ABSTRACT
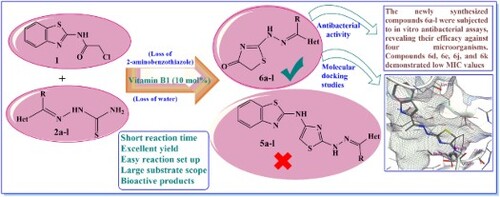
The study focuses on green methodologies in synthetic organic chemistry, emphasizing the use of thiamine hydrochloride (Vitamin B1) as an eco-friendly catalyst for synthesizing hydrazono-thiazolones. These compounds serve as versatile core units with various therapeutic activities, including anti-HIV, anti-tubercular, antifungal, antiviral, and anticancer properties. Building on prior research involving sustainable barium oxide-chitosan nanocomposite catalysts, this novel green synthetic approach contributes to the sustainable synthesis of bioactive heterocycles. The synthesized compounds 6a-l were tested in vitro against different bacterial species, revealing notable activity against Gram-positive and Gram-negative bacteria. The MIC investigation showed that compounds 6d, 6e, 6j, and 6k have broad-spectrum antibacterial potential, with 6j and 6k being especially effective, their MICs near those of ciprofloxacin, highlighting their strong antibacterial capabilities. Molecular docking studies predicting the binding mode of the most potent substances to Escherichia coli DNA gyrase (PDB ID 4DUH) aligned well with in vitro studies, confirming the new ligands’ potency as potential antimicrobial agents. In silico investigations for the most potent substances revealed a favorable ADME profile, suggesting promising pharmacological properties. This research contributes to both sustainable synthesis and potential therapeutic applications of hydrazono- thiazolone derivatives.
Introduction
The susceptibility of bacteria to antimicrobial drugs is a crucial concern in both community and hospital settings (Citation1–3). Infections caused by these bacteria have a widespread global impact, leading to high mortality rates. Consequently, there is a pressing need for effective treatments, driving research efforts toward the development of novel antimicrobials (Citation4–7). Over the years, a variety of antimicrobials have been employed to hinder microbial growth and control infections (Citation6, Citation8–10). In response to this, the production of pharmaceutically effective medications has increasingly relied on the utilization of heterocyclic scaffolds. Thiazolones, a significant family of heterocyclic compounds containing sulfur and nitrogen, play a pivotal role in this context. Hydrazono-thiazolones, as a core unite and crucial building blocks, reflect widespread therapeutic activities such as anti-HIV, anti-tubercular (Citation11), antifungal (Citation12), antiviral (Citation13), and anticancer (Citation14). Also, these scaffolds have been revealed antiproliferative activity against human cervix carcinoma cells (Citation15). It was conjectured that, 4-thiazolone ring is a deputy of the diphosphate moiety present in UDP-N-acetyl enol-pyruvyl glucosamine (Citation16). The pharmacological allure of synthetic thiazolone derivatives has fascinated chemists for decades, prompting exploration of their potential drug applications (Citation6, Citation14).
Green methodologies have prominence concept in modern synthetic organic chemistry due to its multifaceted values such as easy availability, non-toxicity, and cost effectiveness (Citation17, Citation18). These methodologies have been complied with contemporary guidelines for safety and environmental sustainability (Citation19, Citation20). The focal point for efficient green methodologies is based on the performance of biodegradable catalyst. Thiamine hydrochloride (Vit B1), as a natural organo-catalyst, has been emerged to catalyze the synthesis of huge number of heterocyclic compounds under safe and environmentally friendly conditions (Citation21, Citation22). Multicomponent cyclo-condensation reactions were catalyzed by Vit B1, as a biodegradable catalyst, to elaborate isoxazolones (Citation23), thiazolones (Citation24), and polyhydroquinoline derivatives (Citation25). Also, it has recently encompassed in synthesis of a variety series of azoles and azines, containing diverse hydrophobic groups, as bioactive heterocycles (Citation26). Additionally, Vit B1 is an efficient promoter for synthesis of fused pyridines (Citation27, Citation28) in aqueous conditions.
In our previous work (Citation29), we have reported a noteworthy novel synthetic approach for hydrazono-thiazolones in a solitary green methodology from N,N’-(alkane-diyl)bis(2-chloroacetamide) with 2-(arylidinehydrazine)-1-carbothioamide using sustainable barium oxide-chitosan nanocomposite catalyst. Conserving the therapeutic importance of hydrazono-thiazolones, and in continuation of our research interest for green synthesis of bioactive heterocycles (Citation6, Citation14, Citation30–40), we plan in this current study to prepare this target scaffold using thiamine hydrochloride (Vit B1), as a biodegradable efficient green catalyst. Additionally, the molecular docking was performed to scrutinize potential binding interactions of the most potent antimicrobial molecules against the 4DHU protein. This computational technique aids in understanding theoretical compound interactions with macromolecular structures, complementing in vitro antimicrobial results. Importantly, the selection of hetero-atoms is a deliberate choice based on their physiochemical properties and optimization for absorption, distribution, metabolism, and excretion (ADME) in the main therapeutic agents.
Results and discussion
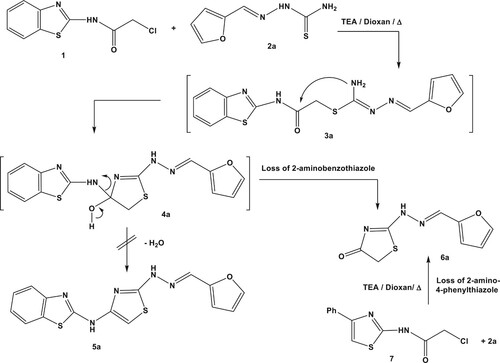
Our strategy in this report to interrogate the reaction pattern of chloroacetamide derivatives with hydrazine-1-carbothioamide moiety. Thus, N-(benzothiazol-2-yl)-2-chloroacetamide (1) (Citation41) was subjected to react with 2-(furan-2-ylmethylene)hydrazine-1-carbothioamide (2a) (Citation42) in dioxane and catalytic drops of triethylamine (Scheme 1). After completion of the reaction, the isolated product was analyzed and revealed molecular ion peak (M+) corresponding to hydrazono-thiazolone (6a) instead of hydrazonothiazole (5a).
The mechanistic pathway of this reaction was inspired and commenced by nucleophilic displacement with dehydrochlorination and intramolecular cyclization to give intermediate 4a. Dehydration of the latter to give compound 5a was excluded based on mass spectrum. On the other hand, loss of amino-benzothiazole moiety afforded the isolated product 6a. To assert the implemented reaction mechanism (Citation43), the authentic sample of hydrazono-thiazolone (6a) was prepared via alternative pathway. Thus, reaction of 2-chloro-N-(4-phenylthiazol-2-yl)acetamide (7) with compound 2a was proceeded via loss of 2-amino-4-phenylthiazole fragment to give compound 6a (Scheme 1).
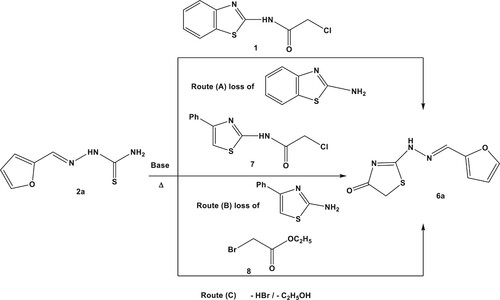
The widespread concern over the adverse consequence for using toxic catalysts influenced on the environment and human health. Thus, green chemistry enlivened researchers to deputize alternative safer catalysts in organic synthesis. Recently, conventional triethylamine was replaced by environmentally eco-friendly basic catalyst in heterocyclic chemistry (Citation6, Citation30, Citation33, Citation36, Citation44–46). In our study, we have illustrated different synthetic pathways (routes A-C, Scheme 2) of 2-[2-((furan-2-yl)methylene)hydrazinyl]thiazol-4(5H)-one (6a) via reaction of 2-(furan-2-ylmethylene)hydrazine-1-carbothioamide (2a) with N-(benzothiazol-2-yl)-2-chloroacetamide (1) (Route A) or 2-chloro-N-(4-phenylthiazol-2-yl)acetamide (7) (Route B) or ethyl bromoacetate (8) (Route C). Also, a comparative study of different basic catalyst (conventional triethylamine and vitamin B1 as a green catalyst) was revealed in .
Table 1. A comparative yield percentage of compound 6a via different synthetic pathways.
As shown in , different concentrations of vitamin B1 (2, 5, and 10 mol%) were used to investigate the catalyst optimization. The yields of the desired product 6a in case of triethylamine were significantly lower than vitamin B1 (entries 1,5,9). The enhancement of the yield percentage was observed by using 2 mol% of vitamin B1 in different routes (A-C) (entries 2,6,10). This observation encouraged us to augment the concentration of vitamin B1 to 5 mol% (entries 3,7,11) and 10 mol% (entries 4,8,12). Further inflating of vitamin B1 resulted into steady for the yield of compound 6a. The overall comparability revealed that vitamin B1 is much superior in terms of yield percentage.
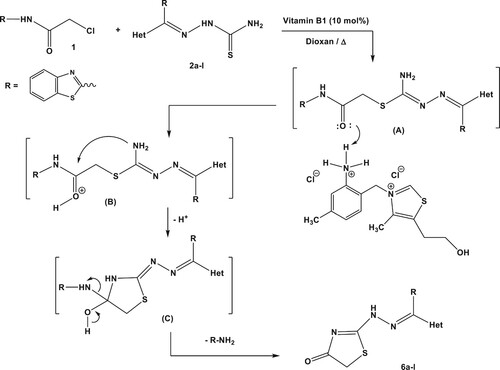
The feasibility of this green synthetic protocol was applied to prepare a series of hydrazono-thiazolones with different heterocycles moieties. Thus, treatment of N-(benzothiazol-2-yl)-2-chloroacetamide (1) with thiosemicarbazones 2a-l in dioxane and 10 mol% of vitamin B1 under thermal conditions afforded the respective hydrazono-thiazolone derivatives 6a-l in excellent yield as shown in Scheme 3 and . The systematic representation of Scheme 3 illustrated that vitamin B1 has conducted as a proton-donner (Citation24, Citation47) which bounded to oxygen atom of amide group on intermediate (A). Thus, the electrophilicity of an amide group will increase and facilitate the intramolecular cyclization (Citation24) of intermediate (B) to give intermediate (C). Removal of 2-aminobenzothiazole from the latter intermediate furnished the isolated products 6a-l.
Table 2. Percentage yields of 2-hydrazonothiazolidin-4(5H)-ones (6a-m).
The antibacterial screening
The newly synthesized compounds 6a-l were assayed in vitro for their antibacterial activity against various bacterial species, both Gram (+) and Gram (−), as well as their susceptibility to standard antibiotic treatments Ciprofloxacin. The data is represented as mean inhibition zone diameters (in millimeters) ± standard deviation measured at a concentration of 10 mM (agar diffusion well method). The bacterial species tested include Gram (+) bacteria such as Staphylococcus aureus (SA), Streptococcus epidermidis (SE), Bacillus subtilis (BS), Staphylococcus pyogenes (SP), and Gram (−) bacteria such as Pseudomonas aeruginosa (PA), Escherichia coli (EC), Klebsiella pneumoniae (KP), and Salmonella typhimurium (ST).
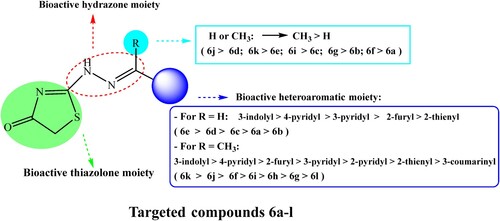
Upon analyzing the data in , several key observations can be made. Firstly, the compounds 6a-l exhibit varying degrees of antibacterial activity against the tested bacterial species. For example, compound 6k demonstrates potent activity against most strains. Conversely, compound 6b shows relatively weaker antibacterial activity with smaller inhibition zone diameters. Additionally, the data reveals differences in the susceptibility of different bacterial species to these compounds, with Gram (-) generally being less susceptible than Gram (+). Compounds 6j and 6k showed excellent activity against all the measured bacterial species. All the measured compounds have no activity towards PA. Compounds 6b, 6c, 6g, 6i, and 6l have no activity towards SP. All the tested compounds give greater activity towards SE than other species.
Table 3. Preliminary antibacterial activity for compounds 6a-l (10 mM).
For Gram (+), compounds 6d, 6f, 6j, and 6k demonstrate particularly high levels of activity, showing comparable or even superior efficacy compared to the standard antibiotic Ciprofloxacin. On the other hand, for Gram (-), compounds 6e, 6j, and 6k stands out as having notable activity against most of the tested strains, with similar or improved effectiveness compared to Ciprofloxacin. These findings suggest that certain compounds within the set (6d, 6f, 6j, and 6k for Gram (+) and 6e, 6j, and 6k for Gram (−)) hold promise as potential antibacterial agents and warrant further investigation. This data provides a preliminary insight into the antibacterial properties of compounds 6a-l, which can serve as a foundation for more comprehensive studies and the development of new antibiotics with improved efficacy and broader spectrum.
Furthermore, the minimum inhibitory concentration (MIC) of the new synthesized compounds 6a-l was determined as the lowest concentration at which no visible growth of bacteria was observed on the plate, as detailed in and .
Table 4. MICs of the synthesized compounds against the tested pathogenic bacteria.Table Footnotea
presents the Minimum Inhibitory Concentrations (MICs) of synthesized compounds against various pathogenic bacteria, revealing a range of antibacterial efficacies. Compounds 6d, 6e, 6j, and 6k show significant activity against both Gram (+) and Gram (-) bacteria, with notably low MIC values, suggesting a broad spectrum of action. In particular, compounds 6j and 6k exhibit exceptionally potent activity, with MIC values approaching those of the standard antibiotic, Ciprofloxacin, especially against Gram (+) strains SA and SE, and Gram (−) bacteria EC and KP. This indicates their potential as effective antibacterial agents. Conversely, compounds 6a, 6b, 6g, 6h, and 6l demonstrate moderate to weak activity. The data underscore the promising therapeutic potential of specific synthesized compounds, particularly 6d, 6e, 6j, and 6k, as potent antibacterial agents with efficacy close to that of Ciprofloxacin against certain pathogens.
Molecular docking studies
Following the identification of active compounds against pathogenic bacterial species, studies employing docking techniques were carried out to elucidate the binding modes. The computational method of molecular docking (Citation34, Citation48) was employed to discern the interaction between ligands and the active pocket of target proteins. This approach facilitates the identification and analysis of how ligands interact with the target proteins, providing valuable insights into their binding mechanisms. Docking of the database of the most potent synthesized compounds, in addition to ciprofloxacin (reference drug), against 4DUH was performed by using Triangle Matcher as placement algorithm and london dG as scoring method. The interactions are summarized in , where overall docking results were compared to ciprofloxacin, revealing interactions with protein residues ().
Table 5. Docking score (kcal/mol), No. of H-bonding, No. of arene interaction, and RMSD of most potent synthesized compounds with 4DUH receptor when compared to Ciprofloxacin.
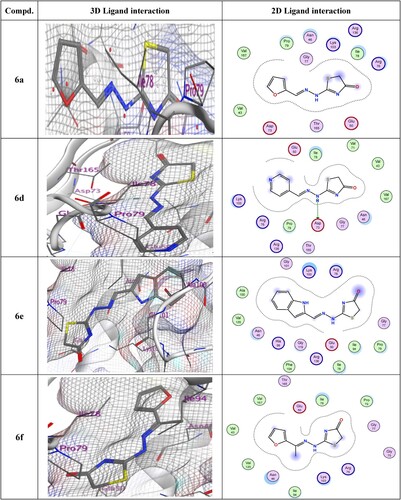
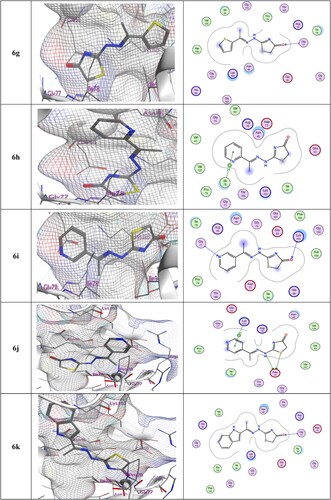
In and , the examination of compound 6d showed a nitrogen atom acting as an H-bond donor with Asp73. The 3D models of compounds 6g and 6k indicated a hydrogen bond acceptor between the oxygen of the thiazolone ring and Gly77. In contrast, the docking of 6i revealed two H-bonding acceptors, one between the oxygen of the thiazolone ring and the residue of Asp103, and another between the nitrogen atom in the pyridine ring and the residue of Gly77. Finally, the docking of 6j, gave two H-bonding donors between the amino acid Asp73 with S of thiazolone and with NH. Additionally, the thiazolone 6j form arene interaction (pi–H) with the residue of Lys103.
Figure 3. 3D and 2D ligand interactions within the binding site of 4DUH for most potent synthesized compounds.
Design and evaluation of physicochemical properties
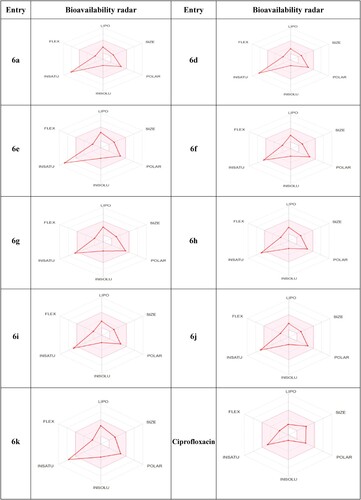
When a molecule adheres to the ‘rule of 5 (Ro5),’ it adopts drug-like characteristics (Citation49). The radar figure in outlines the optimal physicochemical space for oral bioavailability (LIPO: Lipophilicity, SIZE: M.Wt, POLAR: TPSA, INSOLU: Solubility, INSATU: Insaturation, FLEX: Flexibility). Ro5 criteria specify a molecular mass below 500, a log Po/w under 5, and fewer than 5 and 10 hydrogen bond donors and acceptors, respectively (Citation50). According to the Veber rule, molecules with rotatable bonds, polar surface area, and H-bond donors and acceptors of 10, 140, and 12 or fewer, respectively, exhibit superior oral bioavailability (Citation51). These features also influence a molecule’s absorption, distribution, metabolism, excretion, and toxicity (ADMET) (Citation52). Thus, meticulous control of physicochemical features is crucial in determining a molecule’s suitability as a therapeutic candidate and its performance in drug development stages.
In silico physicochemical and ADME properties prediction
In silico pharmacokinetic studies
The Swiss ADME web server (Citation53) was utilized to evaluate physiological parameters such as ADME (Adsorption, Distribution, Metabolism, Excretion) and medication similitude for synthesized thiazolone compounds. The SMILE format of the most potent compounds was inputted into the platform to predict physiological and pharmacokinetic parameters, with the comprehensive results presented in .
Table 6. The physicochemical properties, lipophilicity, pharmacokinetic characteristics, and drug likeness matching of the most potent thiazolidinone derivatives.
All structures examined, were required to adhere to Lipinski’s ‘rule of five.’ The majority of the synthesized thiazolone derivatives exhibited high gastrointestinal (GI) absorption, according to GI absorption data. None of the examined compounds demonstrated blood–brain barrier (BBB) permeability, suggesting an absence of central nervous system (CNS) side effects or suitability for treating CNS infections. In-silico P-glycoprotein (P-gp) substrate inhibition data revealed that the examined thiazolone derivatives, except ciprofloxacin, are not inhibitors of gp-substrate. This non-substrate candidacy for P-glycoprotein is a notable characteristic, as P-gp functions as an efflux transporter. Among the compounds, only 6g inhibited CYP1A2, while none of the examined thiazolone and ciprofloxacin exhibited inhibition of any inhibitors. According to drug likeness data, all examined thiazolones demonstrated significant antimicrobial action, aligning with the criteria set by Lipinski, Ghose, Veber, Egan, and Muegge.
Experimental section
Instruments
The melting points of hydrazono-thiazoloidinone products were measured on Gallenkamp IA 9000, as a digital electrothermal apparatus, (uncorrected). The solid samples were grinded with potassium bromide in the form of discs and injected to Pye-Unicam SP300 instrument to measure IR spectra. Recording of 1H NMR (at 300 MHz) and 13C NMR (at 75 MHz) spectra was manipulated on A Varian Mercury VXR-300 spectrometer using tetramethyl silane as internal standard. Mass spectra of products was recorded on GCMS-Q1000-EX Shimadzu spectrometer and the ionizing voltage was adapted at 70 eV.
General procedure for the preparation of 2-hydrazonothiazol-4(5H)-ones
Method A
Both of N-(benzothiazol-2-yl)-2-chloroacetamide (1) (0.226 g, 1 mmol) and 2-(furan-2-ylmethylene) hydrazine-1-carbothioamide (2a) (0.169 g, 1 mmol) were mingled together and dissolved in 20 mL dioxane containing few drops of triethylamine. The mixture was refluxed for 3 h until all the starting materials were depleted (as monitored by TLC). Eviction of excess solvent was achieved under reduced pressure and the reaction mixture was triturated with methanol. Filtration, washing with methanol, and recrystallization from ethanol are three consecutive processes to obtain the isolated product 6a.
Method B
N-(Benzothiazol-2-yl)-2-chloroacetamide (1) (0.226 g, 1 mmol) was mingled with appropriate 2-(arylidinehydrazine)-1-carbothioamide 2a-m (1 mmol) and dissolved in 20 mL dioxane containing 10 mol% of Vitamin B1. The reaction mixture was thermally heated under constant volume for 3 h until all the starting material was depleted (as monitored by TLC). Ice/water mixture was poured onto the reaction mixture to get rid of catalyst and precipitate the products. Filtration was operated to collect the products which rinsed by methanol, and dried. Recrystallization of the products 6a-l was achieved from ethanol.
Method C
A mixture of 2-(furan-2-ylmethylene)hydrazine-1-carbothioamide (2a) (0.169 g, 1 mmol) and 2-chloro-N-(4-phenylthiazol-2-yl)acetamide (7) or ethyl bromoacetate (8) (1 mmol) was dissolved in 20 mL dioxane containing few drops of triethylamine or 10 mol% Vitamin B1. The mixture was refluxed and treated as described in Method A and Method B.
2-[2-((Furan-2-yl)methylene)hydrazinyl]thiazol-4(5H)-one (6a)
Pale brown powder, mp 233–235°C (Citation54); IR (KBr) υ = 3221 (NH), 1703 (C=O), 1598 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 3.86 (s, 2H, S-CH2), 6.61-6.98 (m, 2H, furan-H), 7.72-7.86 (m, 1H, furan-H), 8.19 (s, 1H, CH=N), 11.93 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 33.1 (CH2), 113.3, 116.9, 141.8, 145.4, 148.2, 159.7 (Ar-C and CH=N), 174.8 (C=O) ppm;MS, m/z (%) 209 (M+, 85). Anal. Calcd. For C8H7N3O2S (209.03): C, 45.93; H, 3.37; N, 20.08; S, 15.32. Found: C, 45.72; H, 3.28; N, 20.11; S, 15.21%.
2-[2-((Thiophen-2-yl)methylene)hydrazinyl]thiazol-4(5H)-one (6b)
Pale yellow powder, mp 233–236°C (Citation54); IR (KBr) υ = 3218 (NH), 1707 (C=O), 1600 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 3.75 (s, 2H, S-CH2), 7.15-7.87 (m, 3H, thiophene-H), 8.31 (s, 1H, CH=N), 11.94 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 32.7 (CH2), 128.3, 129.9, 131.8, 138.4, 151.2, 160.7 (Ar-C and CH=N), 174.5 (C=O) ppm; MS, m/z (%) 225 (M+, 70). Anal. Calcd. For C8H7N3OS2 (225.01): C, 42.65; H, 3.13; N, 18.65; S, 28.46. Found: C, 42.73; H, 3.08; N, 18.52; S, 28.35%.
2-[2-((Pyridin-3-yl)methylene)hydrazinyl]thiazol-4(5H)-one (6c)
Pale yellow powder, mp 276–278°C (Citation54); IR (KBr) υ = 3228 (NH), 1703 (C=O), 1599 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 3.91 (s, 2H, S-CH2), 7.45-8.83 (m, 5H, pyridine-H + CH=N), 12.04 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 32.8 (CH2), 125.3, 130.5, 134.8, 145.4, 151.4, 160.7, 162.3 (Ar-C and CH=N), 174.3 (C=O) ppm; MS, m/z (%) 220 (M+, 83). Anal. Calcd. For C9H8N4OS (220.04): C, 49.08; H, 3.66; N, 25.44; S, 14.56. Found: C, 48.92; H, 3.58; N, 25.31; S, 14.71%.
2-[2-((Pyridin-4-yl)methylene)hydrazinyl]thiazol-4(5H)-one (6d)
Yellow powder, mp 255–257°C (Citation54); IR (KBr) υ = 3231 (NH), 1709 (C=O), 1601 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 3.93 (s, 2H, S-CH2), 7.75-8.81 (m, 5H, pyridine-H + CH=N), 12.09 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 33.1 (CH2), 122.9, 134.8, 145.4, 153.4, 162.7, 165.3 (Ar-C and CH=N), 174.8 (C=O) ppm; MS, m/z (%) 220 (M+, 75). Anal. Calcd. For C9H8N4OS (220.04): C, 49.08; H, 3.66; N, 25.44; S, 14.56. Found: C, 48.91; H, 3.52; N, 25.51; S, 14.68%.
2-[2-((1H-indol-2-yl)methylene)hydrazinyl]thiazol-4(5H)-one (6e)
Pale yellow powder, mp 233–236°C (Citation54); IR (KBr) υ = 3220 (NH), 1704 (C=O), 1600 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 3.85 (s, 2H, S-CH2), 7.13-8.27 (m, 5H, indole-H), 8.51 (s, 1H, CH=N), 11.63 (s, 1H, NH), 11.91 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 32.8 (CH2), 113.7, 121.3, 122.3, 123.8, 125.3, 132.4, 137.2, 144.7, 152.1, 152.6 (Ar-C and CH=N), 174.4 (C=O) ppm; MS, m/z (%) 258 (M+, 72). Anal. Calcd. For C12H10N4OS (258.06): C, 55.80; H, 3.90; N, 21.09; S, 12.41. Found: C, 55.91; H, 3.84; N, 21.28; S, 12.29%.
2-[2-(1-(Furan-2-yl)ethylidine)hydrazinyl]thiazol-4(5H)-one (6f)
Pale brown powder, mp 142–144°C (Citation54); IR (KBr) υ = 3228 (NH), 1703 (C=O), 1598 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.31 (s, 3H, CH3), 3.76 (s, 2H, S-CH2), 6.61-6.98 (m, 2H, furan-H), 7.72-7.86 (m, 1H, furan-H), 11.93 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 15.6 (CH3), 32.9 (CH2), 112.3, 116.8, 141.8, 145.4, 148.3, 159.2 (Ar-C and CH=N), 178.8 (C=O) ppm;MS, m/z (%) 223 (M+, 88). Anal. Calcd. For C9H9N3O2S (223.04): C, 48.42; H, 4.06; N, 18.82; S, 14.36. Found: C, 48.29; H, 3.91; N, 18.71; S, 14.51%.
2-[2-(1-(Thiophen-2-yl)ethylidine)hydrazinyl]thiazol-4(5H)-one (6g)
Orange powder, mp 162–164°C (Citation54); IR (KBr) υ = 3214 (NH), 1709 (C=O), 1599 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.42 (s, 3H, CH3), 3.76 (s, 2H, S-CH2), 7.04-7.41 (m, 3H, thiophene-H), 11.92 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 15.4 (CH3), 33.1 (CH2), 128.3, 129.8, 134.8, 145.4, 156.3, 160.2 (Ar-C and CH=N), 174.5 (C=O) ppm;MS, m/z (%) 239 (M+, 81). Anal. Calcd. For C9H9N3OS2 (239.02): C, 45.17; H, 3.79; N, 17.56; S, 26.79. Found: C, 44.98; H, 3.62; N, 17.38; S, 26.70%.
2-[2-(1-(Pyridin-2-yl)ethylidine)hydrazinyl]thiazol-4(5H)-one (6h)
Pale brown powder, mp 242–244 oC (Citation54); IR (KBr) υ = 3225 (NH), 1701 (C=O), 1601 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.43 (s, 3H, CH3), 3.86 (s, 2H, S-CH2), 7.47-8.12 (m, 4H, pyridine-H), 12.08 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 15.1 (CH3), 33.2 (CH2), 121.3, 124.8, 136.8, 148.4, 156.3, 160.2, 164.2 (Ar-C and CH=N), 174.5 (C=O) ppm; MS, m/z (%) 234 (M+, 75). Anal. Calcd. For C10H10N4OS (234.06): C, 51.27; H, 4.30; N, 23.92; S, 13.68. Found: C, 51.13; H, 4.18; N, 23.78; S, 13.57%.
2-[2-(1-(Pyridin-3-yl)ethylidine)hydrazinyl]thiazol-4(5H)-one (6i)
Pale yellow powder, mp 206–208°C (Citation54); IR (KBr) υ = 3219 (NH), 1709 (C=O), 1600 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.37 (s, 3H, CH3), 3.87 (s, 2H, S-CH2), 7.58-9.00 (m, 4H, pyridine-H), 12.05 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 14.8 (CH3), 33.4 (CH2), 124.3, 133.8, 134.8, 148.5, 151.3, 159.2, 161.2 (Ar-C and CH=N), 174.8 (C=O) ppm; MS, m/z (%) 234 (M+, 70). Anal. Calcd. For C10H10N4OS (234.06): C, 51.27; H, 4.30; N, 23.92; S, 13.68. Found: C, 51.15; H, 4.47; N, 23.82; S, 13.54%.
2-[2-(1-(Pyridin-4-yl)ethylidine)hydrazinyl]thiazol-4(5H)-one (6j)
Pale yellow powder, mp 206–208°C (Citation54); IR (KBr) υ = 3224 (NH), 1708 (C=O), 1603 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.38 (s, 3H, CH3), 3.91 (s, 2H, S-CH2), 7.75-8.64 (m, 4H, pyridine-H), 12.08 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 15.8 (CH3), 33.4 (CH2), 123.3, 148.5, 154.3, 164.2, 169.2 (Ar-C and CH=N), 174.4 (C=O) ppm; MS, m/z (%) 234 (M+, 82). Anal. Calcd. For C10H10N4OS (234.06): C, 51.27; H, 4.30; N, 23.92; S, 13.68. Found: C, 51.39; H, 4.45; N, 24.08; S, 13.54%.
2-[2-(1-(1H-indol-3-yl)ethylidene)hydrazinyl]thiazol-4(5H)-one (6k)
Yellow powder, mp 216–218°C (Citation55); IR (KBr) υ = 3327, 3237 (2NH), 1714 (C=O), 1600 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.46 (s, 3H, CH3), 3.85 (s, 2H, S-CH2), 7.09-7.42 (m, 5H, indole-H), 11.58 (s, 1H, NH), 11.82 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 15.3 (CH3), 32.9 (CH2), 114.7, 121.3, 123.3, 124.8, 125.3, 132.4, 138.2, 145.7, 152.7, 162.6 (Ar-C and CH=N), 174.8 (C=O) ppm.; MS, m/z (%) 272 (M+, 50). Anal. Calcd. For C13H12N4OS (272.07): C, 57.34; H, 4.44; N, 20.57; S, 11.77. Found: C, 57.18; H, 4.28; N, 20.38; S, 11.62%.
2-[2-(1-(2-oxo-2H-chromen-3-yl)ethylidene)hydrazinyl]thiazol-4(5H)-one (6l)
Yellow powder, mp 238–240°C (Citation54); IR (KBr) υ = 3208 (NH), 1738, 1714 (2C=O), 1602 (C=N) cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.46 (s, 3H, CH3), 3.84 (s, 2H, S-CH2), 7.36-8.15 (m, 5H, coumarin-H), 12.01 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ = 16.8 (CH3), 33.4 (CH2), 116.7, 118.1, 119.8, 126.8, 129.8, 133.5, 142.3, 153.2, 161.2 (Ar-C and CH=N), 174.8, 180.2 (C=O) ppm.; MS, m/z (%) 301 (M+, 45). Anal. Calcd. For C14H11N3O3S (301.05): C, 55.81; H, 3.68; N, 13.95; S, 10.64. Found: C, 55.67; H, 3.49; N, 13.88; S, 10.55%.
Antibacterial activity assay
The bacterial strains utilized in this research were sourced from the Regional Center for Mycology and Biotechnology (RCMB) at Al-Azhar University, Cairo, Egypt. To evaluate the antimicrobial efficacy of a series of novel compounds, their activity was determined against specific microbes via the Agar diffusion well method (Citation56). Initially, bacterial cultures reached a density of about 108 cells/mL. Then, wells of 10 mm in diameter were made in agar gel plates, into which 100 µL of each compound (1 mg/mL concentration) was dispensed. These plates were incubated at 37 °C for a period ranging from 24 to 48 h, after which the bacterial growth inhibition was visually assessed by measuring the zones of inhibition around the wells in millimeters, to gauge the compounds’ antimicrobial potency.
Ciprofloxacin served as a benchmark for comparing antibacterial effectiveness, where the extent of the inhibition zone around a well indicated the compound's inhibitory capacity, with wider zones denoting stronger activity. To validate the experimental outcomes, solvent controls with DMSO (Dimethyl sulfoxide), the solvent used for dissolving the compounds under investigation, were also included. These controls showed no inhibition zones, verifying that DMSO had no impact on bacterial growth and ensuring the reliability of the antimicrobial activity observed was solely attributed to the compounds being tested.
MIC determination
The antimicrobial characteristics of the active compounds were further investigated by determining their minimum inhibitory concentration (MIC) values. This assessment utilized a modified version of the agar well diffusion technique, as outlined in earlier studies. We tested concentrations of each active compound ranging from 0.1 to 1000 µg/mL and compared the results with those of conventional drugs. The MIC was defined as the lowest concentration that effectively inhibited the growth of the microorganism within a period of 24 to 48 h.
Docking study
Ligands preparation: Ligands were prepared using the Molecular Operating Environment software, with molecular modeling conducted for potent substances sketched through Chemdraw 12.0. Minimizations were carried out until a root mean square deviation (RMSD) gradient of 0.1 kcal.mol−1.A−1 using Merck Molecular Force Field 94 (Citation57).
Protein preparation: The X-ray crystal structure of the enzyme (PDB ID: 4DUH, resolution: 1.50 Å) was retrieved in PDB format from the Protein Data Bank (Citation58). To prepare the enzyme for docking studies (Citation59), specific steps were undertaken: (1) retaining only chain B and eliminating crystallographic water molecules, (2) introducing hydrogen atoms with standard geometry, reconnecting broken bonds, and addressing potential issues, (3) incorporating dummy atoms into the enzyme structure using Alpha Site Finder for comprehensive site exploration (Citation60). Subsequently, the protein's binding pocket was identified and saved as a Moe file for predicting ligand-enzyme interactions and docking compound molecules. The analysis of the ligand's interactions with active site amino acids involved the use of the Triangle Matcher placement method and the London dG score tool for docking. Following the docking process, visualizations of both 2D and 3D interactions with amino acid residues were conducted in accordance with established protocols, and comprehensive documentation of all docking operations and scoring procedures was maintained (Citation61).
Conclusion
In summary, employing thiamine hydrochloride (Vitamin B1) as a catalyst for hydrazono-thiazolones synthesis marks a noteworthy advancement in eco-friendly and biodegradable methodologies within synthetic organic chemistry. This study not only emphasizes the potential for sustainable production of bioactive heterocycles but also underscores the varied therapeutic applications of hydrazono-thiazolone derivatives 6a-l. Enhancing our knowledge of environmentally conscious synthesis brings us closer to a more sustainable and efficient future for pharmaceutical and chemical industries. The newly synthesized compounds 6a-l underwent antibacterial testing, showing effectiveness against several bacterial species. Specifically, compounds 6d, 6e, 6f, 6j, and 6k demonstrated notable activity against both Gram (+) and Gram (−) bacteria, comparable to the antibiotic Ciprofloxacin. The investigation into their MICs revealed diverse antibacterial strengths. Compounds 6d, 6e, 6j, and 6k, in particular, showed significant activity with low MIC values, suggesting broad-spectrum antibacterial potential. Especially, 6j and 6k were highly effective, with MICs close to Ciprofloxacin's against specific bacteria, indicating their strong antibacterial capabilities. On the other hand, compounds 6a, 6b, 6g, 6h, and 6l exhibited minimal activity. Molecular docking and ADME studies indicated the thiazolone derivatives’ potential for developing new antimicrobial drugs, emphasizing the notable antibacterial potential of 6d, 6e, 6j, and 6k.
Acknowledgements
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Therap. 2015, 40, 277–283.
- PLOS Medicine Editors. Antimicrobial Resistance: Is The World UN Prepared. PLoS Med. 2016, 13, e1002130.
- Khan, S.; Iqbal, S.; Shah, M.; Rehman, W.; Hussain, R.; Rasheed, L.; Alrbyawi, H.; Dera, A.A.; Alahmdi, M.I.; Pashameah, R.A.; Alzahrani, E. Synthesis: In Vitro Anti-Microbial Analysis and Molecular Docking Study of Aliphatic Hydrazide-Based Benzene Sulphonamide Derivatives as Potent Inhibitors of α-Glucosidase and Urease. Molecules 2022, 27, 7129. 10.3390molecules27207129.
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; Yan, Y. Bacteriophage Cocktails Protect Dairy Cows Against Mastitis Caused by Drug Resistant Escherichia coli Infection. Front. Cell. Infect. Microbiol. 2021, 11, 690377. DOI: 10.3389/fcimb.2021.690377.
- Abbas, I.M.; Abdallah, M.A.; Gomha, S.M.; Kazem, M.S.H. Synthesis and Antimicrobial Activity of Novel Azolopyrimidines and Pyrido-Triazolo-Pyrimidinones Incorporating Pyrazole Moiety. J. Heterocycl. Chem. 2017, 54, 3447–3457. 10.1002jhet.2968.
- Edrees, M.M.; Abu-Melha, S.; Saad, A.M.; Kheder, N.A.; Gomha, S.M.; Muhammad, Z.A. Eco-Friendly Synthesis: Characterization and Biological Evaluation of Some Novel Pyrazolines Containing Thiazole Moiety as Potential Anticancer and Antimicrobial Agents. Molecules 2018, 23, 2970.
- Gomha, S.M.; El-Gendy, M.S.; Muhammad, Z.A.; Abdelhamid, A.O.; Abdel-Aziz, M.M. Utility of Bis-Hydrazonoyl Chlorides as Precursors for Synthesis of New Functionalized Bis-Thiadiazoles as Potent Antimicrobial Agents. J. Heterocycl. Chem. 2018, 55, 844–851. 10.1002jhet.3108.
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; Greko, C. Antibiotic Resistance-The Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098.
- Abu-Melha, S.; Muhammad, Z.A.; Abouzid, A.S.; Edrees, M.M.; Abo Dena, A.S.; Nabil, S.; Gomha, S.M. Multicomponent Synthesis, DFT Calculations and Molecular Docking Studies of Novel Thiazolyl-Pyridazinones as Potential Antimicrobial Agents Against Antibiotic-Resistant Bacteria. J. Mol. Str. 2021, 1234, 130180. 10.1016j.molstruc.2021.130180.
- Karam, G.; Chastre, J.; Wilcox, M.H.; Vincent, J.L. Antibiotic Strategies in the Era of Multidrug Resistance. Crit. Care 2016, 20, 1–9.
- Pasha, M.A.; Mondal, S.; Panigrahi, N.; Shetye, G.; Ma, R.; Franzblau, S.G.; Zheng, Y.-T.; Murugesan, S. One-Pot Synthesis of Novel Hydrazono-1,3-Thıazolıdın-4-One Derivatives as Anti-HIV and Anti-tubercular Agents: Synthesis,: Biological Evaluation, Molecular Modelling and Admet Studies. Curr. HIV Res. 2022, 20, 255–271.
- Carradori, S.; Bizzarri, B.; D'Ascenzio, M.; De Monte, C.; Grande, R.; Rivanera, D.; Zicari, A.; Mari, E.; Sabatino, M.; Pasilinakos, A.; Ragno, R.; Secci, D. Synthesis: Biological Evaluation and Quantitative Structure-Active Relationships of 1,3-Thiazolidin-4-One Derivatives. A Promising Chemical Scaffold Endowed with High Antifungal Potency and Low Cytotoxicity. Eur. J. Med. Chem. 2017, 10, 274–292.
- Varluvothu, A.; Vaarla, K.; Vermeire, K.; Leelavathi, P. Microwave-Assisted One-Pot Multicomponent Synthesis of 2-(-4-oxo-2-(1-(2-oxo-2h-Chromen-3-yl) Ethylidene) Hydrazono) Thiazolidin-5-yl) Acetic Acid Derivatives and Their Antiviral Activity. J. Heterocycl. Chem. 2022, 59, 1604–1615. 10.1002jhet.4493.
- Gomha, S.M.; Abdelaziz, M.R.; Kheder, N.A.; Abdel-aziz, H.M.; Alterary, S.; Mabkhot, Y.N. A Facile Access and Evaluation of Some Novel Thiazole and 1,3,4-Thiadiazole Derivatives Incorporating Thiazole Moiety as Potent Anticancer Agents. Chem. Centr. J. 2017, 11, 105.
- Abbas, H.-A.; Abd El-Karim, S.S. Design Synthesis and Anticervical Cancer Activity of New Benzofuran–Pyrazol-Hydrazonothiazolidin-4-One Hybrids as Potential EGFR Inhibitors and Apoptosis Inducing Agents. Bioorg. Chem. 2019, 89, 103035.
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent Developments and Biological Activities of Thiazolidinone Derivatives: A Review. Bioorg. Med. Chem. 2012, 20, 3378–3395.
- Pair, E.; Cadart, T.; Levacher, V.; Brière, J.-F. Meldrum’s Acid: A Useful Platform in Asymmetric Organocatalysis. Chem. Cat. Chem. 2016, 8, 1882–1890. 10.1002cctc.201600247.
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, H.M.; Matar, I.K.; El-Sayed, A.A. Green Synthesis: Molecular Docking and Anticancer Activity of Novel 1,4-Dihydropyridine-3,5-Dicarbohydrazones Under Grind-Stone Chemistry. Green Chem. Lett. Rev. 2020, 13, 6–17. 10.108017518253.2019.1710268.
- Török, B.; Dransfield, T. Green Chemistry: An Inclusive Approach; Elsevier: Amsterdam, 2018.
- Török, M. Contemporary Chemical Approaches for Green and Sustainable Drugs; Elsevier: Amsterdam, 2022.
- Chen, L.; Bao, S.; Yang, L.; Zhang, X.; Li, B.; Li, Y. Cheap Thiamine Hydrochloride as Efficient Catalyst for Synthesis of 4H-Benzo[b]pyrans in Aqueous Ethanol. Res. Chem. Intermed. 2017, 43, 3883–3891. 10.1007s11164-016-2843-x.
- Singh, R.; Ganaie, S.A. An Eco-Compatible Synthesis of Novel Spiro[Acenaphthylene-1,2′[1,3]-Thiazolidine]-2,4′(1H)-Diones Using Thiamine Hydrochloride as Efficient Catalyst in Aqueous Medium. Res. Chem. Intermed. 2017, 43, 45–55. 10.1007s11164-016-2604-x.
- Zhang, D.; Liu, C.; Ren, L.; Li, W.; Luan, B.; Zhang, Y. Vitamin B -Catalyzed Multicomponent Reaction for Efficient Synthesis of an Isoxazolone Compound by Using Ultrasound in a Water and Its Selective Identification of Metal Ions. Chem. Select 2023, 8, e202204658. 10.1002slct.202204658.
- Bhgat, D.S.; Gurnule, W.B.; Rathod, Y.U.; Sankhla, M.S.; Pandit, V.U. Thiamine Hydrochloride (Vitamin B1) Catalyzed Greener Synthesis of Thiazolidin-4-One Derivatives. Mater. Today Proc. 2022, 53, 52–57. 10.1016j.matpr.2021.12.292.
- Gholap, S.; Gunjal, N. Thiamine Hydrochloride (Vit-B1): An Optimized Green Alternative for the Synthesis of Polyhydroquinoline Derivatives. Iran. J. Cat. 2016, 6, 147–152.
- Fahmy, A.F.M.; Rizk, S.A.; Hemdan, M.M.; El-Sayed, A.A.; Hassaballah, A.J. Efficient Green Synthesis and Computational Chemical Study of Some Interesting Heterocyclic Derivatives as Insecticidal Agents. J. Heterocycl. Chem. 2018, 55, 2545–2555. 10.1002jhet.3308.
- Liu, J.; Lei, M.; Hu, L. Thiamine Hydrochloride (VB1): An Efficient Promoter for the One-Pot Synthesis of Benzo[4,5]Imidazo[1,2-a]Pyrimidine and [1,2,4]Triazolo[1,5-a]Pyrimidine Derivatives in Water Medium. Green Chem. 2012, 14, 840–846.
- Siddiqui, I.R.; Rai, P.; Rahila; Srivastava, A.; Srivastava, A.; Srivastava, A. Synthesis of Fused Pyridines in the Presence of Thiamine Hydrochloride as an Efficient and Reusable Catalyst in Aqueous Conditions. New J. Chem. 2013, 37, 3798–3804.
- Khalil, K.D.; Riyadh, S.M.; Bashal, A.H.; Abolibda, T.Z.; Gomha, S.M. Green Synthetic Approaches of 2-Hydrazonothiazol-4(5H)-Ones Using Sustainable Barium oxide-Chitosan Nanocomposite Catalyst. Polymers 2023, 15, 3817. 10.3390polym15183817
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis: Characterization and Application of Copper Oxide Chitosan Nanocomposite for Green Regioselective Synthesis of [1,2,3]Triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937.
- Riyadh, S.M.; Khalil, K.D.; Aljuhani, A. Chitosan-MgO Nanocomposite: One Pot Preparation and Its Utility as an Ecofriendly Biocatalyst in the Synthesis of Thiazoles and [1,3,4]thiadiazoles. Nanomaterials 2018, 8, 928. 10.3390nano8110928.
- Al-Humaidi, J.Y.; Gomha, S.M.; Abd El-Ghany, N.A.; Farag, B.; Zaki, M.E.A.; Abolibda, T.Z.; Mohamed, N.A. Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst. Catalysts 2023, 13, 1311.
- Khalil, K.D.; Riyadh, S.M.; Alkayal, N.S.; Bashal, A.H.; Alharbi, K.H.; Alharbi, W. Chitosan-Strontium Oxide Nanocomposite: Preparation, Characterization, and Catalytic Potency in Thiadiazoles Synthesis. Polymers 2022, 14, 2827. 10.3390polym14142827.
- Gomha, S.M.; Riyadh, S.M.; Alharbi, R.A.K.; Zaki, M.E.A.; Abolibda, T.Z.; Farag, B. Green Route Synthesis and Molecular Docking of Azines Using Cellulose Sulfuric Acid under Microwave Irradiation. Crystals 2023, 13, 260. 10.3390cryst13020260.
- Abdallah, A.M.; Gomha, S.M.; Zaki, M.E.A.; Abolibda, T.Z.; Kheder, N.A. A Green Synthesis, DFT Calculations, and Molecular Docking Study of Some New Indeno[2,1-b]Quinoxalines Containing Thiazole Moiety. J. Mol. Str. 2023, 1292, 136044. 10.1016j.molstruc.2023.136044.
- Alshabanah, L.A.; Gomha, S.M.; Al-Mutabagani, L.A.; Abolibda, T.Z.; Abd El-Ghany, N.A.; El-Enany, W.A.M.A.; El-Ziaty, A.K.; Ali, R.S.; Mohamed, N.A. Cross-Linked Chitosan/Multi-Walled Carbon Nanotubes Composite as Ecofriendly Biocatalyst for Synthesis of Some Composite as Ecofriendly Biocatalyst for Synthesis of Some Novel Benzil Bis-Thiazoles. Polymers 2021, 13, 1728. 10.3390polym13111728.
- Yaccoubi, F.; El-Naggar, M.; Abdelrazek, F.M.; Gomha, S.M.; Farghaly, M.S.; Abolibda, T.Z.; Ali, L.A.; Abdelmonsef, A.H. Pyrido-Pyrimido-Thiadiazinones: Green Synthesis, Molecular Docking Studies and Biological Investigation as Obesity Inhibitors. J. Taibah Uni. Sci. 2022, 16, 1275–1286. 10.108016583655.2022.2159210.
- Said, M.A.; Riyadh, S.M.; Al-Kaff, N.S.; Nayl, A.A.; Khalil, K.D.; Bräse, S.; Gomha, S.M. Synthesis and Greener Pastures Biological Study of Bis-Thiadiazoles as Potential Covid-19 Drug Candidates. Arab. J. Chem. 2022, 15, 104101.
- Al-Humaidi, J.Y.; Badrey, M.G.; Aly, A.A.; Nayl, A.A.; Zayed, M.E.M.; Jefri, O.A.; Gomha, S.M. Evaluation of the Binding Relationship of the RdRp Enzyme to Novel Thiazole/ Acid Hydrazone Hybrids Obtainable Through Green Synthetic Procedure. Polymer 2022, 14, 1594.
- Abu-Melha, S.; Edrees, M.M.; Said, M.A.; Riyadh, S.M.; Al-Kaff, N.S.; Gomha, S.M. Potential COVID-19 Drug Candidates Based on Diazinyl-Thiazol-Imine Moieties: Synthesis and Greener Pastures Biological Study. Molecules 2022, 27, 488.
- Singh, D.C.P.; Hashim, S.R.; Singhal, R.G. Synthesis and Antimicrobial Activity of Some New Thioether Derivatives of Quinoxaline. E. J. Chem 2011, 8, 635–642. DOI: 10.11552011/482831.
- Hernández, W.; Carrasco, F.; Vaisberg, A.; Spodine, E.; Icker, M.; Krautscheid, H.; Beyer, L.; Tamariz-Angeles, C.; Olivera-Gonzales, P. Novel Thiosemicarbazone Derivatives from Furan-2-Carbaldehyde: Synthesis: Characterization, Crystal Structures, and Antibacterial, Antifungal, Antioxidant, and Antitumor Activities. Hindawi J. Chem. 2023, 5413236. DOI: 10.11552023/5413236
- Nayak, S.; Gaonkar, S.L. A New Microwave-Assisted Method for the Synthesis of 2-Substituted-Thiazol-4(5H)-one via Hantzsch Condensation. Rasayan J. Chem. 2022, 15, 310–315. 10.31788RJC.2022.1516578.
- Gomha, S.M.; Badrey, M.G.; Arafa, W.A.A. DABCO-Catalyzed Green Synthesis of Thiazole and 1,3-Thiazine Derivatives Linked to Benzofuran. Heterocycles 2016, 92, 1450–1461.
- Sayed, A.R.; Abd El-lateef, H.M.; Gomha, S.M.; Abolibda, T.Z. L-Proline Catalyzed Green Synthesis and Anticancer Evaluation of Novel Bioactive Benzil Bis-Hydrazones Under Grinding Technique. Green Chem. Lett. Rev. 2021, 14, 180–189. 10.108017518253.2021.1893392.
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activity of Arylazothiazoles and 1,3,4-Thiadiazoles Using Chitosan-Grafted-Poly (4- Vinylpyridine) as a Novel Copolymer Basic Catalyst. Chem. Heterocycl. Compd. 2015, 51, 1030–1038.
- Lei, M.; Ma, L.; Hu, L. A Convenient One-Pot Synthesis of Formamide Derivatives Using Thiamine Hydrochloride as a Novel Catalyst. Tetrahedron Lett. 2010, 51, 4186–4188. 10.1016j.tetlet.2010.06.005.
- Badrey, M.G.; Gomha, S.M.; Zaki, M.E.A.; Farag, B.; El-Reedy, A.A.M. Cyanauric Cloride as a Key Precursor and a Core Component for Three-Armed Triazolopyrimidines: Recent Finding About SARs and Docking Analyses. Res. Chem. 2024, 7, 101337.
- Al-Humaidi, J.Y.; Gomha, S.M.; El-Ghany, N.A.A.; Farag, B.; Zaki, M.E.; Abolibda, T.Z.; Mohamed, N.A. Green Synthesis and Molecular Docking Study of Some New Thiazoles Using Terephthalohydrazide Chitosan Hydrogel as Ecofriendly Biopolymeric Catalyst. Catalysts 2023, 13, 1311. 10.3390catal13091311.
- Kola, I.; Landis, J. Can the Pharmaceutical Industry Reduce Attrition Rates? Nat. Rev. Drug Disc. 2004, 3, 711–716.
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties that Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623.
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the Links Between in Vitro Potency, ADMET and Physicochemical Parameters. Nat. Rev. Drug Disc 2011, 10, 197–208.
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717.
- D'Ascenzio, M.; Bizzarri, B.; De Monte, C.; Carradori, S.; Bolasco, A.; Secci, D.; Rivanera, D.; Faulhaber, N.; Bordon, C.; Jones-Brando, L. Design Synthesis and Biological Characterization of Thiazolidin-4-One Derivatives as Promising Inhibitors of Toxoplasma Gondii. Eur. J. Med. Chem. 2014, 86, 17–30. 10.1016j.ejmech.2014.08.046.
- Abdel-Gawad, H.; Mohamed, H.A.; Dawood, K.M.; Badria, F.A. Synthesis and Antiviral Activity of New Indole-Based Heterocycles. Chem. Pharm. Bull. 2010, 58, 1529–1531. 10.1248cpb.58.1529.
- Smania, A.; Monache, F.D.; Smania, E.F.A.; Cuneo, R.S. Antibacterial Activity of Steroidal Compounds Isolated From Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) Fruit Body. Int. J. Med. Mushrooms 1999, 1, 325–330.
- Abolibda, T.Z.; Fathalla, M.; Farag, B.; Zaki, M.E.; Gomha, S.M. Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase. Molecules 2023, 28, 689. 10.3390molecules28020689.
- Brvar, M.; Perdih, A.; Renko, M.; Anderluh, G.; Turk, D.; Solmajer, T. Structure-Based Discovery of Substituted 4,5′-Bithiazoles as Novel DNA Gyrase Inhibitors. J. Med. Chem. 2012, 55, 6413–6426.
- Ibrahim, M.S.; Farag, B.; Al-Humaidi, J.Y.; Zaki, M.E.A.; Fathalla, M.; Gomha, S.M. Mechanochemical Synthesis and Molecular Docking Studies of New Azines Bearing Indole as Anticancer Agents. Molecules 2023, 28, 3869. 10.3390molecules28093869.
- Labute, P. Protonate3D: Assignment of Ionization States and Hydrogen Coordinates to Macromolecular Structures. Proteins 2009, 75, 187–205.
- Kattan, S.W.; Nafie, M.S.; Elmgeed, G.A.; Alelwani, W.; Badar, M.; Tantawy, M.A. Molecular Docking, Anti-Proliferative Activity and Induction of Apoptosis in Human Liver Cancer Cells Treated with Androstane Derivatives: Implication of PI3 K/AKT/mTOR Pathway. J. Ster. Biochem. Mol. Biol. 2020, 198, 105604.