Abstract
Background: Calluna vulgaris is a woody shrub forming plant communities of economic and international conservation importance. Following winter 2002–2003, there was exceptional Calluna die-back in the central Scottish Highlands.
Aim: To understand the effects and causes of this die-back event at Abernethy Forest Nature Reserve, Cairngorms National Park.
Methods: Patterns of weather, foliage moisture and microclimate were interpreted in relation to patterns of Calluna vulgaris die-back. Vegetation development was investigated over 4 years, based on pre-existing monitoring plots.
Results: February 2003 included a period of exceptionally low air humidity, during which unusually low Calluna shoot moisture readings were made, particularly in areas that subsequently had severe die-back. In monitored areas, Calluna cover declined by a third, with minimal recovery after three growing seasons. Die-back was more severe where Calluna was longer-stemmed and less abundant, and where topography was flatter or more north-facing. These topographical features, and die-back, were correlated with colder winter microclimates. A doubling in Vaccinium myrtillus cover in forest plots was strongly correlated with the pattern of Calluna die-back. Meteorological data showed an eight-fold increase in the variability of winter humidity minima since 1983–1984.
Conclusions: Die-back probably followed severe ‘winter desiccation’, caused by extreme low humidity conditions, combined with low temperatures, lack of snow cover, and Calluna vulnerability due to age. It led to a major vegetation change in the direction of management aims at this site, but contrary to aims at many other Calluna-dominated sites. The importance of climatic variability as a potential driver of major vegetation change is emphasised.
Introduction
Calluna vulgaris (L.) Hull (Ericaceae), henceforth ‘Calluna’, is a woody shrub which forms characteristic plant communities, typical of oceanic climates in north-west Europe (Gimingham Citation1972). These communities often have a high economic value as grazing land and for hunting (Hobbs and Gimingham Citation1987). Calluna is a major component of plant communities of conservation interest, including heathland (Thompson et al. Citation1995) and native Pinus sylvestris L. forest (Steven and Carlisle Citation1959; Summers et al. Citation1999).
An important area of Britain for these communities is the 3800 km2 Cairngorms National Park in the Scottish Highlands (). From spring 2003, Calluna in this area suffered exceptional die-back (M.H. Hancock, pers. obs.; A. MacDonald, unpublished). At Abernethy Nature Reserve, within the National Park (), Calluna die-back was first noticed in late March. Most plants initially showed a mosaic of brown and green shoots, with brown shoots later becoming grey and clearly dead. Other plant species were not obviously affected. Calluna die-back occurred both within woodland and on open heathland. Calluna that was shorter, or had been under snow, seemed less affected (A. MacDonald, unpublished). The timing of die-back, and lack of typical evidence of insect or fungal damage (MacDonald Citation1990), suggested exceptionally severe ‘winter browning’. This is attributed to a combination of low temperatures and water stress (Bannister Citation1964; Watson et al. Citation1966), and is potentially influenced by atmospheric pollutants (Foot et al. Citation1997; Carroll et al. Citation1999; Caporn et al. Citation2000; Sæbo et al. Citation2001). The scale of die-back exceeded anything seen by Reserve staff, some of whom knew the area from the mid-1970s.
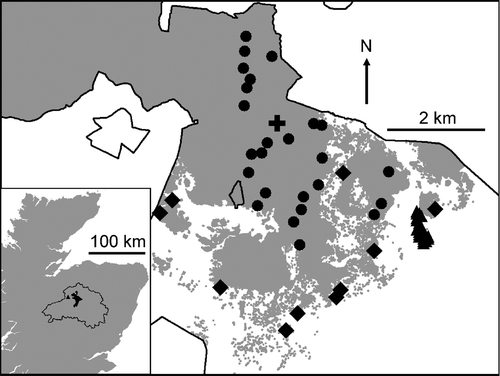
Figure 1 The study area. Main map: central part of Abernethy Nature Reserve, showing Reserve boundary (black line), forest area (grey), study plots (forest plots, circles; forest edge plots, diamonds; heathland plots, triangles (closely clustered at the east side of the map); and Forest Lodge (cross, British National Grid NJ020161; 57°14.2′ N, 3°37.5′ W). Inset: part of Scotland, showing the Cairngorms National Park (black line), Abernethy Reserve (black), and Aviemore meteorological station (triangle; British National Grid NH897143; 57°12.3′ N, 3°49.6′ W).
Although extensive Calluna die-back has occurred in English Breckland and Dutch lowland heaths (Marrs Citation1986, Citation1988, Citation1993; Aerts and Heil Citation1993), nothing similar to this episode has been reported in the literature for upland Britain. Die-back in Dutch heaths has been linked to nitrogen pollution (Heil and Diemont Citation1983; Aerts and Heil Citation1993), and this may have been important in the Breckland heaths (Marrs Citation1993; Woodin and Farmer Citation1993), where nitrogen deposition rates are similar (Pitcairn et al. Citation1995). By contrast, Calluna-dominated habitat at Abernethy lies at middle altitudes in the Cairngorms, where there is little evidence of increasing nitrogen deposition, at least not before 1990 (Pitcairn et al. Citation1995). Although reduced burning at Abernethy was causing the average age of Calluna to rise, rejuvenation by rooting of prostrate stems or layering (Gimingham Citation1988; MacDonald et al. Citation1995) is widespread at the site. This process is rare in some other, drier heaths, such as the Brecklands in southern England (Marrs Citation1988). The shelter and peaty soils of Calluna habitats at Abernethy probably favour layering, which was expected to allow persistent extensive Calluna cover for decades (Scandrett and Gimingham Citation1989; MacDonald et al. Citation1995; Stevenson et al. Citation1996). For these reasons, the extent of die-back in 2003 was unexpected.
In much of its European range (Gimingham Citation1972) maintaining Calluna is a key management aim, for both conservation (Aerts and Heil Citation1993; Thompson et al. Citation1995) and economic (Hobbs and Gimingham Citation1987) reasons. Conversely, in semi-natural pinewoods managed for conservation, such as Abernethy, reduced Calluna dominance could benefit management aims in two different ways. Firstly, it could increase the abundance of the shrub Vaccinium myrtillus L., and hence benefit conservation of the woodland grouse Tetrao urogallus L. (Summers et al. Citation2004). Secondly, the reduction of Calluna could increase Pinus sylvestris seedling establishment (Hancock et al. Citation2005), by providing suitable seed bed sites, and hence benefit forest expansion. To test these hypotheses, different forms of management, all intended to reduce Calluna dominance, are being trialled at Abernethy, and will be reported elsewhere.
A better understanding of severe Calluna die-back events would be valuable in relation to habitats of both conservation and economic importance. Therefore, it was decided to investigate the die-back event, using data from vegetation monitoring plots, originally established as controls for management trials, together with observations in spring 2003, and meteorological and microclimate data. The aims were (1) to infer possible causes of die-back; (2) to assess its consequences, particularly in relation to management aims; and (3) to gauge whether climatic trends may increase the future likelihood of such events.
Methods
Study site
The study site, Abernethy Nature Reserve (), includes c. 4000 ha of Pinus sylvestris-dominated forest, and c. 4500 ha of heathland. Calluna makes up over 50% of forest field-layer cover across a third of the pinewood – type W18 of the British National Vegetation Classification, NVC, Rodwell Citation1991 (Nisbet and Amphlett Citation2003) – and is dominant or co-dominant in the principal adjoining heathland communities (NVC H12, H13 and H19) (Taylor Citation2001). After reserve acquisition in the late 1980s, to promote forest expansion, deer populations were reduced, and sheep grazing and large-scale heathland burning discontinued (Beaumont et al. Citation1995).
Vegetation data were collected at three sets of plots: the ‘forest plots’, ‘forest edge plots’ and ‘heathland plots’ (). Plots were at 250–450 m altitude, on predominately peaty soils. The 25 forest plots were randomly located within ‘old, open forest’ (termed ‘Box 2-3’ by Picozzi et al. Citation1992), in a particular Pinus sylvestris woodland type (NVC W18b), characterised by abundant Calluna and two Vaccinium species. The 10 forest edge and 25 heathland plots were located on heathland areas with little tree regeneration, within 50 m and 250 m, respectively, of mature trees. These were mainly dry heath (NVC H12), with some mire (M19) and grassland (U6). Recording took place at two fine spatial scales which were common to all sets of plots: the sample point (1 m radius) and cell (5 m × 5 m). Each cell contained four sample points, one in each quarter. Recording also took place at the whole-plot scale. Forest edge and heathland plots were 10 m × 10 m in area and contained four cells. Forest plots were 20 m × 20 m in area, and held 16 cells, but recording took place only at alternate cells, making a chequerboard pattern. This study ran from 2002 to 2005, including one growing season before, and three after, the die-back event.
Vegetation and topography
Vegetation surveys took place in August–October each year. At each sample point, a measuring stick was pushed vertically into the moss/litter, until the resistance of the soil/humus was felt. The depth of moss/litter was taken as the maximum height of any piece of moss/litter within 5 cm of the stick. A measure of Calluna maturity, ‘maximum stem length’, was taken as the length from rooting point to tip of the tallest piece of Calluna within 5 cm of the stick. This was considered a more detailed measure of Calluna age and structure than the classic descriptors of Watt (Citation1947) and Gimingham (Citation1972).
Cover estimates within a 1 m radius were made for Calluna, Vaccinium myrtillus, V. vitis-idaea, and combined graminoids (Poaceae, Cyperaceae, Juncaceae). The proportion of Calluna canopy shoots that was recently-dead (brown) or long-dead (grey) was estimated. Vaccinium myrtillus cover estimates at a given stem density are variably affected by defoliation. To compensate for this, defoliation was scored as the proportion of live shoots that were leafless, among the 10 nearest the vertical stick.
All vegetation measures from the forest plots were collected by the author (M.H.). At other plots, recording was by five observers including M.H. Estimates of vegetation cover can differ between observers (Sykes et al. Citation1983). For logistical reasons, it was not possible to have balanced, randomly-allocated samples of plots for different observers, and thus not possible to fit observer as a covariate in analyses. Therefore, scores were adjusted for observer variation using correction factors based on annual photographs taken at heathland plots, where all observers surveyed at least 40 cells. Photographs (standardised oblique views of each cell) were used by M.H. to estimate top cover of live Calluna, dead Calluna, graminoids and Vaccinium species. These scores were arcsine(square-root) or arcsine(fourth-root) transformed, and regressed using reduced major axis regression (Quinn and Keough Citation2002), against corresponding field-recorded top cover scores. Separate regressions were performed for each observer and plant type. Re-arranging regression equations allowed photograph score to be eliminated, and field score ‘as if recorded by M.H.’ to be estimated. These corrections were applied to field scores of total cover, and corrected values used in analyses.
Differences in Calluna die-back between north- and south-facing slopes were expected, due to differences in solar warming or drying. Therefore, an index of exposure to the south was measured, using a clinometer to determine the angle in degrees (α) between the lowest open sky in a southerly direction and the horizontal. Southerly exposure was defined as (90–α). Slope aspect was determined by taking a compass bearing (β) down the steepest slope. Slope aspect in relation to south was defined as -1∗cos(β), giving a value of 1 for south and -1 for north-facing slopes.
Plant recording in February–March 2003
In February–March 2003, when it was suspected that weather conditions might have killed Calluna, fieldwork was underway only at the forest plots. Within 10–40 m of these plots, vegetation moisture content was determined during this period, as part of monitoring for experimental fires (to be reported elsewhere). Calluna moisture content was measured for fine shoots and three diameter-classes of stem. Only shoots with green leaves, and therefore assumed to be alive, were used. Moisture contents were also determined for Vaccinium myrtillus, V. vitis-idaea and Pinus sylvestris. In dry conditions between 10 h and 16 h, a small sample (mean dry weight 2.4 g, range 0.4 –14 g) of material was collected from several plants, and placed in an airtight aluminium can. Samples were oven-dried at 80 °C for 48 h and moisture content calculated as percentage of wet weight. Similar fieldwork also took place at comparable (forest) sites in March–April 2002.
Photographs were taken at the same time as this work. To help estimate the timing of the weather event that caused Calluna die-back, these were later examined. The colouration of Calluna in March–April 2002, February 2003 and March 2003 was compared, by seven different observers, based on 19 triplets of photographs. Each triplet contained one (numbered but not dated) photograph from each time period. Observers were asked to rank the photographs of each triplet in terms of the degree of brown coloration of Calluna plants.
Microclimate measurements
Following Calluna die-back, microclimate measurements were made at die-back locations. Because these took place after die-back, they may have been affected by it. However, structural changes in the vegetation after die-back were slow, reflecting the persistence of dead material in the Calluna canopy (see below). So it was considered that the spatial pattern of microclimate in the winters following die-back could resemble that in the winter during which die-back occurred. Clearly, this was based on the premise that Calluna die-back had little effect on local microclimate, which was not tested. Measurements were made at the forest plots, in winters 2003–2004 and 2004–2005, using 28 temperature loggers. Fourteen plots were selected at random from the 21 with over 50% Calluna cover before die-back. At each of these plots, the sample point with the greatest die-back and that with the least were selected. A temperature logger was placed at each of these points, with the sensor tip pushed c. 2 cm into the moss-litter layer. Loggers were in place from late November until early March, and recorded the maximum temperature within each hour.
Meteorological data
Meteorological data were obtained for the period 1983–2004 from Aviemore meteorological station (228 m a.s.l.), 10–16 km west of the monitoring plots (). Hourly temperature and relative humidity were collated for all days with at least one reading, and daily minimum values of temperature and humidity determined. Daily rainfall and ground frost (grass temperature at 9 am) data were also collated. Snow depth data, from an open area in Abernethy Forest (Forest Lodge, ), were collated for winter 2002–2003.
Methods of analysis
The topographical and vegetation correlates of Calluna die-back were investigated using a generalised linear (mixed) modelling approach (GLM or GLMM: Littell et al. Citation1996). The dependent (y) variable was the proportion of Calluna, present in 2003, which was brown. This was arcsine(fourth-root) transformed (Quinn and Keough Citation2002) at the sample-point scale. Y-values at the cell and plot scales were means of corresponding values at the point and cell scales, respectively. The degree of association between independent (x) variables in a particular model, and Calluna die-back, was tested using an F-test with a threshold P-value of 0.05 (Littell et al. Citation1996). Residuals of final models were plotted against fitted values to check for normality, non-linearity and outliers.
X-variables were six measures of vegetation and topography (), which were chosen by considering a range of factors that might affect die-back, and selecting a small subset that were not strongly inter-correlated. (For the different datasets, the maximum Spearman's correlation coefficient between any two x-variables was 0.60.) Nine models were built, one for each set of plots at each spatial scale. Each model comprised a subset of x-variables, selected using a step-up process, to give a minimally-adequate model (Crawley Citation1993). At the plot scale, this constituted the final model. However, for finer-scale analyses, it was expected that data from grouped sampling units would be correlated. Although this can be modelled in a mixed model (GLMM: Littel et al. Citation1996), step-wise variable selection in such models is not appropriate (Welham and Thompson Citation1997). Therefore, variable selection was carried out initially in a GLM framework just as at the plot level. The expected effect of ignoring spatial correlations at this stage was that more variables would be selected than if correlations were accounted for (Haining Citation2003). Once a subset of x-variables had been selected, their significance was investigated using a mixed model, which also included random effects representing ‘plot’ (and ‘cell’ at the point scale), to model spatial correlation. Any x-variables having a non-significant effect in this model were then deleted to give a final model.
Table 2. Correlates of the spatial pattern of Calluna die-back. Analyses were carried out in a generalised linear modelling framework, with percentage die-back as the dependent variable. The table shows parameter estimates for variables included in minimally-adequate models, selected using a step-wise procedure. Dashes indicate where a variable was not tested, as data were not available at that spatial scale. Standard errors and P-values are given in curved and square brackets respectively. Calluna percentage die-back (the dependent variable) was arcsine (fourth-root) transformed at the point scale, with means taken at higher spatial scales
Similar methods, but without stepwise selection, were used to examine the relationship between Calluna die-back and (1) moisture content; (2) response of Vaccinium myrtillus; and (3) microclimate.
Results
Observations in spring 2003
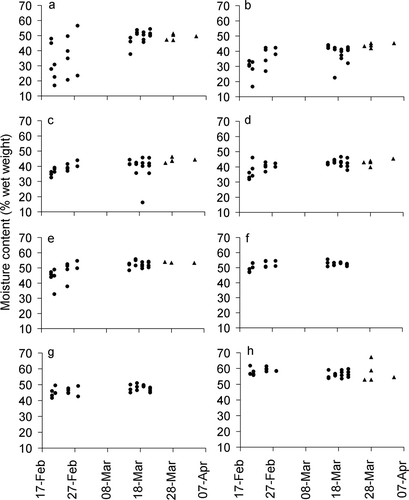
Vegetation moisture contents in February–March 2003, on forest plots, are shown in , with comparable data from five other, similar plots, collected in February–March 2002. In March 2003, and March–April 2002, moisture levels were usually around 50% for Calluna shoots. However, in February 2003, Calluna shoot moisture levels were strikingly variable, with many low readings, half of which were below the lowest value recorded at other times. For other plant species and for Calluna stems, values in February 2003 were less variable, and more similar to their values on other dates.
Figure 2 Moisture contents (as percent fresh weight) of vegetation identified as live, in spring 2002 (triangles) and spring 2003 (circles). Each point represents one vegetation sample at one of the forest plots. (a) Calluna shoots (fine terminal stems and imbricate leaves); (b) Calluna fine stems (0–2 mm diameter); (c) Calluna medium stems (2–5 mm); (d) Calluna large stems (5–10 mm); (e) Vaccinium myrtillus stems; (f) Vaccinium vitis-idaea leaves; (g) Vaccinium vitis-idaea stems; (h) Pinus sylvestris needles. Vaccinium vitis-idaea moisture content was not recorded in 2002.
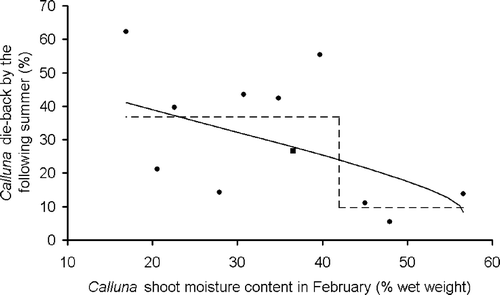
Regression analysis comparing Calluna shoot moisture levels in February 2003, specified as a continuous variable, with later die-back, suggested a significant relationship (, F 1,9 = 3.71, P (one-tailed) = 0.04). Plots with low Calluna moisture contents in February 2003 subsequently had greater die-back (). There was no such relationship with March 2003 moisture contents (F 1,12 = 0.09, P = 0.77). The plotted values for February 2003 suggested an alternative model, with Calluna moisture content specified as a two-level categorical variable, with a ‘threshold’ somewhere between 40% and 44% (, dotted line). Fitting Calluna moisture as a categorical rather than continuous variable resulted in a better model fit, which explained nearly twice the variation with no additional degrees of freedom (R 2 = 0.55 as opposed to 0.29). Similarly, the association between Calluna die-back and the alternative (categorical) moisture content variable was strongly significant (F 1,9 = 10.9, P (one-tailed) < 0.01). Thus, Calluna shoot moistures below 40–44% in February 2003 were strongly associated with greater subsequent die-back.
Table 1. Results of tests of hypotheses relating to the relationship between Calluna die-back and (a) shoot moisture content in spring 2003; (b) increase in Vaccinium myrtillus cover; and (c) microclimate in the following two winters. All results relate to the forest plots. All response variables were arcsine (fourth-root) transformed
Figure 3 Moisture content of green Calluna shoots in February 2003, plotted against subsequent Calluna die-back, as estimated in summer 2003. The data are from the forest plots, with each point representing one plot. At one plot (square symbol) the mean of two moisture readings is used, otherwise each plot is represented by one reading only. The back-transformed fitted relationships are also shown: solid line, moisture content represented by a continuous variable (); dotted line: moisture content represented by a two-level categorical variable. The curve in the solid line results from back-transformation of a linear relationship based on arcsine(fourth-root) transformed die-back scores.
Photographs showed that Calluna was visibly browner in March 2003 than in either February 2003 or March–April 2002 (sign tests, n = 19, P < 0.01 for both). The February 2003 vs. March 2003 comparison gave a similar result when only the earlier March photographs (15 and 17 March) were included (sign test, n = 9, P ≤ 0.01). Calluna colouration differed little between February 2003 and March–April 2002 (sign test, n = 19, P = 0.67). This suggested that browning of Calluna developed between 28 February and 15 March 2003.
The spatial pattern of die-back, summer 2003
At the forest plots, greater die-back was associated, at different spatial scales, with deeper moss/litter, longer Calluna stems, and flat or north-facing topography (). Fitted relationships were examined for each variable, with other variables in the relevant model held at their mean values. Die-back was typically around 5–20% at locations with either shallow moss/litter, short Calluna stems, or sloping or south-facing topography, and about 40% under opposite conditions.
At forest edge plots, at the point and cell scales, greater die-back was associated with lower initial Calluna cover (). Fitted die-back estimates were below 10% at high (near 100%) initial Calluna cover, but 25–40% at low (45–65%) initial cover. Thus die-back tended to accentuate existing fine-scale variation in Calluna cover. At heathland plots, die-back was greater where there was a more northerly aspect (), just as it was at the forest plots (above), ranging from c. 50% at north-facing locations to less than 20% at south-facing locations.
Temporal changes in vegetation following Calluna die-back
Mean live Calluna cover fell considerably between summer 2002 and summer 2003 (, ). In relative terms, compared to initial cover, cover fell by an average of 31%, 24% and 36% for forest, forest edge and heathland plots, respectively (). The mean percentage cover of dead Calluna rose by a factor of 8–22, from 0.9–3.6% in 2002, to 21–30% in 2003 (). Thereafter, dead Calluna cover slowly declined as dead material collapsed into the moss/litter layer. However, 9–22% cover of dead Calluna remained in 2005, 2.5 years after the die-back. There was minimal recovery in Calluna cover after die-back (): the average percentage relative increases across the three groups of plots were 5.4% between 2003 and 2004, and 0.4% between 2004 and 2005.
Figure 4 Temporal changes in mean vegetation percentage cover following Calluna die-back: (a) live Calluna; (b) dead Calluna; (c) combined Vaccinium species; (d) combined graminoids. An arrow indicates the timing of the Calluna die-back event. Boxes show plot-scale medians and inter-quartile ranges, whiskers show 5th and 95th centiles. Means are shown by black circles. White bars: 2002; grey bars: 2003; bold stripe: 2004; fine stripe: 2005.
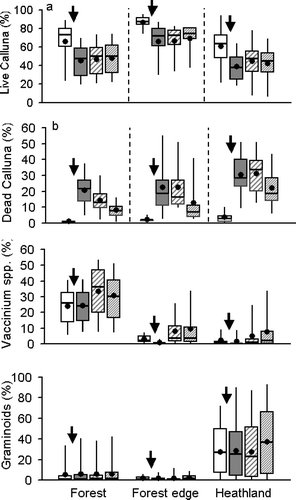
Figure 5 Annual, late-summer photographs illustrating the development of ground vegetation from one year before (2002), to three years after die-back (2005). The vegetation at two example cells, which had initially high Calluna cover, then high Calluna die-back, is shown. Each example consists of a column of four photographs, ordered by year, with 2002 as the upper photograph. Die-back occurred between the first (2002) and second (2003) photograph in each column. (four photographs forming the left-hand column of the figure) and (right-hand column) show a cell at a forest and heathland plot, respectively. Photographs are oblique views of cells, taken from a standing position 2 m from the cell centre, which is marked by a small yellow flag (visible in some of the photographs). Photographs were taken for illustrative rather than mensurative purposes, however an approximate (50 cm) scale for middle distances is given. The photographer's shadow has been digitally lightened in the 2005 photograph of .

Following Calluna die-back, there were increases in Vaccinium species (, ), and graminoids (). Relative increases in cover scores by 2005, compared to 2002, were 18–50% and 28–248% for graminoids and Vaccinium respectively. Increases in Vaccinium myrtillus cover within the forest were of particular interest, as this is a management aim (see Introduction). At the point scale, on forest plots, higher V. myrtillus cover in 2005 was strongly positively associated with greater Calluna die-back, once effects of initial V. myrtillus cover, differences in defoliation and visit date, and non-independence of grouped data points were accounted for (). Associations at higher spatial scales were not significant (cell scale: F 1,135 = 2.78, P = 0.1; plot scale: F 1,20 = 1.46, P = 0.24). Hence there was a strong link, but only at fine spatial scales, between increases in V. myrtillus cover and Calluna die-back. The fitted relationship (at mean initial V. myrtillus cover) suggested a 26%, 93% and 176% relative increase in point-scale Vaccinium myrtillus cover between 2002 and 2005 for zero, mean (26%) and maximum (100%) Calluna die-back, respectively. Effects of Calluna die-back on Pinus sylvestris regeneration would also have been of particular interest, but too few young trees were present in the study plots to allow examination of this.
Microclimate at die-back locations
Due to technical failures, data from 23 and 24 out of 28 loggers were available in winters 2003–2004 and 2004–2005, respectively. Three microclimate variables were collated, each with a hypothesised relationship with Calluna die-back: mean ground temperature T G (colder, more die-back), mean daily temperature range ΔT G (greater range, more die-back) and proportion of days with frost P F (more frequent frost, more die-back). Using the 20 logger locations having data in both winters, these variables were positively correlated between the two winters, though significantly so for only two variables (r = 0.50, 0.59 and 0.23; P = 0.02, 0.01 and 0.34 for T G, ΔT G and P F, respectively). Thus microclimate patterns should be similar in successive winters, at least for T G and ΔT G. This was important if results were to be relevant to the winter in which die-back occurred, which preceded the two measurement winters. However, as microclimate measurements followed die-back, there remains the possibility that the die-back itself affected microclimate at some measurement locations.
The direction of association between each microclimate variable and Calluna die-back was as hypothesised. Greater die-back was strongly associated with lower ground temperatures TG (P ≤ 0.01, ). Similarly, die-back was linked, slightly less strongly, to higher daily temperature ranges ΔTG (F 1,35.9 = 4.32, P (one-tailed) = 0.02), and more frequent frost PF (F 1,30.8 = 3.09, P (one-tailed) = 0.04). At points with greatest die-back, modelled die-back declined by 4.3–11 percentage points (from initial mean of 76%) for each 1 °C increase in mean winter temperature. At points with least die-back, corresponding values were 0.3–1.9 percentage points (from an initial mean of 4.0%). Strong effects of within-plot logger location () showed that there was substantial fine-scale variation that was not explained by measured microclimate temperature variables.
There were correlations between microclimate and topography (), with, for example, flatter and more north-facing areas having lower mean temperatures. Thus the topograhical relationships found in exploratory analyses of the spatial pattern of die-back (above), were consistent with results of microclimate studies.
Table 3. Correlations between microclimate and topography at forest plots. Spearman correlations are given, for the finest spatial scale at which data were available, for each of the two measurement winters. Note that measurement of microclimate took place after die-back occurred, so there is potential for die-back to have affected these microclimate data
Meteorological data
Winter 2002–2003 included various weather events that could have caused Calluna die-back. Particularly cold days, with daily minima exceeding two standard deviations below the long-term mean, occurred on 20 October and 6–8 January (). There were extended periods of ground frost, the longest, of 19 d, ending on 27 February (). Days with particularly low humidity occurred on 19–20 November, 14–20 and 25–26 February, 15–19 and 23–24 March (). There were dry spells (over a week with daily rainfall less than 1.0 mm) from 5–24 December, 5–12 January, 10–28 February, and 13 March to 1 April (). However, the most extreme readings were humidity minima during 14–20 February, which reached five standard deviations below long-term means. This period fell within an 18-day dry spell, when the ground was snow-free (). It followed a period with air temperatures below 0 °C on 15 out of 18 days, and coincided with the most extended ground frost of the winter (). Daily maximum temperatures during this period ranged from 2.9 to 8.4 °C, lower than during the other low humidity periods. This period was characterised by southerly winds, with 74% of hourly readings being in this quarter, as were all strong winds (hourly readings exceeding the long-term mean by more than one standard deviation). It also included many sunny days. However, because die-back was as severe under the forest canopy as in the open, and more pronounced on north-facing slopes, solar radiation and wind were considered unlikely causal factors. Therefore, sunshine and wind data were not collated in detail and are not presented here.
Figure 6 Daily weather readings during winter 2002–2003, when Calluna die-back occurred, and long-term means for the same day across the previous 15 winters. (a) Minimum daily temperature (°C). Black dots indicate winter 2002–2003 values. The greyed area shows the long-term mean daily minimum (central line), plus and minus two standard deviations (based on inter-annual variation for the same date), smoothed using a 30-day running average. Extended periods of ground frost (three or more consecutive days with grass temperature below 0 °C at 9 am GMT) are indicated with horizontal bars. (b) Minimum daily relative humidity (%). Key as . (c) Daily rainfall for winter 2002–2003 (bars) and long-term mean (line) (mm). Horizontal bars indicate periods of snow cover at Abernethy (Forest Lodge: ). All other readings are from the Aviemore weather station ().
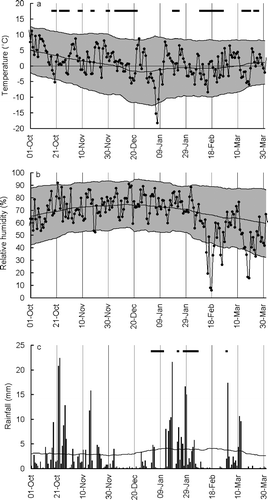
Comparing winter 2002–2003 with the long-term record, the minimum temperature recorded, −18.3 °C, was well within the range of winter minima between 1983–1984 and 2003–2004 (). Comparing two 10-winter samples, 1983–1984–1991–1992 (‘the early period’) and 1992–1993–2003–2004 (excluding 2002/3) (‘the late period’), showed no change in variance for winter minima. Although there was a 19-day period of consecutive morning ground frost in February 2003, there were 11 longer periods of frost in the 21-winter sample. However, the minimum humidity recorded in winter 2002–2003, 5.9%, was much lower compared to the long-term pattern (). Indeed, this reading, and another five hourly readings during 17-18 February 2003, were lower than any other readings in the 21-year dataset. Winter minimum humidity readings were eight times more variable in the late period that the early period (), a difference that was highly significant (F 9,9 = 7.96, P < 0.01). A winter minimum humidity reading below 30% would be expected every 110 years, based on the early period distribution, but every four years, based on the late-period distribution. There were six days with such readings in winter 2002–2003.
Figure 7 Winter (October–March) 2002–2003 weather readings in relation to longer-term data for Aviemore. Vertical lines show individual winters, while bell curves show fitted normal distributions. (a) All-winter minimum temperature; (b) all-winter minimum relative humidity; (c) mean winter daily rainfall. Normal distributions fitted to the longer-term pattern are shown for two 10-winter periods 1983–1984 to 1992–1993 (dotted) and 1993–1994 to 2003–2004, excluding 2002–2003, (solid). The y-axis shows expected relative frequencies. Individual winters (vertical lines), are dotted for 1983–1984 to 1992–1993, and solid for 1993–1994 to 2003–2004. The winter during which Calluna die-back occurred, 2002–2003, is shown in bold and individually labelled.
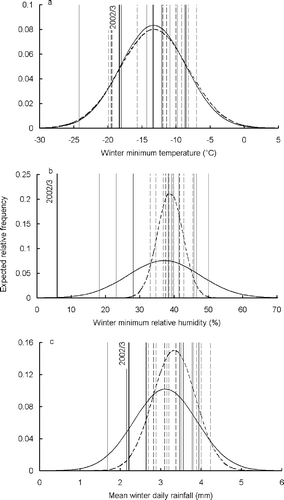
Rainfall in winter 2002–2003 was not exceptional. It was below the long-term average, but there had been drier winters (). It showed higher variance in the late period than the early period, but this difference was not significant (F 9,9 = 2.18, P = 0.13).
Overall, of the various weather parameters investigated, the humidity conditions in winter 2002–2003 were the most unusual in relation to the long-term record. This may be linked to increasing variance in winter humidity extremes.
Discussion
Causes of die-back
‘Winter desiccation’ (Tranquillini Citation1982) provides a likely explanation for the Calluna die-back observed during this study. This occurs when drying conditions are combined with cold temperatures, which cause reduced water transport due to high water viscosity (Kramer Citation1942) or freezing. The mosaic pattern of die-back on individual plants, and the greater variance in moisture contents for shoots than stems in February 2003, fits the model of Tyree and Sperry (Citation1988). They suggested that, for woody species, a proportion of minor branches may die during water stress, allowing the water balance in other minor branches, and the more valuable major branches, to be maintained.
The conditions most likely to have produced Calluna die-back occurred in mid-February 2003. This period had the most unusual weather episode during winter 2002–2003: extremely low humidity, combined with low air temperatures, extended ground frost, absence of snow cover, and low rainfall. Unusually low humidity, combined with frozen soil, were also associated with Calluna die-back by Watson et al. (Citation1966), who focussed on what was probably the most severe die-back event during a long-term study (Green Citation1965; Moss and Miller Citation1976; see also Green Citation1953). Unusually low Calluna shoot moisture readings in February 2003, especially at locations that subsequently had greater die-back, also suggest this was the key period. A comparison with winter (October–March) readings from Bannister (Citation1964), Hinshiri (in Gimingham Citation1972:96–97) and Davies (Citation2006), shows how exceptional these Calluna moisture readings were: Bannister's readings averaged 54% (range 43–61%, n = 20), Hinshiri's averaged 52% (range 41–57%, n = 18, using daily means by site, and Bannister's (Citation1964) mean winter succulence) while Davies’ averaged 49% (range 43–64%, n = 28, using daily means). Comparisons may be affected by methodological differences. However, all daily means of Calluna shoot moisture content readings from this study, for February 2003, fell below the lowest recorded in these other studies (mean 35%, range 23–40%, n = 4). Conversely, daily means from this study for March–April 2002 and March 2003 gave similar averages to those of other studies (March–April 2002: mean 49%, range 47–50%, n = 3; March 2003: mean 48%, range 44–52%, n = 5). The minimum moisture levels recorded by Bannister, Hinshiri and Davies, 41–43%, were similar to the threshold moisture content value of 40–44%, suggested by our data, below which die-back increased (). The unusually low moisture content of green Calluna shoots at Abernethy in February 2003 suggests this as the key period of water stress.
Apart from February 2003, two other weather episodes in winter 2002–2003 were unusual: low humidity in mid-March and low temperatures in early January. However, it is suggested that these events did not cause extensive Calluna die-back, for the following reasons. The low January temperatures occurred after a period of precipitation, when there was snow cover, and normal humidity levels. It was not as extreme, compared to the long-term record, as the February humidity readings. There is experimental evidence that Calluna can tolerate such temperatures (Caporn et al. Citation2000). Photographs suggested that Calluna was no browner in February 2003, well after the low January temperatures, than in March–April 2002. During the low-humidity episode in March, Calluna moisture readings were higher than those in February, and all daily means were within the range of other studies (above). Photographs suggested the Calluna was already appreciably browner, early in the March episode, than it had been earlier. Bannister (Citation1964) has noted that Calluna killed by drought remained green, while waterlogging resulted in browning, and suggested that water might be needed for the colour change. In our study, if most Calluna died during mid-February 2003, the development of browning may have been delayed until warmer temperatures in late February–early March allowed water to return to the damaged tissues. This would be consistent with Watson et al. (Citation1966), who observed that browning in Calluna usually developed 1–2 weeks after the weather conditions thought to have caused damage.
The spatial pattern of die-back, with lower die-back on south-facing slopes, suggests that sunshine had a positive effect on foliage water balance, by warming the soil and plant tissues, and perhaps by melting ice on foliage. Watson et al. (Citation1966) found greater browning in areas facing the prevailing wind. However, in this study, the opposite was found, with greater die-back on north-facing slopes, despite mainly southerly winds during February 2003. Similarly, southerly exposure index was not associated with die-back. The topographical pattern fits better with microclimate data, suggesting that, while low humidity may have been the key wide-scale factor causing water stress, the fine-scale pattern could have resulted from differences in temperature, linked to fine-scale topographical features. Lower temperatures, flatter topography or northerly aspects, and greater Calluna die-back, were all mutually correlated.
Other evidence suggesting low humidity as key to Calluna die-back comes from forest microclimate studies. These show that cold spring temperatures are less likely under coniferous forest canopy than in the open (Aussenac Citation2000; Langvall and Ottosson-Löfvenius Citation2002). If low temperatures were the key driver of die-back, reduced die-back might be expected under the forest canopy. However, in this study, mean die-back at forest plots was intermediate between that of the two sets of plots in open habitats. Conversely, microclimate studies have usually found minimal differences in relative humidity between forested and open areas (Aussenac Citation2000; Gehlhausen et al. Citation2000; Porté et al. Citation2004). Therefore, if low humidity were key to Calluna die-back in this study, minimal attenuation of die-back under the forest canopy would be likely, and this is what was found.
The association between longer Calluna and greater die-back agrees with observations of Watson et al. (Citation1966) and Marrs (Citation1986). The lower root:shoot ratio of older plants (Chapman Citation1970) could make them more vulnerable to desiccation. Winter ground temperatures also reach lower extremes in older Calluna stands (Barclay-Estrup Citation1971). Reduced burning and grazing at Abernethy has probably led to an increase in mean age and stem length of Calluna, perhaps increasing its vulnerability to climatic stress. Higher die-back in forest areas with deep moss/litter could have resulted from competition for water, during climatic stress or subsequent recovery.
Consequences of die-back
The climatic event in winter 2002–2003 was followed by a major change in field-layer vegetation, in both forest and heathland habitats. Calluna, the dominant species, showed a decline in cover of about a third, with minimal recovery after three growing seasons, a change unprecedented in the literature on Calluna in upland habitats. Although Watson et al. (Citation1966) recorded extensive Calluna browning at an upland study site, they noted that a more-or-less complete recovery within one growing season. Conversely, in this study, live Calluna cover showed minimal recovery after three growing seasons.
The potential long-term loss of Calluna seen here shows some parallels with lowland heaths (Marrs Citation1986, Citation1988, Citation1993; Aerts and Heil Citation1993). These studies also took place in Calluna-dominated habitats where grazing and burning management had been reduced. They highlighted the role of factors including climate in triggering Calluna die-back, followed by long-term vegetation change in favour of its competitors. Woodland invasion followed Calluna die-back on some heaths (Marrs Citation1993). Such an outcome would suit management aims at Abernethy, but not at many other heathland sites. This study was not able to measure the effects of Calluna die-back on forest expansion. However, in the short term, within the forest, Vaccinium myrtillus has shown fine-scale increases linked to Calluna die-back. These suit management aims at Abernethy, reflecting the importance of V. myrtillus to the grouse, Tetrao urogallus, a key species at many pinewood sites. Outside the forest, another grouse species, Lagopus lagopus scoticus Lath., may have been adversely affected by Calluna die-back (Miller et al. Citation1966). On heathlands managed for grouse shooting, as is common in Britain, such declines could have serious economic impacts.
On a wider scale, Calluna die-back described here is relevant to the question of how ecosystems might react to increased climate variability. Ciais et al. (Citation2005) have estimated that extreme weather conditions in summer 2003 produced a major reduction in ecosystem primary productivity across Europe, resulting in vegetation being a net source of carbon, and reversing the effects of four years of ecosystem carbon sequestration. This study shows how different climate stresses, earlier in the same year, significantly reduced the abundance of an ecosystem dominant, with potential implications for carbon dynamics. Such effects could be exacerbated if the low moisture content of affected Calluna contributed to more frequent wildfires, such as may have happened in 2003 (Davies in Scott Citation2003), when the number of ‘outdoor vegetation fires’ in Scotland was over twice the long-term mean (Scottish Executive Citation2005). Winter climate stresses affect a range of species: for example Kullman (Citation1991) highlighted how acute winter stress interacted with longer-term climatic trends, leading to stand-scale mortality in Pinus sylvestris. Extreme climatic events may become more common if increased variability is a pronounced feature of climate change (Schär et al. Citation2004). Understanding the effects of such events on widespread plant communities like Calluna heathland and Pinus sylvestris forest is important to the wider issue of terrestrial carbon-cycle feedbacks, a key uncertainty in climate modelling (Meir et al. Citation2006).
How likely are similar events in future?
The weather conditions presumed to have caused Calluna die-back were exceptional when compared to the long-term record. However, low humidity events may be becoming more common: mean values were similar in the two 10-year periods compared in this study, but variance was eight times higher in the more recent period. Katz and Brown (Citation1992) emphasised that the occurrence of extreme events could be more strongly affected by changes in variance of an underlying climate pattern, than by changes in means. Potential changes in the frequency of die-back events should be considered when modelling responses of European heathlands to climate change (e.g. Coquillard et al. Citation2000). In the longer term, climate predictions for the Cairngorms suggest lower snowfall, lower relative humidity and greater variability in rainfall, which might make Calluna die-back more likely, but also increased winter temperatures and rainfall, which might make it less likely (Hulme et al. Citation2002: medium-high emissions, 2080). Across the European range of Calluna, similar winter warming and, particularly in northern Europe, wetting trends are predicted, with changes in extremes exceeding those of means for some climate variables (Räisänen et al. Citation2004). Such trends could result in heathland losses (Peñuelas and Boada, Citation2003), though there may be scope to ameliorate these by altering heathland management (Britton et al. Citation2001).
Calluna can adapt to a wide range of climatic conditions (Gimingham Citation1972). Jackson et al. (Citation1999) found that the physiological condition of Calluna was similar at two sites with contrasting climates, probably because of differences in morphology. Such adaptation by plants may become harder if climate change is characterised by an increase in variability, rather than gradual changes in mean values. More frequent occurrence of exceptional climatic events, such as that responsible for 2003 Calluna die-back at Abernethy, may increasingly drive change in plant communities.
Acknowledgements
I am grateful to R. Summers, C. Legg, A. Amphlett, and J. Wilson for ideas and observations throughout the study; G. Servant, G. Nisbet, H. Swift, R. Setchfield and I. Hutson for substantial contributions to data collection and collation; S. Bierman of BIOSS for advice on methods of analysis, and N. Cowie, G. M. Davies, D. Dugan, C. Egan, V. Egan, C. McClean, R. Proctor, J. Roberts, S. Taylor, and J. Willi for other contributions to the study. UK Meteorological Office weather data were supplied by the British Atmospheric Data Centre, with assistance from B. Robinson. R. Marrs, F. I. Woodward and three anonymous referees provided useful comments on this manuscript.
References
- Aerts , R and Heil , GW . 1993 . Heathlands: patterns and process in a changing environment , Dordrecht : Kluwer Academic Publishers .
- Aussenac , G . 2000 . Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silviculture . Annales of Forest Science , 57 : 287 – 301 .
- Bannister , P . 1964 . The water relations of certain heath plants in relation to their ecological amplitude. II. Field studies . Journal of Ecology , 52 : 481 – 497 .
- Barclay-Estrup , P . 1971 . The description and interpretation of cyclical processes in a heath community. III. Microclimate in relation to the Calluna cycle . Journal of Ecology , 59 : 143 – 166 .
- Beaumont , D , Dugan , D , Evans , G and Taylor , S . 1995 . “ Deer management and tree regeneration in the RSPB reserve at Abernethy Forest ” . In Our pinewood heritage , Edited by: Aldhous , JR . Farnham (UK) : Forestry Commission, RSPB, and Scottish Natural Heritage .
- Britton , AJ , Pakeman , RJ , Carey , PD and Marrs , RH . 2001 . Impacts of climate, management and nitrogen deposition on the dynamics of lowland heathland . Journal of Vegetation Science , 12 : 797 – 806 .
- Caporn , SJM , Ashenden , TW and Lee , JA . 2000 . The effect of exposure to NO2 and SO2 on frost hardiness in Calluna vulgaris. . Environmental and Experimental Botany , 43 : 111 – 119 .
- Carroll , JA , Caporn , SJM , Cawley , L , Read , DJ and Lee , JA . 1999 . The effect of increased deposition of atmospheric nitrogen on Calluna vulgaris in upland Britain . New Phytologist , 141 : 423 – 431 .
- Chapman , SB . 1970 . The nutrient content of soil and root system of a dry heath ecosystem in the south of England . Journal of Ecology , 58 : 445 – 452 .
- Ph , Ciais , Reichstein , M , Viovy , N , Granier , A , Ogée , J , Allard , V , Aubinet , M , Buchmann , N , Bernhofer , C Carrera , A . 2005 . Europe-wide reduction in primary productivity caused by the heat and drought in 2003 . Nature , 437 : 529 – 533 .
- Coquillard , P , Gueugnot , J , Michalet , R , Carnat , A-P and L'Homme , GL . 2000 . Heathlands functioning in a perspective of climate warming – estimation of parameters, elements for discrete event simulation . Plant Ecology , 149 : 107 – 188 .
- Crawley , MJC . 1993 . GLIM for ecologists , Oxford : Blackwell .
- Davies , GM . 2006 . Fire behaviour and impact on heather moorland. Chapter 2. Variation in moisture content of moorland fuels: impacts on fire behaviour [PhD thesis]. , [Edinburgh (UK)] : University of Edinburgh .
- Foot , JP , Caporn , SJM , Lee , JA and Ashenden , TW . 1997 . Evidence that ozone exposure increases the susceptibility of plants to natural frosting episodes . New Phytologist , 135 : 369 – 374 .
- Gehlhausen , SM , Schwartz , MW and Augspurger , CK . 2000 . Vegetation and microclimate edge effects in two mixed-mesophytic forest fragments . Plant Ecology , 147 : 21 – 35 .
- Gimingham , CH . 1972 . Ecology of heathlands , London : Chapman and Hall .
- Gimingham , CH . 1988 . A re-appraisal of cyclical processes in Calluna heath . Vegetatio , 77 : 61 – 64 .
- Green , FHW . 1953 . “ A remarkable low humidity ” . In Weather Vol. 8 , 182 London
- Green , FHW . 1965 . The incidence of low relative humidity in the British Isles . Meteorological Magazine , 94 : 81 – 88 .
- Haining , RP . 2003 . Spatial data analysis: theory and practice , Cambridge : Cambridge University Press .
- Hancock , M , Egan , S , Summers , R , Cowie , N , Amphlett , A , Rao , S and Hamilton , A . 2005 . The effect of experimental prescribed fire on the establishment of Scots pine Pinus sylvestris seedlings on heather Calluna vulgaris moorland . Forest Ecology and Management , 212 : 199 – 213 .
- Heil , GW and Diemont , WM . 1983 . Raised nutrient levels change heathland into grassland . Vegetatio , 53 : 113 – 120 .
- Hobbs , RJ and Gimingham , CH . 1987 . Vegetation, fire and herbivore interactions on heathland . Advances in Ecological Research , 16 : 87 – 173 .
- Hulme , M , Jenkins , GJ , Lu , X , Mitchell , TD , Jones , RG , Lowe , J , Murphy , JM , Hassell , D , Boorman , P McDonald , R . 2002 . Climate change scenarios for the United Kingdom: the UKCIP02 scientific report , Norwich (UK) : Tyndall Centre for Climate Change Research .
- Jackson , GE , Irvine , J and Grace , J . 1999 . Xylem acoustic emissions and water relations of Calluna vulgaris at two climatological regions of Britain . Plant Ecology , 140 : 3 – 14 .
- Katz , RW and Brown , BG . 1992 . Extreme events in a changing climate: variability is more important than averages . Climatic Change , 21 : 289 – 302 .
- Kramer , PJ . 1942 . Species differences with respect to water absorption at low soil temperatures . American Journal of Botany , 29 : 828 – 832 .
- Kullman , L . 1991 . Cataclysmic response to recent cooling of a natural boreal pine (Pinus sylvestris L.) forest in northern Sweden . New Phytologist , 117 : 351 – 360 .
- Langvall , O and Ottosson-Löfvenius , M . 2002 . Effect of shelterwood density on nocturnal near-ground temperature, frost injury risk and budburst date of Norway spruce . Forest Ecology and Management , 168 : 149 – 161 .
- Littell , RC , Milliken , GA , Stroup , WW and Wolfinger , RD . 1996 . SAS system for mixed models , Cary (NC) : SAS Institute .
- MacDonald , A . 1990 . Heather damage: a guide to types of damage and their causes , Peterborough (UK) : Nature Conservancy Council .
- MacDonald , A , Kirkpatrick , AH , Hester , AJ and Sydes , C . 1995 . Regeneration by natural layering of heather (Calluna vulgaris): frequency and characteristics in upland Britain . Journal of Applied Ecology , 32 : 85 – 99 .
- Marrs , RH . 1986 . The role of catastrophic death of Calluna in heathland dynamics . Vegetatio , 66 : 109 – 115 .
- Marrs , RH . 1988 . Vegetation change on lowland heaths and its relevance for conservation management . Journal of Environmental Management , 26 : 127 – 149 .
- Marrs , RH . 1993 . An assessment of change in Calluna heathlands in Breckland, Eastern England, between 1983 and 1991 . Biological Conservation , 65 : 133 – 139 .
- Meir , P , Cox , P and Grace , J . 2006 . The influence of terrestrial ecosystems on climate . Trends in Ecology and Evolution , 21 : 254 – 260 .
- Miller , GR , Jenkins , D and Watson , A . 1966 . Heather performance and Red Grouse populations. I. Visual estimates of heather performance . Journal of Applied Ecology , 3 : 313 – 326 .
- Moss , R and Miller , GR . 1976 . Production, dieback and grazing of heather (Calluna vulgaris) in relation to numbers of red grouse (Lagopus l. scoticus) and mountain hares (Lepus timidus) in north-east Scotland . Journal of Applied Ecology , 13 : 369 – 377 .
- Nisbet , G and Amphlett , A . 2003 . Abernethy Forest Reserve forest field layer mapping project, July–October 2002 , Abernethy (UK) : RSPB .
- Peñuelas , J and Boada , M . 2003 . A global change-induced biome shift in the Montseny mountains (NE Spain). . Global Change Biology , 9 : 131 – 140 .
- Picozzi , N , Catt , DC and Moss , R . 1992 . Evaluation of capercaillie habitat . Journal of Applied Ecology , 29 : 751 – 762 .
- Pitcairn , CER , Fowler , D and Grace , J . 1995 . Deposition of fixed atmospheric nitrogen and foliar nitrogen content of bryophytes and Calluna vulgaris (L.) Hull . Environmental Pollution , 88 : 193 – 205 .
- Porté , A , Huard , F and Dreyfus , P . 2004 . Microclimate beneath pine plantation, semi-mature pine plantation and mixed broadleaved-pine forest . Agricultural and Forest Meteorology , 126 : 175 – 182 .
- Quinn , GP and Keough , MJ . 2002 . Experimental design and data analysis for biologists , Cambridge : Cambridge University Press .
- Räisäsen , J , Hansson , U , Ullerstig , A , Döscher , R , Graham , LP , Jones , C , Meier , HEM , Samuelson , P and Willén , U . 2004 . European climate in the late twenty-first century: regional simulations of two driving global models and two forcing scenarios . Climate Dynamics , 22 : 13 – 31 .
- Rodwell , JS . 1991 . British plant communities , Cambridge : Cambridge University Press .
- Sæbo , A , Aring , Håland , Skre , O and Mortensen , M . 2001 . Influence of Nitrogen and winter climate stresses on Calluna vulgaris (L.) Hull . Annals of Botany , 88 : 823 – 828 .
- Scandrett , E and Gimingham , CH . 1989 . A model of Calluna population dynamics; the effects of varying seed and vegetative regeneration . Vegetatio , 84 : 143 – 152 .
- Schär , C , Vidale , PL , Lüthi , D , Frei , C , Häberli , C , Liniger , MA and Appenzelier , C . 2004 . The role of increasing temperature variability in European summer heatwaves . Nature , 427 : 332 – 336 .
- Scott, K. 2003. Britain ablaze. Guardian newspaper, April 21. Available from www.guardian.co.uk
- Scottish Executive . 2005 . “ Statistical bulletin, criminal justice series ” . In Fire Statistics Scotland 2003 , Edinburgh (UK) : Scottish Executive .
- Steven , HM and Carlisle , A . 1959 . The native pinewoods of Scotland , Edinburgh (UK) : Oliver and Boyd .
- Stevenson , AC , Rhodes , AN , Kirkpatrick , AH and MacDonald , AJ . The determination of fire histories and an assessment of their effects on moorland soils and vegetation . Scottish Natural Heritage Research Survey and Monitoring Report no. 16 . 1996 . Edinburgh (UK): SNH
- Summers , RW , Mavor , R , MacLennan , AM and Rebecca , GW . 1999 . The structure of ancient native pinewoods and other woodland in the Highlands of Scotland . Forest Ecology & Management , 119 : 231 – 245 .
- Summers , RW , Proctor , R , Thornton , M and Avey , G . 2004 . Habitat selection and diet of the Capercaillie Tetrao urogallus in Abernethy Forest, Strathspey, Scotland . Bird Study 51 , : 58 – 68 .
- Sykes , JM , Horrill , AD and Mountford , MD . 1983 . Use of visual cover assessments as quantitative estimators of some British woodland flora . Journal of Ecology , 71 : 437 – 450 .
- Taylor , S . 2001 . Management plan, Abernethy Forest Reserve , Abernethy (UK) : RSPB .
- Thompson , DBA , MacDonald , AJ , Marsden , JH and Galbraith , CA . 1995 . Upland heather moorland in Great Britain: a review of international importance, vegetation change and some objectives for nature conservation . Biological Conservation , 71 : 163 – 178 .
- Tranquillini , W . 1982 . “ Frost drought and its ecological significance ” . In Encyclopedia of Plant Physiology , Edited by: Lange , OL , Nobel , PS Osmond , CB . Vol. 12B , 379 – 400 . Berlin : Springer-Verlag .
- Tyree , MT and Sperry , JS . 1988 . Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model . Plant Physiology , 88 : 574 – 580 .
- Watson , A , Miller , GR and Green , FWH . 1966 . Winter browning of heather Calluna vulgaris and other moorland plants . Transactions of the Botanical Society of Edinburgh , 40 : 195 – 203 .
- Watt , AS . 1947 . Pattern and process in plant community . Journal of Ecology , 35 : 1 – 22 .
- Welham , SJ and Thompson , R . 1997 . Likelihood ratio tests for fixed model terms using residual maximum likelihood . Journal of the Royal Statistical Society (B) , 59 : 701 – 714 .
- Woodin , SJ and Farmer , AM . 1993 . Impacts of sulphur and nitrogen deposition on sites and species of nature conservation importance in Great Britain . Biological Conservation , 63 : 23 – 30 .