ABSTRACT
The current study aims to guarantee eco-friendliness, maximize production, and reduce biodiesel production costs using the new oleaginous fungus Aspergillus terreus KC462061 in the presence of gold-silver core–shell nanoparticles (Au@Ag NPs) as heterogeneous nanocatalysts in the process of biodiesel production. The response surface method (RSM) was used to optimize the production of Au@Ag NPs as heterogeneous nanocatalysts by A. terreus KC46206, supported by ANOVA, to validate the optimal conditions. Au@Ag NPs were characterized using UV-visible spectroscopy (UV-Vis), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR). The process of biodiesel production was optimized by RSM and validated using ANOVA. The maximum biodiesel yield was 43.28% at the optimum conditions of a methanol/oil ratio (4:1), amount of nanocatalyst (1.0 wt.%), reaction temperature (40 °C), reaction time (4.0 h), and shaking speed (600 rpm). The template of fatty acid methyl esters (FAME) in biodiesel displayed significantly higher saturated fatty acids (SFAs) than unsaturated fatty acids (USFAs). The quality of the biodiesel was evaluated, and it was discovered that it met the criteria established by the global specifications, especially in the United States and Europe.
1. Introduction
Biodiesels are forms of diesel extracted from renewable feedstocks such as animal oils, vegetable oils, and oils from microorganisms (bacteria, cyanobacteria, fungi, microalgae, and yeasts). Biodiesel could be the best feasible solution as a promising and distinct green fuel for alternative energy because it is a low-carbon emission and toxin-free fuel (Citation1,Citation2). Biodiesel is gaining more and more popularity around the world as it is cost-effective, environmentally friendly, and biodegradable; nevertheless, it contains little sulfur and aromatic hydrocarbons (Citation3). Oleaginous filamentous fungi are extremely perfect sources for the sustainable biodiesel industry due to their rapid growth rate on a large scale, lack of dependence on light energy, short life cycle, capacity to produce large quantities of lipids using a diversity of carbon sources, and high number of fatty acids (saturated and unsaturated) for high-quality biodiesel production (Citation4). Several oleaginous filamentous fungi are able to be used as biological templates for biodiesel production, such as Aspergillus terreus and A. niger (Citation5), Fusarium oxysporum (Citation6,Citation7), Mucor circinelloides (Citation8), identifying fungal species capable of producing a significant amount of lipids will be highly beneficial for enhancing process economics (Citation9). Due to the complexity of lipid production, it cannot be achieved by just one gene or pathway. Sequence information sheds light on the molecular mechanisms governing fatty acid production based on conserved regions of fatty acid pathways in oleaginous fungi (Citation10,Citation11). The most popular reaction to produce biodiesel from fungal oil is called the transesterification process of triglycerides (TAG) with alcohol (methanol) in the presence of a catalyst mixed with fungal oil to convert them into fatty acid esters and glycerol (Citation12). Produced biodiesel through the triacylglycerides transesterification process utilizing two categories of various catalytic catalysts: (Citation1) classic catalysts such as (i) base catalysts and (ii) acid catalysts; (Citation2) modern catalysts like (i) enzyme catalysts; and (ii) the use of heterogenous nanocatalysts. The advantages and disadvantages of classic catalysts for all these different routes have already been known (Citation13,Citation14). Thus, the challenge is to design an improved catalyst to overcome the disadvantages associated with classic catalysts, combine all the advantages, and add an advantage. Heterogeneous nanocatalysts bridge the gap between two classic types of catalysts and establish a new era in biodiesel production (Citation15,Citation16). Heterogeneous nanocatalysts include the five different types: (i) bimetallic catalysts; (ii) nanozeolites; (iii) nanoferrites; (iv) nanohydrotalcites; and (v) metal organic frameworks (MOFs) (Citation17,Citation18). Au@Ag core–shell nanoparticles belong to bimetallic catalysts with many advantages, such as high catalytic activity, high stability, and efficiency; an efficient surface-to-volume ratio; superior productivity in a short time; Resistant to saponification, the reduced temperature of the reaction (Citation19), high selectivity, enhance longer reusability, be environmentally gentle, cost-effective, and tailor-made (Citation20). To accelerate the progress and development of biodiesel manufacturing, an increasing amount of attention is being paid to the application of advanced nanotechnology for maximum production and low cost (Citation21). In this study, we used RSM to optimize the lipid yield of a novel oleaginous Aspergillus terreus isolate KC462061, and the optimization of the biosynthesis of Au@Ag core–shell nanoparticles, which are used in biodiesel production. RSM was used to specify the optimum factors for biodiesel production in the presence of Au@Ag nanoparticles.
2. Materials and methods
2.1. Fungal isolate
Aspergillus terreus KC462061, isolated from soil date palm from Riyadh city, Saudi Arabia, and registered in GenBank, was used to synthesize Au@Ag core–shell nanoparticles (Citation21). The isolate was maintained on PDA slants and stored at 4°C.
2.2. Mycosynthesis of Au@Ag NPs
The fermentation process included A. terreus KC462061 was grown in 500-ml Erlenmeyer flasks containing 200 ml of potato broth (PB) and glucose (20 g/L) at room temperature and on a rotary shaker for 3 days. The culture was centrifuged at 5000 rpm at 4 °C for 20 min., while the mycelium-free supernatants (MCF) were used for the biosynthesis of Au@Ag NPs. Stock solutions (SE) of Au and Ag solutions were developed by taking 0.003 mol L−1 of the metal precursors (HAuCl4.3H2O and AgNO3) in 10 mL of water (Milli-Q water). 1 mL of MCF from A. terreus KC462061 was incubated with a 1:1 ratio of HAuCl4 and AgNo3 solutions and incubated at a suitable temperature (25°C), pH (Citation6), and time (90) min. The mycosynthesis of Au@Ag NPs is affected by the ratio of AgNO3/HAuCl4.3H2O, the amount of fungal cell-free (mL), the pH of the solution, the reaction time, and the reaction temperature (min) (Citation22).
2.3. Characterization of Au@AgNPs
A double-beam UV-visible spectrophotometer, Milton-Roy, spectronic 1201 (Pennsylvania, USA), was utilized for the primary and definitive characterization of the synthesized Au@AgNPs. X-ray diffraction (XRD) was applied to evaluate the metallic nature of Au@Ag NPs. XRD conditions at 40 KV and 40 mA with Cu-Kα radiation using Shimadzu XRD 6000 (Shimadzu, Japan). Transmission electron microscopy (TEM) was used for acquiring the micrographs of the synthesized Au@AgNPs using JEOL JEM-1010 (Tokyo, Japan). FT-IR analysis was performed to analyze the function-al group of Au@AgNPs ranging from 400 to 4000 cm−1 using the JASCO FTIR-6200 (Tsukuba, Japan). EDAX analysis was completed to analyse the elemental composition of the Au@Ag NPs. For this analysis, we used a QUANTA 250 instrument.
2.4. Experimental design and optimization for mycosynthesis of Au@AgNPs
RSM has been applied to optimize the synthesis parameters of Au@AgNPs with consideration for high yield and also to get economic design and precise prediction for interactions of independent variables to appear in perfect establishing statistical modeling. The influence of independent variables including (X1), (X2), (X3), (X4), and (X5) refers to the ratio of AgNO3/HAuCl4.3H2O, amount of fungal cell-free (mL), pH of the solution, reaction time (min), and reaction temperature, respectively. The ranges and values of each factor are displayed in . It also defines five experimental factors at three levels of value (−1, 0, + 1).
Table 1. The main variables and the related three levels.
2.5. Fermentation medium and optimization process of lipid content
The fermentation medium employed was a modified Czapek Dox Broth medium with a high carbon content and a low nitrogen content to promote the accumulation of lipids. The medium included some salts, such as MgSO4 and FeSO4. Aliquots of 50 ml of media were distributed into 250 ml Erlenmeyer flasks, autoclaved for 15 min at 121 °C, and then inoculated with an A. terreus KC462061 spore solution after cooling. The optimization process was based on seven factors, such as glucose (Y1 g), peptone (Y2 g), NaCl (Y3 g), Na2HPO4 (Y4), pH (Y5), incubation temperature (Y6), and incubation time (Y7), which were analysis in this study to show their developments in lipid production. As shown in .
Table 2. Factors affecting lipid production and their levels.
2.6. Biomass dry weight and lipid content determination
The growing biomass was collected using a Hettich Mikro 22R centrifuge (Tuttlingen, Germany), (5000 rpm, 4°C, 10 min) and then washed 3 times with deionized water to remove the media remains. The fungus’ mycelium was dried for 24 h at 60 °C in an oven to achieve a consistent dry weight. Lipid was extracted from the fungus biomass with a mixture of methanol and chloroform solvent (1:2 v/v) according to (Citation23). Lipid content in dried biomass of A. terreus KC462061 was determined using a sulfo-phospho-vanillin assay (Citation24).
2.7. Biodiesel production using the transestrification process
The transestrification process depends on converting the lipid of the fungal dry biomass to FAME in a one-step according to the method of (Citation23). The biodiesel production was designated to use Au@Ag NPs as a nanocatalyst. The experiment involved extracting fungal lipids and performing reactions in glass-closed vessels with magnetic stirring. The reaction was heated in a thermostatic bath, and the FAME layer was amassed. The crude glycerol was washed five times with n-hexane and water, and the upper organic layers were combined with the first biodiesel layer. The residue containing biodiesel (FAMEs) was used to measure the reaction yield.
The result (biodiesel) was calculated as a percentage of dry biomass and initial lipid according to the following equations:
Biodiesel (% of dry biomass) = Weight of obtained FAME/ Weight of dry biomas X100.
The transesterification process is influenced by five factors, such as the molar ratio of glycerides to alcohol, the amount of nanocatalyst, reaction time, reaction temperature, and shaking speed (Citation25). As shown in , experimental variations were combined with these five parameters at three levels to conduct batch studies for biodiesel production.
Table 3. The main variables and the corresponding three levels affecting biodiesel production.
2.8. Fame analytical method
The gas chromatography-mass spectrometry (GC/MS) analysis of biodiesel was carried out on the Agilent 6890 series GC (Gas Chromatography System) interfaced to an HP 5791A (Santa Clara, USA) equipped with mass-selective ionization (MSI). Column: HP-5 ms capillary column, 30 m x 90.25 mm i.d., 0.25 µm film thickness. The oven temperature: initial temperature of 150°C for 2 min, rate of 4 °C/min to 300 °C, and continuous for 20 min. Detector temperature: 230 °C; injector temperature: 300 °C. Carrier gas: helium, column flow 1.5 mL/min, split ratio 50:1. Injection volume: 1 µL. ChemStation for GC was employed for instrument control and data analysis. Peak-area integration was used to calculate the quantitative determination.
2.9. Characterization of biodiesel
The specifications of the biodiesel produced by A. terreus KC462061 were compared with international specifications EN 14214 (Europe) and ASTM D6751-08 (United States).
3. Results and discussion
3.1. Mycosynthesis of Au@Ag NPs by A. terreus KC462061
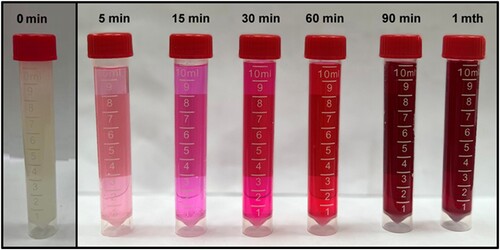
Visually, the biogenic synthesis of Au@Ag NPs appeared through the successive changes in color during the formation of Au@Ag NPs from pink to a dark red in . Biosynthesis of Au@Ag NPs starts after five min. of reaction, is cautious until 90 min. and seems perfectly stable for one month. It is a very rapid, convenient, and safe method of getting Au@Ag NPs.
3.2. UV-Vis spectroscopy analysis
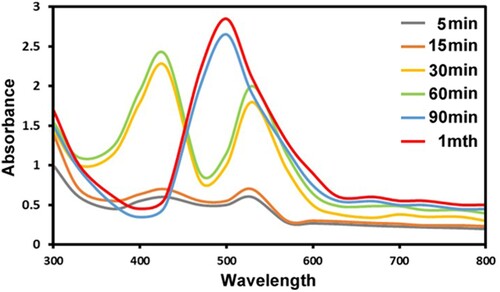
This change in color was analysed by UV-vis spectra to reveal significant surface plasmon resonance (SPR), which mostly reflects on biosynthesized Au@Ag NPs in the colloidal solution . UV-visible spectrometry was used to establish the existence of Au@AgNPs in the range of 300–800 nm. The spectra of the Au@Ag NPs exhibited two separate absorption bands at 430 and 530 nm, connected to the initial step of biogenic Au@Ag NPs. The lack of two bands of Au and Ag and the occurrence of a single broadband at 490 nm indicate the complete formation of Au@Ag NPs.
3.3. Characterization of Au@Ag NPs
3.3.1. TEM analysis
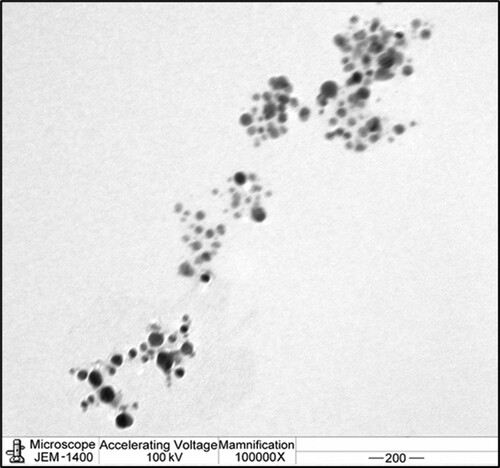
The morphology and size of Au@AgNPs were evaluated using TEM . shows the TEM micrographs of all Au@AgNPs have the core@shell structure, which is also seemingly spherical, well distributed, and has no aggregates. Also, it can be discovered that Ag was founded on the shell and Au was located in the core. A set of Au@AgNPs with the core@shell form can be noticed, where we can differentiate Au-core looks (darker) from Ag-shell looks (lighter), due to the difference in atomic number, which clearly appeared in the shape of the picture.
3.3.2. XRD Analysis of Au@Ag NPs
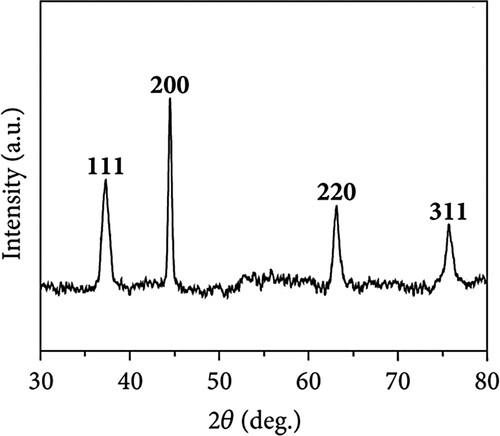
XRD analysis was performed to study the crystalline nature of Au@Ag NPs. indicated four reflections at angles of 37.93, 44.61, 64.29, and 77.18°were assigned to the (111), (200), (220), and (311) planes, respectively.
3.3.3. FT-IR analysis of Au@Ag NPs
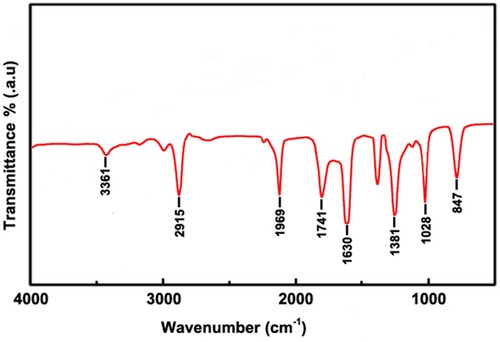
The FT-IR spectrum analysis of Au@Ag NPs is presented in . The FT-IR was used to identify the functional groups responsible for the biosynthesis of Au@Ag NPs. The FT-IR analysis of AgNPs ( and ) showed intensive peaks at 3361, 2915, 1969, and 1747cm−1. These peaks were generated by the O-H stretching of alcohols or phenols, respectively, alkane CH2 stretching of aliphatic compound lipids, C = O frequency of carboxylic acid, and C = O stretch of aldehyde compounds. The rest of the absorption bands are presented in .
Table 4. FTIR wave numbers for different peaks of Au@Ag NPs.
3.3.4. EDAX analysis
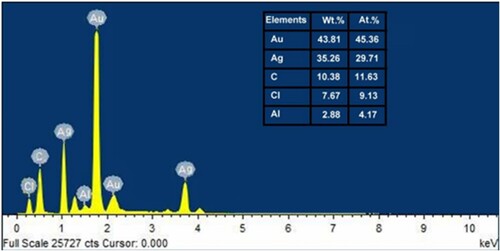
The EDAX analysis was performed to detect the amount of Au@Ag NPs in the sample. The EDAX spectrum () revealed strong signals of Au@Ag NPs in four different places of the sample, ensuring their presence, while weak signals that correspond to other elements such as C, Cl, and Al were also current in the spectrum.
3.4. Screening of the significant variables affecting Au@Ag NPs biosynthesis
The optimization of Au@Ag NPs was studied by performing 24 designed experiments according to RSM . Throughout the experiment, the values of the five different variables were merged in different runs, and the converted form of the experimental size was then fitted to the polynomial mode. The order of the experiments was randomly chosen. Optimization of Au@Ag NPs biosynthesis demonstrated in run 24 (ratio of HAuCl4.3H2O and AgNO3; fungal cell-free amount; pH; time of incubation; and temperature of solution) were the best conditions for Au@Ag NPs biosynthesis based on their response-absorption maximum of the predicted OD, which is represented in .
Table 5. RSM matrix with experimental and predicted responses.
An analysis of variance (ANOVA) was used to demonstrate the models’ suitability, and the estimated optimum conditions for nanoparticle synthesis were determined in . The ratio of the individual term's mean square to the residual's mean square is known as the model F-value. The likelihood of an F-statistics value, or F value, is what is used to test the null hypothesis. The results of our study are statistically consistent with model p values for Au@Ag NPs of 0.0032. The lack-of-fit F-value of 0.2631 and a p value of 98.61% imply that the lack of fit is not significant. The optimized factors for Au@Ag NPs biosynthesis involve the X1 ratio of HAuCl4.3H2O and AgNO3 (mM), the X2 fungal cell-free amount (ml), the X3 pH of the solution, the X4 time of incubation (min) and the X5 temperature of solution. The model's applicability to the biosynthesis of Au@Ag NPs was shown to have a significant well-fitted relationship between the experimental and projected response values using the R2 value. The modified R2 of 0.4178 agrees reasonably with the predicted R2 of 0.2732 from Au@Ag NPs. A strong signal is indicated by the ratio of 4.5096. We can deduce a specific correlation between the input and output variables using this approach. The diagnostic case statistics of the study's predicted coefficient of determination value (Pred R2) and the adjusted coefficient of determination value (Adj R2) confirm the significance of the quadratic model that was applied.
Table 6. ANOVA values and statistical analysis by response surface model fitting.
3.5. Lipid production and optimization
The optimization of A. terreus KC462061 for maximum lipid production by RSM depends on the nutrient and environmental variables, which are very related to the growth medium. This growth medium has varying concentrations of glucose, nitrates, phosphorus, and NaCl at various pHs, incubation times, and temperatures. shows the comparison between the experimental and estimated lipid productivity. The maximum lipid production of more than 40% dry biomass of A. terreus KC462061 was achieved in fermentation process run 19, which contained (50 g/l) glucose (Y1), (1.5 g/l) peptone (Y2), (1.0 g/l NaCl (Y3), 4.0 g/l) Na2HPO4 (Y4), pH 6 (Y5), and was incubated for 4 days at 25°C (Y6). presents evidence that all error values are less than 1%, which further establishes the usefulness of the proposed correlation in calculating the lipid production obtained from the fungal isolate A. terreus KC462061 under the investigated optimization conditions. This demonstrates that the suggested association is accurate and can be utilized successfully to estimate the amount of lipid production within the parameters of the examined impacting variables. Using the ANOVA approach, the importance of the variables influencing A. terreus KC462061 lipid production is determined. It is well known that when a lower P value arises, a variable has a significant influence on lipid production content. The P test shows that the pH factor has a considerable impact on the content of lipid biosynthesis, but glucose has a minor impact, as shown in .
Table 7. Comparison between experimental and estimated lipid production.
Table 8. The optimal variables at maximum lipid production (41.83%).
3.6. Biodiesel production
Biodiesel production studies under experimental conditions demonstrated significant variations in performance in the biodiesel production process. The process efficiency was confirmed by being very dependent on the preferred process conditions in . The difference between the values at the three levels of each factor is the relative influence of the variable. The large variation was related to the stronger influence of the factor. The negative values were disregarded in assessing biodiesel production, but positive values appeared in biodiesel production. The significance of the variables affecting biodiesel production established by Au@Ag NPs as a nanocatalyst is identified using the ANOVA method. It should be noted that the lower P value of a factor reflects the great impact of the factor on biodiesel production. As shown in , the P test indicates that the methanol/oil ratio (mole/mole) factor has a highly meaningful effect on biodiesel production, while the reaction temperature factor has a slight influence on biodiesel production. The relative influence of the factors on biodiesel production can be summarized in the following ascending order: reaction time < reaction temperature, amount of nanocatalyst, and methanol/oil ratio.
Table 9. Factors affecting biodiesel production using transesterification process.
Table 10. ANOVA for response surface methodology.
3.7. Character and analysis fAME
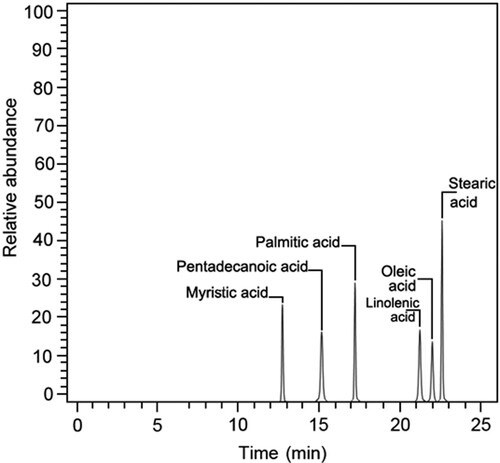
The FAME profile analysis ( and ) showed that the total FAME produced was 98.48%. The fatty acid composition includes myristic acid (17.24%), pentadecanoic acid (10.34%), palmitic acid (18.69%), stearic acid (30.17%), oleic acid (9.36%), and linoleic acid (12.41%). Myristic, pentadecanoic, palmitic, and stearic acids are examples of saturated fatty acids (SFA), whereas oleic and linoleic acids are examples of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) for linoleic acid, respectively.
Table 11. GC-MS analysis of fatty acid esters profile.
3.8. Properties of the produced biodiesel
presents data reports that compare the attributes of the biodiesel produced as a unique green fuel employing Au@Ag NPs as a nanocatalyst against international analytical methodologies for both diesel and biodiesel. The fatty acid profile of biodiesel made using fungal lipids as feedstock clearly indicates its quality. The characteristics of fatty acids, such as their length of carbon chain and degree of unsaturation, greatly affect the properties of biodiesel and, consequently, the efficiency of diesel engines. The FAMEs derived from A. terreus KC462061 exhibit excellent fuel properties. Their superior fuel characteristics suggest that they could be a viable option for biodiesel production. Standard biodiesel parameters, including kinematic viscosity (kv), density (p), iodine value (IV), oxidation stability (OS), cetane number (CN), saponification value (SV), high heating value (HHV), pour point (PP), and cloud point (CP), were evaluated in order to determine the quality of A. terreus KC462061 biodiesel ().
Table 12. Properties of the produced biodiesel compared to international standards.
displays that the high amounts of SFAs, low amounts of MUFAs, and very low amounts of PUFAs fatty acid contents were 76.44, 22.04, and 1.52%, respectively, which were very appropriate for biodiesel production. All standards in biodiesel parameters are compatible with standards for regular fuel. The estimated density of the fungal strain (0.85 g/cm3) in this investigation was in close proximity to the lower bound given by US standards (0.86–0.90 g/cm3). The viscosity of fungal biodiesel was 3.59 mm2/s, which was within the range of biodiesel standards EN14214 and ASTM D6751-08.
compares the catalytic activity of gold-silver core–shell nanoparticles with some of the previous studies. As shown in , in order to obtain a biodiesel yield of more than 85%, high temperature, high methanol-to-oil ratio, or lengthy reaction time is required. In the case of fungal lipid oil, when Au@Ag NPs are used under perfect conditions, biodiesel yields up to 43% in 30 min, compared to 4 h (Citation30). Thus, the present study shows a significant time reaction of biodiesel (30 min.) from fungal lipids by applying a perfect reaction condition. Our method of production of biodiesel is very fast, facilitates, it is very safe, and has a good yield of biodiesel.
Table 13. Biodiesel yield from different oils using various nanocatalysts.
Oleaginous filamentous fungi were discovered as renewable raw materials for the production of biomass, lipids, and biodiesel. Aspergillus spices have promising data for using renewable feedstocks, especially biodiesel production, such as A. niger (Citation30), A. wentii (Citation32), A. caespitosus (Citation33), and A. carneus (Citation34). The improvement in lipid accumulation achieved by oleaginous fungi required the optimization of medium cultivation requirements and nutritional elements (Citation35). Lipid content varied according to the nature of the fungi species and culture conditions like pH of the medium, incubation temperature, incubation time, carbon sources, and nitrogen sources (Citation36). The pH of the medium is a significant factor in lipid accumulation because its influential factors are (1) cell growth, (2) handling the transport of substances from the cells into or out of the cells in the opposite direction, (3) product formation, and (4) sporulation (Citation37). Aspergillus carneus isolate OQ275240 showed maximum lipid content (36.20%), which was achieved at optimal concentrations of 20.82 g/L of glucose and 0.10 g/L of phosphorus (Citation38). In another study, A. caespitosus displayed an extreme lipid content of more than 50% when cultivated in carbon-enriched and nitrogen-limited media (Citation39). High levels of glucose and nitrogen-limiting conditions are suggested to enhance triacylglycerol biosynthesis, which is then retained in liposomes, thereby improving the accumulation of lipids in oleaginous fungi (Citation28). Oleaginous fungi most frequently employ glucose as the most suitable carbon source to stimulate growth and the formation of lipids (Citation38,Citation39). The specific mechanisms of biogenic synthesis of metallic nanoparticles using fungi have not yet been fully elucidated. Fungi secrete large quantities of enzymes and proteins that contribute to reducing metal ions (X+) and producing elemental silver (X0) at the nanoscale. Biomolecules bind to the nanoparticle surfaces to form capping that confers stability and prevents particle aggregation and agglomeration (Citation40,Citation41). The lack of activity of nitrate reductases in fungal isolates leads to a failure to complete reduction processes, which supports the hypothesis of enzyme-based biosynthesis (Citation42).
A hypothetical mechanism for crude fungal extracts containing large amounts of different enzymes and active protein molecules that are able to convert metals and metalloids to nanoparticles (Citation43). It may be hypothesized that phenol-oxidizing enzymes that are found both intra- and extracellularly in fungi cells, which work together to make up a very potent oxidation–reduction system in fungi, are responsible for the reduction of the metal. Furthermore, it has previously been demonstrated that phenol oxidases are found both intra- and extracellularly in fungi (Citation44). Our UV-vis result for Au@AgNPs, supported by (Citation44), confirmed a single absorption band where the maximum location relies on the core size/thickness ratio of the bimetallic particle. Some authors believe that for core–shell systems, the absorption spectra have two bands associated with each of the metals, but in our study, they do not appear (Citation45). The core–shell structure of the (Au) darker nucleus and the (Ag) lighter shell is clearly seen in the TEM image findings. Many publications supported our results about the TEM image revealing variation in the contrast between the dark gold core and the lighter silver shell to synthesize the core–shell nanostructure (Citation7,Citation46). XRD investigations of powdered Au@AgNPs samples revealed the presence of bimetallic Ag-Au, and their patterns revealed that all products were generated as face-centred cubic (fcc) structures with a crystalline nature. The XRD pattern has similar constants corresponding to crystalline planes (111), (200), (220), and (311), located at 2θ diffraction angles. These results were in good agreement with previous studies such as (Citation47,Citation48) which utilized XRD patterns to characterize Au@Ag NPs. FT-IR analysis was performed to identify potential functional groups in the fungal crude extract responsible for the bioreduction processes of Au and Ag in the biosynthesis of Au@AgNPs. The FTIR analysis was done to detect biosynthesized, myco-mediated nanoparticles. The FT-IR analysis was done to present fungi-mediated nanoparticles. The mycelium of fungi contains active biomolecules. Characteristics of functional groups such as the amines of proteins, hydroxyl, ketone compounds, steroids, phenols, etc., which have earlier been reported and most closely matched with our results. The integration and compatibility between these compounds lead to metal ions being converted to nanoparticles through intermediate compounds that undergo oxidation and reduction systems (Citation49). The EDAX analysis was used for the confirmation of the basic elements, purity, and relative quantity of Au@Ag NPs. The EDAX spectra of Au@Ag NPs confirmed that Au and Ag metals are the only major elements. The minor elements have low signals, which are shown as capping organic agents that are bound on the Au@Ag NPs surface. Our results agreement with (Citation50). In this study, RSM has been applied to optimize the variables of Au@Ag NPs biosynthesis to obtain a high yield of nanoparticles, a fast process, and a low concentration of chemicals. RSM is a matrix of mathematical and statistical methods used to design experiments and optimize the responses affected by different variables. RSM is helpful in analysing how process variables interact and in creating a mathematical model to precisely represent the process (Citation51). RSM was significantly successfully used for optimizing all parameters and obtaining perfect modeling in the biosynthesis of myco-mediated Au@Ag NPs research (Citation7,Citation52–55). RSM and ANOVA are used to connect the production of lipids to the nutrition conditions of fungal isolate on growth medium (Citation37,Citation56). The components of medium, incubation time, initial pH, and incubation temperature are the major elements affecting fungal growth and lipid production (Citation57). Fungal cells accumulate lipids through the anabolic pathway, producing fatty acids through β-oxidation. There is powerful proof that carbon and nitrogen sources play a critical role in the biosynthesis, regulation, and accumulation of lipids in fungal isolate biomass (Citation41,Citation58). The biodiesel production parameters (methanol-to-oil mole ratio, catalyst loading, temperature, and reaction time) have various effects on biodiesel production. According to the significance of the previous factors on biodiesel production, temperature was ordered first, followed by time, mole, and catalyst (Citation59). Au@Ag NPs displayed significant catalytic activities for the transesterification process. The maximum biodiesel production from Fusarium solani (oleaginous fungi) was 91.2% which depended on Au@Ag NPs (3%) and a 20:1 methanol-to-oil molar ratio at 70 °C for 30 min (Citation7). Another study used Au@Ag NPs with sunflower oil to produce biodiesel. Reaction parameters guide to a 5% nanocatalyst, a reaction temperature of 65 °C for 2.0 h, and, methanol-to-oil molar ratio of 5:1 to obtain biodiesel yield of 86.9% (Citation45). The major fatty acid compositions in A. terreus are linoleic acid, myristic acid, oleic acid, palmitoleic acid, pentadecanoic acid, and stearic acid (Citation60). A. terreus, the most potent lipid producer, and the fatty acid profile indicated that SFA were more prevalent than USFA. A. terreus was identified as a promising new commercial biodiesel feedstock based on its fatty acid profile (Citation5). The quality of biodiesel is highly correlated with the fatty acid constitution of lipids, which should have lower levels of PUFA and higher levels of SFA and MUFA (Citation61). In general, lipid metabolic genes play a significant function in oleaginous fungi. It implies that the evolutionary concept of lipid metabolic genes was largely dominated by vertical inheritance. Indicating that oleaginous fungi may have gained their lipogenic function during evolution. Based on genome sequencing, mapping of lipogenesis pathway, and major lipid profiling we have provided a semi-complete theory of lipid metabolism in Mortierella alpine (Citation62). The biodiesel properties are totally dependent on the chemical constituents of the used feedstock (Citation63). Biodiesel quality depends on the fatty acid profile, composition of lipids, and the presence of low levels of PUFA and high levels of saturated and MUFA (Citation64). Biodiesel must meet the criteria set up by international standards such as ASTM 6751–3 (USA), EN 14214 (Europe), and the Bureau of Indian Standard (IS 15607-05) for biodiesel (Citation65,Citation66).
4. Conclusions
The study highlights the threefold objectives of (Citation1) developing and utilizing gold-silver core–shell nanoparticles (Au@Ag NPs) as a nanocatalyst for (Citation2) maximize sustainable biodiesel production from A. terreus KC46206; and (Citation3) optimization of lipid productivity by A. terreus KC46206 (a low-cost substrate) on a one-step transesterification reaction. All previous objectives were achieved. First, A. terreus KC46206 grown by fermentation could be utilized for the optimization of myco-synthesized Au@Ag NPs based on physicochemical properties by the RSM method. Second, using the ANOVA approach, proof A. terreus KC46206 was a very promising tool for lipid production. Also, ANOVA of the suggested correlations shows that there is a close match between the experimental and estimated results of lipid production. Finally, the utilization of nanocatalysts (Au@Ag NPs) enhanced biodiesel production from fungal lipids with high amounts of SFAs in a one-step transesterification reaction. The biodiesel quality was assessed and found to meet international specifications EN 14214 (Europe) and ASTM D6751-08 (United States). Our method of production of biodiesel is very fast, facilitates, it is very safe, and has a good yield of biodiesel. In the future, we need more studies about nanocatalysts that could be applied in biodiesel production because their advantages exceed those of the classic methods. Because in the last few years, the good choice of raw material and the perfect catalyst have been critical points in determining the price of biodiesel production in refineries and trying to minimize it.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Miranda da Silva, L.R.; Castro, A.P.; Sanders Lopes, A.A.; Chaves de Lima, R.K.; de Sousa Rios, M.A.; Santos, J.C.S.D. Bioengineering 2022, 9, 539.
- Norjannah, B.; Chyuan Ong, H.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. RSC Adv. 2016, 6, 60034–60055.
- Kotasthan, T. Journal of Marine Science: Research & Development 2017, 7, 223–232.
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microorganisms. 2020, 8, 434–447. doi:10.3390/microorganisms8030434.
- Youssef Ghada, A.; Elrefaey, A.M.; El-Aassar, S. Egypt. J. Bot. 2021, 61, 693–708.
- Sayeda, A.A.; Mohsen, S.A.; Osama, H.E.S. Bulletin of the National Research Centre 2019, 43, 210–225.
- Al-Zaban, M.I.; AlHarbi, M.A.; Mahmoud, M.A.; Bahatheq, A.M. J. Appl. Microbiol. 2022, 132, 381–389.
- Cristiano, E.; Rodrigues, R.; Ana, K.; Furtado, C.; Heitor, B.S.; Bento, B.F. Bioresource Technology Reports 2019, 6, 46–53.
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippousis, A. J. Appl. Microbiol. 2017, 124, 336–367. doi:10.1111/jam.13633.
- Chen, H.; Hao, G.; Wang, L.; Wang, H.; Gu, Z. Liu. Sci. Rep. 2015, 5, 11247. doi:10.1038/srep11247.
- Vorapreeda, T.; Thammarongtham, C.; Cheevadhanarak, S.; Laoteng, K. Microbiology 2012, 158, 217–228. doi:10.1099/mic.0.051946-0.
- Parawira, W. Crit. Rev. Biotechnol. 2009, 29, 82–93.
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. Biotechnol. Bioeng. 2009, 102, 1298–1315.
- Sharma, Y.C.; Singh, B. Renew. Sustain. Energy Rev 2009, 13, 1646–1651.
- Gomes, J.F.P.; Puna, J.F.B.; Goncalves, L.M.; Bordado, J.C.M. Energy 2011, 36, 6770e8.
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Energy 2011, 36, 2693e700.
- Lee, H.V.; Juan, J.C.; Yun Hin, T-Y;; Ong, H.C. Energies 2016, 9, 611.
- Ullah, F.; Dong, L.; Bano, A.; Peng, Q.; Huang, J. J. Energy Inst. 2016, 89, 282–292.
- Fattah, I.M.R.; Noraini, M.Y.; Mofijur, M.; Silitonga, A.S.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C.; Mahlia, T.M.I. Applied Sciences 2020, 10, 6103.
- Saurabh, B.; Somwanshi, S.B.; Somvanshi, P.B.; Kharat, A.J. Phys. Conf. Ser. 2020, 1644, 012046. doi:10.1088/1742-6596/1644/1/012046.
- El-Aziz, A.R.M.A.; Al-Othman, M.R.; Eifan, S.A.; Mahmoud, M.A.; Majrashi, M.; Raju, D.P. Dig. J. Nanomater. Biostructures 2013, 8, 1215–1225.
- Mostafa, E.M.; Abdelgawad, M.A.; Musa, A.; Alotaibi, N.H.; Elkomy, M.H.; Ghoneim, M.M.; Badawy, M.S.E.M.; Taha, M.N.; Hassan, H.M.; Hamed, A.A. Antibiotics 2022, 11, 668.
- Vicente, G.; Bautista, L.F.; Rodriguez, R.; Gutierrez, F.J.; Sadaba, I.; Ruiz, V.R. Biochem. Eng. J. 2009, 48, 22–27.
- Mishra, S.K.; William, I.S.; Wasif, F.; Myounghoon, M.; Anupama, S.; Min, S.P. Bioresour. Technol 2014, 155, 330–333.
- Xu, Q.-Q.; Li, Q.; Yin, J.-Z.; Guo, D.; Qiao, B.-Q. Technol 2016, 144, 37–41.
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C. Appl. Catal., B 2009, 89, 265–272.
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Bioresource Technol 2010, 101, 9570e6.
- Lee, H.V.; Taufiq-Yap, Y.H.; Hussein, M.Z.; Yunus, R. Energy 2013, 49, 12–18.
- Dias, A.P.S.; Bernardo, J.; Felizardo, P.; Correia, M.J.N. Energy 2012, 41, 344–353.
- El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Bioengineering 2016, 3, 14.
- Jambulingam, R.; Shalma, M.; Shankar, V.J. Clean. Prod. 2019, 215, 245–258.
- Shoaib, A.; Bhran, A.; Rasmey, A.H.; Mikky, Y. 3 Biotech. 2018, 8, 417.
- Srinivasan, N.; Thangavelu, K.; Sekar, A.; Sanjeev, B.; Uthandi, S. Microb. Cell Fact. 2021, 20, 179.
- Ibrahim, A.G.; Baazeem, A.; Al-Zaban, M.I.; Fawzy, M.A.; Hassan, S.H.A.; Koutb, M. Sustainability 2023, 15, 6836.
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227.
- Papanikolaou, S.; Komaitis, M.; Aggelis, G. Bioresour. Technol 2004, 95, 287–291.
- Amanullah, A.; McFarlane, C.M.; Emery, A.N.; Nienow, A.W. Biol. Eng 2001, 73, 390–399.
- Jiru, T.M.; Abate, D. Web pub. J. Sci. Res. 2014, 2, 55–65.
- Rasmey, A.H.; Tawfik, M.A.; Abdel-Kareem, M.M. J. Appl. Microbiol 2020, 128, 1074–1085.
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Kahn, M.I.; Kumar, R. Colloids Surf. B Biointerfaces. 2003, 28, 313–318. doi:10.1016/S0927-7765(02)00174-1.
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Nanomedicine 2010, 6, 257–262. doi:10.1016/j.nano.2009.07.002.
- Ottoni, C.A.; Simões, M.F.; Fernandes, S. AMB Expr. 2017, 7, 31–45.
- Sanghi, R.; Verma, P.; Puri, S. Adv. Chem. Engineer. Sci 2011, 1, 154–162.
- Vetchinkina, E.P.; Loshchinina, E.A.; Vodolazov, I.R. Appl. Microbiol. Biotechnol. 2017, 101, 1047–1062.
- Sinha, T.; Ahmaruzzaman, M. Spectrochim. Acta, Part A 2015, 145, 280–288.
- Banerjee, M.; Dey, B.; Talukdar, J.; Kalita, M.C. Energy 2014, 69, 695–699.
- Khan, M.; Al-hamoud, K.; Liaqat, Z.; Shaik, M.R.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Mondeshki, M. Nanomaterials 2020, 10, 1885.
- Jiang, X.; Fan, X.; Xu, W.; Zhang, R.; Wu, G. ACS Biomater. Sci. Eng. 2020, 6, 680−689. doi:10.1021/acsbiomaterials.9b01297.
- Abdel-Hafez, S.I.I.; Nafady, N.A.; Abdel-Rahim, I.R. 3 Biotech. 2016, 6, 199.
- Al-Zaban, M.I.; Mahmoud, M.A.; Alharbi, M.A. Biotechnol. Equip 2021, 35, 341–353. doi:10.1080/13102818.2021.1875876.
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Talanta 2008, 76, 965–977. doi:10.1016/j.talanta.2008.05.019.
- Dasaratrao, S.B.; Salimath, B.; Deshpande, R.; Dhondojirao, B.M.; Krishnamurthy, P.B.; Venkataraman, A. Sci. Technol. Adv. Mater 2008, 9, 035012. doi:10.1088/1468-6996/9/3/035012.
- Fang, H.; Yan, Y.; Ju, Z.; Lian, S.; Pei, X.; Ma, Q.; Qu, Y. Chin. J. Biotechnol. 2019, 35, 2061–2068. doi:10.13345/j.cjb.190160.
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Small 2005, 5, 517–520. doi:10.1002/smll.200400053.
- Mostafa, E.M.; Abdelgawad, M.A.; Musa, A.; Alotaibi, N.H.; Elkomy, M.H.; Ghoneim, M.M.; Badawy, M.S.E.M.; Taha, M.N.; Hassan, H.M.; Hamed, A.A. Antibiotics 2022, 11, 668–678.
- Venkata, S.G.; Venkata, M.S. Fuel 2014, 116, 509–515.
- Wu, G.; Bryant, M.M.; Voitle, R.A.; Roland, D.A. Int. J. Poult. Sci. 2005, 4, 182–186.
- Carvalho, A.K.F.; Bento, H.B.; Izário, F.H.J.; de Castro, H.F. Approaches to Convert Mucor Circinelloides Lipid Into Biodiesel by Enzymatic Synthesis Assisted by Microwave Irradiations. Renewable Energy 2018, 125, 747–754.
- Hossain, M.N.; Siddik Bhuyan, M.S.U.; Md Ashraful Alam, A.H.; Seo, Y.C. Catalysts 2019, 9, 67.
- Ferreira, G.L.; da, R.; Vieira, J.D.G.; D’alessandro, E.B. Res. Soc. Dev. 2022, 11, e37411125049. doi:10.33448/rsd-v11i1.25049.
- Ramos, M.J.; Fernandez, C.M.; Casas, A.; Rodrıguez, L.; Perez, A. Bioresour Technol. 2008, 100, 261–268.
- Coradetti, S.T.; Pinel, D.; Geiselman, G.M.; Ito, M.; Mondo, S.J.; Reilly, M.C.; Cheng, Y.F.; Bauer, S.; Grigoriev, I.V.; Gladden, J.M.; Simmons, B.A.; Brem, R.B.; Arkin, A.P.; Skerker, J.M. Elife 2018, 7, e32110. doi:10.7554/eLife.32110.
- Sorate, K.A.; Bhale, P.V. Renewable Sustainable Energy Rev. 2015, 41, 777-798.
- Verma, P.; Sharma, M.P.; Dwivedi, G. Renewable Sustainable Energy Rev. 2016, 56, 319–333.
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Renewable Sustainable Energy Rev. 2010, 14, 1999.
- Barabás, I.; Todorut, I.-A. Biodiesel- Quality, Emissions and By-Products. InTech 2011.