ABSTRACT
Background: In his 1991 book, Peters criticised the science of ecology, comparing it unfavourably with mainstream science such as physics and chemistry. His points of criticism include: (i) the questions ecologists ask are poorly formulated (ii) there are no fundamental principles (iii) research results seldom provide the basis for prediction and (iv) the science moves forward very slowly, as shown by the sluggish citation performance.
Aims: I revisit Peters’ book and ask whether the criticisms therein still hold.
Methods: Citation metrics are used to track the scientific progress of ecology over the last three decades.
Results: There has been a sharp increase in citations of ecology articles since 1990. There are strong signs that research ecologists are more closely adhering to the Popperian method (hypothesis testing), that they are more concerned with ‘prediction’ and that their work is more often focused on the large questions, such as global change, that humanity faces. More over ecologists today often work within large international networks with scientists from other disciplines, and ecological topics more often appear in general science journals like Nature and Science.
Conclusions: Ecology now has the features of mainstream science and it can be argued that ecology has ‘grown up’.
Introduction
Almost 30 years ago the distinguished limnologist RH Peters published the iconoclastic book A Critique for Ecology, lambasting the science of ecology and those who teach it (Peters Citation1991). Across the western world there were professors who removed the book from library shelves to prevent their students from reading it, lest they became demotivated.
Here, I revisit some important arguments made in the book, examine the main lines of criticism and ask whether they still apply. Was the criticism simply the result of ecology being a ‘young science’, not yet properly grown up? If so, has the subject matured over the years, and are we able to say that ecology provides the ‘predictive power’, which Peters singles out as the hallmark of good science?
Perhaps Peters was harsh on ecology, not taking into account the huge challenges that arise from the sheer complexity of the subject, given that ecosystems are examples of ‘open’ systems and are made up of hundreds of species, each having different traits, with the potential of responding to the others, and to a myriad of physical and chemical stimuli. A chemist or a physicist is able to isolate a small part of the natural world on a bench or in a test tube, adjust one variable and observe the result. Likewise, a medical scientist works with just a single species (Homo sapiens), offers a treatment or intervention to a group of volunteer patients, and records the result to compare against a control group. An ecologist who does the same thing as the physicist, chemist or medical researcher is liable to be criticised for doing ‘unrealistic’ or ‘irrelevant’ work, or work that is ‘not really ecology’ and is far removed from the complexity of ecosystems. In fact, ecologists often do engage in such experimental work using artificial environments, and it is there that some of the most important discoveries are made, from which predictions may be possible.
Nature of the criticism
The question of what is ecology? is only briefly discussed by Peters. He distinguishes ‘mainstream ecology’ defined as that which is tackled by introductory textbooks of ecology and focuses his attention there. These books usually define ecology in relation to Haeckel’s (Citation1866) view that ecology is the study of organisms in relation to their environment. A more focused definition found in many texts and usually attributed to Andrewartha (Citation1961) is: ecology is the scientific study of the distribution and abundance of organisms. However, this ‘scientific study’, in practice, requires consideration of evolution, genetics, physiology and behaviour, and ecology is therefore the ‘mother’ of numerous sub-disciplines including evolutionary ecology, molecular ecology, physiological ecology and behavioural ecology. Peters has largely ignored the sub-disciplines.
Peters criticised these popular textbooks because they present intractable questions, such as ‘what is a community?’ and ‘why do individuals do the things they do?’ and many others of that sort, all of them huge, unanswerable, and quite unlikely to be funded as research topics. It is also true that successive authors of such books have more or less followed each other, after the ‘big three’ (Krebs Citation1972; Colinvaux Citation1973; Ricklefs Citation1973), not wishing to stray from the perceived body of knowledge. There are of course, other possible starting points and different agendas that could have been adopted. Had Eugene Odum’s book (Odum Citation1953) been chosen as the genesis of the academic subject, or Eugen Warming’s, or Charles Elton’s or Arthur Tansley’s, or Ramon Margalef’s, (Warming and Vahl Citation1909; Elton Citation1927; Tansley Citation1939; Margalef Citation1968) then the outcome could have been quite different as these works have different emphases. As it stands, the ‘big three’ Ecology textbooks from which others largely derive were written by early- or mid-career North American scientists, perhaps because in Europe the fusion of Botany and Zoology Departments came later (the first ecology degree offered in Britain was at the University of Edinburgh in 1968), whereas there were ecology degrees in the USA much earlier, and students urgently needed textbooks. The ‘big three’ defined the academic canon. It prevailed. It provoked the questions that Peters deems to be ‘unanswerable’ but which for many years formed the mainstay of examination questions in ecology and biology departments. And it is this textbook ecology, it seems to me, rather than ecology as practiced by most research ecologists now, which Peters mostly criticised when he said ‘Academic ecology poses unanswerable questions’ and he set this view alongside Medawar’s assertion that “science is the art of the soluble” (Medawar). Thus, ipso facto, ecology is not good science.
Peters started his book with a description of the scientific method, including a summary in the form of a diagram (), and reference to Karl Popper and others. Most of us have drawn something like this to explain the scientific method to our students, but most scientists would agree that it can never be taken as a road map for success. Those who speak of how science is done are mostly philosophers (as Popper was) who observe science from afar. Practicing scientists would agree that progress is a good deal more haphazard than suggests, and that inductive thought and ‘reaching out into the unknown’ has a strong role to play. Platt (Citation1964) has argued persuasively that ‘inductive inference’ was key, and that this approach is better developed in some fields of research than others.
Figure 1. The scientific method according to Peters (Citation1991).
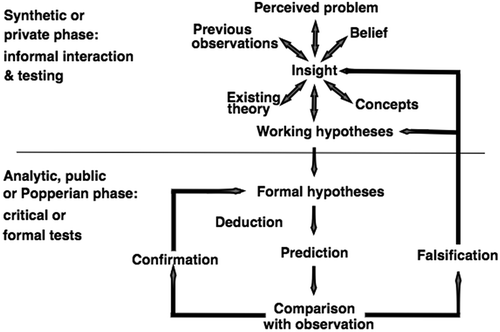
One clear issue is that theory and observations are not always well connected in ecology, perhaps because ‘theory’ is often expressed in the language of mathematics that many ecologists are not comfortable with. The matter of how theory is formed is rarely discussed. Peters says we may use the words ‘hypothesis’ and ‘theory’ as being interchangeable. The theoretical physicist Hawkin (Citation1988) has said more or less the same, in the sense that ‘both are fragile and must be repeatedly tested’, but for most people the ‘theory’ stage comes later – a theory is relatively well-substantiated and regarded as more robust and therefore longer-lasting than a hypothesis (see Ford Citation2000). Ecologists do have their theories: Marquet et al. (Citation2014) has found references to 78 theories in ecology, but challenged the frequent use of the word and suggested these ‘theories’ were merely hypotheses. Notwithstanding this uncertain distinction between theory and hypothesis, Peters has listed his criteria for judging the worth of scientific theories as follows:
Goal definition. According to Peters, most authors in ecology do not define their goal, except in the vaguest and most nebulous of terms such as ‘to investigate’ some process, ‘to illuminate’ some observations. Goals, he says, should be defined in terms that can be judged as having been achieved or not. To test a certain well-stated hypothesis would be an admirably acceptable goal, or (in more modern terms) to reduce the uncertainty in the prediction of a response. In the earliest and more descriptive stages of ecology this criterion would have been unfair, as the valid question was then simply ‘what species are there?’
Relevance. For Peters, ‘Thoughtful scientists choose to study the most important phenomenon within their competence’. He is not, in this case, talking of the relevance to the environmental issues of the day, but the utility of the goal in terms of the field of study – it must lead in fruitful directions, other scientists must find the work vital or at least important, otherwise they will ignore it.
Immediacy. ‘Once a relevant goal has been identified, the theories of first choice are those where this goal is the response variable’. Discarding for now his raccoon example, and using something more topical: if one’s main interest is in the response of growth of forests to elevated CO2 then do not waste time studying foliar biochemistry or longevity of leaves – these alluring topics can be looked into later – they are second order. Peters’ advice would be: go straight in and measure growth in the treatment and control.
Operationalism. If the theory is to be useful to others, it must be couched in terms well-enough defined that the research community can understand and work with. This criterion challenges many of us who work at the interface of disciplines. It is possible, for example, that two different disciplines are working on the same topic, but because of different terms and paradigms they do not notice each other’s work.
Accuracy. The similarity of the observed and predicted values of a response variable measures the accuracy of the theory or hypothesis. Young researchers of today are much more familiar with this idea than their predecessors and more versed in statistical techniques to measure accuracy in these terms.
Generality. It is a human trait to generalise from a small number of observations and scientists are frequently tempted to making hand-waving statements that extrapolate from the particular to the general, in the hope of adding value to their work. Peters does not warn against it, but all-too-briefly considers the generalising power or otherwise of regression analysis.
Quantification. Peters quotes Lord Kelvin ‘If you cannot measure, your knowledge is meagre and unsatisfactory’. Of course, in ecology, it is often hard to measure some of the critical parameters required for testing hypotheses, and researchers often use a proxy measure, or a measure made from a too-small a sample or a biased measure, or one made at the wrong scale of organisation. Such are the inherent pitfalls of the field.
Economy of effort. Peters points out that the response variable may turn out to be easier to measure directly than to predict from the theory, simply because the time taken to acquire the parameters for the model is excessive. His example seems trite, once more taken from a study of raccoons. More generally in ecology the hypotheses are supposed to express, or at least hint at, underlying causal relationships and the goal is to understand these rather than to predict the response variable. In macro-ecology where one variable is plotted against another, a huge economy of effort is suggested, yet the utility is less than it seems because the variables concerned are generally logarithmically transformed (if the antilogarithm is taken, it is clear that the accuracy is poor).
Social elements. Peters make the point that ‘Although the scientific value of our work depends on predictive power, no theory has any value unless it is read and used by the scientific community’. In today’s scientific arena where citations and even downloads are faithfully recorded and expressed as an index, this point is well-recognised by authors and publishers. Moreover, these indices are used in promotion panels, in selecting candidates for employment, and in China they have been used as inputs into an algorithm to determine salary levels. Much emphasis in university courses is therefore placed on expressing research results clearly to one’s peers through seminars, workshops and posters. Thirty years ago, few people consulted the Science Citation Index.
Peters implies that ecologists ought to follow the advice of these nine paragraphs in order to sharpen their science. Years ago, Platt (Citation1964) has observed that some fields of science move forward much more rapidly than others and real advances frequently occur in complex and difficult subjects like molecular biology and high-energy physics. He considered that factors such as the tractability of the subject, the quality or education of those drawn into the subject and the size of the research grants were possible factors, but concluded that these rapidly moving fields were those where a particular method of doing scientific research is systematically used and taught. In fact, my own observation is that until recently most ecology and biology courses did not teach scientific inquiry in a compulsory course – it was expected that students would pick up the ideas by learning from the example of their professors, like a tradesman’s apprentice. One reason for this may have been that the teaching of scientific method in universities often took place in departments of philosophy and were more likely to be taken by students whose field of interest was not science at all. The culture is changing, and now there are guidebooks and advice (Tuomivaara et al. Citation1994; Ford Citation2000; Seppelt et al. Citation2018). There is also much more active debate on the subject of scientific method (see Baker Citation2016).
But how far can one learn how to be a researcher other than by doing it? Is not natural curiosity and the system of trial and error something that is innate in Homo sapiens, but better developed in some people than others, like artistic ability? Possibly it is, but the natural researcher, to become a leading researcher, must learn extra skills, such as how to challenge orthodoxy or to take no notice of it whatsoever.
Some 180 pages of the book then illustrate the weakness of ecology as a science, often in philosophical language that most readers have struggled to grasp (as noted also in the book review of Beal et al. Citation1993), but using the above criteria as a reference point and with emphasis on the weak predictive power of the science. Peters’ most stinging criticism is at the end of the Chapter 9: ‘Ecology now stands in desperate need of ruthless criticism to liberate itself from the accumulated weight of intellectual baggage, so that the brightest researchers will once more be free to engage the tremendous scientific and practical difficulties that confront humanity’.
Citation data are sometimes used, for example Peters shows that ecological works published in the journal Science are less often cited than for other disciplines and that rejection rates for ecology in general are very high compared with the hard sciences such as chemistry and physics (where the standards are rigorously defined and people can follow them, and therefore present acceptable papers more readily) but not as high as for the ‘soft sciences’ such as sociology where rejection rates are even higher because of subjectivity of authors, reviewers and editors.
Peters (Citation1991) has little to say about what exactly makes ecology ‘intractable’. However, most authors would agree that it is indeed ‘intractable’ and deny the existence of anything resembling the exact ‘laws’ of the physical sciences (see Turchin Citation2001). Yet most would expect there to be underlying principles to guide the researcher through the complexities. Grubb (Citation1989) has provided a list of six principles of ecology, and it would be comforting to think that all ecology springs from such a foundation, rather like much of chemistry can be inferred from the Periodic Table, and much of physics can be inferred from the four fundamental forces of nature. All of Grubb’s six principles () were known well before Peters (Citation1991), though not usually referred to implicitly. Like Peter Grubb, John Lawton also proposed ‘three deep universal laws’ and two others that apply to all of ecology Lawton (Citation1999):
The first and second laws of thermodynamics.
The rules of stoichiometry, a particular application of the universal law that matter (in a non-nuclear world) cannot be created or destroyed, and the explanation for why alchemy is a dead profession.
Darwin’s law of natural selection as an explanation for evolution.
The set of general physical principles governing diffusion and transport of gasses and liquids, the mechanical properties of skin and bone, aerodynamics and hydrodynamics, and others that singly or in combination define limits to the performance of individual living organisms, and which underpin the study of plant and animal physiology.
The trivial, but important observation that organisms interact with one another (no species, anywhere in nature, lives in splendid isolation) and with their environment.
Table 1. List of ecological principles proposed by Grubb (Citation1989) and the present author’s comments.
It is of course the fifth ‘law’ which causes trouble for ecologists! The study of an individual species in laboratory conditions is relatively easy, but so many different species interactions can and do occur that the task of studying each one in isolation becomes impossible. Thus, it is indeed hard to predict how climate change will influence whole ecosystems, or to understand how land management might affect biodiversity in the landscape.
It is noteworthy that Grubb’s Law 6 and Lawton’s Laws 1, 2 and 4 are laws borrowed from physics and chemistry, and Grubb’s Law 5 is a mathematical derivation. They are therefore not ecological laws but laws of the universe in general. The remaining Laws can be said truly to belong to ecology, though much of ecology proceeds without reference to any of them.
However, both Grubb’s and Lawton’s ‘laws’ do underpin much ecological thinking – they are dealt with implicitly in various chapters of May and McLean (Citation2007) and reflected in a different viewpoint by the Ecological Society of America’s Report on ecological principles in relation to land management (Dale et al. Citation2000). Perhaps the difficulty of ecology lies in the breadth of its ambition: there is too much to grasp, too many variables, and a plethora of connected phenomena which cross several scales of organisation in space and time, as Fenchel (Citation1987) has stressed. To make matters worse, there has always been schism between animal and plant ecology: unlike most animals, plants are sessile except in the dispersal phase and so there has been a focus on limitations to growth and tolerance of stress (see Körner Citation2018). Evolution has produced a wide diversity of plant forms, with different life cycles, tolerances and fecundities. Despite efforts to produce plant typologies (‘Life forms’ of Raunkiaer Citation1934, ‘Strategies’ of Grime 1979, and ‘Plant Functional Types’ of Diaz and Cabidot 1997 and others) and despite many comparative experiments and ordinations of traits (Grime Citation1965; Harper Citation1977; Tilman Citation1985; Wright Citation2004) there is clearly much remaining work before we can predict how plants will behave in heterogeneous habitats in the face of competition, predation and the hand of humans.
Perhaps slow progress has arisen from the perceived lack of a theoretical, unifying background. Yet the older literature brims with theoretical concepts, mostly derived from studies on animal ecology and behavior: predatory-prey relations (Lotka Citation1925; Volterra Citation1926); species richness (Hutchinson Citation1959); island biogeography (MacArthur and Wilson Citation1967); game theory (Lewontin Citation1961; Maynard Smith Citation1973) and metapopulation theory (Levins Citation1969). Robert May’s book published in (Citation1976) brought much of this together and was a big step forward in bringing a theoretical framework to ecology, demonstrating that many of the dynamic features that we see in nature can be derived from relatively simple relationships, expressed mathematically (May Citation1976). Is all this what Peters called ‘intellectual baggage’? I hope not. Since 1991, when Peters published his book there have been further developments in these subjects, and new topics have emerged, including those discussed by Marquet et al. (Citation2014): optimal foraging theory, metabolic theory of ecology, maximum entropy theory and the neutral theory of biodiversity. May’s book evolved into the much more accessible May and McLean (Citation2007) where theory and observations are brought together; Ägren and Bosatta (Citation1996) presented a theoretical treatise on nutrient cycles in ecosystems.
Perhaps the biggest shift since 1990 has been the re-direction of effort away from the fundamentals of ecology towards data-gathering in relation to environmental change. Many of the best young ecologists have migrated far from the core subject, moving their interests in response to funding opportunities, and occupying academic positions in applied areas such as conservation biology, mathematical modelling, global change and remote sensing (). This often represents shifts of emphasis, shifts of scale, collaboration with scientists from other disciplines and quite often a higher level of engagement with society in areas such as advocacy, nature conservation, consultancy. It seems that contrary to Peters’ stinging criticism, young ecologists are completely ‘free to engage the tremendous scientific and practical difficulties that confront humanity’ and the best of them are in much demand. Another change over the last 30 years has been the strong development of ecology worldwide and especially in southern and central Europe, parts of South America and especially in China, stimulated by the realisation by governments that environmental policy needs to be evidence-based and science-based. There has also been a corresponding proliferation of journals, as new branches of the subject have formed and grown. The long-established journals include Journal of Ecology (founded by the British Ecological Society in 1917), its sister journal, Journal of Animal Ecology (1932), Ecology (by the Ecological Society of America in 1920) and Oikos (1949, by the Nordic Ecological Society). However, ecologists today are just as likely to turn their back on these and submit their work to other even more highly-cited journals such as Global Change Biology (founded in 1995), Conservation Biology (1987) or Ecology Letters (1998). They might consider Nature or Science for something really special. However, the four long-established journals have changed rather little in their editorial policies and may be said to publish ‘mainstream’ and ‘pure’ ecology. But to accommodate the output of the rapidly-growing army of young ecological researchers, new ecology journals have been launched. Here we will add just one relative newcomer to our database for analysis: Functional Ecology (1987, also from the British Ecological Society). We compare some attributes of papers found in the five journals for the year 1990 (when Peters’ book was presumably being written) and 2018. In 1990 the five jointly published 653 papers, but by the end of 2018 the total had risen to 1,124. No-one is capable of reading such a large volume of output. However, search engines have rescued the community from drowning in a tsunami of research reports. One way to test for recent changes in published ecological research 1990–2018 is to examine citation indices using the Web of Science (http://webofknowledge.com) wherein exact search terms may be used as an unbiased research tool to examine a large database of journals.
Citation data
Peters considered that ‘ecology is in a period of eclipse’. He used citation data to prove his point, claiming that for ecology, ‘its papers are cited less frequently than those of other sciences, so its impact is low’. Here we use citation data to examine citation patterns of ecological papers.
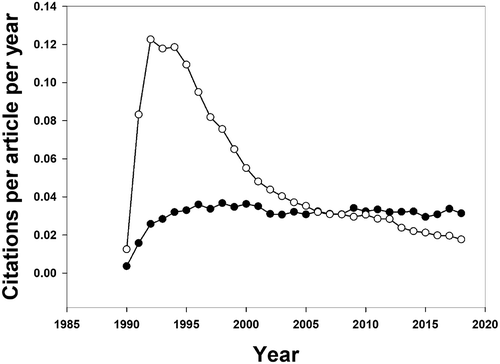
First, we look at papers published in all journals, and compare the performance of papers containing the word ‘ecology’ with papers containing the word ‘genome’ (representing an area of the rapidly-moving science that Peters and others have talked about, and with roughly the same number of papers as ‘ecology’ in 1990). The 934 ‘ecology’ papers from 1990 have by now a mean score of 0.89 citations per article per year. For the 959 ‘genome’ papers published in 1990, there were 1.48 citations per paper per year. An important difference between ‘ecology’ and ‘genome’ is the pattern over the 28-year period (). ‘Ecology’ papers are relatively slow to be cited, but their life is longer. This is exactly what Peters draws attention to: he interprets this long half-life of ecology as ‘stagnation’. But currently (2018) the 1990 ‘ecology’ papers are more often cited per year than the same-age ‘genome’ papers and possibly in years to come the ecology papers will have accumulated a higher total citation. A case of the tortoise and the hare, perhaps.
Figure 3. Citation trends obtained for articles published in 1990, using the search words ‘genome’ (open circles) and ‘ecology (closed circles). The search included all journals on the Web of Science (core collection).
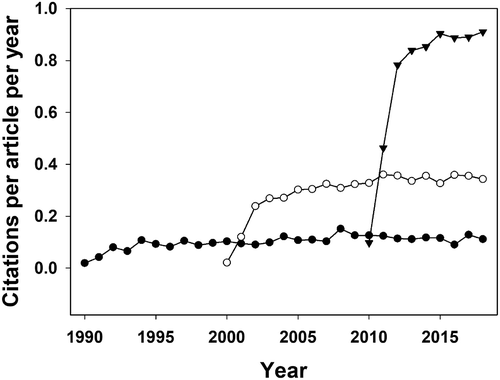
Secondly, we look at papers published in Nature or Science. For both ecology and genome, we may expect to find the highest impact papers (arguably ‘the best’) in these elite journals of general science. For the 1990 papers, ecology had twice as many papers as genome, and a mean citation of 7.4 per paper per year, whereas genome has only 3.8. This is a surprising result given Peters’ comment about the poor impact of ecology papers. If we re-examine the data for papers published in 2000, we find that ecology has advanced to 18.7 cites per paper per year and genome has also advanced, to 17.4, but is still less than ecology.
The usual caution regarding citation data needs to be kept in mind. To some extent the citation data reflect the size of the research community. This may explain the progressive improvement in the citation scores of ecology papers over the decades following Peters’ book (). Another factor may be an increase in the rejection rate as highlighted by Wardle (Citation2012).
Figure 4. Trends of citations for ‘ecology’ papers published in a basket of five top ecological journals in 1990 (closed circles), 2000 (open circles) and 2010 (closed triangles). The journals used in the search were Ecology, Functional Ecology, Journal of Animal Ecology, Journal of Ecology and Oikos.
Hypothesis testing
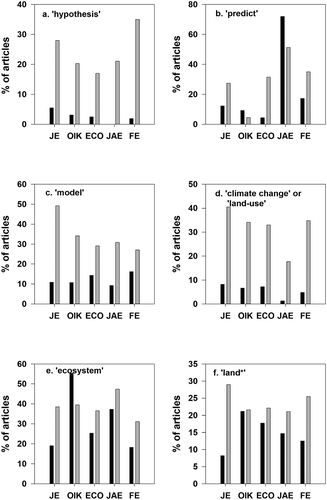
To what extent do authors usually put forward a hypothesis to test? Peters thought they often did not. We can test this by examination of the five mainstream ecology journals by searching for the word ‘hypothesis’. However, we should have in mind that the result is an imperfect test, as some authors do not make an honest a priori hypothesis, but instead invent an a posteriori hypothesis. This practice is unfortunately common in all branches of science, and one of the reasons why Peter Medawar said ‘the scientific paper is a fraud’ (Medawar Citation1964; Howitt and Wilson Citation2014). Putting this thought aside for the moment, we see that the metrics suggest that ecologists are more often offering hypotheses to test than hitherto ()). Now, 24% of papers are found to contain the search term <hypothesis*> whereas in 1990 only 15% did. Oikos presents an interesting case: even in 1990, as many as 21% of the papers included the term, much more than in the other journals (predilections of editors may influence such things). Of course, it is not necessary for every piece of work to be a hypothesis test. For example, the most highly cited paper in Journal of Ecology in 1990 grabbed by the hypothesis search term is about fuzzy data sets relating species and habitat (Equihua Citation1990). The author states: ‘The aim of this paper is to demonstrate that fuzzy sets are a suitable representation of ecological communities, but also that their use requires a philosophical view of the data that is different from the usual approach adopted by ecologists. It can be argued that the human mind tends to filter out fuzziness, which results in a perception of discrete objects, discrete classes of things and discrete events’. Hypothesis testing is a very minor part of this paper; rather, the author puts forward some thoughtful views on the handling of ecological data and demonstrates a new approach. This is a valid activity in science, as reflected in its citation score of 90. On the other hand, the top hypothesis-paper so far in 2018 (the download was 22 January 2019, so citation scores are still low) is indeed a straightforward hypothesis test: the authors conclude that the hypothesis of ‘bet-hedging’ (in the context of carbon storage and drought, see their text) must be rejected, and even included a ‘no’ in the title of the paper to signal the rejection (Bachofen et al. Citation2018). Peters would presumably have approved.
Predictions
Peters repeats the oft-quoted assertion that the hallmark of science is ‘prediction’, illustrating his argument with reference to simple regression. Yet much of scientific activity is not about prediction, and many science disciplines simply are unable to make exact predictions as there are too many variables at work, and insufficient data. Meteorology cannot predict exactly when it will rain, seismology does not tell us when the next eruption of Vesuvius will occur, and astronomy does not predict when or where to expect the next supernova in our galaxy. Prediction sensu stricto can only be made when all the variables that act upon the system are known in infinitesimal detail (as in Laplace’s Demon or Lorenz’s Butterfly). However, all science strives to be able to predict, at least in the rough sense (‘you may expect rain on Thursday’, or ‘it’s likely that an eruption of Vesuvius will occur in the next few years’ or ‘a supernova will occur about twice a century in the Milky Way’).
The criticism from Peters is that ecological research does not attempt prediction. This can be tested on the Web of Science with the search term <predict*> ()). For all five journals tested, the frequency of this term has increased in the 28-year period from a low of 10% of papers in 1990 to a high of up to 49% in Journal of Ecology of 2018. It is more difficult to gauge whether these predictions have been successful or how well they have been tested. Top of the list for the 1990 search in Journal of Ecology is a paper about testing the predictions of the herbivore optimisation theory, in which the authors carry out a set of admirably straightforward field experiments with geese (Hik and Jefferies Citation1990). The hypothesis is adequately tested using Analysis of Variance, yet the authors modestly point out that experimental tests under field conditions are ‘extremely difficult’. Often, as here, the theory does little more than provide the conceptual basis for an experiment, but a rigorous test is impossible. Examining the 2018 top paper we find work on the likely future distribution of the Golden Kelp Laminaria ochroleuca (Franco et al. Citation2018). Controlled experiments show that this alga dies at 24.6 °C and using scenarios of global warming it is possible to predict rather confidently how the species may be found further north as oceans warm. Ecologists in the near future will have to test the prediction.
Several recent papers have emphasised the need for ecology to be more predictive (Clark et al. Citation2001; Houlahan et al. Citation2017; Dietze et al. Citation2018). Clark et al. (Citation2001) have raised the important question of what is inherently predictable, making the point that much of ecology must be regarded as stochastic. Houlahan et al. (Citation2017) have called for a new commitment to prediction through the use of models as a means of demonstrating understanding of ecological behaviours. Dietze et al. (Citation2018) have pointed out that most practical human-related problems require knowledge of what will happen in the short-term (i.e. from months to a few years) yet model predictions by ecologists are usually for changes at the decadal scale.
Peters placed great emphasis on regression as a tool for prediction, but said less about simulation models as tools for prediction. What he did say about them (Peters Citation1991, p. 115–128) is illuminating and justifiably critical considering the state-of-the-art in 1990. By now, 2018, many classes of model have been developed and some of the most effective have been relatively simple representations of the land surface within global climate models, starting with the Simple Biosphere Model (Sellers et al. Citation1986) and leading to a rich assemblage of climate-ecology models reviewed recently by Fisher et al. (Citation2018). A glance at the institutional affiliations of the 20 co-authors of Rosie Fisher’s paper is a good way to demonstrate how ecology is becoming integrated into an emerging new scientific community with physical scientists (a sign of vibrancy in science). Through such models it becomes possible to draw together data sets from plant physiology, demography and remote sensing, and thus ultimately to address the issue of how to scale up from plot to planet (Moorcroft et al. Citation2001). Modeling of a somewhat humbler kind was strongly represented in the journals even in the 1970s, and by 1990 the search term ‘model’ was represented in a quarter to a half of all papers in the five journals (). All journals have increased their emphasis on models by 2018 except for Oikos, which was very high already ().
Models can be beguiling and there exists a natural but dangerous tendency to think that models are the real world, forgetting that they are merely hypotheses, or rather (as in simulation models) sets of hypotheses. Revealing cause-and-effect relationships is at the heart of all scientific endeavour, and this is normally done by experiment. To be realistic, experiments in the topics of societal importance (drought, climate change, biodiversity) need to be done at a large scale (large areas of land, many years of observation), and they are therefore expensive. Yet without them, we rely on mere correlations to inform our models.
From curiosity-driven research to utility research
From browsing the journals, it is clear that most of the earlier papers were driven by the natural curiosity of the researcher, and often involved a study of one or a few species (the ‘autecological approach’). In contrast, many recent papers are motivated by the need to understand and ‘predict’ the impact of some aspect of global change, often climate change or land-use change. This impression is supported by the metrics when search terms <climate change OR land-use> are employed. There has been an enormous increase in climate change research and topics relating to land use change, especially removal of forest. The trend is seen not only in the proliferation of new journals that focus of these topics but also in the mainstream ecology journals. The Journal of Animal Ecology had no such papers in 1990 but they now constitute 21% of published work ()). The shift in emphasis is probably driven by funding agencies. Those writing the research agendas have huge budgets and they perceive a duty to spend it for the public good, especially at the present time when science can be especially powerful in understanding the nature and rate of global change. Grant applications are nowadays scored partly on the basis of societal relevance, although this trend may be stronger in Europe and North America than elsewhere. Many of the early papers in all these journals have no obvious social relevance whatsoever, yet who is to say what such ‘blue sky’ research may deliver in 20 years time?
The ecosystem as the preferred unit of study
The term ecosystem was first used in print by Tansley (Citation1935) to express the notion that plants and animals do not simply operate as communities of organisms, but they interact with their inorganic surroundings, the soil and atmosphere, and respond to environmental cues. Willis (Citation1997) has explained the origin of the term – how a young Oxford researcher Arthur Clapham (later to become Professor AR Clapham) suggested the word during a chance encounter with Tansley. Much later, ecosystem science was taken up by Eugene Odum in his inspirational book Fundamentals of Ecology (Odum Citation1953). It took some while to catch on. But near the time when Peters was writing, Malcolm Cherrett conducted a survey of members of the British Ecological Society, asking which ecological concept they thought to be the most important: top of the list was ecosystem (Cherrett Citation1989). Oddly, Peters barely mentions the concept in his section of ‘Typologies’ (Peters Citation1991, p. 81–82) although more than any other, it has unified ecologists, provided a suitably large-scale unit of study, and aided a holistic view. The citation data show that in 1990 the search term <ecosystem> was infrequent in the leading journals except for Oikos, but by 2018 it has increased ()). The term ‘ecosystem services’ began to be widely used after Grechen Daily’s book (Daily Citation1997) although it was used earlier by Ehrlich and Mooney (Citation1983).
Hypothesis testing with large-scale experiments and research networks
Ecologists have often been criticised for studying processes at such small spatial scales (often no more than a few square metres) that their work could never capture the dynamics of ecological systems given that heterogeneity is always large and many crucial interactions occur across large distances (Wiens Citation1989; Osmond et al. Citation2004). To what extent is it true that today’s researchers think more about landscape scale rather than small sample plots? The search term <land*> may be used to capture anything explicitly related such as landscape or land-use-change ()). Whilst Journal of Ecology and Functional Ecology did show a strong move to ‘land’, the other three journals were less inclined, perhaps because of the proliferation of specialised journals in this area.
Scale issues have frequently emerged as topics for discussion (Wiens Citation1989; Grace et al. Citation1997; Moorcroft et al. Citation2001). In ecological research, not only is the spatial scale frequently too small but also the temporal scale is often insufficient. Ecological processes take place over periods of time much longer than the duration of typical funding. There are well known cases where long-term experiments have been possible, including the Rothamsted Park Grass experiment, started in 1856 (Silvertown et al. Citation2006) and the Hubbard Brook Experimental Forest, from 1955 (Holmes and Likens Citation2016). Ecological systems show unexpected modes of behaviour when viewed over a long period, prompting us to rethink our views on how the ecosystem functions. In the Park Grass experiment, it has been possible to demonstrate some of these phenomena. Several hundred papers have emerged, although the experiment was originally designed by agricultural scientists in order to predict the effect of management on the yield of hay. Only later was the ecological importance recognised and a flurry of papers ensued from the 1970s to the present day. It was possible to show genetic drift, to validate models of carbon accumulation in the soil, and to test several ecological hypotheses that would otherwise have been untestable (Tilman Citation1982; Silvertown et al. Citation2006). However, in the rapidly-moving realm of climate change, ‘long-term’ has come to mean just 5–20 years, which is arguably too short in relation to the behaviour of the climate system. In the era of rapid global change, long-term studies take on a new significance.
Experiments on global change do not rely on ‘nebulous’ hypothetical constructs of the sort described by Peters. Rather, the researcher asks a simple question about the influence of drought, elevated CO2, or nitrogen addition. Frequently those who pose the question and begin the years of work are no longer active scientists when the result is ready for publication! The Swedish Forest Optimum Nutrition Experiment, started by Tamm and others in the late 1960s (Tamm et al. Citation1999) is still providing researchers with answers. In a heroic attempt to bring together all that is known of the impact of climate change on Swedish and other forests, Hyvönen et al. (Citation2007) quite properly called their paper ‘The likely impact of elevated CO2, temperature … ’, acknowledging that much remains to be known about the responsiveness of boreal and temperate forests despite many years of study. Pitfalls remain. The Swedish nitrogen addition experiments are often used to discuss the possible impact of anthropogenic nitrogen reaching the forest from the atmosphere by wet and dry deposition. However, the nitrogen was applied to the soil as a fertilizer, in line with the original intention, overlooking the possibility that direct foliar uptake from the atmosphere may be an important addition, and may influence the ecosystem in profound ways. Indeed, it seems to be the case (Magnani et al. Citation2008; Janssens et al. Citation2010). Thus, experiments should usually be interpreted in relation to their original purpose.
Research in this area often involves large teams and major infrastructure; it is expensive, and leaders of the research community spend much of their time fund-raising on a scale unknown in the earlier period. The possibility of funding large teams of scientists was uncommon before 1990. In the 1990s new funds became available for international co-operation, such as the Large-Scale Biosphere-Atmosphere Experiment in Amazonia, LBA (Nagy et al. Citation2016). In LBA, Brazilian researchers were joined by scientists from USA and Europe. It was a huge stimulus for new science. Likewise, in Europe, substantial funding from the European Union brought major advances, particularly in the quantification of the carbon and nitrogen cycles (Schulze Citation2009; Sutton et al. Citation2011) and aspects of biodiversity (Lung et al. Citation2014).
The CO2 story
Here, we consider the impact of elevated CO2 on vegetation, as an example of how ecological research is providing answers to questions that face humankind. The concentration of CO2 in the atmosphere has been increasing inexorably, from 350 ppm in 1990 to 410 ppm in 2018 (Figueres et al. Citation2018) and is set to continue. The hypothesis to be tested is simply: plants will grow faster in a future high CO2 world. A related question for most ecologists is: how will such conditions influence ecosystems as a whole? A question that follows for climatologists is: will the terrestrial vegetation absorb more of the anthropogenic CO2 and therefore ameliorate global warming? Such questions can only be answered by large controlled environment experiments. The work is relevant not simply for predicting food and timber production but also for running carbon cycle models to enable prediction of behaviour of the earth system under scenarios of change.
CO2 research has a long history. Horticulturists and plant physiologists have long known that the photosynthesis of leaves substantially increases with elevated CO2 (Gaastra Citation1959). Early work by Dutch scientists was quickly translated into commercial practice to enhance growth of glasshouse crops; ecologists and agronomists soon conducted experiments in strictly controlled environmental chambers but using potted plants, often with an unvarying light climate that was rather low and with spectral distribution quite unlike daylight (Grime and Hunt Citation1975; Aoki and Yabuki Citation1977; Bazzaz and Carlson Citation1984). In the quest for a more natural environment, the work was enthusiastically extended using various types of outdoor enclosure, the most popular being the Open Top Chamber (Drake et al. Citation1989; Centritto et al. Citation1999) in which young plants were usually growing in their native soil, in the open or inside a greenhouse, for periods of months or a few years. One of the longest sets of observations was the sour orange experiment started by Idso. It lasted 17 years. The young trees were treated as they would have been in a managed orchard, with an application of nitrogen fertilizer. They grew faster in elevated CO2 and they yielded more oranges consistently (Kimball et al. Citation2007). But all such chamber experiments suffered from a lack of realism: the subjects were individuals of one species. It remained unclear what would happen to plant communities and ecosystems as a whole.
The crowning development in the search for realism was the Free Air Carbon Dioxide Enrichment Experiment (FACE), wherein vegetation could be exposed to high CO2 without the need for any surrounding chamber, retaining the natural microclimate and thus avoiding spurious results. This was achieved by releasing controlled quantities of CO2 from an array of nozzles held on vertical masts, allowing natural turbulence to mix the CO2 with free air. The design, history and development of FACE is described by Norby et al. (Citation2001). One of the longest running of these experiments was probably the Oak Ridge FACE where replicate plots of a sweetgum (Liquidambar styraciflua) plantation were exposed to 550 ppm CO2 from 1998–2009. At first, the Net Primary Productivity was enhanced relative to controls by 24%, but then the enhancement declined, falling to only 8% in 2008. The decline was the result of nitrogen being in short supply, as shown by foliar analysis. Plants need resources other than CO2, most of which are derived from the soil including water and minerals, and Leibig’s Law of the Minimum applies, i.e. when CO2 no longer limits growth, the plant becomes limited by other factors (although plants respond by allocating resources between roots and shoots to compensate for the initial limitation, and so few ecologists would now cite Leibig’s Law, acknowledging that ‘co-limitation’ is common). Ainsworth and Long (Citation2005) asked “What have we learned from 15 years of free-air CO2 enrichment? They identified 120 peer reviewed articles reporting FACE with elevated CO2 concentrations from 475 to 600 ppm. By that time, a wide variety of plant types had been the subjects of experiments. In terms of biomass production, trees showed the largest response in dry matter production (28%), followed by legumes (24%) whilst C3 grasses showed only a 10% increase in above-ground production. Yields of grain crops were far less increased than expected from previous controlled environment studies whilst species with C4 photosynthetic systems (tropical grasses) did not respond at all to elevated CO2.
There are, however, limitations to most of these studies. Researchers seem to have forgotten all that was written about the measurement of plant growth many years ago (Evans Citation1972; Hunt Citation1989). For young trees or developing vegetation, a ‘yield’ increase in relation to a control will always depend on the stage of development as plants tend to grow in an exponential fashion, and so Relative Growth Rate is the correct response variable, not simply ‘yield’ or dry mass. However, the subject moves on, and many others have enlarged upon these results, including the discovery of an increase of nitrogen in the soil when CO2 is elevated (Luo et al. Citation2006), and an increase in the soil carbon with an overall larger ecosystem ‘sink’ of carbon (Hoosebeek and Scarascia-Mungoz Citation2006). Moreover, new work suggests that even the FACE experiments need to continue much longer than most of them run: Reich et al. (Citation2018) has shown that the difference between the responses of C3 and C4 grasses to elevated CO2 was reversed when the experiment was extended for 20 years.
Nearly all of the experiments cited above were still being carried out on young plants of a single species. The response of mature trees to CO2 has rarely been tested (but see Körner et al. Citation2005). The next challenge in FACE experiments has just begun, that is to carry out FACE at a large scale on old-growth forests. Costs escalate, and just four such experiments have by now been planned or have already started, worldwide including a native Eucalyptus forest in Australia (the EucFACE experiment), Amazon rain forests (Manaus, Brazil), a pine forest in southern Sweden (SwedFACE) and an old Oak woodland in England (). The experiments will enable the scope of the work to broaden to the ecologically-meaningful scale, include aspects of animal ecology such as herbivory and pollination. For example, the Australian experiment has now been running for six years, and robust conclusions are possible, for example on the impact of elevated CO2 on the activity of rhizosphere organisms. There was a stimulation of the rhizospere leading to an increased availability of nitrogen and phosphorus to the trees (Hasegawa et al. Citation2016; Ochoa-Hueso et al. Citation2017). Other ecosystem-level data emerging from this important experiment relate to understorey composition, hydrology, insect communities and herbivory.
Figure 6. Free-Air CO2 Enrichment experiment (FACE) on mature oak forest (160-year-old Quercus robur). There are six 30 m-wide rings of fumigation-masts, three control and three elevated CO2. In the centre of the picture is a meteorological and CO2 observation tower. Birmingham Institute of Forest Research (BIFOR), University of Birmingham. Photographic credit: Norbury Estate.

We may conclude from this overview of just one field of ecological research that Peters’ criteria are nowadays fulfilled. In the CO2 work cited above, there has always been a ‘clear hypothesis’, scientist have generally chosen the ‘most important phenomenon within their competence’, the criteria of immediacy and operationalism are clear (researchers have been largely directed by funding opportunities). ‘Accuracy’ as Peters defined it is not relevant here, but in the more general sense results are expressed with uncertainty limits using a variety of statistical tools that had scarcely been developed in 1990. Real accuracy is harder to evaluate – it certainly is likely to be worse than the statistical tests that are reported because the experiments themselves will usually have unknown or hidden flaws, often related to the duration of the experiment, the fact that exposure to high CO2 is only in the day (to save the expense), and the particular species or ecosystems chosen may not be representative. Quantification has been achieved – sometimes to a very high level on a per plant basis but always limited by the amount of replication that is feasible. Generality is expressed, as this is the synthesis of 120 controlled experiments; no attempt is made to over-generalise at this level, as the division of data into broad plant functional types provides the all-important uncertainty limits for each type. The citations of Ainsworth and Long (Citation2005) have been growing over 10 years. Most importantly, the science now enables a level of ‘prediction’ of how much faster plants might grow in the future world of high CO2, in the presence or absence of added nitrogen fertilizer. All this has become possible because ecologists have becoming willing to join networks and to share data, and researchers are in much better contact than hitherto through conferencing and e-mailing. How the world has changed since 1990!
A reviewer of this paper has pointed out that CO2 research may be an exception to the norm in ecology, with higher standards. Is it really ecology? The research area has grown out of forestry and ecology, and researchers consider themselves eco-physiologists or experimental ecologists rather than mainstream ecologists. Moreover, the area has been well-funded, perhaps attracting the best talent. My view is that one could have chosen any of many sub-disciplines of ecology to illustrate substantial progress, and the conclusions would have been the same (the citation data, for example –, e support my view).
Hugely expanded observation base and data mining
As a result of the many years of ecological research a huge data resource is available. Some of it still resides as paper documents in archives, but most of it is freely available in electronic form. Today’s researcher can test hypotheses about global change by interrogating other peoples’ data – i.e. data mining. From earlier times there are impressive examples even though researchers had no computers. For example, Elton and Nicholson (Citation1942) have analysed data from the fur-trading Hudson Bay Company. The data are from counts of hare and lynx pelts traded from different parts of Canada since 1673. This remarkable data set continues to exercise the minds of animal ecologists (Krebs et al. Citation2018). Ecological time series are required for tackling many real-world problems, for example managing fish stocks by using models (Beddington and May Citation1977; Koenigstein et al. Citation2018 but see Pilkey and Pilkey-Jarvis L Citation2007), yet this aspect of ecology – which has been rather successful, and does involve prediction – is not considered by Peters. New lines of time-series data continue to be complied: volunteer organisations in many countries have digitally-archived their observations on distribution and abundance of key taxa, including vascular plants, bryophytes, birds, bats, beetles and butterflies. ‘Citizen science’ adds further data and also inspires a new generation of ecologists. New parts of the world have developed long-term ecological observations: supported by the Chinese Academy of Sciences (CAS), the Chinese Ecosystem Research Network (CERN) was established in 1988, with 36 research stations, some in remote regions of the country, and a Synthesis Centre.
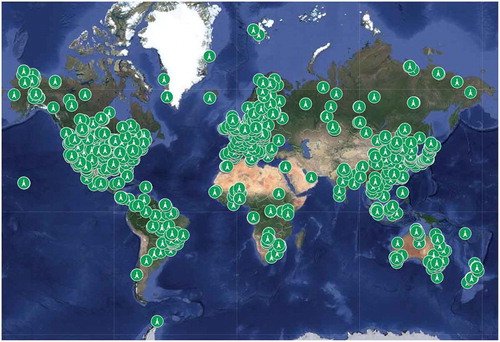
But even more importantly, automatic data collection systems including satellites, cameras and other sensors are silently providing a wealth of ecologically-relevant information from all over the world, which needs analysis. This is not ‘experimentation’ and is usually called ‘monitoring’, but over long periods relationships with patterns of weather can suggest causal relationships. In many cases, data are being collected at a faster rate than can be examined and prepared for publication. For example FLUXNET (), a global network of observation towers that has been in place for over 20 years (Baldocchi et al. Citation2001) collates continuous measurements of exchanges of CO2, H2O and energy between ecosystems and the atmosphere, and groups of researchers use the opportunities to address more general ecological research questions. Some of these sites are additionally equipped with canopy cranes, allowing study of leaf physiology, herbivory and hitherto-neglected components of biodiversity (bryophytes, lichens, arthropods) at close quarters (Shen et al. Citation2018). Young ecologists meanwhile are happily learning data-mining techniques and new types of analysis that were not invented when Peters wrote his book. Although ecological research in the future will rely more-and-more on the analysis of these huge volumes of data, usually by researchers who are far-removed from the sometimes-weary process of collecting the data, it is clear that experimentation will remain crucial for determination of cause-and-effect relationships, as it always has been in most other branches of science.
Figure 7. Distribution of CO2 flux sites 2018 (Burba Citation2019).
Conclusions
Ecology has developed in areas that Peters could not have envisaged. His critique of traditional ecology is not out-of-place: however, it is interesting to reflect on how research-level ecology has left many of the old topics and concepts behind in pursuit of solutions to the problems that mankind is now facing.
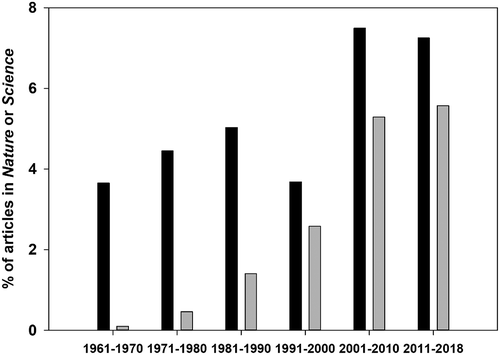
One reviewer of this article has argued that the title ought to be the more tentative: ‘Is ecology growing up?’ rather than ‘Has ecology grown up?’ on the grounds that we can never know whether the fully-grown state has been reached. When is any scientific discipline fully mature? The answer is surely ‘when its best researchers are confident with theory and practice, and able to communicate and collaborate with fellow scientists from other disciplines’. On this criterion, ecology can be said to be grown up. However, if maturity must be judged against being able to answer simple-sounding, but actually highly complex, questions like ‘How is biodiversity maintained?’ or ‘Why are leaves so variable in shape?’ then there is still some way to go. Ecology was in the doldrums in the period before Peters’ book. We may find evidence for this in the number of papers containing ‘ecology’ in the journals Nature and Science, where ecology competes every week with other science disciplines for space. It was a Cinderella science. However, we see a surge in ecology from the year 2000 (). Peters died in 1996, aged only 49. Perhaps he would have been pleased to see that ecology did eventually grow up.
Box 1. CO2 and Biosphere 2
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgements
I thank the three referees and the Editor-in-Chief. All made valuable comments, helping me immensely to improve the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Notes on contributors
John Grace
John Grace is professor emeritus in the School of GeoSciences at the University of Edinburgh. He received his academic degrees from the University of Sheffield, UK. His research interests are: ecophysiology especially water and carbon relations, forest ecology, fluxes of greenhouse gases, global change, urban flora. He is a past President of the British Ecological Society and of the Botanical Society of Scotland. He was the co-founding editor of the British Ecological Society’s journal Functional Ecology.
References
- Ägren GI, Bosatta E. 1996. Theoretical ecosystem ecology – understanding element cycles. Cambridge (UK): Cambridge University Press.
- Ainsworth A, Long S. 2005. What have we learned from 15 Years of free-Air CO2 Enrichment (FACE)? New Phytol. 165:351–371.
- Andrewartha HG. 1961. Introduction to the study of animal populations. Chicago: University of Chicago press.
- Aoki M, Yabuki K. 1977. Studies on the carbon dioxide enrichment for plant growth. VII. Changes in dry matter production and photosynthetic rate of cucumber during carbon dioxide enrichment. Agric Meteorol. 18:475–485.
- Bachofen C, Moser B, Hoch G, Ghazoul J, Wohlgemuth T, Piper F. 2018. No carbon ‘bet hedging’ in pine seedlings under prolonged summer drought and elevated CO2. J Ecol. 106:31–46.
- Baker M. 2016. 1,500 scientists lift the lid on reproducibility. Nature. 533:452–454.
- Baldocchi D, Falge E, Gu LH, Olson, R, Hollınger, D, Running, S, Anthoni, P, Bernhofer, C, Davis, K, Evans, R and Fuentes, J. 2001. FLUXNET: a new tool to study the temporal and spatial scale carbon dioxide, water vapor and energy flux densities. Bull Am Meteorol Soc. 82:2415–2434.
- Bazzaz FA, Carlson RW. 1984. The response of plants to elevated CO2. I. Competition among an assemblage of annuals at different levels of soil moisture. Oecologia. 62:196–198.
- Beal BF, Cartright M, Bethume D, Chaves SA. 1993. Book Review. Limnol Oceanogr. 38:1344–1346.
- Beddington JR, May RM. 1977. Harvesting natural populations in a randomly fluctuating environment. Science. 197:463–465.
- Burba G 2019. Illustrative maps of past and present eddy covariance measurement locations: I. Early update. [accessed 2019 Feb 22]. https://www.researchgate.net
- Centritto M, Lee HSJ, Jarvis PG. 1999. Increase growth in elevated CO2: an early, short-term response?. Glob Chang Biol. 5:623–633.
- Cherrett JM. 1989. Key concepts: the results of a survey of our members’ opinions. In: Cherrett JM, editor. Ecological concepts: the contribution of ecology to an understanding of the natural world. Oxford: Blackwell Scientific Publications; p. p1–16.
- Clark JS, Carpenter SR, Barber M, et al. 2001. Ecological forecasting: an emerging imperative. Science. 5530:657–660.
- Colvinvaux PA. 1973. Introduction to ecology. New York (US): Wiley.
- Daily GC. 1997. Nature’s services: societal dependence on natural ecosystems. Washington: Island Press.
- Dale VH, Brown S, Haeuber RA, Hobbs NT, Huntly N, Naiman RJ, Riebsame WE, Turner MG, Valone TJ. 2000. Ecological principles and guidelines for managing the use of land. Ecol Appl. 10:639–670.
- Darwin C. 1859. On the origin of species. London: John Murray.
- Dietze MC, Fox A, Beck-Johnson LM, Betancourt JL, Hooten MB, Jarnevich CS, Keitt TH, Kenney MA, Laney CM, Larsen LG. 2018. Iterative near-term forecasting: needs, opportunities and challenges. Proc Natl Acad Sci. 115:1424–1432.
- Drake BG, Leadley PW, Arp WJ, Nassiry D, Curtis PS. 1989. An open top chamber for field studies of elevated atmospheric concentration on saltmarsh. Funct Ecol. 3:363–371.
- Ehrlich PR, Mooney HA. 1983. Extinction, substitution and ecosystem services. Bioscience. 33:248–254.
- Elton C. 1927. Animal ecology. London: Sedgewick & Jackson.
- Elton C, Nicholson M. 1942. The ten years cycle in numbers of lynx in Canada. J Anim Ecol. 11:215–244.
- Equihua M. 1990. Fuzzy clustering of ecological data. J Ecol. 78:519–534.
- Evans GC. 1972. The quantitative analysis of plant growth. Oxford: Blackwell Scientific Publications.
- Fenchel T. 1987. Ecology- potentials and limitations. excellence in ecology Volume 1. Luhe (Germany): International Ecology Institute.
- Figueres CC, Le Quere GP, Peters G, Bäte O, Whiteman G, Peters G, Guan D. 2018. Emissions are still rising: ramp up the cuts. Nature. 564:27–30.
- Fisher RA, Koven CD, Anderegg WRL, Christoffersen BO, Dietze MC, Farrior CE, Holm JA, Hurtt GC, Knox RG, Lawrence PJ, et al. 2018. Vegetation demographics in earth system models: a review of progress and priorities. Glob Chang Biology. 24:35–54.
- Ford ED. 2000. Scientific method for ecological research. Cambridge (UK): Cambridge University Press.
- Franco JN, Tuya F, Bertocci I. 2018. The ‘golden kelp’ Laminaria ochroleuca under global change: integrating multiple eco‐physiological responses with species distribution models. J Ecol. 106:47–58.
- Gaastra P. 1959. Photosynthesis of crop plants as influenced by light, carbon dioxide, temperature and stomatal diffusion resistance. Mededelingen van de Landbouwhogeschool. Wageningen. 59:1–86.
- Gause GF. 1934. The struggle for existence. Baltimore: Williams andWilkins.
- Grace J, Van Gardingen PR, Luan J. 1997. Scaling-up from cell to landscape. In: Van Gardingen PR, Foody GM, Curran PJ, editors. Tackling large-scale problems by scaling-up. Cambridge: UK): Cambridge University Press;; p. 7–16.
- Grime JP. 1965. Comparative experiments as a key to the ecology of flowering plants. Ecology. 46:513–515.
- Grime JP, Hunt R. 1975. Relative growth rate: its range and adaptive significance in a local flora. J Ecol. 63:393–422.
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol Rev. 52:107–145.
- Grubb PJ. 1989. Toward a more exact ecology: a personal view of the issues. In: Grubb PJ, Whittaker JB, editors. Toward a more exact ecology. Oxford: Blackwell; p. 3–32.
- Haeckel E. 1866. Generelle Morphologie der Organismen Vol. 2. Berlin: Georg Reimer.
- Harper JL. 1977. Population biology of plants. London: Academic Press.
- Hasegawa S, MacDonald CA, Power SA. 2016. Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited Eucalyptus woodland. Glob Change Biol. 22:1628–1643.
- Hawkin S. 1988. A brief history of time. New York (USA): Bantam Dell.
- Hik DS, Jefferies R. 1990. Increases in the net above-ground primary production of a saltmarsh forage grass: a test of the predictions of the herbivore-optimization model. J Ecol. 78:180–195.
- Holmes RT, Likens GE. 2016. Hubbard Brook: the story of a forest ecosystem. New Haven (CT): Yale University Press.
- Hoosebeek MR, Li YT, Scarascia-Mugnozza GE. 2006. Free atmospheric CO2 enrichment (FACE) increased labile and total carbon in the mineral soil of a short rotation Poplar plantation. Plant Soil. 281:247–254.
- Houlahan JE, McKinney ST, Anderson TM, McGill BJ. 2017. The priority of prediction in ecological understanding. Oikos. 126:1–7.
- Howitt SM, Wilson AN. 2014. Revisiting “Is the scientific paper a fraud”. EMBO Rep. 15:481–484.
- Huffaker CB. 1958. Experimental studies on predation: dispersion factors and predator–prey oscillations. Hilgardia. 27:343–384.
- Hunt R. 1989. Basic Growth Analysis: plant growth analysis for beginners. Berlin: Springer.
- Hutchinson GE. 1959. Homage to Santa Rosalia or why are there so many kinds of animal species? Am Nat. 93:145–159.
- Hyvönen R, Ågren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, et al. 2007. The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal ecosystems a literature review. New Phytol. 173:463–480.
- Janssens IA, Dielean W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, et al. 2010. Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci. 3: 315. 322.
- Kimball BA, Idso SB, Johnson S, RILLIG MC. 2007. Seventeen years of carbon dioxide enrichment of sour orange trees: final results. Glob Chang Biol. 13:2171–2183.
- Koenigstein S, Dahike FT, Stiasny MH, Storch D, Clemmesen C, Pörtner H-O. 2018. Forecasting future recruitment success for Atlantic cod in the warming and acidifying Barents Sea. Glob Chang Biol. 24:526–535.
- Körner C. 2018. Concepts in empirical plant ecology. Plant Ecol Divers. 11:405–428.
- Körner C, Asshoff R, Bignucolo O, Hättenschwiler S, Keel SG, Peláez-Riedl S, Pepin S, Siegwolf RTW, Zotz G. 2005. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science. 309:1360–1362.
- Krebs CJ. 1972. Ecology, the experimental analysis of distribution and abundance. New York (USA): Harper and Row.
- Krebs CJ, Boonstra R, Boutin S. 2018. Using experimentation to understand the 10-year snowshoe hare cycle in the boreal forest of North America. J Anim Ecol. 87:87–100.
- Lawton JH. 1999. Are there general ‘laws’ in ecology? Oikos. 84:177–192.
- Levins R. 1969. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am. 15:237–240.
- Lewontin RC. 1961. Evolution and the theory of games. J Theoret Biol. 1:382–403.
- Lin G, Adams J, Farnsworth B, Wei Y, Marino BDV, Berry JA. 1999. Ecosystem carbon exchange in two terrestrial ecosystem mesocosms under changing atmospheric CO2 concentrations. Oecologia. 119:97–108.
- Lindeman RL. 1942. The trophic-dynamic aspect of ecology. Ecology. 23:399–418.
- Lotka AJ. 1925. Elements of physical biology. Baltimore: Williams and Wilkins.
- Lung T, Meller L Van Teeffelen AJA, Thuiller, W and Cabeza, M. 2014. Biodiversity funds and conservation needs in the EU under climate change. Conserv Lett. 7:309–400.
- Luo YQ, Hui DF, Zong DQ. 2006. Elevated CO2 stimulates net accumulation of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology. 87:53–63.
- MacArthur RH, Wilson EO. 1967. The theory of Island Biogeography. New Jersey: Princeton University Press.
- Magnani F, Mencuccini M, Borghetti M, et al. 2008. Ecologically implausible carbon response? Reply Nature. 451:E3–E4.
- Malthus TR. 1798. An essay on the principle of population. London: J. Johnson.
- Margalef R. 1968. Perspectives in ecological theory. Chicago IL (USA): University of Chicago Press.
- Marino BDV, Odum HT. 1999. Biosphere 2. Introduction and research progress. Ecol Eng. 13:3–14.
- Marquet PA, Allen AP, Brown JH, et al. 2014. On theory in ecology. BioScience. 146:347–353.
- May R. 1976. Theoretical ecology: principles and applications. Oxford: Blackwell Scientific Publishers.
- May R, McLean A. 2007. Theoretical ecology, principles and applications. 3rd ed. Oxford (UK): Oxford University Press.
- Maynard Smith J, Price GR. 1973. Logic of animal conflict. Nature. 246:15–18.
- McGill BJ. 2003. A test of the unified theory of biodiversity. Nature. 442:881–885.
- Medawar PB. 1964. Is the scientific paper a fraud? In: Edge D, editor. Experiment: a series of scientific case histories first broadcast in the BBC third programme. London: British Broadcasting Corporation; p. 7–13.
- Medawar PB. 1967. The art of the soluble. London: Methuen & Co.
- Moorcroft PR, Hurtt GC, Pacala SW. 2001. A method for scaling vegetation dynamics: the ecosystem demography model (ED). Ecol Monogr. 71:557–586.
- Nagy N, Fosberg BR, Artaxo P. 2016. Interactions between biosphere, atmosphere and human land use in the amazon basin. Berlin: Springer.
- Norby RJ, Kimball BA, Kobayashi K. 2001. Rising CO2 –future ecosystems. New Phytol. 150:215–221.
- Ochoa-Hueso R, Hughes J, Delgado-Baquerizo M, Drake JE, Tjoelker MG, Piñeiro J, Power SA. 2017. Rhizosphere-driven increase in nitrogen and phosphorus availability under elevated atmospheric CO2 in a mature Eucalyptus woodland. Plant Soil. 416:283–295.
- Odum EP. 1953. Fundamentals of Ecology. Philadelphia: Saunders.
- Osmond B, Ananyev G, Berry J, Berry, J, Langdon, C, Kolber, Z, Lin, G, Monson, R, Nichol, C, Rascher, U, Schurr, U and Smith, S 2004. Changing the way we think about global change research: scaling up in experimental ecosystem science. Glob Chang Biol. 10:393–407.
- Pegoraro E, Potosnak MJ, Monson RK, Rey A, Barron-Gafford G, Osmond CB. 2007. The effect of elevated CO2, soil and atmospheric water deficit and seasonal phenology on leaf and ecosystem isoprene emission. Funct Plant Biol. 34:774–784.
- Peters RH. 1991. A Critique for Ecology. Cambridge (UK): Cambridge University Press.
- Pilkey OH, Pilkey-Jarvis L. 2007. Useless arithmetic: why environmental scientists can’t predict the future. New York: Columbia University Press.
- Platt JR. 1964. Strong inference. Science. 146:347–353.
- Raunkiaer C. 1934. The life forms of plants and statistical plant geography. Oxford (UK): Oxford University Press.
- Reich PB, Hobbie SE, Lee TD, Pastere MA. 2018. Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science. 360:317–320.
- Ricklefs RE. 1973. Ecology. New York: Chiron Press.
- Schulze E-D, Heinze C, Gash J, Volbers, A, Freibauer, A and Kentarchos, A. 2009. Integrated assessment of the European and North Atlantic Carbon balance. Luxembourg: Office for Official Publications of the European Communities.
- Sellers PJ, Mintz Y, Sud YC, Dalcher A. 1986. A simple biosphere model (SiB) for use within general circulation models. J Atmos Sci. 42:505–531.
- Seppelt R, Beckmann M, Vaclavik T, Volk M. 2018. The art of scientific performance. Trends Ecol Evol. 33:805–809.
- Shen T, Corlett RT, Song L. 2018. Vertical gradient in bryophyte diversity and species composition in tropical and subtropical forests in Yunnan, SW China. J Veget Sci. 29:1075–1087.
- Silvertown J, Poulton P, Johnston E, EDWARDS G, HEARD M, BISS PM. 2006. The park grass experiment 1856-2006: its contribution to ecology. J Ecol. 94:801–814.
- Sutton MA, Howard CM, Erisman JW, Billen, G, Bleeker, A, Grennfelt, P, Van Grinsven, H and Grizzetti, B. 2011. The European nitrogen assessment. Cambridge (UK): Cambridge University Press.
- Tamm CO, Aronsson A, Popovic B, Flower-Ellis JKG. 1999. Optimum nutrition and nitrogen saturation in Scots pine stands. Studia Forestalia Suecia. 266:1–206.
- Tansley AG. 1935. The use and abuse of vegetational concepts and terms. Ecology. 16:284–307.
- Tansley AG. 1939. The British Islands and their vegetation. Cambridge (UK): Cambridge University Press.
- Tilman D. 1982. Resource competition and community structure. Monogr Popul Biol. 17:1–296. Princeton (NJ): Princeton University Press.
- Tilman D. 1985. The resource-ratio hypothesis of plant succession. Am Nat. 125:827–852.
- Tuomivaara T, Hari P, Hannu R, Hakkinen R. 1994. The guide-dog approach: a methodology for ecology. Vol. 11, Helsinki: University of Helsinki, Department of Forest Ecology Publications.
- Turchin P. 2001. Does population ecology have general laws? Oikos. 94:17–26.
- Volterra V. 1926. Variazioni e fluttuazioni del numero d’individui in specie animali conviventi. Mem Acad Lincei Roma. 2:31–113.
- Wardle DA. 2012. On plummeting manuscript acceptance rates by the main ecological journals and the progress of ecology. Ideas Ecol Evol. 5:13–15.
- Warming E, Vahl M. 1909. Oecology of plants – an introduction to the study of plant-communities. Oxford:Clarendon Press.
- Wiens JA. 1989. Spatial scaling in ecology. Funct Ecol. 3:385–397.
- Willis AJ. 1997. The ecosystem: an evolving concept viewed historically Funct. Ecol. 11:268–271.
- Wright IJ. 2004. The world-wide leaf economics spectrum. Nature. 428:821–827.