ABSTRACT
In this scoping review, we summarize the current knowledge of cognitive functioning in adults with cerebral palsy (CP), and identify the neuropsychological tests typically used in this population. 39 studies from the period January 1990 - August 2023 were included in the review, and they differ widely in their aims and approach to studying cognition. Very few studies have cognitive assessment as their core aim and use a neuropsychological test battery. The included studies show great variability in reported intelligence and cognitive functioning in adults with CP, and cognitive deficits have been reported in all cognitive domains. Most of the studies suffer from methodological limitations, and there is ample room for improvement within the field. We conclude by suggesting a number of recommendations that may contribute to increasing our understanding of cognitive impairments in adults with CP.
Introduction
Cerebral palsy (CP) is a condition characterized by motor impairments caused by brain injury in the developing fetal or infant brain.Citation1 Traditionally, research has focused on the motor impairments of CP, but over the last decades, there has been a growing interest in investigating other aspects of the condition. Cognitive functioning can be defined as: “the performance of the mental processes of perception, learning, memory, understanding, awareness, reasoning, judgment, intuition, and language,”Citation2 and they play a significant role in everyday life in relation to e.g., academic learning, communication, social relations, and living an independent life.Citation3 Although it has been recognized for many years that cognition and intelligence may be affected in children with CP,Citation4 only few studies have assessed these functions in adults. Even though CP is a non-progressive disorder, the challenges that each individual experiences may change over time. It is well known that cognitive functions are not static entities but that they develop and mature from infancy into early adulthood.Citation5,Citation6 However, cognitive functioning may not necessarily follow the same developmental trajectories in individuals with CP as in typically developing children: Early brain injury may result in developmental delays, and children with early brain injury may “grow into” cognitive deficits, as cognitive demands increase during childhood.Citation3 It is difficult to predict which cognitive deficits an individual with CP might have, as CP is a very heterogeneous condition, with different lesion sites and different etiologies.Citation7 Furthermore, brain plasticity involves many different mechanisms that may be both adaptive and mal-adaptive, and cognition may also be affected by environmental factors, e.g., social interactions, access to interventions, and social support.Citation3,Citation8,Citation9
Studies from the literature on children with CP have shown that the severity and type of cognitive deficits vary largely. A majority of children with CP have some form of cognitive deficit ranging from a mild deficit in one cognitive domain to severe global impairments across various domains.Citation10 Children with CP may have a range of cognitive deficits, such as deficits in executive functioning, memory, and language comprehension, as well as deficits in visual perception and visuospatial functions. ,Citation11–13 In addition, individuals with CP often have non-cognitive visual impairments such as strabismus, refractive errors, and abnormal contrast sensitivity,Citation14,Citation15 which may affect performance on visuo-perceptual and spatial tasks.Citation16 Although there is an overall correlation between the severity of motor impairment and cognitive functioning in CP, the severity of motor impairment is not an indicator of cognitive functioning at the individual level.Citation10,Citation17,Citation18 Normal cognitive functioning has been documented in children across all levels of gross motor functioning and different subtypes of CP,Citation12 and studies have reported wide ranges of IQ in individuals with CP.Citation12,Citation17 In a recent review on cognitive functioning in children with CP, StadskleivCitation10 pointed out that there are a range of methodological challenges in the CP literature. Some individuals with CP are hard to assess due to e.g., motor and vision impairments, and communication disorders.Citation19,Citation20 This sometimes leads researchers to estimate cognitive functions based on e.g., clinical judgement, school placement, degree of motor impairment or interview with parents, rather than through a detailed neuropsychological assessment. This can lead to imprecise and biased evaluations of the child’s cognitive functioning. It is possible to adapt the response mode of neuropsychological tests to make it possible for even individuals with severe motor and communication disorders to participate in neuropsychological assessment, but when adaptations have been used in clinical studies, they have not always been described in detail.Citation10 This makes it impossible to replicate the results and it also makes it hard to evaluate the validity of the studies, as it is difficult to determine whether and to which degree the adaptations might have had an impact on the results. The literature has primarily focused on assessing cognitive functioning in children, and little is known about cognitive functioning in adults with CP. There is no reason to expect that cognitive dysfunctions disappear as soon as an individual transitions into adulthood, but cognitive challenges may play a different role in adulthood as the demands from society increase. Indeed, cognitive challenges can have consequences in relation to living an independent life, continuing education, career opportunities and in the transition from work to retirement. This has recently been recognized in the Nordic countries with the development of “CPcog-adult,”Citation21 a clinical protocol with recommendations for multiple points of neuropsychological assessment during adulthood, in order to facilitate the right interventions if needed.
The present study was originally planned as a systematic review of the literature on cognitive functioning in adults with CP. However, in conducting the review, it became clear that the review was more suitable as a scoping review, for the following reasons: Firstly, the identified literature used such a wide variety of methods to investigate cognitive functioning that it was only possible to summarize the results narratively. Secondly, the review also focuses on identifying related knowledge gaps in the literature in the area of cognition in adults with CP. As a result, there are four aims of this review: 1) To identify the literature on cognitive functioning in adults with CP. The main focus is on empirical findings related to cognitive functioning; 2) to identify the neuropsychological and cognitive tests used to investigate cognitive functioning in this population; 3) to investigate whether the included studies report adaptations of the included tests; 4) to evaluate the quality of the identified studies, as reports from the child literature on cognitive functioning in CP suggest that a large proportion of studies suffer from a variety of methodological problems.
Method
The protocol for the review was registered on PROSPERO on 14th November 2021, https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=285032. The Psycinfo, PubMed and Web of Science databases were searched for eligible studies using Medical Subject Headings in PubMed, the PsycInfo thesaurus, and text words related to cerebral palsy, neuropsychological assessment, cognition and intelligence. The full search strings are provided in the Supplementary material S1. Reference lists and articles citing the included literature were found through Web of Science and were considered for eligibility. The search was conducted in October 2021 and updated in August 2023. Articles in all languages were considered for inclusion in the review. Google’s translation tool was used to translate abstracts in languages other than English, Danish, Norwegian, Swedish, French, and German but it was not necessary to use the tool to translate any of the included studies. Studies were included if published in a peer-reviewed journal, and if they included measures of neuropsychological or cognitive functioning, or measures of intelligence. In order to be included in the review, at least 50% of the participants had to have a cerebral palsy diagnosis, and at least 50% of the participants had to be above the age of 18 years. In cases where this was unclear, studies were included if the mean age or median age was above 18. These criteria were inspired by previous reviews in the CP literature that also had a criterion stating that at least 50% had to be adults and/or at least 50% of the sample had to have CP.Citation22–24 Papers published before 1990 (when the International Classification of Diseases, 10th revision was published), and literature reviews and papers where full text was not available were not included. We decided to exclude studies that assessed cognition using rating scales. Studies that used a cut off score on a neuropsychological test as an inclusion criterion, but that did not have assessing cognition as a core aim of the study, were also excluded. This decision was taken during the full text review, as these studies were uninformative with regards to the range of cognitive functions that may be found in the population of adults with CP. Data was managed with the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia).
Two reviewers screened the titles and abstracts yielded by the search independently, and full texts were reviewed for records that appeared to meet the inclusion criteria. Studies were included in the analysis if both reviewers agreed that the study met the inclusion criteria. Data were extracted independently by one reviewer using a predefined form. The other reviewer checked the data extraction for each individual study and filled changes into the predefined form. Any disagreement between the reviewers was resolved through discussion. A third reviewer made the final decision in cases where consensus could not be reached. The following information was extracted from each study: Number of participants (incl. % if sample was mixed); age group (incl. % if sample was mixed); diagnosis/diagnoses (incl. % if sample was mixed); CP Classification system, e.g., Gross Motor Function Classification System (GMFCS)Citation25 or Manual Ability Classification System (MACS)Citation26; comparison group (e.g., matched controls, norms); whether cognition was assessed specifically for the study or if results were obtained through other means (e.g., through medical records); adaptations used; cognitive domains studied; instruments used to assess cognitive functions and/or intelligence; and a summary of the main findings.
Quality Assessment
The Newcastle Ottowa Scale (NOS) is a tool that was developed to assess the quality of non-randomized studies in meta-analyses. In this study, it was used to assess the quality of the included studies,Citation27 but the scale was modified slightly to fit better with the types of studies that were included. Only the first two sections on selection and comparability were used, as none of the included studies tested an effect of an exposure. This means that each study could be awarded a maximum total of six points. Four points could be awarded in the selection section, and two points could be awarded in the comparability section. In the comparability section, a study could be awarded up to two points by controlling for two different variables (e.g., age, education, gender): two points were awarded to studies that used age-appropriate norms for all tests; one point was awarded if age-appropriate norms were only used for a subset of the neuropsychological tests. Studies using within-subject design were rated as N/A in the comparability section. For studies that used normative data to assess the performance of participants, the items related to definition and selection of controls on the NOS were rated as N/A. Thus, within-subject studies and studies that used normative data could be awarded a maximum of four points, whilst studies that used control participants could be awarded a maximum of six points. The quality assessment was made independently by two reviewers, and any disagreement was resolved through discussion with a third reviewer.
Results
The selection process is shown in . Of 7111 studies screened for inclusion, 39 studies were included in the review. All of the included studies were originally published in English. These studies are listed in the Supplementary material S2. The age range of the participants in the included studies was six to 62 years, as some of the included studies investigated cognition in both children and adults. Age ranges were provided in 33 of the studies. Only 13 of these studies assessed cognitive functioning in adults above 18 years of age. The rest of the studies included both adults and children, but they were included because the mean or median age of the sample was above 18, or more than 50% of the participants were 18 years or older. All levels of gross motor functioning and manual ability were represented in the studies. Twenty-one studies reported the subtype of CP in their participants (e.g., whether spastic, dyskinetic, ataxic, mixed), and 18 studies did not report this information. When the subtype was reported, spastic and dyskinetic CP were the most common subtypes included in the studies. Not all of the included studies had a core aim of investigating cognitive functioning in adults with CP, but used cognitive tasks, e.g., to investigate skin potential responses as a mean of alternative communication,Citation28 or to investigate the feasibility of measuring single subject event-related potentials in adults with CP.Citation29 Other studies used results of neuropsychological assessment to investigate the relationship between cognitive functioning and factors affecting e.g., quality of life, employability, and adaptive behavior.Citation30–32
Cognitive Functioning
The summary of findings presented in the following section concerns a subset of the 39 studies identified in this review. Despite using cognitive tasks in a group of adults with CP, 15 of the included studies cannot inform us on cognitive functioning in adults with CP in general. Results from the following studies are therefore not included in the summary of findings: Single case studies or case series where scores are reported from a single test, e.g., an IQ testCitation33–35 PPVTCitation36 or digit span forwardCitation37; correlational studies where raw scores are not reportedCitation38,Citation39; studies in which results from CP participants are mixed with participants with other diagnosesCitation40; studies in which the Mini Mental State Examination is used to classify participants according to degree of cognitive impairment, but in which the scores are not reportedCitation41,Citation42; studies of event-related potentials in which cognitive tasks are usedCitation29,Citation43 or studies of skin potential responsesCitation28; studies in which performance of the CP participants is compared to participants with other conditions.Citation39,Citation44,Citation45 Brief descriptions of all 39 studies identified in the review can be found in the Supplementary material S2.
Intelligence
The RCPM was the most used intelligence test, and it was used in all of the studies presented in this section. These studies included both adults and children with CP and were all from the same group of researchers. A small number of participants were included in multiple studies.Citation46–50 IQ scores ranged from 20–129 in participants with primarily bilateral dyskinetic CP.Citation30,Citation46,Citation51–53 Four studies found that participants with dyskinetic CP and spastic CP performed poorer than typically developing controls,Citation47,Citation48,Citation51,Citation53 but mixed results were found when comparing IQ in different subgroups.Citation48,Citation49 Assessment of IQ was also used as a secondary measure in studies on e.g., quality of life, factors that affect employment, and verbal and gestural abilities in adults with CP.Citation30,Citation31,Citation54,Citation55
Attention
Various subdomains of attention have been assessed using a wide range of tests, and studies have reported mixed results. Lemay et al.Citation56 found impaired sustained attention, inhibition and spatial attention in a group of adolescents with CP. This was reflected by more omissions and a greater variability in performance on a reaction time task compared to a group of age-matched controls. In contrast, two studies that used a test of inhibition and sustained attention found no difference between groups of participants with spastic CP, dyskinetic CP and typically developing controls.Citation48,Citation53 Regarding short-term memory, one study found that participants with dyskinetic CP performed poorer than typically developing controls,Citation47 whereas two studies found mixed results when comparing performance between participants with dyskinetic, spastic and mixed CP.Citation49,Citation50
Working Memory
Two studies found emerging evidence for impaired working memory in participants with dyskinetic CP and participants with spastic CP on a group-level compared to typically developing/age- and gender matched controls.Citation48,Citation57 Mixed results were found when comparing performance between participants with different subtypes of CP. Some studies did not find differences between the groups on measures of visual working memory,Citation48,Citation49 but other studies found a tendency for participants with dyskinetic CP to perform significantly better than participants with spastic CP and participants with mixed CP on tests of verbal working memory.Citation49,Citation50 Goble et al. found that 10/11 participants with CP scored below the population mean on the both the longest sequence and the raw score in a test of visual working memory.Citation58
Executive Functions
Executive functions were investigated in multiple studies, with a range of different tests, showing different results. One study found impaired performance in cognitive inhibition,Citation59 and another study found impaired ability to plan ahead on a motor planning test.Citation60 Another study found that impaired executive functions may be associated with greater motor impairment, and may affect performance on other test domains, e.g., long-term memory.Citation47 Some studies also investigated executive functions in adults with CP on a subgroup level. One study found that participants with severe bilateral CP made too many perseverative errors and showed impaired abstract reasoning on a measure of cognitive flexibility.Citation50 In later studies from the same research group, cognitive flexibility was found to be moderately correlated with quality of life, and it was related to white matter integrity in participants dyskinetic CP.Citation30,Citation53 Laporta-Hoyos et al.Citation48 found that a group of participants with dyskinetic CP performed significantly better compared to a group of participants with spastic CP on tests of verbal fluency.
Memory
In terms of memory functioning, three studies did not find significant differences in performance between controls and participants with either dyskinetic, spastic or mixed CP.Citation49,Citation50,Citation61 However, two studies found the opposite result: Ballester-Plané et al.Citation47 found that participants with dyskinetic CP performed significantly worse compared to controls, and Sabbadini et al.Citation62 found impaired memory performance in a small sample of participants with severe CP. Studies have also suggested a link between intelligence and learning mode: In one study, Pueyo et al.Citation63 found that participants with scores within the normal range on RCPM performed significantly better on tests of visual and verbal memory compared to participants who were impaired on RCPM. Icht et al.Citation64 found that recognition of words that were learned by producing the words with the participants’ augmentative and alternative communication systems were better recognized than words that were either read silently or heard.
Visuoperceptual and Visuospatial Functions
On a group level, participants with CP were found to perform worse than controls on visual perceptual and visual spatial tasks. This was reported in a study with a small sample of participants with severe CP,Citation62 and in two studies with participants with bilateral CP. In the latter two studies, they also found impaired visual perceptual and visual spatial performance in mild cases where other cognitive deficits were less prevalent.Citation47,Citation50 When comparing performance between different subtypes of CP (dyskinetic, spastic, and mixed CP) two studies found mixed results.Citation49,Citation50
Language
PPVT, a test of receptive vocabulary, was the most commonly used test across all studies. Taken together, three studies have reported normalized scores on PPVT that ranged from 55 to 119.Citation30,Citation46 Pueyo et al.Citation49,Citation50 did not find any significant differences on a group-level in performance on PPVT between groups with dyskinetic, spastic and mixed CP. Three studies have found that even when controlling for e.g., spelling age, reading age or education, participants with CP perform poorer on various language tests compared to controls.Citation65–67 However, one study found no significant differences between a small group with severe CP and a control group matched for mental age on basic linguistic abilities (phonological, phonemic, lexical and semantic abilities).Citation62 Ballester-Plane et al.Citation47 found no language deficits in a group of participants with dyskinetic CP at GMFCS level I but participants with GMFCS level II showed deficits in receptive vocabulary, basic grammar comprehension and verbal learning compared to typically developing controls. They suggest that this difference was because the group with a gross motor impairment that is equivalent to GMFCS level 2 also had communication difficulties. Lidzba et al.Citation61 found no significant differences on measures of verbal fluency and a word chain task between participants with left- or right hemisphere language compared to controls.
Altogether, the included studies found a great variability on measured of intelligence and cognitive functioning in CP, both when comparing performance to matched controls and normative data and between subtypes of CP. On a group level, impairments have been documented in the domains of working memory,Citation48,Citation57,Citation58 executive functions,Citation48 as well as visual spatial and visual perceptual functionsCitation47,Citation50,Citation62 in participants with CP compared to controls. When comparing subtypes of CP, the results are more mixed, but participants with dyskinetic CP tend to perform better on measures of verbal WMCitation48,Citation49 and verbal fluencyCitation48,Citation61 compared to participants with either spastic or mixed spastic/dyskinetic CP.
Neuropsychological Tests
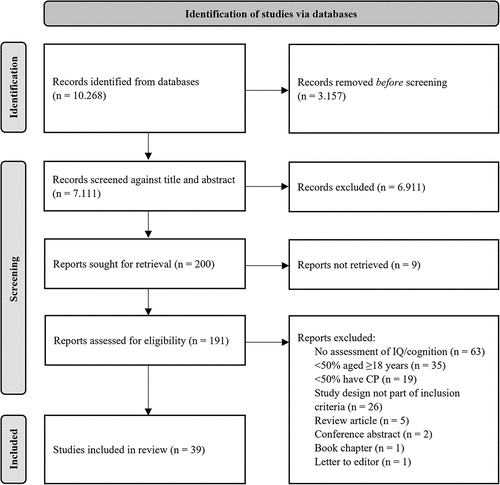
95 different tests were used across the included studies, but only 20 of these tests were used in more than one study. These tests are shown in . The Peabody Picture Vocabulary Test (PPVT),Citation68 a test of receptive vocabulary, was the most widely used test across the studies. This was followed by Raven’s Coloured Progressive Matrices (RCPM),Citation69 a test of logical reasoning, which was used to measure intelligence. Some of the most commonly used tests were from the same research group,Citation30,Citation46–53,Citation55,Citation63 which is why e.g., the receptive part of the Screening Test of Spanish grammar was used in five studies.
As can be seen in and in Supplementary material S2, most of the tests were accuracy-based tests, rather than reaction time-based tests.
Adaptations
In 13 of the included studies, the authors explicitly mentioned that they used adaptations to response mode in some of the cognitive and neuropsychological tests to enable assessment of all participants, regardless of e.g., motor impairments or communication disorders.Citation28,Citation36,Citation37,Citation46–48,Citation50,Citation52,Citation53,Citation55,Citation62,Citation63,Citation65,Citation67 In five other studies, the authors specified that they did not adapt tests but that they instead selected or devised tests/experiments that were well suited for the population.Citation43,Citation58,Citation61,Citation64,Citation66 In one study, the authors only included participants that were able to complete the neuropsychological test.Citation39 Two studies used enlarged print to show visual stimuli,Citation66,Citation67 and in one study, participants could be positioned in different ways.Citation28 The rest of the studies did not mention whether tests were adapted for the population or not. In relation to controlling for visual impairments, only one study made sure that the participants had “sufficient visual and cognitive abilities to complete the task, as determined through a practice session immediately prior to the recording,”Citation57 but the rest of the studies did not test for visual impairments (e.g., visual acuity or contrast sensitivity). However, most studies had inclusion or exclusion criteria related to vision. Inclusion criteria were: “Normal or corrected to normal vision,”Citation32,Citation56,Citation64–66 “sufficient vision to participate,”Citation39,Citation62 “no visual abnormalities,”Citation49,Citation51 or “no sensory disability.”Citation40 Exclusion criteria were “severe visual difficulties that precluded neuropsychological assessment,”Citation30,Citation46,Citation48,Citation52,Citation53,Citation55,Citation63 “severe visual impairment,”Citation41 or “significant uncorrected visual/hearing impairments.”Citation29 Two studies mentioned that they were aware of fatigue in their participants, and they varied the number of sessionsCitation65 or administered tests on different days if it was necessary.Citation66
Quality Assessment
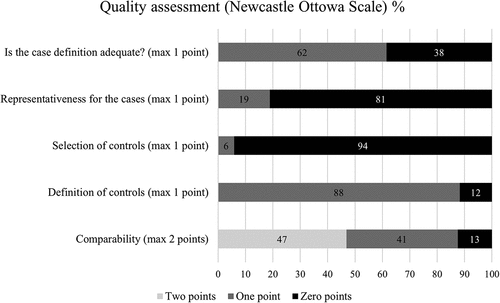
shows an overview of how the studies scored on the NOS. The rating for each study is provided in the Supplementary material S3. Only one study was awarded the maximum amount of points (4/4).Citation52 The main reasons for reduction of points in the quality assessment were related to the lack of representative samples of the participants and the lack of information about how control participants were selected. Here follows a brief summary of the ratings: 1) Adequate case definition. In 15 of the included studies, it was not explicitly mentioned that a diagnosis of CP was confirmed through medical records or by a health care professional; 2) Representativeness of the cases. Two studies were single-case studies, and were therefore rated as N/A. Of the remaining 37 studies, 30 studies did not include a sample of participants that were representative for the population; 3) Selection of controls. Twenty-two studies did not include controls but used norms and were therefore rated “N/A.” 16 of the remaining 17 studies that used control participants did not make it clear whether the controls were representative for the general population; 4) Definition of controls. Of the 17 studies that used controls, two studies did not have inclusion criteria for the control participants, such as no neurological disorders; 5) Comparability. Fifteen studies were awarded two points because they either used appropriate normative data or used a control group that was matched on at least two variables (e.g., age, education, gender). Thirteen studies were awarded one point, either because they did not have appropriate norms for all the tests, or because they only matched the control group on one variable. Four studies were awarded zero points due to inappropriate control groups. In six studies, the comparability item was not applicable as the studies were within-subject design.
Figure 3. Percentage of studies that are awarded 0, 1 or 2 points. “Not applicable” ratings are excluded from the figure.
Overall, the results show a very heterogeneous group of studies, both regarding the aim of the studies, the participants included in the studies, the tests that were used to assess cognitive functioning, and the quality of the studies according to the NOS. However, these results do not necessarily mean that most of the studies are of poor quality, as the studies have very different aims. Different aims might affect e.g., the number of variables that a study controls for, and whether the studies include a representative sample or not.
Discussion
The aims of this review were to: 1) investigate cognitive functioning in adults with CP; 2) to identify the neuropsychological and cognitive tests that are typically used to investigate cognitive functioning in this population; 3) to investigate adaptations used in the studies; and 4) to evaluate the quality of the included studies. Because this is an area with a limited amount of research, we decided to use very lenient inclusion criteria, both in terms of how cognition was assessed, but also by allowing up to 50% of the group to be children. Of the 39 studies identified, only few of the studies aimed both to make a thorough neuropsychological assessment of cognition and to describe cognitive functioning in a group of adults with CP. Instead, we found 39 studies that used various methods to study one or more domains of cognitive functions in different samples of adults with CP or a mix of adults and children with CP.
The results show that there is indeed only scarce knowledge of the cognitive functioning in adults with cerebral palsy. It is clear, however, that cognitive deficits can be found in all cognitive domains, and that there is a great individual variability of performance on cognitive and intelligence tests. The review also shows that there is a lack of consensus as to which tests to use when assessing cognitive functions in adults with CP. Furthermore, there are a range of methodological issues in the literature. There is a strong need for further investigations into the cognitive functioning in adults with CP, and that there are certain methodological problems that could benefit from improvement. In the following sections, findings related to the core aims of the review will be discussed.
Cognitive Functions in Adults with CP
CP is a very heterogeneous condition with many different underlying etiologies and lesion locations in the brain. It is therefore not surprising that cognitive deficits have been found in all domains. In line with previous reviews,Citation11,Citation13 we found that when comparing adults with CP to healthy controls, impairments were predominantly documented in working memory,Citation48,Citation57,Citation58 executive functions,Citation48 as well as visuospatial and visuoperceptual functions.Citation47,Citation50,Citation62 However, the review also shows that at a group level there is a tendency towards individuals with CP having a poorer performance on language tests, even when controlling for factors such as spelling age, reading age or education.Citation65–67 This finding is unexpected, but might be explained by the fact that the three studies that came to this conclusion, all investigated language functions (in terms of e.g., spelling errors,Citation65 phonological processing and reading abilitiesCitation66 as well as lexical decision-making)Citation67 in groups of severely affected individuals with CP that had congenital speech impairments and complex communication needs. This finding might therefore not be generalizable, as many individuals with CP do not have congenital speech impairments.
Most of the 39 identified studies compared performance on tests of cognition and/or intelligence between individuals with CP and healthy controls at a group level. However, differences at the group level can be driven by few individuals with severe deficits and are therefore not always particularly informative. Grouping individuals with CP by e.g., subtype of CP or gross motor difficulties can be useful in some situations, but it is questionable whether this kind of grouping is a good indicator of what to expect in terms of cognitive functioning from an individual with CP. This type of classification can only serve as vague indicators about lesion location and etiology. It has also been demonstrated that some children with severe motor impairments and epilepsy can perform as well as, or even better, on cognitive tests than other children with CP with milder motor impairments and no epilepsy.Citation12 This review also shows that IQ range from 20 to 129 in adults and children with bilateral dyskinetic CP,Citation30,Citation46,Citation51–53 which underlines that CP type alone is not a strong predictor of intelligence or cognitive functioning. It is possible that there are statistical differences between subgroups but more studies with larger samples are required to investigate this further. From a neuropsychological perspective, it would also be interesting to use single case statistics, e.g., Crawford and colleagues’ methodsCitation70,Citation71 to estimate how well each individual participant with CP performs on individual tests instead of only describing performance at a group-level. This could enable the identification of typical cognitive profiles with certain combinations of cognitive deficits that can be seen in CP. Using these methods to describe the frequency of participants with CP whose performance is below expectation, or reporting the cognitive profiles of each individual participants, may be clinically more informative than only reporting results at a group level.
Assessment of Cognitive Functioning
We investigated which neuropsychological tests have been used in the literature on cognitive functioning in adults with CP, as test selection is particularly important for ensuring assessment quality in this population. Not all individuals with CP are able to take part in standard neuropsychological testing due to motor impairments, visual impairments and/or communication disorders.Citation18,Citation62,Citation72 The results show that there is a huge variability in the tests that were used in the included studies, and that there is no consensus in test selection in this area of research. In most cases where tests were used multiple times across studies, the studies were conducted by the same research group. It therefore seems that there is consensus regarding test selection within research groups, but not between research groups. One way to drive the field of neuropsychological assessment of adults with CP forward, could be to create a core battery of neuropsychological tests that can be used across studies. Such a battery has been created for the CPcog-adult follow-up protocol, where tests are selected based on their equivalence to the pediatric CPcog-protocol, their availability of Scandinavian norms, and their current use in clinical practice.Citation21,Citation73 Creating consensus about a core test battery at a broader level could potentially facilitate the implementation of larger international studies, as well as make it easier to compare results across individual studies. This can however also come at a cost, as some specific tests are likely to be more appropriate than others to answer specific hypotheses regarding cognition. Making a test battery that can be used internationally can also be challenging, as few neuropsychological tests are available and validated in all languages with high quality norms.
Although there is no broad consensus on test selection, RCPM and PPVT were the most widely used tests across all studies. Both tests have been used widely to assess individuals with different kinds of disabilities,Citation74 but RCPM has been recommended over PPVT to test intelligence in participants with CP.Citation46 PPVT was originally developed to test receptive language, but it has also been used as a test of verbal intelligence in some of the studies in the current review.Citation30,Citation46,Citation53 This is probably because the test developers have considered it a screening test of intellectual functioning, and because it correlates highly with WISC-III.Citation74 In the adult population, however, correlations with WAIS-III are lower,Citation74,Citation75 and it has been argued that the PPVT-III may underestimate full scale IQ-scores on the WAIS-III to as much as 10 points.Citation75 Strauss et al.Citation74 warn against using the PPVT as an intelligence test but they also acknowledge that it provides a useful estimate of IQ in individuals who cannot communicate verbally.
Many individuals with CP have visual impairments, impairments in hand motor functioning, and/or a communication disorder. It can therefore be assumed that unless all participants only have very mild impairments, there will always be some participants that will need some kind of test adaptation in relation to presentation of stimuli or response mode. According to Alant & Casey,Citation72 tests can be modified, adapted and/or accommodated to test takers with different kinds of impairments. Modifications and adaptations make the tests less equivalent compared to the original assessment tool, as changes are made to e.g., instructions or content of the test. Test accommodations are characterized by changes in ways that the tests are administered and presented, or changes in how to respond to a test, and should not impact the outcomes of the assessment.Citation72 Studies have found that accommodations to tests in the form of changes to response modes do not affect test performance in typically developing children,Citation76,Citation77 but it is unknown if this is the case for all tests and for all patient populations. However, it has been shown that it is possible to test even severely impaired individuals with CP with appropriate changes to response mode.Citation12 In some of the included studies, changes to response mode were described.Citation28,Citation36,Citation37,Citation46–48,Citation50,Citation52,Citation53,Citation55,Citation62,Citation63,Citation65,Citation67 In the study by Sabbadini et al.,Citation62 they recommended that participants should be allowed to give unambiguous responses that could not be influenced by the examiner’s implicit, involuntary messages. They described different ways of responding in a group of participants with CP with severe neuromotor and verbal disabilities.Citation62 Ballester-Plané et al.Citation46 described in their study that they used the recommendations by Sabbadini et al.Citation62 None of the other studies that explicitly described changes to response mode, mentioned whether they used a specific framework or guiding principles for how changes to response mode were made. In five studies, the authors specified that they had selected or devised tests/experiments that were well suited for the population.Citation43,Citation58,Citation61,Citation64,Citation66 In one study, the authors only included participants that were able to complete the neuropsychological test.Citation39 Even though it is well-known that many individuals with CP have visual impairments,Citation11,Citation14,Citation15 only two studies described that they used enlarged print or showed one item pr. page to account for visual impairments.Citation66,Citation67 In one study, they specifically tested for visual functioning in a practice task.Citation57 Most of the included studies had an inclusion or exclusion criterion related to visual functioning,Citation29,Citation30,Citation32,Citation39–41,Citation46,Citation48,Citation49,Citation51–53,Citation55–57,Citation62–66 but that does not necessarily mean that the participants do not have visual problems that might affect performance on cognitive tasks.Citation16 In future studies, it would be beneficial to include more detailed information about visual functioning, including information about any common visual impairments that individuals with CP may have, as e.g., strabismus, refractive errors and abnormal contrast sensitivity, may affect performance on visuo-perceptual and spatial tasks.Citation14–16 Finally, most included tests relied on accuracy rather than reaction time, which is also a way of minimizing the influence of motor difficulties. Although most tests were accuracy-based, the majority of studies did not clearly indicate whether tests had been modified, adapted or accommodated, and whether the participants had been able to perform the tests under optimal conditions. This lack of information makes it difficult to assess the validity of the results and does not enable the replication of studies, as the conditions under which the participants performed the tests are unclear. This finding stresses the importance of describing if and how changes have been made to tests in neuropsychological studies in the CP population, as well as the importance of being aware of any visual impairments, motor impairments and communication disorders, that may influence the results of neuropsychological tests.
Education history is commonly used as an indicator of general cognitive function,Citation10 but was not systematically reviewed in the included studies. For the CP population, educational history is not necessarily a good indicator of intelligence/cognitive functioning, as individuals severely affected by CP might have a potential for higher academic attainments than what they achieve, due to motor and/or speech impairments.Citation78 Thus, using educational history only will not necessarily provide a good estimate of cognitive abilities.Citation10 Although it can be challenging, it is possible to conduct a detailed neuropsychological assessment even of individuals with severe motor impairments and communication disorders,Citation62 and this will provide a better estimate of cognitive abilities.
Methodological Considerations and Quality Assessment
No longitudinal studies on cognitive functioning in adults were identified. This is in contrast to the childhood literature, where StadskleivCitation10 found that nine out of 81 studies on cognitive functioning in children were longitudinal. Longitudinal studies of children with CP have shown that the development of cognitive functions may not follow the same trajectory as typically developing children, ,Citation79–81 but it is unknown how cognitive functioning in adults with CP develop and mature across the lifespan. Adults with CP are at greater risk of stroke compared to others at the same age,Citation82 which in turn can lead to cognitive impairments. One study has reported that more adults with CP get a diagnosis of dementia and that they are diagnosed at an earlier age (below age 65) compared to the general population, although the significant difference disappears when accounting for comorbidities such as sensory impairment, intellectual disability and epilepsy.Citation83 Apart from a greater risk of cognitive impairment, it has also been documented that adults with CP experience more fatigue and pain than the general population, and many experience a deterioration in walking function already at a young age. This knowledge calls for a more close and systematic monitoring of adults with CP by experienced multidisciplinary teams.Citation84
To our knowledge, no scales have been designed to assess the quality of studies using neuropsychological assessments to describe cognitive functioning in reviews. The NOS was selected for this review, as this scale initially seemed to be the most appropriate scale. It turned out that the scale had to be slightly adjusted before it could be used to assess the included studies. Even with the adjustments, it became clear that there were issues with using the scale in a review of neuropsychological studies. One issue with the scale was the importance of using a representative sample, which was defined as “a consecutive or obviously representative series of cases.”Citation27 In this review, only seven of the 39 included studies lived up to this criterion, but none of the included studies aimed to estimate the prevalence of cognitive impairments in adults with CP. It is therefore questionable whether this item on the NOS scale is relevant for this kind of review even though it initially seemed to be the most appropriate scale. A new quality assessment scale for reviews of neuropsychological studies could e.g., focus on how cases and controls are selected, whether the study uses appropriate norms or an appropriate control group, and how results are reported; Crawford et al.Citation85 have made recommendations on how statistics from single case studies should be reported. Similar recommendations would be valuable for group studies in neuropsychology.
Recommendations for Future Studies
Based on this review, it can be concluded that cognitive deficits can be found within all cognitive domains in adults with CP. More importantly, it also shows that there is only sparse literature on the cognitive functioning of adults with CP. More research is needed to understand the cognitive challenges faced by adults with CP and how these challenges develop throughout adulthood. This is both to get more in-depth knowledge of cognitive functioning across the lifespan, but also to find the best assessment methods at different stages of adulthood e.g., in relation to the transition from youth to adulthood or when assessing cognitive functioning in relation to dementia. Future studies that address the following are needed: 1) Population-based studies that can inform us on the prevalence of cognitive impairments in CP; 2) Studies with multiple participants that analyze patterns of cognitive impairments at the individual level; 3) Longitudinal studies that investigate how cognitive functioning evolves across the lifespan of individuals with CP, including into old age; 4) The creation of a core test battery that can be used internationally, and which makes it possible to compare results across studies; 5) Awareness of potential deficits that might influence the test results, e.g., in relation to vision impairments, motor impairment and communication disorders. It should be made clear that these variables have been taken into consideration, as it might affect the validity of the results; 6) An inventory of test-accommodations that can inform researchers and clinicians when assessing individuals with CP. This could potentially also include a standardized way of reporting any changes that have been made to a test.
Supplemental Material
Download Zip (237.4 KB)Acknowledgments
This work was supported by a grant from the Elsass Foundation to RJR (grant no. 20-3-0990). We thank Marie Mathiasen for help with reviewing the articles, data extraction and quality assessment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17518423.2024.2347991.
Additional information
Funding
References
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;109: 8–14. doi:10.1111/j.1469-8749.2007.tb12610.x.
- American Psychological Association A. Cognitive functioning. APA Dictionary of Psychology. Published 2018. https://dictionary.apa.org/cognitive-functioning
- Anderson V, Northam E, Wrennall J, editors. Child neuropsychology - theory and practice. In: Developmental neuropsychology: a clinical approach. 2nd ed. London: Routledge; 2019. pp. 3–26.
- Crowell DH, Crowell DC. Intelligence test reliability for cerebral palsied children. J Consult Psychol. 1954;18(4):276. doi:10.1037/h0062867.
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31(3/5):373–85. doi:10.1023/A:1024190429920.
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–93. doi: 10.1006/nimg.2000.0743.
- Platt MJ, Panteliadis CP, Häusler M. Aetiological factors. In: Panteliadis C. editor. Cerebral palsy: a multidisciplinary approach. 3rd. Springer; 2018. pp. 49–58. doi:10.1007/978-3-319-67858-0_6.
- Johnston MV. Clinical disorders of brain plasticity. Brain Dev. 2004;26(2):73–80. doi:10.1016/S0387-7604(03)00102-5.
- Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20:265–76.
- Stadskleiv K. Cognitive functioning in children with cerebral palsy. Dev Med Child Neurol. 2020;62(3):283–89. doi:10.1111/dmcn.14463.
- Ego A, Lidzba K, Brovedani P, Belmonti V, Gonzalez‐Monge S, Boudia B, Ritz A, Cans C. Visual–perceptual impairment in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2015;57(suppl. 2):46–51. doi:10.1111/dmcn.12687.
- Stadskleiv K, Jahnsen R, Andersen GL, von Tetzchner S. Neuropsychological profiles of children with cerebral palsy. Dev Neurorehabil. 2018;21(2):108–20. doi:10.1080/17518423.2017.1282054.
- Straub K, Obrzut JE. Effects of cerebral palsy on neuropsychological function. J Dev Phys Disabil. 2009;21(2):153–67. doi:10.1007/s10882-009-9130-3.
- Duke RE, Nwachukuw J, Torty C, Okorie U, Kim MJ, Burton K, Gilbert C, Bowman R. Visual impairment and perceptual visual disorders in children with cerebral palsy in Nigeria. Br J Ophthalmol. 2022;106(3):427–34. doi:10.1136/bjophthalmol-2020-317768.
- Rauchenzauner M, Schiller K, Honold M, Baldissera I, Biedermann R, Tschiderer B, Albrecht U, Arnold C, Rostasy K. Visual impairment and functional classification in children with cerebral palsy. Neuropediatrics. 2021;52(5):383–89. doi:10.1055/s-0040-1722679.
- Ibrahimi D, Mendiola-Santibañez JD, Gkaros AP. Analysis of the potential impact of strabismus with and without amblyopia on visual-perceptual and visual-motor skills evaluated using TVPS-3 and VMI-6 tests. J Optom. 2021;14(2):166–75. doi:10.1016/j.optom.2020.04.002.
- Sigurdardottir S, Eiriksdottir A, Gunnarsdottir E, Meintema M, Arnadottir U, Vik T. Cognitive profile in young Icelandic children with cerebral palsy. Dev Med Child Neurol. 2008;50(5):357–62. doi:10.1111/j.1469-8749.2008.02046.x.
- Stadskleiv K, Jahnsen R, Andersen GL, von Tetzchner S. Executive functioning in children aged 6–18 years with cerebral palsy. J Dev Phys Disabil. 2017;29(4):663–81. doi:10.1007/s10882-017-9549-x.
- Hill-Briggs F, Dial JG, Morere DA, Joyce A. Neuropsychological assessment of persons with physical disability, visual impairment or blindness, and hearing impairment or deafness. Arch Clin Neuropsychol. 2007;22(3):389–404. doi:10.1016/j.acn.2007.01.013.
- Coceski M, Hocking DR, Reid SM, Abu-Rayya HM, Reddihough DS, Wrennall J, Stargatt R. Assessing IQ in adolescents with mild to moderate cerebral palsy using the WISC-V. Clin Neuropsychol. 2021;(0):1–20. doi:10.1080/13854046.2021.1928290.
- Stadskleiv K, van Walsem MR, Andersen GL, Bergqvist L, Bøttcher L, Christensen K, Heyerdahl D, Hollung SJ, Høye H, Jahnsen R. et al. Systematic monitoring of cognition for adults with cerebral palsy—the rationale behind the development of the CPCog-adult follow-up protocol. Front Neurol. 2021;12:1–10. doi:10.3389/fneur.2021.710440.
- Philip SS, Guzzetta A, Chorna O, Gole G, Boyd RN. Relationship between brain structure and cerebral visual impairment in children with cerebral palsy: a systematic review. Res Dev Disabil. 2020;99(January):103580. doi:10.1016/j.ridd.2020.103580.
- van Gorp M, Hilberink SR, Noten S, van Gorp M, Benner JL, Stam HJ, van der Slot WMA, Roebroeck ME. Epidemiology of cerebral palsy in adulthood: a systematic review and meta-analysis of the most frequently studied outcomes. Arch Phys Med Rehabil. 2020;101(6):1041–52. doi:10.1016/j.apmr.2020.01.009.
- Benner JL, Noten S, Limsakul C, Van Der Slot WMA, Stam HJ, Selb M, Van Den Berg‐Emons RJG, Roebroeck ME. Outcomes in adults with cerebral palsy: systematic review using the international classification of functioning, disability and health. Dev Med Child Neurol. 2019;61(10):1153–61. doi:10.1111/dmcn.14247.
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood EG, Galuppi B. Reliability of a system, function in children with cerebral palsy. Develop Med Child Neuro. 1997;39(4):214–23. doi:10.1111/j.1469-8749.1997.tb07414.x.
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, Öhrvall A-M, Rosenbaum P. The manual ability classification system (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–54. doi:10.1017/S0012162206001162.
- Wells GA, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottowa scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Accessed October 6, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Tsukahara R, Aoki H. Skin potential response in letter recognition task as an alternative communication channel for individuals with severe motor disability. Clin Neurophysiol. 2002;113(11):1723–33. doi:10.1016/S1388-2457(02)00257-2.
- Lackner CL, Gorter JW, Segalowitz SJ, MyStory Study Group. Cognitive event-related potentials in young adults with cerebral palsy: a proof-of-concept study. Clin EEG Neurosci. 2024;55(1):64–75. Published online 2020. doi:10.1177/1550059420977318.
- Laporta-Hoyos O, Ballester-Plané J, Póo P, Macaya A, Meléndez-Plumed M, Vázquez E, Delgado I, Zubiaurre-Elorza L, Botellero VL, Narberhaus A. et al. Proxy-reported quality of life in adolescents and adults with dyskinetic cerebral palsy is associated with executive functions and cortical thickness. Qual Life Res. 2017;26(5):1209–22. doi: 10.1007/s11136-016-1433-0.
- Tobimatsu Y, Nakamura R. Retrospective study of factors affecting employability of individuals with cerebral palsy in Japan. Tohoku J Exp Med. 2000;192(4):291–99. doi:10.1620/tjem.192.291.
- Warschausky S, Kaufman JN, Evitts M, Schutt W, Hurvitz EA. Mastery motivation and executive functions as predictors of adaptive behavior in adolescents and young adults with cerebral palsy or myelomeningocele. Rehabil Psychol. 2017;62(3):258–67. doi:10.1037/rep0000151.
- Halvey C, Rehfeldt RA. Expanding vocal requesting repertoires via relational responding in adults with severe developmental disabilities. Anal Verbal Behav. 2005;21(1):13–25. doi:10.1007/bf03393007.
- Joseph R. A case analysis in human sexuality: counseling to a man with severe cerebral palsy. Sex Disabil. 1991;9(2):149–59. doi:10.1007/BF01101740.
- Mann J, Foreman DM. Homo-erotomania for a delusional parent: erotomania with Capgras and Fregoli syndromes in a young male with learning difficulties. J Intellect Disabil Res. 1996;40(3):275–78. doi:10.1111/j.1365-2788.1996.tb00630.x.
- Lund SK, Light J. Long-term outcomes for individuals who use augmentative and alternative communication: part I - What is a “good” outcome? AAC Augment Altern Commun. 2006;22(4):284–99. doi:10.1080/07434610600718693.
- Taibo MLG, Iglesias PV, Raposo MDSG, Méndez MS, Del Salvador Gonzalez Raposo M, Méndez MS. An exploratory study of phonological awareness and working memory differences and literacy performance of people that use AAC. Span J Psychol. 2010;13(2):538–56. doi:10.1017/S1138741600002237.
- Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, Burbaud P, Welter M-L, Xie-Brustolin J, Braga MCC. et al. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol. 2009;8(8):709–17. doi: 10.1016/S1474-4422(09)70151-6.
- Alcaide-Aguirre RE, Warschausky SA, Brown D, Aref A, Huggins JE. Asynchronous brain–computer interface for cognitive assessment in people with cerebral palsy. J Neural Eng. 2017;14(6):066001. doi:10.1088/1741-2552/aa7fc4.
- de la Torre-Luque A, Valero-Aguayo L, de la Rubia-Cuestas EJ, de la Torre-Luque A, de la Rubia-Cuestas EJ. Visuospatial orientation learning through virtual reality for people with severe disability. Int J Disabil Dev Educ. 2017;64(4):420–35. doi:10.1080/1034912X.2016.1274022.
- Jaume-I-Capó A, Martínez-Bueso P, Moyà-Alcover B, Varona J. Improving vision-based motor rehabilitation interactive systems for users with disabilities using mirror feedback. Sci World J. 2014;2014:1–9. doi:10.1155/2014/964576.
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12(3):189–98. doi:10.1016/0022-3956(75)90026-6.
- van Elk M, Crajé C, Beeren MEGV, Steenbergen B, van Schie HT, Bekkering H, van Schie HT. Neural evidence for compromised motor imagery in right hemiparetic cerebral palsy. Front Neurol. 2010;1:1–7. doi:10.3389/fneur.2010.00150.
- Ng TKS, Tagawa A, Chun-Man Ho R, Larbi A, Kua EH, Mahendran R, Carollo JJ, Heyn PC. Commonalities in biomarkers and phenotypes between mild cognitive impairment and cerebral palsy: a Pilot exploratory study. Aging (Albany NY). 2021;13(2):1773–816. doi:10.18632/aging.202563.
- Rogers SL, Coe CL, Hartke K. Cognitive impairment after unilateral hemispheric injury of congenital or adult origin. Am J Occup Ther. 2002;56(2 PG–191–201):191–201. doi:10.5014/ajot.56.2.191.
- Ballester-Plané J, Laporta-Hoyos O, Macaya A, Póo P, Meléndez-Plumed M, Vázquez É, Delgado I, Zubiaurre-Elorza L, Narberhaus A, Toro-Tamargo E. et al. Measuring intellectual ability in cerebral palsy: the comparison of three tests and their neuroimaging correlates. Res Dev Disabil. 2016;56:83–98. doi:10.1016/j.ridd.2016.04.009.
- Ballester-Plané J, Laporta-Hoyos O, Macaya A, Póo P, Meléndez-Plumed M, Toro-Tamargo E, Gimeno F, Narberhaus A, Segarra D, Pueyo R. et al. Cognitive functioning in dyskinetic cerebral palsy: its relation to motor function, communication and epilepsy. Eur J Paediatr Neurol. 2018;22(1):102–12. doi: 10.1016/j.ejpn.2017.10.006.
- Laporta-Hoyos O, Ballester-Plané J, Leiva D, Ribas T, Miralbell J, Torroja-Nualart C, Russi ME, Toro-Tamargo E, Meléndez-Plumed M, Gimeno F. et al. Executive function and general intellectual functioning in dyskinetic cerebral palsy: comparison with spastic cerebral palsy and typically developing controls. Eur J Paediatr Neurol. 2019;23(4):546–59. doi: 10.1016/j.ejpn.2019.05.010.
- Pueyo R, Junqué C, Vendrell P. Neuropsychologic differences between bilateral dyskinetic and spastic cerebral palsy. J Child Neurol. 2003;18(12):845–50. doi:10.1177/088307380301801204.
- Pueyo R, Junqué C, Vendrell P, Narberhaus A, Segarra D. Neuropsychologic impairment in bilateral cerebral palsy. Pediatr Neurol. 2009;40(1):19–26. doi:10.1016/j.pediatrneurol.2008.08.003.
- Ballester-Plané J, Schmidt R, Laporta-Hoyos O, Junqué C, Vázquez É, Delgado I, Zubiaurre-Elorza L, Macaya A, Póo P, Toro E. et al. Whole-brain structural connectivity in dyskinetic cerebral palsy and its association with motor and cognitive function. Hum Brain Mapp. 2017;38(9):4594–612. doi: 10.1002/hbm.23686.
- Laporta-Hoyos O, Fiori S, Pannek K, Ballester-Plané J, Leiva D, Reid LB, Pagnozzi AM, Vázquez É, Delgado I, Macaya A. et al. Brain lesion scores obtained using a simple semi-quantitative scale from MR imaging are associated with motor function, communication and cognition in dyskinetic cerebral palsy. Neuroimage Clin. 2018;19:892–900. doi:10.1016/j.nicl.2018.06.015.
- Laporta-Hoyos O, Pannek K, Ballester-Plané J, Reid LB, Vázquez É, Delgado I, Zubiaurre-Elorza L, Macaya A, Póo P, Meléndez-Plumed M. et al. White matter integrity in dyskinetic cerebral palsy: relationship with intelligence quotient and executive function. Neuroimage Clin. 2017;15:789–800. doi:10.1016/j.nicl.2017.05.005.
- Magill-Evans J, Galambos N, Darrah J, Nickerson C. Predictors of employment for young adults with developmental motor disabilities. Work J Prev Assess Rehabil. 2008;31:433–42.
- Pueyo R, Ariza M, Narberhaus A, Ballester-Plané J, Laporta-Hoyos O, Junqué C, Vendrell P. Does verbal and gestural expression ability predict comprehension ability in cerebral palsy? Percept Mot Skills. 2013;116(2):512–27. doi:10.2466/15.10.PMS.116.2.512-527.
- Lemay M, Lê T-T, Lamarre C. Deficits in two versions of a sustained attention test in adolescents with cerebral palsy. Dev Neurorehabil. 2012;15(4):253–58. doi:10.3109/17518423.2012.678020.
- Hoffman RM, Trevarrow MP, Bergwell HR, Embury CM, Heinrichs-Graham E, Wilson TW, Kurz MJ. Cortical oscillations that underlie working memory are altered in adults with cerebral palsy. Clin Neurophysiol. 2021;132(4):938–45. doi:10.1016/j.clinph.2020.12.029.
- Goble DJ, Aaron MB, Warschausky S, Kaufman JN, Hurvitz EA. The influence of spatial working memory on ipsilateral remembered proprioceptive matching in adults with cerebral palsy. Exp Brain Res. 2012;223(2):259–69. doi:10.1007/s00221-012-3256-8.
- Toomela A. Short-term memory in young adults with spastic diplegic cerebral palsy. Dev Neuropsychol. 2012;37(4):317–32. doi:10.1080/87565641.2011.632461.
- Crajé C, van Elk M, Beeren M, van Schie HT, Bekkering H, Steenbergen B, van Schie HT. Compromised motor planning and motor imagery in right hemiparetic cerebral palsy. Res Dev Disabil. 2010;31(6):1313–22. doi:10.1016/j.ridd.2010.07.010.
- Lidzba K, Staudt M, Wilke M, Krägeloh-Mann I. Visuospatial deficits in patients with early left-hemispheric lesions and functional reorganization of language: consequence of lesion or reorganization? Neuropsychologia. 2006;44(7):1088–94. doi:10.1016/j.neuropsychologia.2005.10.022.
- Sabbadini M, Bonanni R, Carlesimo GA, Caltagirone C. Neuropsychological assessment of patients with severe neuromotor and verbal disabilities. J Intellect Disabil Res. 2001;45(2):169–79. doi:10.1046/j.1365-2788.2001.00301.x.
- Pueyo R, Junqué C, Vendrell P, Narberhaus A, Segarra D. Raven’s coloured progressive matrices as a measure of cognitive functioning in cerebral palsy. J Intellect Disabil Res. 2008;52(5):437–45. doi:10.1111/j.1365-2788.2008.01045.x.
- Icht M, Levine-Sternberg Y, Mama Y. Visual and auditory verbal long-term memory in individuals who rely on augmentative and alternative communication. AAC Augment Altern Commun. 2020;36(4):238–48. doi:10.1080/07434618.2020.1852443.
- Hart P, Scherz J, Apel K, Hodson B. Analysis of spelling error patterns of individuals with complex communication needs and physical impairments. AAC Augment Altern Commun. 2007;23(1):16–29. doi:10.1080/07434610600802737.
- Foley BE, Pollatsek A. Phonological processing and reading abilities in adolescents and adults with severe congenital speech impairments. AAC Augment Altern Commun. 1999;15(3):156–73. doi:10.1080/07434619912331278695.
- Smith MM. Simply a speech impairment? literacy challenges for individuals with severe congenital speech impairments. Int J Disabil Dev Educ. 2001;48(4):331–53. doi:10.1080/10349120120094257.
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4th ed. Minneapolis, MN: Pearson Assessment; 2007.
- Raven J. Coloured progressive matrices and Crichton vocabulary scale manual. London: Pearson Assessment; 2008.
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol. 1998;12(4):482–86. doi:10.1076/clin.12.4.482.7241.
- Crawford JR, Garthwaite PH. Comparison of a single case to a control or normative sample in neuropsychology: development of a Bayesian approach. Cogn Neuropsychol. 2007;24(4):343–72. doi:10.1080/02643290701290146.
- Alant E, Casey M. Assessment concessions for learners with impairments. South African J Educ. 2005;25:185–89.
- Bøttcher L, Stadskleiv K, Berntsen T, Christensen K, Korsfelt Å, Kihlgren M, Ödman P. Systematic cognitive monitoring of children with cerebral palsy – the development of an assessment and follow-up protocol. Scand J Disabil Res. 2016;18(4):304–15. doi:10.1080/15017419.2015.1091035.
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. Oxford: Oxford University Press; 2006.
- Bell NL, Lassiter KS, Matthews TD, Hutchinson MB. Comparison of the peabody picture vocabulary test - third edition and wechsler adult intelligence scale - third edition with university students. J Clin Psychol. 2001;57(3):417–22. doi:10.1002/jclp.1024.
- Fiske SI, Haddeland AL, Skipar I, Bootsma JN, Geytenbeek JJ, Stadskleiv K. Assessing language comprehension in motor impaired children needing AAC: validity and reliability of the Norwegian version of the receptive language test C-BiLLT. AAC Augment Altern Commun. 2020;36(2):95–106. doi:10.1080/07434618.2020.1786857.
- Kurmanaviciute R, Stadskleiv K, Visser L. Assessment of verbal comprehension and non-verbal reasoning when standard response mode is challenging: a comparison of different response modes and an exploration of their clinical usefulness. Cogent Psychol. 2017;4(1):1275416. doi:10.1080/23311908.2016.1275416.
- Fluss J, Lidzba K. Cognitive and academic profiles in children with cerebral palsy: a narrative review. Ann Phys Rehabil Med. 2020;63(5):447–56. doi:10.1016/j.rehab.2020.01.005.
- Dahlgren Sandberg A. Reading and spelling abilities in children with severe speech impairments and cerebral palsy at 6, 9, and 12 years of age in relation to cognitive development: a longitudinal study. Dev Med Child Neurol. 2006;48(8):629–34. doi:10.1017/S0012162206001344.
- Gonzalez-Monge S, Boudia B, Ritz A, Abbas-Chorfa F, Rabilloud M, Iwaz J, Bérard C. A 7-year longitudinal follow-up of intellectual development in children with congenital hemiplegia. Dev Med Child Neurol. 2009;51(12):959–67. doi:10.1111/j.1469-8749.2009.03339.x.
- Levine SC, Kraus R, Alexander E, Suriyakham LW, Huttenlocher PR. IQ decline following early unilateral brain injury: a longitudinal study. Brain Cogn. 2005;59(2):114–23. doi:10.1016/j.bandc.2005.05.008.
- Wu CW, Huang SW, Lin JW, Liou TH, Chou LC, Lin HW. Risk of stroke among patients with cerebral palsy: a population-based cohort study. Dev Med Child Neurol. 2016;59(1):52–56. doi:10.1111/dmcn.13180.
- Smith KJ, Peterson MD, Victor C, Ryan JM. Risk of dementia in adults with cerebral palsy: a matched cohort study using general practice data. BMJ Open. 2021;11(1):1–6. doi:10.1136/bmjopen-2020-042652.
- Smith SE, Gannotti M, Hurvitz EA, Jensen FE, Krach LE, Kruer MC, Msall ME, Noritz G, Rajan DS, Aravamuthan BR. et al. Adults with cerebral palsy require ongoing neurologic care: a systematic review. Ann Neurol. 2021;89(5):860–71. doi: 10.1002/ana.26040.
- Crawford JR, Garthwaite PH, Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: rationale, methods, implementations, and proposed reporting standards. Cogn Neuropsychol. 2010;27(3):245–60. doi:10.1080/02643294.2010.513967.