ABSTRACT
Herein, a rapid, clean, less expensive and environmentally friendly route to a novel series of coumarins bearing thiazoles or 1,3,4-thiadiazoles was developed via grinding method under solvent conditions. Thus, 6-bromo-3-(2-bromoacetyl)-2-chromen-2-one was treated with cyanothioacetamide to produce thiazole-2-acetonitrile derivative 2, which was then transformed to iminocoumarins by reacting with hydroxyaldehydes. Hydrolysis of iminocoumarins by conc. HCl furnished coumarins. Furthermore, treatment of compound 2 with phenylisothiocyanate produced thioanilide which interacts with hydrazonoyl chloride and/or α-halocarbonyl compounds afforded 1,3,4-thiadiazole and thiophene derivatives, consequently. All the newly prepared coumarins were screened against six pathogenic microorganisms. The results indicated that compounds 7 and 12 were the most effective against Bacillus pumilis, while compound 4b was highly active against Streptococcus faecalis. Also, compound 5b was highly active against Enterobacter cloacae. Compounds 2 and 7 were higher active compared with reference drug ketoconazole against fungi Saccharomyces cerevisiae.
1. Introduction
In organic synthesis, the use of harmful solvents and catalysts poses risks to human health and causes an environmental threat (Citation1). In addition to the pollution of air, traditional methods have disadvantages such as long reaction time and rising energy consumption. Consequently, there is an increasing demand for developing an efficient protocol, aimed mainly to completely eliminate solvents from organic reaction synthesis or to swap out hazardous solvents with eco-friendly acceptable ones. To address this challenge, green techniques were utilized to help synthetic chemists to overcome the problems of conventional synthesis, such as long reaction time, vigorous conditions, hazardous solvents and catalysts, low yield and high costs (Citation2, Citation3). The green protocols including ultrasound-assisted processes (Citation4), Microwave irradiation (Citation5, Citation6) and ball milling (Citation7). Recently, mechanochemistry, a friendly environmentally technology has gained remarkable momentum for drug manufacturing and large scale organic synthesis (Citation8). Mechanochemistry is a method that uses mechanical force such as grinding and milling, to achieve the chemical reactions. Another green protocol is grinding (Citation9) method in which the reactants were ground together using a mortar and pestle. This method requires no specialized equipment making it economical and eco-friendly.
Coumarins are heterocyclic molecules abundantly available in nature (Citation10, Citation11). Coumarins are regarded to be the most versatile chemical rings for the development and discovery of anticancer drugs. Coumarins have attracted the attention of scientists due to the fact that they have a variety of pharmacological properties, such as antiviral (Citation12–14), antibacterial (Citation15, Citation16), antimicrobial (Citation17), anticancer (Citation18–21), anti-SARS-CoV-2-agents (Citation22), antioxidant (Citation23–25), anti-inflammatory, and anti-HIV (Citation26, Citation27).
On the other side, thiazole derivatives are found in nature as penicillin, thiamine (vitamin B1) and luciferin (Citation28). Thiazole is a good pharmacophore nucleus due to its wide range of therapeutic applications. New thiazole medications were approved in 2019, including the antibiotic cefiderocol, that was effective against a wide range of multidrug-resistant bacteria, and alpelisib, a therapeutic used to treat particular types of breast cancer (Citation29). Lusutrombopag and cobicistat were licenced in 2018 for stimulating platelet production in the treatment of thrombocytopenia and HIV infection, respectively (Citation29). Other approved drugs containing thiazole are ethoxzolamide (carbonic anhydrase inhibitor), meloxicam (anti-inflammatory), tiazofurin, epothilone (antitumor), sulfathiazole and ceftriaxone (antibacterial), ravuconazole (antifungal), pramipexole (antiparkinsonian), mirabegron, thiabendazole (antiparasitic) (Citation30, Citation31).
Literature reviews showed that thiazole and its derivatives possess a broad range of biological activities, such as anticancer (Citation32), antimicrobial (Citation33), antibacterial (Citation34), antiviral (Citation35, Citation36), anti-inflammatory (Citation37, Citation38), antidiabetic (Citation39), and antihypertensive activities (Citation40). Moreover, thiazoles are used to treat Alzheimer’s disease (Citation41, Citation42). Considering the aforementioned evidences and in continuation of our efforts to synthesize new heterocycles using green protocol (Citation43, Citation44), we planned to combine the bioactive thiazole and coumarin moieties by grinding method to create more effective derivatives that may be used as human drugs.
2. Results and discussion
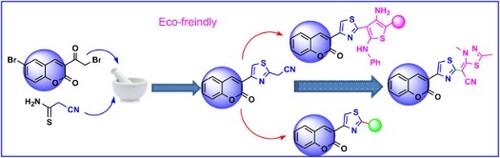
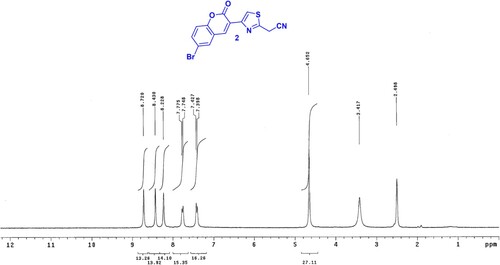
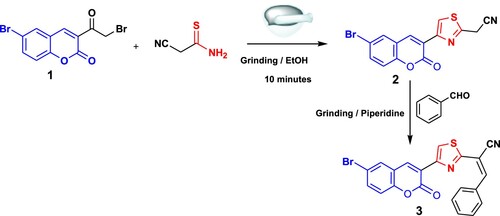
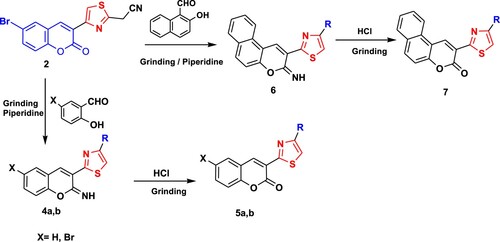
The goal of our research was to design an economical, fast, low cost and clean method for the synthesis of new coumarins. Earlier, Hantzsch’s thiazole synthesis was performed using the traditional method either by refluxing or under stirring conditions α-halocarbonyl compound with cyanoamide. Since, the traditional methods required the presence of organic solvents which are hazardous to human health and the environment, in addition to long reaction time and poor yield; herein we conducted this reaction by grinding method under solvent-free conditions. The synthetic strategy for the target coumarins 2–13 is illustrated in Schemes 1 and 2. Thus, grinding of 6-bromo-3-(2-bromoacetyl)-2H-chromen-2-one (1) (Citation45) and cyanothioacetamide under solid state condition afforded 2-(4-(6-bromo-2-oxo-2H-chromen-3-yl)thiazol-2-yl)acetonitrile (2) in a good yield. The formation of compound 2 was established based on its spectral data. The 1H NMR of 2 in (DMSO-d6) () revealed a singlet signal at δ = 4.45 ppm attributed to CH2 group, besides two doublet signals at δ = 7.31 and 7.74 ppm for CH-coumarin, and three singlet signals at δ = 8.22, 8.45, 8.72 ppm for CH-coumarin and CH-thiazole. Grinding of compound 2 with benzaldehyde in piperidine yielded arylidine derivative 3 ().
On the other hand, cyclocondensation of compound 2 with salicylaldehyde, 5-bromosalicylaldehyde and/or 2-hydroxy-1-naphthaldehyde under grinding condition afforded iminocoumarins 4a, b and 6, respectively, in good yields. The proton nuclear magnetic resonance of 4a in (DMSO-d6) demonstrated characteristic signals at δ 6.07 (s, 1H, thiazole), beside six multiplets in the range of 6.72–8.17 ppm for six coumarin protons as well as four singlet signals δ 8.50, 8.67, 8.74 and 9.06 ppm that corroborated three CH-coumarin, along with NH proton, respectively. Also, the chemical structure of compound 4b was confirmed by the appearance of four doublet signals at δ 6.85, 7.22, 7.47 and 7.83 with J = 8.4, 8.8, 8.8 and 8.3 Hz for CH-coumarin protons along with six singlet signals at δ 7.97, 8.13, 8.55, 8.68, 8.99, 9.24 supporting the presence of CH-thiazole, four CH-coumarin and NH protons, respectively.
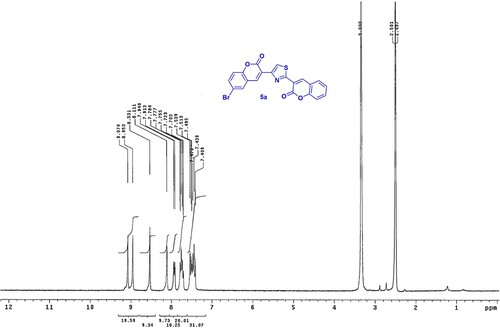
Iminocoumarines 4a, b and 6 underwent hydrolysis producing coumarins 5a, b and 7, respectively upon grinding with three drops of hydrochloric acid (). The structure of the newly prepared coumarins was assured based on 1H NMR spectrum in which confirmed the disappearance of the NH signal. The proton nuclear magnetic resonance of 5a in (DMSO-d6) () as an example, showed no signal for imino proton and displayed two multiplet signals at δ = 7.40–7.53 and 7.70–7.78 ppm for five aromatic protons, in addition to doublet signal at δ = 7.91 ppm and four singlet signals at δ = 8.11, 8.53, 8.95 and 9.07 ppm attributed to CH-thiazole, three CH-coumarin protons, respectively.
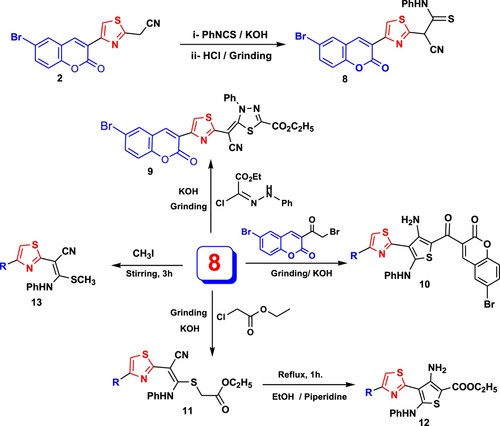
On the next side, grinding of compound 2 with phenyl isothiocyanate and potassium hydroxide gave a nonisolable product which on acidification with HCl afforded thioanilide 8. Grinding of the latter with C-ethoxycarbonyl-N-phenyl hydrazonoyl chloride in potassium hydroxide gave 2,3-dihydro-l,3,4-thiadiazole derivative 9 via elimination of HCl and aniline molecules (). 1H NMR spectrum of 9 showed new signals at δ = 1.42 and 4.43 ppm corresponding to triplet and quartet signals of ethoxy protons with coupling constant J = 7.12 and 7.08 Hz beside two multiplet signals in the region of δ = 6.33–8.24 ppm and two singlet signals at δ = 8.30 and 8.58 ppm. Also, the chemical structure of compound 9 was confirmed by its 13C NMR spectrum where it revealed signals at δ: 9.10 (CH3), 66.81 (CH2), 118.72–131.21 (Ar-C), 152.16, 158.45 (2C=O).
Furthermore, grinding of thioanilid 8 with 6-bromo(2-bromoacetylcoumarin) in the presence of potassium hydroxide led to cyclization affording amino thiophene derivative 10 based on its 1H NMR spectrum. In contrast, the interaction of thioanilid 8 with ethyl chloroacetate at the same reaction condition afforded the open structure 11 which underwent cyclization to amino thiophene derivative 12 after boiling in ethanol and piperidine. The 1H NMR spectrum of compound 10 recorded two doublet signals at δ = 7.20 and 7.64 ppm for two protons and at 7.50–7.55 (m, 9H), in addition to five singlet signals at δ = 7.85, 8.06, 8.48, 8.75, 11.62 ppm due to coumarin, NH and NH2 protons. In the 1H NMR spectrum compound 11, new triplet and quartet signals for ethoxy group at δ = 1.11 and 4.02 ppm and singlet signal at δ = 3.66 ppm for CH2 were observed, beside signals for NH at δ = 11.23 ppm and at δ = 7.14–8.48 ppm for aromatic protons. The structure of compound 12 was supported by its 1H NMR which displayed signals at δ = 1.24 and 4.18 ppm for ester group with J = 7.2 and 6.8 Hz, two triplet at 7.02, 7.33 ppm (3H, J = 7.2, 8 Hz), two doublets at δ = 7.20, 8.22 (3H, J = 7.6, 2.4 Hz), 7.78, 7.81 (dd, 1H, J = 2.4, 2.4 Hz) beside four singlet signals for CH-coumarin and NH at 7.44, 8.45, 8.88 and 9.40 ppm and 7.47 ppm (br, NH2). In addition, 13C NMR spectrum of 12 recorded signals at δ: 14.30 (CH3), 61.90 (CH2), 116.74, 118.60, 119.72, 121.22, 122.29, 125.11, 129.82, 131.21, 134.69, 138.69, 139.19, 152.16 (Ar-C), 165.81, 168.89 (2C=O). Finally, 2-(4-(6-bromo-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-3-(methylthio)-3-(phenylamino)-acrylonitrile (13) was prepared by methylation of thioanilide 8 with methyl iodide in DMF and KOH under stirring condition at room temperature. Its 1H NMR (DMSO-d6) demonstrated signals at δ 2.29 (s, SCH3), 7.21 (s, 1H), 7.40–7.53 (4H, ArH’s), 7.76 (d, 2H, J = 8 Hz), 7.96 (s, 1H), 8.28 (s, 1H), 8.52 (s, 1H), 11.50 (s, 1H, NH).
3. Biological studies
3.1. Antimicrobial activity
The newly synthesized compounds were initially investigated for their antimicrobial activity to elucidate the potential therapeutic utility against various pathogenic microbes using the agar plate diffusion method. The selected bacteria were Bacillus pumilis MTCC-2296 and Streptococcus faecalis MTCC-0459 (G+), Escherichia coli ATCC-25955 and Enterobacter cloacae ATCC-23355 (G−), while, Saccharomyces cerevisiae ATCC-9763 and Candida albicans ATCC-10231 were the chosen fungi. Penicillin G and ciprofloxacin were used in the positive and negative bacterial controls, respectively, while the fungi were controlled by the fungicidal drug ketoconazole. The resulting inhibition zone diameters were measured in millimeters (). According to the screening results, compounds 7 and 12 were the most effective against B. pumilis with inhibition zone diameters of 24 and 23 mm compared to penicillin G (26 mm). Also, compounds 4b, 7, 11 and 12 were highly active against S. faecalis with inhibition zone values of 22, 20, 20 and 20 mm. On the other hand, compounds 7 and 11 showed significant activities toward E. coli with growth inhibition values of 22 and 22 mm compared to ciprofloxacin (28 mm). With respect to E. cloacae compounds 5a and 5b were most active with inhibition values of 22 and 24 mm compared to ciprofloxacin (28 mm). For the fungi, compounds 2 and 7 showed significant growth inhibition zones of 22 and 21 mm against S. cerevisiae. These values are higher than that of reference drug ketoconazole (20 mm). Additionally, compounds 5a, 5b, 10 and 12 were highly active against S. cerevisiae with inhibition zones of 18, 17, 19 and 17 mm, respectively. In the same context, we found that the compounds 4a, 4b, 5b, 6, 7, 9, 11 and 12 were the most active against C. albicans with inhibition zones of 18, 18, 19, 19, 18, 17, 17 and 19 mm compared to ketoconazole (24 mm). The remaining compounds showed moderate to low activity against the tested microbes.
Table 1. In vitro preliminary antimicrobial activity of the newly synthesized compounds against pathogenic bacteria and fungi.
3.2. MIC and MBC
Our in vitro antimicrobial activity assay results led us to the conclusion that the majority of the target compounds had strong antibacterial activity. To find the lowest concentration of a molecule that can prevent or eliminate harmful microbial, we investigated the MIC, MBC, and MFC values for the most effective compounds against the tested microorganisms. From the results, we observed that nearly all compounds have low MIC values (1.95–31.3 µg/ml) against the tested bacteria ().
Table 2. In vitro MIC and MBC for the newly synthesized compounds.
3.3. MIC and MFC
The results for the minimum inhibitory concentrations (MIC) and the minimum fungicidal concentrations (MFC) were illustrated in . By studying the results we found that compounds 5a, 5b, 7, 8, 10 showed the lowest MIC values against S. cerevisiae (3.91–15.6 µg/ml). Whilst, compounds 5b, 7 and 9 showed the lowest MIC values of 15.6 µg/ml with C. albicans. Based on the MIC data, we deduced that several our target compounds have a strong potential to function as effective antibacterial agents.
Table 3. In vitro MIC and MFC of the synthesized compounds.
4. Experimental part
4.1. Chemistry
Melting points of all the prepared heterocyclic compounds were measured on an electrothermal apparatus and may be uncorrected. IR spectra of these derivatives were determined after conversion to disc with potassium bromide using a spectrophotometer (Pye-Unicam SP300). Moreover, a solution of deuterated DMSO-d6 was utilized for measurements of the spectra of 1H NMR on a Varian Gemini 400 NMR spectrometer. Mass spectra were carried out at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar Univeristy, Nasr City, Cairo, Egypt, on Direct Probe Controller Inlet part to Single Quadropole mass analyzer in Thermo Scientific GC-MS model ISQ LT. Antimicrobial activity was performed at the Department of Microbiology, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11754, Egypt.
4.1.1. 2-(4-(6-Bromo-2-oxo-2H-chromen-3-yl)thiazol-2-yl)acetonitrile (2)
A mixture of compound (1) (3.46 g, 10 mmol), cyanothioacetamide (1.00 g, 10 mmol) and few drops of ethanol were ground with a pestle in a mortar for 15 min. The brown solid was collected and recrystallized from AcOH to give 2. Yield 80%; m.p. 218–220°C; 1H NMR (DMSO-d6) δ 4.45 (s, CH2), 7.31 (d, 1H, J = 8.0 Hz, Ar-H), 7.74 (d, 1H, J = 8.0 Hz, Ar-H), 8.22 (s, 1H), 8.45 (s, 1H), 8.72 (s, 1H). Anal. calcd. for C14H7BrN2O2S (347.19): C, 48.43; H, 2.03; Br, 23.01; N, 8.07; S, 9.24%. Found: C, 48.55; H, 2.17; Br, 23.13; N, 8.20; S, 9.37%.
4.1.2. Synthesis of thiazole derivatives 3, 4a, b and 6
Acetonitrile derivative 2 (0.347 g, 1 mmol) and the appropriate of benzaldehyde, salicylaldehyde, 5-bromosalicylaldehyde or 2-hydroxynaphthalene-l-carbaldehyde (1 mmol) were ground with a pestle in a mortar in the presence of few drops of piperidine for 15 min. The colored solid was collected and recrystallized from suitable solvent giving the desired products 3, 4a, b and 6, respectively.
4.1.2.1. 2-[4-(6-Bromo-2-oxo-2H-chromen-3-yl)-l,3-thiazol-2-yl]-3-phenylacrylonitrile (3)
Brown powder (AcOH); Yield 80%; m.p. 230–232°C; 1H NMR (DMSO-d6) δ 7.21–7.82 (m, 10H, ArH’s and thiazole H-5), 8.21 ppm (s, 1H, CH-vinyl). Anal. calcd. for C21H11BrN2O2S (435.29): C, 57.94; H, 2.55; Br, 18.36; N, 6.44; S, 7.37%. Found: C, 57.87; H, 2.65; Br, 18.42; N, 6.53; S, 7.30%.
4.1.2.2. 6-Bromo-3-(2-(2-imino-2H-chromen-3-yl)thiazol-4-yl)-2H-chromen-2-one (4a)
Beige powder (DMF); Yield 80%; m.p. 240–242°C; IR (cm−1): 3414 (NH), 3022 (CH), 1704 (C=O), 1605 (C=N); 1H NMR (DMSO-d6) δ 6.07 (s, 1H, CH-thiazole), 6.72–6.80 (m, 1H, Ar-H), 6.88–6.97 (m, 1H, Ar-H), 7.10–7.15 (m, 1H, Ar-H), 7.24–7.34 (m, 1H, Ar-H), 7.43–7.56 (m, 1H, Ar-H), 8.15–8.17 (m, 1H, Ar-H), 8.50 (s, 1H), 8.67 (s, 1H), 8.74 (s, 1H), 9.06 (s, NH). Anal. calcd. for C21H11BrN2O3S (451.29): C, 55.89; H, 2.46; Br, 17.71; N, 6.21; S, 7.11%. Found: C, 55.79; H, 2.36; Br, 17.82; N, 6.33; S, 7.01%.
4.1.2.3. 6-Bromo-3-[2-(6-bromo-2-imino-2H-chromen-3-yl)-thiazol-4-yl]-chromen-2-one (4b)
Yellow powder (DMF); Yield 82%; m.p. >300°C; IR (cm−1): 3427 (NH), 3052 (CH), 1721 (C=O), 1621(C=N); 1HNMR (DMSO-d6) δ 6.85 (d, 1H, J = 8.4 Hz, Ar-H), 7.22 (d, 1H, J = 8.8 Hz, Ar-H), 7.47 (d, 1H, J = 8.8 Hz, Ar-H), 6.83 (d, 1H, J = 2 Hz, Ar-H), 7.97 (s, 1H), 8.13 (s, 1H), 8.55 (s, 1H), 8.68 (s, 1H), 8.99 (s, 1H), 9.24 (s, NH). Anal. calcd. for C21H10Br2N2O3 (530.19): C, 47.57; H, 1.90; Br, 30.14; N, 5.28; S, 6.05%. Found: C, 47.67; H, 1.81; Br, 30.21; N, 5.39; S, 6.17%.
4.1.2.4. 7-Bromo-3-[2-(3-imino-3H-benzo[f]chromen-2-yl)-thiazol-4-yl]-chromen-2-one (6)
Brown powder (DMF); Yield 88%; m.p. >300°C; 1H NMR (DMSO-d6) δ 7.46 (d, 1H, J = 8.4 Hz, Ar-H), 7.63 (s, 1H), 7.80 (d, 2H, J = 8.4 Hz, Ar-H), 8.04 (d, 1H J = 8 Hz, Ar-H), 8.14 (d, 1H, J = 8 Hz, Ar-H), 8.29 (s, 1H), 8. 45 (s, 1H), 8.57 (s, 1H), 8.69–8.75 (t, 1H, J = 7.4 Hz, Ar-H), 9.08 (s, 1H), 9.25 (s, 1H), 9.41 (s, NH). Anal. calcd. for C25H13BrN2O3S (501.35): C, 59.89; H, 2.61; Br, 15.94; N, 5.59; S, 6.40%. Found: C, 59.98; H, 2.70; Br, 15.84; N, 5.49; S, 6.50%.
4.1.3. Synthesis of coumarins 5a, 5b and 7
Imino coumarins 4a, 4b or 6 (10 mmol) and three drops of concentrated hydrochloric acid were ground with a pestle in a mortar for 10 min. The generated solid was collected, washed with water, and recrystallized from DMF affording the desired products.
4.1.3.1. 3-[4-(6-Bromo-2H-chromen-3-yl)-thiazol-2-yl]-chromen-2-one (5a)
Yellow powder; Yield 89%; m.p. >300°C; 1H NMR (DMSO-d6) δ 7.40–7.53 (m, 3H), 7.70–7.78 (m, 2H, Ar-H), 7.91 (d, 1H, J = 8.4 Hz, Ar-H), 8.11 (s, 1H), 8.53 (s, 1H), 8.95 (s, 1H), 9.07 (s, 1H). Anal. calcd. for C21H10BrNO4S (452.28): C, 55.77; H, 2.23; Br, 17.67; N, 3.10; S, 7.09%. Found: C, 55.67; H, 2.12; Br, 17.77; N, 3.21; S, 7.20%.
4.1.3.2. 3-(4-(6-Bromo-2-oxo-2H-chromen-3-yl) thiazol-2-yl)-6-bromo-2H-chromen-2-one (5b)
Yellow powder; Yield 90%; m.p. >300°C; 1H NMR (DMSO-d6) δ 6.87 (d, 1H, J = 8.4 Hz), 7.24 (d, 1H, J = 8.8 Hz, Ar-H), 7.50 (d, 1H, J = 8.8 Hz, Ar-H), 6.81 (d, 1H, J = 2 Hz, Ar-H), 7.95 (s, 1H), 8.16 (s, 1H), 8.53 (s, 1H), 8.70 (s, 1H), 8.97 (s, 1H). Anal. calcd. for C21H9Br2NO4S (531.17): C, 47.48; H, 1.71; Br, 30.09; N, 2.64; S, 6.04%. Found: C, 47.58; H, 1.82; Br, 30.18; N, 2.74; S, 6.19%.
4.1.3.3. 2-[4-(7-Bromo-2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-benzo[f]chromen-3-one (7)
Brown powder; Yield 89%; m.p. >300°C; 1H NMR (DMSO-d6) δ: 7.38–7.8.11 (m, 10H, Ar-H), 8.14, 8.35 (2s, 2H); Anal. calcd. for C25H12BrNO4S (502.34): C, 59.77; H, 2.41; Br, 15.91; N, 2.79; S, 6.38%. Found: C, 59.87; H, 2.50; Br, 15.81; N, 2.70; S, 6.48%.
4.1.3.4. 2-[4-(6-Bromo-2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-2-cyano-N-phenyl-thioacetamide (8)
Acetonitril derivative 2 (3.47 g, 10 mmol), phenyl isothiocyanate (1.37 g, 10 mmol) and KOH (0.56 g, 10 mmol) were ground with a pestle in a mortar for 15 min. The resulting mixture was poured onto water and acidified with conc. HCl. The product was collected, washed with water, dried and recrystallized from acetic acid to give thioanilide 8.
Brown powder; Yield 90%; m.p. 250–252°C; 1H NMR (DMSO-d6) δ: 7.02–8.01 (m, 9H, Ar-H), 8.78, 8.94 (2s, 2H), 11.42 (s, NH). Anal. calcd. for C21H12BrN3O2S2 (482.37): C, 52.29; H, 2.51; Br, 16.56; N, 8.71; S, 13.29%. Found: C, 52.30; H, 2.60; Br, 16.66; N, 8.80; S, 13.37%.
4.1.4. Synthesis of compounds 9–11
A mixture of thioanilid 8 (0.482 g, 1 mmol), the appropriate of C-ethoxycarbonyl-N-phenyl hydrazonoyl chloride, 6-bromo(2-bromoacetylcoumarin) or ethyl chloroacetate (1 mmol) and KOH (0.28 g, 5 mmol) were ground with a pestle in a mortar for 15 min. The solid was collected, washed with water, dried and recrystallized from suitable solvent furnishing 9–11, respectively.
4.1.4.1. 5-{[4-(6-Bromo-2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-cyano-methylene}-4-phenyl-4,5-dihydro-[1,3,4]thiadiazole-2-carboxylic acid ethyl ester (9)
Red powder (DMF); Yield 80%; m.p. >300°C; 1H NMR (DMSO-d6) δ: 1.42 (t, 3H, J = 7.12 Hz), 4.43 (q, 2H, J = 7.08 Hz,), 6.33–6.39 (m, 1H, Ar-H), 7.08–8.24 (m, 7H, Ar-H), 8.30 (s, 1H), 8.58 (s, 1H); 13C NMR (DMSO-d6) δ: 9.10 (CH3), 66.81 (CH2), 118.72, 121.22, 124. 28, 129.11, 129.82, 130.7, 131.21(Ar-C), 152.16, 158.4 (2C=O). Anal. calcd. for C25H15BrN4O4S2 (579.44): C, 51.82; H, 2.61; Br, 13.79; N, 9.67; S, 11.07%. Found C, 51.92; H, 2.75; Br, 13.91; N, 9.77; S, 11.20%.
4.1.4.2. 3-Amino-4-(4-(6-bromo-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-5-(phenylamino)-2-(6-bromo-2-oxo-2H-chromen-3-yl-carbonyl) thiophene (10)
Yellow powder (DMF); Yield 85%; m.p. >300°C; 1H NMR (DMSO-d6) δ 7. 20 (d, 1H, J = 7.8 Hz), 7.50–7.55 (m, 9H, Ar-H), 7.64 (d, 1H, J = 8 Hz, Ar-H), 7.85 (s, 1H), 8.06 (s, 1H), 8.48 (s, 1H), 8.75 (s, NH), 11.62 ppm (s, NH2). Anal. calcd. for C32H17Br2N3O5S2 (747.43): C, 51.42; H, 2.29; Br, 21.38; N, 5.62; S, 8.58%. Found: C, 51.50; H, 2.20; Br, 21.30; N, 5.70; S, 8.68%.
4.1.4.3. {2-[4-(6-Bromo-2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-2-cyano-1-phenylamino-vinylsulfanyl}-acetic acid ethyl ester (11)
Green powder (dioxane); Yield 88%; m.p. 234–235°C; 1H NMR (DMSO-d6) δ: 1.11 (t, 3H, J = 7.2 Hz), 3.66 (s, 2H), 4.02 (q, 2H, J = 6.8 Hz), 7.14 (t, 1H, J = 7.2 Hz, Ar-H), 7.35 (t, 2H J = 8 Hz, Ar-H), 7.41–7.44 (m, 3H, Ar-H), 7.76, 7.79 (dd, 1H, J = 2.4, 2.4 Hz, Ar-H), 8.00 (d, 1H, J = 2 Hz, Ar-H), 8.30 (s, 1H), 8.48 (s, 1H), 11.23 (s, NH); 13C NMR (DMSO-d6) δ: 14.98 (CH3), 66.81 (2CH2), 118.21, 122.29, 125.11, 130.01, 131.48, 134.76, 138.79, 152.16 (Ar-C), 158.77, 160.55 (2C=O). Anal. calcd. for C25H18BrN3O4S2 (568.46): C, 52.82; H, 3.19; Br, 14.06; N, 7.39; S, 11.28%. Found: C, 52.92; H, 3.31; Br, 14.20; N, 7.27; S, 11.40%.
4.1.4.4. Ethyl-3-amino-4-(4-(6-bromo-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-5-(phenylamino)-thiophene-2-carboxylate (12)
To a solution of compound 11 (0.568 g, 1 mmol) in 15 ml ethanol, two drops of piperidine were added and the mixture was boiled under reflux for 1 h. The yellow solid was collected and recrystallized from DMF giving 12. Yellow powder; Yield 87%; m.p. >300°C; 1H NMR (DMSO-d6) δ: 1.24 (t, 3H, J = 7.2 Hz), 4.18 (q, 2H, J = 6.8 Hz), 7.02 (t, 1H, J = 7.2 Hz, Ar-H), 7.20 (d, 2H, J = 7.6, Ar-H), 7.33 (t, 2H, J = 8 Hz, Ar-H), 7.44 (s, 1H), 7.47 (br, NH2), 7.78, 7.81 (dd, 1H, J = 2.4, 2.4 Hz, Ar-H), 8.22 (d, 1H, J = 2.4 Hz, Ar-H), 8.45 (s, 1H), 8.88 (s, 1H), 9.40 (s, NH); 13C NMR (DMSO-d6) δ: 14.30 (CH3), 61.90 (CH2), 116.74, 118.60, 119.72, 121.22, 122.29, 125.11, 129.82, 131.21, 134.69, 138.69, 139.19, 152.16 (Ar-C), 165.81, 168.89 (2C=O). Anal. calcd. for C25H18BrN3O4S2 (568.46): C, 52.82; H, 3.19; Br, 14.06; N, 7.39; S, 11.28%. Found: C, 52.90; H, 3.10; Br, 14.16; N, 7.47; S, 11.20%.
4.1.4.5. 2-(4-(6-Bromo-2-oxo-2H-chromen-3-yl)thiazol-2-yl)-3-(methylthio)-3-(phenylamino)-acrylonitrile (13)
Equimolar amounts of thioanilide 8 (0.482 g, 1 mmol), phenyl isothiocyanate (0.137 g, 1 mmol), and KOH (0.28 g, 5 mmol) in N,N-dimethylformamide (15 mL) were stirred for 2 h at room temperature. Methyl iodide (0.71 g, 0.32 mL) was added with stirring. The resulting solid was collected and recrystallized from dioxane to give 13.
Yellow powder; Yield 89%; m.p. 245–246°C; 1H NMR (DMSO-d6) δ 2.29 (s, SCH3), 7.21 (s, 1H), 7.40–7.53 (4H, ArH’s), 7.76 (d, 2H, J = 8 Hz, Ar-H), 7.96 (s, 1H), 8.28 (s, 1H), 8.52 (s, 1H), 11.50 (s, NH). Anal. calcd. for C22H14BrN3O2S2 (496.40): C, 53.23; H, 2.84; Br, 16.10; N, 8.46; S, 12.92%. Found: 53.30; H, 2.90; Br, 16.20; N, 8.56; S, 12.82%.
4.2. Antimicrobial activity
Evaluation of the antimicrobial activity of the prepared compounds was performed against four bacteria and two fungal species using the agar plate diffusion method (Citation46). Penicillin G and ciprofloxacin were used as reference drugs for gram-positive and gram-negative bacteria, while ketoconazole was used as a standard antifungal drug.
4.3. Evaluation of MIC, MBC and MFC
The minimum inhibitory concentrations (MICs), the minimum bactericidal concentrations (MBCs) and the minimum fungicidal concentrations (MFCs) were determined using the microdilution method (broth dilution) (Citation47).
5. Conclusions
In this paper, we reported a green route for the synthesis of novel coumarins bearing thiazoles or 1,3,4-H thiadiazoles via grinding method. 6-Bromo-3-(2-bromoacetyl)-2-chromen-2-one (1) was treated with cyanothioacetamide to produce thiazole-2-acetonitrile derivative 2. The latter was used as a starting material for synthesis of coumarins, 1,3,4-thiadiazole and thiophene derivative. All the newly prepared coumarins were screened against two (G+), two (G−) bacteria and two fungal species. The results indicated that, compounds 7 and 12 were the most effective against B. pumilis, while compound 4b was highly active against S. faecalis. Compound 5b was highly active against E. cloacae and compounds 2 and 7 displayed activity against fungi S. cerevisiae higher than reference drug ketoconazole.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rubab, L.; Anum, A.; Al-Hussain, S.A., Irfan, A.; Ahmed, S.; Ullah, S.; Al-Mutairi A.A. and Zaki, M.E.A. Catalysts 2022, 12, 1329. https://doi.org/10.3390/catal12111329
- Abdel-Aziem, A. Int. J. Pharm. Pharm. Sci. 2015, 7 (1), 61.
- Abdel-Aziem, A.; Rashdan, H.R.M.; Ahmed, E.M.; Shabaan, S.N. Green Chem. Lett. Rev. 2019, 12 (1), 9.
- Harish, K.K.; Nesaragi, A.R.; Kalagatur, N.K., Naik, P.; Madegowda, M.; Pandith, A.; Dahlous, K.A.; Mohammad, S.; Shivarudrappa, H.P.; Sharanakumar, T.M. and Guddappa, H. J. Photochem. Photobiol. A Chem. 2024, 452, 115565.
- Nesaragi, A.R.; Gasti, T.; Metre, T.V., Anand, A.; Kamble, R.R.; Chougale, R.B. and Keri, R.S. Chem. Sel. 2022, 7, e202200604. https://doi.org/10.1002/slct.202200604
- Nesaragi, A.R.; Kamble, R.R.; Dixit, S., Kodasi, B.; Hoolageri, S.R.; Bayannavar, P.K.; Dasappa, J.P.; Vootla, S.; Joshi, S.D. and Kumbar, V.M. Bioorg. Med. Chem. Lett. 2021, 43, 128112.
- Leitch, J.A.; Smallman, H.R.; and Browne, D.L.,. J. Org. Chem. 2021, 86, 14095–14101.
- Geib, R.; Colacino, E.; Gremaud, L. ChemSusChem. 2024, e202301921.
- Abdel-Aziem, A.; El-Sawy, E.R.; Kirsh, G. Polycycl. Arom. Comp. 2020, 42(5), 2617–2631. https://doi.org/10.1080/10406638.2020.1848890.
- Guerra, A.R.; Duarte, M.F.; Duarte, I.F. J. Agric. Food Chem. 2018, 66, 10663.
- Molnar, M.; Loncˇaric, M.; Kovacˇ, M. Curr. Org. Chem. 2020, 24, 4–43.
- Mishra, S.; Pandey, A.; Manvati, S. Heliyon 2020, 6, e03217.
- Hwu, J.R.; Kapoor, M.; Gupta, N.K., Tsay, S.; Huang, W.; Tan, K.; Hu, Y.; Lyssen, P. and Neyts, J. Eur. J. Med. Chem. 2022, 232, 114164.
- Chattopadhyay, D.S.K. Int. J. Res. Anal. Rev. 2018, 5, 11.
- Abdel-Aziem, A.; Baaiu, B.S.; Elbazzar, A.W.; Elabbar, F. Synth. Commun. 2020, 50 (16), 2522–2530.
- Abdel-Aziem, A.; Baaiu, B.S.; El-Sawy, E.R. Polycycl. Arom. Comp. 2022, 42, 4809–4818. https://doi.org/10.1080/10406638.2021.1916543
- Abdel-Aziem, A. J. Heterocycl. Chem. 2015, 52, 251–259.
- Nesaragi, A.R.; Algethami, J.S.; Alsaiari, M., Alsareii, S.A.; Mathada, B.S.; Ningaiah, S.; Sasidhar, B.S.; Harraz, F.A. and Patil, S.A.. J. Mol. Struct. 2024, 1302, 137478.
- Yerer, M.B.; Dayan, S.; Han, M.I.; Sharma, A.; Tuli, H.S.; Sak, K. Anticancer Agents Med. Chem. 2020, 20 (15), 1797–1816.
- Bhattara, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Mini Rev. Med. Chem. 2021, 21, 2996–3029. https://doi.org/10.2174/1389557521666210405160323
- Abdel-Aziem, A.; Abdelhamid, A.O. Polycycl. Arom. Comp. 2022, 42, 7310–7320. https://doi.org/10.1080/10406638.2021.1998152
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.L.; Malak, L.G.; Khallaf, I.S.A.; Bringmann, G.; Farag, S.F. RSC Adv. 2021, 11, 16970–16979.
- Basappa, V.C.; Penubolu, S.; Achutha, D.K.; Kariyappa, A.K. J. Chem. Sci. 2021, 133, 55.
- Kecel-Gunduz, S.; Budama-Kilinc, Y.; Bicak, B., Gok, B.; Belmen, B.; Aydogan, F. and Yalacon, C. Arab. J. Chem. 2023, 16 (2), 104440.
- Couttolenc, A.; Díaz-Porras, Á; Espinoza, C.; Medina, M.E.; Trigos, Á. J. Phys. Org. Chem. 2020, 33 (1), 1.
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Fitoterapia 2021, 150, 104863.
- Tao, L.; Zhuo, Y.-T.; Qiao, Z.-H.; Li, J.; Tang, H.-X.; Yu, Q.-M.; Liu, Y.-Y.; Liu, Y.-P. Nat. Prod. Res. 2022, 36 (10), 2526–2533.
- Hananya, N.; Shabat, D. Angew. Chem. Int. Ed. 2017, 56, 16454–16463.
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Molecules 2021, 26, 3166.
- Borcea, A.-M.; Ionut, I.; Crisan, O.; Oniga, O. Molecules 2021, 26, 624.
- Scott, K.A.; Njardarson, J.T. Top. Curr. Chem. (Z) 2018, 376 (1), 5.
- El-Naggar, A.M.; El-Hashash E, M.A.; Elkaeed, B. Bioorg. Chem. 2021, 108, 104615.
- Shabaan, S.N.; Baaiu, B.S.; Abdel-Aziem, A.; Abdel-Aziz, M.S. Green Chem. Lett. Rev. 2021, 14 (4), 679–688.
- Mishra, I.; Mishra, R.; Mujwar, S.; Chandra, P.; Sachan, N. J. Heterocycl. Chem. 2020, 57, 2304.
- Singh, I.P.; Gupta, S.; Kumar, S. Med. Chem. 2020, 16, 4.
- Abu-Melha, S.; Edrees, M.M.; Riyadh, S.M.; Abdelaziz, M.R.; Elfiky, A.A.; Gomha, S.M. Molecules 2020, 25, 4565.
- Liaras, K.; Fesatidou, M.; Geronikaki, A. Molecules 2018, 23, 685.
- Abdelall, E.K.A.; Kamel, G.M. Eur. J. Med. Chem. 2016, 118, 250–258.
- Wang, G.; Peng, Z.; Gong, Z.; Li, Y. Bioorg. Chem. 2018, 78, 195–200.
- Sowjanya, C.; Seetaram, S.S.; Gomathi, S.; Ashok, B.K. Int. J. Adv. Res. Med. Pharm. Sci. 2016, 1, 6.
- Sagar, S.R.; Singh, D.P.; Das, R.D.; Panchal, N.B.; Sudarsanam, V.; Nivsarkar, M.; Vasu, K.K. Bioorg. Chem. 2019, 89, 102992.
- Sahin, Z.; Ertas, M.; Bender, C.; Bülbül, E.F.; Berk, B.; Biltekin, S.N.; Yurttas, L.; Demirayak, S. Drug Dev. Res. 2018, 79, 406–425.
- Abdel-Aziem, A.; Abdelhamid, A.O. Int. J. Adv. Res. 2013, 1, 717.
- Shabaan, S.N.; Baaiu, B.S.; Abdel-Aziem, A.; Abdel-Aziz, M.S. Green Chem. Lett. Rev. 2021, 14, 679–688.
- Vijesh, A.M.; Isloor, A.M.; Prabhu, V.; Ahmad, S.; Malladi, S. Eur. J. Med. Chem. 2010, 45 (11), 5460–5464.
- Bauer, A.W.; Kirby, W.W.M.; Sherris, J.C.; Turck, M. Am. J. Clin. Pathology 1966, 45, 493–496.
- Rodriguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; Richardson, M.; Verweij, P.E. Clin. Microbiol. Infect. 2003, 9 (8), i–viii.