Abstract
Background: The Rhynie chert, Aberdeenshire, encapsulates the ecophysiological and anatomical information on all components, including plants, animals and micro-organisms, of an early terrestrial ecosystem as it existed some 400 million years ago (Early Devonian), as preserved by siliceous waters emanating from a hot spring system.
Aims: This paper concentrates on the higher plants (tracheophytes) and brings together information on the habitats of the plants and the environmental pressures that they endured to answer issues relating to their endemism and their ecophysiology.
Methods & Results: The synthesis includes detailed information on the palaeo-environments recorded in the chert, plus anatomical and autecological data from the plants themselves, and makes comparisons with the abiotic and biotic data obtained from an extant analogue, the alkali–chloride geothermal systems at Yellowstone National Park. Particular attention is paid to the physiological basis and evolution of osmotic and chemical tolerance of halophytes, the dominant colonisers of Yellowstone's wetlands and, to a lesser extent, metallophytes.
Conclusions: The Rhynie plants colonised wetlands at the low temperature fringes of a hot spring system and were versatile, but physiologically highly specialised, capable of withstanding osmotic and chemical stresses in a dynamic environment, but were probably out-competed by mesophytic vegetation elsewhere.
Introduction
Hot springs active at Rhynie, Aberdeenshire, Scotland in the Early Devonian preserved the oldest and most complete example of an early terrestrial ecosystem (e.g. Trewin and Rice Citation2004). The ensuing siliceous rock, the Rhynie chert, provides a key palaeontological data point that underpins much of our understanding of the evolution of interactions between micro-organisms, plants and animals. The diverse, early land plant flora includes the earliest well-documented plant of lycophyte affinity (Asteroxylon), rhyniophytes (e.g. Rhynia), zosterophylls (e.g. Trichopherophyton) and those of less certain relationships (e.g. Nothia, Horneophyton, Aglaophyton). Plants were preserved in great numbers, anatomically in three-dimensions and to the cellular level, as silica-rich geothermal fluids from hot springs permeated plant structures and cells. Frequently plants were preserved in situ, often still in growth position. Allochthonous plant material within chert beds also underwent early silicification following extremely short distance transport events leading to exceptional preservation.
This phenomenal preservation has allowed detailed reconstruction of the morphology and anatomy of the sporophytic plants, insights into the complete life cycles from spores to gametes, as well as fungal and bacterial interactions in life and death. The plants allow many inferences on the palaeo-ecophysiology of early land plants plus the broader evolutionary patterns in basal tracheophytes. The chert contains the earliest body-fossil evidence of certain groups of insects (e.g. Engel and Grimaldi Citation2004; Ross and York Citation2004), continental aquatic crustaceans (e.g. Anderson et al. Citation2004), algae (e.g. Dotzler et al. Citation2007), arachnids (e.g. Dunlop et al. Citation2004), lichens (e.g. Taylor et al. Citation1995) and fungi (e.g. Taylor et al. Citation2004). Interactions between the various elements of the biota, including early evidence of parasitism (chytrid fungi on aquatic algae (e.g. Taylor et al. Citation1992)), mutualism (mycorrhizal fungi within plants (e.g. Remy et al. Citation1994)), symbioses (lichen (Taylor et al. Citation1995)), herbivory (the mite Palaeotydeus devonicus Dubinin and coprolites (Habgood Citation2004)), saprotrophism (chytrid fungi (e.g. Taylor et al. Citation2004)), detritivory (collembolans, myriapods and coprolites (Habgood Citation2004)) and predation (trigonotarbid arachnids and centipedes (e.g. Anderson and Trewin Citation2003, Fayers et al. Citation2004)), provide a snapshot picture of a relatively complex and, in many respects, modern-looking trophic-web (Habgood et al. Citation2004).
However, just how representative the higher plants were of terrestrial vegetation some 400 million years ago remains conjectural. The hot spring environmental and taphonomic setting of the Rhynie chert is unique within this period of geological time, and rare throughout the rock record. The value of preservation of biota by hot springs is a double-edged sword. It is one of a limited number of environments where organisms are preserved in situ by permineralisation and thus may be compared with coal balls that reflect vegetation composition of peat in a mire. In the case of the Rhynie plants, there is also anchorage to the substrate by which their ‘rooting systems’ interacted in life and in an environment where palaeontological, sedimentological, mineralogical and geochemical features of the rocks allows pinpointing of physical and chemical parameters of the environment. However, as we illustrate in this paper, the hot spring environment and the habitats it provides have a suite of features, particularly relating to water and chemical stress, that are combined in few if any other terrestrial settings. It might thus be considered that the ecosystem preserved at Rhynie (and also those in younger fossil hot spring deposits) was atypical and individual taxa endemic and specialised. An alternative scenario is that whilst the environment was special, its inhabitants were more widespread and possessed adaptations to other more common habitats and this, by way of pre-adaptation, allowed colonisation (Wellman Citation2004, Citation2006).
It is the aim of this synthesis to address these various hypotheses by assembling information on the geological, geochemical and botanical data on the Rhynie chert together with that on environments and plants at Yellowstone National Park, USA as an extant hot spring system analogue. The major limitation, that the Rhynie plants, with one exception, have no extant representatives, is counterbalanced by the fact that the physical, chemical and hydrological parameters that influence the distribution of life in active geothermal areas have probably remained unchanged despite the elapse of 400 million years. Thus the stresses endured by extant plants and the adaptations/tolerances required provide an excellent analogue for the Rhynie plants. However, until recently comparatively little attention has been paid to the variety of microhabitats available for colonisation in the geothermal system (but see Trewin et al. Citation2003; Fayers and Trewin Citation2004; Trewin and Wilson Citation2004), because of the lack of outcrop in the Rhynie area and information on their spatial distribution. Some studies (e.g. Remy and Hass Citation1996; Kerp et al. Citation2001) have provided very detailed associations of organisms within individual chert beds/blocks and touched on the ecophysiology of plants. To date, discussion has been limited to preferences of substrate, tolerances of dryland versus wetland conditions with discussions on the key attributes of the Rhynie plants (cuticle, stomata, conducting tissues and ‘rooting systems’) in terms of terrestrial environments (but see Edwards et al. Citation1998).
A picture is emerging that the Rhynie environment was much wetter than traditionally viewed, being prone to flooding both from thermal springs and the local river system (e.g. Channing Citation2001, Citation2003; Trewin et al. Citation2003; Fayers and Trewin Citation2004; Trewin and Wilson Citation2004; Channing and Edwards Citation2009). Given this new view of the Rhynie depositional and preservational environment there is a crucial need to re-evaluate the ecophysiological tolerances and stresses of the inhabitants.
We base the discussion in this paper on observations of Yellowstone National Park, but the observations could also apply to other active geothermal areas we have investigated e.g. the Taupo Volcanic Zone, New Zealand; El Tatio, Atacama Desert, Chile, and Haukadalur, Iceland. Yellowstone is selected as a modern analogue for the Rhynie deposit for a number of reasons. It has the world's largest concentration of active hot springs and geysers. The geothermal resources of the Park are protected by law and are located within wilderness that is not impacted heavily by human intervention. Many of the Park's geothermal features are typified by alkali–chloride thermal waters (expanded below) and deposit silica sinter. They are thus suitable geochemical analogues for springs active at Rhynie. The hot springs of Yellowstone show great variety of form (e.g. Bryan Citation1995) and thus offer a vast array of hot spring sub-environments, plus plant habitats and preservational environments. The situation at Yellowstone contrasts with thermal areas of New Zealand and Iceland where water extraction for geothermal energy impacts many hot spring areas. This process reduces the local water table and rising alkali–chloride waters boil and turn to steam in the sub-surface. This process alters water chemistry to acid–sulphate compositions (White et al. Citation1988). Most New Zealand and Icelandic geothermal areas are thus dominated by steam heated thermal ground and acidic springs, although exceptions occur. Yellowstone has limitations climatically compared with low latitude Rhynie (located at the time of chert formation at c. 28 °S) as it is located at c. 45 °N and most thermal areas occur on the Yellowstone Plateau at an average elevation of c. 2400 m (e.g. Channing and Butler Citation2007). This means that the area is typified by short summers and long winters. Summer air temperatures may reach in excess of 30 °C but average maximum temperatures are nearer 22–23 °C (Channing and Butler Citation2007). Daily maximum air temperatures generally remain below freezing from November to March each year and in winter, snow pack covers much of the area (Channing and Butler Citation2007). With the exception of New Zealand, the world's other major geothermal areas suffer similarly (Channing and Butler Citation2007). Thus cold winters occur on Iceland at high latitude where they are accompanied each year by a long period of total darkness. At El Tatio, situated on a volcano at 4300 m in the Atacama Desert in the Chilean Andes freezing conditions occur overnight and high solar radiation characterises daylight hours. The African Rift area (e.g. Lake Bogoria, Kenya) is equatorial and not subjected to freezing processes. However, many hot springs deposit calcium carbonate (travertine) rather than silica sinter, and the region is so heavily impacted by evaporation that evaporite minerals, zeolites and magadiite (which do not occur at Rhynie) are associated with silica deposition (e.g. Renaut and Tiercelin Citation1994).
The geothermal system and habitats at Yellowstone
General setting
The major geothermal basins of Yellowstone occur within a large volcanic caldera and associated fault systems created c. 600,000 years ago by the last major eruption of the Yellowstone volcano. Geothermal activity dominantly occurs at an elevation of 2400 m on the formely glaciated Yellowstone Plateau (e.g. White et al. Citation1988; Bryan Citation1995). This is surrounded to the north, east and south by the Rocky Mountains which reach elevations of over 4000 m. Geothermal basins are variously situated in the broad alluvial valleys containing the Yellowstone, Gibbon, Firehole and Madison rivers (e.g. the Norris, Upper, Lower and Midway basins), on the shores of lakes (e.g. West Thumb Basin at Yellowstone Lake) or in deeper and narrower hydrothermal valleys (e.g. Heart Lake Basin (Shovic Citation1996)). Current geothermal activity and the majority of ‘sub-fossil’ hot spring deposits of the Park have formed since the end of the last ice age (c. 10,000 years ago), though older sinter and travertine deposits document thermal activity over the last c. 600,000 years (White et al. Citation1988; Hinman and Walter Citation2005). Sedimentary and geomorphic influences observed in and around the current thermal areas include reworking of volcanic products, glacial/periglacial, alluvial, fluvial and lacustrine processes. The account below provides a snapshot of the deposition and vegetation in a temporally dynamic setting.
In active hot spring areas of Yellowstone National Park, a suite of hot spring sub-environments provides habitats for communities of higher plants (e.g. Despain Citation1990; Channing Citation2001, 2003; Stout and Al-Niemi Citation2002; Trewin et al. Citation2003; Channing et al. Citation2004; Channing and Edwards Citation2009). These are set against a number of often overlapping and cyclically fluctuating physical gradients i.e. from wet to dry and from hot to cold. Other habitat boundaries are much more ‘hard edged’ i.e. high vs. low pH, dry vs. wet, or heated vs. unheated water or substrates.
Here we describe a typical set of hot spring sub-environments that develop around point sources of geothermal fluid discharge. For a review of a typical hot spring basin including background geological processes and an account of the diversity of thermal features and their morphological and chemical/physical characteristics see White et al. (Citation1988).
Vent pools
Water temperatures associated with sinter-depositing spring vent pools usually exceed the upper temperature limit for eukaryotic organisms and fluids are habitat only for archaea and extremophile bacteria (e.g. Brock Citation1994). Distinctive silica precipitation fabrics develop in vent pools including well-documented botryoidal, pustular, digitate, shrubby, columnar, spicular, domical and lily-pad stromatolites, pitted sinter and silica oncoids and pisoids (e.g. Cady and Farmer Citation1996; Jones and Renaut Citation2003a; Lowe and Braunstein Citation2003). Cooler vent pool conditions are encountered when pools undergo periods of dormancy (e.g. Trewin et al. Citation2003) and/or when they come under the influence of ‘normal’ water sources e.g. flooding during storms, or by local streams and rivers, or by influxes of water from shallow non-thermal groundwater (e.g. White et al. Citation1988; Vitale et al. Citation2008). During these periods pools may form habitats for communities of aquatic and semi-aquatic plants and animals (Trewin et al. Citation2003). It should be noted that additions of meteoric non-thermal waters and mixing are likely to alter water composition (to a dilute acid composition (White et al. Citation1988)) and reduce dissolved Si concentrations to below saturation precluding sinter formation and permineralisation. A resumption of thermal activity resulting in influxes of alkali–chloride thermal water will kill any low-temperature biota – possibly leading to preservation. Such events would be recorded in the rock record by mineral precipitation fabrics as outlined above that are distinctive from those formed in low-temperature environments.
Sinter aprons
Water erupted from the vent pool is rich in dissolved silica, which, as it cools, precipitates. This forms mound shaped aprons of opaline sinter adjacent to the vent margin. These accrete vertically and laterally over time and may reach many hundreds of metres diameter (e.g. Walter et al. Citation1996). Depending on eruption style and frequency, apron morphology may develop surface sub-environments. Where apron surfaces have a shallow dip and where sheet flow eruptions are common, sets of shallow apron terraces (from a few millimetres to tens of centimetres high) form (e.g. Walter et al. Citation1996; Guidry and Chafetz Citation2003). Water, in this area of the apron, cools to temperatures which allow colonisation by diverse mat-forming bacterial communities (e.g. Cady and Farmer Citation1996; Walter et al. Citation1996; Jones et al. Citation1998a). When these interact with precipitating silica they form strongly laminated sinter deposits, which alternate with shallow pool features containing shrubby and/or domical stromatolites and oncoids created by phototactic growth of microbes (e.g. Jones et al. Citation1998a). Growth and silicification of microbial colonies at terrace margins forms dams that create rimstone pools. Where water erupts from isolated points at the margins of vents, run-off streams and channels develop. These often have raised lateral margins (also created by silicification of microbial colonies) that prevent water flowing to other areas of the apron. Within run-off streams and in areas of constant sheet-flow, filamentous microbes entrained in the flowing water are silicified to create distinctive streamer fabrics that are indicators of local flow direction (e.g. White et al. Citation1989). The biosedimentary features encountered on the surfaces of aprons commonly enter the fossil/rock records and vertical sections through fossil apron deposits typically have distinctive laminated to bedded internal structure with common vertically orientated shrubby microbial fabrics (e.g. White et al. Citation1989; Walter et al. Citation1996; Guido and Campbell Citation2009).
While some hot springs erupt constantly (from our own observations of Big Blue Hot Spring, Yellowstone (Channing and Edwards Citation2009)) over periods of decades, most (and nearly all geysers) erupt cyclically with intervals between eruptions spanning from minutes (e.g. Channing and Edwards Citation2004) to many years. Sinter aprons undergo periodic flooding and drying and, depending on the life conditions of the local vegetation (e.g. wetland vs. dryland), disturbance events related to water availability are a systematic part of life in these areas.
Dry sinter aprons
Dry areas of sinter aprons offer a poor substrate for higher plant growth (e.g. Channing et al. Citation2007). They are essentially a hard, but relatively porous, monomineralic rock that proves difficult for roots to penetrate (Channing Citation2001). As the only organic matter present in these areas comprises low volumes of microbial material, they have poor water retention properties and low nutrient availability. In Yellowstone, colonisation of abandoned areas of aprons is a slow process, bryophytes and lichens are early colonisers (Despain Citation1990; Powell et al. Citation2000a) and alkali- and salinity-tolerant bunch grasses are not uncommon (e.g. Puccinellia nuttalliana (JA Schultes) AS Hitchc.). However, aprons generally remain essentially barren on the scale of decades rather than years (e.g. Channing Citation2001). Similar features are observed around New Zealand hot spring areas where plants, including trees, eventually become established on old, weathered and eroded apron surfaces (Jones and Renaut Citation2003b).
Wet sinter aprons
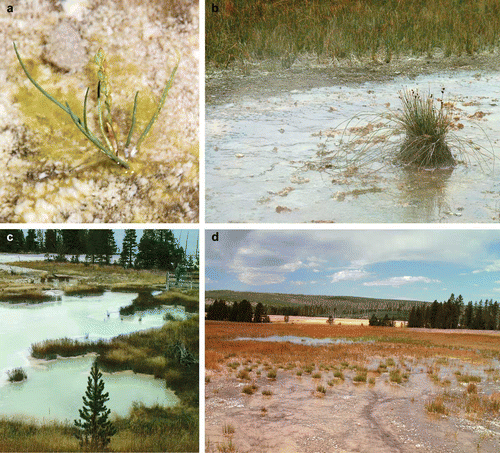
These are also hostile environments for higher plant growth. In this sub-environment, high water temperatures in areas proximal to vents prevent colonisation (Channing and Edwards Citation2009). Below c. 40 °C higher plants may be able to tolerate temperature but are again faced with the problem of rooting to a hard substrate (Channing and Edwards Citation2009). One species that overcomes this problem is Triglochin maritimum L. which has been observed to find attachment via microbial mats () (Channing Citation2003). Other plants present in high temperature areas of wet aprons grow (as on dry areas) on islands of bison dung (). In this setting they produce stolons and propagate with varied success (Channing and Edwards Citation2009). High geothermal gradients are another feature of hot spring basins. Sinter aprons may lie only centimetres above the local geothermal water table and are heated from below, substrate temperatures in excess of 40 °C are not uncommon. This adds to the factors that prevent higher plant colonisation of aprons and can also exacerbate plant decay by raising temperature and thus increasing microbial activity.
Figure 1. (a) Stunted Triglochin maritimum growing on partially silicified microbial mat on the wet sinter apron surface of the alkali–chloride hot spring, Grand Prismatic, Midway Geyser Basin. (b) Eleocharis rostellata and Juncus tweedyi growing on a bison dung island surrounded by steam drifting from the vent pool of the alkali–chloride hot spring, Big Blue, Elk Park, Norris Geyser Basin. Stolons from E. rostellata radiate onto the sinter surface where the shallow film of water is in excess of 50 °C. Stolons in this environment fail to develop clones, whereas plants growing below c. 45 °C may. Areas of plant growth beyond the apron are beyond the influence of high temperature hot spring water. (c) Acid–sulphate pool at West Thumb Geyser Basin. Stands of Juncus tweedyi grow on islands within the pool which, as it precipitates clays rather than silica has a low potential for preserving plant fossils. Note the lack of sinter apron development cf. alkali–chloride hot springs. (d) Looking down-apron towards the geothermally influenced wetland of Big Blue Hot Spring. The bare surface in the foreground is the margin of the sinter apron, the scattered green plants beyond this are T. maritimum. E. rostellata forms a denser cover to the wetland beyond. The standing water pool in the centre of the image contains abundant filamentous chlorophyte algae. (e) T. maritimum growing within a run-off stream at Big Blue Hot Spring. Small microbially mediated sinter rims form on brecciated sinter at the stream margin. (f) Geothermally influenced wetland at Big Blue. The substrate in the bottom right of the image comprises silica sinter, to the top and left the wetland surface is covered in an organic ‘soup’ comprising clumps of microbes and algae plus flocculated silica particles. Arching stolons connect adjacent clones of E. rostellata. A litter comprising fallen and partially silicified aerial stems (some still attached to living plants) is visible in the bottom right.

Thermal ground and acid sulphate hot springs
In other areas where volcanic or sedimentary deposits are topographically higher than the geothermal water table, boiling of water in the subsurface creates steam that condenses to create acidic soil conditions and acidic hot spring pools (e.g. White et al. Citation1988). These are recognised by the sparseness or absence of vegetation, the reddish or whitish colour of the soil, and the frequent occurrence of patches of sulphur crystals encrusting rocks and soil particles (e.g. White et al. Citation1988). In these areas ground temperatures may exceed 70 °C and deposition of silica sinter is limited or absent. The silica-dominated mineral assemblage typical of alkali–chloride areas is replaced by deposition of kaolinitic clays, native sulphur and potassium sulphate minerals such as jarosite and alunite (e.g. Jones et al. Citation1999, Citation2000). Wet acidic environments (∼30 °C, pH 2.5–2.8) are typically colonised by species of Juncus (), including the endemic J. tweedyi Rydb., Tweedy's rush, and Eleocharis flavescens var. thermalis (Rydb.) Cronq., warm springs spikerush, (Channing Citation2001, 2003). Drier acid areas and thermal ground are habitat for grasses, including Agrostis rossiae Vasey, A. scabra, Dichanthelium lanuginosum (Elliot) Gould, Panicum capillare L. (e.g. Stout and Al-Niemi Citation2002). Recorded root temperatures of these grasses have been in excess of 50 °C. Thermal tolerance appears to be provided by the plants’ association with VAM fungi (Stout and Al-Niemi Citation2002). Where sinter deposition has been recorded around acid thermal features, there is some evidence that fungi rather than cyanobacteria dominate the microbial community (Jones et al. Citation1999, Citation2000).
Geothermal wetlands
The colonisation and taphonomic trends evident in areas proximal to vent pools are reversed at the periphery of many sinter aprons and in low-lying areas within and at the fringes of the hot spring basins of Yellowstone (Channing and Edwards Citation2009). Here, where water temperatures drop below c. 35 °C, extensive tracts of thermally influenced wetland develop that are habitats for large numbers of emergent aquatic plants (). Compared with adjacent mesic dryland settings of Yellowstone these areas are typified by a very low diversity (Channing and Edwards Citation2009). Two plants are dominant. Triglochin maritimum, seaside arrowgrass, is common in areas of deep (c. 10–20 cm) standing to sluggishly flowing water and on drying areas on the interfluves of run-off streams entering wetland areas (). On interfluves it may be joined by the alkali- and salinity-tolerant grasses found within apron areas. In shallower areas of wetland Eleocharis rostellata (Torr.) Torr., beaked spikerush, () forms monotypic carpets and ‘quaking-mats’ (Channing and Edwards Citation2009).
Mesic environments
Approximately 80% of Yellowstone National Park is forested. On the Yellowstone Plateau, the location of the major hot spring basins, 80% of forested landscape is covered by lodgepole pine, Pinus contorta Dougl. ex. Loud. Approximately 15% of the Park is covered with grassland. Elevation differences within the Park produce a range of plant communities, from semi-arid steppe to alpine tundra. More than 1700 species of vascular plants have been recorded. Two of these species, Ross's bentgrass, Agrostis rossiae, and Yellowstone sand verbena, Abronia ammophila Green, are endemic (United Nations Environment Program Report Citation2008).
Sharp boundaries between mesic and stressed (aquatic and/or geothermally influenced) vegetation types are evident in the Park (Channing and Edwards Citation2009). Lodgepole pine forest surrounding geothermal areas is adapted to growth on low-nutrient, monomineralic soils. It is a coloniser of abandoned sinter aprons (Channing Citation2001). However, the species is intolerant of high temperatures and is commonly killed by increases in ground temperature and by subsurface gas influxes (). It is also intolerant of flooding, thus resumption of hot spring activity leads to drowning of the trees (). The progradation of sinter aprons and geothermally influenced wetlands across mesic environments causes drowning of broad areas of lodgepole forest and meadows (parkland). When inundation of forest areas occurs, drowned trees may remain standing and bases of trunks and root systems may become silicified by permeating silica. However, geothermal wetland environments quickly develop between stumps ().
Figure 2. (a) Acid–sulphate thermal area North of Norris Geyser Basin. A linear zone of brown, dying conifers and billowing steam marks the location of newly created fumarole-vents. (b) Margin of thermal wetland at Big Blue Hot Spring that has encroached into former forest area drowning and killing Pinus contorta. Stands of conifers beyond the current wetland fringe occur above the local water table. (c) Wetland conditions developed around the base of a still standing, but dead, Pinus stump. Adjacent is a fallen and decaying branch or trunk. The white base of the standing trunk is evidence of wicking, or capillary action drawing silica-rich water into the wood structure partially permineralising it. (d) Sinter apron surface in the Back Basin, Norris Geyser Basin. Alkalinity- and salinity-tolerant grasses form domes with a crude radial structure where silica-rich waters have invaded and encrusted plants growing on the formerly dry apron surface. Plants on the right hand margin of the image are maintaining growth despite partial encrustation and the presence of shallow films of brackish thermal water. (e) Stunted (5–10 cm high) Eleocharis rostellata with fallen and silica encrusted aerial stems on the apron of Big Blue Hot Spring. (f) Block collected from a Holocene sub-fossil sinter outcrop adjacent to the Firehole River and Lower Geyser Basin. Vertically orientated basal stems occur below a top surface crowded with silicified fragments of aerial stems of E. rostellata.


Relationship between habitat and preservation at Yellowstone
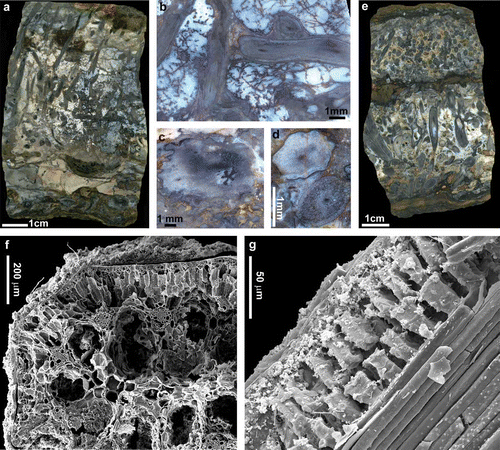
The Rhynie chert () is world-renowned for the excellent cellular preservation that is seen in most published accounts of the plants (e.g. Kidston and Lang Citation1917a,Citationb, Citation1920a,Citationb, Citation1921a,Citationb; Lyon and Edwards Citation1991; Remy and Hass Citation1991a–d, Citation1996; Powell et al. Citation2000b; Kerp et al. Citation2001, Citation2004). It could be argued that there has been a bias to the selective use of fossils with good preservation in taxonomic and anatomical investigations of the Rhynie plants. In reality a spectrum of preservation states are present (e.g. Trewin Citation1996). The exceptional preservation of the Rhynie plants has been used to imply extremely rapid preservation of plants in a more normally drying terrestrial setting by short-lived and ‘accidental’ or chance flooding events (e.g. Trewin Citation1994; Powell et al. Citation2000a). More contrived explanations for the exceptional preservation have suggested that the Rhynie hot spring waters contained more dissolved silica than those of active springs (e.g. Trewin et al. Citation2003). Whilst it is true that dissolved silica concentration varies from hot spring to hot spring and that concentration in a single spring may fluctuate markedly over time, silica concentration and other physical and chemical parameters are remarkably consistent for active sinter depositing features (e.g. White et al. Citation1988; Ball et al. Citation1998; Jones et al. Citation1998b, 2001; Channing Citation2001; Jones and Renaut Citation2003a; Channing and Edwards Citation2004, Citation2009, Channing et al. Citation2004; McCleskey et al. Citation2005, Owen et al. Citation2008). From our taphonomic experiments conducted in Yellowstone (Channing and Edwards Citation2004, Citation2009) we have demonstrated that the time period required for tissue stabilisation leading to silicification in a range of hot spring settings is in the order of several months rather than being a ‘flash’ process (e.g. cf. Powell et al. Citation2000a; Trewin et al. Citation2003; Kerp et al. Citation2004). Critically in relation to the Rhynie plants, we have observed that drying of an environment prior to tissue stabilisation results in low-quality cell and tissue preservation or total taphonomic removal. Thus for exceptional preservation (particularly of parenchymatous tissues, which dominate the construction of the Rhynie plants) to take place, the onset of silicification must precede or accompany plant death, and immersion must be protracted and sustained.
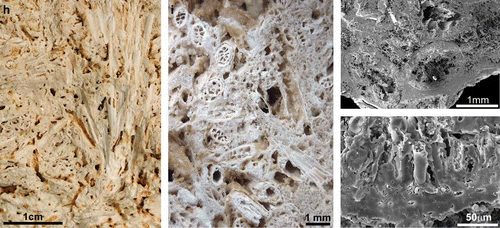
Figure 3. (a–e) Rhynie chert plants and preservation features. (a) Block of Rhynie chert with horizontal and vertical axes of Rhynia surrounded by a meshwork of microbial filaments. (b) Open space between axes and filaments of the meshwork is filled with later phases of white to clear chert. At the base of the block a silicified clastic horizon contains degraded and poorly preserved axes of Asteroxylon (c). (d) Two adjacent axes of Rhynia from the upper chert horizon illustrated in (e). Cellular preservation typifies the lower axis whilst the upper axis lacks discernable cell structures. (e) Composite chert bed with lower, central and upper clastic horizons. Basal chert lens contains horizontal and vertical axes of Aglaophyton and less common horizontal axes of Rhynia. The upper lens contains dominantly horizontal axes of Rhynia. (f) Scanning electron microscope (SEM) image of a transverse fracture section through the stem of an Eleocharis sub-fossil. Opal-A containing microbes encrusts the stem surface. Opal-A films and particle aggregates line inner and outer cell-wall surfaces within the stem fixing them within a mineral matrix. (g) SEM image of oblique longitudinal fracture section through the outer cortex and fibre sheath surrounding the vascular bundle of a sub-fossil Eleocharis. The parenchymatous cells of the outer cortex exhibit three-dimensional preservation typical of well-preserved Rhynie plants. (h–k) Pleistocene to Early Holocene geothermal wetland specimens from Yellowstone. (h) Fracture section reveals vertical and horizontal stems of Eleocharis. Porosity in the matrix and plant stems is yet to be filled by silica. This process, which accompanies burial and the diagenetic transformations that convert opal-A to chert, has been halted by the cessation of thermal activity in the area of plant preservation. (i) Numerous horizontal stems of Eleocharis with well-preserved anatomy. (j) SEM image of Eleocharis stems in wetland matrix. Stems are encrusted by opal-A and microbial meshworks span between adjacent stems. (k) Transverse fracture section through part of the stem of Eleocharis. Centre and top of image reveal the outer cortex where parenchymatous cells are preserved three dimensionally and intercellular space is almost completely filled with opal-A, whilst intracellular space is partially filled with opal-A aggregates but remains open. Lower margin of image is the site of the epidermis and bundles of fibres. Almost all inter- and intracellular space in these tissues is occluded.
Many of the environments listed above (vent pools, dry sinter aprons, thermal ground and mesic settings) have very low potential for plant preservation. The difficulties inherent in the colonisation of those influenced by thermal activity mean that they generally support only a sparse low-diversity vegetation. This low initial abundance of living material is further exacerbated by low preservation potentials. In the drying environments of aprons, the likelihood of decay is dominant over preservation. In acid thermal areas characterised by thermal ground and acid–sulphate pools, the dominant mineral assemblage is not conducive to sinter–apron formation or the process of permineralisation. The strong partitioning of mesic and hot spring influenced vegetation means that the vast majority of mesophytes are unlikely to come into contact with fluids capable of preserving them.
Taphonomy experiments in Yellowstone vent pools illustrate that they are a good environment for three-dimensional permineralisation of plants (Channing and Edwards Citation2004). However, they are in general of limited lateral extent and the mechanisms by which plants may be deposited in them convoluted. Certainly in situ and in life habit preservation is unlikely in such settings.
In our observations of sinter aprons in Yellowstone, higher plant megafossils are limited to the disseminules of nearby forest vegetation (e.g. fallen and transported leaves of angiosperm trees, needles and cones of conifers and blown fragments of grasses. Fallen and decaying tree trunks and branches may litter the surface of apron margins and, due to the relatively high stability of lignin these may be incorporated into sinter deposits. In situ preservation is generally of restricted occurrence and limited to the preservation of prostrate (i.e. flooded and killed) grasses (). It should be noted that in Yellowstone the grass species commonly colonising dry and wet sinter aprons are generally alkalinity- and salinity-tolerant.
In apron settings, encrustation of the surface of plant remains by silica is dominant over permineralisation of tissues. Decay prevails over cellular preservation which often falls short of the excellent three-dimensional preservation of parenchymatous tissues seen in the Rhynie chert. We have observed that similar taphonomic features are typical of fossil sinter apron deposits ranging in age from the Pleistocene of the Western USA and New Zealand, to the Jurassic of Patagonia and to the only other Palaeozoic hot spring deposits yet discovered in the Drummond Basin, Queensland, Australia (and see e.g. White et al. Citation1989; Walter et al. Citation1996, Citation1998).
Taphonomic potential of plants increases dramatically in geothermal wetland environments. Water flowing from vents and across aprons has little time in which precipitation may take place, thus water entering areas of plant growth is often still saturated with respect to opal-A. Plants thus spend their life in water, which, on their death, can rapidly preserve their cells and tissues (Channing and Edwards Citation2009). The annual growth of Eleocharis rostellata means that at the end of each short growing season its culms collapse to the wetland surface and are immersed in silicifying fluids (Channing and Edwards Citation2009). The floor of wetland areas is typified by a litter of partially decayed but three-dimensionally preserved silicified stems (, ,). Rapid accretion of wetland sediments allied with early silicification preserves many plants, particularly basal stems and their shallow roots in situ and in growth position (). Geothermal wetlands are one of the few active settings we have observed where this most striking taphonomic feature of the Rhynie chert is replicated. Further, observation of exposures of wetland surfaces with well-preserved and in situ sub-fossil plant material and vertical sections through sub-fossil wetland profiles (, ,), also with abundant, in situ and well-preserved root systems and basal stems, lead us to hypothesise that such hot spring sub-environments were important for preservation at Rhynie. Preservation is favoured in geothermal wetlands because it is a setting where plants grow and die in intimate association with silica-rich fluids and are subsequently systematically, rather than fortuitously, preserved. The environment is singly the most important plant preservation environment at Yellowstone, both in terms of area and numbers of plants preserved, and also in terms of quality of preservation. It is also the only environment where in situ in growth position preservation is the norm not the exception. Our conclusion, having made observations of active hot spring areas worldwide, is that wetland conditions are a requirement for ‘Rhynie-style preservation’ and thus most plants preserved around hot springs live in areas with a high water table, on saturated substrates and are flooding tolerant.
From facies to palaeo-environmental reconstructions – the Rhynie geothermal landscape
General setting
Trenching and drilling combined with sedimentological observations on coeval clastic outcrops and chert float blocks in the Rhynie area, have shown stratigraphic and lithological aspects of the Rhynie deposit. These form the basis for our understanding of the general setting of the hot spring complex and of the distribution and extent of hot spring influence within the palaeo-landscape.
Cores through the Rhynie deposit show a stacked sequence of more than 45 in situ chert beds (Powell et al. Citation2000a). These occur interbedded within a much thicker (2–400 m; Rice and Ashcroft Citation2004) sequence of grey–green shales and buff–blue sandstones (Powell et al. Citation2000a). Deposition of the Rhynie sedimentary sequence was rapid. The entire thickness of preserved strata occurs within a single spore assemblage biozone (Wellman Citation2006). The general palaeo-environment at Rhynie during the deposition of the cherts was an alluvial plain with an axial river system that drained north to what is now the Moray Firth (Trewin and Rice Citation1992; Powell et al. Citation2000a). Current interpretations of the structure of the basin view it as a possible pull-apart structure related to strike-slip fault movement (Rice and Ashcroft Citation2004). Sediments within the basin were dominated by the products of overbank river deposition of mud and silt onto the local floodplain and into small ephemeral floodplain lakes (Fayers and Trewin Citation2004). River flooding events are indicated by various interbedded associations of sands representing levee banks and crevasse bank–breach deposits. Coarsening upward sequences of sand-rich lithofacies which occur interbedded within the grey–green lacustrine shales appear to represent small floodplain-lake deltas, possibly being fed by crevasse channels (Fayers and Trewin Citation2004).
Drying conditions during deposition of the clastic sequence are indicated by desiccation cracks on the surfaces of some sand- and mud-rich beds (e.g. Powell Citation2000a). However, desiccation features are often filled by sand deposited by a subsequent flooding event rather than removed by erosion perhaps indicating a high flood frequency. Occasional vertisol soil development and carbonate cements and calcrete nodules are observed in the Rhynie cores. These indicators of a generally semi-arid climate occur in the Upper Shales and Pre-Lava Sandstones (Rice et al. Citation2002) above and below the chert-bearing strata. Their presence is in accordance with the semi-arid climate proposed for this area during the Early Devonian (Clarke and Parnell Citation1999). Vertisol development occurs in modern environments when there are cyclic and quite marked variations in soil water content (Marriott and Wright Citation1993). Vertisol horizons thus may indicate periods of flooding and drought and/or distinct wet and dry seasons. In modern settings the vertisol features of the Rhynie Basin develop over decadal timescales, whilst calcite cements and nodules develop over considerably longer time periods (Marriot and Wright 1993). The lack of such features actually within the chert-bearing sequence could thus imply either protracted periods of waterlogging or a flood repeat frequency on sub-decadal timescales. Positive evidence of high water tables in the clastic beds occurring between chert horizons include; the reduced nature of the sedimentary sequence and the absence of red-beds that would signify drying and oxidising conditions, the common presence of early diagenetic framboidal pyrite (sometimes observed to be replacing plant material) and preserved (but compacted and degraded) organic matter in the subsurface (e.g. Fayers Citation2003; Trewin and Wilson Citation2004). The latter again indicate reducing and saturated subsurface conditions rather than drying and oxidising.
Thermally influenced settings
Flooding in the Rhynie Basin also occurred from the hot springs and geysers themselves and is represented by the chert beds and silicified clastic horizons. The chert subcrop and presence of thermal alteration minerals indicate that sporadic hot spring activity occurred along an approximately 2–4 km long section of the Rhynie Fault Zone which acted as the ‘conduit’ for up-flow of geothermal waters (e.g. Rice et al. Citation2002; Barron et al. 2004). Outflow of thermal waters created a suite of hot spring influenced environments that have been distinguished by geological, sedimentological and palaeontological attributes. The Rhynie cherts have, however, proved difficult to categorise in terms of sub-environments. Many of the classic ‘marker fabrics’ used in investigations of fossil hot spring deposits are absent or poorly represented and the lack of outcrop severely hampers interpretation of the distribution of hot spring sub-facies. Flow-paths and hence chemical and physical gradients that are plainly evident in active hot spring areas (e.g. Channing and Edwards Citation2009) and many other fossil hot spring deposits (e.g. Walter et al. Citation1996; Guido and Campbell Citation2009; Guido et al. in press) are cryptically evident at Rhynie at best.
Vent pools
Vent pool settings are poorly represented in currently available cores and trenches, but one float block from the Windyfield locality, which lies to the north of the Rhynie locality adjacent to the Rhynie fault zone, appears to preserve botryoidal textures comparable to those formed in the splash-zone of active geysers (e.g. Trewin Citation1994, Citation1996; Fayers and Trewin Citation2004).
Sinter aprons
Broad outcrops comprising strongly laminated sinter apron fabrics characterise the bulk of available exposure at many of the fossil hot spring deposits we have investigated e.g. those of the Drummond Basin, Queensland (and see White et al. Citation1989; Walter et al. Citation1996) and the Deseado Massif, Patagonia where apron complexes are observed to extend many hundreds of metres along strike (see Guido and Campbell Citation2009; Guido et al. in press) and are seen to merge down apron slope and hence down the temperature and physicochemical gradient outlined for Yellowstone hot spring areas described earlier, with peripheral geothermal wetlands where Rhynie style plant preservation is common (Guido et al. in press). The distinctive laminated and microstromatolitic marker-fabrics, which would provide definitive evidence for such environments are poorly represented at Rhynie. Laminated fabrics are recorded in chert blocks, cores and trenches and have been interpreted as sinter deposited in apron settings. The extent of such environments does, however, appear to be restricted relative to many active and other fossil deposits. Drying of sinter aprons at Rhynie is similarly difficult to prove conclusively. The presence of plants on sinter substrates is presented as evidence for a subaerial situation at the time of plant growth, in general on the basis of the homoiohydric characteristics of the plants, rather than on the basis of sedimentological features within the chert. This makes discussions of the presence of wet vs. dry apron settings at Rhynie problematic. We report occurrences below with the interpretation of the original authors using the terms proximal apron (near vent, high-temperature) and distal apron (cooler apron settings).
Proximal sinter aprons
As with vent pools, high-temperature ‘proximal’ sinter apron deposits (represented by laterally extensive, laminated chert lacking plant fossils) are present but apparently poorly represented (e.g. Trewin Citation1996; Fayers and Trewin Citation2004). Most evidence for apron environments comes from the Windyfield locality. Fayers and Trewin (Citation2004) described samples of chert that exhibit alternations of chert laminae microtexture from clear-chert, to chert containing abundant microbial filaments with phototactic orientations, to small chert-lenses containing abundant coprolites of aquatic crustaceans. These are interpreted as alternations between abiotic silica precipitation from water in excess of 73 °C (proximal wet-apron) to silicification of cyanobacteria on apron surfaces at temperatures below c. 59 °C (wet mid-apron or subsequent decrease in temperature of vent-fluid flowing across the apron), to ponding of cool water in shallow terrace pools (Fayers and Trewin Citation2004).
Distal sinter aprons
Barren laminated chert horizons that alternate with plant bearing chert appear to represent areas of apron where shallow-angled sinter terraces occurred in close association with areas of plant growth (e.g. Trewin Citation1996; Powell et al. Citation2000a; Fayers and Trewin Citation2004). Run-off streams flowing across aprons are represented by float blocks discovered from the Windyfield locality that contain in situ, but brecciated, laminated apron sinter plus allochthonous sinter and sand clasts (Fayers and Trewin Citation2004). Such sinter intraclast breccias (which form the interfluve banks of distal run-off streams in Yellowstone) result, in part, from drying and cracking of dried sinter apron surfaces (e.g. Walter et al. Citation1996). Again, these are uncommon at the Rhynie chert locality (e.g. Powell et al. Citation2000a; Fayers and Trewin Citation2004).
Low temperature apron margins and geothermal wetland
At the Rhynie locality, most chert beds are intimately associated with the carbonaceous sandy clastic sediments of the local floodplain, which occur above and below most chert beds and as thin partings within composite chert lenses (e.g. Powell Citation2000a). The lateral extent of chert horizons at Rhynie appears to extend to tens of metres which led Trewin and Wilson (Citation2004, p. 81) to conclude that the Rhynie locality was a ‘low-relief area at the cool end of an outwash (sinter) apron’ with a mosaic of plant habitats. Composite lenticular cherts at the Rhynie locality (e.g. Trewin Citation1994, 1996; Powell Citation1994; Powell et al. Citation2000a) display successions of alternating sand with carbonaceous material and sinter deposition, indicating that there was low-relief and very little topographic height difference between floodplain and sinter depositional environments. Trewin (Citation1996) suggested pool/depression depths of c. 15 cm. In these depressions, chert lens formation and plant permineralisation clearly indicates the presence of silica-rich fluids at least over the scale of several months to a year, the period required for plant silicification. Organic carbon preservation in the thin intervening sands suggests continued waterlogging following river sediment deposition and/or insufficient time for oxidation of organic material prior to resumption of sinter deposition. Trewin and Wilson (Citation2004) considered that river flooding frequency, which was viewed as halting sinter deposition by dilution, was sufficiently high to prevent the development of mesophyte climax communities (but see below). The emerging consensus interpretation of this setting (e.g. Trewin et al. Citation2003; Trewin and Wilson Citation2004) is one of a low-angle hot spring outwash apron in a distal region of sinter deposition; in other words, a peripheral geothermally influenced wetland (Channing Citation2003; Channing et al. Citation2004; Channing and Edwards Citation2009; Greb et al. Citation2006). The water table in this environment was close to, or at, the surface (e.g. Powell et al. Citation2000a; Channing et al. Citation2004) and water entered the area both by discharge from alkali–chloride, silica-depositing hot springs, via subsurface flow and from overbank flooding from the nearby river system.
The vast majority of chert recovered from the Rhynie deposit and the majority of chert horizons within cored sections contain plant material (e.g. Powell et al. Citation2000a; Trewin and Wilson Citation2004). Thus distal, low-temperature apron to geothermally influenced wetland environments appear to have dominated the Rhynie geothermal landscape.
Geothermally influenced ephemeral pools, ponds and lakes
Further thermally-influenced, wetland to fully-aquatic environments have been described. The Windyfield locality is dominated by metre-scale pods of chert formed in topographic depressions on an uneven ground surface (Fayers and Trewin Citation2004). Chert pods contain a diverse aquatic fauna (aquatic crustaceans) and flora (charophytes) and comprise composite cherts with partings of carbonaceous sandstone. A massive to vuggy (more porous) chert-matrix micro-texture occurs at the base of many chert lenses surrounding well preserved in situ and upright plants at the Rhynie locality (e.g. Trewin Citation1994, 1996; Powell Citation1994; Powell et al. Citation2000a; Fayers and Trewin Citation2004). Channing et al. (Citation2004) considered that these formed in geothermal wetland settings, where pools of standing water, derived primarily from alkali–chloride hot springs, were the site of silica precipitation from the water column via a range of colloidal mechanisms and accumulation of unconsolidated silica sediment. At the Windyfield locality such massive cherts contain clotted organic masses reminiscent of mulm, the organic-rich amorphous material found at the bottom of modern ponds (Trewin et al. Citation2003; Fayers and Trewin Citation2004) suggesting massive to diffusely bedded chert fabrics may indeed be formed in aquatic settings. We note that similar material is commonly present on the floors of geothermal wetland pools throughout Yellowstone (Channing and Edwards Citation2009). Open microbial meshworks in other Windyfield samples indicate deposition and silicification in an aquatic setting with no interval of emergence and drying between the two events (Trewin et al. Citation2003; Fayers and Trewin Citation2004).
Flooding of peripheral clastic environments
Local flooding of clastic sediments by silica-rich fluids was also commonplace at both Rhynie and Windyfield and occurred both as water flowed across the land surface and laterally and vertically in the sub-surface. These processes created cherty sandstones, which are the dominant lithology between chert horizons (e.g. Powell et al. Citation2000a; Trewin and Wilson Citation2004). They, because silica was precipitated, appear to indicate an at least periodical presence of a relatively high geothermal water table (as opposed to a high ‘normal’ groundwater table which would have acted to dilute silica concentrations). Rhizolith-like chert nodules which contain plant “rooting system” and stem fragments are a common feature in cherty sandstone horizons. Rhizolith formation has been observed in hot spring areas of New Zealand where it occurs in ‘marshy’ areas where the geothermal water table intersects the ground surface (Jones et al. Citation1998b). At Rhynie there are no elements of the flora that are found solely in chert nodules within silicified clastics. If contemporary ‘mesophytes’, which had greater volumes of lignified cells in their construction, were growing in this setting it appears logical that they, rather than the typical Rhynie assemblage should be recorded in this taphofacies. Their absence perhaps suggests that the clastic environments immediately adjacent to hot spring influenced areas and extending to the fluvial system were again sufficiently hostile to exclude mesophytes. Sub-fossil rhizolith horizons, formed around the roots of members of the Cyperaceae, have recently been described from lakeshore delta areas influenced by hot spring activity within the East African Rift (Owen et al. Citation2008), indicating that a range of wetland environments are favourable to the formation and entry of such horizons into the fossil record.
Ecophysiological influences at Yellowstone
From the brief review of habitats and vegetation above it is clear that in Yellowstone plants in the wetlands are those most likely to be preserved by silicification. The tolerances of such vegetation of active thermal areas find their closest analogy in coastal and estuarine salt-marshes subject to periodic flooding, and indeed, at c. 2400 m elevation and straddling the continental divide, the commonest plants in Yellowstone geothermal wetlands are salt-marsh colonisers Eleocharis rostellata and Triglochin maritimum (e.g. Channing and Edwards Citation2009).
Water chemistry and physical parameter gradients at Yellowstone
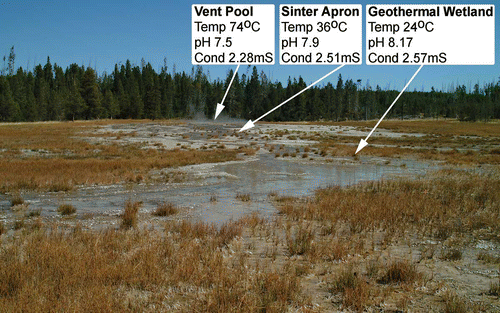
Typical physical and chemical parameters of a geothermal vent–apron–wetland complex are provided in and illustrated in . A typical sinter-depositing, alkali–chloride hot spring has vent fluid approaching boiling point (70–100 °C), with a circum-neutral to alkaline pH (6.5–8) that contains Na (300–450 ppm), Cl (500–650 ppm) and Si (200–750 ppm) plus a suite of trace elements including heavy metals and metalloids (). As water flows from the vent pool it cools, degasses and begins to precipitate supersaturated elements, ions and minerals. Degassing removes carbon monoxide and carbon dioxide from the water forcing an increase in pH. Loss of solvent water by condensation and evaporation proceeds faster than precipitation of dissolved elements. This results in an increase in salinity. Water flowing into areas of plant growth is thus brackish (c. 1–1.5 ppt salinity), of relatively high pH (8–9) and temperature (c. 35 °C) and containing potentially phytotoxic concentrations of heavy metals (e.g. Channing Citation2003; Channing and Edwards Citation2009).
Table 1. Physical parameters associated with three alkali–chloride, sinter-depositing hot springs (a–c) and thermally influenced sections of the Madison River watershed (d), Yellowstone National Park, USA
Table 2. Chemical composition of waters from sinter-depositing hot springs, geothermally influenced wetlands, geothermally influenced rivers plus recent sinter samples and Rhynie chert
Figure 4. Chemical and physical parameter distribution along a thermal out-flow gradient in the Yellowstone National Park; USA. Image taken looking up thermal gradient, across the sinter apron and towards the vent pool of Big Blue Hot Spring, from the peripheral geothermal wetland. Foreground vegetation is dominated by Eleocharis rostellata with fringes of Triglochin maritimum. The white sinter apron in the centre of the image has sparse T. maritimum plus small alkali- and salinity-tolerant grasses on interfluves between braided run-off streams. Lodgepole pine (Pinus contorta) dominated forest forms a backdrop that emphasises partitioning of vegetation across a sharp boundary that occurs between the low-lying, thermally influenced environments conducive to plant preservation and topographically higher and therefore less preservation-prone mesophyte communities.
Stresses in the most common environment of plant growth and preservation in Yellowstone therefore are:
-
chemical, including heavy metals
-
fluctuating flooding and evaporation
-
waterlogging/anaerobiosis
-
temperature (subaerial/ground).
The above account of hot spring water properties and the physical and chemical values in and provide snapshots of environmental parameters in a dynamic fluctuating system in both time and restricted space, which contrasts markedly with the majority of more equable swamp/wetland habitats but is comparable with those in coastal salt marshes. Extrinsic features of the Yellowstone climate system add further stresses to those most obvious in geothermally influenced wetlands. Following outflow into the environment fluids may undergo strong evaporation and concentration of dissolved elements, ions and minerals, leading to both actual and physiological drought, or be diluted by meteoric/pluvial water. The account is by necessity much simplified and could be taken to imply static conditions of water parameters. In reality, hot springs undergo erratic but cyclic phases of eruption and quiescence, high- to low-volume discharge, and heating and cooling over hourly to decadal, even millennial timescales. Where wetland areas receive the discharge from a number of springs, mixing of source waters creates further complexity. However, from our observations at Yellowstone (data logging and repeat measurements conducted over the scale of days to weeks and periodically over the last 10 years) it is apparent that the presence of water already in wetland areas acts to some degree to buffer and mitigate against wholesale physicochemical change by subsequent water influxes (Channing and Edwards Citation2009). Alkali–chloride geothermal wetland water chemistry parameters, irrespective of such a dynamic input system, all seem to cluster about a common composition range as evidenced by the presence park-wide of a similar flora. This observation extends beyond the boundaries of Yellowstone, to other lesser thermal areas of the western USA (e.g. Mosley Citation1995).
Transition and heavy metals
Transition metals (e.g. Fe, Cu) and heavy metals (e.g. Hg, Sb, Tl), and As that may be toxic at high concentrations are common constituents of hot spring waters, sinter and geothermal soils. Detailed consideration of tolerance mechanisms to maintain growth (Clemens Citation2001, Citation2006) are beyond the scope of this paper and lack of physiological research on Yellowstone plants prevents the assumption that those growing in the vicinity of the vents may be metallophytes. There is however limited and indirect evidence that Eleocharis rostellata and Triglochin sp. growing within the Gibbon-Madison-Firehole watershed (Yellowstone's watershed most heavily influenced by geothermal inputs) contained very high concentrations of As (16.2 and 28.5 mg/kg dry weight respectively), although the precise locations of the plants were not given (Kocar et al. Citation2004). There is some evidence from experimental work on acid drainage from abandoned mine workings in Japan that Eleocharis acicularis (L.) Roem. & Schult. is a hyperaccumulator of Pb and accumulator in decreasing amounts of Fe, Cr, Cu, Ni, and Mn, all of which were thought to have been in the form of sulphides (Ha et al. Citation2009). Studies on hot springs and mine workings in Mexico indicate that Eleocharis sp. is a hyperaccumulator of As (Flores-Tavizon Citation2003). This work on hot springs provides further evidence for the ubiquity of heavy metal tolerances in these stressed environments and indeed has led to the suggestion that the initial selection pressures came from colonisation of environments influenced by As-rich hot springs early in the evolution of land plants (Meharg and Hartley-Whitaker Citation2002) with the consequence that mechanisms involved in Cd and As detoxification are ubiquitous, even though throughout plant history most habitats are now free of these elements. However, as Clemens (Citation2001, Citation2006) has pointed out, there are other (and probably far more likely) biochemical explanations, relating in part to essential metal homeostasis, especially as there is no evidence that hot springs were critical to early land plant evolution.
From our observations of wetland vegetation at Yellowstone and other active hot spring areas we see no drastic lateral changes or zonation in the composition of vegetation related to temperature or soil chemistry gradients in wetlands settings. Instead, we see a temperature threshold at about 45 °C and below this temperature higher plants become a component of the hot spring biota. This contrasts markedly with the situation in dryland settings where temperature zonation and soil chemistry are apparent. In both New Zealand (Boothroyd Citation2009) and Yellowstone (Despain Citation1990) bare ground at high temperatures gives way to bryophyte zones and thence higher plant growth.
Salinity
The influences of salinity stress in Yellowstone communities on sinter aprons and surrounding wetlands are evident in the composition of the vegetation itself, which is almost exclusively dominated by monocots characteristic of salt-marsh communities. Indeed from numerous publications on halophytes (e.g. Flowers and Colmer Citation2008) can be drawn parallels with the colonisers of arguably these even more dynamic (but localised) habitats at Yellowstone (see e.g. Waisel Citation1972; Flowers et al. Citation1986).
Thus it can be assumed that taxa such as Eleocharis rostellata and Triglochin maritimum are similarly physiologically adapted in that there is limited transport of Na+ and Cl− to the shoots accompanied by compartmentalisation of these ions in vacuoles which assures the maintenance of lower concentrations in cytoplasm (Munns and Tester Citation2008). However, of particular interest here is adaptation of the Yellowstone plants to a combination of chemical (salt and metal toxicity) and water stress, the latter produced on flooding and drought. Such interactions are now attracting increasing attention in their relevance to salinisation, drought and irrigation/flooding of crop plants such as wheat and rice (see references in Munns Citation2002; Colmer and Flowers Citation2008). Physiological adaptation to salt marsh environments also occurs at the intraspecific level with populations originating in salt marshes having greater salt tolerance (Rozema et al. Citation1978) and flooding tolerance (Davies and Singh Citation1983) than populations originating from non-saline or non-flooded habitats. In the hot spring context, the ability of halophytes to continue to regulate salt relations rapidly (Flowers Citation1985) when flooded, and more importantly waterlogged, is of relevance, as the latter, associated with anoxia, can reduce salt tolerance in glycophytes (e.g. Yeo et al. Citation1999; Barrett-Lennard Citation2003). Indeed early experiments on the effects of salinity and flooding/drainage on salt marsh species of the grass Spartina suggested that their distribution was more closely related to flooding disturbance than salinity (Parrondo et al. Citation1978). Considering anatomical adaptations associated with water-logging and possible anoxia, in this study, very extensive aerating systems are present in Eleocharis shoots and roots but other adaptations have not yet been studied at Yellowstone. In particular we need to establish the degree of intraspecific divergence that has occurred in the Yellowstone hot spring populations of species of Eleocharis and Triglochin maritimum compared with species outside these habitats.
The above account reflects on the range of stresses that might be encountered by Yellowstone and, with the exception of seasonal temperature extremes, the Rhynie plants. The previously overlooked effects of the most obvious element, Si, in such hot spring scenarios are surprisingly controversial and will be examined in a little depth.
The conundrum of silicon: a diversion
Silicon is exceeded only by oxygen in its abundance in the Earth's crust and is present in most soils as silicic acid, typically in the range of 50 to 100 ppm, yet its role in plant growth development and metabolism is still debated (Epstein Citation1994, Citation1999). Even when present in quantities as high as 300 ppm as seen in the Yellowstone wetlands, it is non-toxic. That silicon is an essential element in algae such as diatoms, and in higher plants, particularly Equisetum, is uncontestable. Although functions can be listed for it, its essentiality in other plants remains controversial, despite increasing evidence that it is beneficial to growth and development (Epstein Citation1994, Citation1999). A possible explanation of past uncertainty is based on the demonstration that deficiency symptoms in tomato plants grown in a medium lacking silica, appeared only after the first flower buds had developed suggesting a role for silica in reproduction (Takahashi and Miyake Citation1977). This assumes that in earlier experiments, plants had not been allowed to reach reproductive maturity. Other experiments on rice (Matoh et al. Citation1986; Yeo et al. Citation1999), wheat (Savant et al. Citation1999), sugarcane (Savant et al. Citation1999) and barley showed increased productivity on application of silica (particularly in rice) in the reproductive phase (Takahashi and Miyake Citation1977) such that in rice Si-containing fertilizers are often applied (Pereira et al. Citation2004). Indeed a recent change in the definition of essentiality as proposed by Epstein and Bloom (Citation2005) includes changes in productivity.
Functions of silicon in plants
-
Incorporation into the cell walls of leaves as silica (SiO2 n H2O – opal) recorded in ferns (Parry et al. Citation1985) and monocots leads to increased rigidity and, particularly in the latter group, increases the productivity of the plant by enhanced light capture via structural, as opposed to physiological, means. In addition, experiments with rice (noted above) where over-use of nitrogen fertilizer has reduced both support tissues and disease resistance, the addition of silica increased productivity by strengthening walls (Takahashi and Miyake Citation1977). As Raven (Citation1983) indicated the use of silica rather than lignin in wall strengthening is more energy efficient. Whether or not silicon actually modulates lignin synthesis or increases wall elasticity by interacting with polyphenols remains conjectural.
-
Deposition of silica on or within cell walls forms a physical barrier against pathogens and where occurring in root endodermal cell walls, creates an apoplastic resistance to entry into the xylem (Lux et al. Citation2003). Silica also affords a degree of physical protection from phytophagous insects (e.g. Epstein Citation1994) and in the form of phytoliths, spines, prickles acts as a deterrent against herbivory (e.g. Massey et al. Citation2006).
-
Silicon increases tolerance to elements in excess including metals such as Al, Mn, As, Fe, Cu (Liang et al. Citation2007) in a variety of ways. Common external mechanisms include release of phenolic compounds resulting in formation of metal-phenolic complexes, changes in pH and co-deposition of silica and metals in growth media (Liang et al. Citation2007, ). Internally, in many cases, silicon forms metallosilicates, e.g. Zn silicate or aluminosilicate that decrease toxicity in the cytoplasm while their subsequent breakdown in the vacuole (such complexes are relatively unstable) isolates the metallic ion and results in deposition of silica (Neumann and zur Nieden Citation2001; Neumann and De Figueiredo Citation2002). Silicon can also act indirectly. In rice, tolerance to high Mn and Fe concentrations in the roots is achieved by increased rigidity and volume of aerenchyma in the shoots and roots and leads to enhanced oxidation of the toxic reduced Fe and Mn compounds (e.g. Korndörfer et al. Citation1999). Experiments with rice, grown in increasing concentrations of NaCl, showed that application of silica greatly decreased the drop in productivity of plants grown in saline solutions (e.g. Matoh et al. Citation1986).
Of direct relevance to our discussion on Yellowstone – and indeed the Rhynie chert – are studies on the Si contents within plants in relation to concentration of silica in the available water. The vast majority of eudicots, which are termed non-accumulators, contain c. 0.5% dry weight of silicon indicating the uptake from the soil solution is passive or even prevented (Takahashi and Miyake Citation1977). Some of the highest concentrations in dicots are in heavy metal tolerant species where Si has been shown to ameliorate the effects of the heavy metals via complexing. In these plants, silica crystals have been illustrated in vacuoles as well as walls (Neumann et al. Citation1997). Silica accumulators fall into two groups. Cyperaceae and wet-land grasses including rice contain 10–15% dry mass (DM) silicon, with maximum values (30%) in certain organs such as Schoenus seeds. By contrast dryland grasses including cereals can contain 1–3% DM Si. Takahashi and Miyake (Citation1977) surveyed the Si content of 175 species under the same soil conditions, and found that Si was one of the most variable elements present and was present in excess (i.e. Si accumulators) in plants of diverse affinity including bryophytes (two species), ferns (representatives of six families including the Schizaeaceae and ‘Polypodiaceae’), lycophytes (four species) and sphenophytes (two species) as well as angiosperms. However, in a phylogenetic survey of the Si content of shoots of 735 species based on the literature and covering all major clades, Hodson et al. (Citation2005) found that in general bryophytes and Equisetum had higher values than ferns, gymnosperms and angiosperms, with Acerales and Poales having greater concentrations than in the non-commelinoid monocot clades. The high internal values in the Si accumulators that are far greater than those expected based on concentrations in the bathing media indicate active uptake of H4SiO4 and, since they are usually recorded in shoots, are suggestive of transport in the apoplastic pathway, with active input into the xylem (this metabolic activity in the roots being decreased by inhibitors). Moreover, experiments with barley shoots show that accumulation is independent of transpiration rate because more SiO2 was taken up under conditions of high humidity and therefore is a function of root metabolism. Precipitation of Si is close to the ends of the transpiration stream in the walls and surfaces of leaf cells where saturation points are reached and exceeded.
Hydrochemistry of the Rhynie hot springs and geysers
At Yellowstone we have direct measurements of water chemistry and physical parameters and can place upper-lower limits on these parameters. At Rhynie such direct measurements are not possible because we have only the product of precipitation, the cherts. However, substantial indirect evidence of water chemistry may be drawn from the cherts themselves and the alteration chemistry of local country rocks. Elements recorded in chert horizons and silicified carbonaceous sandstones at the margin of the chert beds (e.g. Rice and Trewin Citation1988) include; Au, Ag, As, Sb, Bi, Cu, Pb, Zn, Ba, Mn, Ti, Al, Fe, Mg, Ca and K (). Whilst these elements and the recorded concentrations may in part reflect later overprinting of concentrations present at the surface at the time of deposition, comparison with recently precipitated sinters (: Iceland and New Zealand) suggests that, cautiously, the figures may be used to reflect potential chemical stressors in the environment during plant growth. Investigations of trapped fluid inclusions within chert beds indicate that chert was deposited at temperatures of less than 40–50 °C (Barron et al. 2004). The majority of fluid inclusions were of saline composition (10 ppt) containing c. 1 wt% NaCl eq. (Barron et al. 2004). Analogy between the Rhynie hot springs and active sinter depositing springs appears soundly based on the observation that the water chemistry and sinter major and trace element concentrations associated with alkali–chloride sinter depositing springs worldwide remain relatively consistent (: cf. Iceland and New Zealand).
Historically, derived largely on data from the lithological log from Tait's trench (Horne et al. Citation1916) which had at its base a bed of white clay, Kidston and Lang (Citation1921b) proposed the Rhynie chert as being analogous to deposits associated with Javanese fumaroles where “vegetation of xerophytic construction is rooted in hot, acidic soil of white clays” (Kidston and Lang Citation1921b, p. 895). This implied an acid–sulphate, steam-dominated setting for growth and preservation of the Rhynie plants. However, the setting of the Rhynie chert (in a fault controlled environment associated with andesitic lavas and tuffs), mineralisation (chert- quartz- and minor carbonate-bearing breccias and veins), alteration (pervasive alteration of sediments and lavas to quartz, K-feldspar, calcite and illitic/chloritic clays), geochemistry (local Au, As, Hg, Sb and Tl anomalies (: Rhynie chert) and deposition of surficial chert deposits all suggest that the hot springs of Rhynie were the surface expression of a low-sulphidation (alkali–chloride) epithermal system (e.g. Rice and Trewin Citation1988; Rice et al. Citation1995; Channing Citation2003; Baron et al. Citation2004). The geothermal system was recharged by deeply circulating meteoric fluids with a possible minor input of high-temperature magmatic fluid (Baron et al. Citation2004). Hydrothermal fluids at depth in the geothermal system thus had an alkali- and chloride-rich rather than acid- and sulphate-rich character (e.g. Panteleyev Citation1996a cf. Panteleyev Citation1996b). These were transported to the surface along the Rhynie Fault Zone (along which the epithermal alteration is concentrated) and flowed into surface environments (e.g. Barron et al. 2004).
A number of mechanisms are recognised to alter alkali–chloride fluids to acid–sulphate fluids during their ascent to the surface from deep reservoirs. These include near surface mixing with shallow recharging meteoric sources and pH reductions brought about either by separation of a steam phase during shallow subsurface boiling and chemical or microbial (e.g. White et al. Citation1988) oxidation of H2S or SO4 2- (e.g. White et al. Citation1988). At the surface acid–sulphate hot spring activity creates diagnostic minerals such as kaolinite clays and sulphate minerals of the alunite-jarosite group and native sulfur (e.g. Jones et al. Citation2000). The landscape features typical of acid–sulphate thermal areas include fumaroles, steam-vents, mud pots and volcanoes and barren thermal ground (e.g. White et al. Citation1988). To our knowledge none of these diagnostic mineral occurrences or geothermal sub-environments are reported at Rhynie. We thus conclude that the Rhynie hot springs and their associated sub-environments were characterised by alkali–chloride waters. This hypothesis is further supported by the observation that the sediment sequence containing the Rhynie cherts has a regional alteration signature (Baron et al. Citation2004), rather than epithermal signature, thus precluding the presence of such processes even in the shallow subsurface. Subsequent research has thus demonstrated that Kidston and Lang (Citation1921b) actually evoked an environment, that given current evidence, appears absent from the Rhynie landscape.
In the preceding sections we have outlined evidence of the geological, chemical and physical environment of active hot spring systems and demonstrated that the environments of plant growth and preservation at Rhynie were closely comparable. Continuing the analogy naturally leads to the realisation that the Rhynie plants were probably subject to an identical set of stresses as extant plants. The key point in this respect is that the Rhynie plants grew in an environment systematically inundated by alkali–chloride hot spring waters. The dominance of alkali–chloride, silica-rich geothermal fluids has major implications for the physical and chemical parameters of water that flows into areas peripheral to spring systems and as a consequence any local biota. Broadly, in active alkali–chloride geothermal wetlands plants grow in fluids with temperatures up to 45 °C that have circum-neutral to alkaline pH (recorded pH between 6.5 and 9.1), are brackish (salinity c. 1.5 ppt, conductivity up to 3.5 mS cm−1) and contain dissolved silica at or above saturation concentrations.
Reconstructing the ecology and palaeo-ecophysiology of Rhynie plants
The excellence of preservation in silica at the cellular level combined with in situ growth of many plants allows more confidence in recreating their physiology in relation to habitat than for any other early land plant ecosystem. Surprisingly, Kidston and Lang (Citation1917b, Citation1920a,Citationb, Citation1921a,Citationb) did not adopt this approach in depth, although they were clearly interested in where the plants grew (e.g. 1921b; see Reconstructing the palaeo-ecophysiology of the Rhynie plants, below). In the introduction to their account on Rhynia gwynne-vaughanii Kidston & Lang (now known to be a mixture of Rhynia and Aglaophyton) when they compared its gross morphology to that in Psilotum, they mentioned “xerophytic aerial stems” (Kidston and Lang Citation1917b, p. 766) arising from delicate rootless rhizomes but did not provide the qualifying anatomical evidence. Perhaps they were referring to the prominent epidermis with thick outer walls, strongly developed cuticle and sparse stomata that they noted in the succeeding description.
Direct evidence for in situ growth/autochthonous preservation
Perhaps the outstanding feature of the Rhynie chert (and other younger fossiliferous hot spring deposits) is the prima facie evidence of linkage to their environment provided by large numbers of plants preserved in life position on the substrate on which they grew. Powell et al. (Citation2000a) encountered all of the Rhynie plants except Ventarura in life position in drill-core 19C, in which in situ plants occurred in 29 of the 45 in situ chert beds. In situ rhizomes with aerial axes were recorded for Rhynia, Aglaophyton, Horneophyton and Asteroxylon, only Nothia and Trichopherophyton were not found as in situ rhizomes. From this account it can be seen that in situ preservation was normal, rather than a chance happening at Rhynie. Below we highlight some key examples of the types of evidence for in situ growth and autochthonous preservation.
-
Rhizomes bearing rhizoids may traverse the surface of a substrate with rhizoids usually on the lower surface (e.g. Nothia aphylla). In the case of Nothia hundreds of rhizomes are similarly orientated with rhizoidal ridges in the same position (Kerp et al. Citation2001). The rhizomes associated with Trichopherophyton bear outgrowths interpreted as rhizoids on all surfaces and these are inferred to have grown through the substrate, be it litter or organic-rich sand (Lyon and Edwards Citation1991). The former, usually associated with grains of sediment, but sometimes described as a peat, could have been washed in and subsequently supported growth.
-
Many plant growth horizons at Rhynie comprise horizontal rhizomatous axes attached to vertically aligned but hollow axes ‘straws’ (e.g. Trewin Citation1996). Cavities within the vertical axes may be filled with geopetal structures (sediment and/or mineral precipitates that indicate the influence of gravity). Such straws have traditionally been viewed as evidence for partial and incomplete silicification due to cessation and removal of permineralising fluids. We envisage they actually record a palaeo-wetland water table where preservation occurs below the water surface and normal microbial decay progresses above (Channing and Edwards Citation2009).
-
Aerial axes orientated in parallel show curvature suggestive of having been bent over by currents on flooding, but with basal regions anchored in the substrate. Others, which are straight and erect, occur in large numbers of one species (e.g. Rhynia gwynne-vaughanii) are interpreted as ‘rooted’ clones and thought unlikely to have drifted.
-
Gametophytes of a number of taxa completely cover surfaces indicating mass germination producing localised almost pure stands of a single taxon (Remy et al. Citation1993).
Ecology
Whole plant reconstructions and autecology
Nothia aphylla Lyon ex. El-Saadawy et Lacey. Kerp et al. (Citation2001) reconstructed N. aphylla as possessing persistent long-lived subterranean rhizomes that colonised a sandy soil containing strongly developed plant litter, that were capable of multiple regeneration from dormant growing points. The latter developed into short lived aerial shoots which the authors considered only moderately capable of adaptation to seasonality involving drier periods.
Aglaophyton major (Kidston and Lang) D. Edwards. In A. major, the basal regions of the upright axes show extensive thickenings of the peripheral tissues (i.e. extending into the hypodermis) particularly in substomatal areas (Remy and Hass Citation1996). The authors considered this an adaptation to prevent attack from aquatic fungi in plants whose habitats were periodically flooded. As evidence for this they compared the buds on rhizomes that were free from chytrid attack, while the aerial apices, which lacked thickenings, were often infected. Further evidence for an aquatic environment came from the association of the charophyte Palaeonitella with the Aglaophyton rhizomes and of chytrids with germinating spores of the higher plants. The presence of numerous arrested apices (buds) with enhanced potential for regrowth was also considered suggestive of colonisation of an unstable environment experiencing periods of sedimentation, flooding, overcrowding and drought.
Kerp et al. (Citation2004); and see Taylor et al. Citation2005b, illustrated the almost complete life-cycle of Aglaophyton and its gametophyte Lyonophyton from the ‘white chert’ an algae-rich chert, clearly of wetland origin, discovered in trenches at the Rhynie locality and first described by D.S. Edwards and Lyon (Citation1983). A single block contained dozens of well-preserved axes, some bearing archegonia, occurring in situ and with the plants standing more or less in vertical position. Amongst the axes were numerous germinating spores plus poorly preserved antheridiophores of Lyonophyton. The authors, having observed dense Palaeonitella mats in the same block suggested that aquatic conditions prevailed before and during silicification.
Rhynia
Trewin et al. (Citation2003, fig. 6) illustrated crowded stands of Rhynia preserved in an upright position and surrounded by horizontal, laminated bacterial sheets. Interspersed with the bacterial sheets, which occurred at various levels through the full depth of a chert block c. 15 cm deep, were bubbles created by trapping of gasses formed during microbial photosynthesis. The authors considered that the association of the plants plus bacterial sheets and the lack of any features within the microbial laminae other than dispersed spores, indicated that they were both growing in water and that the bacterial mats provided a support to the plant axes as they grew.
Kerp et al. (Citation2004) illustrated numerous Rhynia gametophytes (Remyophyton delicatum) preserved in situ growing on a sinter surface containing filamentous microbes and overlying a monotypic stand of Rhynia. Germinating spores and germlings occurred dispersed amongst rhizoid-bearing parts of the gametophyte. Laminated and microbe-rich chert horizons surround and cover the clumps of gametophytes to a depth of c.12–14 mm. This situation indicates germination, growth to maturity and preservation on an apparently wet sinter apron.
Rhynia/Aglaophyton/Asteroxylon vs. Horneophyton
Kidston and Lang (Citation1921b) examined the axial surfaces close to the junctions between aerial stems and rhizomes and noted that, apart from Horneophyton, stomata were present in the proximal regions of the aerial axes. This led to the inference that these three taxa were not subjected to surface flooding but this did not preclude the possibility that the soils themselves were waterlogged. Asteroxylon possessed leafless rhizomes from which bifurcating root-like structures penetrated up to 20 cm into the relatively dry sandy humus-rich soils. By contrast, the bulbous rhizomes of Horneophyton were inferred to have grown in shallower pools on peat surfaces as further evidenced by an associated abundance of aquatic invertebrate remains. Kerp et al. (Citation2004) noted large numbers of fertile Horneophyton axes in the ‘white chert’ block that contained Aglaophyton/Lyonophyton and Palaeonitella.
These autecological approaches provide evidence for adaptations to survival in unstable environments in which flooding and drought were contributing factors.
Plant communities based on associations of plants with hot spring fabrics and sediments
Although Kidston and Lang's series of papers are noted principally for the anatomical studies of plants and animals, the first (1917b) and final publications (1921b) provide valuable insights on the occurrences of ‘peats’ containing the four vascular plant taxa and the intervening siliceous and cherty sandstones. We write peats in inverted commas because, as Kidston and Lang noted, the accumulations of organic matter show no increased compression or decay with depth and often contain considerable amounts of sediments implying some clastic transport with litter although there are ‘pure’ beds which indicate an autochthonous origin. Plant-rich chert beds rarely exceed 30 cm in thickness (Kidston and Lang Citation1917b; Powell et al. Citation2000a) and represent accumulations over relatively few years. Tasch (Citation1957) considered that chert bed formation occurred over a period of 150 ± 50 years. Reported sinter accretion rates in Yellowstone include 5–10 mm/month for shrubby microbial microfabrics in pools and 3.2–10.0 cm/year for sinter apron deposits (e.g. White et al. Citation1988) would severely reduce this estimate. The Kidston and Lang analyses, based on a trench through c. 3 m depth of section as well as on loose blocks, c. 60 cm in diameter, which were thought to have derived from the same horizons, led them to conclude that there was considerable lateral variation in the composition of assemblages with little constantly occurring associations. Tasch (Citation1957) and more recently Bateman (Citation1991) reproduced the original log with comments on the associations of plants present. Tasch further deliberated on the chemical nature of the ‘pre-peat’ surface, the timing of silicification and the duration of accumulations of ‘peat’, with a more detailed evaluation of the distribution and flooding history of Rhynia gwynne-vaughanii.
Exploration interests led by Rice and co-workers in Aberdeen on the Rhynie cherts as a potential gold deposit, stimulated studies on interpretation of chert fabrics as components of a hot spring system (e.g. Rice and Trewin Citation1988; Trewin and Rice Citation1992; Trewin Citation1994) and, following the acquisition of seven cores, the longest being 35.41 m, presented the opportunity for a more systematic analysis of the relationship between silicified rocks and organic accumulations in terms of plant communities and their substrates (Powell et al. Citation2000a). The latter, coupled with a succession of anatomical and autecological studies of individual taxa, conducted by Remy and his team in Münster has resulted in a far greater refinement of plant distribution around hot springs than previously available (e.g. cf. El-Saadawy Citation1966; Powell Citation1994; Trewin Citation1996; Powell et al. Citation2000a).
El-Saadawy (Citation1966) considered that the Rhynie plants grew in ephemeral pools which appeared or reappeared on a barren landscape. Trewin and Rice (Citation1992) considered that the plants generally grew on sandy substrates or sinter surfaces and close to pools on the fluvial floodplain prior to inundation by hot spring water. Following field visits to Yellowstone, where a range of geothermal wetland environments were investigated in collaboration with the present authors, and following the discovery of the Windyfield locality, output of the Aberdeen group has trended towards a wetter Rhynie setting (e.g. Trewin et al. Citation2003; Fayers and Trewin Citation2004; Trewin and Wilson Citation2004). This process which in part arises to accommodate the clearly aquatic/wetland settings of the Windyfield locality, has also impacted on the inferred substrate preferences of the plants. An example is Aglaophyton, which in earlier interpretations was a plant that tolerated flooding (e.g. Remy and Hass Citation1996; Powell et al. Citation2000a), but later papers propose that it was a plant associated with damp environments that tolerated drying (e.g. Fayers and Trewin Citation2004).
Powell (1994) logged in detail the 35.41 m long, 54 mm diameter core and identified 53 chert beds (45 in situ), some a single unit, others composite, most with plants, but some (though few) were barren. These were separated by fine-grained, carbonaceous, micaceous chert-cemented sandstones, and rarer thin silicified shales and mudstones. She then identified the cherts as fossilised subaerial sinters in the vicinity of hot spring vents, with laminated cherts typical of deposition on sinter terraces and massive and lenticular cherts containing Palaeonitella and crustaceans as deposits in ponds.
Poorly preserved coalified remains were recovered from carbonaceous sandstones and shales suggesting that plants colonised the alluvial floodplains subject to flooding. The plant remains in cherts represent autochthonous and allochthonous accumulations of litter, both colonised by living communities with various types of rhizomes often bearing rhizoids. Many communities were monotypic, others consisted of two taxa; rare examples comprised three taxa on a single surface within the core which was only 54 mm in diameter. Powell et al. (Citation2000a) acknowledged the limitations of working with isolated cores in clearly heterogeneous and dynamic environments in the spatial reconstruction of ecosystems. However, cores represent temporal evolution. Changes of substrates/cherts can be detected as hydrodynamic changes occur e.g. a transition from a hot to cool pool, or drainage and infilling plus development of soils. They found no consistent successional patterns nor indications of a climax community (Trewin and Wilson Citation2004), although detection of the latter in such dynamic systems involving variations of water table, water chemistry, pH may be unrealistic and indeed has been disputed for unstable habitats such as salt marshes (Waisel Citation1972). Nevertheless, some tentative conclusions relating to preferences of taxa for certain substrates were reached. Rhynia and Horneophyton often formed monotypic stands in basal parts of organic-rich profiles, the former first noted by Kidston and Lang (Citation1917b), but very rarely are the two taxa found together because of different substrate preference. The buried tubiferous rhizomes of Horneophyton grew in litter, but their rhizoids anchored them on surfaces of sinters, which, because of their hygroscopic nature, maintained a moist substrate (Powell et al. Citation2000a). Such a wet habitat had already been suggested by Kidston and Lang (Citation1921b) on the basis of decay, while Remy and Hass (Citation1991c) provided evidence of aquatic chytrids as its causal agent. By contrast, Rhynia is postulated as a primary coloniser of drier sandy substrates, and persisted as organic material accumulated. Its association with other taxa as humus built up suggests that Rhynia was a generalist capable of colonisation of a wide range of drier and disturbed environments. Thus Rhynia stands alternating with Horneophyton in composite cherts suggests adaptation to variations in water-table levels during flooding.
Aglaophyton, considered by Remy and Hass (Citation1996) to possess anatomical modifications resulting in tolerance to flooding, was surprisingly never found on sinter surfaces by Powell et al. (Citation2000a) but far more frequently occurred in litter accumulations than in life position on organic substrates. However, Kerp et al. (Citation2004) reported sporophytes and gametophytes in life position with Horneophyton and the alga Palaeonitella in the ‘white chert’ (D.S. Edwards and Lyon Citation1983) an algae-rich chert created in an aquatic environment where little clastic influence of the environment is evident. Aglaophyton is associated with all four common taxa in the cherts.
Asteroxylon, like Aglaophyton, appeared to have a preference for substrates comprising accumulations of litter. Its extensively developed ‘rooting’ systems penetrate presumably transported litter produced by two or more of the common Rhynie taxa, and may penetrate sandstones below. The very limited information available on Trichopherophyton (Lyon and Edwards Citation1991) suggests it was part of a diverse community colonising humus-rich substrate and hence is interpreted as a late coloniser. Nothia was not recorded as a monotypic stand in growth position, nor as a sole member of allochthonous/autochthonous litter, but provided a substrate for the growth of Rhynia, Aglaophyton and Asteroxylon. Its absence above mineral soils suggests it was not a primary coloniser, but required organic matter in substrates for establishment (Powell et al. Citation2000a). In a similar approach, but based on isolated blocks of chert, Kerp et al. (Citation2001) described cherty sandstone containing nodules with hundreds of small well-preserved, similarly orientated rhizomes preserved in growth position in decomposed and compressed plant debris, which contained a considerable proportion of highly decomposed Nothia aerial stems. They described excellence in preservation in the debris and poor preservation in the litter layers. The nodular cherts also contain considerable numbers of ‘rooting’ systems of Asteroxylon and some rhizomes of Horneophyton and axes of Aglaophyton, with the rhizomes and ‘roots’ showing a similar orientation. Rooting systems of Asteroxylon and rhizomes of Nothia penetrate the usually highly decayed rhizomes of Horneophyton, which led to the inference by Kerp et al. inference that Nothia and Asteroxylon lived on sandy soils containing plant litter. This supports the more limited data from the core, and suggests that, although clonal in growth, Nothia was gregarious.
Fungi and fungal associations of the Rhynie plants as wetland indicators
Three major fungal groups are recorded from the Rhynie chert; the Chytridiomycota, Zygomycota and Ascomycota (Taylor et al. Citation2004). These have been used as indicators of wetland versus dryland conditions (e.g. Remy and Hass Citation1996; Trewin et al. Citation2003; Taylor et al. Citation2004; Trewin and Fayers Citation2004). Thus, chytrids within chert blocks are taken to signify aquatic conditions and plant associations with mycorrhizal fungi and ascomycetes suggest terrestrial, drying substrates (e.g. Fayers Citation2003; Trewin et al. Citation2003; Taylor et al. Citation2004). Below we briefly review the tolerances of extant fungal groups relevant to the Rhynie fungi as their distribution in extant environments and in living plants is an indication of the habitats of the latter.
Chytrids
Chytrids in the Rhynie chert are associated with the sporophytes Aglaophyton, Horneophyton (and its gametophyte Lyonophyton) and Nothia where attached to rhizoids, as well as the alga Palaeonitella and plant and fungal spores (e.g. Taylor et al. Citation2004). The presence of chytrids has been used to imply freshwater conditions in pools at Rhynie as many extant forms prefer water with low osmotic potential. However, extant chytrids are tolerant of many of the physicochemical conditions of geothermal wetlands. Some genera grow in brackish estuaries and others are parasitic on marine algae. Laboratory studies have shown that many freshwater and soil chytrids can grow on media supplemented with 10 ppt NaCl but not with 20 ppt NaCl (e.g. Gleason et al. Citation2006, Citation2008). Estuarine chytrids, however, tolerate a much broader range of NaCl concentrations. The upper temperature limits for most chytrid species coincide with upper temperatures of plant growth we observe in Yellowstone, some species grow at temperatures over 35 °C, however, none are recorded that grow at temperatures above 45 °C (e.g. Gleason et al. Citation2008 and references therein). Many chytrids isolated from freshwater, soil, brackish and marine environments are thought to be obligate aerobes, and their growth rates are greatly inhibited by low dissolved oxygen concentrations (e.g. Barr Citation2001; Gleason et al. Citation2007a). A few facultative anaerobes isolated from stagnant waters tolerate low dissolved oxygen concentrations (e.g. Emerson and Natvig Citation1981; Whisler Citation1987). Intact thalli, sporangia and possibly zoospore cysts of some species (particularly those in the Blastocladiales and Spizellomycetales) are resistant to drying (e.g. Couch Citation1945; Willoughby Citation2001; Gleason et al. Citation2004; Gleason et al. Citation2007b; Hoffman et al. Citation2008). Thus at best chytrids are indicative of wet conditions ranging from damp soils to free water but are not exclusive to freshwater and can tolerate chemical stress and hypoxia.
Zygomycetes
The second common group of fungi in the Rhynie chert, the zygomycetes, occur as rhizome (and stem) mutualists or symbionts. Since mycorrhizal fungi in the chert have latterly (e.g. Fayers Citation2003; Trewin et al. Citation2003) been considered to represent drying or at least aerated soils they will be considered in some detail here.
Mycelia: Glomites rhyniensis is an arbuscular mycorrhiza-forming species that is known from both the sporophyte Aglaophyton and the plants gametophyte Lyonophyton rhynienesis. Glomites rhyniensis occurs as an intercellular endophyte in A. major's prostrate axes and becomes intracellular exclusively within a distinct zone of the cortex (i.e. the mycorrhizal arbuscule zone), where it forms arbuscules (Krings et al. Citation2007). The fungus is strikingly similar to the extant arbuscular mycorrhizal fungus Glomus.
Of the unnamed endophytes described from prostrate axes of Nothia (Krings et al. Citation2007) one fungus (number three) produces wide aseptate hyphae with intercellular vesicles, similar to those produced by G. rhyniensis in Aglaophyton, and narrow hyphae with thick-walled spores similar to many extant endomycorrhizal fungi.
Spores: Scutellosporites devonicus Dotzler, M. Krings, T.N. Taylor & Agerer related to the extant genus Scutellospora occurs in degraded aerial axes of Asteroxylon mackiei Kidston & Lang (Dotzler et al. Citation2006). Acaulosporoid forms (Dotzler et al. Citation2009) considered to be related to one of the extant acaulosporoid spore-forming genera Kuklospora, Acaulospora, Otospora, Ambispora or Archaeospora occur in axes of Aglaophyton major. Palaeomyces gordoni Kidst. & W.H. Lang resembles Scutellosporites devonicus and has been described from degraded tissues of Asteroxylon, Horneophyton and Rhynia, though germination shields have not been recognised (Dotzler et al. Citation2006). Glomites sporocarpoides Karatygin, Snigirevskaya, K. Demchenko et Zdebska, a Glomus-like fungus, is reported from Aglaophyton, Rhynia and Nothia (Karatygin et al. Citation2006).
The inference that the presence of mycorrhiza in chert plants indicates dry, aerated soils in part reflects the formerly widespread view that arbuscular mycorrhiza fungi are confined to dryland plant species. However, recent studies have shown that AM fungi are commonly present in wetland areas (including salt-affected marshes). Indeed, the extant genera Glomus and Scutellospora, those considered most likely modern relatives of the Rhynie forms, dominate in such settings (e.g. Bohrer et al. Citation2004; Radhika and Rodrigues Citation2007 and references therein) and thus do not preclude wet substrates for the Rhynie plants.
Ascomycetes
The ascomycetes present in the chert such as the perithecial ascomycete Paleopyrenomycites devonicus Taylor, Hass, Kerp, M. Krings & Hanlin (Taylor et al. Citation2005a) that occurs beneath the epidermis and on the enation bases of Asteroxylon mackiei stems (Taylor et al. Citation2004, Citation2005a) was considered a parasite and compared with extant pyrenomycetes. Many extant members of this group are reported from wetlands (e.g. Shearer et al. Citation2007; Raja et al. Citation2009 and references therein). Indeed, the acscomycetes are particularly important in standing decay of modern salt marsh plants where they occupy similar positions within epidermal tissues (e.g. Newell, Citation1996; Buchan et al. Citation2003). Ascomycetes (along with zygomycetes and deuteromycetes) have been isolated from tufa deposits and wetland soils from the hot spring influenced margins of Mono Lake, California (Steiman et al. Citation2004). Their presence in Asteroxylon may thus be indicative of wetland or dryland environments and fresh or saline waters.
Algal and bacterial associations with growing Rhynie plants as indicators of wetland ecology
A number of the Rhynie plants are recorded in situ and in growth position with algae, bacteria and biofilms (e.g. Fayers and Trewin (Citation2004): in Windyfield chert pods). These associations with growing plants emphasise tolerance of the higher plants of wetland conditions and wetland ecology (see p. 114).
The charophyte Palaeonitella cranii (Kidston & Lang) Pia is commonly observed in life position in massive to vuggy cherts (e.g. Trewin et al. Citation2003; Fayers and Trewin Citation2004) often in association with aquatic crustaceans and chytrid fungi. In many examples stands of the alga are preserved with common alignments suggestive of preservation whilst entrained in flowing water (e.g. Fayers and Trewin Citation2004). Kerp et al. (Citation2004) report the alga in dense mats in the ‘white chert’. In this environment it is reported in associated with well-preserved and in situ carpets of vertically aligned Aglaophyton (D.S. Edwards and Lyon Citation1983) and its gametophyte Lyonophyton plus large numbers of fertile Horneophyton (Kerp et al. Citation2004). Although Palaeonitella is commonly reported as representing a freshwater environment, extant charophytes also occur in brackish waters (Kelman et al. Citation2004) and are often early colonisers of disturbance-prone environments. In Yellowstone the extant charophyte Chara globularis Thuill. occurs in hot spring run-off streams and apron pools where water temperatures of c. 30 °C and pH 8.66–8.99 are recorded (Kelman et al. Citation2004).
Dotzler et al. (Citation2007) reported the first occurrence of prasinophycean alga (of the genus Cymatiosphaera) from ‘freshwater’ noting that earlier examples were primarily recorded from marine settings. D.S. Edwards and Lyon (Citation1983) recorded filamentous chlorophyte algae (Mackiella and Rhynchertia) from the ‘white chert’ which were commonly associated with stands of Rhynia.
Cyanobacteria are another common element of aquatic environments of Rhynie. D.S. Edwards and Lyon (Citation1983) described coccoid sheet-forming cyanobacteria (Rhyniococcus). Kidston and Lang (Kidston and Lang Citation1921b) described two species of Oscillatoria-like cyanobacteria Archaeothrix oscillatoriformis Kidston et W.H. Lang and A. contexta Kidston et W.H. Lang. Croft and George (Citation1959) reported three filamentous cyanobacteria Kidstoniella fritschi Croft et George, Langiella scourfieldi Croft et George and Rhyniella vermiformis Croft et George. Archaeothrix contexta was observed to occur within stems of Rhynia (Citation1921b) and more recently an Archaeothrix-like cyanobacterial endophyte has also been reported from degrading sporangia of Aglaophyton (Taylor and Krings Citation2005). Further evidence of putative symbiotic endophytic cyanobacteria (again Oscillatoria) was reported from the chert within in situ prostrate mycorrhizal axes of Aglaophyton which host abundant cyanobacterial filaments in the subepidermal and cortical tissues (Krings et al. Citation2009). Infection of the host occurred as cyanobacteria entered the plant via stomata.
Mat-forming cyanobacteria in the chert are represented by Croftalania venusta M. Krings (Krings et al. Citation2007) that colonised aquatic substrates and also formed sheaths with tufted projections on submerged plant axes. Less well preserved microbial sheaths are an extremely common feature surrounding the axes of higher plants (particularly Rhynia and Aglaophyton) in the wetland environments of the Windyfield locality (e.g. Fayers and Trewin Citation2004) and are also reported from the Rhynie locality (Trewin et al. Citation2003, , 6). Comparable features (e.g. microbial sheaths and mats) are commonly observed in the geothermal wetlands of Yellowstone (e.g. Trewin et al. Citation2003; Channing and Edwards Citation2009).
Preservational environments
Whilst the growth environments of the Rhynie plants have been viewed as a range of dryland to wetland habitats, the preservation environments, because of the need for large volumes of silica-rich water in the formation of chert and in the process of exceptional preservation, have universally been viewed as wetland environments. A number of the ecological and autecological features and biotic associations we have highlighted above e.g. growth of gametophytes on wet sinter aprons and sporophytes and gametophytes in pools surrounded by algae, suggest that they are often one and the same. A direct example of the temporal scale of flooding events is provided by the periods required for germination and growth to reproductive maturity of extant charophytes. These are not inconsiderable. Casanova and Brock (Citation1999) report mean establishment times (from oospore to shoot growth > 3 cm) for five charophyte species in tropical wetlands of New South Wales, Australia that range from 40–306 days. The observation by Kerp et al. (Citation2004) that beds of Palaeonitella, plus mature stands of Horneophyton and Aglaophyton, were growing in an aquatic environment prior to silicification provide a clear indication of the tolerance of protracted periods of immersion in the higher plants. Further direct evidence for tolerance of protracted flooding includes the observation that Rhynie plants are commonly preserved with erect axes. This shows an apparent resistance to wilting, that given the period required for tissue stabilisation by silica would be manifest in extant dryland plants.
We consider that the most parsimonious conclusions based on these many lines of evidence are that: (1) the plants were at least flooding tolerant, and (2) that as flooding events were often protracted the plants coped with fluids that for many dryland plants would be toxic.
Palaeo-ecophysiology
Evidence from anatomy
Stomata
Stomatal and particularly sub-stomatal characteristics in Rhynia gwynne-vaughanii and Aglaophyton major indicate high water use efficiency (Edwards et al. Citation1998). In both taxa, but especially well illustrated in Aglaophyton, there is a substomatal channel widening into a substomatal cavity with thickening and probable cutinisation of the walls of hypodermal and cortical cells adjacent to the channel. The hypodermal cells partially underpin or cradle the guard cells which possess pronounced stomatal ledges (Edwards et al. Citation1998, fig. 5). The deeply-seated substomatal chamber is lined with parenchymatous cells which often possess extensions such that there is an extensive intercellular space system forming a tissue that is inferred to have been the site of photosynthesis. These hypodermal and outer cortical cell adaptations are thought to have reduced transpiration as would have the very low stomatal densities (also noted in Nothia and Horneophyton: Edwards Citation1998; Edwards et al. Citation1998). A similar conclusion was reached by Roth-Nebelsick and Konrad (Citation2003), using a porous model approximation involving these anatomical features, with Aglaophyton showing a lower transpiration rate than Rhynia and both smaller than in extant plants. They agreed with Edwards et al. (Citation1998) that these anatomical characters reflected “an optimisation strategy with a fine tuning of gaseous exchange” (p. 153), feasible because of the relatively high carbon dioxide concentrations in the atmosphere. Roth-Nebelsick and Konrad suggest that the high water-use efficiency or ‘water-conserving strategy’ was an adaptation to the relatively low capacity for water uptake in the limited rhizomatous system, as was also the whole plant morphology of many of these aerial axes.
A further adaptation to reduce water loss is seen in the sunken stomata of Asteroxylon which are borne only on stems with short spiny and distal leaves in the fertile zone (Edwards et al. Citation1998). These contrast with the more conventional stomata found on both stems and the central parts of leaf bases where the long, fleshy leaves are oval in cross section. It is tempting to conclude that these leaves would have allowed an increase in humidity over the stomata (Münster University Palaeobotanical Research Group Citation2000), thus reducing transpiration rate in contrast to the situation in the spiny leaves with exposed but sunken stomata. Whether or not the two types occur on the same plant or even belong to the same species remains conjectural. The second certainly represents a xeromorphic character in the lycophyte. However, the frequency of stomata in Asteroxylon is an order of magnitude greater than in the remaining Rhynie chert plants and led Kerp (Münster University Palaeobotanical Research Group Citation2000) to suggest that Asteroxylon had a greater capacity to regulate water-loss than the axial vascular plants (and hence was able to colonise drier environments).
Intercellular space systems
The preservation of intercellular air-space systems in the Rhynie chert fossils provides a unique opportunity to deliberate on gaseous diffusion in early land plants. In addition to the putative photosynthetic tissue mentioned above, air spaces are developed to varying extents throughout the cortex. Thus, in Rhynia gwynne-vaughanii the cells of the inner cortex have “fairly large intercellular spaces” and were putatively associated with photosynthesis (Kidston and Lang Citation1917b, 112). They do not occur in the stele, but in the zosterophyll Ventarura lyonii Powell, Edwards & Trewin a conspicuous intercellular air-space system which occupies a considerable volume characterises the innermost zone of a three-layered cortex (Powell et al. Citation2000b). A similar zone occurs in sterile axes that are associated with the fertile axes and, in that they bear outgrowths similar to rhizoids, are interpreted as rhizomes. In a second zosterophyll Trichopherophyton, the inner cortex of the aerial axes comprises a tissue with well-developed intercellular spaces and a tendency to radial alignment (Lyon and Edwards Citation1991). This is accentuated in the vertical lamellae noted in the trabeculate zone in the inner cortex of the aerial shoots of Asteroxylon, but not in its rooting systems (Kidston and Lang Citation1920b). The latter are sometimes characterised by large cavities, but here as in other examples (Aglaophyton (Kidston and Lang Citation1917b, Plate IV, fig. 19)) their association with very poorly preserved cells suggests decay rather than the presence of a large aerating system. Thus the abundant air spaces that characterise many plants growing in water-logged conditions today are absent in aerial axes and rhizomes of the axial Rhynie chert plants. That those in aerial stems of Asteroxylon had this function as suggested by Kidston and Lang (Citation1920b) is more appealing to us than the possibility that they indicate ‘cheap’ construction! (Bateman Citation1991). Indeed, work in progress on an Upper Devonian hot spring locality in Australia indicates that there geothermal wetlands were dominated by a lycopod with abundant air spaces.
Giant cells
The epidermis in rhizomes of Nothia aphylla contains cells 250–500 μm long, 70–80 μm wide and 95–125 μm deep, which are much longer than the remainder. They may occur in short rows or individually. Kerp et al. (Citation2001) explored a number of possible functions (viz. excretion, crystal or water storage, hinge cells) as found in similar cells in extant plants and concluded they were probably associated with water storage.
Horneophyton lignieri (Kidston & Lang) Barghoorn & Darrah has long epidermal cells sometimes attaining a millimetre in length distally. These cells are extremely prominent in longitudinal section because their width is up to nine times greater than that of the sectioned guard cells, and they appear equally as gigantic when compared with the more or less isodiametric cells of the cortex and narrow elongate cells of the hypodermis (Edwards et al. Citation1998, fig. –G). These authors did not discuss the functions of such cells, but a water storage function inter alia again seems feasible.
Rhizoids
Rhizoids are often found as unicellular outgrowths on the lower surface of a rhizome (where usually taken to infer a subaerial habit) or more rarely around the entire periphery. Their presence on the basal regions of closely packed aerial axes of Rhynia gwynne-vaughanii led Kerp (Münster University Palaeobotanical Research Group Citation2000) to suggest that they were involved in absorption of atmospheric moisture because the rhizomatous areas “probably had real difficulties in absorbing sufficient water”. Kidston and Lang (Citation1921a) had much earlier suggested that the protuberances (often bearing the rhizoids) were an adaptation to excessive atmospheric moisture.
Periphery with thick-walled cells
The aerial axes of Nothia aphylla are characterised by a ‘multi layered epidermis’ of thick-walled cells which Kerp et al. (Citation2001) postulated would have reduced transpiration. However, the taxon possessed stomata that were exposed to the atmosphere on the apices of hemispherical emergences that had only a thickened epidermal layer, and this combination, when factored into the model developed by Roth-Nebelsick and Konrad (Citation2003), resulted in a transpiration rate in excess of that calculated for Rhynia and Horneophyton.
These adaptations, vis. putatively to reduce water loss and hence increase water use efficiency and/or for water storage and absorption of atmospheric water, all suggest that either water was not consistently present in the substrate (i.e. drought), or if present may not have been readily available to the higher plants.
Putative ecophysiology of Rhynie plants
Given the tolerances that allow survival in the wetlands associated with hot springs at Yellowstone, it is considered highly likely that the Rhynie chert plants possessed similar tolerances for drought and salinity and were capable of responding to variations in stress by altered metabolism. Relevant here is the probability that the genetic underpinning for such tolerances occurs in all higher plants (Bartels and Sunkar Citation2005). Thus even Arabidopsis which, for example, does not tolerate extremes of salt stress shows sufficient reactions to increased salinity (Zhu Citation2000) that it can be used to elucidate stress response pathways, although the latter are perhaps better investigated using extreme drought (e.g. Bartels and Salamini Citation2001) and salinity tolerant plants (e.g. Zhu Citation2001). Such studies reinforce the concept that no unique genes have been acquired for tolerance, but are activated at different thresholds, that the complexes involved are ubiquitous and the enzymes themselves are as intolerant of high salt concentrations in halophytes as are those in glycophytes (Munns Citation2002). Supporting evidence comes from similarities of initial responses to increased drought and salinity stress. These involve cellular dehydration production of reactive oxygen species and increases in abscisic acid (Bartels and Sunkar Citation2005). It is beyond the scope of this paper to report on the disentangling of the various regulatory circuits, including stress sensors, transcription factors and proteins involved, but stress responsive gene families have been isolated from a number of angiosperms. Of particular interest here would be the demonstration of their presence in spore-producing embryophytes. The recent publication of the genome of the moss Physcomitrella patens (Hedw.) Bruch & Schimp. (Rensing et al. Citation2008) has allowed comparison with those of angiosperms and unicellular green algae. It shows the presence of desiccation tolerant genes which are shared with those in liverworts, as might be anticipated in a poikilohydric lifestyle, but are not expressed in the vegetative cells of homoiohydric plants. Access to the complete genomes of lycophytes (e.g. Selaginella), horse-tails and ferns and hence demonstration of the genes associated with water and salt stress is essential for assessing the ubiquity and hence antiquity of sequences in vascular plants. The almost complete absence of these groups and most bryophytes on saltmarshes today raises the possibility that tolerance of salt toxicity might have been acquired later than that for more general osmotic stress. Indeed a polyphyletic origin of halophytes was postulated by Flowers et al. (Citation1977) in listing their occurrence in orders of higher plants. However the environments in which the Rhynie chert plants flourished strongly suggest the possession of tolerance to water and salt stress in these early vascular plants.
Were Rhynie chert plants typical of coeval Lower Devonian vegetation or were they highly adapted endemics?
Accepting our postulate that the Rhynie plants must have been able to endure water and chemical stress, the question now arises as to whether there were highly specialised plants restricted to such hot-spring environments and similarly stressed ones (e.g. salt marshes), or were common in the vegetation that colonised water-side habitats throughout the Old Red Sandstone continents. The impact of Kidston and Lang's series of papers had been two-fold. First, there was the phenomenal anatomical preservation and secondly the architectural simplicity of the plants themselves, which for many years were considered to be Middle Devonian (Kidston and Lang Citation1921a). It was not until 1967 that Richardson proposed a Siegenian–Emsian age based on comparative palynology with the Midland Valley of Scotland. Reasons for this simplicity had been long debated. Scott (Citation1920) in acknowledging that Kidston and Lang's Psilophytales and particularly the Rhyniaceae were by far the simplest vascular plants then known expressed reservations that all their characters were primitive. He reiterated Kidston and Lang's comments on the possible xerophytic status of ‘Rhynia’, based here on stomatal numbers, and postulated that it, together with Horneophyton, were secondarily reduced.
Leclercq (Citation1954) in a paper entitled ‘Are the Psilophytales a starting or a resulting point’ proposed a phylogenetic/phyletic explanation on the basis that these axial plants represented a lineage that evolved in parallel to the other major groups; Darrah (Citation1960) additionally suggested that the simplicity resulted from growth under ‘peculiar’ ecological conditions; Knoll (Citation1985) in a similar approach but comparing the affinities of Rhynie chert plants and coeval vegetation, interpreted the Rhynie chert as a peat-forming swamp that might be compared with later Carboniferous swamp floras in which the latter were considered as relictual because they contained higher numbers of taxa that might have been significant elements of the earlier floodplain floras. Thus he called the swamps ‘evolutionary museums’ in the sense that the plants were unlikely to have produced new groups which would have become ecologically widespread.
Of course these speculations were published before the recent resurgence of interest in the Rhynie chert, the demonstration of greater sophistication of growth form in taxa such as Rhynia and Nothia, and the discovery of the two zosterophylls, but provide a historical insight into the ongoing debate on the uniqueness of the Rhynie plants.
The problem is compounded by taphonomy – the Rhynie plants were essentially of parenchymatous construction relying on turgor for support in an environment where water was almost always present. By contrast the ‘Old Red Sandstone plants’ grew in seasonally-influenced habitats and possessed abundant structural/lignified tissues of much higher fossilisation potential. Thus on preservational grounds the Rhynie plants are unlikely to be preserved in clastic depositional environments, and in rare cases where, for example, compression fossils show similar sporangial shapes to Rhynie plants (e.g. Aglaophyton/Salopella: Edwards and Richardson Citation1974), they lack the anatomy for confident identification, especially as there is undoubtedly convergence in sporangial form in these early land plants.
However, even though the ground tissues, both vegetative and reproductive, of Rhynie plants might have rapidly decayed and escaped fossilisation outside the influence of siliceous fluids, their sporopollenin-impregnated spores would have been far more resilient to degradation, as is evidenced by the abundant dispersed spore record in associated clastic rocks (Wellman Citation2004, Citation2006). Knowledge of in situ spores integrated with the latter should thus inform on the presence of a plant even in the absence of its megafossils. This was the approach adopted by Wellman (Citation2004, Citation2006), who initially surveyed the in situ spores of all the Rhynie taxa () and has, with Kerp and Hass, produced more detailed accounts of spores of Horneophyton (Wellman et al. Citation2004) and Aglaophyton (Wellman et al. Citation2006). All the in situ spores are present in the rocks between the cherts recovered from cores, and all, at least at the generic level, are found in coeval rocks elsewhere on the Old Red Sandstone Continent. Considering the former, the dispersed spore flora might have been produced by vegetation in the vicinity of rivers and thus might have colonised typical ‘mesophytic’ habitats or vegetation surrounding hot springs elsewhere. However looking in more detail at the spores themselves with respect to wider distribution by the plants, Retusotriletes and Apiculiretusispora species have been recorded in a number of sporangia of diverse plant types (Edwards and Richardson Citation1996), indeed smooth retusoid forms appear to characterise the zosterophyll lineage, and thus are not unequivocal evidence for the presence of the Rhynie taxa elsewhere (e.g. Aglaophyton in Wellman et al. Citation2004). The spores of Horneophyton, Emphanisporites decoratus Allen are far more promising, because the spore is highly distinctive (although with some variation in size and distribution of distal ornament) and in addition to being recovered from the clastic beds between the cherts is also found throughout the Old Red Sandstone Continent (e.g. Anglo-Welsh and Ardennes-Rhenish basins, Canada, Spitsbergen), sometimes occurring abundantly in rocks assigned to the polygonalis-emsiensis spore biozone (Pragian-basal Emsian). This led Wellman et al. (Citation2004) to suggest a similar distribution for Horneophyton and gave support to Wellman's hypothesis that the Rhynie plants were not ‘highly specialised or adapted’ to the hot-spring environment, but were the only members of a diverse and widespread flora able to tolerate that environment, ‘i.e. they were pre-adapted’ (Wellman Citation2004, Citation2006). The absence of Horneophyton outside the chert reflected a taphonomic bias. Some support for Wellman's taphonomic hypothesis came from a solitary sporangium, identified as cf. Horneophyton and containing Emphanisporites cf. micrornatus Richardson & Lister from the Lochkovian of the Welsh Borderland (Edwards and Richardson Citation2000). This specimen was encrusted by a microbial film which perhaps explains its coherence (Wellman Citation2004) but since other sporangia in the assemblage are charcoalified may point to the plant's scarcity in the assemblage. The almost complete absence of the spore in coprolites at the locality indicated a paucity of spores and particularly spore clusters in the litter, which was transported together with a highly diverse embryophytic assemblage, and perhaps points to cf. Horneophyton colonising niches outside the catchment area of the stream or river.
Table 3. Rhynie chert plants with in situ spores whose names were originally erected for dispersed spore taxa (based on Wellman 2004)
We are of the opinion that given our conclusions on the habitats of the plants in wetlands influenced by siliceous fluids – an essential prerequisite for fossilisation – that the plants themselves were adapted anatomically and, from Yellowstone observations, physiologically to highly stressed conditions. That these tolerances were not universally present is evidenced by the absence in the chert of coeval plants that typify riparian habitats in other parts of the Old Red Sandstone Continent (). Whether or not the Rhynie plants were also part of this more widespread vegetation is less clear. A striking feature of Yellowstone vegetation is the very marked distinction in composition of communities influenced by siliceous waters and those of drier substrates watered by pluvial/meteoric sources and dominated by typical mesophytes/glycophytes. While the latter clearly lack the adaptation to withstand the physical environmental stresses in the vicinity of hot springs, it is thought that halophytes cannot survive in non-saline conditions because of competition (Waisel Citation1972). There is little experimental evidence to prove this for halophytes in natural vegetation. Indeed monocot halophytes today grow optimally in the absence of or very low concentrations of salt (Glenn et al. Citation1999), but some support comes from crop plants (Waisel Citation1972). Thus we would suggest that a similar situation existed for the Rhynie plants which might have flourished in more unstable stressed environment, e.g. in areas of evaporation around lakes and lagoons in seasonally-dry semi-arid climates and even in coastal salt marshes. The latter might well have provided the environmental stimuli which resulted in the evolution of extreme tolerance to water and salt stress as is postulated for angiosperms but would not explain heavy metal tolerances. In this scenario, the adapted plants would have migrated inland and flourished in stressed environments such as provided by the hot springs. The alternative is that all early vascular plants possessed the gene families which may well have had already evolved as they acquired their homoiohydric lifestyle, but have been variably expressed depending on the degree of stress in the environment.
Table 4. Lists of taxa from localities in the polygonalis-emsiensis spore zone.
To a certain extent this fits in with the evolutionary scenario of Pierce et al. (Citation2005), who postulated that the cellular stress response common to all cells (including single-celled organisms) in their possession of a minimal stress proteome (c. 300 proteins) was augmented by multicellularity and concomitant morphological complexity which was exhibited by the early vascular plants.
Such drought tolerance (as opposed to suspension of activity that evolved earlier in mosses (sic)) was considered to have been driven by nutrient-limited ecosystems, i.e. the vascular system and stomata were considered as adaptations for controlled nutrient uptake and transport (Pierce et al. Citation2005). We would argue that the major selection pressure would have been limited or ephemeral water supplies and evaporation. Then, as Munns (Citation2002, p. 248) commented, “any improvements in drought resistance would make a plant more adapted to saline soil”, which would have involved reduction in salt uptake and cellular compartmentalisation to avoid toxicity. Thus we would postulate that the basal embryophytes, like bryophytes today and the earliest tracheophytes, lacked salt tolerance (Flowers et al. Citation1986). Exactly where and when this acquisition occurred is debatable. Possible environmental drivers are salt marshes, intertidal areas, evaporites around lakes, calcretes, and, of course, hot springs systems, and such adapted plants would have possessed the genetic plasticity to respond to various stress conditions in hostile subaerial habitats.
Synthesis
-
From observations of the Rhynie chert, where drill cores contain up to c. 53 stacked and generally richly fossiliferous chert lenses, it is clear that preservation at the locality occurred in a widespread and commonly reoccurring setting where plant immersion in silica-rich waters was a frequent almost systematic and inherent feature of the environment. After nearly 100 years of research only seven sporophyte plant species have been detected from the locality of which four (Rhynia, Aglaophyton, Horneophyton and Asteroxylon) might be considered to dominate the flora. Sedimentological evidence supports the growth of the various plants on substrates ranging from sandy/muddy clastic surfaces, to organic-rich sands, of various humus-content, to sinter apron surfaces. As these few plants consistently colonised and were preserved, whilst plants that might be viewed as typical Lower Devonian mesophytes never became preserved, and as taphonomic studies indicate a close correlation between growth in environments influenced by large scale and protracted influxes of geothermal waters, we consider it parsimonious to view the plants as inhabitants of geothermal wetlands. Specifically, we identify environments at the periphery of alkali–chloride hot springs subject to the continual replenishment of silica-rich fluids which are prerequisite in large volumes for creation of sinter, the chert matrix, and exceptional preservation of plants.
-
Plants show considerable anatomical adaptations to reduce evapotranspiration and increase water-use efficiency. Rarer evidence exists for possible water storage, e.g. in the giant epidermal cells of Horneophyton and postulated hinge cells in Nothia. Are these adaptations to fluctuations in water table or an indication of ‘physiological drought’? In that halophytes once thought of as ‘physiological xerophytes’ are now known to transpire at similar rates to glycophytes when growing in fresh water and those of salt marshes have far fewer xerophytic adaptations than those on dunes or foreshore habitats (Crawford Citation1989), the former may have been in the case for the Rhynie plants. However, detailed analysis of potential habitats and the conclusion realised in (1) above indicate that the plants were irrigated by water rich in a variety of chemicals, not the least salt, and hence must have been tolerant of chemical stress. Recent molecular genetic studies indicate that extreme stress tolerant plants do not possess unique genes and that initial responses to salt and water stress are similar (Munns Citation2002; Bartels and Sunkar Citation2005) thus it is tempting to conclude that such genes were acquired very early in the evolution of land plants, perhaps initially in drought tolerance associated the evolution of homoiohydry followed by very early acquisition of salt-toxicity tolerance.
-
Plants were probably both halophytic and alkaliphilic with reliance on turgor (and possibly even silica) for support, and thus were probably more widespread in the Lower Devonian in stressed environments such as salt marshes (and perhaps margins of lagoons or lakes), where substrates are more hostile to mesophytes. It is noteworthy that with one exception, the prominent peripheral support tissues with polyphenols-impregnated thick walls that characterises zosterophyllophytes (Edwards Citation1970) and trimerophytes (Edwards et al. Citation1997) are not recorded in the Rhynie chert. The exception is seen in the rare zosterophyll Ventarura (Powell et al. Citation2000b) where a thick-walled zone is present in the middle cortex. The sterome is thought to have been an important support tissue in plants growing in river banks to interfluvial habitats that would have been subjected to periods of water stress in the postulated semi-arid and probably seasonal climate (e.g. Clarke and Parnell Citation1999; Fayers and Trewin Citation2004). Whether or not the Rhynie plants were able to survive under such conditions, but were not preserved, remains debatable, but returning to the Yellowstone analogy, there is a very strong demarcation between the ‘hot spring’ wetland assemblages and the more typical mesophytic herbaceous vegetation raising the probability that the former were outcompeted by the mesophytes at Rhynie. Direct experimental evidence for this in living plants is scarce, being concentrated on crop plants and negligible for wild halophytes (Waisel Citation1972).
-
Previous speculations on the ecophysiology of the Rhynie plants have overlooked the roles of silica in the amelioration of both salt and heavy-metal toxicity. The former has been more widely investigated in studies on agricultural crops (e.g. Matoh et al. Citation1986; Neumann and zur Nieden Citation2001; Ma Citation2004). High concentrations of arsenic recorded in Eleocharis and Triglochin species at Yellowstone (Kocar et al. Citation2004) infer a similar role for silica in the hot spring environment. To date we have failed to demonstrate high concentrations of heavy metals in the Rhynie chert plants, although analyses of chert and sediment samples (Rice and Trewin Citation1988; Rice et al. Citation1995; Baron et al. Citation2004) indicate the possibility of toxic concentrations in irrigating fluids. Here the postulated ubiquity and antiquity of heavy-metal-tolerant genes strongly suggest their presence in the Rhynie plants, but not necessarily their origin.
-
Raven (Citation1983) in reviewing the role of silica in structural support in terrestrial plants mentioned the difficulties of detecting the distribution of silica in early vascular plants whose subsequent preservation relied on silicification. Here we introduce the possibility based on comparisons with Yellowstone plants that silica accumulations provided support in addition to that traditionally assigned to turgor. As mentioned in (3) above, lignification is very limited in the Rhynie plants, while silicified walls would have resulted in substantial energy saving (Raven Citation1983).
-
If silica accumulators, the Rhynie chert plants would seem to have been predisposed to fossilisation although Siever and Scott (Citation1963) considered that silica deposition in tissues was not a factor in their silicification. In contrast Owen et al. (Citation2008) considered that silica deposition by plants in-life was a contributing factor to preservation in rhizolith horizons around the Kenyan Rift lakes. Additionally, the presence of silica as a ‘coherent external skeleton’ in the glumes and lemmas of two Tertiary prairie grass genera Stipidium and Berriochloa was thought of as a facilitator of their preservation as fossils (Elias Citation1942), while other parts e.g. leaves and awns had insufficient amounts of ‘siliceous impregnation’ to preserve them. In the hot spring environment parallels might be drawn from observations on Eleocharis, where the presence of silica in the living plant facilitates additional precipitation and hence rapid stabilisation and prevention of collapse of soft tissues (Channing and Edwards Citation2004, Citation2009) although complete silicification is a much longer process involving continual inundation with silica-rich fluids over many months. It is perhaps no coincidence that Eleocharis sub-fossils occur in the Quaternary deposits at Yellowstone, whereas Triglochin maritimum, also frequently found in the wetlands but not a silica accumulator (De Bakker et al. Citation1999), is seldom found fossilised.
We are convinced that the Rhynie plants were physiologically specialised to withstand osmotic and chemical stress. Whether or not all were endemics remains equivocal. Evidence from extant halophytes, which are very rarely endemics, suggests not, as does the widespread distribution of certain spores (e.g. Emphanisporites decoratus) sometimes in abundance in coeval clastic rocks. However, we consider it unlikely that they were common elements in the mesophytic vegetation that included plants not able to survive in the hot spring environment, but instead were confined to water-stressed habitats.
Notes on contributors
Alan Channing is a palaeontologist and geobiologist interested in the geochemistry and biotas of hot spring areas and the physical and chemical challenges faced by thermally influenced ecosystems. He has investigated active hot spring areas worldwide and is currently investigating the biota of newly discoved fossil hot spring ecosystems ranging in age from the early Holocene to Lower Palaezoic.
Dianne Edwards is a Distinguished Research Professor at Cardiff University, Wale who specialises in the thorough morphological and anatomical descriptions of fossils of early land plants using a wide range of techniques and uses such a data base to speculate more confidently on their affinities, evolution, palaeoecology, palaeophysiology and phytogeography and roles in early terrestrial ecosystems.
Acknowledgements
The invitation to publish a paper as part of John Raven's retirement celebrations has provided an opportunity not only to bring together all the published observations on the habitats and functioning of the Rhynie chert plants for the first time but also to indulge in some highly speculative inferences that have been based on our studies at Yellowstone and physiological and molecular work on Recent plants. It was the integration of the physiological and quantitative anatomical data by John Raven that initiated interest in the evolution of homoiohydry, acquisition of characters and functioning of early vascular plants, and encouraged DE to broaden her palaeobotanical studies beyond anatomy and morphology. For this, she is exceedingly grateful. Both authors wish to thank Professor Nigel Trewin, the staff of Yellowstone National Park, and Stuart Davies, Hans Kerp and two anonymous reviewers for critical reading of the manuscript. Funding for this research was provided by The Leverhulme Trust (Grant Number F/00 407/S) and NERC (Grant Number NE/F004788/1).
References
- Anderson , LI , Crighton , WRB and Hass , H. 2004 . A new univalve crustacean from the Early Devonian Rhynie chert hot spring complex . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 355 – 369 .
- Anderson , LI and Trewin , NH. 2003 . An Early Devonian arthropod fauna from the Windyfield chert, Aberdeenshire, Scotland . Palaeontology , 46 : 467 – 510 .
- Ball JW, Nordstrom DK, Jenne EA, Vivit DV. 1998. Chemical analyses of hot springs, pools, geysers, and surface waters from Yellowstone National Park, Wyoming, and Vicinity, 1974–1975. USGS Open-File Report 98–182. Available at http://wwwbrr.cr.usgs.gov/projects/GWC_chemtherm/pubs/ofr 98-182.pdf (Accessed: 10 October 2009 ).
- Baron , M , Hillier , S , Rice , CM , Czapnik , K and Parnell , J. 2004 . Fluids and hydrothermal alteration assemblages in a Devonian gold-bearing hot-spring system, Rhynie, Scotland . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 309 – 324 .
- Barr , DJS. 2001 . “ Chytridiomycota ” . In The Mycota , Edited by: McLaughlin , DJ , McLaughlin , EG and Lemke , PA . Vol. VII , 93 – 112 . New York : Springer-Verlag . 5Part A
- Barrett-Lennard , EG. 2003 . The interaction between water logging and salinity in higher plants: causes, consequences and implications . Plant and Soil , 253 : 35 – 54 .
- Bartels , D and Salamini , F. 2001 . Desiccation tolerance in the resurrection plant Craterostigma plantagineum. A contribution to the study of drought tolerance at the molecular level . Plant Physiology , 127 : 1346 – 1353 .
- Bartels , D and Sunkar , R. 2005 . Drought and salt tolerance in plants . Critical Reviews in Plant Sciences , 24 : 23 – 58 .
- Bateman , RM. 1991 . “ Palaeoecology ” . In Plant fossils in geological Investigation: the Palaeozoic. Chichester (UK) , Edited by: Cleal , CJ . 34 – 116 . Ellis Horwood Ltd .
- Bohrer , KE , Friese , CF and Amon , JP. 2004 . Seasonal dynamics of arbuscular mycorrhizal fungi in differing wetland habitats . Mycorrhiza , 14 : 329 – 337 .
- Boothroyd , IKG. 2009 . Ecological characteristics and management of geothermal systems of the Taupo Volcanic Zone, New Zealand . Geothermics , 38 : 200 – 209 .
- Brock , TD. 1994 . Life at high temperatures. Yellowstone National Park (USA) , Yellowstone Association for Natural Science, History & Education, Inc .
- Bryan , ST. 1995 . The geysers of Yellowstone. Niwot (CO) , University Press of Colorado .
- Buchan , A , Newell , SY , Butler , M , Biers , EJ , Hollibaugh , JT and Moran , MA. 2003 . Dynamics of bacterial and fungal communities on decaying salt marsh grass . Applied Environmental Microbiology , 69 : 6676 – 6687 .
- Cady , SL and Farmer , JD. 1996 . “ Fossilisation processes in siliceous thermal springs: trends in preservation along the thermal gradient ” . In Evolution of hydrothermal ecosystems on Earth (and Mars?). Ciba Foundation Symposium 202. Chichester (UK) , Edited by: Bock , GR and Goode , J . 150 – 173. . John Wiley & Sons .
- Casanova , MT and Brock , MA. 1999 . Life histories of charophytes from permanent and temporary wetlands in Eastern Australia . Australian Journal of Botany , 47 : 383 – 397 .
- Channing , A. 2001 . Processes and environments of vascular plant silicification , Unpublished PhD thesis, Cardiff University (UK) .
- Channing , A. 2003 . The Rhynie chert early land plants: palaeo-ecophysiological and taphonomic analogues . Transactions of the Institution of Mining and Metallurgy (Section. B: Applied Earth Science) , 112 : B170 – B171 .
- Channing , A and Butler , IB. 2007 . Cryogenic silica precipitation at Yellowstone hot springs: Earth and Planetary Science Letters , 257 : 121 – 131 .
- Channing , A and Edwards , D. 2004 . Experimental taphonomy: silicification of plants in Yellowstone hot spring environments . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 503 – 521 .
- Channing , A and Edwards , D. 2009 . Silicification of higher plants in geothermally influenced wetlands: Yellowstone as a Lower Devonian Rhynie analog . Palaios , 24 : 505 – 521 .
- Channing , A , Edwards , D and Sturtevant , S. 2004 . A geothermally influenced wetland containing unconsolidated geochemical sediments . Canadian Journal of Earth Sciences , 41 : 809 – 827 .
- Channing , A , Zamuner , AB and Zuñiga , A. 2007 . A new Middle–Late Jurassic flora and hot spring chert deposit from the Deseado Massif, Santa Cruz province . Argentina. Geological Magazine , 144 : 401 – 411 .
- Clarke , P and Parnell , J. 1999 . Facies analysis of a back-tilted lacustrine basin in a strike-slip zone, Lower Devonian, Scotland. Palaeogeography, Palaeoclimatology, Palaeoecology. , 151 : 167 – 190 .
- Clemens , S. 2001 . Molecular mechanisms of plant metal homeostasis and tolerance . Planta , 212 : 475 – 486 .
- Clemens , S. 2006 . Toxic metal accumulation responses to exposure and mechanisms of tolerances in plants . Biochimie , 88 : 1707 – 1719 .
- Colmer , TD and Flowers , TJ. 2008 . Flooding tolerance in halophytes . New Phytologist , 179 : 964 – 974 .
- Couch , JN. 1945 . Observations on the genus Catenaria . Mycologia , 37 : 163 – 193 .
- Crawford , RMM. 1989 . Studies in plant survival , Oxford : Blackwell Scientific Publications .
- Croft , WN and George , EA. 1959 . Blue-green algae from the Middle Devonian of Rhynie, Aberdeenshire . Bulletin British Museum of Natural History, Geology , 3 : 341 – 353 .
- Darrah , WC. 1960 . Principles of paleobotany , New York : The Ronald Press Company .
- Davies , MS and Singh , AK. 1983 . Population differentiation in Festuca rubra L. and Agrostis stolonifera L. in response to soil waterlogging . New Phytologist , 94 : 573 – 583 .
- De Bakker , NVJ , Hemminga , MA and Van Soelen , J. 1999 . The relationship between silicon availability and growth and silicon concentration of the salt-marsh halophyte Spartina anglica . Plant and Soil , 215 : 19 – 27 .
- Despain , DG. 1990 . Yellowstone Vegetation: consequences of environment and history in a natural setting . Roberts Rinehart, Boulder Colorado USA. p. , : 239
- Dotzler , N , Krings , M , Taylor , TN and Agerer , R. 2006 . Germination shields in Scutellospora (Glomeromycota: Diversisporales, Gigasporaceae) from the 400 million-year-old Rhynie chert . Mycological Progress , 5 : 178 – 184 .
- Dotzler , N , Taylor , TN and Krings , M. 2007 . A prasinophycean alga of the genus Cymatiosphaera in the Early Devonian Rhynie chert . Review of Palaeobotany and Palynology , 147 : 106 – 111 .
- Dotzler , N , Walker , C , Krings , M , Hass , H , Kerp , H , Taylor , TN and Agerer , R. 2009 . Acaulosporoid glomeromycotan spores with a germination shield from the 400-million-year-old Rhynie chert . Mycological Progress , 8 : 9 – 18 .
- Dunlop , JA , Anderson , LI , Kerp , H and Hass , H. 2004 . A harvestman (Arachnida: Opiliones) from the Early Devonian Rhynie cherts, Aberdeenshire, Scotland . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 341 – 354 .
- Edwards , D. 1970 . Further observations on the Lower Devonian plant, Gosslingia breconensis Heard . Philosophical Transactions of the Royal Society of London B , 258 : 225 – 243 .
- Edwards , D. 1998 . Climate signals in Palaeozoic land plants . Philosophical Transactions of the Royal Society of London B , 353 : 141 – 157 .
- Edwards , D , Ewbank , G and Abbot , GD. 1997 . Flash pyrolysis of the outer cortical tissues in Lower Devonian Psilophyton dawsonii . Botanical Journal of the Linnean Society , 124 : 345 – 360 .
- Edwards , D , Kerp , H and Hass , H. 1998 . Stomata in early land plants: an anatomical and ecophysiological approach . Journal of Experimental Botany , 49 : 255 – 278 . Special Issue
- Edwards , D and Richardson , JB. 1974 . Lower Devonian (Dittonian) plants from the Welsh boarderland . Palaeontology , 17 : 1 – 324 .
- Edwards , D and Richardson , JB. 1996 . “ Review of in situ spores in early land plants ” . In Palynology: principles and application, Volume 1 Principles. American Association of Stratigraphic Palynologists Foundation , Edited by: Jansonius , J and McGregor , DC . 391 – 407 . Salt Lake City, UT : Publishers Press .
- Edwards , D and Richardson , JB. 2000 . “ Progress in reconstructing vegetation on the Old Red Sandstone Continent: two Emphanisporites producers from the Lochkovian sequence of the Welsh Borderland ” . In New perspectives on the Old Red Sandstone , Edited by: Friend , PF and Williams , BPJ . Vol. 180 , 355 – 370 . London : Special Publications of the Geological Society of London .
- Edwards , DS and Lyon , AG. 1983 . Algae from the Rhynie chert . Botanical Journal of the Linnean Society , 86 : 37 – 55 .
- Elias , MK. 1942 . Tertiary prairie grasses and other herbs from the high plains. Geological Society of America . Special Paper , 41 : 1 – 176 .
- El-Saadawy , W. 1966 . Studies on the flora of the Rhynie chert , Unpublished PhD thesis, University of Wales (UK) .
- Emerson , R and Natvig , DO. 1981 . “ Adaptation of fungi to stagnant waters ” . In The fungal community. Its organization and role in the ecosystem , Edited by: Wicklow , DT and Carroll , GC . 109 – 128 . New York : Marcel Dekker .
- Engel , MS and Grimaldi , DA. 2004 . New light shed on the oldest insect . Nature , 427 : 627 – 630 .
- Epstein , E. 1994 . The anomaly of silicon in plant biology . Proceedings of the National Academy of Sciences, USA , 91 : 11 – 17 .
- Epstein , E. 1999 . Silicon. Annual Review of Plant Physiology . Plant Molecular Biology , 50 : 641 – 644 .
- Epstein , E and Bloom , AJ. 2005 . “ Mineral nutrition of plants: principles and perspectives ” . In Sunderland (MA) , 2nd , Sinauer .
- Fayers , SR. 2003 . The biota and palaeoenvironments of the Windyfield chert, Early Devonian, Rhynie, Scotland , Unpublished PhD thesis, University of Aberdeen (UK) .
- Fayers , SR , Dunlop , JA and Trewin , NH. 2004 . A new Early Devonian trigonotarbid arachnid from the Windyfield chert, Rhynie, Scotland . Journal of Systematic Palaeontology , 2 : 269 – 284 .
- Fayers , SR and Trewin , NH. 2004 . A review of the palaeoenvironments and biota of the Windyfield chert . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 325 – 339 .
- Flores-Tavizón , E , Alarcón-Herrera , MT , González Elizondo , S and Olguin , EJ. 2003 . Arsenic tolerating plants from mine sites and hot springs in the semi-arid region of Chihuahua, México . Acta Biotechnologia , 23 : 113 – 119 .
- Flowers , TJ. 1985 . Physiology of halophytes . Plant and Soil , 89 : 41 – 56 .
- Flowers , TJ and Colmer , TD. 2008 . Salinity tolerances in halophytes . New Phytologist , 179 : 945 – 963 .
- Flowers , TJ , Hajbagheri , MA and Clipson , NJW. 1986 . Halophytes . Quarterly Review of Biology , 61 : 313 – 337 .
- Flowers , TJ , Troke , PF and Yeo , AR. 1977 . The mechanism of salt tolerance in halophytes . Annual Review of Plant Physiology , 28 : 89 – 121 .
- Gleason , FH , Kagami , M , Lefevre , E and Sime-Ngando , T. 2008 . The ecology of chytrids in aquatic ecosystems: roles in food web dynamics . Fungal Biology Reviews , 22 : 17 – 25 .
- Gleason , FH , Letcher , PM and McGee , PA. 2004 . Some Chytridiomycota in soil recover from drying and high temperatures . Mycological Research , 108 : 583 – 589 .
- Gleason , FH , Midgley , DJ , Letcher , PM and McGee , PA. 2006 . Can soil Chytridiomycota survive and grow in different osmotic potentials? Mycological Research , 110 : 869 – 875 .
- Gleason , FH , Mozley-Standridge , SE , Porter , D , Boyle , DG and Hyatt , A. 2007a . Preservation of Chytridiomycota in culture collections. Mycological Research , 111 : 129 – 136 .
- Gleason , FH , Peter , M , Letcher , PM and McGee , PA. 2007b . Some aerobic Blastocladiomycota and Chytridiomycota can survive but cannot grow under anaerobic conditions . Australasian Mycologist , 26 : 57 – 64 .
- Glenn , EP , Brown , JJ and Blumwald , E. 1999 . Salt tolerance and crop potential of halophytes . Critical Reviews in Plant Sciences , 18 : 227 – 255 .
- Goldstein , JN , Hubert , WA , Woodward , DF , Farag , AM and Meyer , JS. 2001 . Naturalized salmonid populations occur in the presence of elevated trace element concentrations and temperatures in the Firehole River, Yellowstone National Park, Wyoming, USA . Environmental Toxicology and Chemistry , 20 : 2342 – 2352 .
- Greb , SF , DiMichele , WA and Gastaldo , RA. 2006 . “ Evolution of wetland types and the importance of wetlands in earth history ” . In Wetlands Through Time , Edited by: Greb , S and DiMichele , WA. Vol. 399 , 1 – 40 . Geological Society of America Special Papers .
- Guido , DM and Campbell , KA. 2009 . Jurassic hot-spring activity in a fluvial setting at La Marciana, Patagonia, Argentina . Geological Magazine , 146 : 617 – 622 .
- Guido , DM , Channing , A , Campbell , K and Zamuner , A . In press . Jurassic geothermal landscapes and ecosystems at San Agustín, Patagonia, Argentina . Journal of the Geological Society of London. , doi: 10.1144/0016-76492009-109
- Guidry , SA and Chafetz , HS. 2003 . Siliceous shrubs in hot springs from Yellowstone National Park, Wyoming, USA . Canadian Journal of Earth Sciences , 40 : 1571 – 1583 .
- Ha , NTH , Sakakibara , M , Sano , S , Hori , RS and Sera , K. 2009 . The potential of Eleocharis acicularis for phytoremediation: case study at an abandoned mine site . CLEAN - Soil, Air, Water , 37 : 203 – 208 .
- Habgood , KS , Hass , H and Kerp , H. 2004 . Evidence for an early terrestrial food web: coprolites from the Early Devonian Rhynie chert . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 371 – 389 .
- Hinman , NW and Walter , MR. 2005 . Textural preservation in siliceous hot spring deposits during early diagenesis: Examples from Yellowstone National Park and Nevada, USA . Journal of Sedimentary Research , 75 : 200 – 215 .
- Hodson , MJ , White , PJ , Mead , A and Broadley , MR. 2005 . Phylogenetic variation in the silicon composition of plants . Annals of Botany , 96 : 1027 – 1046 .
- Hoffman , Y , Aflalo , C , Zarka , A , Gutman , J , James , TY and Boussiba , S. 2008 . Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota) parasitic on the green alga Haematococcus . Mycological Research , 111 : 70 – 81 .
- Horne , J , Mackie , W , Flett , JS , Gordon , WT , Hickling , G , Kidston , R , Peach , BN and Watson , M. S. 1916 . “ The plant-bearing cherts at Rhynie, Aberdeenshire ” . In Report of the eighty-sixth meeting of the British Association for the Advancement of Science , 206 – 216 . London : John Murray .
- Jones , B and Renaut , RW. 2003a . Petrography and genesis of spicular and columnar geyserite from the Whakarewarewa and Orakeikorako geothermal areas, North Island, New Zealand . Canadian Journal of Earth Sciences , 40 : 1585 – 1610 .
- Jones , B and Renaut , RW. 2003b . Hot spring and geyser sinters: the integrated product of precipitation, replacement, and deposition . Canadian Journal of Earth Sciences , 40 : 1549 – 1569 .
- Jones , B , Renaut , RW and Rosen , MR. 1998a . Influence of microbes on the fabrics of siliceous microbialites: case study from Ohaaki Pool hot-spring system, North Island, New Zealand . Journal of Sedimentary Research , 68 : 413 – 434 .
- Jones , B , Renaut , RW and Rosen , MR. 1999 . Role of fungi in the formation of siliceous coated grains, Waiotapu geothermal area, North Island, New Zealand . Palaios , 14 : 475 – 492 .
- Jones , B , Renaut , RW and Rosen , MR. 2000 . Stromatolites forming in acidic hot-spring waters, North Island, New Zealand . Palaios , 15 : 450 – 475 .
- Jones , B , Renaut , RW and Rosen , MR. 2001 . Biogenicity of gold- and silver-bearing siliceous sinters forming in hot (75°C) anaerobic spring-waters of Champagne Pool, Waiotapu, North Island, New Zealand . Journal of the Geological Society , 158 : 895 – 911 .
- Jones , B , Renaut , RW , Rosen , MR and Klyen , L. 1998b . Primary siliceous rhizoliths from Loop Road Hot-Springs, North Island, New Zealand . Journal of Sedimentary Research , 68 : 115 – 123 .
- Karatygin , IV , Snigirevskaya , NS and Demchenko , KN. 2006 . Species of the genus Glomites as plant mycobionts in early Devonian ecosystems . Paleontological Journal , 40 : 572 – 579 .
- Kelman , R , Feist , M , Trewin , NH and Hass , H. 2004 . Charophyte algae from the Rhynie chert . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 445 – 455 .
- Kerp , H , Hass , H and Mosbrugger , V. 2001 . “ New data on Nothia aphylla Lyon, 1964 ex El Saadawy et Lacey, 1979: a poorly known plant from the Lower Devonian Rhynie chert ” . In Plants invade the land: evolutionary and environmental perspectives , Edited by: Gensel , PG and Edwards , D . 52 – 82 . New York : Columbia University Press .
- Kerp , H , Trewin , NH and Hass , H. 2004 . New gametophytes from the Early Devonian Rhynie chert . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 411 – 428 .
- Kidston , R and Lang , WH. 1917a . On Rhynia gwynne-vaughani . Report British Association for the Advancement of Science (Newcastle upon Tyne 1916) , : 493
- Kidston , R and Lang , WH. 1917b . On Old Red Sandstone plants showing structure, from the Rhynie chert bed, Aberdeenshire. Part I. Rhynia gwynne-vaughani, Kidston and Lang . Transactions of the Royal Society of Edinburgh , 51 : 761 – 784 .
- Kidston , R and Lang , WH. 1920a . On Old Red Sandstone plants showing structure, from the Rhynie chert bed, Aberdeenshire. Part II. Additional notes on Rhynia gwynne-vaughani Kidston and Lang; with descriptions of Rhynia major, n.sp., and Hornia lignieri, n.g., n.sp . Transactions of the Royal Society of Edinburgh , 52 : 603 – 627 .
- Kidston , R and Lang , WH. 1920b . On Old Red Sandstone plants showing structure, from the Rhynie chert bed, Aberdeenshire. Part III. Asteroxylon mackiei, Kidston and Lang . Transactions of the Royal Society of Edinburgh , 52 : 643 – 680 .
- Kidston , R and Lang , WH. 1921a . On Old Red Sandstone plants showing structure, from the Rhynie chert bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion of their bearing on the general morphology of the pteridophyta and the origin of the organisation of land-plants . Transactions of the Royal Society of Edinburgh , 52 : 831 – 854 .
- Kidston , R and Lang , WH. 1921b . On Old Red Sandstone plants showing structure, from the Rhynie Cert bed, Aberdeenshire. Part V. The Thallophyta occurring in the peat-bed; the succession of the plants throughout a vertical section of the bed, and the conditions of accumulation and preservation of the deposit . Transactions of the Royal Society of Edinburgh , 52 : 855 – 902 .
- Knoll , A. 1985 . Exceptional preservation of photosynthetic organisms in silicified carbonates and silicified peats . Philosophical Transactions of the Royal Society, London , 311B : 111 – 122 .
- Kocar , BD , Garrott , RA and Inskeep , WP. 2004 . Elk exposure to arsenic in geothermal watersheds of Yellowstone National Park, USA . Environmental Toxicology and Chemistry , 23 : 982 – 989 .
- Korndörfer , GH , Datnoff , LE and Corrêa , GF. 1999 . Influence of silicon on grain discoloration and upland rice growth in four savannah soils of Brazil . Journal of Plant Nutrition , 22 : 93 – 102 .
- Krings , M , Hass , H , Kerp , H , Taylor , TN , Agerer , R and Dotzler , N. 2009 . Endophytic cyanobacteria in a 400-million-yr-old land plant: A scenario for the origin of a symbiosis? Review of Palaeobotany and Palynology , 153 : 62 – 69 .
- Krings , M , Kerp , H , Hass , H , Taylor , TN and Dotzler , N. 2007 . A filamentous cyanobacterium showing structured colonial growth from the Early Devonian Rhynie chert . Review of Palaeobotany and Palynology , 146 : 265 – 276 .
- Leclercq , S. 1954 . Are the Psilophytales a starting or a resulting point . Svensk Botanisk Tidskrift , 48 : 301 – 315 .
- Liang , Y , Sun , W , Zhu , Y-G and Christie , P. 2007 . Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review . Environmental Pollution , 147 : 422 – 428 .
- Lowe , DR and Braunstein , D. 2003 . Microstructure of high-temperature (>73 oC) siliceous sinter deposited around hot springs and geysers, Yellowstone National Park: the role of biological and abiological processes in sedimentation . Canadian Journal of Earth Sciences , 40 : 611 – 1642 .
- Lux , A , Luxova , M , Abe , J , Tanimoto , E , Hattori , T and Inanaga , S. 2003 . The dynamics of silicon deposition in the sorghum root endodermis . New Phytologist , 158 : 437 – 441 .
- Lyon , AG and Edwards , D. 1991 . The first zosterophyll from the Lower Devonian Rhynie chert, Aberdeenshire . Transactions of the Royal Society of Edinburgh: Earth Sciences , 82 : 323 – 332 .
- Ma , JF. 2004 . Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses . Soil Science and Plant Nutrition , 50 : 11 – 18 .
- Marriott , SB and Wright , VP. 1993 . Palaeosols as indicators of geomorphic stability in two Old Red Sandstone alluvial suites, South Wales . Journal of the Geological Society, London , 150 : 1109 – 1120 .
- Massey , FP , Ennos , AR and Hartley , SE. 2006 . Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder . Journal of Animal Ecology , 75 : 595 – 603 .
- Matoh , T , Kairusmee , P and Takahashi , E. 1986 . Salt-induced damage to rice plants and alleviation effect of silicate . Soil Science and Plant Nutrition , 32 : 295 – 304 .
- McCleskey , RB , Ball , JD , Nordstrom , K , Holloway , JM and Taylor , HE. 2005 . Water-chemistry data for selected hot springs, geysers, and streams in Yellowstone National Park, Wyoming, 2001-2002 U.S. Geological Survey Open-File Report , : 2004 – 1316 .
- McKenzie , EJ , Brown , KL , Cady , SL and Campbell , KA. 2001 . Trace metal chemistry and silicification of microorganisms in geothermal sinter, Taupo volcanic zone, New Zealand . Geothermics , 30 : 483 – 502 .
- Meharg , AA and Hartley-Whitaker , J. 2002 . Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species . New Phytologist , 152 : 29 – 43 .
- Moseley , RK. 1995 . The ecology of geothermal springs in south-central Idaho , Unpublished report on file at: Boise (ID): Idaho Department of Fish and Game, Conservation Data Center .
- Munns , R. 2002 . Comparative physiology of salt and water stress . Plant, Cell and Environment , 25 : 239 – 250 .
- Munns , R and Tester , M. 2008 . Mechanisms of salinity tolerance . Annual Review of Plant Biology , 59 : 651 – 681 .
- Münster University Palaeobotanical Research Group. 2000. The Rhynie chert and its flora Available at www.uni-muenster.de/GeoPalaeontologie/Palaeo/Palbot/erhynie.html (Accessed: 10 October 2009 ).
- Neumann , D and De Figueiredo , C. 2002 . A novel mechanism of silicon uptake . Protoplasma , 220 : 59 – 67 .
- Neumann , D , Lichtenberger , O , Schwieger , W and zur Nieden , U . 1997 . Silicon storage in selected dicotyledons . Botanica Acta , 110 : 282 – 290 .
- Neumann , D and zur Nieden , U . 2001 . Silicon and heavy metal tolerance of higher plants . Phytochemistry , 56 : 685 – 692 .
- Newell , SY , Porter , D and Lingle , WL. 1996 . Lignocellulolysis by ascomycetes (fungi) of a saltmarsh grass (smooth cordgrass) . Microscopy Research and Technique , 33 : 32 – 46 .
- Owen , RA , Owen , RB , Renaut , RW , Scott , JJ , Jones , B and Ashley , GM. 2008 . Mineralogy and origin of rhizoliths on the margins of saline, alkaline Lake Bogoria, Kenya Rift Valley . Sedimentary Geology , 203 : 143 – 163 .
- Panteleyev , A. 1996a . “ Epithermal Au-Ag-Cu: High Sulphidation ” . In Selected British Columbia mineral deposit profiles, volume 2 – metallic deposits , Edited by: Lefebure , DV and H[otilde]y , T . 37 – 39 . British Columbia Ministry of Employment and Investment Open File 1996-13 .
- Panteleyev , A. 1996b . “ Epithermal Au-Ag: Low Sulphidation ” . In Selected British Columbia mineral deposit profiles, volume 2 – metallic deposits , Edited by: Lefebure , DV and H[otilde]y , T . 41 – 44 . British Columbia Ministry of Employment and Investment Open File 1996-13 .
- Parrondo , RT , Gosselink , JG and Hopkinson , CS. 1978 . Effects of salinity and drainage of the growth of three salt marsh grasses . Botanical Gazette , 139 : 102 – 107 .
- Parry , DW , Hodson , MJ and Newman , RH. 1985 . The distribution of silicon deposits in the fronds of Pteridium aquilinum L . Annals of Botany , 55 : 77 – 83 .
- Pereira , HS , Korndörfer , GH , Vidal , AD and de Camargo , MS. 2004 . Silicon sources for rice crop . Scientia Agricola , 61 : 522 – 528 .
- Pierce , S , Vianelli , A and Cerabolini , B. 2005 . From ancient genes to modern communities: the cellular stress response and the evolution of plant strategies . Functional Ecology , 19 : 763 – 776 .
- Powell , CL. 1994 . “ The palaeoenvironments of the Rhynie cherts ” . Unpublished PhD thesis. University of Aberdeen (UK) .
- Powell , CL , Edwards , D and Trewin , NH. 2000b . A new vascular plant from the Lower Devonian Windyfield chert, Rhynie, N.E. Scotland . Transactions of the Royal Society of Edinburgh, Earth Sciences , 90 : 331 – 349 .
- Powell , CL , Trewin , NH and Edwards , D. 2000a . “ Palaeoecology and plant succession in a borehole through the Rhynie cherts, Lower Old Red Sandstone, Scotland ” . In New Perspectives on the Old Red Sandstone , Edited by: Friend , PF and Williams , BPJ . Vol. 180 , 439 – 457 . Special Publications of the Geological Society of London .
- Preston , LJ , Benedix , GK , Gengea , MJ and Sephton , MA. 2008 . A multidisciplinary study of silica sinter deposits with applications to silica identification and detection of fossil life on Mars . Icarus , 198 : 331 – 350 .
- Radhika , KP and Rodrigues , BF. 2007 . Arbuscular mycorrhizae in association with aquatic and marshy plant species in Goa, India . Aquatic Botany , 86 : 291 – 294 .
- Raja , HA , Schmit , JP and Shearer , CA. 2009 . Latitudinal, habitat and substrate distribution patterns of freshwater ascomycetes in the Florida Peninsula . Biodiversity Conservation , 18 : 419 – 455 .
- Raven , JA. 1983 . The transport and function of silicon in plants . Biological Reviews , 58 : 179 – 207 .
- Remy , W , Gensel , PG and Hass , H. 1993 . The gametophyte generation of some early Devonian land plants . International Journal of Plant Science , 154 : 35 – 38 .
- Remy , W and Hass , H. 1991a . Ergänzende Beobachtungen an Lyonophyton rhyniensis . Argumenta Palaeobotanica , 8 : 1 – 27 .
- Remy , W and Hass , H. 1991b . Kidstonophyton discoides nov. gen., nov. spec., ein Gametophyt aus dem Chert von Rhynie (Unterdevon, Schottland) . Argumenta Palaeobotanica , 8 : 29 – 45 .
- Remy , W and Hass , H. 1991c . Langiophyton mackiei nov. gen., nov. spec., ein Gametophyt mit Archegoniophoren aus dem Chert von Rhynie (Unterdevon, Schottland) . Argumenta Palaeobotanica , 8 : 69 – 117 .
- Remy , W and Hass , H. 1991d . Gametophyten und Sporophyten im Unterdevon – Fakten und spekulationen . Argumenta Palaeobotanica , 8 : 193 – 223 .
- Remy , W and Hass , H. 1996 . New information on gametophytes and sporophytes of Aglaophyton major and inferences about possible environmental adaptations . Review of Palaeobotany and Palynology , 90 : 175 – 193 .
- Remy , W , Taylor , TN , Hass , H and Kerp , H. 1994 . Four hundred-million-year-old vesicular arbuscular mycorrhizae . Proceedings of the National Academy of Sciences of the United States of America , 91 : 11841 – 11843 .
- Renaut , RW and Tiercelin , J.-J. 1994 . “ Lake Bogoria, Kenya Rift valley: a sedimentological overview ” . In Sedimentology and Geochemistry of Modern and Ancient Saline Lakes , Edited by: Renaut , RW and Last , WM . Vol. 50 , 101 – 123 . SEPM Special Publication .
- Rensing , SA , Lang , D , Zimmer , AD , Terry , A , Salamov , A , Shapiro , H , Nishiyama , T , Perroud , P-F , Lindquist , EA , Kamisugi , Y and al , et . 2008 . The Physcomitrella genome reveals evolutionary insights into the conquest of the land by plants . Science , 319 : 64 – 69 .
- Rice , CM and Ashcroft , WA. 2004 . Geology of the northern half of the Rhynie Basin, Aberdeenshire, Scotland . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 299 – 308 .
- Rice , CM , Ashcroft , WA , Batten , DJ , Boyce , AJ , Caulfield , JBD , Fallick , AE , Hole , MJ , Jones , E , Pearson , MJ , Rogers , G and al , et . 1995 . A Devonian auriferous hot spring system, Rhynie, Scotland . Journal of the Geological Society, London , 152 : 229 – 250 .
- Rice , CM and Trewin , NH. 1988 . A Lower Devonian gold-bearing hot-spring system, Rhynie, Scotland . Transactions of the Institute of Mineralogy and Metallurgy , 97 : B141 – B144 .
- Rice , CM , Trewin , NH and Anderson , LI. 2002 . Geological setting of the Early Devonian Rhynie cherts, Aberdeenshire, Scotland: an early terrestrial hot spring system . Journal of the Geological Society, London , 159 : 203 – 214 .
- Richardson , JB. 1967 . Some British Lower Devonian spore assemblages and their stratigraphic significance . Review of Palaeobotany and Palynology , 1 : 111 – 129 .
- Ross , AJ and York , PV. 2004 . A catalogue of the type and figured specimens of Hexapoda from the Rhynie chert (Early Devonian) at the Natural History Museum, London . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 391 – 395 .
- Roth-Nebelsick , A and Konrad , W. 2003 . Assimilation and transpiration capabilities of rhyniophytic plants from the Lower Devonian and their implications for paleoatmospheric CO2 concentration . Palaeogeography, Palaeoclimatology, Palaeoecology , 202 : 153 – 178 .
- Rozema , J , Rozema-Dijst , E , Freysen , AHJ and Huber , JJL. 1978 . Population differentiation within Festuca rubra L. with regard to soil salinity and soil water . Oecologia , 34 : 329 – 341 .
- Savant , NK , Korndörfer , GH , Datnoff , LE and Snyder , GH. 1999 . Silicon nutrition and sugarcane production: a review . Journal of Plant Nutrition , 22 : 1853 – 1903 .
- Scott , DH. 1920 . “ Studies in fossil botany ” . In Pteridophyta , 3rd , Vol. 1 , London : A & C Black Ltd .
- Shearer , CA , Descals , E , Kohlmeyer , B , Kohlmeyer , J , Marvanova , L , Padgett , D , Porter , D , Raja , HA , Schmit , JP , Thorton , H and al , et . 2007 . Fungal biodiversity in aquatic habitats . Biodiversity Conservation , 16 : 49 – 67 .
- Shovic , H. 1996 . Landforms and associated surficial materials of Yellowstone National Park . Yellowstone Center for Resources, Yellowstone National Park, Wyoming. , Report YCR-NRSR-96-3
- Siever , R and Scott , RA . 1963 . “ Organic geochemistry of silica ” . In Organic geochemistry. International series of monographs on earth sciences , Edited by: Breger , IA . 579 – 595 . New York : Perganon .
- Steiman , R , Ford , L , Ducros , V , Lafond , JL and Guirau , P. 2004 . First survey of fungi in hypersaline soil and water of Mono Lake area (California) . Antonie van Leeuwenhoek , 85 : 69 – 83 .
- Stout , RG and Al-Niemi , TS. 2002 . Heat-tolerant flowering plants of active geothermal areas in Yellowstone National Park . Annals of Botany , 90 : 259 – 267 .
- Takahashi , E and Miyake , Y. 1977 . Silica and plant growth. Proceedings of the International Seminar on soil environment and fertility management in intensive agriculture 603 – 611 . Tokyo
- Tasch , P. 1957 . Flora and fauna of the Rhynie chert: a palaeoecological evaluation of published evidence . University of Wichita Bulletin , 32 : 3 – 24 .
- Taylor , TN , Hass , H , Kerp , H , Krings , M and Hanlin , RT . 2005a . Perithecial ascomycetes from the 400 million year old Rhynie chert: an example of ancestral polymorphism . Mycologia , 97 : 269 – 285 .
- Taylor , TN , Hass , H , Krings , M , Klavins , SD and Kerp , H. 2004 . Fungi in the Rhynie chert: a view from the dark side . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 457 – 473 .
- Taylor , TN , Hass , H and Remy , W. 1992 . Devonian fungi: interactions with the green alga Palaeonitella . Mycologia , 84 : 901 – 910 .
- Taylor , TN , Hass , H , Remy , W and Kerp , H. 1995 . The oldest fossil lichen . Nature , 378 : 244
- Taylor , TN , Kerp , H and Hass , H. 2005b . Life history of early land plants: deciphering the gametophyte phase . Proceedings of the National Academy of Sciences USA. , 102 : 5892 – 5897 .
- Taylor , TN and Krings , M. 2005 . Fossil microorganisms and land plants: associations and interactions . Symbiosis , 40 : 119 – 135 .
- Trewin , NH. 1994 . Depositional environment and preservation of biota in the Lower Devonian hot-springs of Rhynie, Aberdeenshire, Scotland . Transactions of the Royal Society of Edinburgh: Earth Sciences , 84 : 433 – 442 .
- Trewin , NH. 1996 . “ The Rhynie cherts: an Early Devonian ecosystem preserved by hydrothermal activity ” . In Evolution of hydrothermal ecosystems on Earth (and Mars?). Ciba Foundation Symposium 202 , Edited by: Bock , GR and Goode , J . 131 – 149 . Chichester, , UK : John Wiley & Sons .
- Trewin , NH , Fayers , SR and Kelman , R. 2003 . Subaqueous silicification of the contents of small ponds in an Early Devonian hot spring complex, Rhynie, Scotland . Canadian Journal of Earth Sciences , 40 : 1697 – 1712 .
- Trewin , NH and Rice , CM. 1992 . Stratigraphy and sedimentology of the Devonian Rhynie chert locality . Scottish Journal of Geology , 28 : 37 – 47 .
- Trewin , NH and Rice , CM . 2004 . The Rhynie Hot Springs System: geology, biota and mineralisation . Transactions of the Royal Society of Edinburgh, Earth Sciences , 94 : 246
- Trewin , NH and Wilson , E. 2004 . Correlation of the Early Devonian Rhynie chert beds between three boreholes at Rhynie, Aberdeenshire, Scotland . Scottish Journal of Geology , 40 : 73 – 81 .
- United Nations Environment Program Report, World Conservation Monitoring Centre. 2008. Yellowstone National Park, Wyoming, United States of America. Available at http://www.unep-wcmc.org/sites/wh/pdf/Yellowstone.pdf (Accessed: 10 October 2009 ).
- Vitale , MV , Gardner , P and Hinman , NW. 2008 . Surface water–groundwater interaction and chemistry in a mineral-armored hydrothermal outflow channel, Yellowstone National Park, USA . Hydrogeology Journal , 16 : 1381 – 1393 .
- Waisel , Y. 1972 . Biology of halophytes , 395 New York and London : Academic Press .
- Walter , MR , Des Marais , D , Farmer , JD and Hinman , NW. 1996 . Lithofacies and biofacies of mid-Paleozoic thermal spring deposits in the Drummond Basin, Queensland, Australia: Palaios , 11 : 497 – 518 .
- Walter , MR , McLoughlin , S , Drinnan , AN and Farmer , JD. 1998 . Palaeontology of Devonian thermal spring deposits, Drummond Basin, Australia . Alcheringa , 22 : 285 – 314 .
- Wellman , CH. 2004 . Palaeoecology and palaeophytogeography of the Rhynie chert plants: evidence from integrated analysis of in situ and dispersed spores . Proceedings of the Royal Society of London, B , 271 : 985 – 992 .
- Wellman , CH. 2006 . Spore assemblages from the Lower Devonian ‘Lower Old Red Sandstone’ deposits of the Rhynie outlier, Scotland . Transactions of the Royal Society of Edinburgh: Earth Sciences , 97 : 167 – 211 .
- Wellman , CH , Kerp , H and Hass , H. 2004 . Spores of the Rhynie chert plant Horneophyton lignieri (Kidston & Lang) Barghoorn & Darrah, 1938 . Transactions of the Royal Society of Edinburgh: Earth Sciences , 94 : 429 – 443 .
- Wellman , CH , Kerp , H and Hass , H. 2006 . Spores of the Rhynie chert plant Aglaophyton (Rhynia) major (Kidston and Lang). D.S. Edwards, 1986 . Review of Palaeobotany and Palynology , 142 : 229 – 250 .
- Whisler , HC. 1987 . “ Isolation and culture of the water molds: the Blastocladiales and Monoblepharidales ” . In Zoosporic Fungi in Teaching and Research. Athens (GA) , Edited by: Fuller , MS and Jaworski , A . 121 – 124 . Southeastern Publishing .
- White , DE , Hutchinson , RA and Keith , TEC. 1988 . The geology and remarkable thermal activity of Norris Geyser Basin, Yellowstone National Park, Wyoming . USGS Professional Paper , 1456 : 84
- White , NC , Wood , DG and Lee , MC. 1989 . Epithermal sinters of Paleozoic age in north Queensland, Australia . Geology , 17 : 718 – 722 .
- Willoughby , LG. 2001 . The activity of Rhizophlyctis rosea in soil: some deductions from laboratory observations . Mycologist , 15 : 113 – 117 .
- Yeo , AR , Flowers , SA , Rao , G , Welfare , K , Senanayake , N and Flowers , TJ. 1999 . Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow . Plant, Cell and Environment , 22 : 559 – 565 .
- Zhu , J-K. 2000 . Genetic analysis of plant salt tolerance using Arabidopsis . Plant Physiology , 124 : 941 – 948 .
- Zhu , J-K. 2001 . Plant salt tolerance . Trends in Plant Science , 6 : 66 – 71 .