ABSTRACT
Worldwide, rhamnolipids have become the ‘green, eco-friendly’ alternative to traditional chemical surfactants. Rhamnolipids are widely applicable in a variety of industries, such as bioremediation, food, petroleum, and agriculture. The most capable producer of rhamnolipids is still Pseudomonas aeruginosa; nevertheless, due to its pathogenicity, large-scale production and application may pose safety and health risks. The antibacterial, antifungal, and antiviral characteristics of biosurfactants derived from bacteria make them particularly interesting for use in medicine and other biological settings. It is anticipated that the review will deepen our knowledge of rhamnolipid's effects on the environment and offer crucial insights to inform the expanding application of this intriguing chemical in remediation. One thing that appears to be neglected a lot is the biodegradation of rhamnolipids. Providing a thorough understanding of rhamnolipid biosynthesis and biodegradability is the aim of this review.
1. Introduction
Surfactants are an important class of chemical products with high capacity use in a great variety of industrial and household applications. In 2007, it was projected that they produced about 10 million tons annually (Citation1). Most of these surfactants come from petroleum and are made chemically. But the widespread adoption of environmentally friendly technologies has accelerated the hunt for naturally occurring biodegradable substances (Citation2). An estimated 13 million tonnes of surfactant compounds are produced annually worldwide for industrial application; the majority of these compounds are produced by the synthesis of organic compounds using petrochemicals (Citation3). The development of sustainable products has led to a rapid evolution of environmental legislation. This has motivated modern scholars to investigate natural resources for use in both home and industrial settings. Surface-active chemicals are among the many synthetic products that are used extensively around the world (Citation4). Biosurfactants are becoming more and more popular than chemical surfactants because of their versatility, environmental friendliness, ability to be produced on a large scale, selectivity, effectiveness in small quantities under harsh conditions, production from renewable sources, and possible uses in environmental protection (Citation5). As manufacturers create environmentally acceptable biosurfactants from various natural and sustainable sources, biosurfactants (BS) are currently gaining popularity as a viable substitute on the market. Early studies on hydrocarbon fermentation, beginning in the late 1960s, identified BS as extracellular amphiphilic molecules. Because of their excellent biodegradability and safety, BS were first identified as ‘alter-native surfactants’ when they were first discovered. The past ten years have seen the discovery of some special characteristics of BS, such as biological activities, which are completely absent from traditional chemical surfactants. As a result, BS are drawing more and more attention as multipurpose materials for the twenty-first century in a variety of industries (Citation6). Extreme conditions, such as variations in salinity, increased UV exposure, restricted food availability, and variations in temperature and pH, have been shown to enable marine microorganisms to produce biosurfactants (Citation7). Numerous marine bacterial species, typically found in oil-contaminated waters, have been shown to synthesize biosurfactants. These species include those in the genera Alteromonas, Halomonas, Pseudoalteromonas, and Alcanivorax (Citation8).
Glycolipids are the most common class of biosurfactants produced by bacteria, and the best-characterized subclass is called rhamnolipids (RLs) (Citation9). Industry perception of RLs as appealing substitutes for synthetically produced surfactant chemicals is growing, with uses in the food, pharmaceutical, home care, and petrochemical industries (Citation10). The annual growth rate indicates the widespread use and demand for biosurfactants across a range of global sectors. Because of its high purity and rhamnolipids functionality, the market for glycolipids is anticipated to expand at a faster compound annual growth rate among the other biosurfactants (Citation11). The primary barrier to the widespread use of biosurfactants is costs, since their synthesis can be up to 50 times expensive than that of chemically generated surfactants (Citation12). At present, commercial production of BioSs in bulk quantities is restricted as the wholesale total production cost is still very high (Citation13).
The aim of this review is to provide a comprehensive overview of the applications of rhamnolipids in various fields. The review focuses on the biosynthesis of rhamnolipids. The review explores the properties of biosurfactants and specifically the structure and properties of rhamnolipids and its biodegradability. It further discusses the biosynthesis of rhamnolipids and the microorganisms involved in the production. It includes information about nature and chemical structure of rhamnolipids. The review enumerates the difficulties facing rhamnolipid production research. Previous research, which has been published at different times, has concentrated on a specific area of bio-based rhamnolipid synthesis. Nonetheless, this review offers thorough information about advancements in the subjects covered under various sections. Researching the genetic control mechanisms and biosynthesis of rhamnolipid synthesis could aid in the creation of mutant strains with higher rhamnolipid production capacities. This review discusses more specifics in this aspect, which is uncommon.
2. Surfactants and biosurfactants
Surfactants are structurally amphiphilic organic compounds that consist of both hydrophilic and hydrophobic segments, or polar and hydrophilic heads and nonpolar hydrophobic tails. They exhibit a very noticeable inclination to diffuse and adsorb at the interfaces between liquids and solids, gases, and liquids (Citation14). Historically, petroleum or its derivatives have been used to manufacture the chemically derived surfactants. Because of these surfactants’ numerous uses in the food, pharmaceutical, textile, and oil industries, they have been widely used (Citation15). This is because surfactants have a unique property that allows them to lower surface tension between two fluid surfaces. However, because petroleum-based surfactants are effective at lowering surface tension, modern society has long ignored the negative impacts of these chemicals. The ecosystem will suffer greatly from these detrimental effects, and human health will suffer as well. Much research is being done to find more environmentally friendly and biobased biosurfactant solutions made from microbes in order to address these problems and replace the extensive use of petroleum-based surfactant (Citation16). Synthesized by plants (saponins), microorganisms (bacteria, actinobacteria, yeasts, and filamentous fungi), and higher organisms (bile salts), biosurfactants are an intriguing substitute for synthetic surfactants because they are nontoxic and biodegradable, improving surface active or bio-emulsifier molecules. They also exhibit antimicrobial, antitumor, and larvicidal or insecticidal potential (Citation17).
Biosurfactants have several advantages over chemical surfactants, such as low toxicity, biodegradability, and activity throughout a broad pH, salinity, and temperature range (Citation18). Biosurfactants are used in many industrial areas, much like synthetic surfactants already are used. Being less toxic, non-hazardous, environmentally friendly, and biodegradable gives biosurfactants various advantages over synthetic ones. Furthermore, they exhibit enhanced foaming characteristics and increased selectivity (Citation19). Benefits of biosurfactants’ versatility in terms of production and use are also present. By altering the organism's surroundings, microbes may produce biosurfactants with different structures and compositions. Therefore, by modifying the microorganism's habitat, the qualities of the biosurfactants it produces can be customized for a specific need (Citation20). Because of their versatile characteristics, biosurfactants have also found application in agriculture, primarily as a substitute for synthetic surfactants in pesticide and agrochemical formulations. This has facilitated the growth of ‘green chemistry’ in this industry in response to the need to lessen or eliminate harmful effects on the environment and public health resulting from the overuse of chemical compounds (Citation21).
3. Classification and chemical nature of biosurfactants
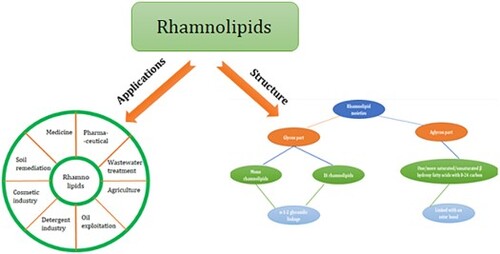
Based on their chemical makeup, biosurfactants are broadly divided into five major types.: as illustrated in .
Figure 1. Classification of biosurfactants with examples for each category (Citation22).
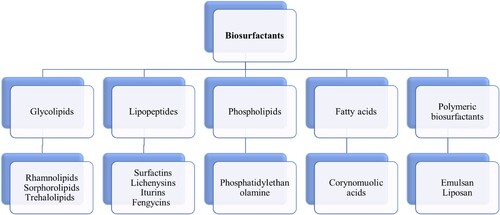
Glycolipids, lipopeptides and lipoproteins, phospholipids and fatty acids, polymeric surfactants, and particle surfactants are the different chemical categories into which biosurfactants are divided. The most well-known glycolipids include rhamnolipids, trehalolipids, and sophorolipids. Glycolipids are composed of various sugars connected by an ester group to linear or branched alkyl groups. The most well-known families of lipopeptides include surfactin, iturin, and fengysin. Lipopeptides are categorized as either cyclic or linear molecules and are made up of fatty acids coupled with peptide residues. High molecular weight biopolymers, such as lipoproteins, proteins, polysaccharides, lipopolysaccharides, or complex combinations of these substances, can be the building blocks of polymeric biosurfactants. Particulate biosurfactants form as vesicles on the extracellular membrane, forming a microemulsion that affects how well microbial cells absorb alkanes (Citation23). Most often, the chemical makeup and microbiological source of microbial surfactants are used to classify them (Citation24).
Additionally, biosurfactants are categorized according to their charges and molecular structures. They might be neutral, cationic, or anionic (Citation25). Fatty acids are typically found in the hydrophobic domains, while functional groups like alcohol, organic acids, amino acids, or carbohydrates make up the hydrophilic portion. Biosurfactants can be categorized as high molecular weight molecules (such as polymeric and particulate biosurfactants) or low molecular weight molecules (such as lipopeptides, glycolipids, and phospholipids) based on their chemical structures (Citation26).
Straight-chain fatty acids, neutral lipids, phospholipids, glycolipids, and lipopeptides are examples of low molecular weight biosurfactants. Particulate surfactants facilitate the emulsification process, and heterosaccharide-containing proteins (polymeric surfactants) are examples of high molecular weight biosurfactants (Citation27). Glycolipids are the low molecular weight biosurfactant class that has been studied the most. The hydrophilic carbohydrate moiety of a glycolipid molecule is joined to hydrophobic fatty acid chains of varying lengths by an ester group (Citation28). Microbial surfactants are amphipathic molecules with hydrophilic and hydrophobic domains that enable the partitioning of two fluid phases with different polarity levels. Depending on their molecular weight, they are divided into two major categories: biosurfactants and bioemulsifiers. Phosphates, carboxylic acids, proteins, amino acids, and alcohol motifs make up the hydrophobic portion of biosurfactants. The carbon atoms that bind the fatty acids together to form ester groups, ethers, and amides constitute the lipophilic portion of the biosurfactant. They are divided into four different groups: cationic, anionic, non-ionic, and zwitterionic, depending on the charge and mass (Citation29). By joining biological activities like ethers (C–O–C), amides (N–C––O), and esters (O–C––O), both molecular components are put together. BS are often categorized as glycolipids, lipopolysaccharides, lipopeptides, phospholipids, or fatty acids according on the type of each component; each group has distinct physiological functions and physicochemical characteristics (Citation30). Biosurfactants are amphipathic substances that contain both hydrophobic (saturated, unsaturated, branched, linear fatty acids) and hydrophilic (carbohydrate, cyclic peptide, amino acid, carboxylic acid, alcohol, or phosphate) moiety. This helps to lessen surface tension and interfacial tensions between two immiscible phases with varying degrees of polarity and hydrogen bonding, such as the oil and water interphase. Because of these qualities, BSs are great foaming, dispersing agents and emulsifier (Citation31).
4. Rhamnolipids
Since their discovery in Pseudomonas aeruginosa cultures in the late 1940s, rhamnolipids (RLs), surface-active chemicals, have been the subject of much research. However, the first report of a rhamnolipid molecule's structure did not appear until the middle of the 1960s (Citation32). P. aeruginosa's secondary metabolites, known as rhamnolipids, were initially identified in 1949 and were discovered to be crucial for the bacterium's development on hydrophobic carbon sources (Citation33). Because of these beneficial properties, rhamnolipids make 4p one of the most interesting classes of biosurfactants (Citation34). Pseudomonas aeruginosa produces surface-active substances called rhamnolipid biosurfactants. Pseudomonas is well renowned for its ability to generate large amounts of glycolipids. These biosurfactants are categorized as Rhamnolipids and can lower water surface tension to 25–30 mN/m, depending on the various microbial sources and the medium's pH and salinity levels. Their CMC (critical micelle concentration) ranges from 10–230 mg/L (Citation35). They can stabilize emulsions, lessen surface tension, encourage foaming, and are typically non-toxic, non-hazardous, and biodegradable (Citation36). An essential property of surfactants is their capacity to form aggregates called micelles, that only begin to develop above what is referred to as the critical micelle concentration (CMC). To effectively perform the function of dispersing insoluble molecules in a solution, the surfactant needs micelles to be present. Temperature, pH, and ionic nature are some of the variables that affect a surfactant's CMC (Citation37).
5. Rhamnolipid structure and properties
The lipid moieties (aglycon component) and rhamnose moieties (glycon part) that make up RLs are connected to one another by an O-glycosidic bond (Citation36). One of the most researched microbial amphipathic biosurfactants is RLs, which Bergstrom et al. (1946) referred to as ‘oily glycolipids’ (Citation38). depicts rhamnolipid moieties.
Figure 2. Illustration of rhamnolipid moieties (Citation39).
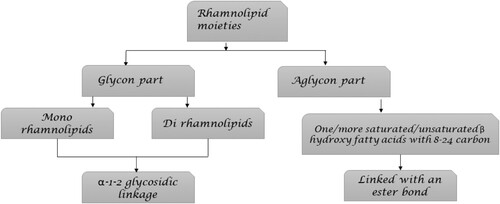
There are two of them: The hydrophobic portion of a rhamnolipid is made up of two (or, less frequently, one) saturated or unsaturated hydroxy fatty acids with varying chain lengths (C8–C24), while the hydrophilic portion is made up of one (mono-rhamnolipids) or two (di-rhamnolipids) rhamnose molecules (Citation40). The molecular structure of mono-rhamnolipids or di-rhamnolipids is illustrated in .
Figure 3. Molecular structure of (a) Mono rhamnolipids and (b) Di rhamnolipids (Citation41).
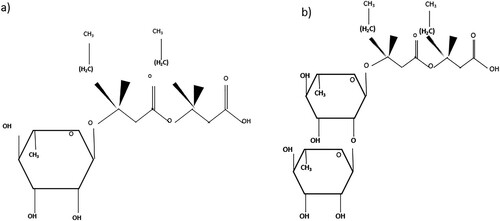
The amount of rhamnose groups in the molecular structure varies between mono- and di-rhamnolipids. Rhamnolipids vary according to the growth and environmental conditions in terms of chain length, degree of branching, and degree of unsaturation in the fatty acid chains. Compared to P. aeruginosa, certain Burkholderia species have been demonstrated to synthesize longer alkyl chain rhamnolipids. Additionally, other substrates – including alkenes, citrates, glucose, fructose, and olive oil – have been used to create biosurfactants with various characteristics (Citation42). A wide pool of RLs congeners, estimated to be over 60 structures, are produced by the variances in chemical structures, and the main source of these differences is the modification of the aglycon portion. A mono- or disaccharide made of l-rhamnopyranosyl units trans-1,2-O-glycosylates the lipid unit (R,R)-β-hydroxyalkanoyl-β-hydroxyalkanoic acids (two esterified β-hydroxy fatty acids) with different chain lengths (C6-C14) to produce the generalized structure of biosynthesized rhamnolipid (Citation43).
Rhamnolipids are made up of one or two rhamnose units connected to one to three fatty acid chains. These chains can be unsaturated or saturated, and they can range in length from C8 to C16. Rha-C10-C10 and Rha-Rha-C10-C10 are the two main congeners that make up rhamnolipids, which are typically a mixture of other congeners. The range of rhamnolipids that P. aeruginosa produces is largely influenced by the strain specifics, carbon sources, culture conditions, and isolation technique (Citation44). Many types of bacteria make glycolipidic biosurfactants called rhamnolipids. Originally discovered as byproducts of the opportunistic pathogen Pseudomonas aeruginosa, they were identified as a combination of four congeners: α-L-rhamnopyranosyl-α-L-rhamnopyranosyl-β-hydroxydecanoyl-β-hydroxydecanoate (Rha-Rha-C10-C10), α-L-rhamnopyranosyl-α-L-rhamnopyranosyl-β-hydroxydecanoate (Rha-Rha-C10), and their mono-rhamnolipid congeners Rha-C10-C10 and Rha-C10. Burkholderia species have been shown to produce rhamnolipids that have longer alkyl chains than those produced by P. aeruginosa (Citation8). The quantity of rhamnose rings, chain length, and fatty acid moiety saturability vary amongst them. The four common homologues of rhamnolipid are Rha-C10-C10, Rha-C10, Rha2-C10C10, and Rha2-C10, in that order (Citation45). Rhamnolipids have a low minimum surface tension (30–32 mN/m), a high emulsifying activity, and a greater affinity for organic molecules that are hydrophobic, making them ideal surfactants (Citation46).
6. Biodegradability of rhamnolipids and biodegradation by rhamnolipids
Compared to synthetic surfactants, biosurfactants are more biodegradable, have a highly selective surface activity, and are resistant to harsh environments (Citation47). The enzymatic activities of various microorganisms break down these surface-active molecules by first cleaving and then inactivating the surfactant monomers. It is known that a number of enzymes can break down surfactant monomers. For example, the polysaccharide backbone of emulsions is broken by emulsan polymerase, rendering the molecule inactive (Citation48). The fact that rhamnolipids are readily biodegradable is a widely acknowledged benefit concerning their environmental safety (Citation43). It is reasonable to assume that within the complex microbiota found in a certain niche, there will always be microbes capable of degrading rhamnolipids due to their chemical structure. ‘Everything is everywhere, but the environment selects’ is a widely recognized general principle in microbial ecology (Citation49). When anaerobic conditions prevailed, rhamnolipids decomposed notably more slowly than when aerobic conditions were met (Citation50). Another study demonstrated that during the biodegradation of solubilized HOCs, bacteria are capable of degrading rhamnolipids (Citation51). Given that biosurfactants used in personal care or household detergents would likely end up in sewage effluents as the primary means of disposal, wastewater treatment facilities will probably be the primary sink. But overall, biosurfactants have been shown to be sufficiently biodegradable, therefore it is assumed that the presence of those compounds in wastewater treatment plant effluents won't have any negative effects on aquatic life (Citation52). The phrase ‘substrates are freely available to cross microbial cell membrane from the medium the microorganism inhabits at a given time’ is crucial to understanding biodegradation. When hydrophobic organic compounds (HOCs) are biodegraded, they break down into three different states: aqueous HOCs (dissolution as a molecular state), micellar HOCs (pseudo-solubilization), and sorbed or non-aqueous phase liquids (NAPL)-state HOCs at the interface. As small repositories of HOCs, the co-aggregates of rhamnolipids and HOCs are known to improve mass transfer to microbial cells. Rhamnolipids can therefore be added to increase the sorbed or NAPL-state HOCs’ bioavailability (Citation53). The impact of rhamnolipid as a biosurfactant on thermoplastics’ microbial colonization and biodegradation was studied. The study investigated the potential of a consortium of microorganisms, consisting of Penicillium raperi, Aspergillus flavus, Penicillium glaucoroseum, and Pseudomonas spp., to biodegrade mixed plastics (polyethylene (PE), polystyrene foam (PS), and polyethylene terephthalate (PET)) that have been exposed to ultraviolet light (UV) and when biosurfactant (rhamnolipid) is present. The study suggested that polystyrene foam (PS) could be biodegraded more quickly by combining rhamnolipid with UV pre-treatment (Citation54).
A rhamnolipid mixture (Rh-mix) and Triton X-100 (TX-100) were tested at varying concentrations to see how surfactants affected the biodegradation of trifluralin and atrazine (by Streptomyces PS1/5) and coumaphos (by degrading consortia from a contaminated cattle dip) in liquid cultures and soil slurries. At high concentrations of both surfactants, the degree of trifluralin biodegradation in liquid culture was enhanced. When either surfactant was present, the rate of atrazine breakdown decreased. Surfactant micelles can prevent pollutants partitioned in the micellar phase from biodegrading because of their molecular structure. This is because the surfactant has the potential to impede microorganisms from interacting with the pollutant partitioned in the micellar core by forming a barrier on the hydrophilic micellar surface or by causing irreversible damage to the cellular envelop. Despite being basically harmless and biodegradable in comparison to synthetic surfactants, biosurfactants may exhibit unique compatibilities with the cell envelope structures of the bacteria that create them. As a result, they may have inhibitory effects on the cell structures of other microorganisms (Citation55). A model co-contaminated system was created to test the hypothesis that rhamnolipid, a metal-complexing biosurfactant, could lessen metal toxicity and improve organic biodegradation by a Burkholderia sp. that was isolated from soil. When rhamnolipid was added, cadmium toxicity was eliminated at a concentration ten times higher than cadmium (890 μM), reduced at an equimolar quantity (89 μM), and had no impact at a dosage ten times lower (8.9 μM) (Citation56). Various properties of different type of rhamnolipids is illustrated in .
Table 1. Illustration of sources, properties and types of rhamnolipids.
7. Other properties of biosurfactants
As seen in , biosurfactants offer outstanding surface and interface activity, biodegradability, anti-adhesiveness, emulsifying qualities, low toxicity, and thermal endurance, among other special qualities (Citation66).
Figure 4. Properties of biosurfactants making it useful in wide variety of applications (Citation67).
Biosurfactants can be generically classified as low molecular weight or high molecular weight based on their average molecular mass, which varies between 500 and 1500 Da. Although higher molecular weight biosurfactants work best for stabilizing oil-in-water emulsions, low molecular weight biosurfactants are more successful at lowering surface tension at the air–water interface and interfacial tension at the oil–water interface (Citation48).
7.1. Micelle formation
Micelles are characterized as aggregates of molecules that have both hydrophilic and hydrophobic groups, with the hydrophobic groups facing oil and the hydrophilic groups facing water (Citation65). One process that leads to the production of a nanostructure is the equilibrium process of micelle formation. Surfactants typically form micelles in aqueous solutions when they aggregate at increasing concentrations. Using hydrophobic and van der Waals interactions, they self-assemble to create micelles. In addition to promoting emulsification and stability, micelle production also plays a significant role in reducing surface activity. Additionally, the creation of micelles preferentially facilitates the solubilization of environmental pollutants at concentrations lower than critical micelle concentration (CMC) and the mobilization of environmental toxins at concentrations above CMC (Citation68). They have the same capacity to self-assemble and create micelles as chemically synthesized surfactants. Their specificity is increased and their ability to have anatomically distinct structures from one another is enabled. Their potential to reduce interfacial and surface tension qualifies them for commercial use (Citation69). The key to unlocking surfactant/biosurfactant's industrial perspectives is to know its CMC value. Its CMC values and molecular structure dictate its cleaning, detergency, and solubilization capabilities (Citation70).
7.2. Surface and interface activity
Biosurfactants are highly effective at reducing the interfacial activity and surface tension between two surfaces, either liquid or solid. When compared to chemical surfactants, biosurfactants are thought to be far more effective due to their capacity to lower surface tension at much lower concentrations (Citation71). The interfacial tension between the two phases of a system is decreased when biosurfactants are added because they adsorb at the liquid–air or liquid–liquid contact. The reason for this decrease in interfacial tension is because the hydrophobic tails of the biosurfactant molecules in the polar phase (such as water) and the hydrophilic heads in the nonpolar phase (such as oil) are aligned. Increased stability and homogeneity result from this orientation's reduction of the unfavorable interactions between the two immiscible phases (Citation13, Citation72, Citation73). The capacity of biosurfactants to reduce interfacial tension offers multiple benefits across a range of applications. With less interfacial tension, finely distributed droplets of one phase can more easily form and stabilize in another, resulting in emulsions that are more stable over time (Citation74, Citation75). Biosurfactants are also useful emulsifiers due to their distinct surface and interface activity.
7.3. Emulsifying agents
Emulsifiers are used to control the emulsification process and stability. A suitable emulsifying agent can increase the stability of emulsions because they are thermodynamically unstable. While mineral oils with HLB values less than 8, such as liquid paraffin, are used to generate w/o emulsions, surfactants with HLB (hydrophilic lipophilic balance) values more than 8, such as spans and tweens (non-ionic surfactants), are used to form o/w emulsions (Citation76). In order for an emulsion to be successful, emulsifiers are essential because they help lipid droplets form during homogenization and make them more stable. Because they may regulate globulse clustering, emulsifiers extend product useful life and reduce phase separation, which promotes the stability of heterogeneous systems (Citation77). Although high molecular weight biosurfactants are well known for their ability to emulsify, they are not very good at lowering surface tension (Citation78). The decrease in surface tensions at the oil/water interface is a sign of the amphiphilic emulsification behavior of BSs. They vary from their competitors (synthetic surfactants) by displaying biological (plants and bacteria) and renewable origins. When compared to their synthetic equivalents, biosurfactants have been shown to have significantly reduced cytotoxicity, superior emulsification tendencies, and function across a wide temperature range (Citation79).
7.4. Low toxicity
In general, biosurfactants have low toxicity. There are numerous research that have looked into the toxicity of biosurfactants in aquatic life, plants, and human cell lines. In aquatic environments, a naturally occurring mono rhamnolipid and a synthetic mono rhamnolipid with an EPA rating of ‘slightly toxic’ both obtained EC50 level scores (Citation43). Biosurfactants are beneficial in industrial, food, pharmaceutical, and cosmetic goods because of their low toxicity profile (Citation80). Nevertheless, elevated levels of biosurfactants may pose a hazard to microorganisms, impeding their growth and influencing the effectiveness of biodegradation (Citation81).
8. Applications of biosurfactants
The use of biosurfactants is growing in a variety of commercial sectors, including the oil industry for oil recovery and bioremediation of environments, as well as the medical and pharmaceutical fields for their antimicrobial, surface-active, anti-adhesive, and anti-biofilm qualities (Citation82). Because of their ability to generate emulsions and stabilize mixtures, surfactants are already often utilized as components in food formulations. Furthermore, surfactants enhance the texture and durability of products; these characteristics apply to both synthetic and biosurfactants (Citation83). Because of its excellent degradability, ability to eliminate hydrophobic strains, and neutral performance, biosurfactant is most in demand in the detergent industry among these uses (Citation10). Because of their surface activity, biosurfactants can also be used in remediation procedures to bind or emulsify contaminants (Citation84). Numerous biosurfactants are important adjuvants for antigens in vaccines and gene therapy, and they have been shown to have potential uses in immunomodulatory and antimicrobial (anti-fungal, anti-viral, and anti-malarial) activities as well as sticky and anti-adhesive properties (Citation85). states the applications of various classes biosurfactants.
Table 2. List of applications of various classes biosurfactants obtained from different microbes.
Farming, food processing, water and soil restoration, oil recovery based on microorganisms, biomedicine, nanotechnology, and a number of other diverse fields – including cleaning agents – benefit greatly from the usage of biosurfactants (Citation86).
9. Applications of rhamnolipids
Numerous possible uses for rhamnolipids have been reported. It has been demonstrated that they possess antibacterial properties against rival microorganisms and are optional in the biological regulation of zoosporic phytopathogens (Citation94). The most popular biosurfactants in the bioremediation industry are rhamnolipids, which can greatly increase the water solubility of saturated and aromatic hydrocarbons (Citation95). The discovery of a bacterial biosurfactant with hemolytic and antibiotic properties led to the first reports of the application of biosurfactants in 1949 and 1968 (Citation96). The primary obstacle to the extensive commercialization of rhamnolipids is the expense associated with their manufacture. If the majority of chemical surfactants cost between $1 and $6 kg−1, rhamnolipids can cost up to thirty times as much (Citation97). Although rhamnolipid has several industrial uses, its use is restricted because of its high cost of manufacture (Citation98).
9.1. Soil remediation
Petroleum-contaminated soil is a global issue that will soon require the application of innovative and environmentally friendly technology. Microbial surface-active compounds (SACs) have demonstrated a number of benefits over fossil-based surfactants, in light of their toxicity. These benefits include high hydrocarbon remobilization, low toxicity, biodegradability, sustainability, and others. The primary grievances they raise are related to the expenses incurred in the downstream process (such as extraction and purification), the pathogenic nature of certain biosurfactant producers, the poor yield attained, and heterogeneity (i.e. the quantity and variety of congeners). Low concentrations of surfactants have been shown to be very promising for the effective and controlled bioremoval of pollutants from porous soil medium without endangering the autochthonous microbial communities or the degraders of pollutants (Citation99).
This study examined how rhamnolipid biosurfactants and natural mineral slow-release nutrients combined to improve the bioremediation of an intertidal zone contaminated by heavy oil. Slow-release nutrients were resistant to seawater dilution and performed well over an extended period of time. The metabolic activity of native microorganisms and the biodegradation efficiency of heavy oil degraders were both significantly increased by the simultaneous application of slow-release nutrients and rhamnolipid biosurfactants (Citation100). Another study looked into the aqueous rhamnolipid solution's ability to extract crude oil from polluted sand. Sixty-seven percent of the crude oil in the sand samples was removed by rhamnolipid washing, indicating the product's potential risk in oil spill cases (Citation101). From initial solubilization tests to the microbial breakdown of hydrocarbons in a fixed bed of soil, rhamnolipids have been used extremely frequently for a variety of applications. Rhamnolipids, as an example, more effectively facilitate the mobilization of hexadecane and phenanthrene in soils than do artificial surfactants like SDS and Tween 80 (Citation5).
9.2. Pollutant removal/heavy metal contamination
Rhamnolipids and surfactins are two types of biosurfactants that have been effectively demonstrated to work in this process in conjunction with an extra precipitant (Citation102). Over the course of a 24-hour monitoring period, it was shown that rhamnolipid biosurfactant not only improved the extraction of Cr(III) from kaolinite-contaminated soils but also made 100% reduction of Cr(VI) to Cr(III) possible (Citation100).
Metals have been successfully removed from waste water streams, sewage sludge, industrial effluents, and mine water using microbially generated surfactants, or ‘bio-surfactants.’ This article provides experimental findings that assess the efficacy of rhamnolipids in improving heavy metal removal from water systems that are contaminated with heavy metals. The inclusion of a bio-surfactant aims to complexate the free form of the metal present in solution, which may facilitate the desorption of heavy metals from contaminated water. This leads to direct contact between the bio-surfactant and the sorbed metal and, consequently, increases desorption by reducing the metal's solution-phase activity. The mobility of biosurfactant-metal complexes is influenced by the size and charge of the biosurfactant structure. Furthermore, charge and structural size will have an impact on how easily biosurfactants enter filter pores (Citation103). A study investigated the possibility of removing cadmium from an aqueous solution using foam flotation and a sample of rhamnolipid biosurfactant. With minor changes, it appears that the use of rhamnolipid biosurfactant can be promising in the removal of heavy metals from wastewaters by foam flotation, despite the relatively low removals. According to these findings, biosurfactants like rhamnolipid may be crucial to ion flotation and have positive effects on the environment (Citation104). Using foam flotation and a rhamnolipid biosurfactant as a dye collector, Methylene blue was effectively extracted from an aqueous solution. Because of its strong ability to bind metal ions and its high surface activity and foamability, rhamnolipid biosurfactant, which is generated from the Pseudomonas aeruginosa bacteria, is a well-known bioproduct that is effectively employed in the treatment of wastewater and the remediation of heavy metal-contaminated soils (Citation105).
9.3. Waste water treatment
The development of a compact clogging layer, also known as activated sludge, is a common issue impeding the removal of Extracellular polymeric substances (EPS) pollutants. In order to remove clogging in-situ by dissolving or dispersing the clogging materials, an appropriate method that is safe and effective must be found. The biosurfactant rhamnolipid (RL) enhanced the solubilization of blocking deposits in-situ in a CW plant. By using this method, clogged deposits were much more evenly distributed and soluble. Extracellular polymeric substances (EPS) are coated with enzymes that can be released to improve the elimination of pollutants. RL increases the dispersion and dissolution of EPS (Citation106).
One promising method for treating wastewater is bioremediation. In this study, Pseudomonas aeruginosa (MTCC 1688) was used in an experimental setting to develop a biosurfactant that would be used to remove TCS and Ibuprofen (IBU) from household wastewater (Citation107). Worldwide, oily wastewater from many businesses poses a serious ecological threat. The conventional approaches to treating oily wastewater are expensive and ineffective. Surfactants disperse oil into an aquatic environment, which can accelerate the biodegradation of petroleum hydrocarbons. In this work, we utilized a cell-free culture broth containing rhamnolipids to improve the biodegradation of lubricating oil and crude oil in a traditional aerobically-activated sludge system. Rhamnolipids’ increased solubility and decreased interfacial tension were primarily responsible for the increased hydrocarbon elimination (Citation108). Rhamnolipids were used to remove heavy metals from dewatered sludge; the sludge came from an urban wastewater treatment facility. Domestic wastewater makes up 60% of the total wastewater treated by the wastewater treatment plant, which also handles industrial wastewater. Using rhamnolipids at a concentration of 2 g/L and a 10/1 liquid/solid ratio for 24 h, the removal efficiencies of Cu, Zn, Cr, Pb, Ni, and Mn from dewatered sludge were 16%, 15%, 12%, 5%, 14.01%, and 6.11%, respectively (Citation109).
9.4. Medicine
In the medical field, rhamnolipids biosurfactant is still in the trial stage. However, most experimental results show that while they are safer than synthetic medications, they are very successful. Their anti-inflammatory, immune-modulating, anticarcinogenic, antimicrobial, and anticancer properties have all been documented (Citation110). Biosurfactants such as Rhamnolipids have been shown to have antibacterial applications and non-cytotoxic anti-tumergenic characteristics against colon cancer cell line, as demonstrated in Pseudomonas (Citation111).
It has been reported that rhamnolipid, a glycolipid biosurfactant, possesses antibacterial qualities and is used in drug delivery. Their use in medication distribution gets around the drawback of the medication's poor blood–brain barrier solubility (Citation112). The human pathogen Pseudomonas aeruginosa is known to create rhamnolipids, which are virulence factors. Nonetheless, a number of studies claimed that rhamnolipids were crucial in the management of medical conditions in people. Researchers discovered that using one or more rhamnolipids as active components would enable them to function as beneficial immune modulators. Psoriasis and lichen rubber planus are two dermatological autoimmune disorders for which a number of clinical evidence indicated that rhamnolipids had outstanding therapeutic therapy (Citation113). Because they cause permeabilization, which weakens the bacterial plasma membrane, glycolipids and rhamnolipids have antibacterial qualities. This operates similarly to synthetic cationic surfactants in that it modifies hydrophobicity and impacts cell surface charge. Because they can stop biofilms from forming, they can also make bacteria more vulnerable to antimicrobial treatments (Citation38).
10. Rhamnolipid producing organisms
In the past, Pseudomonas aeruginosa, the primary bacterium for rhamnolipid research, was the source of the first description of rhamnolipids, which occurred 70 years ago (Citation96). The strains of Pseudomonas species are renowned producers of rhamnolipids with low molecular mass and superior surfactant qualities (Citation114). P. aeruginosa produces extracellular glycolipids composed of L-rhamnose and 3-hydroxyalkanoic acid (rhamnolipids). In Pseudomonas aeruginosa, rhlA is necessary for the synthesis of 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids, a new biosurfactant that promotes swarming motility. Numerous marine bacterial species, typically found in oil-contaminated waters, have been shown to create biosurfactants. These species include those in the genera Alteromonas, Halomonas, Pseudoalteromonas, and Alcanivorax (Citation7). Pseudomonas is not the only organism capable of rhamnolipid production. Acinetobacter calcoaceticus, Enterobacter asburiae, Enterobacter hormaechei, Nocardioides sp., Pseudoxanthomonas sp., and an Antarctic strain of Pantoea sp. were among the bacteria that were discovered to synthesis rhamnolipids (Citation115). Distinct bacterial strains have occasionally been reported to possess the ability to produce RL through horizontal gene transfer, such as Pseudomonas chlororaphis NRRL B-30761. Although Pseudomonas putida has been utilized as a heterologous host for the manufacture of this biosurfactant with reasonably acceptable yields, P. aeruginosa, the ubiquitous opportunistic pathogenic bacterium, is the best producer of rhamnolipids (Citation116). P. putida, P. chlororaphis, P. fluorescens, B. plantarii, B. Kururiensis, B. glumae, and other strains were among those whose rhamnolipid production was documented. Still, Pseudomonas aeruginosa's pathogenicity prompted the investigation of other, safe rhamnolipid manufacturers (Citation117). Though the rhamnolipids generated differ from those by P. aeruginosa, two similar but nonpathogenic bacteria have recently been identified as makers of rhamnolipids. Pseudomonas chlororaphis alone generates mono-rhamnolipid, while Burkholderia thailandensis mostly generates di-rhamnolipid, which has longer alkyl chains than P. aeruginosa (Citation118).
When one or two 3-hydroxy fatty acids of different lengths are joined to a mono- or dirhamnose molecule, a complex combination of congeners known as rhamnolipids is created. Generally speaking, L-rhamnosyl-beta-hydroxydecanoyl-beta-hydroxydecanoate and L-rhamnosyl-Lrhamnosyl-beta-hydroxydecanoyl-beta-hydroxydecanoate are the two most prevalent rhamnolipids (Citation116). One of the many extracellular virulence factors that contribute to opportunistic human infections in immunocompromised and cystic fibrosis patients is the production of rhamnolipids (Citation119).
11. Biosynthesis of rhamnolipid
A biosurfactant that can replace conventional oil-based surfactants is rhamnolipid, a secondary metabolite generated by certain bacteria. Beginning with two b-hydroxy fatty acids, biosynthesis generates the dimer 3-(3-hydroxyalkanoyloxy) alkanoate (HAA). This is then coupled to one or more rhamnose units to produce mono- and di-rhamnolipids (mRL and dRL). These arise spontaneously as combinations with different mRL to dRL ratios and hydroxyl fatty acid chain lengths (Citation120). The synthesis of rhamnolipids is crucial for biofilm development, assimilation of hydrophobic carbon sources, and cell motility. The production of rhamnolipids reaches its peak during the stationary phase of growth. The RsmA/RsmZ system modulates transcriptionally, while quorum-sensing circuitry regulates transcription (Citation121). In general, biosynthesis relies on core metabolic processes, like the synthesis of sugar and fatty acids, which is followed by the rhamnosylation of fatty acid chains to produce a final rhamnolipid product. Bacterial rhamnolipids’ aglycone portion is most likely synthesized denovo, despite the potential involvement of homologous enzymes in several bacterial producers. Nevertheless, it is still necessary to identify and use the process's similarities and differences in a targeted rhamnolipid manufacturing manner. Its byproducts are also diffusible substances that can pass through different channels. Thus, it has been discovered that the synthesis of lipopolysaccharides, polyhydroxyalkanoates, 4-hydroxy-2alkylquinolines, or alginates is linked to rhamnolipid formation (Citation122). More recent research has concentrated on developing novel carbon sources or growing environments to increase the economy of rhamnolipid biosynthesis, such as by employing inexpensive raw materials from waste streams. The well-established host organisms used in these experiments, such as P. putida KT2440, were modified to use ethanol, pyrolysis oil, or substitute sugars such xylose and arabinose as components of lignocellulosic hydrolysates or from agricultural leftovers (Citation123).
Burger et al. (1963) first postulated the steps of rhamnolipid biosynthesis, which include the following order for the production of di-rhamnolipids (Rha-RhaC10C10):
2β-hydroxydecanoyl-CoA→β-hydroxydecanoyl-β-hydroxydecanoate + 2CoA-SH.
TDP-1-rhamnose+β-hydroxydecanoyl-β-hydroxydecanoate → TDP + 1-rhamnoyl-β-hydroxydecanoyl-β-hydroxydecanoate.
TDP-1-rhamnose + 1-rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate→TDP + 1-rhamnosyl-1-rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate. (Citation124).
RhlA, RhlB, and RhlC catalyze the synthesis of rhamnolipids from these precursors. In order to create 3-HAAs, RhlA has to link two molecules of (R) 3-hydroxydecanoyl-ACP, which are intermediates in the de novo route. The possibility that RhlA plays a part in switching (R)-β-hydroxyacyl-ACP from de novo fatty acid synthesis to the rhamnolipid biosynthesis pathway has also been identified. RhlB is a membrane-bound rhamnosyltransferase that forms a mono-rhamnolipid by joining the first dTDP-L-rhamnose molecule to the rhamnosyltransferase. RhlC is an extra rhamnosyltransferase II that uses an α(12) glycosidic bond to attach to another dTDP-L-rhamnose molecule in order to catalyze the synthesis of di-rhamnolipid from mono-rhamnolipids. In P. aeruginosa, RhlA and RhlB form an operon with rhlI and rhlR, and the strain has two distinct RhlC genes that are situated differently in the genome (Citation43).
A bacterial population can coordinate its behavior by secreting signal molecules called auto-inducers, which are used in bacterial quorum sensing (QS), a communication system. In many gram-negative bacteria, the signal synthase, signal receptor protein, and signal molecule comprise the QS system (Citation38). Under the guidance of two interconnected quorum sensing systems, namely las and rhl, Pseudomonas sp. manufacture rhamnolipids, which are significant microbially derived surface active agents (Citation125). Additional regulators regulate quorum sensing (QS) circuits las and rhl, modifying their responses by compensating modulation (Citation126).
The lux family includes the QS network of B. thailandensis, which has three sets of luxI-luxR type genes: btaI1-btaR1 (QS-1), btaI2-btaR2 (QS-2), and btaI3-btaR3 (QS-3). Additionally, there are three orphan luxR homologs: btaR4 (BTH_II2087) (QS-4), btaR5 (BTH_II1681) (QS-5), and btaR6 (BTH_II1275) (QS-6) with no related luxI. N-acyl-homoserine lactones (AHLs), diffusible molecules produced by the btaI synthetase, are sequestered at the N terminal of transcriptional regulators btaR, activating the C terminal to engage with RNA polymerase (α and σ subunits) and/or DNA fragments (lux box-like sequences). While QS-2 encodes and responds to N-3-hydroxy-decanoyl-HSL (3OH C10-HSL) as well as 3OHC8-HSL, QS-1 and QS-3 encode and respond to N-octanoyl-homoserine lactone (C8-HSL) and N-3-hydroxy-octanoyl-HSL (3OH C8-HSL), respectively. It is discovered that these three QS systems are linked, coordinating transcriptionally and post-transcriptionally each other's expression during bacterial growth. When the stationary phase begins, QS-3 is engaged, whereas QS-1 and Q-2 are activated during the exponential phase (Citation38). Given its GRAS designation, well-established genome manipulation tools, and comprehension of its genome, Escherichia coli could be a desirable host for the creation of recombinant rhamnolipids. When RhlAB was first observed, E. coli did not produce rhamnolipids. This was assumed to be because of insufficient activated rhamnose availability productivity (Citation97).
12. Genetic regulation of rhamnolipid production
Rhamnolipid synthesis is generally under metabolic control of a complex control network consisting of transcriptional regulation by quorum sensing (QS) systems, sigma factors (RpoN, RpoS), post- transcriptional modification by small non-coding RNAs, as well as physiological and environmental stimuli (Citation127).
Rhamnolipids can be created by heterologous expression of RhlA, RhlB, and RhlC genes in (appropriate) host organisms able to synthesize the necessary precursors and withstand high concentrations of the enzymes essential for complicated regulation, pathogenicity, or to improve other aspects of production. It is possible to achieve growth-independent synthesis of these otherwise secondary metabolites by cloning an inducible promoter upstream of the rhamnosyltransferase genes, which allows the user to control when the culture synthesizes rhamnolipids. Given its GRAS designation, well-established knowledge of its genome, and methods for manipulating it, Escherichia coli could be a desirable host for the creation of recombinant rhamnolipids (Citation97). Many attempts have been made to use Escherichia coli producing the RhlAB operon to generate mono-rhamnolipid, but only a tiny amount of this product has been successful. By over-expressing the RhlAB operon, rhamnolipids have also been generated in other heterologous hosts, such as P. putida and P. fluorescens. These Pseudomonas species are known to create rhamnolipids in small but noteworthy amounts, however the limiting mechanism for their production was not identified. A plausible explanation for the low levels of rhamnolipids generated by these heterologous hosts could be the restricted synthesis of the necessary rhamnolipid precursors in these bacteria. When the RhlAB operon is present in E. coli, the overproduction of dTDP-L-rhamnose leads to an increase in the production of mono-rhamnolipids (Citation128).
A study successfully identified and characterized KT1115, a wild-type Pseudomonas aeruginosa strain that can convert rapeseed oils into di-rhamnolipids, a class of biosurfactants with a wide range of potential applications. When the genomes of P. aeruginosa PAO1 and KT1115 were analyzed for the gene linked to rhamnolipid synthesis, the amino acid sequences encoded by RhlI, RhlA, RmlA, and RmlC were found to differ slightly. Within P. aeruginosa's RhlI-RhlR quorum sensing system, RhlI was an enzyme that regulated the expression of lipase genes and the rhamnolipid production pathway.
The mutant RhlI in strain KT1115 may have an impact on the expression of lipase genes and the rhamnolipid synthesis pathway. The amino acid substitutions of RhlI were the cause of their activities and changed substrate specificities. As a consequence, strain KT1115 has more HAAs [3-(3-hydroxyalkanoyloxy) alkanoate synthetase] than strain PAO1 to supply rhamnolipid synthesis, possibly due to the amino acid difference of RhlA (at position 165) that had a positive influence on RhlA catalysis. Additionally, it is possible that the specificity of RmlA and RhlC will improve the availability of dTDP-l-rhamnose, which would account for the large yield of di-RLs in KT1115 (Citation129).
The NtrB/C-RpoN cascade, drives transcription of the nitrogen-regulated sRNA (NrsZ), which in turn activates the expression of RhlA. This increases the production of rhamnolipids by nitrogen limitation. Limitation has demonstrated that NtrB/C controls a number of genes, such as those involved in carbon catabolism, glutamine synthetase, porin, amino acid transporter, and urea absorption (Citation130). Plasmids carrying PAO1 genes, which are involved in the biosynthesis of this biosurfactant, or the RhlR gene, which codes for the QSR transcriptional regulator, were introduced into the strain in order to increase ATCC 9027 mono-RL production. The PAO1 strain was then used to compare the effects of these genetic manipulations. Research revealed that whereas RhlA and RhlAB expression had a minimal impact on strain ATCC 9027s ability to produce mono-RL, strain PAO1's ability to produce RL was severely reduced when RhlA was expressed. This outcome was most likely caused by the interaction between the PHA synthesis pathway and the RL biosynthesis route (Citation131). It appears to be very difficult to enhance rhamnolipid production in specific native producers, which are characterized as non-pathogenic like the P. aeruginosa strain ATCC 9027 or species like P. chlororaphis, B. thailandensis, as their regulation is one of the bottlenecks for the high-yield production of rhamnolipids (Citation132).
13. Limitations
The physicochemical characteristics of these rhamnolipids are influenced not only by their source but also by the methods of manufacturing and purification. Accurately determining their industrial usage requires an understanding of these characteristics. A number of bacterial species other than P. aeruginosa have been reported to produce RLs, but it's important to keep in mind that many of these species haven't had their ability to produce RLs confirmed using highly accurate analytical techniques, nor have they been positively identified. Because P. aeruginosa is an opportunistic bacterium with a high intrinsic resistance to antibiotics and poses a significant health risk, using it to produce these chemicals on an industrial scale is subject to stringent limitations. In an attempt to get around the challenge of generating RL through extensive P. aeruginosa cultivation, efforts have been made to generate these surfactants using naturally occurring RL producers. But in the majority of these situations, the amount of RL produced is less. Rhamnolipids are produced by P. aeruginosa, however the complexity of the genetic network makes it difficult to create strains with increased levels of these biosurfactant production. Despite the many appealing qualities and benefits of biosurfactants, their industrial production has not been feasible because of significant investment costs that prevent them from being competitively priced with synthetic surfactants. Commercialization of some biosurfactants, like surfactin, sophorolipids, rhamnolipids, and mannosylerythritol lipids, is becoming increasingly restricted.
14. Future scope
Indeed, the broad use of rhamnolipids in a variety of industries will be fueled by additional research into improving production techniques, investigating novel uses, and comprehending their mechanisms of action. By continuing to invest in research, industry will be able to fully utilize the advantages of rhamnolipids and open the door to a future that is more ecologically conscious and sustainable. Through genetic engineering, rhamnolipid manufacturing yield and quality can be increased. To increase yield and lower manufacturing costs, new tactics and technologies are needed. Comprehensive cost–benefit analyses of biosurfactant recovery techniques are required. Large-scale rhamnolipid manufacturing using wastes as raw materials is required to support the waste valorization idea.
15. Conclusion
Biosurfactants are categorized into multiple groups, such as polymeric substances (emulsan, alasan), lipopeptides (surfactin, iturin), and glycolipids (rhamnolipids, sophorolipids, trehalose lipids). Because of their superior physicochemical characteristics, rhamnolipids have been investigated the most out of all of them. Rhamnolipids are regarded as ‘green’ products since they are derived from renewable resources and are generally thought to be less harmful and more biodegradable than the synthetic surfactants that are currently in use. Numerous possible uses for rhamnolipids have been reported. They have drawn interest from a variety of industries, including food, pharmaceuticals, healthcare, and petrochemicals, because of their low surface tension, high biodegradability, and lack of toxicity. Therefore, it is anticipated that rhamnolipids will be taken into consideration as a viable substitute with a number of industrial uses.
The field of quorum sensing (QS) has drawn a lot of interest in recent years. A biosurfactant called rhamnolipid is produced, and this process is one that is controlled by quorum sensing molecules. For rhamnolipids to be produced at a reasonable price, a high-quality strain is essential. In order to obtain non-pathogenic strains with high rhamnolipid productivity, a variety of techniques have been used. These techniques include the isolation of potentially rhamnolipid-producing strains from the environment, fermentation condition optimization, and strain improvement through the use of different mutagenesis and metabolic engineering techniques. One of the main obstacles to creating strains of P. aeruginosa with higher production of these biosurfactants is the intricacy of the genetic network involved in rhamnolipid synthesis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Van Bogaert, I.N.; Saerens, K.; De Muynck, C.; Develter, D.; Soetaert, W.; Vandamme, E.J. Microbial Production and Application of Sophorolipids. Appl. Microbiol. Biotechnol. 2007, 76, 23–34.
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in Utilization of Renewable Substrates for Biosurfactant Production. AMB. Express. 2011, 1, 5. doi:10.1186/2191-0855-1-5.
- Marchant, I.; Banat, I.M. Biosurfactants: A Sustainable Replacement for Chemical Surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. doi:10.1007/s10529-012-0956-x.
- Nandwani, S.K.; Saxena, N.; Kumar, A. Enhanced Oil Recovery Potential Analysis Through Simulation of a Bio-Based Surfactant Using CFD. J. Mol. Liq. 2024, 396, 124112.
- Negrete, P.S.; Ghilardi, C.; Pineda, L.R.; Pérez, E.; Herrera, M.L.; Borroni, V. Biosurfactant Production by Rhodococcus ALDO1 Isolated from Olive Mill Wastes. Biocatal. Agric. Biotechnol. 2024, 57, 103106.
- Kitamoto, D.; Isoda, H.; Nakahara, T. Functions and Potential Applications of Glycolipid Biosurfactants – From Energy-Saving Materials to Gene Delivery Carriers. J. Biosci. Bioeng. 2002, 94, 187–201. doi:10.1016/S1389-1723(02)80149-9.
- De Carvalho, C.C.C.R. Marine Biofilms: A Successful Microbial Strategy with Economic Implications. Front. Mar. Sci. 2018, 5, 1–11. doi:10.3389/fmars.2018.00126.
- Tripathi, L.; Twigg, M.S.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Biosynthesis of Rhamnolipid by a Marinobacter Species Expands the Paradigm of Biosurfactant Synthesis to a new Genus of the Marine Microflora. Microb. Cell Fact 2019, 18, 164. doi:10.1186/s12934-019-1216-8.
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of Structures, Microbial Origins and Roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. doi:10.1007/s00253-010-2498-2.
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial Biosurfactants Production, Applications and Future Potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. doi:10.1007/s00253-010-2589-0.
- Ambaye, T.M.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, Characterization and Application of Biosurfactant in Various Industries: A Critical Review on Progress, Challenges and Perspectives. Environ. Technol. Innov. 2021, 24, 102090. doi:10.1016/j.eti.2021.102090.
- Deleu, M.; Paquot, M. From Renewable Vegetables Resources to Microorganisms: New Trends in Surfactants. Comptes Rendus Chim. 2004, 7, 641–646. doi:10.1016/j.crci.2004.04.002.
- Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant Production by Bacillus Subtilis B30 and its Application in Enhancing Oil Recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. doi:10.1016/j.colsurfb.2013.09.022.
- Verma, C.; Hussain, C.M.; Quraishi, M.A.; Alfantazi, A. Green Surfactants for Corrosion Control: Design, Performance and Applications. Adv. Colloid Interface Sci. 2023, 311, 102822. doi:10.1016/j.cis.2022.102822.
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, Remediation and Green Surfactants. Environ. Chem. Lett. 2014, 12, 275–287. doi:10.1007/s10311-014-0466-2.
- Wu, Y.S.; Ngai, S.C.; Goh, B.H.; Chan, K.G.; Lee, L.H.; Chuah, L.H. Anticancer Activities of Surfactin Potential Application of Nanotechnology Assisted Surfactin Delivery. Front. Pharmacol. 2017, 8, 1–22. doi:10.3389/fphar.2017.00761.
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, Natural Alternatives to Synthetic Surfactants: Physicochemical Properties and Applications. Adv. Colloid Interface Sci. 2020, 275, 2061. doi:10.1016/j.cis.2019.102061.
- Rajitha, K.; Nancharaiah, Y.V.; Venugopalan, V.P. Inhibition of Biofilm Formation and Settlement of Barnacle Larvae by a Biosurfactant Produced from a Marine Biofilm-Forming Exiguobacterium sp. R58. Int. Biodeterior. Biodegrad. 2024, 187, 105724.
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental Applications of Biosurfactants: Recent Advances. Int. J. Mol. Sci. 2011, 12, 633–654. doi:10.3390/ijms12010633.
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 8, 102340. doi:10.1016/j.cis.2020.102340.
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. doi:10.3389/fpls.2019.00845.
- Banat, I.M.; Joshi, S.J. Biosurfactants: Production and Potential Applications in Microbial Enhanced oil Recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. doi:10.1016/j.bcab.2018.01.010.
- Domínguez Rivera, A.; Martínez Urbina, M.A.; López y López, V.A. Advances on Research in the use of Agro-Industrial Waste in Biosurfactant Production. World J. Microbiol. Biotechnol. 2019, 35, 1–18. doi:10.1007/s11274-019-2729-3.
- Adetunji, A.I.; Olaniran, A.O. Production and Potential Biotechnological Applications of Microbial Surfactants: An Overview. Saudi J. Biol. Sci. 2021, 28, 669–679. doi:10.1016/j.sjbs.2020.10.058.
- Wang, F.; Shang, J.; Zhang, Q.; Lu, T.; Li, Y.; Wang, X.; Farooq, U.; Qi, Z. Influence of Surfactant Molecular Features on Tetracycline Transport in Saturated Porous Media of Varied Surface Heterogeneities. Water Res. 2024, 255, 121501.
- Chen, Y.; Ma, F.; Wu, Y.; Tan, S.; Niu, A.; Qiu, W.; Wang, G. Biosurfactant from Pseudomonas Fragi Enhances the Competitive Advantage of Pseudomonas but Reduces the Overall Spoilage Ability of the Microbial Community in Chilled Meat. Food Microbiol. 2023, 115, 104311.
- Chafale, A.; Kapley, A. Biosurfactants as Microbial Bioactive Compounds in Microbial Enhanced oil Recovery. J. Biotechnol. 2022, 352, 1–15. doi:10.1016/j.jbiotec.2022.05.003.
- Sarubbo, L.A.; Da Gloria, M.; Silva, C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, Properties, Applications, Trends, and General Perspectives. Biochem. Eng. J. 2022, 181, 108377. doi:10.1016/j.bej.2022.108377.
- Safdel, M.; Anbaz, M.A.; Daryasafar, A.; Jamialahmadi, M. Microbial Enhanced oil Recovery, a Critical Review on Worldwide Implemented Field Trials in Different Countries. Renew. Sustain. Energy Rev. 2017, 74, 159–172. doi:10.1016/j.rser.2017.02.045.
- Henkel, M.; Hausmann, R. Chapter 2 – Diversity and Classification of Microbial Surfactants. In Biobased Surfactants, Second Ed.; AOCS Press, 2019, pp 41–63.
- Kumari, K.; Nandi, A.; Sinha, A.; Ghosh, A.; Sengupta, S.; Saha, U.; Singh, P.K.; Panda, P.K.; Raina, V.; Verma, S.K. The Paradigm of Prophylactic Viral Outbreaks Measures by Microbial Biosurfactants. J. Infect. Public Health 2023, 16, 575–587. doi:10.1016/j.jiph.2023.02.016.
- Dubeau, D.; Déziel, E.; Woods, D.E.; Lépine, F. Burkholderia Thailandensis Harbors Two Identical Rhl Gene Clusters Responsible for the Biosynthesis of Rhamnolipids. BMC Microbiol. 2009, 9, 263. doi:10.1186/1471-2180-9-263.
- Li, Z.; Zhang, Y.; Lin, J.; Wang, W.; Li, S. High-Yield Di-Rhamnolipid Production by Pseudomonas Aeruginosa YM4 and its Potential Application in MEOR. Molecules 2019, 24. doi:10.3390/molecules24071433.
- Ansari, M.M.; Singh, T.; Majhi, B.; Misra, S.; Chauhan, P.S. Chapter 21 – Biosurfactant producing plant growth–promoting bacteria: eco-friendly approaches for charcoal rot management. In Macrophomina Phaseolina, Academic Press, 2023, pp 313–321.
- Souza, E.C.; Vessoni-Penna, T.C.; De Souza Oliveira, R.P. Biosurfactant-enhanced Hydrocarbon Bioremediation: An Overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. doi:10.1016/j.ibiod.2014.01.007.
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential Commercial Applications of Microbial Surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. doi:10.1007/s002530051648.
- Ree, L.C.; de la Hunty, M.; Moret, S.; Chadwick, S. An Investigation Into the Effect of Surfactants on Iron Oxide Powder Suspension Formulations for Fingermark Development. Forensic Sci. Int. 2024, 358, 112019.
- Chong, H.; Li, Q. Microbial Production of Rhamnolipids: Opportunities, Challenges and Strategies. Microb. Cell Fact 2017, 16, 1–12. doi:10.1186/s12934-017-0753-2.
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging Trends and Promising Strategies in the Field of Biotechnology and Biomedicine. Microb. Cell Fact 2021, 20, 1–15. doi:10.1186/s12934-020-01497-9.
- Gudiña, E.J.; Rodrigues, A.I.; De Freitas, V.; Azevedo, Z.; Teixeira, J.A.; Rodrigues, L.R. Valorization of Agro-Industrial Wastes Towards the Production of Rhamnolipids. Bioresour. Technol. 2016, 212, 144–150. doi:10.1016/j.biortech.2016.04.027.
- Chebbi, A.; Franzetti, A.; Gomez Tovar, F.H.; Sbaffoni, S.; Vaccari, M. Potentials of Winery and Olive oil Residues for the Production of Rhamnolipids and Other Biosurfactants: A Step Towards Achieving a Circular Economy Model. Waste Biomass Valoriz 2020, 12, 4733–4743.
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and Applications in Drug Delivery. Biotech. Ecotoxic. Bioeng 2021, 8. doi:10.3390/bioengineering8080115.
- Hogan, D.E.; Tian, F.; Malm, S.W.; Olivares, C.; Palos Pacheco, R.; Simonich, M.T.; Hunjan, A.S.; Tanguay, R.L.; Klimecki, W.T.; Polt, R.; Pemberton, J.E.; Curry, R.M. Biodegradability and Toxicity of Monorhamnolipid Biosurfactant Diastereomers. J. Hazard. Mater. 2019, 364, 600–607. doi:10.1016/j.jhazmat.2018.10.050.
- Ma, K.Y.; Sun, M.Y.; Dong, W.; He, C.Q.; Chen, F.L.; Ma, Y.L. Effects of Nutrition Optimization Strategy on Rhamnolipid Production in a Pseudomonas Aeruginosa Strain DN1 for Bioremediation of Crude oil. Biocatal. Agric. Biotechnol. 2016, 6, 144–151. doi:10.1016/j.bcab.2016.03.008.
- Liu, Y.; Zeng, G.; Zhong, H.; Wang, Z.; Liu, Z.; Cheng, M.; Liu, G.; Yang, X.; Liu, S. Effect of Rhamnolipid Solubilization on Hexadecane Bioavailability: Enhancement or Reduction? J. Hazard. Mater. 2017, 322, 394–401. doi:10.1016/j.jhazmat.2016.10.025.
- Helmy, Q.; Gustiani, S.; Mustikawati, A.T. Application of rhamnolipid biosurfactant for bio-detergent formulation. In IOP Conference Series: Mat. Sci.Eng 2020, 823, 012014.
- Yalaoui-Guellal, D.; Fella-Temzi, S.; Djafri-Dib, S.; Sahu, S.K.; Irorere, V.U.; Banat, I.M.; Madani, K. The Petroleum-Degrading Bacteria Alcaligenes Aquatilis Strain YGD 2906 as a Potential Source of Lipopeptide Biosurfactant. Fuel 2021, 285, 119112. doi:10.1016/j.fuel.2020.119112.
- Soares Dos Santos, A.; Pereira, N.J.; Freire, D.M.G. Strategies for Improved Rhamnolipid Production by Pseudomonas Aeruginosa PA1. Peer J. 2016, 4, e2078. doi:10.7717/peerj.2078.
- Parus, A.; Ciesielski, T.; Woźniak-Karczewska, M.; Ślachciński, M.; Owsianiak, M.; Ławniczak, Ł; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Basic Principles for Biosurfactant-Assisted (bio)Remediation of Soils Contaminated by Heavy Metals and Petroleum Hydrocarbons – A Critical Evaluation of the Performance of Rhamnolipids. J. Hazard. Mater. 2023, 443, 130171. doi:10.1016/j.jhazmat.2022.130171.
- Mohan, P.K.; Nakhla, G.; Yanful, E.K. Biokinetics of Biodegradation of Surfactants Under Aerobic, Anoxic and Anaerobic Conditions. Water Res. 2006, 40, 533–540. doi:10.1016/j.watres.2005.11.030.
- Oberbremer, A.; Müller-Hurtig, R.; Wagner, F. Effect of the Addition of Microbial Surfactants on Hydrocarbon Degradation in a Soil Population in a Stirred Reactor. Appl. Microbiol. Biotechnol. 1990, 32, 485–489. doi:10.1007/BF00903788.
- Johann, S.; Weichert, F.G.; Schröer, L.; Stratemann, L.; Kämpfer, C.; Seiler, T.B.; Heger, S.; Töpel, A.; Sassmann, T.; Pich, A.; Jakob, F. A Plea for the Integration of Green Toxicology in Sustainable Bioeconomy Strategies – Biosurfactants and Microgel-Based Pesticide Release Systems as Examples. J. Hazard. Mater. 2022, 426, 127800. doi:10.1016/j.jhazmat.2021.127800.
- Zeng, Z.; Liu, Y.; Zhong, H.; Xiao, R.; Zeng, G.; Liu, Z.; Cheng, M.; Lai, C.; Zhang, C.; Liu, G.; Qin, L. Mechanisms for Rhamnolipids-Mediated Biodegradation of Hydrophobic Organic Compounds. Sci. Total Environ. 2018, 634, 1–11. doi:10.1016/j.scitotenv.2018.03.349.
- Taghavi, N.; Zhuang, W.Q.; Baroutian, S. Effect of Rhamnolipid Biosurfactant on Biodegradation of Untreated and UV-Pretreated non-Degradable Thermoplastics: Part 2. J. Environ. Chem. Eng 2022, 10, 107033. doi:10.1016/j.jece.2021.107033.
- Mata-Sandoval, J.C.; Karns, J.; Torrents, A. Influence of Rhamnolipids and Triton X-100 on the Desorption of Pesticides from Soils. Environ. Sci. Technol. 2002, 36, 4669–4675. doi:10.1021/es011260z.
- Sandrin, T.R.; Chech, A.M.; Maier, R.M. A Rhamnolipid Biosurfactant Reduces Cadmium Toxicity During Naphthalene Biodegradation. Appl. Environ. Microbiol. 2000, 66, 4585–4588. doi:10.1128/AEM.66.10.4585-4588.2000.
- Aleksic, I.; Petkovic, M.; Jovanovic, M.; Milivojevic, D.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Anti-biofilm Properties of Bacterial Di-Rhamnolipids and Their Semi-Synthetic Amide Derivatives. Front. Microbiol. 2017, 8, 1–16. doi:10.3389/fmicb.2017.02454.
- Patel, R.M.; Desai, A.J. Surface-active Properties of Rhamnolipids from Pseudomonas Aeruginosa GS3. J. Basic Microbiol. 1997, 37, 281–286. doi:10.1002/jobm.3620370407.
- Haba, E.; Abalos, A.; Jáuregui, O.; Espuny, M.J.; Manresa, A. Use of Liquid Chromatography-Mass Spectroscopy for Studying the Composition and Properties of Rhamnolipids Produced by Different Strains of Pseudomonas Aeruginosa. J. Surfactants Deterg. 2003, 6, 155–161. doi:10.1007/s11743-003-0260-7.
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical Characterization and Antimicrobial Properties of Rhamnolipids Produced by Pseudomonas Aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. doi:10.1002/bit.10474.
- Deepika, K.V.; Ramu Sridhar, P.; Bramhachari, P.V. Characterization and Antifungal Properties of Rhamnolipids Produced by Mangrove Sediment Bacterium Pseudomonas Aeruginosa Strain KVD-HM52. Biocatal. Agric. Biotechnol. 2015, 4, 608–615. doi:10.1016/j.bcab.2015.09.009.
- Prabakaran, G.; Hoti, S.L.; Rao, H.S.P.; Vijjapu, S. Di-rhamnolipid is a Mosquito Pupicidal Metabolite from Pseudomonas Fluorescens (VCRC B426). Acta Trop. 2015, 148, 24–31. doi:10.1016/j.actatropica.2015.03.003.
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; García, F.; Manresa, A. Physicochemical and Antimicrobial Properties of new Rhamnolipids Produced by Pseudomonas Aeruginosa AT10 from Soybean oil Refinery Wastes. Langmuir 2001, 17, 1367–1371. doi:10.1021/la0011735.
- Li, D.; Tao, W.; Yu, D.; Li, S. Emulsifying Properties of Rhamnolipids and Their In Vitro Antifungal Activity Against Plant Pathogenic Fungi. Molecules 2022, 27. doi:10.3390/molecules27227746.
- Costa, S.G.V.A.O.; Nitschke, M.; Lépine, F.; Déziel, E.; Contiero, J. Structure, Properties and Applications of Rhamnolipids Produced by Pseudomonas Aeruginosa L2-1 from Cassava Wastewater. Process Biochem. 2010, 45, 1511–1516. doi:10.1016/j.procbio.2010.05.033.
- Abbot, V.; Paliwal, D.; Sharma, A.; Sharma, P. A Review on the Physicochemical and Biological Applications of Biosurfactants in Biotechnology and Pharmaceuticals. Heliyon 2022, 8, e10149. doi:10.1016/j.heliyon.2022.e10149.
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An Overview of Current Research and Developments in Biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. doi:10.1016/j.jiec.2021.05.017.
- Ben Ayed, H.; Jemil, N.; Maalej, H.; Bayoudh, A.; Hmidet, N.; Nasri, M. Enhancement of Solubilization and Biodegradation of Diesel oil by Biosurfactant from Bacillus Amyloliquefaciens An6. Int. Biodeterior. Biodegrad. 2015, 99, 8–14. doi:10.1016/j.ibiod.2014.12.009.
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, Properties, and Applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. doi:10.1016/j.cocis.2020.03.013.
- Satpute, S.K.; Płaza, G.A.; Banpurkar, A.G. Management Systems in Production Engineering Biosurfactants ‘ Production from Renewable Natural Resources : Example of Innovative and Smart Technology. Manag. Syst. Prod. Eng. 2017, 25, 46–54.
- Joy, S.; Rahman, P.K.S.M.; Sharma, S. Biosurfactant Production and Concomitant Hydrocarbon Degradation Potentials of Bacteria Isolated from Extreme and Hydrocarbon Contaminated Environments. Chem. Eng. J. 2017, 317, 232–241. doi:10.1016/j.cej.2017.02.054.
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. doi:10.3390/ijms17030401.
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not Biosurfactants and Require Different Screening Approaches. Front. Microbiol. 2015, 6, 245. doi:10.3389/fmicb.2015.00245.
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. Biomed Res. Int. 2023, 1, 2375223. doi:10.1155/2023/2375223.
- Nikolova, C.; Gutierrez, T. Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives. Front. Bioeng. Biotechnol. 2021, 9. doi:10.3389/fbioe.2021.626639.
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in Food Industry. Trends Food Sci. Technol. 2007, 18, 252–259. doi:10.1016/j.tifs.2007.01.002.
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety – A Review. Agronomy 2022, 12, 1–35. doi:10.3390/agronomy12030662.
- Ghosh, M.; Singh, D.; Singh, A. Recent Advancements in Nanocarrier Based Therapy Against Acne: The Role of Biosurfactants and Status of Patents. Heal. Sci. Rev. 2023, 7, 100088. doi:10.1016/j.hsr.2023.100088.
- Sandeep, L. Biosurfactant: Pharmaceutical Perspective. J. Anal. Pharm. Res. 2017, 4, 1–2. doi:10.15406/japlr.2017.04.00105.
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Biosurfactant from Candida Sphaerica UCP0995 Exhibiting Heavy Metal Remediation Properties. Process Saf. Environ. Prot. 2016, 102, 558–566. doi:10.1016/j.psep.2016.05.010.
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2. doi:10.3390/colloids2030035.
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Rita de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. 2021. Production of Green Surfactants: Market Prospects. Elect. J. Biotech 2021, 51, 28–39.
- Fink, R.; Potočnik, A.; Oder, M. Plant-based Natural Saponins for Escherichia Coli Surface Hygiene Management. Lwt 2020, 122, 109018. doi:10.1016/j.lwt.2020.109018.
- Tiehm, A. Degradation of Polycyclic Aromatic Hydrocarbons in the Presence of Synthetic Surfactants. Appl. Environ. Microbiol. 1994, 60, 258–263. doi:10.1128/aem.60.1.258-263.1994.
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential Applications in Medicine. J. Antimicrob. Chemother 2006, 57, 609–618. doi:10.1093/jac/dkl024.
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant Production: Emerging Trends and Promising Strategies. J. Appl. Microbiol 2019, 126, 2–13. doi:10.1111/jam.14057.
- Wang, X.T.; Liu, B.; Li, X.Z.; Lin, W.; Li, D.A.; Dong, H.; Wang, L. Biosurfactants Produced by Novel Facultative-Halophilic Bacillus sp. XT-2 with Biodegradation of Long Chain n-Alkane and the Application for Enhancing Waxy oil Recovery. Energy 2022, 240, 122802. doi:10.1016/j.energy.2021.122802.
- Mishra, A.; Sawood, G.M.; Gautam, S.B.; Trivedi, R.K. Optimization of Process Inputs for the Synthesis of Waste Rice Bran oil Isolated Pseudomonas Aeruginosa MTCC 424 Biosurfactant Using Response Surface Methodology for oil Recovery Applications. Bioresour. Technol. Reports 2021, 14, 100653. doi:10.1016/j.biteb.2021.100653.
- Gidudu, B.; Chirwa, E.M.N. The Combined Application of a High Voltage, low Electrode Spacing, and Biosurfactants Enhances the bio-Electrokinetic Remediation of Petroleum Contaminated Soil. J. Clean. Prod. 2020, 276, 122745. doi:10.1016/j.jclepro.2020.122745.
- Liu, Q.; Niu, J.; Yu, Y.; Wang, C.; Lu, S.; Zhang, S.; Lv, J.; Peng, B. Production, Characterization and Application of Biosurfactant Produced by Bacillus Licheniformis L20 for Microbial Enhanced oil Recovery. J. Clean. Prod. 2021, 307, 127193. doi:10.1016/j.jclepro.2021.127193.
- Jia, K.; Yi, Y.; Ma, W.; Cao, Y.; Li, G.; Liu, S.; Wang, T.; An, N. Ion Flotation of Heavy Metal Ions by Using Biodegradable Biosurfactant as Collector: Application and Removal Mechanism. Miner. Eng. 2022, 176, 107338. doi:10.1016/j.mineng.2021.107338.
- Zeng, F.; Zhou, H.; Lin, X.; Li, Y.; Liang, Y.; Xie, Q.; Atakpa, E.O.; Shen, C.; Zhang, C. Enhanced Remediation of Fracturing Flowback Fluids by the Combined Application of a Bioflocculant/Biosurfactant-Producing Bacillus sp. SS15 and its Metabolites. Chemosphere 2022, 302, 134870. doi:10.1016/j.chemosphere.2022.134870.
- Ribeiro, B.G.; De Veras, B.O.; Dos Santos Aguiar, J.; Medeiros Campos Guerra, J.; Sarubbo, L.A. Biosurfactant Produced by Candida Utilis UFPEDA1009 with Potential Application in Cookie Formulation. Electron. J. Biotechnol. 2020, 46, 14–21. doi:10.1016/j.ejbt.2020.05.001.
- Stanghellini, M.E.; Miller, R.M. Their Identity and Potential Efficacy in the Biological Control of Zoosporic Plant Pathogens. Plant Dis. 1997, 81, 4–12. doi:10.1094/PDIS.1997.81.1.4.
- Kiran, G.S.; Ninawe, A.S.; Lipton, A.N.; Pandian, V.; Selvin, J. Rhamnolipid Biosurfactants: Evolutionary Implications, Applications and Future Prospects from Untapped Marine Resource. Crit. Rev. Biotechnol. 2016, 36, 399–415. doi:10.3109/07388551.2014.979758.
- Jarvis, F.G.; Johnson, M.J. A Glyco-Lipide Produced by Pseudomonas Aeruginosa. J. Am. Chem. Soc. 1949, 71 (12), 4124–4126.
- Blunt, W.; Blanchard, C.; Morley, K. Effects of Environmental Parameters on Microbial Rhamnolipid Biosynthesis and Bioreactor Strategies for Enhanced Productivity. Biochem. Eng. J. 2022, 182, 108436. doi:10.1016/j.bej.2022.108436.
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; Taherzadeh, M.J.; Ngo, H.H.; Chang, J.S.; Wong, J.W.; You, S.; Teixeira, J.A.; Bui, X.T. Bio-based Rhamnolipids Production and Recovery from Waste Streams: Status and Perspectives. Biores. Tech 2021, 319, 124213.
- Chebbi, A.; Franzetti, A.; Formicola, F.; Ambaye, T.G.; Gomez, F.H.; Murena, B.; De Marco, E.; Beltrani, T.; Sbaffoni, S.; Vaccari, M. Insights Into Rhamnolipid-Based Soil Remediation Technologies by Safe Microorganisms: A Critical Review. J. Clean. Prod. 2022, 367, 133088. doi:10.1016/j.jclepro.2022.133088.
- Dai, X.; Lv, J.; Zhang, Z.; Wang, H. Bioremediation of Heavy Oil-Contaminated Intertidal Zones Using Slow-Release Nutrients and Rhamnolipid Biosurfactants. J. Env. Chem. Eng. 2023, 11, 109323.
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Structure and Applications of a Rhamnolipid Surfactant Produced in Soybean oil Waste. Appl. Biochem. Biotechnol. 2010, 160, 2066–2074. doi:10.1007/s12010-009-8707-8.
- Massara, H.; Mulligan, C.N.; Hadjinicolaou, J. Effect of Rhamnolipids on Chromium-Contaminated Kaolinite. Soil Sediment Contam. 2007, 16, 1–14. doi:10.1080/15320380601071241.c.
- Elouzi, A.A.; Akasha, A.A.; Elgerbi, A.M.; El-Baseir, M.; El Gammudi, B.A. Removal of Heavy Metals Contamination by bio-Surfactants (Rhamnolipids). J. Chem. Pharm. Res. 2012, 4, 4337–4341.
- Bodagh, A.; Khoshdast, H.; Sharafi, H.; Shahbani Zahiri, H.; Akbari Noghabi, K. Removal of Cadmium(II) from Aqueous Solution by ion Flotation Using Rhamnolipid Biosurfactant as an ion Collector. Ind. Eng. Chem. Res. 2013, 52, 3910–3917. doi:10.1021/ie400085t.
- Mahmoodabadi, M.; Khoshdast, H.; Shojaei, V. Efficient dye Removal from Aqueous Solutions Using Rhamnolipid Biosurfactants by Foam Flotation. Iran. J. Chem. Chem. Eng. 2019, 38, 127–140.
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Ayodele, O.B.; Mohammed, I.Y.; Olupinla, S.F.; Daud, W.M.W. Insight Into Wastewater Decontamination Using Polymeric Adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672. doi:10.1016/j.jece.2018.02.019.
- Jayalatha, N.A.; Devatha, C.P. Experimental Investigation for Treating Ibuprofen and Triclosan by Biosurfactant from Domestic Wastewater. J. Environ. Manage. 2023, 328, 116913. doi:10.1016/j.jenvman.2022.116913.
- Zhang, H.; Long, X.; Sha, R.; Zhang, G.; Meng, Q. Biotreatment of Oily Wastewater by Rhamnolipids in Aerated Active Sludge System. J. Zhejiang Univ. Sci. B. 2009, 10, 852–859. doi:10.1631/jzus.B0920122.
- Tang, J.; He, J.; Liu, T.; Xin, X. Extraction and Environmental Risk Assessment of Heavy Metal in the Municipal Dewatered Sludge Using Rhamnolipid Treatment. Hum. Ecol. Risk Assess. An Int. J. 2017, 23, 1522–1538. doi:10.1080/10807039.2017.1331728.
- Kumar, R.; Das, A.J. Rhamnolipid Biosurfactant: Recent Trends Prod. Appl. 2018. doi:10.1007/9789811312892.
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics. 2020, 12. doi:10.3390/pharmaceutics12111099.
- Kumari, K.; Nandi, A.; Sinha, A.; Panda, P.K.; Ghosh, A.; Gouda, S.K.; Suar, M.; Verma, S.K.; Raina, V. Biosurfactant-functionalized Silver Nanoparticles Infer Intrinsic Proximal Interaction via Lysine and Glutamic Acid for Reduced in Vivo Molecular Biotoxicity with Embryonic Zebrafish Through Oxidative Stress and Apoptosis. J. Environ. Chem. Eng. 2023, 11, 110147. doi:10.1016/j.jece.2023.110147.
- Chen, J.; Wu, Q.; Hua, Y.; Chen, J.; Zhang, H.; Wang, H. Potential Applications of Biosurfactant Rhamnolipids in Agriculture and Biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. doi:10.1007/s00253-017-8554-4.
- Master, N.G.; Markande, A.R.; Patel, J.K. Comparative Negation of Amphiphile Production Using Nutrition Factors: Amyloids Versus Biosurfactants. Int. J. Biol. Macromol. 2024, 265, 130909.
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipids and PHAs: Recent Reports on Pseudomonas-Derived Molecules of Increasing Industrial Interest. Process Biochem. 2011, 46, 621–630. doi:10.1016/j.procbio.2010.12.012.
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids Produced by Pseudomonas: From Molecular Genetics to the Market. Microb. Biotechnol. 2021, 14, 136–146. doi:10.1111/1751-7915.13700.
- Funston, S.J.; Tsaousi, K.; Rudden, M.; Smyth, T.J.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Characterising Rhamnolipid Production in Burkholderia Thailandensis E264, a Non-Pathogenic Producer. Appl. Microbiol. Biotechnol 2016, 100, 7945–7956. doi:10.1007/s00253-016-7564-y.
- Marchant, R.; Banat, I.M. Microbial Biosurfactants: Challenges and Opportunities for Future Exploitation. Trends Biotechnol. 2012, 30, 558–565. doi:10.1016/j.tibtech.2012.07.003.
- Daniels, R.; Vanderleyden, J.; Michiels, J. Quorum Sensing and Swarming Migration in Bacteria. FEMS Microbiol. Rev 2004, 28, 261–289. doi:10.1016/j.femsre.2003.09.004.
- Déziel, E.; Lépine, F.; Dennie, D.; Boismenu, D.; Mamer, O.A.; Villemur, R. Liquid Chromatography/Mass Spectrometry Analysis of Mixtures of Rhamnolipids Produced by Pseudomonas Aeruginosa Strain 57RP Grown on Mannitol or Naphthalene. Biochim. Biophys. Acta 1999, 1440, 244–252. doi:10.1016/s1388-1981(99)00129-8.
- Loeschcke, A.; Thies, S. Engineering of Natural Product Biosynthesis in Pseudomonas Putida. Curr. Opin. Biotechnol. 2020, 65, 213–224. doi:10.1016/j.copbio.2020.03.007.
- Jirku, V.; Cejkova, A.; Schreiberova, O.; Jezdik, R.; Masak, J. Multicomponent Biosurfactants–A “Green Toolbox” Extension. Biotechnol. Adv. 2015, 33, 1272–1276. doi:10.1016/j.biotechadv.2015.03.005.
- Bator, I.; Wittgens, A.; Rosenau, F.; Tiso, T.; Blank, L.M. Comparison of Three Xylose Pathways in Pseudomonas Putida KT2440 for the Synthesis of Valuable Products. Front. Bioeng. Biotech. 2020, 7, 480.
- Lovaglio, R.B.; Silva, V.L.; Ferreira, H.; Hausmann, R.; Contiero, J. Rhamnolipids Know-how: Looking for Strategies for its Industrial Dissemination. Biotechnol. Adv. 2015, 33, 1715–1726. doi:10.1016/j.biotechadv.2015.09.002.
- Dusane, D.H.; Zinjarde, S.S.; Venugopalan, V.P.; McLean, R.J.C.; Weber, M.M.; Rahman, P.K.S.M. Quorum Sensing: Implications on Rhamnolipid Biosurfactant Production. Biotechnol. Genet. Eng. Rev. 2010, 27, 159–184. doi:10.1080/02648725.2010.10648149.
- Juhas, M.; Eberl, L.; Tümmler, B. Quorum Sensing: The Power of Cooperation in the World of Pseudomonas. Environ. Microbiol. 2005, 7, 459–471. doi:10.1111/j.1462-2920.2005.00769.x.
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids Produced by Pseudomonas: From Molecular Genetics to the Market. Microb. Biotech. 2021, 14, 136–146.
- Toribio, J.; Escalante, A.E.; Soberón-Chávez, G. Rhamnolipids: Production in Bacteria Other Than Pseudomonas Aeruginosa. Eur. J. Lipid Sci. Tech. 2010, 112, 1082–1087.
- Liu, S.; Xu, N.; Liu, H.; Zhou, J.; Xin, F.; Zhang, W.; Qian, X.; Jiang, M.; Dong, W. Genome Characterization of Pseudomonas Aeruginosa KT1115, a High Di-Rhamnolipid-Producing Strain with Strong Oils Metabolizing Ability. Curr. Microbiol. 2020, 77, 1890–1895.
- Warner, N.R.; Christie, C.A.; Jackson, R.B.; Vengosh, A. Impacts of Shale gas Wastewater Disposal on Water Quality in Western Pennsylvania. Environ. Sci. Technol. 2013, 47 (20), 11849–11857.
- Grosso-Becerra, M.V.; González-Valdez, A.; Granados-Martínez, M.J.; Morales, E.; Servín-González, L.; Méndez, J.L.; Delgado, G.; Morales-Espinosa, R.; Ponce-Soto, G.Y.; Cocotl-Yañez, M.; Soberón-Chávez, G. Pseudomonas Aeruginosa ATCC 9027 is a non-Virulent Strain Suitable for Mono-Rhamnolipids Production. Appl. Microbiol. Biotech. 2016, 100, 9995–10004.
- Wittgens, A.; Rosenau, F. Heterologous Rhamnolipid Biosynthesis: Advantages, Challenges, and the Opportunity to Produce Tailor-Made Rhamnolipids. Front. Bioeng. Biotech. 2020, 8, 594010.