Abstract
Purpose: This systematic review evaluated the efficacy of therapeutic interventions on improving swallow, respiratory, and cough functions in Parkinson's disease (PD).
Method: A PRISMA systematic search was implemented across six databases. We selected studies reporting pre- and post-assessment data on the efficacy of behavioural therapies with a swallow or respiratory/cough outcome, and excluded studies on medical/surgical treatments or single-session design. Cross-system outcomes across swallow, respiratory, and cough functions were explored. Cochrane’s risk of bias tools were utilised to evaluate study quality.
Result: Thirty-six articles were identified and further clustered into four treatment types: swallow related (n = 5), electromagnetic stimulation (n = 4), respiratory loading (n = 20), and voice loading (n = 7) therapies. The effects of some behavioural therapies were supported with high-quality evidence in improving specific swallow efficiency, respiratory pressure/volume, and cough measures. Only eleven studies were rated with a low risk of bias and the remaining studies failed to adequately describe blinding of assessors, missing data, treatment adherence, and imbalance assignment to groups.
Conclusion: Behavioural therapies were diverse in nature and many treatments demonstrated broad cross-system outcome benefits across swallow, respiratory, and cough functions. Given the progressive nature of the condition, the focus of future trials should be evaluating follow-up therapy effects and larger patient populations, including those with more severe disease.
Introduction
Parkinson’s disease (PD) is a progressive neuromotor degenerative disorder affecting six million people globally (GBD 2016 Neurology Collaborators, Citation2018). Four out of five people with PD develop swallowing difficulties based on instrumental assessment findings (Kalf et al., Citation2012). Impaired cough function is also common including dysfunction in motor components (cough intensity) and cough reflex sensitivity (Ebihara et al., Citation2003). PD related swallow and cough dysfunctions are associated with many clinical complications including requirements for increased medication, malnutrition, dehydration, and aspiration with or without pneumonia. These complications negatively affect the quality of life of individuals with PD and increase morbidity and mortality rates (Beyer et al., Citation2001; Ebihara et al, Citation2003) with aspiration pneumonia reported to be one of the leading causes of death in PD (Beyer et al., Citation2001).
Pharmacological and surgical PD interventions have been extensively covered in previous reviews, and demonstrate only limited effects on laryngopharyngeal functions (Allen & Miles, Citation2020; Baijens & Speyer, Citation2009; Trail et al., Citation2008). In recent literature, nonpharmacological behavioural therapies have begun to demonstrate positive outcomes. Yet, previous systematic reviews (Baijens & Speyer, Citation2009; Gandhi & Steele, Citation2022; Kim & Kim, Citation2023; López-Liria et al., Citation2020; Park et al., Citation2019; van Hooren et al., Citation2014) have focused on swallowing specific behavioural therapies in treating dysphagia, excluding the nonswallow therapies that also demonstrate cross-system effects on swallow and cough function. Recent literature on behavioural techniques emphasise the spread effects from one system to another. For example, respiratory focused activities such as expiratory muscle strengthening training (EMST) demonstrate not only respiratory system improvements in cough function but also improved swallow function (Pitts et al., Citation2009; Troche et al., Citation2010). Furthermore, a number of clinical trials have been published in recent years (Cocks et al., Citation2022; Di Benedetto et al., Citation2009; Plaza & Ruviaro Busanello-Stella, Citation2022; Stegemöller, Hibbing, et al., Citation2017; Stegemöller, Radig, et al., Citation2017; Tamplin et al., Citation2019; Tamplin et al., Citation2020; Wang et al., Citation2018) which were not included in previous systematic reviews (Baijens & Speyer, Citation2009; López-Liria et al., Citation2020; van Hooren et al., Citation2014). To date, no systematic reviews have covered efficacy of interventions for both swallow and cough despite their critical interrelationship in determining airway protection. Therefore, in addition to traditional swallow interventions, this review targeted all behavioural therapies that report swallow and respiratory/cough outcome measures. The study aimed to systematically identify randomised controlled trials (RCT) and non-RCTs that report efficacy of therapeutic interventions in (a) improving swallow and respiratory/cough physiological function, (b) improving functional swallowing outcomes, (c) reducing events of aspiration, and (d) change in pulmonary health in individuals with PD. We also aimed to extract key intervention components such as delivery, dose, intensity, and timing to provide an overview to aid in clinical decision-making.
Method
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Moher et al., Citation2010) and was registered on the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020185622).
Selection criteria for studies
We included RCTs and non-RCTs that investigated the efficacy of therapies that reported swallow, respiratory, or cough outcomes in individuals with idiopathic PD. Studies were excluded if the intervention was solely based on pharmacological or surgical therapies. Several studies were identified where intervention was provided on only a single occasion (Supplementary Appendix 1). Studies with single case/case series or single-session designs were also excluded, as they did not demonstrate long-term treatment effects nor follow-up measures. Two studies were excluded as they described only compensatory strategies (Supplementary Appendix 1).
Studies recruiting individuals with all stages and severity levels of PD and all severity levels of swallowing difficulties, including no self-reported swallowing problems, were included. Objective and/or subjective measures of the selected studies were categorised as: (a) instrumental swallow measures (videofluoroscopy, endoscopy, electromyography); (b) instrumental cough/respiratory measures (spirometer, manometer, respiratory function test); (c) functional swallow outcomes based on functional change in diet and/or change in quality of life (QOL) by validated, subjective, self-administered questionnaires; (d) events of aspiration and change in pulmonary health chest x-ray reports, temperature charts, or records of aspiration pneumonia; change in the incidence of aspiration rated by the Penetration-Aspiration Scale score; change in residue severity measured by videofluoroscopy or endoscopy); and (e) adverse effects associated with intervention such as deterioration in function and discomfort. We included respiratory training studies with no cough parameters as recent evidence suggests that functional respiratory measures were correlated with cough strength measures (Curtis et al., Citation2020; Curtis et al., Citation2022; Jo & Kim, Citation2016; Kaneko et al., Citation2019). Inclusion and exclusion criteria are presented in .
Table I. Selection criteria of the studies included in this review.
Search strategy
A specialist university librarian was consulted for advice on databases and search terms. A comprehensive search strategy was designed using the Medical Subject Headings (MeSH) terms as relevant to the research questions and piloted on MEDLINE (Supplementary Appendix 2). The search strategy was adapted for searches in other databases (Embase, Scopus, BIOSIS, Web of Science, and the Cochrane Controlled Trial Register [CCTR]) and we completed the search in March 2021. We updated our search again in March 2022. Broad search terms used included “Parkinson’s disease,” “swallowing disorders,” “cough,” “respiration,” “exercise,” “training,” “therapies,” and “treatment.”
Data collection and synthesis
Selected studies were saved in Endnote (Clarivate Analytics, Philadelphia) and duplicates were removed. Titles of studies retrieved were screened against exclusion criteria () then abstracts were screened for potentially eligible studies. A hand search of the reference list of related review articles and all articles that met inclusion criteria was performed to identify any additional eligible studies. After the initial screening, full texts of eligible studies were reviewed by all three authors for inclusion or exclusion in the final study. Any disagreements during the eligibility review were resolved by discussion.
Data extraction
The data extraction template published by the Cochrane group was adapted to include the selected study data. The adapted data extraction sheet included general study information, study characteristics, patient characteristics, a detailed description of the intervention based on the TIDieR checklist (Hoffmann et al., Citation2014), and outcome measures were recorded as per the CONSORT 2010 statement (Moher et al., Citation2010). Data extraction was performed by the first author, and further amendments and expansions were undertaken by the second author. Any disagreements were resolved by discussion.
Assessment of risk of bias and reporting of study quality
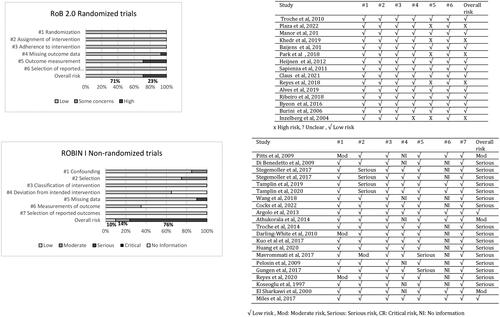
Cochrane Collaboration’s tool for assessing the risk of bias (RoB2; Sterne et al., Citation2019) was used to assess the methodological quality of the clinical trials for randomised trials under five main domains and graded as low, high, or some concerns according to the designed algorithm for suggested judgement. Risk of Bias in Non-Randomised Studies - of Interventions (ROBINS-I; Sterne et al., Citation2016) was used to rate the non-randomised trials included in this review under five criteria and graded as low, moderate, serious, critical risk, or no information. All data were reviewed by the second author at both study selection and quality appraisal stages. Discrepancies were resolved through consensus.
Data synthesis and analysis
Types of therapies were broadly grouped based on the focus of treatment: swallow-related, electromagnetic stimulation, respiratory loading, and voice loading therapies. Each study’s intervention protocol, study design, and participants’ demographics were tabulated in a summary table. Mean values for participants’ age, disease duration, and disease severity stage were calculated from the raw data where it was not otherwise specified. After reviewing the results of the included studies, the studies did not meet the requirements for meta-analysis as there were significant variation between assessment protocols, heterogeneity of study designs, and a lack of poolable data for the outcome measures of interest. The results of the studies were summarised descriptively for all swallow measures, cough/respiratory measures, and intervention type, reporting effect sizes and statistical significance where available. An evidence hierarchy classification model was used to summarise the overall findings (where Level 1a = meta-analysis of RCTs and Level IV = expert option/clinical experience only; Supplementary Appendix 3).
Result
The initial search yielded a total of 709 citations (MEDLINE = 184, Embase = 182, BIOSIS = 106, PubMed = 45, Scopus = 99, CCTR = 93). A PRISMA flow chart describing the study selection is presented in . Thirty-six studies met all review criteria and were included in the final synthesis: 15 randomised controlled trials, 7 non-randomised clinical trials, and 14 pre/post non-controlled studies. summarises types of intervention and their outcomes, and Supplementary Appendix 4 provides a comprehensive description of each study’s methodological design, study population, intervention, results, risk of bias, and level of evidence. In total, studies included 1151 individuals with PD and 816 received treatment. Mean age was 69 years (range 55–75 years), mean disease duration was 7 years (range 5–11 years), and Hoehn and Yahr Scale disease severity stage ranged from 2 to 3 in most of the studies. Overall male:female ratio was 2:1. Sample size varied widely, from eight to 109 participants ().
Figure 1. Adapted PRISMA flow diagram, presenting different phases of study selection for the systematic review.
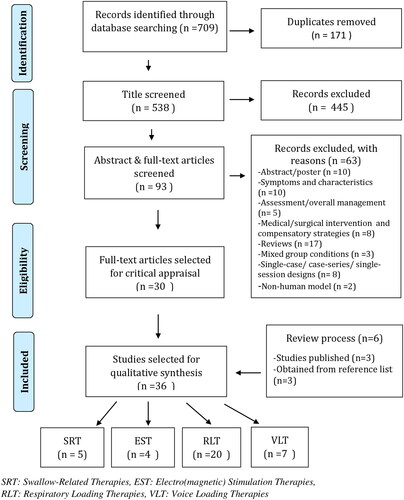
Table II. Types of interventions and outcomes.
Table III. Participant characteristics.
Dosage and intensity
The length and frequency of therapy varied across studies (). All the studies relied on interventions that were two or more weeks in duration (range 2–16 weeks) with the exception of ParkinSong, which lasted for 52 weeks. Fifty-eight percent of the studies had four or more sessions in a week and training session duration varied from 30 to 120 min. Interestingly, 71% of studies (n = 25/36) reported interventions that required specialist skills for delivery. Treatment adherence was reported in 67% of the studies (n = 24/36). Three-quarters of studies applied interventions during the "on" state of medication dosing. Maintenance of therapeutic effects was reported in 10 of the 36 studies (28%).
Table IV. Dosage and intensity of the interventions.
Study characteristics
A diverse range of physical therapies were identified: swallow-related (n = 5), electromagnetic stimulation (n = 4), respiratory loading (n = 20), and voice loading (n = 7) therapies. Flexible endoscopic evaluation of swallowing (FEES; n = 5), videofluoroscopic swallow study (VFSS; n = 11), and surface electromyography (sEMG; n = 4) were widely used to measure swallow function. The most commonly reported objective respiratory/cough outcome measures were pressure manometer, spirometer, and pulmonary function tests measures. Validated questionnaires were utilised to provide patient reported swallow-related quality of life measures (Supplementary Appendix 4).
Swallow-related therapies
Of the five studies grouped under the swallow-related therapies, three studies targeted treatment combinations of a range of movement exercises, tongue pressure exercises, and swallow manoeuvres (Argolo et al., Citation2013; Plaza & Ruviaro Busanello-Stella, Citation2022; Wang et al., Citation2018). The other two studies focused on biofeedback based swallow training and skill training approaches (Athukorala et al., Citation2014; Manor et al., Citation2013). Motor swallow exercises (Argolo et al., Citation2013) demonstrated significant reduction in pharyngeal residue, piecemeal deglutition, and improvement in bolus control but did not significantly change the swallow safety scores. Plaza and Ruviaro Busanello-Stella (Citation2022) reported tongue isometric pressure exercises resulted in significant improvement in Functional Oral Intake Scale (FOIS) score, and large effects on tongue muscle strength (d = 2.13) and suprahyoid muscle activity (d = 6.33). Also, there was a strong significant correlation between suprahyoid muscle activity and tongue strength (r = 0.994, p < .001). Combination of effortful swallow manoeuvre and tongue range of movement exercises (Wang et al., Citation2018) demonstrated improvements in laryngeal excursion amplitude. Athukorala et al. (Citation2014) reported large effects (d > 1) in swallow timing measures (pre-motor time, pre-swallow time, duration of submental muscle contraction) and time per swallow (d = 0.72) after biofeedback in strength and skill training (BiSSkiT). Video assisted skill training (VAST) study trial (Manor et al., Citation2013) showed significant reduction in pharyngeal residue, yet significant change in the swallow safety score was not evident. Significant improvement was reported in Swallowing Quality of Life (SWAL-QOL) and Eating Assessment Tool (EAT-10) scores (Argolo et al., Citation2013; Athukorala et al., Citation2014; Manor et al., Citation2013; Plaza & Ruviaro Busanello-Stella, Citation2022). VAST study showed follow-up improvement in QOL at 6 months (Manor et al., Citation2013). Maintenance effects of the swallow functions were evident for some treatments (tongue isometric pressure exercise for 4 weeks and BiSSkiT for 2 weeks).
Electromagnetic stimulation therapies
Of the four studies addressing the effects of electrical/magnetic stimulation, one study evaluated the effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on advancing PD with dysphagia (Khedr et al., Citation2019). The other three studies focused on combined treatments with the inclusion of electrical stimulation (surface electrical stimulation [SES]; Baijens et al., Citation2013) and neuromuscular electrical stimulation (NMES; Heijnen et al., Citation2012; Park et al., Citation2018) alongside behavioural therapies in the intervention group and dysphagia therapies alone in the control group (Baijens et al., Citation2013; Heijnen et al., Citation2012; Park et al., Citation2018). Significant effects (p < .05) were reported in swallow duration measures of hyoid movement and pharyngeal transit time following rTMS, but no effects demonstrated for swallow safety scores (Khedr et al., Citation2019). Significant reduction in piecemeal deglutition and initiation of pharyngeal reflex were evident following SES (Baijens et al., Citation2013). Large effects were evident in displacement of hyoid (d = 0.9) and swallow safety score was significantly reduced following NMES in combination with effortful swallow and conventional dysphagia therapy (Park et al., Citation2018). No effects on FOIS score (Heijnen et al., Citation2012) were reported after NMES. There was a significant reduction in Arabic Dysphagia Handicap Index (A-DHI) and Unified Parkinson's Disease Rating Scale (UPDRS III) scores after rTMS therapy (Khedr et al., Citation2019). No change was demonstrated between groups for Dysphagia Severity Scale (DSS) and SWAL-QOL after NMES (Heijnen et al., Citation2012). Treatment effects were maintained at 3 months for rTMS (Khedr et al., Citation2019). Self-reported outcomes were maintained for NMES (for 3 months; Heijnen et al., Citation2012) and rTMS (for a month; Khedr et al., Citation2019). None of the studies reported maintenance effects on objective outcomes.
Respiratory loading therapies
The 20 studies categorised under respiratory loading therapies targeted respiratory muscle strength training (inspiratory and/or expiratory; Claus et al., Citation2021; Cocks et al., Citation2022; Darling-White & Huber, Citation2017; Huang et al., Citation2020; Inzelberg et al., Citation2005; Kuo et al., Citation2017; Pitts et al., Citation2009; Reyes et al., Citation2018; Sapienza et al., Citation2011; Troche et al., Citation2010), general strength training (Alves et al., Citation2019; Mavrommati et al., Citation2017), functional training exercises (Pelosin et al., Citation2009), pulmonary exercises (Güngen et al., Citation2017), and breath-stacking or intensive spirometry (Ribeiro et al., Citation2018). Some studies used combination of approaches (Burini et al., Citation2006; Byeon, Citation2016; Reyes et al., Citation2020). Studies with EMST training had significant effects (p < .05) on voluntary cough measures (Pitts et al., Citation2009; compression phase duration, expiratory phase rise time, and cough volume acceleration; p < .05), large (d = 0.89; Reyes et al., Citation2018) to moderate effects (d = 0.77; Cocks et al., Citation2022; Reyes et al., Citation2020) on voluntary peak cough flow (PCF), and small effects on reflexive PCF (d = 0.27; Reyes et al., Citation2018; Reyes et al., Citation2020). A combination of EMST plus air stacking training had large effects on both voluntary (d = 1.00) and reflexive (d = 1.34) PCF measures (Reyes et al., Citation2020). A number of EMST studies demonstrated positive effects on swallow outcomes, which are explained in further detail below, under cross-system effects (Byeon, Citation2016; Claus et al., Citation2021; Cocks et al., Citation2022; Pitts et al., Citation2009; Troche et al., Citation2010). EMST training studies had significant effects on maximum expiratory pressure (MEP; Claus et al., Citation2021; Cocks et al., Citation2022; Darling-White & Huber, Citation2017; Huang et al., Citation2020; Inzelberg et al., Citation2005; Kuo et al., Citation2017; Pitts et al., Citation2009; Reyes et al., Citation2018; Reyes et al., Citation2020; Sapienza et al., Citation2011; Troche et al., Citation2010) and one follow-up study reported improved MEP was maintained after 3 months as the change was not significant when compared to immediate post-treatment values (Troche et al., Citation2014). Maximum inspiratory pressure (MIP) measure showed only small effect size (d = 0.42) following EMST training (Huang et al., Citation2020; Reyes et al., Citation2018; Reyes et al., Citation2020). Inspiratory muscle training (Inzelberg et al., Citation2005) also had significant effects (p < .05) on MIP and peak pressure in one study. No change in respiratory measures was reported in between group comparison of a dual arm respiratory muscle training study (with inspiratory and expiratory muscle training; Huang et al., Citation2020). Full-body exercises had significant positive effects on respiratory function (Mavrommati et al., Citation2017; Pelosin et al., Citation2009) and inspiratory pressure measures (Alves et al., Citation2019), while no change in expiratory pressures was noted (Alves et al., Citation2019). Pulmonary techniques (Güngen et al., Citation2017; Köseoğlu et al., Citation1997) and aerobic exercises (Burini et al., Citation2006) had significant positive effects on some of the pulmonary function test measures. Qigong technique alone did not affect any of the tested measures (Burini et al., Citation2006). Significant improvement was shown in tidal volume and minute ventilation after using breath-stacking and incentive spirometry techniques (Ribeiro et al., Citation2018). Treatment effects were maintained at 1 month for treadmill (Pelosin et al., Citation2009), 2 months for aerobic training (Burini et al., Citation2006), and 3 months for EMST (Claus et al., Citation2021; Troche et al., Citation2014). Pulmonary physio (Güngen et al., Citation2017) and full-body exercises (Pelosin et al., Citation2009) study trials had significant effects on disease severity measures, but this was not reported in other studies (Alves et al., Citation2019; Inzelberg et al., Citation2005; Kuo et al., Citation2017). Furthermore, two studies, each with two treatment arms, did not report any significant effects between groups on disease severity scores (Burini et al., Citation2006; Inzelberg et al., Citation2005).
Voice loading therapies
While vocal therapies were not the focus of this review, seven studies used intervention approaches specifically focused on improving voicing in PD, included voice and choral singing treatment (VCST; Di Benedetto et al., Citation2009), Lee Silverman voice treatment (LSVT; El Sharkawi et al., Citation2002; Miles et al., Citation2017), therapeutic singing (Stegemöller, Hibbing, et al., Citation2017; Stegemöller, Radig, et al., Citation2017), and ParkinSong (Tamplin et al., Citation2019; Tamplin et al., Citation2020). These intensive therapy programs incorporated vocal exercises with speech activities and/or singing. These studies met criteria for inclusion in this review as they demonstrated positive effects on swallow and respiratory/cough outcomes, which are explained in further detail below, under cross-system effects. It is outside the scope of this review to provide a detailed analysis of voice outcomes for these studies and we refer the reader to the scientific papers for these details (summary of the findings were listed in ).
Table V. Studies with cross-system effects.
Cross-system outcomes
Sixty-seven percent of the studies (n = 24/36) used more than one treatment strategy and one-third of the studies (n = 11/36) investigated the spread effects of a treatment specifically focused on a voice, swallowing, or respiratory activity to another body function (swallow, cough, respiratory). summarises the outcomes of the interventions that demonstrated cross-system effects.
Swallow outcomes
Only one electrical/magnetic stimulation study (using rTMS alone) showed significant improvement (p < .05) in swallowing measures, with improved duration of hyoid movement and pharyngeal transit time for solid swallows in the intervention group when compared to the sham group (Khedr et al., Citation2019). However, no significant change was reported for liquid bolus trials. Other electrical stimulation studies (SES, NMES) with combination of swallow therapies did not demonstrate significant improvement in swallow measures in between-groups comparison, although, significant changes were reported in pre- and post-session comparisons within groups.
Amongst respiratory loading therapies, five of the expiratory muscle strength training studies evaluated the effects in objective swallow measures (Byeon, Citation2016; Claus et al., Citation2021; Cocks et al., Citation2022; Pitts et al., Citation2009; Troche et al., Citation2010). Studies using EMST found significant (p < .05) positive effects on swallow timing (Pitts et al., Citation2009; Troche et al., Citation2010), residue score (Claus et al., Citation2021), and PAS score (Pitts et al., Citation2009; Troche et al., Citation2010). However, Claus et al. (Citation2021) found no significant change in PAS scores after EMST, as all patients lacked penetration and aspiration at baseline. Byeon (Citation2016) reported significant reduction in overall VFSS score following EMST. A 3 months follow-up study of EMST with 10 participants by Troche et al. (Citation2014) reported that change of PAS score was not significant when compared to immediate post-treatment. In questionnaire metrics, Swallowing Disturbance Questionnaire (Claus et al., Citation2021) and SWAL-QOL (Cocks et al., Citation2022; Troche et al., Citation2010) scores were significantly improved after EMST therapy.
Of the voice loading studies, two small non-randomised LSVT studies demonstrated significant change (p < .05) in swallow temporal measures (El Sharkawi et al., Citation2002; Miles et al., Citation2017), bolus residue (El Sharkawi et al., Citation2002; Miles et al., Citation2017), and maximum displacement of pharyngoesophageal sphincter (PES; Miles et al., Citation2017) on the VFSS findings. Treatment effects were maintained at 6 months for LSVT (Miles et al., Citation2017). No significant change was evident in hyoid timing and hyolaryngeal displacement measures after pre/post comparison of LSVT (Miles et al., Citation2017). A study utilising therapeutic singing (Stegemöller, Hibbing, et al., Citation2017) reported significant changes (p < .05) in electromyography study for submental muscles amplitude and submental muscle fall time for thick liquid trials in between group comparison of low and high frequency treatment groups. In contrast, VCST showed no significant change in vocal fold movements, tremor, and glottic closure in videostroboscopy ratings (Di Benedetto et al., Citation2009). There was a significant reduction (improvement; p < .05) in Eating Assessment Tool-10 and Parkinson’s Disease Questionnaire-8 scores after LSVT (Miles et al., Citation2017). Other authors report no significant change in SWAL-QOL score, but significant improvement in UPDRS scores after therapeutic singing (Stegemöller, Radig, et al., Citation2017).
Respiratory/cough outcomes
Five of the voice loading therapies (Di Benedetto et al., Citation2009; Miles et al., Citation2017; Stegemöller, Hibbing, et al., Citation2017; Stegemöller, Radig, et al., Citation2017; Tamplin et al., Citation2019; Tamplin et al., Citation2020) reported objective respiratory/cough indices as one of the primary outcome measures (). Significant improvement (p < .05) in reflexive cough measures (peak expiratory flow rate and peak expiratory flow rise time) were reported after LSVT and the effects were maintained at 6 months (Miles et al., Citation2017). VCST showed significant improvement in functional residual capacity and maximum inspiratory and expiratory pressure measures (Di Benedetto et al., Citation2009). Therapeutic singing treatment showed significant improvements in MIP and MEP measures despite a dosage difference between groups (Stegemöller, Radig, et al., Citation2017). ParkinSong treatment showed significant effects (p < .05) on MEP measure after 3 months, but the change was not significant at 12 months post-treatment (Tamplin et al., Citation2020; Tamplin et al., Citation2019).
Methodological quality and risk of bias
presents the best evidence statements of behavioural therapies from the 11 studies rated low risk of bias. Risk of bias ratings of the 15 randomised controlled trials are displayed in for each of the RoB2 criteria (Sterne et al., Citation2019). Twenty-three percent of studies were categorised as exhibiting high risk of bias. No information on blinding of the assessors was rated as an area of high risk in five of the RCTs as it is most likely that assessment was influenced by knowledge of intervention received. A high percentage of missing data reported in one study was categorised as a significant risk, resulting in half of the allocated participants being excluded from the final analysis (Inzelberg et al., Citation2005).
Table VI. Best evidence statement of behavioural therapies in improving swallow and respiratory/cough measures in individuals with Parkinson’s disease.
shows the calculations for each of the ROBINS-I criteria (Sterne et al., Citation2016) for the 21 non-randomised trials. Significant limitations were found in the majority of the studies leading to an overall risk rating of moderate to serious risk in 90% of the non-randomised studies. Treatment adherence and blinding of assessors were not reported in many studies. Participant recruitment processes were not clearly defined in three of the studies (Darling-White & Huber, Citation2017; Huber et al., Citation2003; Pitts et al., Citation2009; Reyes et al., Citation2020). Five studies reported on imbalanced assignment of participants to intervention and control groups (no power calculation was provided to gauge the sensitivity of the study to detect an effect) and this was categorised as selection bias (Mavrommati et al., Citation2017; Stegemöller, Hibbing, et al., Citation2017; Stegemöller, Radig, et al., Citation2017; Tamplin et al., Citation2019; Tamplin et al., Citation2020). Two studies were rated serious risk for missing data, as more than 20% of the participants dropped out before final analysis and no reasons were given (Güngen et al., Citation2017; Mavrommati et al., Citation2017).
Discussion
This review has evaluated a substantial number of studies that support the efficacy of behavioural therapies in improving swallow, respiratory, and cough functions in individuals with PD. A total of 36 studies met the selection criteria and interventions were clustered into four thematic treatment types: swallow-related therapies, electrical/magnetic stimulation therapies, respiratory-loading therapies, and voice loading therapies. Behavioural therapies are gaining a strong body of supporting evidence in PD rehabilitation and represent as an important intervention modality that is feasible, safe, and beneficial. Most of these studies evaluated treatment effects using objective, instrumental measures of swallowing and cough/respiratory functions and several therapies were rated Level 1b evidence for positive swallowing, cough, and QOL outcomes. Our aims to evaluate the effects of therapies on aspiration and pulmonary health were not the focus of published papers. Many therapies require high-quality RCT replication to add strength to their evidence-base, such as video assisted swallow training and strength and skill-based swallow programs. Evidence for electrical/magnetic stimulation therapies remains in its infancy. EMST and LSVT have the advantage of improving both swallowing and respiratory/cough function. Our critical review of study methodology and results was consistent with previous systematic reviews (Baijens & Speyer, Citation2009; Gandhi & Steele, Citation2022; Kim & Kim, Citation2023; López-Liria et al., Citation2020; Park et al., Citation2019; van Hooren et al., Citation2014). However, we provide an updated and novel critical appraisal of studies examining cross-system effects of other therapy programs, which have not previously been reported.
Cross-system effects
Respiration, cough, and swallow functions are physiologically interrelated through shared anatomy and functional interdependence. There is a critical interrelationship through cortical control coordinating breathing and swallowing (Martin-Harris et al., Citation2022; Troche et al., Citation2010). Recent studies have investigated the correlational aspects of respiration and cough parameters, respiratory/cough-swallow coordination, and the interrelationship of cortical control of these functions. Study findings (Jo & Kim, Citation2016; Kaneko et al., Citation2019) demonstrated a significant correlation between respiratory parameters such as forced vital capacity (FVC), MIP, MEP, and cough peak flow. Curtis et al. (Citation2020) demonstrated a significant interrelationship between tidal breathing and respiratory-swallow coordination in people with PD.
With this in mind, respiration-swallow cross-system approaches are beginning to be utilised to treat patients with swallow dysfunction (Martin-Harris et al., Citation2017). Though EMST primarily focuses on strengthening the expiratory muscles, studies with high-quality evidence show promising effects on swallow efficiency measures that are in line with previous reviews (Kim & Kim, Citation2023; López-Liria et al., Citation2020; Mancopes et al., Citation2020; van Hooren et al., Citation2014). Seven studies (LSVT, therapeutic singing, ParkingSong, and voice and choral singing) categorised under voice training also demonstrate statistically significant improvement in selected swallow and respiratory/cough measures. However, none of these studies as yet have proven the effects with high-level of evidence and six of these studies (Di Benedetto et al., Citation2009; El Sharkawi et al., Citation2002; Stegemöller, Hibbing, et al., Citation2017; Stegemöller, Radig, et al., Citation2017; Tamplin et al., Citation2019; Tamplin et al., Citation2020) have methodological limitations that prevent the drawing of strong conclusions. This review, however, provides promising evidence that many of the treatment options available for improving respiratory and cough functions demonstrate promising spread effects benefitting swallowing function. Clinically, swallow management in PD needs to be extended beyond traditional dysphagia therapy and utilise a combination of approaches to achieve best outcomes for airway protection, as well as swallowing efficiency, particularly given the lack of benefit from pharmacological treatments.
Methodological quality and best level of evidence
Although the selected 36 studies demonstrate positive gains following a variety of behavioural therapies, only a small number of studies were rated as low risk of bias with high-quality evidence. This reflects the difficulty in performing such interventional studies, particularly longitudinally, and in finding suitable comparison groups. In this review, nine randomised controlled trials with low-risk bias ratings have provided the best level of evidence regarding improved swallow and respiratory/cough functions in individuals with PD (). Among swallow-related therapies, video assisted swallow therapy (Manor et al., Citation2013) is supported with high-quality evidence showing reduction in pharyngeal residue. Surface electrical stimulation study (Baijens et al., Citation2013) also showed improvement in swallow efficiency parameters through combining traditional swallow and logopaedic therapy. The scarcity of other controlled trials in swallow therapies perhaps hinders generalisations of the findings.
In comparison, EMST studies (Byeon, Citation2016; Claus et al., Citation2021; Sapienza et al., Citation2011; Troche et al., Citation2010) demonstrate consistent improvements in instrumental swallowing measures (total score and durational measures for VFSS; total and residue scores for FEES) as well as significant improvements in maximum expiratory pressure measures, which supports its role in cough augmentation (Jo & Kim, Citation2016). Miles and colleagues’ pilot LSVT study (Citation2017) demonstrated improvement in VFSS findings for pharyngoesophageal sphincter durational and displacement measures, reduction in paste residue, and improvement in reflexive cough peak expiratory flow measures. Yet these findings need validation with high-level evidence and larger cohorts with varying disease severity to generalise to the wider PD population. Full-body exercises (Alves et al., Citation2019), aerobic training (Burini et al., Citation2006), and breath-stacking or incentive spirometry (Ribeiro et al., Citation2018) techniques were all effective treatments to improve pulmonary measures in PD and were supported by high-quality evidence.
Combination of treatment approaches
Interestingly, use of a combination of treatment approaches is gaining support in recently published literature. Many studies included in this review used a combination of treatment approaches. In swallow behavioural therapies, specific swallow manoeuvres were combined with the range of movement exercises (Argolo et al., Citation2013; Wang et al., Citation2018) and resistance pressure exercises were combined with the range of movement exercises (Plaza & Ruviaro Busanello-Stella, Citation2022). Combining biofeedback therapy (Athukorala et al., Citation2014; Manor et al., Citation2013) along with individualised swallow skill/behavioural training also demonstrated promising effects on swallow efficiency scores. Similar approaches are now being explored for cough, with emerging studies exploring skill training and voluntary upregulation of reflex cough with biofeedback approaches (Brandimore et al., Citation2017; Curtis et al., Citation2020; Curtis & Troche, Citation2020; Hegland et al., Citation2012). Combination therapies, while common, make it difficult to draw conclusions on efficacy of individual therapies.
All the electromagnetic swallow therapies (except rTMS) were coupled with traditional logopaedic therapies (Baijens et al., Citation2013; Heijnen et al., Citation2012; Park et al., Citation2018). Respiratory training interventions targeted combining breathing and body exercises (Burini et al., Citation2006; Köseoğlu et al., Citation1997; Mavrommati et al., Citation2017) or resistance pressure training and air stacking (Reyes et al., Citation2020). EMST plus air stacking approaches demonstrated large effects on voluntary and reflexive cough parameters (Reyes et al., Citation2020). However, further RCTs with low risk of bias ratings are warranted. Another RCT targeted combining EMST and swallow postural techniques (Byeon, Citation2016), demonstrating improved VFSS parameters when compared to EMST alone. All the voice training interventions incorporated more than one voice training/singing or functional speech activity in their protocol, with minimum 50 min per session. Further studies with high-quality evidence, low bias, with detailed study protocols and objective outcomes are needed to further understand the effects on respiratory/cough and/or swallow function.
Study limitation and future directions
This review included only original articles published in English. It is possible that some eligible studies were missed due to this search strategy (Supplementary Appendix 2), although every effort was made to perform a comprehensive database search. Data extraction, analysis, and risk of bias assessments were conducted manually by one author and cross-checked with a second author. We included articles that had limitations to methodological quality. Common concerns found in most of the studies were a lack of information on blinding of assessors, treatment adherence, participant recruitment processes, and imbalanced assignment of the participants to groups. High drop-out rate was a risk factor for bias reported in one study. Lack of blinding of personnel and therapists was commonly found across the studies; however, we acknowledge it is difficult to blind clinicians in manual therapy experimental studies. Most of the studies included predominantly mild to moderate disease stage patients and excluded patients having comorbidities, which limit the generalisation of the results to the wider PD population. Heterogeneity of the quantitative (swallow) measures used across studies also prevented reliable comparison of treatment effects and ability to make stronger conclusions. Only a few studies reported measures of treatment effect size, therefore, by highlighting studies with p < .05, where power was not achieved, improvement reported may not be clinically significant. It is critical that researchers report beyond p values and provide effect sizes in order for us to evaluate treatment efficacy and potential impact on functional performance. Many of the studies’ baseline measures did not show impairments in the evaluated symptoms, which may mute the actual positive effects. Most studies do not demonstrate significant effects on swallow safety as many of the participants included in these studies had mild baseline impairments (low aspiration rates and high FOIS scores) leading to a ceiling effect. In this review, we excluded single case studies and single session studies, perhaps missing some newer therapies without a substantive evidence-base yet. These methodological limitations may affect the strength of recommendations, nevertheless, in health research, we understand well-designed RCTs are difficult to perform. Although PD is a progressive neurological condition, only a few studies reported maintenance effects or long-term follow-up. When developing targeted preventive intervention protocols, researchers must focus on identifying the longitudinal effects in reducing the swallow and respiratory/cough morbidity rates to support evidence-based practice. Future studies should use strong methodology and design to reduce the above-mentioned limitations, where possible, and provide additional substantive evidence for the currently available therapies.
Conclusion
PD is a multidimensional, complex, progressive neurodegenerative disease that provides a challenge in optimising rehabilitation. A diverse range of evidence-based manual therapies have been reported in the literature and evaluated in this review. They show broad positive therapy effects, often involving or extending to more than one body system. The range of therapies identified suggests multiple options are available to improve swallow and respiratory/cough function. Varied modalities were used, such as muscle strength and resistance training exercises, biofeedback-based therapies, electromagnetic stimulation therapies, device-driven treatments, and voice training singing therapies. Combined therapeutic approaches are also supported by this literature, and effective combinations should be considered and evaluated further. Given the progressive nature of PD, future studies should focus on well-designed randomised controlled trials using larger samples sizes that include more severe disease levels, long-term outcomes, and maintenance therapy.
Supplemental Material_1st Aug 2022.docx
Download MS Word (127 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allen, J. E., & Miles, A. (2020). Parkinson disease. In P. A. Weissbrod & D. O. Francis (Eds.), Neurologic and neurodegenerative diseases of the larynx (pp. 143–159). Springer International Publishing.
- Alves, W. M., Alves, T. G., Ferreira, R. M., Lima, T. A., Pimentel, C. P., Sousa, E. C., Abrahin, O., & Alves, E. A. (2019). Strength training improves the respiratory muscle strength and quality of life of elderly with Parkinson disease. The Journal of Sports Medicine and Physical Fitness, 59(10), 1756–1762. https://doi.org/10.23736/S0022-4707.19.09509-4
- Argolo, N., Sampaio, M., Pinho, P., Melo, A., & Nóbrega, A. C. (2013). Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson’s disease? NeuroRehabilitation, 32(4), 949–955. https://doi.org/10.3233/NRE-130918
- Athukorala, R. P., Jones, R. D., Sella, O., & Huckabee, M. L. (2014). Skill training for swallowing rehabilitation in patients with Parkinson’s disease. Archives of Physical Medicine and Rehabilitation, 95(7), 1374–1382. https://doi.org/10.1016/j.apmr.2014.03.001
- Baijens, L. W., & Speyer, R. (2009). Effects of therapy for dysphagia in Parkinson’s disease: systematic review. Dysphagia, 24(1), 91–102. 10. https://doi.org/10.1007/s00455-008-9180-1
- Baijens, L. W., Speyer, R., Passos, V. L., Pilz, W., van der Kruis, J., Haarmans, S., & Desjardins-Rombouts, C. (2013). Surface electrical stimulation in dysphagic Parkinson patients: a randomized clinical trial. The Laryngoscope, 123(11), E38–E44. https://doi.org/10.1002/lary.24119
- Beyer, M. K., Herlofson, K., Arsland, D., & Larsen, J. P. (2001). Causes of death in a community-based study of Parkinson’s disease. Acta Neurologica Scandinavica, 103(1), 7–11. https://doi.org/10.1034/j.1600-0404.2001.00191.x
- Brandimore, A. E., Hegland, K. W., Okun, M. S., Davenport, P. W., & Troche, M. S. (2017). Voluntary upregulation of reflex cough is possible in healthy older adults and Parkinson’s disease. Journal of Applied Physiology, 123(1), 19–26. https://doi.org/10.1152/japplphysiol.00612.2016
- Burini, D., Farabollini, B., Iacucci, S., Rimatori, C., Riccardi, G., Capecci, M., Provinciali, L., & Ceravolo, M. G. (2006). A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Europa Medicophysica, 42(3), 231–238.
- Byeon, H. (2016). Effect of simultaneous application of postural techniques and expiratory muscle strength training on the enhancement of the swallowing function of patients with dysphagia caused by Parkinson’s disease. Journal of Physical Therapy Science, 28(6), 1840–1843. https://doi.org/10.1589/jpts.28.1840
- Claus, I., Muhle, P., Czechowski, J., Ahring, S., Labeit, B., Suntrup-Krueger, S., Wiendl, H., Dziewas, R., & Warnecke, T. (2021). Expiratory muscle strength training for therapy of pharyngeal dysphagia in Parkinson’s disease. Movement Disorders, 36(8), 1815–1824. https://doi.org/10.1002/mds.28552
- Cocks, N., Rafols, J., Embley, E., & Hill, K. (2022). Expiratory muscle strength training for drooling in adults with Parkinson’s disease. Dysphagia, 37(6), 1525–1531. https://doi.org/10.1007/s00455-022-10408-6
- Curtis, J. A., Dakin, A. E., & Troche, M. S. (2020). Respiratory-swallow coordination training and voluntary cough skill training: A single-subject treatment study in a person with Parkinson’s disease. Journal of Speech, Language, and Hearing Research, 63(2), 472–486. https://doi.org/10.1044/2019_JSLHR-19-00207
- Curtis, J. A., Huber, J. E., Dakin, A. E., & Troche, M. S. (2022). Effects of bolus holding on respiratory-swallow coordination in Parkinson’s disease. American Journal of Speech-Language Pathology, 31(2), 705–721. https://doi.org/10.1044/2021_AJSLP-21-00044
- Curtis, J. A., & Troche, M. S. (2020). Effects of verbal cueing on respiratory-swallow patterning, lung volume initiation, and swallow apnea duration in Parkinson’s disease. Dysphagia, 35(3), 460–470. https://doi.org/10.1007/s00455-019-10050-9
- Darling-White, M., & Huber, J. E. (2017). The impact of expiratory muscle strength training on speech breathing in individuals with Parkinson’s disease: A preliminary study. American Journal of Speech-Language Pathology, 26(4), 1159–1166. https://doi.org/10.1044/2017_AJSLP-16-0132
- Di Benedetto, P., Cavazzon, M., Mondolo, F., Rugiu, G., Peratoner, A., & Biasutti, E. (2009). Voice and choral singing treatment: a new approach for speech and voice disorders in Parkinson’s disease. European Journal of Physical and Rehabilitation Medicine, 45(1), 13–19.
- Ebihara, S., Saito, H., Kanda, A., Nakajoh, M., Takahashi, H., Arai, H., & Sasaki, H. (2003). Impaired efficacy of cough in patients with Parkinson disease. Chest, 124(3), 1009–1015. https://doi.org/10.1378/chest.124.3.1009
- El Sharkawi, A., Ramig, L., Logemann, J. A., Pauloski, B. R., Rademaker, A. W., Smith, C. H., Pawlas, A., Baum, S., & Werner, C. (2002). Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. Journal of Neurology, Neurosurgery, and Psychiatry, 72(1), 31–36. https://doi.org/10.1136/jnnp.72.1.31
- Gandhi, P., & Steele, C. M. (2022). Effectiveness of Interventions for Dysphagia in Parkinson disease: A systematic review. American Journal of Speech-Language Pathology, 31(1), 463–485. https://doi.org/10.1044/2021_AJSLP-21-00145
- GBD 2016 Neurology Collaborators. (2018). Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurology, 17(11), 939–953. https://doi.org/10.1016/s1474-4422(18)30295-3
- Güngen, B., Aydemir, Y., Aras, Y., Güngen, A., Kotan, D., & Bal, S. (2017). The effects of a pulmonary rehabilitation program on exercise tolerance, quality of life, sleep quality and emotional status in the patients with Parkinson’s disease. Biomedical Research, 28(2), 786–790.
- Hegland, K. W., Bolser, D. C., & Davenport, P. W. (2012). Volitional control of reflex cough. Journal of Applied Physiology, 113(1), 39–46. https://doi.org/10.1152/japplphysiol.01299.2011
- Heijnen, B. J., Speyer, R., Baijens, L. W., & Bogaardt, H. C. (2012). Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson’s disease and oropharyngeal dysphagia: effects on quality of life. Dysphagia, 27(3), 336–345. https://doi.org/10.1007/s00455-011-9371-z
- Hoffmann, T. C., Glasziou, P. P., Boutron, I., Milne, R., Perera, R., Moher, D., Altman, D. G., Barbour, V., Macdonald, H., Johnston, M., Lamb, S. E., Dixon-Woods, M., McCulloch, P., Wyatt, J. C., Chan, A.-W., & Michie, S. (2014). Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348, g1687. https://doi.org/10.1136/bmj.g1687
- Huang, C.-C., Lai, Y.-R., Wu, F.-A., Kuo, N.-Y., Tsai, Y.-C., Cheng, B.-C., Tsai, N.-W., & Lu, C.-H. (2020). Simultaneously improved pulmonary and cardiovascular autonomic function and short-term functional outcomes in patients with Parkinson’s disease after respiratory muscle training. Journal of Clinical Medicine, 9(2), 316. https://doi.org/10.3390/jcm9020316
- Huber, J. E., Stathopoulos, E. T., Ramig, L. O., & Lancaster, S. L. (2003). Respiratory function and variability in individuals with Parkinson disease: pre- and post-Lee Silverman Voice Treatment. Journal of Medical Speech–Language Pathology, 11, 185.
- Inzelberg, R., Peleg, N., Nisipeanu, P., Magadle, R., Carasso, R. L., & Weiner, P. (2005). Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. The Canadian Journal of Neurological Sciences, 32(2), 213–217. https://doi.org/10.1017/s0317167100003991
- Jo, M. R., & Kim, N. S. (2016). The correlation of respiratory muscle strength and cough capacity in stroke patients. Journal of Physical Therapy Science, 28(10), 2803–2805. https://doi.org/10.1589/jpts.28.2803
- Kalf, J. G., de Swart, B. J., Bloem, B. R., & Munneke, M. (2012). Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism & Related Disorders, 18(4), 311–315. https://doi.org/10.1016/j.parkreldis.2011.11.006
- Kaneko, H., Suzuki, A., & Horie, J. (2019). Relationship of cough strength to respiratory function, physical performance, and physical activity in older adults. Respiratory Care, 64(7), 828–834. https://doi.org/10.4187/respcare.06490
- Khedr, E. M., Mohamed, K. O., Soliman, R. K., Hassan, A. M. M., & Rothwell, J. C. (2019). The effect of high-frequency repetitive transcranial magnetic stimulation on advancing Parkinson’s disease with Dysphagia: Double blind randomized clinical trial. Neurorehabilitation and Neural Repair, 33(6), 442–452. https://doi.org/10.1177/1545968319847968
- Kim, J. Y., & Kim, H. (2023). Effects of behavioural swallowing therapy in patients with Parkinson’s disease: A systematic review. International Journal of Speech-Language Pathology, 25(2), 269–280. https://doi.org/10.1080/17549507.2022.2045356
- Köseoğlu, F., Inan, L., Ozel, S., Deviren, S. D., Karabiyikoğlu, G., Yorgancioğlu, R., Atasoy, T., & Oztürk, A. (1997). The effects of a pulmonary rehabilitation program on pulmonary function tests and exercise tolerance in patients with Parkinson’s disease. Functional Neurology, 12(6), 319–325.
- Kuo, Y. C., Chan, J., Wu, Y. P., Bernard, J. R., & Liao, Y. H. (2017). Effect of expiratory muscle strength training intervention on the maximum expiratory pressure and quality of life of patients with Parkinson disease. NeuroRehabilitation, 41(1), 219–226. https://doi.org/10.3233/NRE-171474
- López-Liria, R., Parra-Egeda, J., Vega-Ramírez, F. A., Aguilar-Parra, J. M., Trigueros-Ramos, R., Morales-Gázquez, M. J., & Rocamora-Pérez, P. (2020). Treatment of Dysphagia in Parkinson’s disease: A systematic review. International Journal of Environmental Research and Public Health, 17(11), 4104. https://doi.org/10.3390/ijerph17114104
- Mancopes, R., Smaoui, S., & Steele, C. M. (2020). Effects of expiratory muscle strength training on videofluoroscopic measures of swallowing: A systematic review. American Journal of Speech-Language Pathology, 29(1), 335–356. https://doi.org/10.1044/2019_AJSLP-19-00107
- Manor, Y., Mootanah, R., Freud, D., Giladi, N., & Cohen, J. T. (2013). Video-assisted swallowing therapy for patients with Parkinson’s disease. Parkinsonism & Related Disorders, 19(2), 207–211. https://doi.org/10.1016/j.parkreldis.2012.10.004
- Martin-Harris, B., Kantarcigil, C., Reedy, E. L., & McFarland, D. H. (2022). Cross-System Integration of Respiration and Deglutition: Function, Treatment, and Future Directions. Dysphagia. https://doi.org/10.1007/s00455-022-10538-x
- Martin-Harris, B., Garand, K. L. F., & McFarland, D. (2017). Optimizing respiratory-swallowing coordination in patients with oropharyngeal head and neck cancer. Perspectives of the ASHA Special Interest Groups, 2(13), 103–110. https://doi.org/10.1044/persp2.SIG13.103
- Mavrommati, F., Collett, J., Franssen, M., Meaney, A., Sexton, C., Dennis-West, A., Betts, J. F., Izadi, H., Bogdanovic, M., Tims, M., Farmer, A., & Dawes, H. (2017). Exercise response in Parkinson’s disease: insights from a cross-sectional comparison with sedentary controls and a per-protocol analysis of a randomised controlled trial. BMJ Open, 7(12), e017194. https://doi.org/10.1136/bmjopen-2017-017194
- Miles, A., Jardine, M., Johnston, F., de Lisle, M., Friary, P., & Allen, J. (2017). Effect of Lee Silverman Voice Treatment (LSVT LOUD®) on swallowing and cough in Parkinson’s disease: A pilot study. Journal of the Neurological Sciences, 383, 180–187. https://doi.org/10.1016/j.jns.2017.11.015
- Moher, D., Hopewell, S., Schulz, K. F., Montori, V., Gøtzsche, P. C., Devereaux, P. J., Elbourne, D., Egger, M., & Altman, D. G. (2010). CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ, 340, c869. https://doi.org/10.1136/bmj.c869
- Park, J.-S., Oh, D.-H., Hwang, N.-K., & Lee, J.-H. (2018). Effects of neuromuscular electrical stimulation in patients with Parkinson’s disease and dysphagia: A randomized, single-blind, placebo-controlled trial. NeuroRehabilitation, 42(4), 457–463. https://doi.org/10.3233/NRE-172306
- Park, M. S., Choi, J. Y., Song, Y. J., Choi, H., Park, E. J., & Ji, E. S. (2019). Systematic review of behavioral therapy to improve swallowing functions of patients with Parkinson’s disease. Gastroenterology Nursing, 42(1), 65–78. https://doi.org/10.1097/SGA.0000000000000358
- Pelosin, E., Faelli, E., Lofrano, F., Avanzino, L., Marinelli, L., Bove, M., Ruggeri, P., & Abbruzzese, G. (2009). Effects of treadmill training on walking economy in Parkinson’s disease: A pilot study. Neurological Sciences, 30(6), 499–504. https://doi.org/10.1007/s10072-009-0141-8
- Pitts, T., Bolser, D., Rosenbek, J., Troche, M., Okun, M. S., & Sapienza, C. (2009). Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest, 135(5), 1301–1308. https://doi.org/10.1378/chest.08-1389
- Plaza, E., & Ruviaro Busanello-Stella, A. (2022). Effects of a tongue training program in Parkinson’s disease: Analysis of electrical activity and strength of suprahyoid muscles. Journal of Electromyography and Kinesiology, 63, 102642. 10–1016. https://doi.org/10.1016/j.jelekin.2022.102642
- Reyes, A., Castillo, A., & Castillo, J. (2020). Effects of expiratory muscle training and air stacking on peak cough flow in individuals with Parkinson’s disease. Lung, 198(1), 207–211. https://doi.org/10.1007/s00408-019-00291-8
- Reyes, A., Castillo, A., Castillo, J., & Cornejo, I. (2018). The effects of respiratory muscle training on peak cough flow in patients with Parkinson’s disease: a randomized controlled study. Clinical Rehabilitation, 32(10), 1317–1327. https://doi.org/10.1177/0269215518774832
- Ribeiro, R., Brandão, D., Noronha, J., Lima, C., Fregonezi, G., Resqueti, V., & Dornelas de Andrade, A. (2018). Breath-stacking and incentive spirometry in Parkinson’s disease: Randomized crossover clinical trial. Respiratory Physiology & Neurobiology, 255, 11–16. https://doi.org/10.1016/j.resp.2018.04.011
- Sapienza, C., Troche, M., Pitts, T., & Davenport, P. (2011). Respiratory strength training: Concept and intervention outcomes. Seminars in Speech and Language, 32(1), 21–30. https://doi.org/10.1055/s-0031-1271972
- Stegemöller, E. L., Hibbing, P., Radig, H., & Wingate, J. (2017). Therapeutic singing as an early intervention for swallowing in persons with Parkinson’s disease. Complementary Therapies in Medicine, 31, 127–133. https://doi.org/10.1016/j.ctim.2017.03.002
- Stegemöller, E. L., Radig, H., Hibbing, P., Wingate, J., & Sapienza, C. (2017). Effects of singing on voice, respiratory control and quality of life in persons with Parkinson’s disease. Disability and Rehabilitation, 39(6), 594–600. https://doi.org/10.3109/09638288.2016.1152610
- Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., Henry, D., Altman, D. G., Ansari, M. T., Boutron, I., Carpenter, J. R., Chan, A.-W., Churchill, R., Deeks, J. J., Hróbjartsson, A., Kirkham, J., Jüni, P., Loke, Y. K., Pigott, T. D., … Higgins, J. P. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ, 355, i4919. https://doi.org/10.1136/bmj.i4919
- Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., Cates, C. J., Cheng, H.-Y., Corbett, M. S., Eldridge, S. M., Emberson, J. R., Hernán, M. A., Hopewell, S., Hróbjartsson, A., Junqueira, D. R., Jüni, P., Kirkham, J. J., Lasserson, T., Li, T., … Higgins, J. P. T. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ, 366(1136), l4898–10. https://doi.org/10.1136/bmj.l4898
- Tamplin, J., Morris, M. E., Marigliani, C., Baker, F. A., Noffs, G., & Vogel, A. P. (2020). ParkinSong: Outcomes of a 12-month controlled trial of therapeutic singing groups in Parkinson’s disease. Journal of Parkinson’s Disease, 10(3), 1217–1230. https://doi.org/10.3233/JPD-191838
- Tamplin, J., Morris, M. E., Marigliani, C., Baker, F. A., & Vogel, A. P. (2019). ParkinSong: A controlled trial of singing-based therapy for Parkinson’s disease. Neurorehabilitation and Neural Repair, 33(6), 453–463. https://doi.org/10.1177/1545968319847948
- Trail, M., Protas, E. J., & Lai, E. C. (2008). Neurorehabilitation in Parkinson’s disease: An evidence-based treatment model. SLACK Incorporated.
- Troche, M. S., Okun, M. S., Rosenbek, J. C., Musson, N., Fernandez, H. H., Rodriguez, R., Romrell, J., Pitts, T., Wheeler-Hegland, K. M., & Sapienza, C. M. (2010). Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology, 75(21), 1912–1919. https://doi.org/10.1212/WNL.0b013e3181fef115
- Troche, M. S., Rosenbek, J. C., Okun, M. S., & Sapienza, C. M. (2014). Detraining outcomes with expiratory muscle strength training in Parkinson disease. Journal of Rehabilitation Research and Development, 51(2), 305–310. 10. https://doi.org/10.1682/JRRD.2013.05.0101
- van Hooren, M. R., Baijens, L. W., Voskuilen, S., Oosterloo, M., & Kremer, B. (2014). Treatment effects for dysphagia in Parkinson’s disease: a systematic review. Parkinsonism & Related Disorders, 20(8), 800–807. https://doi.org/10.1016/j.parkreldis.2014.03.026
- Wang, C.-M., Shieh, W.-Y., Ho, C.-S., Hu, Y.-W., & Wu, Y.-R. (2018). Home-based orolingual exercise improves the coordination of swallowing and respiration in early Parkinson disease: A quasi-experimental before-and-after exercise program study. Frontiers in Neurology, 9, 624. https://doi.org/10.3389/fneur.2018.00624