?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Novel bio-based electrode polyurethane (PU) fabricated using various conducting materials such as graphene oxide (GO), gold (Au), and carbon nanotube (CNT) as the primary source of a charge carrier. Initially, PU/GO, PU/Au, and PU/CNT polymers were synthesized using a pre-polymerization approach. Therefore, the application of conductive materials modifies and increases the conductivity of PU. The connection between PU and the conductive materials (GO, Au, and CNT) was identified using Fourier transform infrared (FTIR), whereby some important functional groups obtained in this spectrum investigation, such as the presence of amide (-NH) and carbonyl urethane(-C = O), following the absence of diisocyanate groups (-NCO) revealed the synthesis was satisfactory. The glass transition temperatures (Tg) of PU/GO, PU/Au, and PU/CNT were analyzed using differential scanning calorimetry (DSC). In contrast, the mass loss during the heat treatment was observed using thermogravimetric analysis (TGA). Furthermore, thermal studies have proven that PU/GO has higher thermal stability compared to PU/Au and PU/CNT. The field emission scanning electron microscopy (FESEM) presents satisfactory results between PU composites and pure PU. The conductivity of PU and PU composites was examined using electrochemical impedance spectroscopy (EIS) and showed that PU/GO has a better electrical conductivity (σ) at 3.68 × 10−3 S cm−1.
1. Introduction
Among polymers employed in the industry (electrochemical devices, batteries, coating, biomedical, etc.), polyurethanes (PUs) are among the most appealing polymers. PUs have better resistance, biocompatibility, and mechanical properties than other polymers (Citation1). Furthermore, applying PUs in numerous materials is well-established (Citation2). The characteristics of PU allow for the tailoring of a broad range of particular properties, yet owing to the weak conductivity of PU, several drawbacks occur during PU performance. However, the weakness of PU can be modified by augmenting the conductive substances, namely, metal nanoparticles and carbon-based materials (Citation3).
Researchers have made numerous attempts to increase the thermal and mechanical strength and chemical properties, and the essential part is the conductivity of PU (Citation4). Polyurethane (PU) has been employed in several industry polymers due to its chemical stability, satisfactory thermal, better mechanical strength, good elasticity, and easy production (Citation5). Furthermore, a good connection between conductive materials and PU increases the conductivity of PU (Citation6). Generally, PU is divided into two significant compounds: soft segments, which come from a polyol, and hard segments, which come from diisocyanate (Citation7). Complex components are applied to create an intermolecular hydrogen bonding between the complicated elements of urethane linkages. Furthermore, PU crystallinity can be determined based on the content of the hard segment (urethane group) (Citation8,Citation9). Numerous PU productions have been reported for preparing polymer electrolytes in developing electrochemical instruments (Citation10–12).
Currently, polymers can be generated from renewable origins. Specifically, plant oils, as well as palm kernel oil (PKO), jatropha oil, soybean, and rapeseed, have triggered many researchers to use them owing to their advantages such as price, not being harmful to the environment, can be obtained easily in colossal amounts (Citation13–15). These plant oils have attracted researchers and factories to use these materials to produce green bio-polyurethane. Bio-based polyurethane has been reported for various applications such as adhesives, composite applications, electrolytes, and coating (Citation16–18).
Various industrial polymers have been produced, such as polyacetal (PA), polysulfone (PS), polylactic acid (PLA), polyethylene (PE), polyglycolic acid (PGA), polymethylmethacrylate (PMMA) and polyurethane (PU) (Citation19,Citation20). For decades, PU has become the most famous polymer applied in the world. PUs have been used widely in many areas, such as the coating industry, sensor development, construction materials, and biomedical applications (Citation20). Urethane is the main compound in the PU structure due to the reaction between isocyanates (-NCOs) attacked by the hydroxyl (-OH) groups. PU has many functional groups, such as urea, ether, ester, and aromatic compounds (Citation22).
Several methods have been reported to invent PU, such as via pre-polymerization, quasipolymerization, and single-step polymerization. They all have different approaches where the single-step polymerization is applied when the diisocyanate, polyol, and catalyst are combined, adding solvent and chain extender (polyethylene glycol). In contrast, the pre-polymerization technique has two imperative actions. The first action uses urethane production via the reaction between diisocyanate and polyol, and the last action is the addition of the chain extender, where the urethane compound reacts with diol or water to form PU. A reaction between diisocyanate and polyol with a higher content of free isocyanate at 15–30% in the urethane prepolymer is called the quasipolymerization approach (Citation23,Citation24).
The PU has been produced and validated by Munir et al. (Citation25) using palm kernel oil (PKO) as the monoester polyol. The polymer was also obtained using the pre-polymerization method. Nevertheless, the challenge of this study was to improve the conductivity of PU so it could be utilized to develop an electrochemical device for subsequent research. This study will apply several conducting materials, such as graphene oxide (GO), gold (Au), and carbon nanotube (CNT), to the PU film. Furthermore, the findings observed by previous studies showed that effort must be made to find the best-conducting material to improve PU conductivity. Therefore, this study aims to develop and evaluate several conducting materials, as mentioned before. The present study analyzed the conductivity, physical, thermal, and polymer structure using several instruments such as differential scanning calorimetry (DSC), thermogravimetry analysis (TGA), field emission scanning electron microscopy (FESEM), Fourier transform infrared spectrometer (FTIR) to investigate the major functional groups, whereas the application of electrochemical impedance spectroscopy (EIS) to examine the conductivity of materials applied.
2. Experimental procedures
2.1. Materials applied
The palm kernel oil–based monoester polyol (PKO-p) was obtained from PT. Chemie Mitra Indonesia, where the PKO-p has an OHV of about 370 mg KOH/g and a moisture content of less than 0.1%. The polymeric isocyanate of 4,4-diphenylmethane diisocyanate (MDI) manufactured by Sanche was acquired from PT. Chemie Mitra Indonesia has an NCO content of 32%. Several chemicals used in this study were purchased from Sigma Aldrich Indonesia, such as graphene oxide (GO), gold (Au), carbon nanotube (CNT), technical grade acetone (≥99.5%), and polyethylene glycol (PEG) of 400 Da molecular weight. The Group of Chemistry, Universitas Kebangsaan Malaysia, successfully validated and developed the polyurethane film applied in this work. The polymer has three significant components: MDI, PKO-p, and PEG 400. All chemicals with analytical grades were employed during experiments. Solutions were obtained using deionized water and stored at 4°C.
2.2. Fabrication of PU with various conducting materials
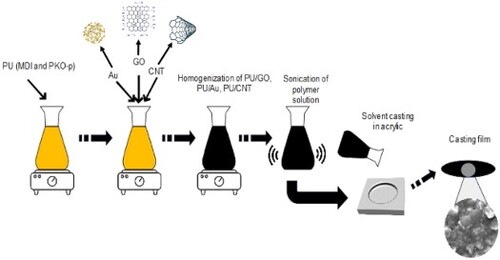
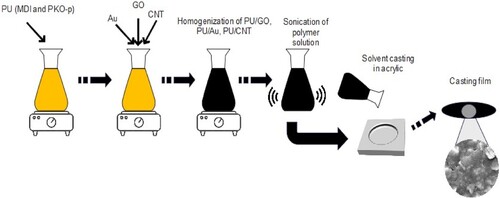
The homogeneous and transparent solutions of PU were prepared according to Munir et al. (Citation22) in a mixture of MDI, PKO-p, and PEG 400, whereby the ratio was 100: 100: 40, grams, respectively. Afterward, the GO, Au, and CNT with the various levels (1, 2, 5, and 10 wt%, respectively) were added to the PU solution separately and dispersed ultrasonically for one hour to prepare the PU/GO, PU/Au, and PU/CNT solutions. All these different sonicated solutions were immediately cast at ambient temperature. The casting films were fabricated using PU/GO, PU/Au, and PU/CNT composite solution with a transparent acrylic plate and stored in a vacuum oven at 60°C for three hours to ensure solvent removal, as shown in .
2.3. Chemical, physical characterization, and conductivity study of PU/GO, PU/Au, and PU/CNT composites
Before testing and analysis, the films were conditioned for 24 h at ambient conditions. Only cured samples were analyzed. The characterization of pure PU and PU composites was conducted using several approaches. The thermal properties were measured using a TGA and DSC analyzer, whereas the FTIR spectroscopy was used to identify the presence of some important functional groups. The FESEM was applied to study the PU film surface. Unless otherwise indicated, all analyses and tests were performed at room temperature (23°C ± 2°C). Furthermore, the surface morphology of the polymer composites was investigated using a Field-emission scanning electron microscope (FESEM, JSM-6510 LA) equipped with Energy Dispersion X-ray (EDX) spectroscopy. The thermal properties of polymers were studied using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).
The TGA analysis was employed using the Perkin Elmer Pyris, where 5 mg of sample was weighed, and the temperature applied was 35°C until 800°C with a temperature rate of 15°C/min under an N2 gas (50 mL min−1). The DSC analysis was at a heating rate of 10°C/min from 25°C until 200°C, on a DSC-60 Plus Shimadzu. The procedure of DSC was as follows: 5 mg of polymers scaled and sealed. The samples were heated from 25°C to 150°C for 1 min, rapidly cooled from 150°C to −100°C for 1 min, and then heated to 200°C at 10°C/min. The alteration of heat capacity referred to the inflection point chosen as glass transition temperature (Tg). The chemical structures of polymer composites were identified using FTIR (Thermo Scientific Nicolet iS10 using Diamond Attenuation Total Reflectance (DATR)). Several important functional groups should be investigated to verify whether the PU synthesis works or vice versa, such as -NH, -CH, -NCO, and -C = O.
Furthermore, the electrochemical impedance spectroscopy (EIS) analysis was studied using an Electrochemical Workstation (Sebelas Maret University, Indonesia). The conductivity study used three electrodes: the PU/GO, PU/Au, and PU/CNT as working electrodes (1 cm2 exposure area), Ag/AgCl applied as a reference electrode, and platinum wire performed as an auxiliary electrode. The experiment was conducted at ambient temperature. The EIS analysis was done to study the resistance and conductivity of each polymer composite. The EIS detection was performed at open circuit potential (OCP). A Potentiostat instrument was applied for the EIS detection at OCP, and the frequency was selected between 10 mHz to 10 kHz with 10 mV amplitude (peak to zero). The Zview Analyzer Software was applied to fit the electrochemical outcomes from the EIS test. The OCP data of each sample was investigated during 24 h of immersion in the test electrolyte.
3. Results and discussions
The investigation of the chemical structure of polyurethane and polyurethane with other conducting materials confirmed that the polymer synthesis was successfully synthesized, as shown in . Based on their study, Ramezani and Monroe (Citation26) reported that polyols (palm kernel oil and PEG) must react with the NCO in MDI to produce urethane polymers. Moreover, according to , the traces of -NCO in this spectrum cannot be found at ∼2265 cm−1, denoting complete synthesis between hydroxyl groups of polyols with isocyanate groups of MDIs. Numerous functional groups in these polymer composites by GO, Au, and CNT were verified. There were countless functional groups, such as C = O and NH formation of the PU in addition to OH, while the NCO depletion was also investigated.
Figure 2. Characterization of polymer films such as polyurethane (PU), polyurethane/gold (PU/Au), polyurethane/graphene oxide (PU/GO), and polyurethane/carbon nanotube (PU/CNT).
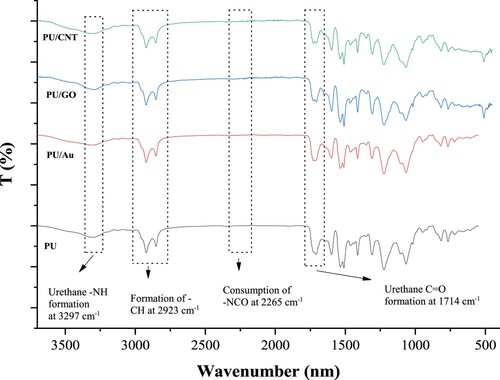
The functional groups of amide (-NH) and carbonyl urethane (-C = O) are obtained in this spectrum. All polymers have similar wavenumber at ∼3297 cm−1 and ∼1714 cm−1, respectively. The wavenumber shows around ∼1712 cm−1, indicating the functional group of carbonyl urethane (Citation27). The urea peak at ∼1690 cm−1 also did not form during the polymer synthesis, signifying that the urea as the by-product of the polymerization synthesis did not exist (Citation28).
The wavenumber at ∼3297 cm−1 in this spectrum denoted the formation of -NH in urethane formation, whereas a similar wavenumber around ∼3442 cm−1 reported by Mendiburu-Valor et al. (Citation24) indicating the presence of -NH functional group. Several atoms in MDI that act as hard segments, such as O, N, and H, can form dipoles owing to their powerful electrostatics that will react with other atoms (Citation29).
The urethane polyurethane formed from nucleophilic substitution, where the amine acts as a nucleophile. Furthermore, the amine attacked the isocyanate (-N–C = O) of MDI. Different nucleophilic substitution occurred during the reaction between atom O of PEG, acts as a nucleophile, and struck the -NCO of MDI. Nevertheless, atom N in palm kernel oil (PKO) is more electropositive than atom O in PEG, causing the amine to be more reactive with the isocyanate compared to the hydroxyl group of the PEG (Citation30). Thus, the alkalinity of amine causes the functional group to react with atom C in the -NCO of MDI (Citation31). Some studies reported that the polyol compound is more reactive than PEG during the synthesis with the MDI. Furthermore, the intermediate complex can be formed due to the stabilization of polyol, even if it has a very long chain. However, the PEG application increases the PU chain length and avoids the by-products that occur, such as the formation of water and urea (Citation29).
According to , there are no significant distinctions of peaks in their spectrums. This happens because the conductive substances used in this study did not irritate the peak of PU, although the particles applied were entrapped inside the PU pores. The pores in the PU are formed due to the application of acetone as a solvent during the combination of MDI, PKO-p, and PEG 400; once all materials are spined into the homogenous solution, the acetone diffuses into the fibers. It causes the fiber to shrink and become porous after the acetone evaporates during the drying process (Citation33).
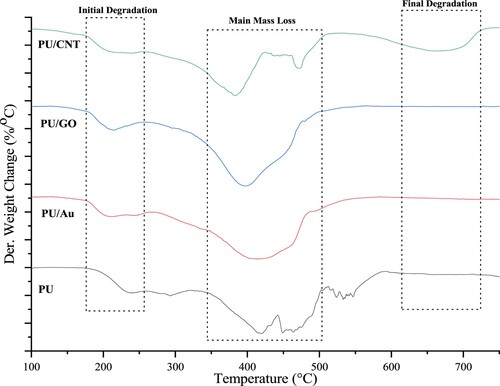
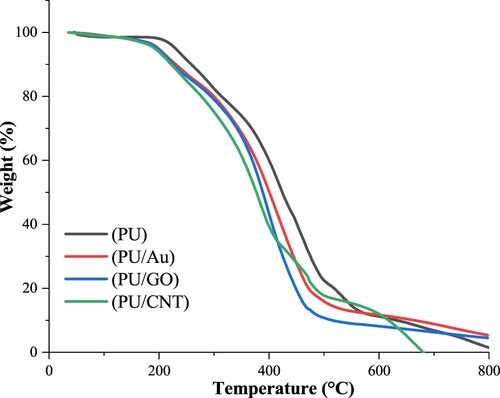
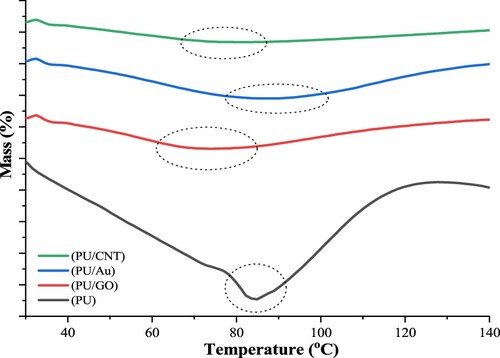
Many studies have reported that PU has various properties regarding its behavior and stability. The TGA analysis was applied to study the thermal resistance of PU, PU/Au, PU/GO, and PU/CNT. and display the DTG and TG curves of a series of polymers, respectively. lists the thermal decomposition temperatures of PU, PU/Au, PU/GO, and PU/CNT acquired from TGA. Tmax is the temperature at the maximum rate of thermal degradation, and Ti is the first degradation temperature (Citation33,Citation34).
Table 1. TGA degradation of PU, PU/Au, PU/GO, and PU/CNT.
According to , two significant steps occurred during the thermal degradation of polyurethane. The initial degradation begins at 242°C with ±3% mass loss, related to the preliminary decomposition of PU, namely the breakdown of branched-chain and terminal groups. Furthermore, the separation of PU macromolecules occurred at 416–500°C, causing the significant mass loss of PU. Likewise, the leading crosslinking network of polyurethane and the macromolecule segments are mostly decomposed. Moreover, the carbon residue of the polyurethane was 18.3%.
Furthermore, the initial decomposition of PU combined with various conducting materials has started below 220°C; nevertheless, the primary loss of PU/Au, PU/GO, and PU/CNT is at 415.11, 400.01°C, and 382.51°C, with the carbon residue at 7.2, 5.2, and 6.2%, respectively.
After the heat temperature is approximately 200°C, the degradation process of PU/Au and PU/GO only remains one major mass loss step, whereas, for PU/CNT, there are two significant mass loss steps. Applying CNT as a composite polymer causes the mass loss to increase by several steps. According to , it can be seen that the final mass loss occurs at 670°C. A study reported that the CNT application indicates that the polyurethane matrix increases in terms of thermal stability; thus, the combination of PU and CNT enhances the heat transfer rate and results in several degradation steps (Citation35).
FTIR, DTG, and TGA outcomes considered that the initial breakdown of PU and PU composites, such as degradation of macromolecules, took place approximately at 200°C. That makes the film structure easily destroyed, and (Citation33) reported that it causes the tensile properties of PU to decline. The subsequent analysis of PU and others using the DSC to study the glass transition temperature (Tg). displays the DSC graphs of PU, PU/GO, PU/Au, and PU/CNT.
During the heating treatment, shows that merely one specific peak can be referred to as the glass transition (Tg) when the temperature of a series of PUs is applied on DSC at 25–200°C. The glass transition temperatures on PU, PU/GO, PU/CNT, and PU/Au are 78.09, 81.4, 82.5, and 88.6°C, respectively. Furthermore, the DSC approach generally analyzes the glass transitions (Citation36).
According to the discussion about PU degradation, when a specific temperature is applied, it causes the degradation of the unstable part of PU. However, the unstable substances, namely the branched chain of PU and functional groups, are enormously flexible. Thus, when the specific temperature is applied, the flexible segments at lower Tg will degrade; nevertheless, the application of conducting materials causing the higher Tg obtained can be seen in the PU/Au curve. This occurs owing to an escalation of molecular motion inside the PU film with graphene as a conducting material. The purpose was to investigate not merely the impact of the curing conditions on the physical and mechanical properties of the PUs but also to measure the Tg that can intensify when the specific temperature is applied after the first low-temperature curing conditions. The Tg detection is a general technique to describe the mechanical properties and curing propagation: as curing advances, the Tg increases, whereas the molecular network mobility decreases or vice versa (Citation37).
The pure PU and PU composite morphologies were investigated using FESEM. The SEM micrograph images for the PU, PU/Au, PU/GO, and PU/CNT specimens at a magnification of 100× until 10,000× and 100 until 1 µm are presented in (a-l), respectively. After adding various conducting materials, the figures of PU reveal different morphological characteristics compared to pure PU. (j-l) presents the conducting materials applied in this study: GO, Au, and CNT.
Figure 6. FESEM images of PU, PU/GO, PU/Au, and PU/CNT with various magnifications. (a) PU with 100×, (b) PU/Au with 100×, (c) PU/GO with 100×, (d) PU/CNT with 100×, (e), PU with 5000×, (f) PU/Au with 5000×, (g) PU/GO with 5000×, (h) PU/CNT with 5000×, (i) PU with 10,000×, (j) PU/Au with 10,000×, (k) PU/GO with 10,000× and (l) PU/CNT with 10,000×.
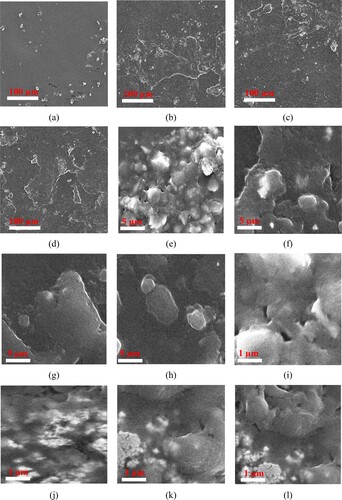
(j-l) illustrates the fragility of the film owing to the conductive particles clinging inside the PU, which is related to the smooth surface properties after the addition of conducting materials. Furthermore, pure PU ((a)) has a uniformly smooth surface morphology compared to other PU composites since pure PU has more ductile properties than PU composites.
The conductivity of PU/Au, PU/CNT, and PU/GO was measured by the alternating current (ac) EIS technique, as presented in . The frequency of the EIS method ranged from 1 Hz to 10 MHz, 100 mV amplitude (25°C, 1 atm). The PU and PU composites were analyzed using impedance spectroscopy in PBS (0.01 mol·L−1) at pH 7. The Zviewer Analyzer Software was applied to validate the circuit model of EIS data. Furthermore, the electrical conductivity (σ) of PU composites was estimated using EquationEquation 1(1)
(1) .
(1)
(1) Rb is the bulk resistance obtained from the equivalent circuit analysis; the polymer film thickness (l, 0.1 mm) was estimated using the thickness gauge calipers, and A is the contact area of the thin film, approximately 1 cm2.
Figure 7. Spectra of PU and PU composites impedance in PBS (0.01 mol L−1) at pH 7 and each polymer's electrical conductivity (σ).
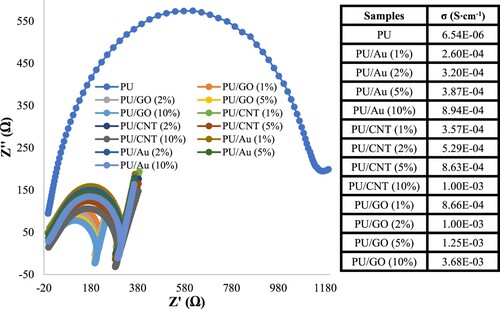
Based on , each sample has a different bulk resistance (Rb) value after being plotted by the Zviewer Software. Several studies reported that the Rb value influences the electrical conductivity value if the film thickness and contact area are constant (Citation22,Citation38,Citation39). The lower the Rb value, the better the conductivity of the polymer. This is because the resistance increases and the current decreases, provided all other factors, such as the film thickness and surface contact, are kept constant. According to this study, the PU/GO with a concentration of 10% GO obtained the best electrical conductivity, at 3.68 × 10−3 S cm−1, compared to other materials and concentrations. Whereas PU can be considered to have a low conductivity, this is related to some studies that reported PUs are thermal insulation and insufficient to act as a conductive material, a specific porous structure causing the PU to have low conductivity (Citation40). Graphene oxide (GO) as a conducting material is commonly applied owing to graphene’s extraordinary properties, such as excellent electric and mechanical, compared to gold (Au) and carbon nanotube (CNT) (Citation41). Furthermore, the porosity of PU causes the conducting materials to be entrapped inside the PU film.
The electrochemical parameters were acquired from establishing the EIS data by fitting the best equivalent circuits using the Zview Analyzer Software presented in . In the circuits, several elements have been investigated, such as resistance (Rs), charge transfer resistance (Rct), coating resistance (Rc), constant phase element of double layer (Qdl), and constant phase element of coating (Qc).
Table 2. The outcomes of the EIS data after fitting using the Zview Analyzer Software for PU and PU composites in the phosphate buffer solution.
In , Rc, Rct, CPEc and CPEdl are the resistance, capacitance, and constant phase element of the film modified that formed by the polyurethane composites (GO, Au, and CNT) with various concentrations. As shown in , all the semicircle diameters in Nyquist plots have been established using the Zview Analyzer Software; this also indicates the resistance bulk (Rb) for each sample, as shown in . Nevertheless, each modified polyurethane film gave various Rb values, which caused the variation of each sample's electrical conductivity (σ), as presented in . The change of the resistance value in this study is a consequence of the diffusion of aggressive solution and conducting materials beneath the polyurethane film that causes the formation of corrosion products at the coating interface, which can lead to the detachment of the coating layer. The coating disbondment may escalate the electrode surface area exposure to the electrolyte, reducing the Rct or charge transfer resistance.
4. Conclusions
A series of conducting materials, such as graphene oxide (GO), gold (Au), and carbon nanotubes (CNT), have been applied to polyurethane. Their chemical structure, thermal properties, morphological surface, and conductivity were compared. The polymerization approach has produced the synthesis of polymer with the conducting materials. We found that the FTIR spectrums of PU and PU composites were similar and concluded that the addition of materials did not influence the chemical structure of PU. The glass transition temperature of the PU and the composites prepared from other materials differed, with the PU/GO giving the higher glass transition temperature. According to the DTG curves, there were two phase separations of the complex and soft segments; the first loss at low temperatures was the hard segment, and the second loss was the soft segment that occurred at higher temperatures. The FESEM observed the adherence properties of the matrix with the conducting materials. The outcome showed satisfactory adhesion when using the conducting materials. The FESEM observation was applied to study the polyurethane's uniform Au, GO, and CNT dispersion. The EIS was performed to examine the conductivity of PU and PU composites and revealed that PU/GO 10% has the best electrical conductivity (σ).
Acknowledgments
The authors thank the Research Group of Solid State Chemistry and Catalysis, Chemistry Department, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, for providing research facilities and financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cho, D.H.; Cho, K.G.; An, S.; Kim, M.S.; Oh, H.W.; Yeo, J.; Yoo, W.C.; Hong, K.; Kim, M.; Lee, K.H. Self-healable, Stretchable, and Nonvolatile Solid Polymer Electrolytes for Sustainable Energy Storage and Sensing Applications. Energy Storage Mater. 2022, 45, 323–331.
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, Characterization, and Antioxidant Activity of a Novel Electroactive and Biodegradable Polyurethane for Cardiac Tissue Engineering Application. Mater. Sci. Eng. C 2014, 44, 24–37.
- Gu, W.; Li, F.; Liu, T.; Gong, S.; Gao, Q.; Li, J.; Fang, Z. Recyclable, Self-healing Solid Polymer Electrolytes by Soy Protein-based Dynamic Network. Adv. Sci. 2022, 9 (11), 2103623.
- Mohit, N.Y.; Hashmi, S.A. High Energy Density Solid-sate Supercapacitors Based on Porous Carbon Electrodes Derived from Pretreated Bio-waste Precursor Sugarcane Bagasse. J. Energy Storage 2022, 55, 105421.
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode Materials for Lithium-ion Batteries: A Review. Appl. Surf. Sci. Adv. 2022, 9, 100233.
- Zhang, W.; Zuo, H.; Cheng, Z.; Shi, Y.; Guo, Z.; Meng, N.; Thomas, A.; Liao, Y. Macroscale Conjugated Microporous Polymers: Controlling Versatile Functionalities Over Several Dimensions. Adv. Mater. 2022, 34 (18), 2104952.
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10 (1), 107017.
- Jamil, R.; Silvester, D.S. Ionic Liquid gel Polymer Electrolytes for Flexible Supercapacitors: Challenges and Prospects. Curr. Opin. Electrochem. 2022, 35, 101046.
- Yadav, D.; Karki, S.; Ingole, P.G. Current Advances and Opportunities in the Development of Nanofiltration (NF) Membranes in Wastewater Treatment, Water Desalination, Biotechnological and Pharmaceutical Applications. J. Environ. Chem. Eng. 2022, 10 (4), 108109.
- Guo, K.; Li, S.; Chen, G.; Wang, J.; Wang, J.; Wang, Y.; Xie, X.; Xue, Z. One-pot Synthesis of Polyester-Based Linear and Graft Copolymers for Solid Polymer Electrolytes. CCS Chem. 2022, 4 (9), 3134–3149.
- He, C.; Xu, P.; Zhang, X.; Long, W. The Synthetic Strategies, Photoluminescence Mechanisms and Promising Applications of Carbon Dots: Current State and Future Perspective. Carbon. N. Y. 2022, 186, 91–127.
- Li, Q.; Shen, H.X.; Liu, C.; Wang, C.F.; Zhu, L.; Chen, S. Advances in Frontal Polymerization Strategy: From Fundamentals to Applications. Prog. Polym. Sci. 2022, 127, 101514.
- Alfonso, A.T.; Sanchez, M.C.; Franco, J.M. A Review of the Sustainable Approaches in the Production of Bio-based Polyurethanes and their Applications in the Adhesive Field. J. Polym. Environ. 2020, 28, 749–774. doi:10.1007/s10924-020-01659-1.
- Carrico, C.S.; Fraga, T.; Carvalho, V.E.; Pasa, V.M.D. Polyurethane Foams for Thermal Insulation Uses Produced from Castor oil and Crude Glycerol Biopolyols. Molecules 2017, 22 (7), 1091–1104.
- Mustapa, S.R.; Aung, M.M.; Ahmad, A.; Mansor, A.; TianKhoon, L. Preparation and Characterization of Jatropha-oil-based Polyurethane as Non-aqueous Solid Polymer Electrolyte for Electrochemical Devices. Electrochim. Acta 2022, 222, 293–302.
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent Advances in the Conversion of Waste Cooking Oil into Value-added Products: A Review. Fuel 2022, 324, 124539.
- Gu, J.; Zhong, H.; Chen, Z.; Shi, J.; Gong, Z.; Yang, Y. Advances in Sulfide-based all-solid-state Lithium-sulfur Battery: Materials, Composite Electrodes and Electrochemo-mechanical Effects. Chem. Eng. J. 2023, 454, 139923.
- Vinodh, R.; Atchudan, R.; Kim, H.J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFc) Applications: A Critical Review. Polymers. (Basel) 2022, 14 (2), 300–321.
- Aziz, S.B.; Ali, F.; Anuar, H.; Ahamad, T.; Kareem, W.O.; Brza, M.A.; Kadir, M.F.Z.; Ali, O.A.A.; Saleh, D.I.; Asnawi, A.S.F.M.; Hadi, J.M.; Alshehri, S.M. Structural and Electrochemical Studies of Proton Conducting Biopolymer Blend Electrolytes Based on MC: Dextran for EDLC Device Application with High Energy Density. Alexandria Eng. J. 2022, 61 (5), 3985–3997.
- Mohanty, A.K.; Wu, F.; Micheca, R.; Hakkarainen, M.; Raquez, J.M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable Polymers. Nat. Rev. Methods Primers 2022, 2, 46.
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213.
- Munir, M.A.; Badri, K.H.; Heng, L.Y.; Inayatullah, A.; Nurinda, E.; Estiningsih, D.; Fatmawati, A.; Aprilia, V.; Syafitri, N. The Application of Polyurethane-LiClO4 to Modify Screen-Printed Electrodes Analyzing Histamine in Mackerel Using a Voltammetric Approach. ACS Omega 2022, 7 (7), 5982–5991.
- An, Z.W.; Xue, R.; Ye, K.; Zhao, H.; Liu, Y.; Li, P.; Chen, Z.M.; Huang, C.X.; Hu, G.H. Recent Advances in Self-healing Polyurethane Based on Dynamic Covalent Bonds Combined with Other Self-healing Methods. Nanoscale. 2023, 15 (14), 6505–6520.
- Ozsevinc, A.; Alkan, C. Ethylene Glycol Based Polyurethane Shell Microcapsules for Textile Applications Releasing Medicinal Lavender and Responding to Mechanical Stimuli. Colloids Surf., A 2022, 625, 129888.
- Munir, M.A.; Badri, K.H.; Heng, L.Y.; Inayatullah, A.; Badrul, H.A.; Emelda, E.; Dwinta, E.; Kusumawardani, N.; Wulandari, A.S.; Aprilia, V.; Supriyono, R.B.Y. Design and Synthesis of Conducting Polymer Bio-based Polyurethane Produced from Palm Kernel oil. Int. J. Polym. Sci. 2022, 2022, 6815187.
- Ramezani, M.; Monroe, M.B.B. Biostable Segmented Thermoplastic Polyurethane Shape Memory Polymers for Smart Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4 (3), 1956–1965.
- Mendiburu-Valor, E.; Calvo-Correas, T.; Martin, L.; Harismendy, I.; Pena-Rodriguez, C.; Eceiza, A. Synthesis and Characterization of Sustainable Polyurethanes from Renewable and Recycled Feedstocks. J. Cleaner Prod. 2023, 400, 136749.
- Pongmuksuwan, P.; Harnnarongchai, W. Synthesis and Characterization of Soft Polyurethane for Pressure Ulcer Prevention. Polym. Test. 2022, 112, 107634.
- Karunarathna, B.; Jayakody, R.S.; Karunanayake, L.; Govender, K.K. Computational Development and Validation of a Representative MDI-BDO-Based Polyurethane Hard Segment Model. J. Mol. Model. 2021, 27, 37.
- Lv, Z.; Tang, Y.; Dong, S.; Zhou, Q.; Cui, G. Polyurethane-based Polymer Electrolytes for Lithium Batteries: Advances and Perspectives. Chem. Eng. J. 2022, 430, 132659.
- Chen, H.; Chauhan, P.; Yan, N. “Barking” Up the Right Tree: Biorefinery from Waste Stream to Cyclic Carbonate with Immobilization of CO2 for Non-isocyanate Polyurethanes. Green Chem. 2020, 22 (20), 6874–6888.
- Heshmat, M.; Leven, M.; Linker, O.; Sebastian, M.; Gurtler, C.; Machat, M.R. A DFT-Metadynamics Study Disclosing key Properties of Ring-opening Polymerization Catalysts to Produce Polyethercarbonate Polyols from Cyclic Ethylene Carbonate as Part of an Emerging CCU Technology. Phys. Chem. Chem. Phys. 2023, 25 (30), 20485–20494.
- He, Y.; Wu, J.; Qiu, D.; Yu, Z. Experimental and Numerical Analyses of Thermal Failure of Rigid Polyurethane Foam. Mater. Chem. Phys. 2019, 233, 378–389.
- Santana, J.S.; Cardoso, E.S.; Triboni, E.R.; Politi, M.J. Polyureas Versatile Polymers for New Academic and Technological Applications. Polymers. (Basel) 2021, 13 (24), 4393.
- Pourmohammadi-Mahunaki, M.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Koosha, M.; Yazdi, M. Preparation of Polyurethane Composites Reinforced with Halloysite and Carbon Nanotubes. Polym. Compos. 2020, 42 (1), 450–461.
- Michel, M.; Ferrier, E. Effect of Curing Temperature Conditions on Glass Transition Temperature Values of Epoxy Polymer Used for Wet Lay-up Applications. Constr. Build. Mater. 2020, 231, 117206.
- Ferrier, E.; Agbossou, A. Temperature Effects on the Shear Capacity of External Bonded Fiber Reinforced Polymer on Concrete. Constr. Build. Mater. 2017, 152, 333–344.
- Bonomo, M.; Zarate, A.Y.S.; Fagiolari, L.; Damin, A.; Galliano, S.; Gerbaldi, C.; Bella, F.; Barolo, C. Unreported Resistance in Charge Transport Limits the Photoconversion Efficiency of Aqueous Dye-sensitised Solar Cells: An Electrochemical Impedance Spectroscopy Study. Mater. Today Sustainability 2023, 21, 100271.
- Wang, Y.; Zhao, G. A Comparative Study of Fractional-order Models for Lithium-ion Batteries Using Runge Kutta Optimizer and Electrochemical Impedance Spectroscopy. Control. Eng. Pract. 2023, 133, 105451.
- Tao, J.; Yang, F.; Wu, T.; Shi, J.; Zaho, H.B.; Rao, W. Thermal Insulation, Flame Retardancy, Smoke Suppression, and Reinforcement of Rigid Polyurethane Foam Enabled by Incorporating a P/Cu-Hybrid Silica Aerogel. Chem. Eng. J. 2023, 461, 142061.
- Afroj, S.; Tan, S.; Abdelkader, A.M.; Novoselov, K.S.; Karim, N. Highly Conductive, Scalable, and Machine Washable Graphene-based E-textiles for Multifunctional Wearable Electronic Applications. Adv. Funct. Mater. 2020, 30 (23), 1–10.