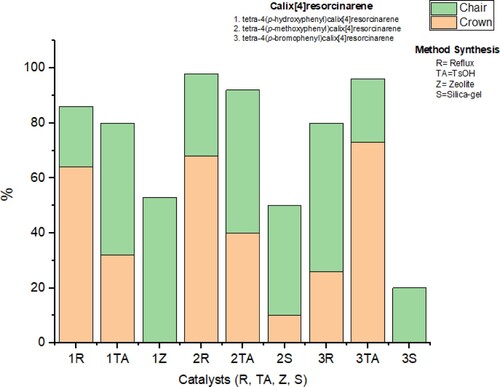
In this way, for the reaction between resorcinol and p-hydroxybenzaldehyde in presence of p-toluenesulphonic acid (, bars 1R, 1TA, and 1Z), the HPLC analysis shows the generation of the conformational mixture with a decrease in the composition of the crown isomer, that is, the result is similar to that observed when the reaction is carried out in solution. When the reaction is carried out in the presence of zeolite as an acid catalyst, it is found to be selective for the chair conformer as the only product (, bar 1Z), while when using silica gel as a catalyst, the formation of conformers was not observed.
Similar results can be seen when the process is carried out via a solvent-free reaction of resorcinol and p-methoxybenzaldehyde (2R, 2TA, and 2S in ). The use of p-toluenesulphonic acid as the catalyst allows a high degree of conversion of the reactants, finding that the chair conformer is the majority product of the process, and this trend is maintained when the reaction is carried out with silica gel as the catalyst. In the process that used zeolite as a catalyst, the conformers were not observed.
Finally, similar results can be observed when the reaction is carried out between resorcinol and p-bromobenzaldehyde (, 3R, 3TA, and 3S). For these processes, the results suggest that the macrocycle products were only observed when p-toluenesulphonic acid or silica gel were used as the catalyst; however, when zeolite was used as the catalyst, the formation of any of the expected conformers was not evident: possibly oligomers were generated that are not easy to characterize from the complex mixture obtained. For this reaction, it was evident that when the acid catalysis was carried out with silica gel, only the chair conformer was the main product of the reaction (, bar 3S). On the other hand, when comparing the reaction catalyzed by p-toluenesulphonic with the conventional methodology, it was found that the crown conformer is obtained with higher yield than chair conformer.
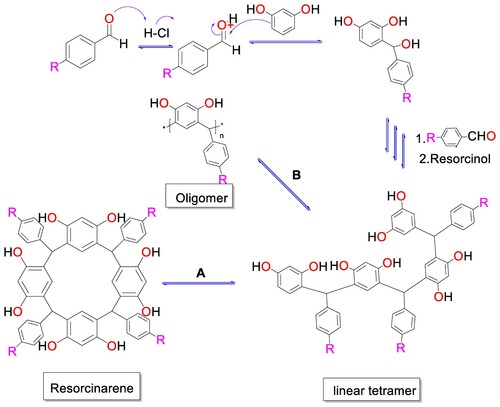
According to the results observed, some general topics can be drawn. The first is that employed a strong acid or relatively strong as catalyst (p-toluenesulphonic acid or HCl), there is conversion to cyclic tetramers with high yields, while if the catalyst is of lower acidity (zeolite or silica gel), the conversion towards cyclic tetramers decreases. A possible explanation for this behavior can be found in the cyclocondensation reaction between resorcinol and an aldehyde. According to what has been described in the literature (Citation14), the process begins with the generation of an electrophilic species by means of the protonation of the aldehyde in the oxygen atom of the carbonyl group. The electrophilic species formed is susceptible to the nucleophilic attack of resorcinol, allowing the formation of a sigma bond between the two reacting species. This process involves the loss of a water molecule. Similarly, the reaction proceeds to form a linear tetramer (). At this point, there are two possibilities: cyclization (route A) or continuation of the formation of oligomers (route B). Therefore, what the results show is that cyclization will be favored if a high acidity catalyst is used. If the catalyst is of low acidity, there is competition in the formation of the two types of products ().
Another consideration to take into account is changes in the conformational composition between the two methods. These changes can be explained by the change in the Gibbs free energy during the reaction process. In accordance with what has been shown for similar processes, it can be stated that in the solvent-free method a linear behavior of the Gibbs free energy is to be expected as the reaction progresses, which implies that conformational equilibria are not generated. When the reaction is carried out in solution, minimum points can be expected in the Gibbs free energy values that can be associated with conformational equilibria where ΔG = 0. Additionally, in solution, secondary interactions are favored, especially hydrogen bonds between the hydroxyls of tetra(aryl)resorcin[4]arene and the hydrogen atoms of water or ethanol molecules. The lack of hydrogen bonding interactions for solvent-free reactions probably causes the higher proportion of chair conformer.
From the comparison made between the two types of methodologies, it stands out that each synthesis carried out by the solvent free process, applied the twelve fundamental principles of green chemistry (Citation26, Citation27); within which several aspects can be highlighted. The first has to do with the fact that the use of solvents was significantly reduced, both for the synthesis process and for the purification of the products. Secondly, less use was made of dangerous reagents such as HCl, which can be exchanged for substances that are easy to handle and that were used in a comparatively smaller proportion. Finally, it can be argued that the processes are energetically economical since the reaction times are short. Additionally, the processes carried out by the solvent free methodology, allowed making the stereoselective reactions and with simpler purification protocols since the macrocyclic products can be easily extracted.
Experimental section
Materials
Resorcinol 99.9%, p-hydroxybenzaldehyde 98%, p-methoxybenzaldehyde, p-bromobenzaldehyde 98%, absolute ethanol, distilled and deionized water type 1, hydrochloric acid 37%, p-toluenesulfonic acid, DMSO-d6, were purchased from Sigma-Aldrich. Clinoptilolite zeolite manufactured by CNL Chile laboratory was also used.
Equipment used for the characterization
1H and 13C-NMR spectra were recorded at 400 MHz on a Bruker advance 400 instrument. Chemical shifts were reported in ppm making use of the residual solvent signal. Molar mass was determined with a Thermo Scientific Hybrid Quadrupole-Orbitrap mass spectrometer. IR spectra were taken using a Thermo Nicolet iS5 FT-IR spectrometer with ATR accessory, spectral range ZnSe 4000–400 cm¹ as well as absorption in cm−1. RP-HPLC analyses were performed with a C-18 column (Merck, Kenilworth, NJ, USA, 50 mm) on a Hitachi 1210 auto-sampler.
Conventional process for the synthesis of tetra(aryl)resorcin[4]arenes
The synthetic process is based on carrying out a cyclocondensation reaction catalyzed by mineral hydrochloric acid at 37%. Thus a solution of resorcinol and of aromatic aldehyde is formed in molar proportions 1:1 with ethanol as a solvent, and HCl (37%) is added dropwise. The reaction flask is protected from light and heated at reflux for 4–8 h (monitoring by TLC). After this time, a precipitate forms and is filtered under reduced pressure and washed with water until obtaining neutral pH in the filtrate. Finally, the solid is dried at 45 °C for 5 h (Citation1–3).
General technique for the separation of conformers from tetra(aryl)resorcin[4]arenes
The separation of the different conformers was carried out with 100 mg of conformational mixture of tetra(aryl)resorcin[4]arene and adding 2 mL of dimethylsulfoxide. This solution was agitated at 250 rpm at 60 °C for 60 min. Afterwards, it was allowed to cool and filtered under reduced pressure and dried to obtain the first conformer, which is generally the chair, due to its low solubility in the mentioned solvent. Subsequently at filtered of the separation is added the same proportion of water, forming a significant precipitate, which is filtered under reduced pressure, washed with distilled water and dried.
The synthesized tetra(aryl)resorcin[4]arene were characterized using FT-IR, 1H NMR, and HPLC, as shown below.
2,4,6,8-tetrakis(4-hydroxyphenyl)-1,3,5,7(1,3)-tetrabenzenacyclooctaphan-14,16,34,36,54,56,74,76-octaol (Crown): Light pink solid obtained with 74% yield. As for its melting point, it is higher than 250 °C, decomposing at that temperature. IR-TF (KBr/cm−1) 3384.56 (O-H), 1071.31 (C–O). NMR−1H DMSO-d6 δ (ppm): 5.52 (s, 4H, ArCH), 6.08 (s, 4H, ArH, ortho to OH), 6.48 (d,8H, ArH, J = 8 Hz), 6. 50 (s, 4H, ArH, meta a OH), 6.64 (d, 8H, ArH, J = 8 Hz), 8.45 (s, 8OH, ArOH resorcinol), 8.85 (4OH, p-OHAr). ESI-MS (m/z): 857.2557 [M + H]+, Calc. mass for M: 856.25.
2,4,6,8-tetrakis(4-hydroxyphenyl)-1,3,5,7(1,3)-tetrabenzenacyclooctaphan-14,16,34,36,54,56,74,76-octaol (Chair): Creamy-white solid with a yield of 42%. Melting point > 250 °C (desc.). FT-IR (KBr/cm−1): 3401 (O-H), 1077 (C–O); 1H-NMR, DMSO-d6, δ (ppm): 5.39 (s, 4H, ArCH), 5.88 (s, 2H, ArH, ortho to OH), 6.06 (s, 2H, ArH, ortho to OH), 6.24 (s, 2H, ArH, meta to OH), 6.27 (s, 2H, ArH, meta to OH), 6.28 (d, 8H, ArH, J = 8 Hz), 6.38 (d, 8H, ArH, J = 8 Hz), 8.31 (s, 4OH, ArOH resorcinol), 8.35 (s, 4OH, ArOH resorcinol), 8.61 (s, 4 OH, 4-OHAr). ESI-MS (m/z): 857.2555 [M + H]+, Calc. mass for M: 856.25.
2,4,6,8-tetrakis(4-methoxyphenyl)-1,3,5,7(1,3)-tetrabenzenacyclooctaphan-14,16,34,36,54,56,74,76-octaol (Crown): A light pink solid with a yield of 42% was obtained, with a melting point > 250 °C (decomposition). FT-IR (KBr/cm−1): 3389 (O-H), 3002 and 1607 (ArH), 2970 (CH3), 1113 (C–O); 1H-NMR, DMSO-d6, δ (ppm): 3.70 (s, 12H, CH3), 5.58 (s, 4H, ArCH), 6.12 (s, 4H, ArH, ortho to OH), 6.49 (s, 4H, ArH, meta to OH), 6.53 (d, 8H, ArH, J = 8 Hz), 6.60 (d, 8H, ArH, J = 8 Hz), 8.49 (s, 8OH, ArOH resorcinol). ESI-MS (m/z): 913.3182 [M + H]+, Calc. mass for M: 912.31.
2,4,6,8-tetrakis(4-methoxyphenyl)-1,3,5,7(1,3)-tetrabenzenacyclooctaphan-14,16,34,36,54,56,74,76-octaol (Chair): A creamy-white solid was obtained with 60% yield. Melting point > 250 °C (decomposition). FT-IR (KBr/cm−1): 3362 (O-H), 1605 (ArH), 1113 (C–O); NMR-1H, DMSO-d6, δ (ppm): 3.59 (s, 12H, CH3), 5.42 (s, 4H, ArCH), 5.52 (s, 2H, ArH, ortho to OH), 6.07 (s, 2H, ArH, ortho to OH), 6.25 (s, 2H, ArH, meta to OH), 6.26 (s, 2H, ArH, meta a OH), 6.40 (d, 8H, ArH, J = 8 Hz), 6.47 (d, 8H, ArH, J = 8 Hz), 8.35 (s, 4OH, ArOH resorcinol), 8.43 (s, 4OH, ArOH resorcinol) ESI-MS (m/z): 913.3181 [M + H]+, Calc. mass for M: 912.31.
2,4,6,8-tetrakis(4-bromophenyl)-1,3,5,7(1,3)-tetrabenzenacyclooctaphan-14,16,34,36,54,56,74,76 octaol (Crown): Orange solid obtained with a 30% yield. As for its melting point, it is higher than 250 °C, decomposing at that temperature. FT-IR (KBr/cm−1): 3523 (O-H), 1614 (ArH), 1152 (C–O) 822 (C–Br) 1H- NMR, DMSO-d6 δ (ppm): 5.60 (s, 8H, ArH meta to OH, ArH ortho to OH), 6.16 (s, 4H, ArCH), 6.56 (d, 8H, J = 8.5 Hz), 7.19 (d,8H, J = 8.5 Hz ArH), 8.73 (s, 8OH, ArOH resorcinol) ESI-MS (m/z): 1104.9003 [M + H]+, Calc. mass for M: 1103.91.
2,4,6,8-tetrakis(4-bromophenyl)−1,3,5,7(1,3)-tetrabenzenacyclooctaphan-14,16,34,36,54,56,74,76 octaol (Chair): Yellow solid obtained with a 70% yield. As for its melting point, it is higher than 250 °C, decomposing at that temperature. FT-IR (KBr/cm−1): 3362 (O-H), 1605 (ArH), 1113 (C–O), 777 (C–Br) 1H-NMR-, DMSO-d6, δ (ppm): 5.49 (t, 4H, ArCH), 6.24 (m, 8H, ArCH meta to OH and ArH ortho to OH), 6.55 (d, 8H, ArH ortho to Br, J = 8.4 Hz), 7.20 (d, 8H, ArH meta to Br, J = 6.7 Hz), 8.65 (s, 8OH, ArOH resorcinol) 8.75 (d, 8OH, ArOH resorcinol, J = 4.3 Hz) ESI-MS (m/z): 1104.9003 [M + H]+, Calc. mass for M: 1103.91.
General method solvent-free for the synthesis of tetra(aryl)resorcin[4]arenes
Equimolar amounts of resorcinol and aldehyde were mixed, adding acidic catalyst (p-toluenesulfonic acid (TsOH), silica gel, or clinoptilolite-type zeolite with a catalytic amount of 5% w/w for all experiments) All was mixed in a reaction vial, taking it to an oil bath at approximately 80 °C. After about 5 min, a paste was formed, which was washed with 1:1 of ethanol:water, filtered under reduced pressure, and then dried.
Chromatographic analysis
RP-HPLC analyses were performed on a Chromolith RP-18e (50 × 4.6 mm) using an Agilent 1200 liquid chromatograph (Agilent, Omaha, NE). A gradient ranging of solvent B (acetonitrile with 0.05% TFA) in solvent A (water with 0.05% TFA) was performed as follows: 20/20/100/100/20% B at 0/18/18/22/22.1/ min. Detection was performed at 210 nm, and the flow rate was 2 mL/min.
Conclusions
We employed three solvent-free protocols for the synthesis of tetra(aryl)resorcin[4]arenes under mild conditions in the presence of p-toluensulphonic acid, a commercial zeolite clay, and silica gel. Compared with the traditional method, this method has several advantages, such as that the energy is harmless, the reaction is performed in the absence of organic solvents, the time is short, and in some cases the reaction is stereoselective. The catalytic reaction is sensitive to the acidity of the catalyst; thus when HCl acid in solution or p-toluensulphonic acid in solvent free condition is employed, the formation of macrocycles predominates. When zeolite or silica gel is employed, the increase in the yield of linear oligomers is remarkable.
Acknowledgements
We gratefully acknowledge to Universidad Nacional de Colombia
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Jiang, J.; Xiao, F.; He, W.M.; Wang, L. The Application of Clean Production in Organic Synthesis. Chin. Chem. Lett. 2021, 32 (5), 1637–1644. https://doi.org/10.1016/j.cclet.2021.02.057.
- Kharissova, O.V.; Kharisov, B.I.; González, C.M.O.; Méndez, Y.P.; López, I. Greener Synthesis of Chemical Compounds and Materials. R. Soc. Open. Sci. 2019, 6. https://doi.org/10.1098/rsos.191378.
- Ballini, R. Eco-Friendly Synthesis of Fine Chemicals 2010, 132. https://doi.org/10.1021/ja1000483.
- Murti, Y.; Pathak, D.; Pathak, K. Green Approaches to Synthesize Organic Compounds and Drugs. In Nanotechnology in the Life Sciences; Springer Science and Business Media B.V., 2020; pp 191–222.
- Wang, G.W. Mechanochemical Organic Synthesis. Chem. Soc. Rev. 2013, 42 (18), 7668–7700. https://doi.org/10.1039/c3cs35526h.
- de la Hoz, A.; Díaz-Ortiz, À; Moreno, A. Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects. Chem. Soc. Rev. 2005, 34 (2), 164–178. https://doi.org/10.1039/b411438h.
- Ahluwalia, V.K.; Kidwai, M. New Trends in Green Chemistry; Springer: Netherlands, 2004.
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis 2000.
- Grajewski, J. Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles. Molecules 2022. MDPI February 1. https://doi.org/10.3390/molecules27031004.
- Liu, Z.; Nalluri, S.K.M.; Fraser Stoddart, J. Surveying Macrocyclic Chemistry: From Flexible Crown Ethers to Rigid Cyclophanes. Chem. Soc. Rev. Royal Society of Chemistry May 2017, 7, 2459–2478. https://doi.org/10.1039/c7cs00185a.
- Crini, G.; Fourmentin, S.; Fenyvesi, É; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Environ. Chem. Lett. December 15, 2018, 1361–1375. Springer Verlag. https://doi.org/10.1007/s10311-018-0763-2.
- Castillo-Aguirre, A.; Maldonado, M. Preparation of Methacrylate-Based Polymers Modified with Chiral Resorcinarenes and Their Evaluation as Sorbents in Norepinephrine Microextraction. Polymers (Basel) 2019, 11 (9). https://doi.org/10.3390/polym11091428.
- Castillo-Aguirre, A.; Esteso, M.A.; Maldonado, M. Resorcin[4]Arenes: Generalities and Their Role in the Modification and Detection of Amino Acids. Curr. Org. Chem. 2020, 24 (21), 2412–2425. https://doi.org/10.2174/1385272824999200510232141.
- Jain, V.K.; Kanaiya, P.H. Chemistry of Calix[4]Resorcinarenes. Russ. Chem. Rev. 2011, 80 (1), 75–102. https://doi.org/10.1070/RC2011v080n01ABEH004127.
- Li, W.; Ma, J.; Zhou, Y.; Sun, X.; Gao, D. The Application of Sulfonated Tetraphenyl Calix[4] Resorcinarene as a Novel, Multi-Functional and Eco-Friendly Ligand in Zirconium Tanning System. J. Clean. Prod. 2021, 280, 124337. https://doi.org/10.1016/j.jclepro.2020.124337.
- Castillo-Aguirre, A.; Maldonado, M.; Esteso, M.A. Removal of Toxic Metal Ions Using Poly(BuMA–Co–EDMA) Modified with C-Tetra(Nonyl)Calix[4]Resorcinarene. Toxics 2022, 10 (5). https://doi.org/10.3390/toxics10050204.
- Jain, V.K.; Pillai, S.G.; Kanaiya, P.H. Synthesis of Calix[4]Resorcinarene Based Dyes and Its Application in Dyeing of Fibres. E-J. Chem. 2008, 5 (SUPPL. 1), 1037–1047. https://doi.org/10.1155/2008/980290.
- Castillo-Aguirre, A.; Rivera-Monroy, Z.; Maldonado, M. Selective O-Alkylation of the Crown Conformer of Tetra(4-Hydroxyphenyl)Calix[4]Resorcinarene to the Corresponding Tetraalkyl Ether. Molecules 2017, 22 (10). https://doi.org/10.3390/molecules22101660.
- Hayashi, Y.; Maruyama, T.; Yachi, T.; Kudo, K.; Ichimura, K. Synthesis and Fluorescence Behavior of Calix[4]Resorcinarenes Possessing Pyrenyl Group(s). J. Chem Soc. Perkin Transactions 1998, 2 (4), 981–987. https://doi.org/10.1039/a704762b.
- Castillo-Aguirre, A.A.; Pérez-Redondo, A.; Maldonado, M. Influence of the Hydrogen Bond on the Iteroselective O-Alkylation of Calix[4]Resorcinarenes. J. Mol. Struct. 2020, 1202. https://doi.org/10.1016/j.molstruc.2019.127402.
- Castillo-Aguirre, A.A.; Rivera Monroy, Z.J.; Maldonado, M. Analysis by RP-HPLC and Purification by RP-SPE of the C -Tetra(p -Hydroxyphenyl)Resorcinolarene Crown and Chair Stereoisomers. J. Anal. Methods. Chem. 2019. https://doi.org/10.1155/2019/2051282.
- Velásquez-Silva, B.A.; Castillo-Aguirre, A.; Rivera-Monroy, Z.J.; Maldonado, M. Aminomethylated Calix[4]Resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface. Polymers (Basel) 2019, 11 (7). https://doi.org/10.3390/polym11071147.
- Castillo-Aguirre, A.A.; Velásquez-Silva, B.A.; Palacio, C.; Baez, F.; Rivera-Monroy, Z.J.; Maldonado, M. Surface Modification of Poly(GMA-Co-EDMA-Co-MMA) with Resorcarenes. J. Braz. Chem. Soc. 2018, 29 (9), 1965–1972. https://doi.org/10.21577/0103-5053.20180074.
- Castillo-Aguirre, A.A.; Rivera Monroy, Z.J.; Maldonado, M. Analysis by RP-HPLC and Purification by RP-SPE of the C -Tetra(p -Hydroxyphenyl) Resorcinolarene Crown and Chair Stereoisomers. J. Anal. Methods. Chem. 2019. https://doi.org/10.1155/2019/2051282.
- Castillo-Aguirre, A.; Maldonado, M. Preparation of Methacrylate-Based Polymers Modified with Chiral Resorcinarenes and Their Evaluation as Sorbents in Norepinephrine Microextraction. Polymers (Basel) 2019, 11 (9), 1–21. https://doi.org/10.3390/polym11091428.
- Ahluwalia, V.K. Green Chemistry Environmentally Benign Reactions, 3rd ed.. 2021, 12 (3), 6–17.
- Ahluwalia, V.K.; Kidwai, M. New Trends in Green Chemistry; Springer, 2004.

![Scheme 1 . Synthesis of C-tetra(aryl)resorcin[4]arenes](/cms/asset/6fcd011b-3602-4d6d-b399-b827a9674f5a/tgcl_a_2290847_f0005_oc.jpg)