Abstract
Purpose
This invited commentary addresses the importance of the senses in human communication, outlines advances achieved with cochlear implants, and new research directions to improve neural prostheses.
Result
In severely deaf people, cochlear implants restore speech understanding and enable children to achieve spoken language. Research in neural prostheses is advancing the restoration of hearing, vision, tactile senses, movement and the management of epilepsy. Bio-inspired stimulation strategies incorporating temporal and spatial characteristics of neural responses may deliver improved speech, vision and tactile perception using prostheses. To achieve stable long-term stimulation, chronic inflammation at the brain-electrode interface may be reduced using ROCK/Rho signalling pathway inhibitors and materials with brain-mimicking properties.
Conclusion
This commentary paper addresses two Sustainable Development Goals: industry, innovation and infrastructure (SDG 9) and good health and well-being (SDG 3).
Introduction
Good hearing is a key element that facilitates auditory-verbal communication and is thus important for the good health and well-being (Sustainable Development Goal 3, SDG 3, United Nations, Citation2015) of many people who rely on spoken language in their day-to-day lives. The World Health Organisation (WHO) (World Health Organization, Citation2021) estimated there are 430 million people, or 5.5% of the world population, living with moderate to severe hearing loss. This can lead to loneliness and lost educational and employment opportunities (Dammeyer et al., Citation2019). The WHO considers this costs the world economy US$980 billion. Before the invention of the multi-channel cochlear implant (bionic ear) in the 1970s, it was thought impossible to restore hearing to people with significant hearing loss. This important discovery has since inspired industry, innovation and infrastructure developments (SDG 9, United Nations, Citation2015) for the remediation of other senses, such as restoring sight to those who are legally blind and sensation to those with spinal cord injuries. Therefore, it is evident that innovations aiming to restore the senses would help to achieve SDG targets that focus on healthcare accessibility (Target 3.8), inclusive industrialisation (Target 9.2) and scientific research (Target 9.5) (United Nations, Citation2015).
Overview of the cochlear implant
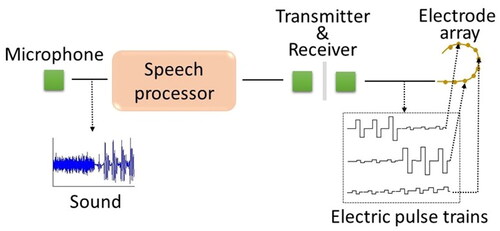
The University of Melbourne’s implantable receiver-stimulator used for the first bionic ear was, at the time, the most complex package of electronics implanted in a patient (Clark, Black, et al., Citation1978). The multi-channel cochlear implant has an external component (speech processor) that sends signals and power to the internal component (receiver-stimulator). The receiver-stimulator transmits electrical pulses along an electrode array implanted in the cochlea to excite appropriate auditory nerve fibres that send signals to frequency-specific sites in the brain. The prosthesis thereby partially reproduces the coding of sound normally undertaken by the inner ear.
A multi-channel cochlear implant was first inserted into a profoundly deaf adult at the Royal Victorian Eye & Ear Hospital in 1978 (Clark, Black, et al., Citation1978). Subsequently, there was a “eureka” moment when this patient heard vowel sounds when frequency-specific regions of the auditory nerve for the key formant frequencies of the vowels were stimulated. That led to the first clinically successful speech coding strategy for a cochlear implant (Tong et al., Citation1980).
Since being developed industrially by Cochlear Limited, the cochlear implant has restored hearing and speech understanding safely to hundreds of thousands of people in more than 100 countries. A world study of the cochlear implant on pre-linguistically deaf children (Clark et al., Citation1987) led, in 1990, to it becoming the first cochlear implant of any type to be approved for children by the US FDA or any world regulatory body. It was a second “eureka” moment when we discovered that children have improved speech and language outcomes when implanted at an early age. This has been confirmed by many subsequent studies (Cupples et al., Citation2018; Dettman et al., Citation2016, Karltorp et al., Citation2020). Early implantation is associated with increased educational and employment levels later in life (Dammeyer et al., Citation2019). There are also demonstrated benefits for older recipients of cochlear implants, including reduced risk of cognitive decline (Sarant et al., Citation2019), and improved dementia screening test (DemTect) results (Issinget al., Citation2021). We refer the interested reader to the book by Stinson and Buckley (Citation2013) for anecdotes from 15 cochlear implant recipients, which highlights their lived experiences post-implantation and its impact on their social engagement.
Signal analyses for neural prostheses
Contemporary neural prostheses have been used as substitutes for hearing, vision and touch, activating paralysed muscles and improving conditions such as epilepsy and Parkinson’s disease. Other examples include implantable devices for pain and bladder control.
Auditory prostheses
Cochlear implants restore functional hearing using electrical stimulation to activate auditory nerve fibres (ANFs). The tonotopic organisation of surviving ANFs allows stimulation of distinct neural populations, encoding spectral information using electrode positions, illustrated in .
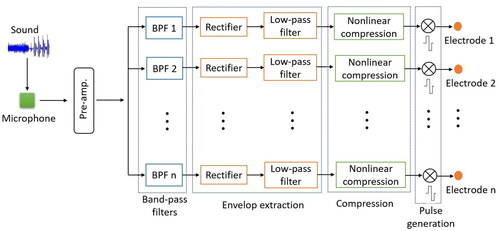
Sound coding strategies map microphone signals to electrical stimulation across the implanted electrodes. Early commercial stimulation strategies for multi-electrode cochlear implants were spectral feature extraction strategies, where the voice pitch (fundamental frequency, F0) and important frequency information (formants F1, F2 and high-frequency band-pass filter outputs) were extracted. The frequencies were conveyed by stimulating appropriate electrodes while F0 controlled the pulse repetition rate. The F0/F1/F2 and Multipeak strategies were implemented in the Cochlear WSP-III and MSP wearable speech processors in the 1980s (Cohen et al., Citation1993).
In early research, the importance of temporal cues in cochlear implant sound strategies alone was considered, and the concept of spectral analysis using band-pass filters was proposed (Clark, Black, et al., Citation1978). In the 1990s, spectral maxima sound processing (SMSP) (McKay et al., Citation1992) and continuous interleaved sampling (CIS) (Wilson et al., Citation1991) largely replaced spectral feature extraction strategies due to better speech perception performance (Wouters et al., Citation2015). In these spectral analysis strategies, the temporal envelopes of the filtered waveforms are extracted, and the compressed envelope outputs used to modulate the electrode stimulation levels, shown in . To avoid simultaneous electrical field interference, simulation pulses are rapidly delivered to one electrode at a time so that no simultaneous stimulation occurs between the electrodes.
The commonly used strategies in modern cochlear implants are (Wouters et al., Citation2015):
Advanced Combination Encoder (ACE), implemented in most Cochlear Limited systems, which is based upon the SMSP strategy and is one of the most widely used speech processing strategies, producing more spectral detail and better speech understanding than the CIS strategy for implants with many electrodes.
Fine Structure Processing (FSP), implemented in MED-EL systems, aims to represent the temporal fine structure in the lowest frequencies of the audio signals by delivering bursts of stimulus pulses on one or more corresponding electrodes.
HiRes120, implemented in Advanced Bionics systems, applies current steering by stimulating two or more electrodes simultaneously to generate virtual channels, aiming to increase apparent numbers of electrodes.
Ongoing research seeks to better represent spectral and temporal cues to deliver improved music perception and speech recognition in noisy environments. Current steering and current focussing reduce interference between electrodes and increase effective spectral channels. More recently, a bio-inspired coding strategy incorporating tempo-spectral characteristics of responses of ANFs to stimulation has been developed (CitationTabibi et al., 2020).
Vision prostheses
Vision prostheses aim to provide vision to those who are blind using electrodes implanted near the retina or visual cortex. Because of the two-dimensional nature of vision, two-dimensional arrays of electrodes are used that may contain tens to thousands of electrodes, creating a significant challenge for signal analysis. Strategies with 16–60 electrodes have used non-simultaneous pulsatile stimulation, similar to cochlear implants, with stimuli on electrodes conveying brightness (Ayton et al., Citation2014; Edwards et al., Citation2018). For greater electrode numbers, simultaneous stimulation is required. Existing approaches use independent stimulators for each electrode activated by incoming light levels but have results similar to arrays with fewer electrodes (Wilke et al., Citation2011). Current research is investigating the control of the pattern of electric fields generated across the array using simultaneous electrode activation, called “neural activity shaping” (Spencer et al., Citation2019). This strategy estimates neural responses generated by electrodes and combines these to create complex stimulation patterns.
Typically, neural prostheses are connected to the external environment, which leads to some challenges in signal transmission, such as robustness and security of signal transmission between neural prostheses and external systems, autonomy, and power efficiency (Loeb, Citation2018). Improvements in task performance, usability, embodiment, and bi-directional communication between prostheses and neurons are the primary goals in development of neural prostheses. To this end, closed-loop neural prostheses have begun to emerge that use recording of neural responses to fine-tune delivery of stimulation; these offer an opportunity to produce high fidelity neural responses in a new generation of neural prostheses (Levi et al., Citation2018).
Interfacing signals to the central nervous system (CNS)
Fundamental to the clinical success of neuro-prostheses and achieving SDG 3 is the ability to effectively integrate information with the neural circuitry. However, over time, these devices have progressively degraded signals and unreliable stimulation. For example, fibrous tissue encapsulation of cochlear implant electrodes can result in increased impedance, thus contributing to reduced device performance. Likewise, studies of central neuro-prostheses have reported variable stimulation thresholds and losses in signal detection. A major contributing factor is chronic inflammation that destabilises the brain-electrode interface (Salatino et al., Citation2017).
Inflammation of the CNS in response to neuro-electrodes
Inflammation within the brain from electrode implantation consists of a complex cascade of biological reactions involving immune cells. It begins with an initial migration of microglia and macrophages to the injury site, followed by the release of pro-inflammatory cytokines. Additionally, glial cells, specifically astrocytes, proliferate around the injury whilst simultaneously increasing their expression of filament proteins. These processes result in the formation of a glial scar that acts to protect the brain parenchyma from infiltration of inflammatory cells and foreign proteins, thus preventing secondary degeneration. The astrocytic response then becomes trophic, supporting reconstruction of brain circuits. However, the persistence of reactive astrocytes (often called glial scarring) can thwart this trophic phase and prevent axons from entry to the injury site (Maclean et al., Citation2016).
Most neuro-electrodes currently under investigation in clinical trials or primate studies are constructed from silicon, which has an elastic modulus of ∼100 GPa. Conversely, the human brain has a modulus of ∼1 kPa (Salatino et al., Citation2017). This mechanical mismatch means that any movement of the electrode or pulsation of the brain results in micro-trauma, and consequently glial scarring, at the electrode-brain interface long after the initial implantation injury has occurred. Glial scarring creates a cytotoxic environment that induces the death of adjacent neurons and also increases resistance and capacitance around the electrode surface. The compounded effect of increased tissue resistance and greater distances between the electrode and neurons reduces stimulation currents and impairs neural excitability (Salatino et al., Citation2017).
Future directions
There are two key targets for improving the brain-electrode interface when designing implants. The first target is the electrode material, which should closely mimic the mechanical and structural properties of the CNS tissue. One promising development is flexible mesh electronics. This device consists of small, exposed electrodes interconnected through a network of polymer-insulated metal lines to the input/output pads situated at opposite ends of the mesh, as shown in .
Figure 3. (I)16-channel mesh electronics with electrodes (bold black square) and input/output (I/O) pads. (II) Mesh electronics probe (arrow) in aqueous saline solution. (III) Mesh electronics partially ejected through glass needle (inner diameter = 95µm). Reprinted with permission from Hong et al. (Citation2018); Copyright 2018 American Chemical Society.
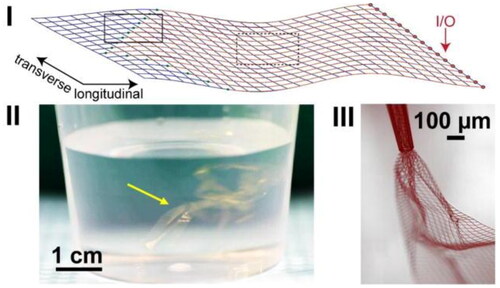
Fabrication of mesh electronics utilises standard lithographic techniques and can be injected into the CNS via syringe. The advantages of open-mesh electronics are: (a) they exhibit tissue-like flexibility thereby reducing mechanical mismatch; (b) the electrode components are fabricated to sizes comparable to neuron soma to allow for better targeting of specific neuron subtypes; (c) the device demonstrates superior macro-porosity, allowing easier diffusion of neurites around the electrodes (Hong et al., Citation2018) whilst preventing accumulation of pro-inflammatory cytokines at the electrode surface. For applications such as cochlear implants, flexible electrodes can help achieve finer temporo-spatial responses as the electrodes could sit closer to ANFs.
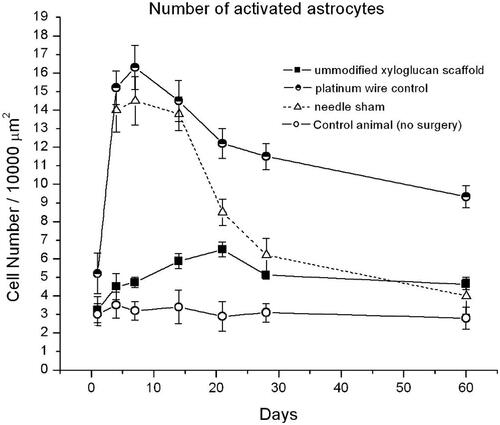
Although these novel neuronal probes are minimally invasive and demonstrate advantageous mechanical properties, there is still a need to further attenuate chronic inflammation. Therefore, biochemical inhibition of the reactive astrocytes should also be targeted when designing neuro-prostheses. Recent investigations have revealed that galectins, signalling proteins that bind to galactose, will modulate and terminate reactive gliosis via the ROCK/Rho pathway (Maclean, Ims et al., Citation2018). They are also important in the transition to brain-derived neurotrophic factor (BDNF) production and the trophic inflammatory phase, which preserve neural cells such as spiral ganglion (Clark, Citation2003). To explore how ROCK/Rho inhibitors attenuate the reactive astrocyte response, the striatum of Wistar rats were implanted with galactose moieties attached to hydrogel materials (xyloglucan grafted with poly-d-lysine, PDL) (Nisbet et al., Citation2010). When compared with the inflammatory response in rats modelling both a simple stab wound and platinum wire implant, it was found that the xyloglucan significantly suppressed the reactive astrocyte response, shown in . This highlights the possibility of using galactose or other ROCK/Rho inhibitors to suppress not only the glial scar around central neuro-protheses, but also the fibrous tissue response around cochlear implants. Encapsulation of electrodes with galactose moieties could be achieved utilising polyelectrolyte multilayer films for example (Maclean et al., Citation2018).
Figure 4. Number of astrocyte cells observed within 100µm of scaffold or controls in Wistar rat brains. Reprinted with permission from Nisbet et al. (Citation2010). Copyright 2010 Mary Ann Liebert, Inc.
In summary, combining astrocyte suppression with ultra-flexible mesh electronics that impart a degree of brain mimicry presents a potential pathway to achieve superior bio-integration, reduced impedance, and stimulation stability.
Summary and conclusions
This paper has discussed how biomedical engineering advances can improve our senses and allow many people to obtain a greater appreciation of the world around them. Cochlear implants can also reduce the risk of dementia in adults with a severe hearing loss (Sarant et al., Citation2019). Furthermore, they can restore hearing in children, allowing them to achieve normal spoken language educational milestones (Dettman et al., Citation2016; Karltorp et al., Citation2020) and lead to increased employment opportunities. To achieve SDG 3 and 9, there is a great need for a multifaceted solution that includes, but is not limited to, making the cochlear implant available to all peoples, improving the accessibility of rehabilitation infrastructure and providing ongoing support to patients.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ayton, L.N., Blamey, P.J., Guymer, R.H., Luu, C.D., Nayagam, D.A.X., Sinclair, N.C., … Allen, P.J. (2014). First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS ONE, 9, e115239. doi:10.1371/journal.pone.0115239
- Clark, G.M. (2003). Cochlear implants: Fundamentals and applications. Springer-Verlag, New York, Inc.
- Clark, G.M., Black, R., Forster, I.C., Patrick, J.F., & Tong, Y.C. (1978). Design criteria of a multiple-electrode cochlear implant hearing prosthesis. Journal of the Acoustical Society of America, 63, 631–633. doi:10.1121/1.2033198
- Clark, G.M., Blamey, P.J., Busby, P.A., Dowell, R.C., Franz, B.K.-H.G., Musgrave, G.N., … Seligman, P.M. (1987). A multiple-electrode intracochlear implant for children. Archives of Otolaryngology, 113, 825–828. doi:10.1001/archotol.1987.01860080031010
- Clark, G.M., Tong, Y.C., Bailey, Q., Black, R., Martin, L.F., Millar, J.B., … Pyman, B.C. (1978). A multiple-electrode cochlear implant. Journal of the Oto-Laryngological Society of Australia, 4, 208–212. http://hdl.handle.net/11343/28714
- Cohen, N.L., Waltzman, S.B., & Fisher, S.G. (1993). A prospective randomized study of cochlear implants. New England Journal of Medicine, 328, 233–237. doi:10.1056/nejm199301283280403
- Cupples, L., Ching, T.Y.C., Button, L., Seeto, M., Zhang, V., Whitfield, J., … Marnane, V. (2018). Spoken language and everyday functioning in 5-year-old children using hearing aids or cochlear implants. International Journal of Audiology, 57, S55–S69. doi:10.1080/14992027.2017.1370140
- Dammeyer, J., Crowe, K., Marschark, M., & Rosica, M. (2019). Work and employment characteristics of deaf and hard-of-hearing adults. Journal of Deaf Studies and Deaf Education, 24, 386–395. doi:10.1093/deafed/enz018
- Dettman, S.J., Dowell, R.C., Choo, D., Arnott, W., Abrahams, Y., Davis, A., … Briggs, R.J. (2016). Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicentre study. Otology & Neurotology, 37, e82–e95. doi:10.1097/MAO.0000000000000915
- Edwards, T.L., Cottriall, C.L., Xue, K., Simunovic, M.P., Ramsden, J.D., Zrenner, E., & MacLaren, R.E. (2018). Assessment of the electronic retinal implant alpha AMS in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology, 125, 432–443. doi:10.1016/j.ophtha.2017.09.019
- Hong, G., Viveros, R.D., Zwang, T.J., Yang, X., & Lieber, C.M. (2018). Tissue-like neural probes for understanding and modulating the brain. Biochemistry, 57, 3995–4004. doi:10.1021/acs.biochem.8b00122
- Issing, C., Baumann, U., Pantel, J., & Stöver, T. (2021). Impact of hearing rehabilitation using cochlear implants on cognitive function in older patients. Otology and Neurotology, 42, 1136–1141. doi:10.1097/MAO.0000000000003153
- Karltorp, E., Eklöf, M., Östlund, E., Asp, F., Tideholm, B., & Löfkvist, U. (2020). Correlates of orthographic learning in swedish children with cochlear implants. Acta Paediatrica, 109, 332–341. doi:10.1111/apa.14954.
- Levi, T., Bonifazi, P., Massobrio, P., & Chiappalone, M. (2018). Closed-loop systems for next-generation neuroprostheses. Frontiers in Neuroscience, 12, 26. doi:10.3389/fnins.2018.00026
- Loeb, G.E. (2018). Neural prosthetics: A review of empirical vs. systems engineering strategies. Applied Bionics and Biomechanics, 2018, 1435030. doi:10.1155/2018/1435030
- Maclean, F.L., Ims, G.M., Horne, M.K., Williams, R.J., & Nisbet, D.R. (2018). A programmed anti‐inflammatory nanoscaffold (PAIN) as a 3D tool to understand the brain injury response. Advanced Materials, 30, 1805209. doi:10.1002/adma.201805209
- Maclean, F.L., Williams, R.J., Horne, M.K., & Nisbet, D.R. (2016). A commentary on the need for 3D-biologically relevant in vitro environments to investigate astrocytes and their role in central nervous system inflammation. Neurochemical Research, 41, 589–592. doi:10.1007/s11064-015-1697-8
- McKay, C.M., McDermott, A., Vandali, A., & Clark, G.M. (1992). A comparison of speech perception of cochlear implantees using the Spectral Maxima Sound Processor (SMSP) and the MSP (Multipeak) processor. Acta Oto-Laryngologica, 112, 752–761. doi:10.3109/00016489209137470
- Nisbet, D.R., Rodda, A.E., Horne, M.K., Forsythe, J.S., & Finkelstein, D.I. (2010). Implantation of functionalized thermally gelling xyloglucan hydrogel within the brain: Associated neurite infiltration and inflammatory response. Tissue Engineering Part A, 16, 2833–2842. doi:10.1089/ten.tea.2009.0677
- Salatino, J.W., Ludwig, K.A., Kozai, T.D.Y., & Purcell, E.K. (2017). Glial responses to implanted electrodes in the brain. Nature Biomedical Engineering, 1, 862–877. doi:10.1038/s41551-017-0154-1
- Sarant, J., Harris, D., Busby, P., Maruff, P., Schembri, A., Dowell, R., & Briggs, R. (2019). The Effect of Cochlear Implants on Cognitive Function in Older Adults: Initial Baseline and 18-Month Follow Up Results for a Prospective International Longitudinal Study. Frontiers in Neuroscience, 13, 789 10.3389/fnins.2019.00789PMC: 31427915
- Spencer, M.J., Kameneva, T., Grayden, D.B., Meffin, H., & Burkitt, A.N. (2019). Global activity shaping strategies for a retinal implant. Journal of Neural Engineering, 16, 026008. doi:10.1088/1741-2552/aaf071
- Stinson, M. & Buckley, G. (Eds). (2013). New beginnings: Acquiring and living with a cochlear implant. Rochester, NY: RIT Press.
- Tabibi, S., Kegel, A., Lai, W.K., & Dillier, N. (2020). A bio-inspired coding (BIC) strategy for cochlear implants. Hearing Research, 388, 107885. doi:10.1016/j.heares.2020.107885
- Tong, Y.C., Clark, G.M., Seligman, P.M., & Patrick, J.F. (1980). Speech processing for a multiple-electrode cochlear implant hearing prosthesis. Journal of the Acoustical Society of America, 68, 1897–1898. doi:10.1121/1.385184
- United Nations. (2015). Sustainable Development Goals: 17 goals to transform our world. https://www.un.org/sustainabledevelopment/sustainable-development-goals/
- Wilke, R.G.H., Moghadam, G.K., Lovell, N.H., Suaning, G.J., & Dokos, S. (2011). Electric crosstalk impairs spatial resolution of multi-electrode arrays in retinal implants. Journal of Neural Engineering, 8, 046016. doi:10.1088/1741-2560/8/4/046016
- Wilson, B.S., Finley, C.C., Lawson, D.T., Wolford, R.D., Eddington, D.K., & Rabinowitz, W.M. (1991). Better speech recognition with cochlear implants. Nature, 352, 236–238. doi:10.1038/352236a0
- World Health Organization. (2021). World report on hearing. https://apps.who.int/iris/handle/10665/339913
- Wouters, J., McDermott, H.J., & Francart, T. (2015). Sound coding in cochlear implants: From electric pulses to hearing. IEEE Signal Processing Magazine, 32, 67–80. doi:10.1109/MSP.2014.2371671