ABSTRACT
Aim
To describe hand use development in children with unilateral cerebral palsy who did/did not participate in constraint-induced movement therapy (CIMT) before 7 years of age.
Method
The study included 334 participants (18 months–12 years) who were assessed with 1,565 Assisting Hand Assessments (AHAs) and categorized into no intensive training (NIT), CIMT (18 months–7 years), and Baby-CIMT (<18 months) groups.
Results
AHA performance at 18 months (AHA-18) was positively associated with development regardless of training. The CIMT group had lower AHA-18 performance than the NIT group (p = .028), but higher stable limit (p = .076). The age when 90% of development was reached was highest in the CIMT group (p = .014). Although non-significant, the Baby-CIMT group had higher mean curve than NIT and CIMT combined (AHA-18 p = .459, limit p = .477).
Conclusion
The CIMT group improved more over time than the NIT group. Intensive training extended the window of development, and Baby-CIMT might promote early development.
Introduction
The positive effects of intensive training programs for the upper extremities in children with unilateral cerebral palsy (UCP) are well established. Common intensive training programs include constraint-induced movement therapy (CIMT), bimanual intensive training, and goal-directed training.Citation1–5 In Sweden and Norway, intensive training as an addition to standard pediatric rehabilitation programs is primarily offered to preschool and young school-aged children as well as, more recently, infants. However, the long-term effects of intensive training have not been thoroughly investigated. The typical follow-up time employed in the literature is 3–12 months after training,Citation2 with only a few small studies utilizing longer follow-up periods.Citation6,Citation7 The extent to which this type of training influences children’s long-term development is more important than knowledge of its short-term effects. Although children’s outcomes are often assumed to be related to the available rehabilitation services, both the type and severity of the brain lesion, as well as genetic factors, are also known to strongly influence development.Citation8,Citation9
The current evidence for upper limb training is predominantly based on controlled studies; thus, insufficient knowledge exists regarding the long-term effects of training performed in clinical practice. In addition, to understand how environmental factors influence development, it is important to consider the services that are available in countries with distinct cultures and social structures before the results can be generalized. Sweden and Norway have numerous similarities as welfare states. All children with UCP have access to standard pediatric rehabilitation services that encompass free systematic monitoring of motor functioning during childhood and interventions that include parental guidance on home training. Upper limb interventions in Scandinavia generally comprise activity-based functional skills training, most often conducted as individualized preschool or home-based programs supervised by an occupational therapist.Citation10 Intensive hand-training programs are intended to be performed at the most feasible time in the child’s everyday setting when sufficient health care resources are available. In this study, most of the included children were enrolled in CIMT training as part of standard rehabilitation services, although some had participated in research programs provided by local occupational therapists.Citation11 In line with the CIMT protocol,Citation11 intensive training was defined as a period of intensive, structured training based on motor learning theory and emphasizing active engagement in purposeful activity, motivation, repetition, and intensity of practice.
The aim of this study was to describe the developmental trajectories of hand use, as measured with the Assisting Hand Assessment (AHA), of children with UCP between 18 months and 12 years of age, comparing those who had participated in a CIMT program before 7 years of age with those who had received standard care only.
Materials and Methods
Design
This study employed a longitudinal cohort design and included a merged convenience sample of data from Norway and Sweden. The Norwegian data were retrieved from the Norwegian Quality and Surveillance Registry for Cerebral Palsy (NorCP) on March 31, 2020, while the Swedish data were collected from a convenience sample in the Stockholm region.
Participants
Children in the Norwegian cohort were included if they were registered in the NorCP with two or more AHAs and if information was available regarding whether they had participated in intensive hand training. The Swedish cohort comprised children who had participated in previous longitudinal data collection beginning in 2004, with continuous inclusion over subsequent years. Many of the participants were included in previous studies (n = 166 in Klevberg et al.,Citation12 n = 96 in Nordstrand et al.,Citation7 and n = 55 in Eliasson et al.Citation11,Citation13,Citation14) All participants had access to standard pediatric rehabilitation services, which included spasticity-reducing treatment (e.g. botulinum toxin A).
Parents signed informed consent upon registration in the NorCP or inclusion in the Swedish studies. The study was approved by the Data Protection Officer of Oslo University Hospital, the Regional Committee for Medical and Health Research Ethics of South East Norway (reg. nr. 2019/30715), and the Swedish Ethical Review Authority (reg. nr 2003–151 and reg. nr. 2003, 03–151, 2015/2281–32).
Assessments
The AHA is a criterion-referenced standardized test for children with UCP aged 18 months to 18 years that measures the spontaneous use of the affected hand during bimanual play.Citation15 It consists of a semi-structured, video-based play session in which performance is rated on a 4-point scale for 22 items and transformed through Rasch analysis to an AHA-unit scale of 0 to 100 points. A higher score indicates better performance. The inter-rater reliability of the AHA has been found to be good.Citation16
The Manual Ability Classification System (MACS) was developed to describe the ability to handle objects during everyday activities on a 5-level ordinal scale. Children with UCP are classified as levels I – III.Citation17 The Mini-MACS has been available since 2016 for children under 4 years of age.Citation18
The classification of brain injury was based on magnetic resonance imaging (MRI) and characterized as normal, predominantly white matter diffusion injury (WMDI), predominantly gray matter/focal injury, other injury, or missing.Citation19
Intensive Hand Training
CIMT was administered for approximately 2 hours a day over a 2-month period (approximately 80–120 hours in total). Baby-CIMT was usually administered as two 6-week blocks of training, with 30 minutes of training per day (approximately 30–40 hours in total). Older children wore a fabric glove with a built-in stiff plastic volar splint or similar apparatus on the dominant hand during CIMT while infants wore a soft restraint during Baby-CIMT. Five children who had performed bimanual intensive training, administered similarly to CIMT, were also included, as previous reports have shown the short-term effects of bimanual training to be similar to CIMT.Citation3 The training occurred during the preschool period, either at home or at the preschool, and parents and teachers were the providers of the training, which was typically supervised by occupational therapists at least once a week. No intensive training (NIT) indicated children who had access to standard pediatric rehabilitation services, including contact with an occupational therapist and physical therapist, without a structured intensive training program targeting hand function.
Procedure
In the Norwegian cohort, the AHAs were administered and scored by occupational therapists at regional pediatric rehabilitation units as part of the standard NorCP protocol, in which children with UCP are followed with yearly assessments until 7 years of age and, thereafter, annually or every second year depending on their MACS level. In the Swedish cohort, the AHA sessions were conducted by occupational therapists at a local rehabilitation center or by the research team at Karolinska Hospital. The assessments were primarily rated by the research team. The children were generally invited for data collection once a year until 8 years of age and, thereafter, every second year or when suitable for the families. All raters were certified in scoring the AHA but not blinded, as the tests were part of clinical practice. All AHAs completed for participants between 18 months and 12 years of age were included. Participants’ MACS levels were classified during the final assessments and retrieved from the NorCP for the Norwegian cohort and from medical records or discussion with caregivers for the Swedish cohort. Descriptive information was gathered from the NorCP for the Norwegian participants and during data collection for the Swedish participants. MRIs were classified by pediatricians in the rehabilitation units or in-patient pediatric hospital units.
Three groups were analyzed: 1) children who participated in intensive training between 18 months and 7 years of age (CIMT), 2) children not included in any intensive hand training (NIT), and 3) children who participated in Baby-CIMT before 18 months of age (Baby-CIMT). Most children in the CIMT group had one period of intensive training, though some children had multiple bouts. For the Baby-CIMT group we know that 27 children participated in additional CIMT before 7 years of age. Data on multiple training bouts were not systematically collected and thus not explored in the analysis.
Data Analysis
Descriptive analyses of the study population were conducted using IBM SPSS Statistics version 25. As indicated in , Welch’s independent samples t-test was used to compare continuous variables and Pearson’s chi-squared and Fisher’s exact tests were used to compare categorical variables between the countries. For comparisons between the training groups, one-way between-groups ANOVA and Tukey’s test were used. All statistical tests were performed as two-sided tests, and test results were considered statistically significant if p-values were<0.05.
Table 1. Description of the study participants.
Developmental trajectories from repeated AHA measures were estimated with a stable limit nonlinear mixed-effects model (SLM). The SLM has two parameters corresponding to the asymptotic limit and rate of change in AHA units as a function of age (for further details, see Klevberg et al.Citation12: AHA = limit − (limit − start) × exponent (−rate × age). Both parameters were entered into the model as fixed effects and random effects by patient ID with a diagonal covariance structure. In addition, a second random effect for the limit by country was included after it was found to improve the model fit (according to likelihood-ratio tests with significance threshold of 0.05). A country-specific random effect for the rate parameter did not improve the model fit and was thus not included in the final model. Thus, three different nested models were fitted to the data: model 1 without any random effect for country, model 2 with only a random effect for the limit by country, and model 3 with random effects for both limit and rate parameters by country. The model fits were compared by likelihood-ratio tests between the following pairs of nested models Citation20: 1 vs 2 (likelihood ratio LR = 5.91, p-value = .015) and 2 vs 3 (LR = 0.01, p-value = .92). Please note that the results of likelihood-ratio tests for mixed models should be treated with caution, since the test statistic is only approximately chi-squared-distributed. In addition, model fit was also measured by AIC (model 1: AIC = 10862; model 2: AIC = 10858; model 3: AIC = 10860).
The limit represented the maximum AHA performance level the model estimated would be achieved and sustained over time. To describe how quickly the children improved their performance before reaching their limit, a rate parameter was estimated. For easier interpretability, the rate was transformed into the Age-90 parameter, which indicated the age in months at which the children reached 90% of their limit on the AHA. The AHA-18 represented the observed or estimated AHA values for the participants at 18 months of age.
The estimated developmental trajectories were compared between the training groups and described according to participants’ MACS levels. Corresponding parameter estimates were described by their means and 95% confidence intervals and compared with two-sided Welch’s t-tests.
The nonlinear mixed-effects models were fitted in the statistical software R version 4.1.1,Citation21 using the R package nlme version 3.1–152.Citation22 The R script with the complete data analysis is available from the corresponding author on request.
Results
The study included 334 children between 18 months and 12 years of age and 1,565 AHAs (median of four AHAs per participant, range 2–14). The children were distributed across the entire age range, although a majority of AHAs were completed between 2 and 6 years of age (n = 956, 61%; ). The Norwegian cohort (n = 178) included more children than the Swedish cohort (n = 156) as well as a higher proportion of children classified as MACS level II. There was no significant difference between the countries in age at inclusion (p = .342), but the Swedish children were assessed with more AHAs, were monitored over a longer period, and were older at their final assessment (p < .001 for all). Additional characteristics of the cohorts are described in . As the mean curves for the two countries were similar, the cohorts were combined in the subsequent calculations of developmental trajectories. When investigating the characteristics of the treatment groups (), the children in the NIT group were followed for a shorter period, were given fewer assessments, and were older at their first assessment compared to the CIMT and Baby-CIMT groups (p ≤ .032 for all). The Baby-CIMT group was the smallest of the three groups (n = 51, 15%), and Swedish children made up the largest proportion of this group. Due to the Baby-CIMT group’s sample size, the primary results presented will be from the comparison of the CIMT (n = 144) and NIT (n = 139) groups, with trends in development described for the Baby-CIMT group.
Table 2. Descriptive participant information: age, number of AHA assessments, monitoring duration, and Manual Ability Classification System (MACS) levels for each training group.
Children’s Development Over Time in the CIMT and NIT Groups
The developmental trajectories of the different groups are illustrated in and described according to the participants’ MACS levels in . Children at all MACS levels improved over time regardless of whether they participated in intensive training, as illustrated by the increase in mean AHA units from AHA-18 to the stable limit in all groups (). The NIT group initially had higher AHA-18 performance than the CIMT group (mean difference 4.0 units, p = .028); however, although the mean AHA value remained higher in the NIT group throughout the developmental trajectory, there was no significant difference between the two groups at the stable limit (mean difference 2.9 units, p = .076). The NIT group included a larger proportion of children at MACS level I (27%) than the CIMT group (13%; χ2 = 10.96, n = 270, p = .004).
Figure 1. Developmental trajectories of hand use in three training groups of children aged 18 months to 12 years old, where intensive training occurred before 7 years of age. The vertical dotted lines represent the group-wise Age-90 values and corresponding 95% CIs. Note that the black (NIT) and red (Baby-CIMT) curves lie very close to each other.
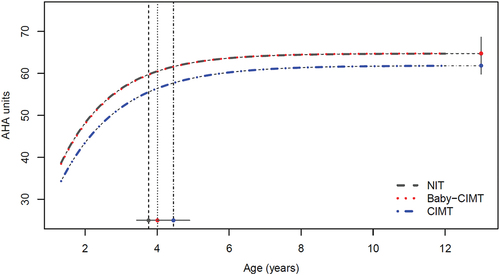
Table 3. Developmental rates, limits, and Age-90 for each training group according to Manual Ability Classification System (MACS) level.
Table 4. Developmental trajectories compared between training groups.
3.2 Intensive training extends the window of development
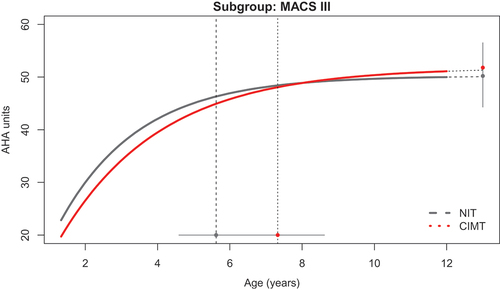
Children in the CIMT group achieved the Age-90 an average of 8.3 months later than the NIT group (p = 0.014; , ). The latest Age-90 occurred in children classified as MACS level III and was observed an average of 20.5 months later in the CIMT group (n = 28) than in the NIT group (n = 27; p = 0.048; ). The only curve crossover occurred between the CIMT group and the NIT group for children classified as MACS level III (, ). The CIMT group performed at an average of 1.4 AHA units lower than the NIT group at AHA-18 (p = 0.649), yet reached a stable limit that was an average of 1.6 units higher
Figure 2. Developmental trajectories of hand use between 18 months and 12 years of age, comparing children classified as MACS level III who had not performed intensive training (NIT) and those who had performed constraint-induced movement therapy (CIMT). The vertical dotted lines represent the group-wise Age-90 values and corresponding 95% CIs.
Trends in the Effects of Baby-CIMT on Early Development
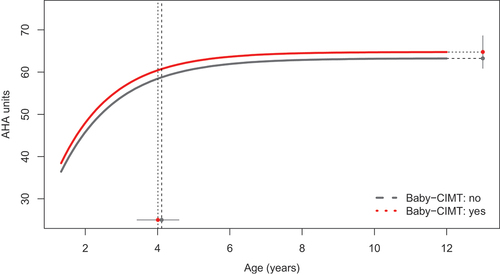
The Baby-CIMT group did not differ significantly from the other groups in developmental characteristics (, ). A weak positive trend in AHA performance for children in the Baby-CIMT group was observed, however, as the Baby-CIMT group (n = 51) performed 1.95 units higher at AHA-18 (p = .0459) and reached a stable limit that was 1.53 units higher than the CIMT and NIT groups combined (p = .477; ).
Figure 3. Developmental trajectories of hand use between 18 months and 12 years of age in children who had participated in Baby-CIMT before 18 months (n = 51) and children who had not participated in intensive training before 18 months of age (n = 283). The vertical dotted lines represent the group-wise Age-90 values and corresponding 95% CIs.
Brain Lesion Type Did Not Influence the Long-Term Response to Treatment
In general, children with WMDI demonstrated better development than children with gray matter/focal infarcts, as indicated by their higher AHA-18 (mean difference 5.9 units, p = .006) and higher stable limit (mean difference 5.0 units, p = .008) scores. When comparing the development of children with WMDI and gray matter/focal infarcts within each training group, no significant differences appeared for AHA-18, stable limit, or Age-90 (Supplementary ). Data on brain lesion type were missing for 130 children.
Discussion
For children in all groups, the AHA value at 18 months predicted the development of hand use. This association has been previously shown in children classified at different functional levels,Citation7,Citation12 and we demonstrated in this study that intensive training does not change this association. The significant difference already present between the primary groups (i.e. CIMT and NIT) at 18 months of age indicates an intervention by indication bias and makes the comparison of the long-term influence of intensive training difficult. However, while the NIT group performed significantly higher than the CIMT group at 18 months of age, the difference between the groups weakened over time. When the stable limit was reached, the CIMT group had somewhat caught up with the NIT group, as indicated by the difference between groups being no longer significant.
Which Children are Included in Intensive Training Programs?
Higher-functioning children were less likely to participate in intensive training programs, as evidenced by the significantly higher AHA values at 18 months of age in the NIT group compared to the CIMT group. In addition, there was a higher proportion of children classified as MACS level I in the NIT group, though there were large variations in function in both groups. We can only speculate on the possible reasons children were included or not included in intensive training. First, as Baby-CIMT and CIMT are not standard treatments, limited resources may result in fewer intensive intervention opportunities for the least-affected children. The paramount principle of need and solidarity governs the health care system in Scandinavia and may influence the treatment options available for children classified as MACS level I, who may be considered mildly impaired and thus less likely to be offered interventions. Furthermore, opinions regarding the effectiveness of CIMT may have varied between clinicians during this period, and pediatric rehabilitation services face several challenges when incorporating new methods into clinical practice.Citation23,Citation24
The Window of Development Was Influenced by Intensive Training
As previously shown, the age at which children approach their stable limit of development (Age-90) varies by their MACS level, with children classified at MACS level III having a longer developmental period than children at MACS levels I and II.Citation7,Citation12 The results of this study revealed that intensive training extended the window of development for children in the CIMT group, particularly for children classified as MACS level III. As the results for children at MACS level III were different between the CIMT and NIT groups, this finding cannot be attributed to the severity of manual impairment alone. This finding is clinically relevant, as it indicates that children’s developmental limits appear to be influenced by training and that activity-dependent neural plasticity enables learning over a longer period if intensive training is provided. This further relates to the development of the cognitive strategies required for bimanual hand use that are known to develop at later agesCitation25 and is perhaps particularly important for children with more limited ability.
Early Intensive Training Might Be Beneficial
There was a weak trend supporting early intervention, with a somewhat higher mean AHA-18 value observed in children in the Baby-CIMT group compared to children who had not participated in intensive training before 18 months of age (the CIMT and NIT groups combined). We perceive AHA-18 performance to be the most important parameter indicating the effect of Baby-CIMT. Due to the small sample size of the Baby-CIMT group, further analysis was not relevant. Nevertheless, our results may contribute information to the growing body of literature supporting early intervention. Our findings align with evidence that brain development is activity dependent and occurs most rapidly at early ages and support the usefulness of the recently published practice guidelines.Citation26–30
Limitations
Although the Norwegian cohort included data from the national NorCP registry, which includes more than 90% of the children with CP in Norway,Citation31 it only comprised 25% of the population with UCP due to a lack of repeated AHAs.Citation12 This study must thus be regarded as having a convenience clinical sample, and differences between the Norwegian and Swedish cohorts might be due to differing recruitment procedures. Additionally, the Norwegian data were scored by therapists that follow the children with annual follow-ups in clinical practice, while the Swedish data were scored by only a few very experienced raters. Despite these differences, the statistical modeling showed no reason to separate the cohorts during analysis.
A further limitation was the use of MACS levels that were classified at the children’s most recent assessments, as this might bias the group divisions. However, MACS have been shown to be fairly stable over time,Citation32 and we thus assumed that most of the children were likely to remain at the same MACS level throughout the study, regardless of training group. Nevertheless, we do not know whether the MACS levels influenced the results.
Our inability to control for other types of interventions, including common interventions such as functional goal-directed training or botulinum neurotoxin injections, is a further limiting factor. We chose to only include CIMT in this study because the dose for this intervention is clearly defined, and dosage is known to be the most important factor in improvement.Citation33 There is a recommended dose of 120 hours for CIMT programs employed in both Sweden and Norway, though this may vary by individual. Nevertheless, the 40-hour minimum dose for intensive treatment, as recommended by Jackman et al.,Citation33 was most likely exceeded for all children in this study. The exact timing of the interventions and whether some children had repeated periods of training were not reported in this study. Instead, we chose to use specific time frames, defined as from 18 months to 7 years of age for CIMT and before 18 months of age for Baby-CIMT. By restricting the timing of the training to these periods, we could use the period between 7 and 12 years of age to investigate the long-term influence of training. Due to the small sample size for the Baby-CIMT group, we could not report on separate analyses for the children who performed only Baby-CIMT and those who performed both Baby-CIMT and later CIMT. Taking repeated interventions and their timing into account would require more detailed data registration and more sophisticated statistical methods. As we were primarily interested in the long-term perspective, we found the defined periods for intensive training to be sufficient to achieve the purposes of this study.
Clinical Implications and Interpretations
We believe that interventions for this population are important, though this is difficult to prove because all children in high-income countries have access to some kind of intervention and support, and long-term randomized controlled studies with non-treatment control groups are unethical. Nevertheless, we can learn more about the efficacy of interventions from low-income countries, where children have less access to interventions. For example, a study from Moldova showed that children involved in early intervention had significantly fewer contractures than those who were not provided any intervention.Citation34 Furthermore, in a study from a rural area in Uganda where children had no access to treatment, lower scores in functional skills and slower rates of gross motor development was seen compared to findings reported from similar studies in high-income countries.Citation35–37 Comparisons with low-income countries further remind us that the results of this study are specific to this Scandinavian cohort and cannot be generalized to countries where services and interventions are governed by other health care policies or based on other theoretical perspectives.Citation38
We additionally believe in the value of intensive hand training at different periods of development. We know from animal models that very early activity-based training is important for the development of the corticospinal tract, but also that neural plasticity remains significant at later ages as well, making intervention important for both younger and older children. At least two periods for intensive training have been described by Friel and colleagues.Citation39 The most effective intervention occurs very early, at approximately 6–12 months in humans, which is a critical period during which the corticospinal tract establishes spinal connections. However, a robust capacity to repair the central nervous system by eliminating aberrant ipsilateral projections remains over some years, as does the representational plasticity of the motor cortex. This suggests the importance of initiating interventions early and continuing them throughout childhood. According to our findings, this may be particularly important for children with more significant limitations in hand function. Despite the need for additional long-term studies with controlled research designs, it is encouraging to report findings that indicate the most promising development among the children who underwent early intervention.
Conclusion
The developmental trajectories of children with UCP in this Scandinavian cohort were related to their AHA values at 18 months of age, regardless of manual ability levels and training programs. However, CIMT extended the window of development, particularly in children classified at MACS level III. CIMT additionally appears to facilitate development, as the CIMT group caught up with the NIT group by the time they reached their stable limit. For Baby-CIMT, only a weak positive trend was observed.
Supplemental Material
Download MS Word (33.6 KB)Acknowledgments
We would like to thank all the children and their families who participated in this research. We are also truly grateful for the work and effort of all the collaborating therapists who have helped us in recruiting participants and collecting data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17518423.2023.2193256.
Additional information
Funding
References
- Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2007;49(11):830–38. doi:10.1111/j.1469-8749.2007.00830.x.
- Hoare BJ, Wallen MA, Thorley MN, Jackman ML, Carey LM, Imms C. Constraint-induced movement therapy in children with unilateral cerebral palsy. Cochrane Database Syst Rev. 2019;4 Cd004149. doi:10.1002/14651858.CD004149.pub3.
- Klepper SE, Krasinski DC, Gilb MC, Khalil N. Comparing unimanual and bimanual training in upper extremity function in children with unilateral cerebral palsy. Pediatr Phys Ther. 2017;29(4):288–306. doi:10.1097/pep.0000000000000438.
- Myrhaug HT, Ostensjo S, Larun L, Odegaard-Jensen J, Jahnsen R. Intensive training of motor function and functional skills among young children with cerebral palsy: a systematic review and meta-analysis. BMC Pediatr. 2014;14(1):292. doi:10.1186/s12887-014-0292-5.
- Vroland-Nordstrand K, Eliasson AC, Jacobsson H, Johansson U, Krumlinde-Sundholm L. Can children identify and achieve goals for intervention? A randomized trial comparing two goal-setting approaches. Dev Med Child Neurol. 2016;58(6):589–96. doi:10.1111/dmcn.12925.
- Eliasson AC, Holmefur M. The influence of early modified constraint-induced movement therapy training on the longitudinal development of hand function in children with unilateral cerebral palsy. Dev Med Child Neurol. 2014;57(1):89–94. doi:10.1111/dmcn.12589.
- Nordstrand L, Eliasson AC, Holmefur M. Longitudinal development of hand function in children with unilateral spastic cerebral palsy aged 18 months to 12 years. Dev Med Child Neurol. 2016;58(10):1042–48. doi:10.1111/dmcn.13106.
- Holmefur M, Kits A, Bergstrom J, Krumlinde-Sundholm L, Flodmark O, Forssberg H, Eliasson AC. Neuroradiology can predict the development of hand function in children with unilateral cerebral palsy. J Neurol Rehabil. 2013;27(1):72–78. doi:10.1177/1545968312446950.
- Michael-Asalu A, Taylor G, Campbell H, Latashia-Lika L, Kirby RS. Cerebral palsy: diagnosis, epidemiology, genetics, and clinical update. Adv Pediatr. 2019;66:189–208. doi:10.1016/j.yapd.2019.04.002.
- Klevberg GL, Ostensjo S, Elkjaer S, Kjeken I, Jahnsen R. Hand function in young children with cerebral palsy: current practice and parent-reported benefits. Phys Occup Ther Pediatr. 2017;37(2):222–37. doi:10.3109/01942638.2016.1158221.
- Eliasson AC, Shaw K, Berg E, Krumlinde-Sundholm L. An ecological approach of constraint induced movement therapy for 2–3-year-old children: a randomized control trial. Res Dev Disabil. 2011;32(6):2820–28. doi:10.1016/j.ridd.2011.05.024.
- Klevberg GL, Jahnsen R, Elkjaer S, Zucknick M. Hand use development in children with unilateral cerebral palsy. Dev Med Child Neurol. 2021;63(12):1462–68. doi:10.1111/dmcn.14957.
- Eliasson AC, Krumlinde-Sundholm L, Shaw K, Wang C. Effects of constraint-induced movement therapy in young children with hemiplegic cerebral palsy: an adapted model. Dev Med Child Neurol. 2005;47:266–75. doi:10.1017/s0012162205000502.
- Eliasson AC, Nordstrand L, Ek L, Lennartson F, Sjostrans L, Tedroff K, Krumlinde-Sundholm L. The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral-cerebral palsy; an explorative study with randomized design. Res Dev Disabil. 2018;72:191–201. doi:10.1016/j.ridd.2017.11.006.
- Louwers A, Beelen A, Holmefur M, Krumlinde-Sundholm L. Development of the Assisting Hand Assessment for Adolescents (Ad-AHA) and validation of the AHA from 18 months to 18 years. Dev Med Child Neurol. 2016;58(12):1303–09. doi:10.1111/dmcn.13168.
- Holmefur M, Aarts P, Hoare B, Krumlinde-Sundholm L. Test-retest and alternate forms reliability of the Assisting Hand Assessment. J Rehabil Med. 2009;41(11):886–91. doi:10.2340/16501977-0448.
- Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(07):549–54. doi:10.1017/s0012162206001162.
- Eliasson AC, Ullenhag A, Wahlstrom U, Krumlinde-Sundholm L. Mini-MACS: development of the Manual Ability Classification System for children younger than 4 years of age with signs of cerebral palsy. Dev Med Child Neurol. 2017;59(1):72–78. doi:10.1111/dmcn.13162.
- Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42(12):816–24. doi:10.1017/S0012162200001511.
- Pinhero JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000.
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Authors EISPACK, Heisterkamp S, VanWilligen B, Ranke J, Core Team R. Linear and nonlinear mixed effects models. R Package Version. 2019:3.1–141. https://CRAN.R-project.org/package=nlme.
- R Core Team. A language and environment for statistical computing. 2019. https://www.R-project.org/.
- Andersen JC, Majnemer A, O’grady K, Gordon AM. Intensive upper extremity training for children with hemiplegia: from science to practice. Semin Pediatr Neurol. 2013;20(2):100–05. doi:10.1016/j.spen.2013.06.001.
- Shikako-Thomas K, Fehlings D, Germain M, Gordon AM, Maynard D, Majnemer A. Current practice “constraints” in the uptake and use of intensive upper extremity training: a Canadian perspective. Phys Occup Ther Pediatr. 2018;38(2):143–56. doi:10.1080/01942638.2017.1303802.
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001;20(1):385–406. doi:10.1207/S15326942DN2001_5.
- Morgan C, Fetters L, Adde L, Badawi N, Bancale A, Boyd R, Chorna O, Cioni G, Damiano D, … Novak I. Early intervention for children aged 0 to 2 years with or at high risk of cerebral palsy: international clinical practice guideline based on systematic reviews. JAMA Pediatr. 2021;175(8):846–58. doi:10.1001/jamapediatrics.2021.0878.
- Friel KM, Kuo HC, Carmel JB, Rowny SB, Gordon AM. Improvements in hand function after intensive bimanual training are not associated with corticospinal tract dysgenesis in children with unilateral cerebral palsy. Exp Brain Res. 2014;232(6):2001–09. doi:10.1007/s00221-014-3889-x.
- VB de Graaf-Peters, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82(4):257–66. doi:10.1016/j.earlhumdev.2005.10.013.
- Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, Cioni G, Damiano D, Darrah J, … Badawi N. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):897–907. doi:10.1001/jamapediatrics.2017.1689.
- Nordstrand L, Holmefur M, Kits A, Eliasson AC. Improvements in bimanual hand function after baby-CIMT in two-year old children with unilateral cerebral palsy: a retrospective study. Res Dev Disabil. 2015;41-42:86–93. doi:10.1016/j.ridd.2015.05.003.
- Hollung SJ, Vik T, Wiik R, Bakken IJ, Andersen GL. Completeness and correctness of cerebral palsy diagnoses in two health registers: implications for estimating prevalence. Dev Med Child Neurol. 2017;59(4):402–06. doi:10.1111/dmcn.13341.
- Öhrvall AM, Krumlinde-Sundholm L, Eliasson AC. The stability of tHe manual ability classification system over time. Dev Med Child Neurol. 2014;56(2):185–89. doi:10.1111/dmcn.12348.
- Jackman M, Lannin N, Galea C, Sakzewski L, Miller L, Novak I. What is the threshold dose of upper limb training for children with cerebral palsy to improve function? A systematic review. 2020;67(3):269–80. doi:10.1111/1440-1630.12666.
- Bufteac EG, Andersen GL, Spinei L, Jahnsen R. Early intervention and follow-up programs among children with cerebral palsy in Moldova: potential impact on impairments? BMC Pediatr. 2020;20(1):29. doi:10.1186/s12887-020-1931-7.
- Andrews C, Namaganda L, Eliasson A-C, Kakooza-Mwesige A, Forssberg H. Functional development in children with cerebral palsy in Uganda: population-based longitudinal cohort study. Dev Med Child Neurol. 2022;64(1):70–79. doi:10.1111/dmcn.14996.
- Burgess A, Boyd RN, Chatfield MD, Zivani J, Sakzewski L. Self-care performance in children with cerebral palsy: a longitudinal study. Dev Med Child Neurol. 2020;62(9):1061–67. doi:10.1111/dmcn.14561.
- Smits DW, Gorter JW, Riddell CA, Voorman JM, Rosenbaum PL, Palisano RJ, Walter SD, Hanna SE, van Wely L, Ketelaar M. Mobility and self-care trajectories for individuals with cerebral palsy (aged 1–21 years): a joint longitudinal analysis of cohort data from the Netherlands and Canada. Lancet Child Adolesc Health. 2019;3(8):548–57. doi:10.1016/s2352-4642(19)30122-1.
- Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A, Langdon K, Mc Namara M, Paton MCB, … Badawi N. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 2020;20(2):3. doi:10.1007/s11910-020-1022-z.
- Friel KM, Williams PTJA, Serradj N, Chakrabarty S, Martin JH. Activity-based therapies for repair of the corticospinal system injured during development. Front Neurol. 2014;5:229. doi:10.3389/fneur.2014.00229.
