?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: Exploring prescribing trends and economic burden of chronic low back pain (cLBP) patients prescribed buprenorphine buccal film (Belbuca®) or transdermal patches. Methods: In the MarketScan® commercial insurance claims (employees and their spouses/dependents, 2018–2021), the first film or patch prescription date was an index event. The observation covered 6-month pre-index and 12-month post-index periods. Results: Patients were propensity-score matched (708 per cohort). Buprenorphine initiation had stable cost trends in buccal film and increasing trends in transdermal patch cohort. Between-cohort comparisons of healthcare expenditures, cost trends and resource utilization showed significant differences, mostly in favor of buccal film. Buccal film also had higher daily doses and wider dosing range. Conclusion: Buprenorphine film is more cost-effective cLBP treatment with more flexible dosing.
Plain language summary
What is this article about?
This retrospective study included patients with chronic low back pain (cLBP) and commercial insurance in the USA. Only patients treated with Belbuca®, a buprenorphine buccal film, or a buprenorphine transdermal patch were included. Patients were observed 6 months prior to and 12 months after the first buprenorphine prescription. Healthcare costs, cost trends, resource use and buprenorphine treatment characteristics were explored.
What were the results?
Patients with cLBP on buccal film had lower costs, stable cost trends and less healthcare resources used. Also, they had higher buprenorphine daily doses.
What do the results mean?
The results imply that buccal film is less costly for cLBP patients than patches. The buccal film had more flexible dosing with higher daily doses, which might be associated with better pain control.
This retrospective commercial claims study analyzed US patients diagnosed with chronic low back pain (cLBP) and prescribed buprenorphine buccal film or buprenorphine transdermal patches.
Index date was the first buprenorphine prescription claim with an observation of 6 months pre-index and 12 months post-index periods and a propensity-score matching analysis based on the population characteristics (708 per cohort).
The analysis of healthcare cost trends (6-months pre-index vs. 6-months post-index) showed stable expenditures after buccal film initiation with a $56 saving in cLBP-related ED costs (p = 0.025).
Introduction of buprenorphine patch led to increasing trends in total all-cause and cLBP-related healthcare costs ($3989 [p < 0.001] and $1337 [p = 0.043], respectively), driven by cost increase in the outpatient setting ($1734; p < 0.001).
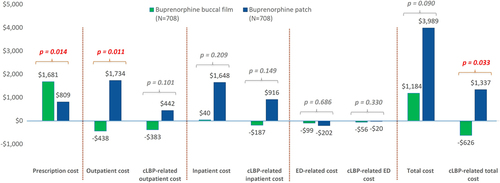
The significant between-group differences were reported in all-cause outpatient cost changes ($438 saving in buccal film vs. $1734 increase in a transdermal patch; p = 0.011) and cLBP-related total cost changes ($626 saving in buccal film vs. $1337 increase in a transdermal patch; p = 0.033).
During the 6-month follow-up, the buccal film was associated with lower cLBP-related ED ($29 vs. $73 [p = 0.038], respectively) and total cLBP-related costs ($2909 vs. $4124 [p = 0.047], respectively).
Buprenorphine film patients had lower cLBP-related healthcare costs and resource utilization measures in the ED setting over the 12-month follow-up.
The difference in pre-index outpatient costs ($7979 buccal film vs. $6332 transdermal patch; p = 0.027) diminished during the follow-up.
Although there were no differences in buprenorphine prescription counts and treatment duration, buccal film patients had a much higher average buprenorphine daily dose than transdermal patch patients (502.0 vs. 236.7 mcg; p < 0.001).
Monthly analysis also showed a wider distribution of buccal film patients across different average buprenorphine daily dose categories (<100 to ≥2000 mcg groups) compared with a narrower dosing range in transdermal patch patients (100–800 mcg).
1. Background
Chronic low back pain (cLBP) is a musculoskeletal condition associated with chronic pain, muscle tension or stiffness that is usually located between the lower ribs and the gluteal fold. Sciatica symptoms may also occur in cLBP patients [Citation1,Citation2]. According to the most recent Global Burden of Disease data, there were 568.4 million cases in 2019. The age-standardized point prevalence estimate on the global level was 6,972.5 per 100,000 population. One of the countries with the highest burden was the USA, alongside Denmark and Switzerland [Citation3].
The cLBP diagnosis has a lifetime prevalence that reaches 40% among US adults [Citation4]. It represents one of the main disability causes with 12.8% of patients receiving disability benefits [Citation5]. Highly affecting patients' quality of life, cLBP patients are usually heavily treated, leading to a high economic burden from the payer's perspective [Citation6]. Data suggests that total cLBP-related healthcare costs reach $1.8 billion within the first year of the diagnosis, mainly driven by outpatient service and medication prescription costs. Non-surgical cLBP cases with physical therapy, imaging procedures and epidural steroidal injections had $5,868 annual per patient cost, while cases without these procedures reported only $359 annual per patient cost [Citation7].
US guidelines for cLBP recommend different treatment options depending on the severity of chronic pain. Non-invasive procedures such as psychotherapies, lifestyle changes, clinician-directed exercise programs and over-the-counter medications (i.e., NSAIDs, topical capsaicin products, etc.) are suggested as a first-line treatment choice for patients with chronic pain of low-to-moderate severity. On the other hand, chronic patients with a severe pain require more optimized treatment, with combination of nonopioid analgesics, schedule II and schedule III opioids, adjuvant analgesics (i.e., antidepressants, antipsychotics, anticonvulsants, etc.), or surgical interventions [Citation8–10]. Opioids (short- and long-acting) are commonly prescribed to cope with severe chronic pain in clinical practice, especially for patients without effective pain management using only nonopioid medications [Citation11]. Although opioids are considered as the effective treatment option, common use is frequently followed by severe and serious adverse events including overdose and opioid misuse [Citation12,Citation13]. Additionally, there seems to be insufficient evidence supporting the clinical benefits of longstanding Schedule II opioid use [Citation13].
Buprenorphine is a Schedule III opioid analgesic due to a low-to-moderate possibility for achieving physical and/or psychological dependence [Citation14]. The analgesic effects of buprenorphine are mainly based on the μ-opioid receptor agonism, providing similar efficacy as Schedule II medications [Citation15]. However, buprenorphine has a potential to minimize opioid-rewarding effects and euphoria, and also to mitigate the risk of life-threatening serious adverse events [Citation16]. Hence, buprenorphine is considered a long-term treatment option for chronic pain management, as its efficacy and safety characteristics have the potential to result in an improved risk-benefit profile compared with other opioids [Citation17].
Although the bioavailability of per-os buprenorphine is very low (10–15%), transdermal and buccal formulations were designed to overcome this issue [Citation16,Citation18]. Despite these buprenorphine formulations demonstrated valuable effects on chronic pain relief and patients' quality of life, there were considerable differences in clinical trial results regarding responder rates in chronic pain patients [Citation15]. To the best of our knowledge, there are no publicly available studies that evaluated and directly compared treatment benefits of these novel formulations, including economic aspects of their use within the real-world setting.
The study objective was to explore healthcare costs, resource use and buprenorphine dosing trends of cLBP patients treated with buprenorphine buccal film (Belbuca®) or transdermal buprenorphine patches using US commercial insurance claims.
2. Methodology
The research was performed and results were reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) cohort studies recommendations [Citation19].
This analysis is a continuation of the previously published study that focused on the concomitant use of oral Schedule II opioid and nonopioid rescue medications in a similar sample of cLBP patients treated with buprenorphine buccal film and transdermal patches [Citation20]. Hence, there are certain similarities between the Methodology sections of the two publications.
2.1. Data source
This retrospective real-world evidence analysis was performed using the Merative MarketScan® database. The database contains insurance claims (medical and prescription data) of over 273 million individuals, encompassing employees, their spouses and their dependents who are covered by employer-sponsored private health insurance in USA [Citation21]. The database complies with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), protecting patient's privacy and ensuring the confidentiality of personal data. In addition, only de-identified data was used in the study, whereby approval of Institutional Review Board for conducting the research was not required.
2.2. Study population
The defined inclusion and exclusion criteria were used to identify the study population and ensure a homogenous cLBP sample.
2.2.1. Inclusion criteria
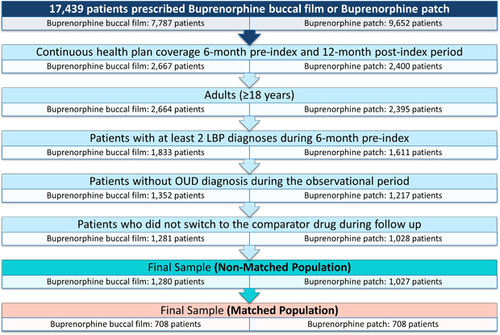
Patients prescribed buprenorphine buccal films or transdermal buprenorphine patches (based on the most relevant National Drug Code [NDC] codes, Supplementary Table S3)
Adult patients (≥18 years of age)
At least two low back pain claims based on the International Classification of Diseases – Clinical Modification (ICD-10-CM) codes (Supplementary Table S1) in a 6-month period prior to the first buprenorphine prescription
2.2.2. Exclusion criteria
An insurance gap during the observational period
Opioid use disorder (OUD) diagnosis during the observational period (Supplementary Table S2)
Switch between buprenorphine buccal film and transdermal patch during the observational period
2.3. Study design
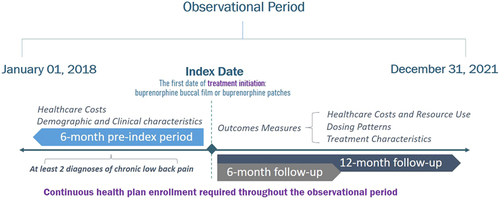
This retrospective study was performed on the Merative MarketScan commercial claims data captured in a period from 1 January 2018 to 31 December 2021. Patients with cLBP and prescribed buprenorphine buccal film or transdermal patch were captured using NDC codes (Supplementary Table S3). The index date was defined as the first buprenorphine prescription date. Based on the buprenorphine administration route, patients were stratified into two cohorts (buccal film vs. transdermal patch). Patients were observed during the 6-month pre-index and 12-month post-index periods. Demographic characteristics were captured on the index date, while clinical characteristics were assessed during the pre-index period. The study design is presented in .
Outcome measures were assessed after buprenorphine initiation. Healthcare cost trends were compared between 6-month pre-index vs. 6-month post-index periods among the study cohorts separately. Additionally, annual healthcare costs and resource utilization were evaluated over prolonged follow-up period (12-month post-index period). Buprenorphine dosing trends and patterns were evaluated monthly during the initial 6 months of follow-up, while average treatment duration, number of buprenorphine prescriptions and daily dose were assessed within the 12 months after index date.
2.4. Outcome measures
2.4.1. Healthcare costs & resource use
Healthcare costs were analyzed over 6-month pre-index and 6-month post-index periods for both cohorts, with the aim to explore the trends (cost increase or saving) before and after the buprenorphine treatment initiation. Changes in expenditures were explored during equal periods of time to ensure a valid within-group comparison. All-cause healthcare costs were estimated as a sum of expenditures for all provided services, while cLBP-related costs were estimated as a sum of expenditures for services claimed with cLBP diagnosis. All-cause and cLBP-related costs were stratified by healthcare settings into categories of prescription costs (all outpatient prescription costs), outpatient-, emergency department (ED)- and inpatient-related costs and compared between the cohorts.
Additionally, the annual healthcare costs (all-cause and cLBP-related) were assessed over 12 months of post-index period and analyzed between the cohorts to fully depict the economic burden of the cLBP patients. Resource utilization (all-cause and cLBP-related services) was also evaluated on an annual basis. The number of outpatient, ED and inpatient visits, as well as the total duration of hospital stays, were the main outcomes that reflected the resource consumption.
2.4.2. Buprenorphine dosing trends
Several prescription characteristics, such as formulation strength, number of units dispensed and corresponding days of medication supply from the database were used to calculate the buprenorphine average daily dose (BADD; mcg/day) per prescription. The following formulae were used to extract the BADD per prescription among buprenorphine buccal film and transdermal patch patients:
The additional formula was used to calculate BADD per patient:
In the formulae, N represents a total number of prescriptions per patient.
The mean and median BADD per patient over the 12-month post-index period are reported and compared between the study cohorts. Additionally, the monthly BADD trends per patient were presented for the first 6 months of the follow-up period. All patients that had at least 1 day of supply with buprenorphine during the observed month of the follow-up were stratified in the corresponding dosing category regarding their BADD. Patient stratification was performed within buprenorphine buccal film and transdermal patch cohorts separately.
2.5. Statistical analysis
Categorical variables were presented as numbers and corresponding proportions, while continuous variables were summarized and presented in a mean and standard deviation format.
Independent t-test was performed to explore the significance between the comparable cohorts for continuous outcome measures, while chi-square test of independence was employed to test statistical significance for categorical outcomes. Furthermore, a paired sample T-test was used to assess the healthcare cost trends (differences between the 6-month pre-index and 6-month post-index periods) within the cohorts. Two-sided p-values lower than 0.05 were considered statistically significant.
Propensity-score matching (PSM) with the nearest-neighbor matching algorithm was performed to minimize the selection bias. Propensity scores were retrieved from the multivariable logistic regression, with all relevant confounders included. The study sample characteristics were used as a basis for the matching process.
All analyses in this research were performed using the IBM SPSS Statistics for Windows, Version 23.0. (NY, USA).
3. Results
There have been 2307 patients identified in the final non-matched sample after applying inclusion and exclusion criteria (1280 buccal film and 1027 transdermal patch patients). PSM analysis yielded the final matched sample of 1416 patients (708 patients in both cohorts). The flow diagram of patient selection process is presented in .
3.1. Non-matched population
The mean patient's age was 50 years in both cohorts, with 36.6% of males in buprenorphine buccal film and 32.8% in the transdermal patch groups. The higher number of patients insured by consumer-driven health plan was noted in buccal film (14.1 vs. 10.2%; p = 0.005), while substantially more patients insured by comprehensive health plan was observed in the transdermal patch cohort (2.5 vs. 5.6%; p < 0.001). It was denoted that a significantly higher number of patients treated with buprenorphine patch resided in north central and west regions (13.8 vs. 21.3%; p < 0.001 and 7.9 vs. 16.3%; p < 0.001, respectively), while buccal film patients were more dominant in the south region (72.3 vs. 55.9%; p < 0.001). Demographic characteristics of a non-matched study sample are shown in Supplementary Table S4.
There were no significant differences observed in the mean Charlson Comorbidity Index (CCI) scores between the cohorts. Among CCI items, the difference was noted only in patients with peptic ulcer disease with a higher proportion in the buprenorphine patch group (0.8 vs. 2.4%; p = 0.001). Also, there were significantly higher proportions of patients in the buprenorphine patch cohort diagnosed with other comorbidities such as depression (24.4 vs. 29.3%; p = 0.008), diabetic neuropathy (7.9 vs. 11.4%; p = 0.004) and fibromyalgia (14.1 vs. 17.2%; p = 0.041). Clinical characteristics of a non-matched study sample are shown in Supplementary Table S5.
3.2. Matched population
PSM analysis was employed with the aim of balancing differences in study population characteristics between the cohorts and diminishing their influence on the main study outcomes. The analysis used the nearest neighbor matching algorithm to match the study cohorts in a 1:1 ratio using a pre-defined caliper of 0.001. The matching process was performed based on the following pre-index characteristics: gender, region of residence, type of health plan, CCI, malignancy, peptic ulcer disease, depression, diabetic neuropathy and congestive heart failure.
There were no differences observed among the matched population in terms of any demographic and clinical characteristics (Supplementary Table S6 & S7). The mean patient's age in the matched population was approximately 49 years, with 31% of males. Most patients resided in the South region (∼68%) and had preferred provider organization type of health plan (∼60%).
A significant increase in healthcare costs 6 months after the buprenorphine buccal film initiation was reported in prescription costs compared with the pre-index period ($4091 vs. $5772; p < 0.001). Significant cost-savings were noted in the cLBP-related ED cost category ($86 vs. $29; p = 0.025). There is a notable decrease in total cLBP-related healthcare costs after buprenorphine buccal film initiation, but without statistical significance ($3535 vs. $2909; p = 0.331). Except for all-cause inpatient costs, the cost-savings trend was observed in all other cost categories (outpatient, ED and inpatient), with numerically lower healthcare costs in a 6-month period after buccal film initiation, but without statistical significance. All-cause total healthcare costs remained stable during the 6-month post-index period ().
Table 1. Healthcare costs trends among buprenorphine buccal film and transdermal patch patients (6-month pre- vs. 6-month post-index).
Among buprenorphine patch-treated patients, a substantial increase in healthcare costs 6 months after buprenorphine patch initiation vs. pre-index period was shown in total all-cause and cLBP-related healthcare costs ($14,806 vs. 18,796; p < 0.001 and $2,787 vs. $4,124; p = 0.043, respectively). The increase in all-cause total healthcare costs was mainly driven by the significant increase in prescription costs ($4478 vs. $5288; p = 0.002) and all-cause outpatient costs ($6332 vs. $8066; p < 0.001). Except for ED-related costs, the healthcare expenditure increases were denoted in all other cost categories over the 6-month period after the buprenorphine patch initiation, but without statistical significance ().
Buprenorphine buccal film had significantly greater savings in all-cause outpatient and cLBP-related total healthcare costs (-$438 vs. $1734; p = 0.011 and -$626 vs. $1,337; p = 0.033; respectively). However, there was also a significantly higher increase in outpatient prescription costs ($1681 vs. $809; p = 0.014) compared with the buprenorphine patch cohort (). Increase in all-cause total healthcare costs was numerically lower in buprenorphine buccal film than in buprenorphine patch cohort ($1184 vs. $3989; p = 0.090), but without statistical significance observed.
Figure 3. Healthcare cost changes from 6-month pre- to 6-month post-index period compared between study cohorts.
Notes: Positive values represent an increase in healthcare costs, while negative are related to cost-savings. Independent t-test was performed to test differences between the cohorts (mixed model ANOVA was employed to validate those differences).
cLBP: Chronic low-back pain; ED: Emergency department.
There were no observed differences in pre-index healthcare costs among buprenorphine buccal film vs. transdermal patch patients (), except in all-cause outpatient healthcare costs, which were significantly higher in buccal film cohort ($7979 vs. $6332; p = 0.027). However, all-cause and cLBP-related total healthcare costs were numerically higher among buprenorphine buccal film patients, but statistically similar to buprenorphine transdermal patch cohort. On the other hand, over the 6-month follow-up period difference in all-cause outpatient costs diminished and significantly lower expenditures in buccal film vs. transdermal patch cohorts were reported in cLBP-related ED and total cLBP-related costs ($29 vs. $73; p = 0.038 and $2909 vs. $4124; p = 0.047). The healthcare costs in all other cost categories were equally distributed between the cohorts during the 6-month period after treatment initiation ().
Table 2. Side-by-side comparison during the 6-month pre- and post-index periods.
To fully depict healthcare expenditures, all-cause and cLBP-related total healthcare costs were evaluated during the 12-month post-index period (Supplementary Table S8). The results were numerically lower among buprenorphine buccal film-treated patients but without statistical significance observed. The main difference was demonstrated in the cLBP-related ED cost category, with significantly lower healthcare costs in the buccal film cohort ($49 vs. $122; p = 0.017).
A substantially lower number of all-cause ED visits was shown in buprenorphine buccal film vs. transdermal patch cohorts (0.8 vs. 1.1; p = 0.011), with a lower proportion of patients who experienced at least one ED visit during the 12-month post-index period (36.3 vs. 42.5%; p = 0.017). The same trend was denoted in the cLBP-related ED visits, with a significantly lower number of visits among patients treated with buccal films (0.06 vs. 0.09; p = 0.042) as well as a significantly lower proportion of patients with one or more cLBP-related ED visit (4.7 vs. 7.3%; p = 0.034). There were no observed differences between the study cohorts in terms of all-cause and cLBP-related hospitalizations and outpatient visits during the 12-month period after the buprenorphine treatment initiation ().
Table 3. Resource use during the 12-month follow-up period.
An equal average number of buprenorphine prescriptions (∼5 in both cohorts) was observed among buccal films and the transdermal patches. A significantly higher mean buprenorphine dose was reported in the buccal film cohort (502.0 mcg vs. 236.7 mcg; p < 0.001). The same trend was demonstrated in a sub-sample of patients with three or more buprenorphine prescriptions. Buprenorphine dosing trends over 12-month follow-up are ().
Table 4. Buprenorphine dosing trends during the 12-month follow-up period.
An increasing trend in average buprenorphine daily dose over the 6-month follow-up was noted among buprenorphine buccal films (Supplementary Table S9). The lowest buccal film dose captured in the first month was 392.7 mcg, and the highest dose in the last month of the follow-up was 825.5 mcg. Within the buprenorphine patch cohort, it was observed that the doses rose in a much narrower range, from 214.4 mcg (month 1) to 292.4 mcg (month 6). Buprenorphine buccal film patients were on significantly higher mean buprenorphine daily doses than transdermal patch patients at each month of the follow-up (all p < 0.001). A significant difference between the proportions of patients treated with buccal film or transdermal patch on a monthly level was observed only at Month 2 (72.9 and 60.3%, respectively; p < 0.001).
During the first month of buprenorphine initiation, the highest proportion of buprenorphine buccal film patients were reported in the 300–399 mcg dosing category (36.7%), while transdermal patch patients were in the 100–199 mcg dosing category (46.2%). In the final month of follow-up, the highest proportion of buccal film patients was observed in the 800–1400 mcg dosing category (33.8%) and in the 200–299 mcg dosing category for the buprenorphine patch cohort (34.7%) ().
Table 5. Patient stratification by average buprenorphine daily dose for each month during the 6-month follow-up period.
4. Discussion
To our knowledge, this is the first retrospective, commercial claims analysis of the US population that fully depicts and compares the healthcare expenditures of buprenorphine buccal films and transdermal patches in cLBP patients. The study denoted several advantages of buccal films over transdermal systems between well-balanced cohorts. Buprenorphine buccal film was associated with a more favorable 6-month healthcare cost trend after treatment initiation than the transdermal patches. Buccal film introduction led to a stable expenditure trend, insignificantly decreasing inpatient and outpatient healthcare costs, while a statistical difference was reported in the ED setting. On the other hand, buprenorphine patch initiation significantly increased outpatient and total healthcare costs. The cost increase was also observed in the inpatient setting with marginal insignificance (p = 0.057). Additionally, the observed average duration of buprenorphine treatment during the 12-month post-index period was approximately 150–160 days, and patients had ∼5 buprenorphine prescriptions on average. Hence, it could be considered that established cost savings and increases over the 6-month follow-up period were highly impacted by the type of buprenorphine treatment. Most of those differences in healthcare costs between the cohorts diminished during the long-term follow-up (12 months), probably due to the small proportion of patients who had continued buprenorphine treatment after a 6-month post-index period (between month 7 and month 12). However, stratification of estimated expenditures and resource utilization by type of healthcare setting showed significantly better long-term economic outcomes in ED among buccal films.
Previously conducted studies mainly focused on the economic burden of cLBP or healthcare costs of opioid-treated patients in general. Retrospective analysis by Gore et al. [Citation22] included a large sample of 101,294 patients with a cLBP diagnosis and matched controls in the US. The study reported a significantly greater economic burden of cLBP compared with non-cLBP controls. Opioids were the most prescribed pain medications (36.5% received short-acting opioids and only 3.8% long-acting opioids). Outpatient services were mostly utilized in the cLBP cohort, with all patients having at least one service, a median number of 14 visits per patient, and a mean total outpatient cost of $4922 [Citation22]. Another large sample claims analysis by Spears et al. [Citation23] included more than 50,000 cLBP patients and also demonstrated the highest utilization of outpatient services. The study reports that 50% of patients have had >4 cLBP-related outpatient visits during the first year of follow-up, and 68.5% of patients over the second year. Average annual healthcare costs of cLBP patients were similar in the first and second follow-up years ($10,796 and $10,069). The costs were mainly driven by all-cause outpatient costs ($7214 in the first year and $6361 in the second year), while cLBP-related outpatient costs were much lower ($2586 in the first year and $1638 in the second year). Pain medication costs increased after the index event from $431 at baseline to $533 in the first year and $595 in the second year. Most of these costs were related to opioid use, which showed a similar trend ($352 at baseline, $427 in the first year, and $491 in the second year) [Citation23].
Neither of these real-world analyses reported a healthcare cost specifically related to buprenorphine treatment and stratified it by product type. The only captured study with this data was a review by Hale et al. which reported the average monthly pharmaceutical costs of buprenorphine buccal films and transdermal patches in the US ($352–857 and $313–817, respectively) [Citation24]. However, the pharmaceutical economic burden greatly depends on several crucial factors in a real-world setting, such as effectiveness and safety, dosing regimen, insurance coverage, product availability, discounts, etc.
The dose-dependent treatment effect is a well-known characteristic of all opioid medications. A meta-regression analysis of randomized controlled trials (RCTs) including buprenorphine and Schedule II opioids reported a significant effect of opioid daily dose on pain control [Citation25]. Buprenorphine buccal films are currently available in a much wider dosing range (75–900 mcg) than buprenorphine patches (5–20 mcg/h). Buccal film formulation is also characterized by greater bioavailability of 46–65% compared with transdermal systems (∼15%) [Citation26]. Our real-world evidence study reported that the BADD was almost twice higher in buccal films than in transdermal patches with similar buprenorphine treatment characteristics (treatment duration and the number of prescriptions). The difference in BADD between study cohorts was even greater within a subsample of patients with at least three buprenorphine prescriptions. After the initial titration phase (2–3 months), most buccal film-treated patients had 800–1,400 mcg average dose, with some having more than 2000 mcg. On the other hand, patients on buprenorphine patches mostly had around 200–300 mcg average dose and neither of them exceeded 800 mcg.
These differences between formulation characteristics and maximal daily doses allow for an individualized pain management approach by providing more flexible dose titration and reaching higher buprenorphine doses for optimal pain control. Therefore, buprenorphine transdermal patches may have limited efficacy in patients with severe pain who require higher daily doses. The higher daily dose could be related to a more beneficial economic profile of patients treated with buprenorphine buccal films vs. transdermal patches, in terms of substantially lower ED and total cLBP-related healthcare costs as shown over the 6-month post-index period. Those differences in buprenorphine daily doses were observed in the indirect comparison of similar RCTs as a greater proportion of opioid-experienced chronic pain patients achieved ≥30% and ≥50% reduction in pain intensity and had lower dropout rates due to a lack of efficacy when treated with buccal film than a buprenorphine patch [Citation15,Citation26]. However, the evidence of buccal film superiority to transdermal systems regarding pain control is inconsistent as a more recently published meta-analysis showed insignificantly different efficacy between the formulations in chronic pain management [Citation27]. Hence, head-to-head comparisons are required to substantiate any differences in efficacy between buprenorphine formulations.
Several study limitations related to the inherent characteristics of real-world data were coped by several investigational techniques, such as data cleaning, precise patient selection process and PSM analysis. The main study limitation originates from the nature of the insurance claims database and coding systems obstacles. The primary purpose of collecting retrospective insurance claims is billing, therefore, errors in data entry and miscoding, duplicate, or negative-cost claims may have appeared. Primarily, the study outcomes were assessed during 6-month pre-index vs. 6-month post-index periods with the aim to reflect healthcare cost trends and changes in the timeframes of the same duration (prior vs. after buprenorphine initiation). Additionally, the economic burden 12 months after the treatment was evaluated to depict annual trends. Hence, the study population was required to have continuous healthcare and pharmaceutical coverage during the observation (6-month pre-index and 12-month post-index periods). Another limitation is a lack of data that specifies the severity of chronic low back pain. There is a potential risk that pain intensity differed between the buprenorphine cohorts as it could not be assessed within the database. Also, it was challenging to precisely define the cLBP, as there is a lack of specific coding that would describe low back pain chronicity. Thus, the inclusion criteria of having at least two low back pain diagnoses within a 6-month period prior to the buprenorphine treatment initiation was used to ensure homogeneity among patients regarding this issue. Finally, the findings have limited generalizability as this analysis was performed in a sample of US patients with commercial insurance.
5. Conclusion
Buprenorphine buccal film showed the potential for overall healthcare cost reduction as compared with transdermal patches and was associated with significantly lower cLBP-related total healthcare costs after buprenorphine initiation. The long-term benefits of buccal film treatment during the 12-month follow-up were mostly in the ED category (service utilization measures and associated healthcare costs).
These study findings indicate that buprenorphine buccal film use in cLBP patients may lead to more cost-efficient disease management than transdermal patch treatment, with buccal film patients experiencing more flexible dosing while consuming less healthcare resources. Future studies may extend the evidence by comparing the economic burden of buprenorphine treatment to other opioids in different chronic pain conditions.
Author contributions
V Zah, F Stanicic, D Vukicevic and D Grbic equally contributed to this research. All authors were responsible for the conception and design of the work, the acquisition, analysis and interpretation of data for the work, drafting the work and revising it critically for important intellectual content, providing the final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial disclosure
This study was funded by Collegium Pharmaceutical, Inc. ZRx Outcomes Research Inc. received financial support for conducting the research. V Zah, F Stanicic, D Vukicevic and D Grbic are employees of ZRx Outcomes Research Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was funded for manuscript preparation.
Supplementary Tables S1-S9
Download MS Word (46.7 KB)Acknowledgments
All steps in conducting this research were supervised and critically reviewed by experts from Collegium Pharmaceutical, Inc.
Supplemental materials
Supplemental data for this article can be accessed at https://doi.org/10.1080/17581869.2024.2348989
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers. 2018;4(1):52. doi:10.1038/s41572-018-0052-1
- Urits I, Burshtein A, Sharma M, et al. Low back pain, a comprehensive review: pathophysiology, diagnosis, and treatment. Curr Pain Headache Rep. 2019;23(3):23. doi:10.1007/s11916-019-0757-1
- Chen S, Chen M, Wu X, et al. Global, regional and national burden of low back pain 1990–2019: a systematic analysis of the Global Burden of Disease study 2019. J Orthop. 2022;32:49–58. doi:10.1016/j.jot.2021.07.005
- Manchikanti L, Singh V, Falco FJE, et al. Epidemiology of low back pain in adults. Neuromodulation. 2014;17:3–10. doi:10.1111/ner.12018
- Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res. 2016;68(11):1688–1694. doi:10.1002/acr.22890
- Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4(2):e2037371. doi:10.1001/jamanetworkopen.2020.37371
- Kim LH, Vail D, Azad TD, et al. Expenditures and health care utilization among adults with newly diagnosed low back and lower extremity pain. JAMA Netw Open. 2019;2(5):e193676. doi:10.1001/jamanetworkopen.2019.3676
- Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi:10.7326/M16-2367
- North American Spine Society (NASS). Evidence-based clinical guidelines for multidisciplinary spine care: diagnosis and treatment of low back pain. 2020 [ updated 2020 Aug 10; cited 2024 Mar 15]. Available from: http://www.spine.org/Portals/0/assets/downloads/ResearchClinicalCare/Guidelines/LowBackPain.pdf
- Department of Veteran Affairs (VA) and Department of Defense (DoD). VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain v3.0. 2022 [ updated 2023 May 11; cited 2024 Mar 15]. Available from: http://www.healthquality.va.gov/guidelines/pain/lbp/
- Fine PG, Mahajan G, McPherson ML. Long-acting opioids and short-acting opioids: appropriate use in chronic pain management. Pain Med. 2009;10(Suppl. 2):S79–S88. doi:10.1111/j.1526-4637.2009.00666.x
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review. Ann Intern Med. 2015;162(4):276–286. doi:10.7326/M14-2559
- Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain: evidence report/technology assessment no. 218. AHRQ. 2014; 14-E005-EF. doi:10.23970/AHRQEPCERTA218
- Drug Enforcement Administration (DEA). Schedules of controlled substances: rescheduling of buprenorphine from schedule V to schedule III. Final rule. Fed Regist. 2002;67(194):62354–62370.
- Pergolizzi JV Jr, Raffa RB. Safety and efficacy of the unique opioid buprenorphine for the treatment of chronic pain. J Pain Res. 2019;12:3299–3317. doi:10.2147/JPR.S231948
- Gudin J, Fudin J. A narrative pharmacological review of buprenorphine: a unique opioid for the treatment of chronic pain. Pain Ther. 2020;9(1):41–54. doi:10.1007/s40122-019-00143-6
- Webster L, Gudin J, Raffa RB, et al. Understanding buprenorphine for use in chronic pain: expert opinion. Pain Med. 2020;21(4):714–723. doi:10.1093/pm/pnz356
- Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44(7):661–680. doi:10.2165/00003088-200544070-00001
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl. 1):S31–S34. doi:10.4103/sja.SJA_543_18
- Stanicic F, Grbic D, Vukicevic D, Zah V. Treatment characteristics of chronic low back pain patients treated with buprenorphine buccal film or transdermal patch. Pain Manag. 2024;14(1):35–48. doi:10.2217/pmt-2023-0124
- Merative. Merative™ MarketScan® Research Databases. 2023 [ cited 2024 April 15]. Available from: https://www.merative.com/real-world-evidence
- Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. 2012;37(11):E668–E677. doi:10.1097/BRS.0b013e318241e5de
- Spears CA, Hodges SE, Kiyani M, et al. Health care resource utilization and management of chronic, refractory low back pain in the United States. Spine. 2020;45(20):E1333–e1341. doi:10.1097/BRS.0000000000003572
- Hale M, Gimbel J, Rauck R. Buprenorphine buccal film for chronic pain management. Pain Manag. 2020;10(4):213–223. doi:10.2217/pmt-2020-0013
- Abdel Shaheed C, Maher CG, Williams KA, et al. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958–968. doi:10.1001/jamainternmed.2016.1251
- Hale M, Garofoli M, Raffa RB. Benefit-risk analysis of buprenorphine for pain management. J Pain Res. 2021;14:1359–1369. doi:10.2147/JPR.S305146
- Wong SSC, Chan TH, Wang F, et al. Analgesic effect of buprenorphine for chronic noncancer pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2023;137(1):59–71. doi:10.1213/ANE.0000000000006467