ABSTRACT
Background
Dark septate endophytes (DSEs) represent a form-group of ascomycetous fungi that inhabit the roots of a wide range of plant species, but our knowledge on their interaction with the host plants is still limited.
Aims
This study was conducted to examine the effect of DSEs on the nutrition and growth of tomato (Solanum lycopersicum) in order to assess their potential application in horticultural plant production.
Methods
The capacity of two model DSE species, Periconia macrospinosa and Cadophora sp. to mobilise different forms of N and P (organic and inorganic) was analysed, and an in vitro bio assay with tomato plantlets was applied to screen the compatibility of these fungi with the plant. Pot-culture experiments with and without compartments were conducted to study the effects of these DSEs on the growth and nutrient uptake of tomato plants grown with organic and inorganic N and P sources.
Results
Periconia macrospinosa, but not Cadophora sp., increased the root and shoot biomass of tomato plants when organic nutrient resources were present, and both DSEs promoted shoot growth when cultivated with inorganic fertilisers. Analysis of N and P concentrations indicated that the growth response of tomato with inorganic fertilisation was not based on DSE-improved plant nutrition. However, P. macrospinosa improved N uptake from organic sources.
Conclusion
The positive effects of DSEs seem to be due to nutrient mobilisation rather than to hyphal transport to the plant.
Introduction
The vast majority of plants live in association with fungal endophytes (Card et al. Citation2016), which colonise plant tissues without causing recognisable symptoms (Petrini Citation1991; Wilson Citation1995; Saikkonen et al. Citation1998). In addition to well-known mycorrhizal fungi, dark septate endophytes (DSEs) are abundant root-colonising fungi commonly found in diverse ecosystems and environmental conditions (Mandyam and Jumpponen Citation2005). Despite the common occurrence of DSEs, their ecological functions and interactions with their host plants are not well understood.
The effect of DSEs on plant performances and nutrient contents can range from positive to negative (Jumpponen Citation2001; Mandyam and Jumpponen Citation2005; Newsham Citation2011; Mayerhofer et al. Citation2013). Although some studies showed that DSEs can have negative effects on their host (Terhonen et al. Citation2016; Wilcox and Wang Citation1987; Tellenbach et al. Citation2011; Reininger et al. Citation2012), a meta-analysis focusing on controlled experiments showed that DSEs generally had positive effects on plants (Newsham Citation2011). DSEs positively affected different parameters of tomato plants and fruit quality in greenhouse conditions (Andrade-Linares et al. Citation2011b) and enhanced tomato plant growth when an organic N source was present (Mahmoud and Narisawa Citation2013). DSE inoculation also helped pine seedlings absorb more P and N (Jumpponen et al. Citation1998). It has been suggested that DSEs mineralise proteins and peptides in the soil, making N more available for uptake by roots (Upson et al. Citation2009). Although the reciprocal nutrient transport between DSEs and plants has been proven (Usuki and Narisawa Citation2007; Porras-Alfaro and Bayman Citation2011), and the contribution of DSEs to N uptake has been studied in detail (Usuki and Narisawa Citation2007; Upson et al. Citation2009; Porras-Alfaro and Bayman Citation2011; Vergara et al. Citation2017, Citation2018), the mechanisms behind the effects of those root colonisers on their hosts are not well understood. Generally, root-associated fungi can mobilise different nutrients and help the uptake of these nutrients by plants or bridge nutrient depletion zones and transport the nutrients via hyphae to the roots (Behie and Bidochka Citation2014; Souza et al. Citation2015).
DSE fungi are frequent in harsh environments such as arid and semiarid ecosystems (Barrow Citation2003; Zhang et al. Citation2010; Knapp et al. Citation2012). Two characteristic, frequent and common DSEs of such ecosystems are Periconia macrospinosa and Cadophora sp., which generally colonise grass and non-grass hosts, respectively (Knapp et al. Citation2012). The complete genomes of both DSEs have recently been sequenced and analysed (Knapp et al. Citation2018). When isolates of P. macrospinosa were used in in vitro experiments with Arabidopsis thaliana, the effect of the DSE varied according to the plant ecotype (Mandyam et al. Citation2013).
Both P. macrospinosa and Cadophora sp. have a wide range of enzymatic activities (Knapp and Kovács Citation2016) and are rich in carbohydrate-active enzymes (CAZymes) (Knapp et al. Citation2018), suggesting these DSEs have a potential role in nutrient mobilisation, which might be beneficial for their host plant. Thus, a primary function of DSEs in nutrient-limited environments may be the facilitation of nutrient uptake by their host plants (Jumpponen and Trappe Citation1998; Mandyam and Jumpponen Citation2005; Knapp and Kovács Citation2016).
Under controlled conditions in horticultural practices, it is advantageous for mineral fertilisers to be reduced or replaced by organic fertilisers (Roy et al. Citation2006). This reduction or exchange could be complemented by the use of microbes as inoculants (Mei and Flinn Citation2010). As generalist root colonisers, DSEs could potentially be used in horticulture as inoculants. Previous analyses have noted that experimental conditions and the type of nutrient supply (organic vs. inorganic sources of elements and the presence of a carbon source) have significant effects on plant-DSE interactions (Newsham Citation2011; Mayerhofer et al. Citation2013).
One of the most important vegetable crops is tomato, which is associated with different root endophytic fungi, including DSEs (Andrade-Linares et al. Citation2011a). The effect of inoculations with DSEs isolated from tomato or rice has already been tested (Andrade-Linares et al. Citation2011b; Vergara et al. Citation2017). In our study presented here, we analysed (i) whether the two model DSE species P. macrospinosa and Cadophora sp. influence tomato growth and nutrient uptake in poor soils where N and P are present in different (organic and inorganic) forms and (ii) whether DSEs could mobilise nutrients from compartments unreachable by roots and make those nutrients available to the plants.
Materials and methods
Cultivation of DSEs
The two DSE species (P. macrospinosa ‘Isolate DSE2036’ and Cadophora sp. ‘Isolate DSE1049’) were isolated from semiarid sandy grasslands in Hungary (Knapp et al. Citation2012). Cadophora sp. isolate DSE1049 is most likely conspecific with the recently described C. meredithiae (Walsh et al. Citation2018). Both isolates were maintained on Pontecorvo’s complete medium (Pontecorvo et al. Citation1953). Fungal inocula were prepared by transferring three to five fungal plugs from freshly growing cultures to a liquid complete medium. Cultures were grown for 3–4 weeks on a shaker (110 rpm) at 26°C. Later, mycelia were collected, filtered, washed and suspended in distilled water and mixed for 45 s using a blender (Model D72, Moulinex, Leipzig, Germany) at minimal speed. The number of propagules (i.e. hyphal fragments) was calculated by spreading 1:1000 to 1:100,000 dilutions of each suspension on complete medium Petri plates supplied with ampicillin. The initiating fungal colonies were counted 3–6 days after inoculation and grown at room temperature.
Testing the effect of organic and inorganic N and P sources on fungal growth
To check whether there are differences in the growth of the two DSEs on organic and/or inorganic N and P sources, plugs from the cultures (5 mm diameter) were placed in the centres of multi-purpose beakers (Roth, Karlsruhe, Germany) containing 50 ml medium. The medium contained basic salts (KCl 0.5 g/L; MgSO4×7H2O 0.5 g/l), microelements (MnCl2×4H2O 5 mg/L; H3BO4 14 mg/L; ZnSO4×7H2O 22 mg/L; FeSO4×7H2O 5 mg/L; (NaH4)6Mo7O24×4H2O 1.1 mg/L; CuSO4×5H2O 1.6 mg/L; CoCl2×5H2O 1.6 mg/L; Na2EDTA×2H2O 51 mg/L), glucose 10.25 g/L and agar 15 g/L (Roth, Karlsruhe, Germany), and the pH was adjusted to 6.7 using NaOH. In medium (a), NaNO3 5 g/L and KH2PO4 1.85 g/L were added as inorganic sources of N and P, in medium (b), 0.84 g/L casein and 1.6 g/L phytate were used to achieve the same concentrations of the two elements, in medium (c), organic N was combined with inorganic P, and in medium (d), inorganic N was combined with organic P. All growth tests were performed in three replicates. Cultures were incubated at 24°C for 2 weeks in the dark. To assess fungal biomass, beakers were heated in the microwave until the agar melted, fungal mycelia were collected and dried in an oven at 60°C for 24 h, and then the dry weight was measured.
In vitro co-cultivation of DSEs and tomato plants
To study the effect of DSEs on the growth of tomato seedlings in vitro, PNM medium allowing the growth of plants and fungi (Johnson et al. Citation2011) was poured into tissue culture glass bottles, and a mesh membrane (20 µm) was placed on top of the medium. One 5 mm plug of either P. macrospinosa or Cadophora sp. was transferred to the top of the mesh and kept at 26°C for 1 week. Tomato seeds (Solanum lycopersicum cv ‘Hildares F1’, Hild Samen GmbH, Marbach, Germany) were surface sterilised by soaking for 1 min in 70% ethanol and for 4 min in 2.5% NaClO. After washing five times with sterilised water, seeds were germinated on MS medium (Murashige and Skoog Citation1962) at 4°C for 48 h in the dark, followed by 8 days in a phytochamber (Adaptis A1000, Conviron Germany GmbH, Berlin) set on a 16/8 h day/night cycle at 23/21°C under a light intensity of 110 μmol/m2/s. Thereafter, the seedlings were transferred to the bottles with 1-week-old fungal inoculations. Each of the glass bottles contained four tomato seedlings and either P. macrospinosa or Cadophora sp. or a fungus-free agar plug as a negative control. Each experimental treatment had four replicates. After 5 weeks under the above described light chamber conditions, the plants in each bottle were pooled, and the total fresh weight of the plants was measured. To check for DSE colonisation, the fungi were re-isolated from inoculated roots. The root system of one seedling per bottle were cut into 1 cm fragments, which were surface sterilised by immersing in 70% ethanol for 1 min, followed by an immersion of 4 min in 2.5 NaClO. After washing at least three times over one min with sterile distilled water, the root fragments were placed on a PDA Petri dish (Sigma-Aldrich, Taufkirchen, Germany) supplied with ampicillin (100 mg/L), and fungal out-growth from the fragments was monitored.
Pot experiments
Greenhouse pot experiments were carried out in Großbeeren, Germany (52°N, 13°E). Tomato seeds were surface sterilised by soaking for 4 min in 2.5% NaClO followed by five washes with sterilised water and germinated on sand in the greenhouse for 2 weeks. The tomato seedlings were transplanted into pots after being inoculated with the DSEs by dipping the roots overnight in a propagule suspension of either P. macrospinosa or Cadophora sp. or in a mock solution (autoclaved mixture of a propagule suspension of the two fungi). The concentrations of the suspensions were 1.3 × 105 cfu/ml for P. macrospinosa and 0.9 × 105 cfu/ml for Cadophora sp.
Substrates were prepared by mixing nutrient solutions with subsoil obtained from the C-horizon of a Luvisol from Weihenstephan, Southern Germany (48°25ʹN, 11°50ʹE). The subsoil is classified as loamy sand (45.2% sand, 42.0% silt, 13% clay) and contained (mg kg−1): 5.2 and 3.4 CaCl2 (0.0125 M)-extractable NH4 + and NO3 −, respectively. The organic matter content was 0.3% in dry soil, with a substrate pH (CaCl2) of 7.7 and a CaCO3 equivalent of 23%. The substrate contained (in mg kg−1) 6.5 acetate lactate-extractable P after heat sterilisation (Schüller Citation1969), 65.7 CAL-extractable K, and 1.9 (Fe), 15.0 (Mn), 0.3 (Zn), 0.9 (Cu), 0.09 (B) and 0.04 (Mo) CAT-extractable micronutrients (Alt and I Citation1992). Soil characteristics and plant available nutrients were analysed by LUFA Rostock according to VDLUFA 2007 (Baumgärtel et al. Citation2007).
For the first pot experiment, nutrients were added to achieve the following concentrations of each element in the soil: N: 99 mg/kg, K ≈ 200 mg/kg, P: 50 mg/kg, Mg: 99 mg/kg, Ca: 13 mg/kg, Fe: 7 mg/kg, Zn: 10 mg/kg, Cu: 10 mg/kg, S: 184 mg/kg or 211 mg/kg. N and P were added either as organic forms using casein for N and phytate for P or as inorganic forms using NH4NO3 for N and KH2PO4 for P. Organic treatments were also enriched with 40 mg/kg C added as glucose. Pots were filled with a total of 350 g of substrates before the seedlings were transplanted. The experiment was carried out with 11–12 replicates per treatment.
In the second pot experiment, soil compartments were set in the pots with a 20-µm mesh through which only fungal hyphae could pass. The pots with the plants contained 300 g of soil enriched with inorganic nutrients, and the compartment was filled with 50 g of soil enriched with organic nutrients. Inorganic and organic soil/nutrient mixtures were prepared as described above. The experiments were carried out with 12 replicates per treatment.
All pots in all experiments contained a single plant each and were irrigated daily or every second day with equal amounts of osmotic water (25–100 ml) to maintain the substrate moisture. Greenhouse day/night temperatures were 19.8/16.15°C, with a relative humidity of 57%/55% and a mean daily light radiation of 33 mol m−2 day−1. Plants were harvested 7 weeks after inoculation. The fresh and dry weights of the shoots and roots were measured.
Determination of total N, P, and C content of shoots and soil
To determine the P contents of plant shoots and soil samples, flow injection analysis was performed by means of colorimetric detection following ISO/EN/DIN 15,681–1. Shoots were dried in a drying oven at 60°C, and, after measuring the shoot dry weight, samples were ground to a fine powder. A total of 200–500 mg per sample (shoots or soil) was analysed to determine the total phosphorus content. First, microwave digestion was carried out after adding 65% nitric acid (HNO3) and 30% hydrogen peroxide (H2O2). The resulting ortho-phosphate was treated with molybdate in acidic medium to produce phosphomolybdate, which was reduced to molybdenum blue by zinc-(II)-chloride/hydroxylamine. The dye intensity was detected by absorbance measurement at 700 nm using an EPOS 5060 analyser (Eppendorf, Hamburg Germany). For the quantification of N and C, 10 mg of the powder was analysed in an elemental analyser (Elementar Vario EL, Elementar, Germany) based on the DUMAS method. Due to the small size of plants grown on the organic soil, two-three plants were pooled in known proportions to achieve a sufficient amount of plant material for the measurement.
Microscopy
Light microscopy was applied to check for the presence of DSE structures in 100 root segments of three plants per treatment in both experiments after staining with trypan blue (Phillips and Hayman Citation1970).
Furthermore, rRNA fluorescence in situ hybridisation (FISH) was used for the detection and identification of DSE structures (Vagi et al. Citation2014). Roots of three plants per treatment from the first pot experiment and from three plants from P. macrospinosa and two plants from Cadophora sp. treatments from the second pot experiment (with the compartment) were checked for the presence of the fungi using in planta rRNA FISH. Roots were washed from soil particles with tap water, cut into ≈ 1 cm fragments and vacuum infiltrated at room temperature in CSK buffer (5.84 g/L NaCl, 102.6 g/L sucrose, 0.6 g/L MgCl2 × 6 H2O, 6.05 g/L PIPES, 1% Triton X100 and 0.38 g/L EGTA, pH 6.8) for 30 min. Root fragments were then transferred to 4% paraformaldehyde in phosphate-buffered saline (PBS) and incubated at room temperature for at least 1 h. The fixed root fragments were washed in PBS three times and stored in 70% ethanol. Prior to hybridisation, samples were washed three times in distilled water. Six to 10 fragments were placed in one PCR tube containing 50 µl hybridisation buffer (Vagi et al. Citation2014). The hybridisation buffer consisted of 50 µl 20× SSC buffer (175.33 g/L NaCl, 77.42 g/L Na3C6H5O7, pH = 7.5), 5 µg dextran sulphate, 5 ng bovine serum albumin (Sigma-Aldrich, Taufkirchen, Germany), 0.125 µl RiboLock (40 u/µl, Thermo Scientific, Waltham, USA), 7.5 µl salmon sperm DNA (10 mg/mL in H2O, Sigma-Aldrich, Taufkirchen, Germany) and 2.5 µg of the DNA probe labelled with Alexa Fluor 488 (Eurofins Genomics, Ebersberg, Germany). The probe sequences were designed as specific to either P. macrospinosa (5′-[A488] gta tgt caa ccc cga g-3′) or Cadophora sp. (5′-[A488] gag agg agc cac att ccc aa −3′). Hybridisation experiments were carried out overnight at 36°C for P. macrospinosa and at 38°C for Cadophora sp. On the following day, the labelled root fragments were washed three times with washing buffer (hybridisation buffer without RiboLock, salmon sperm DNA and DNA probe). In the case of Cadophora sp., the washing buffer contained 50% dimethylformamide. Root fragments were mounted in FluoroShield (Sigma-Aldrich, Taufkirchen, Germany) and checked by fluorescence microscopy.
PCR analysis of soil compartments
DNA was extracted from the soils of the compartments using the PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, USA) to check for the presence of P. macrospinosa. Eight soil samples from P. macrospinosa-inoculated pots were analysed. The PCR reaction (20 µL total volume) contained 10−50 ng of the template DNA, 200 μM dNTPs, 0.5 units of Taq polymerase (Bioline, Luckenwalde, Germany) and 0.5 μM of the P. macrospinosa-specific ITS primers (forward: ttc ggc gct cct tat aca c; reverse: ggt caa gtc gtg aaa ggt ct). DNA extracted from P. macrospinosa cultures was used as a positive control. DNA was also extracted from eight soil compartments from the control treatment. The PCR programme started with 95°C for 10 min, followed by 35 cycles of amplification (94°C for 30 s, 65°C for 30 s, and 72°C for 30 s) and a final 10 min extension at 72°C. PCR products were run on 2 or 1.5% agarose gel followed by visualisation under UV after ethidium bromide staining. PCR amplicons from the soil DNA were cloned into the pGEM®-T vector (Promega, Mannheim, Germany) and sequenced (Eurofins Genomics, Ebersberg, Germany) to confirm whether the amplified fragments represented P. macrospinosa.
Statistical analyses
All statistical analyses were performed using STATISTICA programme version 11 (StatSoft Inc., Tulsa, Oklahoma, USA). The normality of the datasets was tested using Kolmogorov-Smirnov’s test, and the homogeneity of variance was tested using Levene’s test. In the pot experiment of organic-inorganic N and P mobilisation, shoot growth parameters were log-transformed before analysis of variance (ANOVA) to meet the test’s assumptions. Data from this experiment were analysed by two-way ANOVA or with the Kruskal-Wallis test when data did not exhibit homogeneity of variance after using common statistical transformations. In the compartment-experiment, a post hoc Tukey’s test at α = 0.05 was applied after the one-way ANOVA.
Results
In vitro growth of DSEs and tomato seedling inoculations
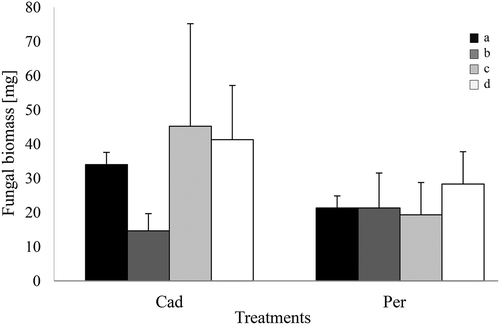
Both DSEs, P. macrospinosa and Cadophora sp., could grow on the different media containing organic and inorganic N and P forms. Significant differences in the growth of the DSEs were not detected ().
Figure 1. In vitro growth of DSEs on different forms of N and P. Periconia macrospinosa (Per) and Cadophora sp. (Cad) were grown for 2 weeks on solid media. N and P were both supplied in inorganic forms (a) and organic forms (b). N was supplied in organic form, and P was supplied in inorganic form (c). P was supplied in organic form, and N was supplied in inorganic form (d). Two-way ANOVA (P = 0.05, n = 3) was carried out on the square-root-transformed data, resulting in no significant impact of either the ‘media’ or ‘DSE’ factor and an absence of interactions.
No visible disease symptoms were observed in the roots and shoots of the tomato plantlets during the in vitro bioassay. After the 5-week experiment, no significant differences in plant biomass were detected between the control and inoculated plants. Only Cadophora sp. could be re-isolated (in one case of the four inoculated replicates) from the roots.
The effect of DSEs on plant growth and nutrition with inorganic and organic fertilisation
In the pot-culture experiment, when N and P were supplied in organic and inorganic forms, no visible disease symptoms could be observed in any plant during the 7 weeks of cultivation.
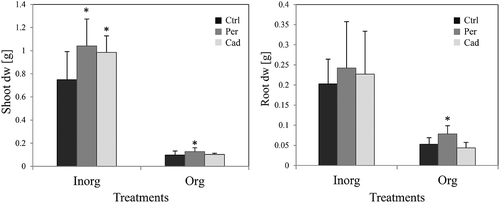
Plant growth was inhibited by organic fertilisation in comparison to the inorganic treatment (). A positive effect of DSE inoculation on the growth of the plants was detected (). Both DSEs increased the shoot dry weight in the presence of inorganic N and P (). When organic sources of N and P were applied, only P. macrospinosa had a significant positive effect on the shoot and root fresh (data not shown) and dry biomass ().
Figure 2. Impact of DSEs on tomato vegetative growth (first pot-experiment). Tomato plants were non-inoculated (Ctrl), inoculated with Periconia macrospinosa (Per) or inoculated with Cadophora sp. (Cad) and were grown in organically fertilised (Org) or inorganically fertilised (Inorg) soils. The dry weight of roots and shoots was measured. After log-transformation, two-way ANOVA (P = 0.05, n = 11–12) was carried out, showing that the factor ‘DSE’ had a significant impact on the dry weight (dw) of shoots, but there was no interaction between ‘DSE’ and ‘fertilisation’. The Kruskal–Wallis test (P = 0.05, n = 11–12) showed that root dry weight (dw) was also influenced by the factor ‘DSE’, and all plant growth parameters were influenced by the factor ‘fertilisation’. Significant differences between inoculated and non-inoculated plants are indicated by asterisks (Kruskal–Wallis test; P = 0.05, n = 11–12).
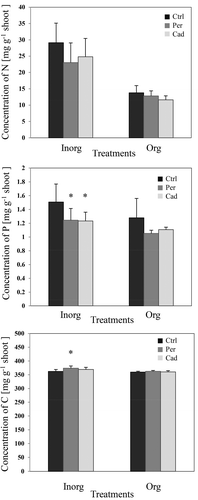
There was a significant decrease in the P concentration in the shoots inoculated with DSEs and treated with inorganic N and P (). Periconia macrospinosa inoculation had a positive significant effect on the C mass fractions in the presence of inorganic nutrients ().
Figure 3. Impact of DSEs on the concentrations of nitrogen (N), phosphorus (P) and carbon (C) (first pot experiment). Tomato plants were non-inoculated (Ctrl), inoculated with Periconia macrospinosa (Per), or inoculated with Cadophora sp. (Cad) and grown in organically fertilised (Org) or inorganically fertilised (Inorg) soils. Concentrations of elements were measured. The Kruskal–Wallis test (P = 0.05, n = 11–12 for ‘Inorg’, 3–5 for ‘Org’) showed an impact of the factor ‘fertilisation’ on the concentrations of both elements in plant shoots. Two-way ANOVA (P = 0.05, n = 11–12 for ‘Inorg’, 3–5 for ‘Org’) showed an impact of the factor ‘inoculation’ on the concentration of P. Significant differences between inoculated and non-inoculated plants are indicated by asterisks (Tukey’s test; P = 0.05, n = 11–12 for ‘Inorg’, 3–5 for ‘Org’).
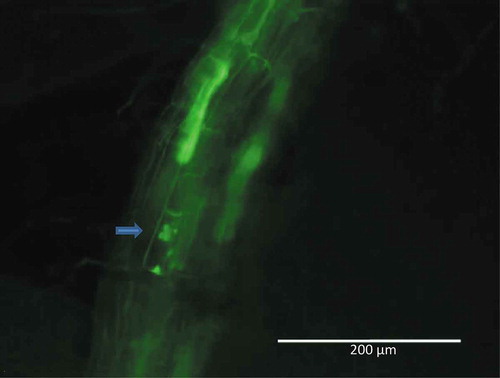
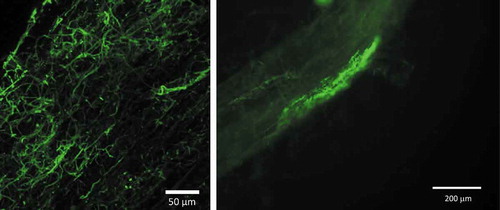
Using traditional staining and light microscopy, we could not detect microsclerotia or microsclerotia-like structures in the roots of plants inoculated with either P. macrospinosa or Cadophora sp. When in planta FISH was applied, we detected very low colonisation and a few probably juvenile microsclerotia in one plant inoculated with P. macrospinosa (2 out of 100 inspected root cross sections) grown in organic soil ().
The contribution of DSEs to plant N and P uptake via hyphal transport
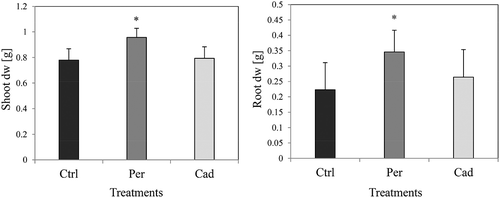
In the pot experiment with compartments, similar to all experiments, plants developed normally and showed no disease symptoms. In this experiment, inoculation with P. macrospinosa had significant positive effects on the fresh (data not shown) and dry biomass () of roots and shoots.
Figure 4. Effect of DSE inoculation on tomato growth (pot experiment with compartments). Tomato plants were non-inoculated (Ctrl), inoculated with Periconia macrospinosa (Per), or inoculated with Cadophora sp. (Cad) and grown in the presence of compartments. Pots contained inorganic forms of N and P, and compartments contained organic forms of N and P. The dry weight (dw) of roots and shoots was measured. One-way ANOVA (P = 0.05, n = 12) followed by Tukey’s post-hoc test showed significant differences between treatments. Significant differences between inoculated and non-inoculated plants are indicated by asterisks.
Inoculation with Cadophora sp., however, had a significant effect only on root fresh weight, with no significant effect on the N and P concentrations in the shoots. The N, P and C concentrations were also measured in the compartments at the end of the experiments. No significant differences were detected between treatments with and without the DSEs (data not shown).
No microsclerotia could be detected in the roots using traditional staining and light microscopy. Only hyphae of Cadophora sp. could be detected using FISH in two of the roots studied, and these hyphae were detected at a low rate (2 out of 100 root segments) (). When DNA was extracted from the soil of the compartments, two out of eight soil samples from pots inoculated with P. macrospinosa were positive for the DSE based on PCR analysis, and the sequencing of cloned fragments confirmed that the amplicon represented P. macrospinosa.
Discussion
Diverse cohorts of root-associated fungi can facilitate plant growth and nutrient uptake via different mechanisms. Both DSE species used in our experiments were able to grow in vitro on different media with organic and inorganic sources of N and P. This indicates the enzymatic capabilities of these DSEs to degrade and utilise complex forms of N and P, which agrees with previous findings about different DSE species suggesting their potential to help plants via the mobilisation of nutrients from organic sources (Caldwell et al. Citation2000). Mandyam et al. (Citation2010) have also shown that DSEs, including different isolates of P. macrospinosa, are able to utilise both organic and inorganic sources of N. Knapp and Kovács (Citation2016), using enzyme assays, have shown that different DSE fungi, including P. macrospinosa and Cadophora sp., have a broad range of enzymes and can use a wide range of carbon and other sources of nutrients.
Periconia macrospinosa and Cadophora sp. have relatively large genomes with a high number of predicted proteins, including large numbers of genes encoding carbohydrate-active enzymes (CAZymes) and plant cell wall degradation enzymes (PCWDE) (Knapp et al. Citation2018). These genes play crucial roles in the breakdown of complex plant polysaccharides, which might be important for both the saprophytic and endophytic lifestyles of fungi. The genomes also contain secreted peptidases and lipases (Knapp et al. Citation2018). Presumably, such extracellular peptidases are important for the cycling of N from organic materials in the soil.
In vitro co-inoculation systems used to study plant-fungi interactions are frequently established with media containing inorganic nutrients (Kageyama et al. Citation2008; Junker et al. Citation2012). In the in vitro inoculation system, we applied an experimental test suggested by Johnson et al. (Citation2011) to investigate the possible plant growth-promoting effect of the DSEs on tomato seedlings. As shown for the model root endophyte Serendipita indica (Piriformospora indica), this system helped result in remarkable alternations observed in the root morphology of inoculated seedlings via an effect leading to improved growth that was assumed to be governed by auxin biosynthesis (Lee et al. Citation2011). Andrade-Linares et al. (Citation2011a) have isolated 14 root-colonising fungi from tomato roots, 8 of which were considered DSEs, and have found that inoculation with nine representatives of the isolates had no significant effect on the shoot development of tomato plantlets in a bioassay system similar to that of the present study; these results agree with the results of our experiment with two DSEs.
Several studies have suggested DSEs have a role in plant nutrition by improving the nutrient content and growth of inoculated plants (Haselwandter and Read Citation1982; Jumpponen et al. Citation1998; Newsham Citation2000). We hypothesised that the effects of DSEs on plants might be more important when organic N and P sources are present, as these sources are inaccessible by the plants, leading to low growth.
Our data showed that inoculation with P. macrospinosa had a positive effect on shoot and root biomass in the presence of organic nutrients. While P concentrations slightly decreased in the growth-promoted plants, the N concentrations of inoculated and control plants did not differ. These results indicate that P. macrospinosa supported more N uptake than P uptake and are in accordance with the general findings that DSEs have significant effects on plant biomass when organic N is present (Newsham Citation2011). On the other hand, inoculation with Cadophora sp. had a positive effect on tomato only when the nutrient sources were present in inorganic form. This result correlates with the fungal growth tests on different media, in which Cadophora sp. seemed to more efficiently use inorganic N and P than organic N and P. However, Cadophora sp. have showed a more diverse and stronger enzyme degrading capacity than P. macrospinosa when the enzyme capacities of these DSEs were tested using different enzyme assays (Knapp and Kovács Citation2016).
In the pot experiment with compartments, both DSEs promoted the growth of tomato plants grown on inorganic sources. If this result were correlated with a positive effect on nutrient uptake, the nutrient concentrations would not be expected to change. However, the N and P concentrations decreased, indicating that the growth-promoting effect was not positively correlated with better N or P nutrition, as these two elements were diluted in the plants with more biomass. In addition to exhibiting an increase in dry weight, the plants showed an increase in C concentration in inorganic soils when inoculated with P. macrospinosa. C assimilation has been shown to be enhanced by mycorrhizal fungi (Caravaca et al. Citation2003; Birhane et al. Citation2012; Zhu et al. Citation2012) and the root endophyte S. indica (Rai et al. Citation2008). In the case of mycorrhizal plants, P supply has been suggested to play a role in improved photosynthesis (Jacob and Lawlor Citation1992). This cannot explain the increased C concentration of P. macrospinosa-inoculated plants, as the P fraction tended to be lower in these plants than in non-inoculated plants. The presence of P. macrospinosa in the soil might have influenced the water relations of the P. macrospinosa-inoculated plants. AM fungi were shown to play a role in the formation of soil aggregates (Rillig and Mummey Citation2006) and changing the hydraulic properties of soil (Bitterlich, Franken, et al. Citation2018; Bitterlich et al. Citation2018). AMF also affect soil water retention (Auge et al. Citation2001; Daynes et al. Citation2013). Although DSE fungi are frequent in arid and semiarid ecosystems, the effect of these fungi on the drought stress resistance of plants has seldom been addressed in experiments (Li et al. Citation2018).
Mechanisms of plant growth promotion, if not related to the support of nutrient uptake, need to be tested in future, although a meta-analysis showed that the mineralisation of N sources could be the main reason for the positive effect of DSEs on their hosts (Newsham Citation2011).
In the experiment with compartments, we aimed to test the hypothesis that DSEs help plants acquire nutrients via their hyphal networks, thus reaching sources typically unreachable by the plant. In mycorrhizal interactions, nutrients (e.g. P) can be taken up by the AM fungal hyphae and transferred to the host roots from compartments (George et al. Citation1992; Kikuchi et al. Citation2016). Using a compartment system in vitro, the nitrogen transfer from Heteroconium chaetospira (now: Cladophialophora chaetospira) DSE fungus to non-mycorrhizal Chinese cabbage has been proven previously (Usuki and Narisawa Citation2007). In our compartment system, Periconia macrospinosa increased several biomass parameters, similar to the results of the first pot-culture experiment, without decreasing N and P concentrations.
The impact of P. macrospinosa on the plants impelled us to detect the fungus in the compartments and determine the ability of P. macrospinosa to expand the plant nutrient acquisition range beyond the reach of the roots. Due to its possible saprophytic lifestyle, however, we were uncertain whether the fungus could interact with the roots directly and transport nutrients from organic resources towards the plant. Nutrient analyses of compartment soils were not able to detect a decrease in N or P. This indicates that the hyphal transport of nutrients mobilised from organic sources through the soil towards the plant does not play a significant role in plant-DSE interactions, as it does in mycorrhizal interactions (Hawkins et al. Citation2000). We suggest that the support of plant nutrition is based on the root uptake of nitrogen mobilised by DSEs in the vicinity of roots.
The hyphae of P. macrospinosa were barely detected inside the roots using microscopic techniques, and structures most likely representing forming microsclerotia were observed in few of the samples examined by FISH (). In the case of Cadophora sp. (), root-associated hyphae were detected at a very low rate. DSEs are known for their wide host range (Jumpponen and Trappe Citation1998), and the two studied DSE species are characteristic of either gramineous hosts or non-grass plants (Knapp et al. Citation2012). If the main effect of DSEs on plant growth is not derived from the transport of nutrients into the hosts but is based on the mobilisation of sources in the vicinity of roots, colonisation would not be a requirement. This has also been highlighted by Newsham (Citation2011), who indicated ‘that DSE probably do not influence plant growth through direct contact with roots’. Additionally, Ruotsalainen and Kytöviita (Citation2004) have shown that the DSE Phialocephala fortinii had several positive effects on Gnaphalium norvegicum without colonisation of the roots of the host plant, most likely because of the mobilisation of different sources.
Conclusions
In the current study, we carried out experiments to assess the impacts of two DSE species on the nutrient uptake and growth of tomato plants. A better understanding of the benefits that DSEs can confer to plants will be of high value in the understanding of their ecological function and the use of DSEs as potential inoculants in agricultural and horticultural practices. Our experiments showed that tomato plant biomass increased when two distinct DSEs were applied as inoculum with limited amounts of inorganic fertilisers, but this increase was not based on improved nutrient uptake. In the case of tomato, such an increase has previously been observed in response to one of three DSEs isolated from tomato roots (Andrade-Linares et al. Citation2011b), but there was no such increase in response to isolates obtained from rice roots (Vergara et al. Citation2017). Regarding the organic nutrient supply, plant growth promotion could be based on increased N uptake, similar to previous findings with numerous DSE-plant combinations, or the result of nutrient mobilisation rather than direct nutrient transport towards the plant. In summary, we found that tomato growth can be positively affected by root endophytes isolated from non-agricultural environments and that this response does not depend on the mode of fertilisation. These results can be important for horticultural practices where DSEs could be elements in synthetic communities used as inocula in various production systems.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Wael Yakti
Wael Yakti has studied biotechnology and completed his PhD on root-fungus interactions; his research focuses on plant–DSE interactions.
Gábor M. Kovács
Gábor M. Kovács is a mycologist with an interest in the functioning and diversity of plant-associated fungi and root-colonising endophytes, especially in semiarid areas, with an emphasis on fungal evolution.
Pál Vági
Pál Vági explores the formation, dynamics, possible competition and cooperation in plant/fungus interactions, with experience in light and electron microscopy of plant pathogenic and hyperparasitic fungi and viruses.
Philipp Franken
Philipp Franken is a molecular phytopathologist interested in root-fungus interactions concerning plant nutrition and plant health in horticultural production systems.
References
- Alt D , I P. 1992. Die CaCl2/DTPA-Methode zur Untersuchung gärtnerischer Erden auf Mengen- und Spurenelemente [The CaCl2/DTPA method to analyse bulk and trace elements in horticultural soils]. Agribio Res. 45(4):215–224, German.
- Andrade-Linares DR , Grosch R , Franken P , Karl HR , Kost G , Restrepo S , Mcc DG , Maximova E. 2011a. Colonization of roots of cultivated Solanum lycopersicum by dark septate and other ascomycetous endophytes. Mycologia. 103(4):710–721.
- Andrade-Linares DR , Grosch R , Restrepo S , Krumbein A , Franken P . 2011b. Effects of dark septate endophytes on tomato plant performance. Mycorrhiza. 21(5):413–422.
- Auge RM , Stodola AJW , Tims JE , Saxton AM . 2001. Moisture retention properties of a mycorrhizal soil. Plant Soil. 230(1):87–97.
- Barrow JR . 2003. Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza. 13(5):239–247.
- Baumgärtel G , Breitschuh G , Ebertseder T , Eckert T , Gutser R , Hege U , Herold L , Wiesler F , Zorn W . 2007. VDLUFA-Standpunkt “Nährstoffbilanzierung im landwirtschaftlichen Betrieb” [Verband Deutscher Landwirtschaftlicher Untersuchungsund Forschungsanstalten (VDLUFA), Spyer, Germany - viewpoint “Nutrient balance in agriclture”. In: Selbstverlag , editor. https://www.vdlufa.de/Dokumente/Veroeffentlichungen/Standpunkte/10-Naehrstoffbilanzierung.pdf
- Behie SW , Bidochka MJ . 2014. Nutrient transfer in plant–fungal symbioses. Trends Plant Sci. 19(11):734–740.
- Birhane E , Sterck FJ , Fetene M , Bongers F , Kuyper TW . 2012. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 169(4):895–904.
- Bitterlich M , Franken P , Graefe J . 2018. Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Front Plant Sci. 9:301.
- Bitterlich M , Sandmann M , Graefe J . 2018. Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front Plant Sci. 9:154.
- Caldwell BA , Jumpponen A , Trappe JM . 2000. Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia. 92(2):230–232.
- Caravaca F , Diaz E , Barea JM , zcon-Aguilar C , Roldan A . 2003. Photosynthetic and transpiration rates of Olea europaea subsp sylvestris and Rhamnus lycioides as affected by water deficit and mycorrhiza. Biol Plant. 46(4):637–639.
- Card S , Johnson L , Teasdale S , Caradus J . 2016. Deciphering endophyte behaviour: the link between endophyte biology and efficacious biological control agents. FEMS Microbiol Ecol. 92(8):fiw114–fiw114.
- Daynes CN , Field DJ , Saleeba JA , Cole MA , Pa M . 2013. Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol Biochem. 57(SupplementC4):683–694.
- George E , K-U H , Vetterlein D , Gorgus E , Marschner H . 1992. Water and nutrient translocation by hyphae of Glomus mosseae . CanJ Bot. 70(11):2130–2137.
- Haselwandter K , Read DJ . 1982. The significance of a root-fungus association in two Carex species of high-alpine plant communities. Oecologia. 53:352–354.
- Hawkins HJ , Johansen A , George E . 2000. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil. 226(2):275–285.
- Jacob J , Lawlor DW . 1992. Dependence of photosynthesis of sunflower and maize leaves on phosphate supply, ribulose-1,5-bisphosphate carboxylase oxygenase activity, and ribulose-1,5-bisphosphate pool size. Plant Physiol. 98(3):801–807.
- Johnson JM , Sherameti I , Ludwig A , Nongbri PL , Sun C , Lou Ea B . 2011. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation—a model system to study plant beneficial traits. J Endocytobiosis Cell Res. 21(2011):101–113, International Society of Endocytobiology zs.thulb.uni-jena.de/content/main/journals/ecb/info.xml.
- Jumpponen A . 2001. Dark septate endophytes - are they mycorrhizal? Mycorrhiza. 11(4):207–211.
- Jumpponen A , Mattson KG , Trappe JM . 1998. Mycorrhizal functioning of Phialocephala fortinii with Pinus contorta on glacier forefront soil: interactions with soil nitrogen and organic matter. Mycorrhiza. 7(5):261–265.
- Jumpponen A , Trappe JM . 1998. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol. 140(2):295–310.
- Junker C , Draeger S , Schulz B . 2012. A fine line – endophytes or pathogens in Arabidopsis thaliana . Fungal Ecol. 5(6):657–662.
- Kageyama S.A., Mandyam K.G., Jumpponen A. 2008. Diversity, Function and Potential Applications of the Root-Associated Endophytes. In: Varma A. (eds) Mycorrhiza. Springer, Berlin, Heidelberg; P. 29-57.
- Kikuchi Y , Hijikata N , Ohtomo R , Handa Y , Kawaguchi M , Saito K , Masuta C , Ezawa T . 2016. Aquaporin-mediated long-distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: application of virus-induced gene silencing. New Phytol. 211(4):1202–1208.
- Knapp DG , Kovács GM . 2016. Interspecific metabolic diversity of root-colonizing endophytic fungi revealed by enzyme activity tests. FEMS Microbiol Ecol. 92:12.
- Knapp DG , Németh JB , Barry K , Hainaut M , Henrissat B , Johnson J , Kuo A , Lim JHP , Lipzen A , Nolan M . 2018. Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Sci Rep. 8(1):6321.
- Knapp DG , Pintye A , Kovacs GM . 2012. The dark side is not fastidious - Dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS One. 7(2):8.
- Lee YC , Johnson JM , Chien CT , Sun C , Cai DG , Lou BG , Oelmuller R , Yeh KW . 2011. Growth promotion of Chinese Cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesised auxin. Mol Plant-Microbe Interact. 24(4):421–431.
- Li X , He X , Hou L , Ren Y , Wang S , Su F . 2018. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci Rep. 8(1):7896.
- Mahmoud RS , Narisawa K . 2013. A new fungal endophyte, Scolecobasidium humicola, promotes tomato growth under organic nitrogen conditions. PLoS One. 8(11):8.
- Mandyam K , Jumpponen A . 2005. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud Mycol. 53:173–189.
- Mandyam K , Loughin T , Jumpponen A . 2010. Isolation and morphological and metabolic characterization of common endophytes in annually burned tallgrass prairie. Mycologia. 102(4):813–821.
- Mandyam KG , Roe J , Jumpponen A . 2013. Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biol. 117(4):250–260.
- Mayerhofer MS , Kernaghan G , Harper KA . 2013. The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza. 23(2):119–128.
- Mei C , Flinn BS . 2010. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol. 4(1):81–95.
- Murashige T , Skoog F . 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 15(3):473–497.
- Newsham KK . 2000. Phialophora graminicola, a dark septate fungus, is a beneficial associate of the grass Vulpia ciliata ssp. Ambigua. New Phytol. 144(3):517–524.
- Newsham KK . 2011. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 190(3):783–793.
- Petrini O. 1991. Fungal endophytes of tree leaves In: Microbial Ecology ofleaves (Andrews, JH and Hirano, SS, Eds.).
- Phillips JM , Hayman DS . 1970. Improved procedure for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 55:158–161.
- Pontecorvo G , Roper JA , Hemmons LM , Macdonald KD , Bufton AWJ . 1953. The genetics of Aspergillus nidulans . Adv Genet Incorp Mol Genet Med. 5:141–238.
- Porras-Alfaro A, Bayman P. 2011. Hidden fungi, emergent properties:endophytes and microbiomes. Annual review of phytopathology, 49, pp.291-315.
- Rai MK , Shende S , Strasser RJ . 2008. JIP test for fast fluorescence transients as a rapid and sensitive technique in assessing the effectiveness of arbuscular mycorrhizal fungi in Zea mays: analysis of chlorophyll a fluorescence. Plant Biosyst. 142(2):191–198.
- Reininger V , Grünig CR , Sieber TN . 2012. Host species and strain combination determine growth reduction of spruce and birch seedlings colonized by root-associated dark septate endophytes. Environ Microbiol. 14(4):1064–1076.
- Rillig MC , Mummey DL . 2006. Mycorrhizas and soil structure. New Phytol. 171(1):41–53.
- Roy RN , Finck A , Blair GJ , Tandon HLS . 2006. Plant nutrition for food security. A guide for integrated nutrient management. Rome: FAO. FAO Fertilizer and Plant Nutrition Bulletin.
- Ruotsalainen AL , Kytöviita -M-M . 2004. Mycorrhiza does not alter low temperature impact on Gnaphalium norvegicum . Oecologia. 140(2):226–233.
- Saikkonen K , Faeth SH , Helander M , Sullivan TJ . 1998. Fungal endophytes: A continuum of interactions with host plants. Annu Rev Ecol Syst. 29:319–343.
- Schüller H . 1969. Die CAL-Methode, eine neue Methode zur Bestimmung des pflanzenverfügbaren Phosphates in Böden [The CAL method, a new method to determinine plant-available phosphate in soils]. Zeitschrift für Pflanzenernährung und Bodenkunde. 123(1):48–63, German.
- Souza R , Ambrosini A , Passaglia LMP . 2015. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 38:401–419.
- Tellenbach C , Grünig CR , Sieber TN . 2011. Negative effects on survival and performance of Norway spruce seedlings colonized by dark septate root endophytes are primarily isolate-dependent. Environ Microbiol. 13(9):2508–2517.
- Terhonen E , Sipari N , Asiegbu FO . 2016. Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Bioll Control. 99:53–63.
- Upson R , Read DJ , Newsham KK . 2009. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza. 20(1):1–11.
- Usuki F , Narisawa H . 2007. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia. 99(2):175–184.
- Vagi P , Knapp DG , Kosa A , Seress D , Horvath AN , Kovacs GM . 2014. Simultaneous specific in planta visualization of root-colonizing fungi using fluorescence in situ hybridization (FISH). Mycorrhiza. 24(4):259–266.
- Vergara C , Araujo KEC , Alves LS , Srd S , LA S , Santa-Catarina C , Kd S , Gmd P , Gr X , Jé Z . 2018. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz J Microbiol. 49(1):67–78.
- Vergara C , Araujo KEC , Urquiaga S , Schultz N , Fdc B , Ps M , LA S , Gr X , Je Z . 2017. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front Microbiol. 8(2437).
- Walsh E , Duan W , Mehdi M , Naphri K , Khiste S , Scalera A , Zhang N . 2018. Cadophora meredithiae and C. Interclivum, new species from roots of sedge and spruce in a western Canada subalpine forest. Mycologia. 110(1):201–214.
- Wilcox HE , Wang CJK . 1987. Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7-month-old tree seedlings. Can J For Res-Revue Canadienne De Recherche Forestiere. 17(8):884–899.
- Wilson D . 1995. Endophyte: the evolution of a term, and clarification of its use and definition. Oikos. 73(2):274–276.
- Zhang H , Tang M , Chen H , Wang Y , Ban Y . 2010. Arbuscular mycorrhizas and dark septate endophytes colonization status in medicinal plant Lycium barbarum L. In arid Northwestern China. Afr J Microbiol Res. 4(18):1914–1920.
- Zhu XC , Song FB , Liu SQ , Liu TD , Zhou X . 2012. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. Under drought stress. Plant Soil Environ. 58(4):186–191.