Abstract
Background: This systematic review and meta-analysis assessed the benefits of an automatic lancing device compared with a manual lancet or a hypodermic needle in neonates. Materials & methods: We followed the Cochrane Handbook methodology, used the RoB-2 tool for risk of bias assessment, RevMan 4.1 for meta-analysis and GRADE framework for certainty assessment. We searched the databases and gray literature on 15 November 2023. Results: Six eligible studies enrolling 539 neonates were included. An automatic lancing device reduced pain scores during and after heel prick, sampling time and the need for repeat puncture. The certainty of evidence was very low to moderate. Conclusion: An automatic lancing device is preferred for heel pricks in neonates, given less pain and higher efficiency.
PROSPERO registration number: CRD42023483189
Plain language summary
What is this article about?
The heel prick is a common painful procedure in neonates. It is performed either with a hypodermic needle or a lancet (manual or automatic lancing device). Few studies have shown that an automatic lancing device, with depth regulation, causes less pain. We reviewed the available literature to assess the benefits and harms of different sampling methods.
What were the results?
We found six studies comparing these interventions for heel prick in neonates. There was a significant reduction in pain score, sampling time and need for repeated pricks when using an automatic lancing device.
What do the results of the study mean?
The automatic lancing device causes less pain (safer) and reduces the time required for sampling and repeated pricks (more effective) when used for heel pricks in neonates.
This meta-analysis included six studies enrolling 539 neonates and compared the benefits of automatic lancing devices with either manual lancet or hypodermic needles.
An automatic lancing device reduced pain during and after the heel prick, the time taken for sample collection and the need for repeat pricks.
The purpose of heel prick varied across studies, from blood glucose estimation (requires a single drop of blood) to dried blood spot for newborn screening and bilirubin or blood gas estimation (requires several drops of blood).
The certainty of evidence varied from very low to moderate, as two studies were at a high risk of bias.
Despite a lower certainty of evidence, an automatic lancing device may be preferred over a hypodermic needle or manual lancet for heel prick.
Future studies should be considered to confirm the benefits of using automatic lancing devices for heel pricks in neonates of different gestational ages (term and preterm) and for varied purposes.
1. Background
With a reduction in neonatal mortality and improving survival [Citation1], the focus of neonatal care has been shifting to improve the quality of care and long-term outcomes [Citation2]. Recent evidence suggests that developmental supportive care (DSC) can have several long-term neurodevelopmental and behavioural benefits [Citation3]. The various components of DSC include providing a healing environment, partnering with families, proper positioning and handling, safeguarding sleep, minimizing stress and pain, protecting skin and optimizing nutrition [Citation3]. Despite several advances in various components of DSC, pain in neonates remains under-recognized and inadequately managed [Citation4]. Neonates admitted to the hospital undergo several painful procedures, which can affect the maturation of neuronal pathways and predispose them to long-term adverse neurodevelopmental outcomes [Citation5].
Among the various procedures in neonatal care, heel lance or prick accounts for about 20% of the painful procedures [Citation6]. The use of pharmacological and non-pharmacological analgesic measures to decrease pain during heel prick has been extensively studied [Citation7,Citation8]. The procedure of heel prick includes several components: the site, the method, the device used, prewarming of the heel, squeezing and other aspects that can potentially affect the severity of pain experienced by the neonate. However, the various aspects affecting heel prick are sparsely studied. Few studies have shown that using automatic lancing devices can potentially reduce pain due to heel prick, as the depth is regulated [Citation9,Citation10]. On the other hand, using the automatic lancing device may require more squeezing and pricks, thereby offsetting this benefit.
With this background, we performed a systematic review and meta-analysis to assess the benefits and harms of using an automatic lancing device instead of a manual lancet or a hypodermic needle for heel pricks in neonates.
2. Materials & methods
2.1. Inclusion criteria
We included all randomized and quasi-randomized trials in which the automatic lancing device was compared with a manual lancet or a hypodermic needle during heel prick in neonates and had an assessment of pain scores as one of the outcomes.
2.2. Literature search
The review protocol was prospectively registered in the PROSPERO database with the registration number CRD42023483189 and can be accessed at www.crd.york.ac.uk/PROSPERO/. We searched the databases of PubMed (inception to 15 November 2023), Embase (inception to 15 November 2023), Cochrane Library (inception to 15 November 2023), Web of Science and Scopus (see Supplementary material for the search strategy). The reference lists of the included studies and published reviews were searched to identify relevant studies. We consulted experts working in this field. To identify ongoing trials, we searched ClinicalTrials.gov and the ISRCTN registry. We did not use language or date restrictions.
2.3. Selection of articles & data extraction
Two reviewers (RPA and EAR) independently performed the title and abstract screening and full-text screening. The data extraction from the included studies was performed by two reviewers (RPA and EAR) in a blinded manner. Any disagreements were resolved by mutual discussion. We extracted the following data: gestational age, birth weight, exclusion criteria, details of heel prick procedure, details of devices used for lancing (automatic lancing device, manual lancet and hypodermic needles), pain scores used and outcome measures of pain scores, sample collection time, number of squeezes and pricks needed and adverse effects like redness and bruising.
2.4. Risk of bias assessment
We assessed the risk of bias in each study using Cochrane's Risk of Bias tool, version 2 (RoB2) [Citation11]. We assessed the studies in the domains of random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the outcome assessment, selection of the reported result and other possible sources of bias.
2.5. Statistical analysis
We decided to conduct a meta-analysis if there were at least two studies comparing similar interventions and comparators and measuring the primary outcome (the pain score). The difference in pain scores was presented as a standardized mean difference as the pain scales used varied across the studies. The other continuous outcomes were expressed as mean differences, with 95% confidence intervals. To convert the median and interquartile range to mean and standard deviation, we used the conversion formulae provided by Wan et al. [Citation12]. The categorical outcomes were expressed as odds ratios, with 95% confidence intervals.
Heterogeneity was explored by considering the study populations (e.g. differences in gestational age), comparators (e.g. manual lancet or hypodermic needle), outcome definitions and, in statistical terms, the I2 statistic. The I2 statistic, with a level of >50%, was considered to indicate moderate levels of heterogeneity and I2 >80% was considered a significant heterogeneity [Citation13]. We used a fixed-effects model when heterogeneity was minimal or a random-effects model when heterogeneity was substantial to combine the results [Citation14].
Studies at a low risk of bias were pooled separately. We explored the causes for heterogeneity when the I2 statistic was >50%. Subgroup analyses for different gestational age groups (i.e., preterm/≤36 weeks and 6 days gestational age versus term/≥37 weeks gestational age) and different comparators (i.e., manual lancet versus hypodermic needle) were planned a priori.
We assessed the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [Citation15]. The certainty of evidence was downgraded when the heterogeneity was unexplained. We assessed the publication bias by using the funnel plots.
3. Results
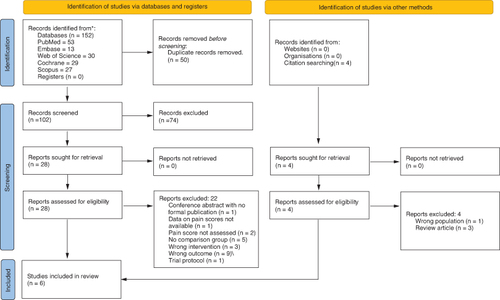
In total, 156 citations were identified through the electronic search (n = 152) and other sources (n = 4). After removing 50 duplicate citations, we evaluated 106 studies by reading titles and abstracts. In all, 78 studies were excluded as they did not meet the inclusion criteria. Full-text screening was performed for 32 articles. A total of 22 studies were excluded (see Supplementary Table S1 for the list of excluded studies). Six studies were included in the meta-analysis [Citation10,Citation16–20]. The PRISMA flow diagram is shown in .
The characteristics of the included studies are shown in Supplementary Table S2. The characteristics of the participants included in these studies are shown in . Of the six included studies, five were randomized clinical trials (RCT), while one trial was a quasi-RCT [Citation20], where the group allocation was based on the enrollment date (odd or even numbers). Two studies included only preterm neonates (<37 weeks gestational age) [Citation18,Citation19], two studies included only full-term neonates (≥37 weeks gestational age) [Citation17,Citation20] and the other two studies included both gestational age groups [Citation10,Citation16]. Four studies used manual lancets in the control group [Citation16–18,Citation20] and three used hypodermic needles for heel prick [Citation10,Citation16,Citation19].
Table 1. Participant characteristics.
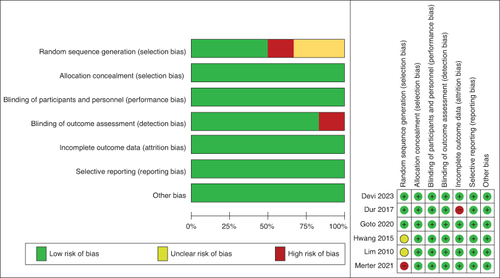
The risk of bias assessment is shown in . Two studies were at a high risk of bias, one due to not blinding the outcome assessors [Citation17] and another because of inappropriate randomization methods [Citation20].
3.1. Primary outcome
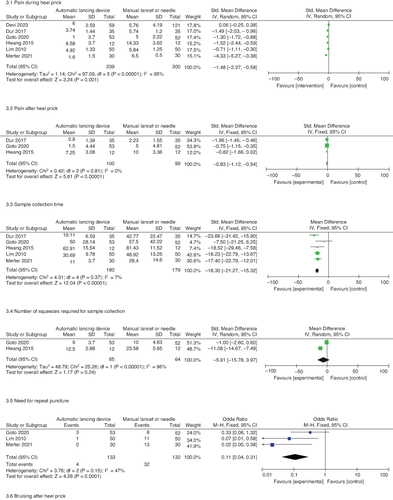
On meta-analysis, using an automatic lancing device, compared with a hypodermic needle or a manual lancet, significantly reduced the pain during (six studies, 539 neonates, standardized mean difference [SMD] -1.48, 95% CI: -2.37, -0.58; I2 = 95%) the procedure.
3.2. Secondary outcomes
There was a significant reduction in the pain post heel prick (three studies, 199 neonates, SMD -0.83, 95% CI: -1.12, -0.54; I2 = 0%) on using an automatic lancing device. There was also a reduction in sample collection time (five studies, 359 neonates, mean difference -18.3 s, 95% CI: -21.27, -15.32 s, I2 = 7%), a need for repeat puncture (three studies, 265 neonates, odds ratio [OR] 0.11, 95% CI: 0.04, 0.31, I2 = 47%) and bruising (three studies, 265 neonates, OR 0.15, 95% CI: 0.03, 0.71, I2 = 60%) by using an automatic lancing device. Although three studies reported the number of squeezes required [Citation10,Citation16,Citation18], we could meta-analyze only two studies [Citation10,Citation18]. There was no statistically significant reduction in the number of squeezes required. The results are shown in and the forest plots are shown in .
Table 2. Meta-analysis and GRADE assessment.
3.3. Sensitivity analysis
On the sensitivity analysis after excluding studies at high risk of bias (see Supplementary Figure S1), there was a significant reduction in the pain during heel prick on using an automatic lancing device (four studies, 409 neonates, SMD -0.81, 95% CI: -1.54, -0.08; I2 = 91%).
3.4. Subgroup analysis
The pain during heel prick was significantly lesser with an automatic lancing device in both preterm neonates (three studies, 179 neonates, SMD -0.73, 95% CI: -1.29, -0.17, I2 = 62%) and full-term neonates (two studies, 130 neonates, SMD -2.88, 95% CI: -5.66, -0.10; I2 = 98%), with no significant intergroup difference (test for subgroup differences: Chi2 = 2.22, degree of freedom = 1, P = 0.14, I2 = 55%). The automatic lancing device resulted in less pain than both manual lancets (four studies, 272 neonates, SMD -1.78, 95% CI: -3.48, -0.07; I2 = 97%) and hypodermic needles (three studies, 598 neonates, SMD -1.24, 95% CI: -2.03, -0.45, I2 = 91%), with no significant intergroup difference (test for subgroup differences: Chi2 = 1.38, degree of freedom = 1, P = 0.24, I2 = 27.4%). The results are shown in the Supplementary Figure S2.
3.5. Publication bias
The funnel plot did not show evidence of publication bias for the pain during and after the heel prick (see Supplementary Figure S3).
3.6. Certainty of evidence
The certainty of the evidence was moderate for sample collection time and the need for repeat puncture, low for pain score after heel prick and number of squeezes and very low for pain score during heel prick and bruising ().
3.7. Cost–effectiveness
The significant decrease in the need for repeat puncture indicates that using automatic lancing devices can be cost-effective. When the meta-analysis findings are extrapolated to 100 neonates undergoing heel prick, only three would require a repeat puncture when an automatic lancing device is used (3% chance) and 24 would require one when a manual lancet/hypodermic needle is used (24% chance). Significant benefits were noted when we estimated the cost–effectiveness (see Supplementary material for estimation of cost–effectiveness).
4. Discussion
This systematic review and meta-analysis included six studies enrolling 539 neonates. Five studies were randomized trials and another was a quasi-randomized trial. Two studies were at high risk of bias. The findings of this review indicate that using an automatic lancing device instead of a manual lancet or a hypodermic needle for heel prick reduced pain scores during and after the procedure. There is also a reduction in the sample collection time and the need for repeat puncture and bruising. There was no significant reduction in the number of squeezes required for sampling. No significant harm was noted.
This is the first meta-analysis assessing the type of device for heel prick in neonates. Lancing is widely used in neonatal care to collect capillary blood samples and assess blood sugar, serum bilirubin, newborn screening with dried blood spots and capillary blood gas. A review of various capillary blood collection techniques by Hoffmann et al. highlighted the differences in sample quality, volume and pain when various techniques were used [Citation21]. The various factors shown to influence pain levels include hypodermic needle diameter, penetration depth, speed at penetration and actuation. With automatic lancing devices, the penetration depth is regulated and shallow, while the speed at penetration is higher, thus resulting in lesser pain.
Several automatic lancing devices are available in the market. While this meta-analysis clearly shows that automatic lancing device use is better than manual lancet or hypodermic needle, we did not compare the various available devices head-to-head. Few studies have compared the different automatic lancing devices for heel pricks in neonates [Citation22–26]. The Tenderfoot® device by Accriva Diagnostics was the least painful in two studies [Citation23,Citation26], while two other studies found QuikHeel® Lancet from Becton Dickinson beneficial [Citation22,Citation24].
The findings of this meta-analysis are clinically significant and applicable to clinical practice. As heel prick is among the most common painful interventions in neonates, a change in practice in neonatal units could significantly decrease the overall pain experienced by neonates during the hospital stay. This feasible approach has been used effectively in quality improvement initiatives targeting a reduction in pain in neonates [Citation27]. While bruising and repeat punctures are definite benefits of using the automatic lancing device, additional analgesic measures must be considered for optimal pain reduction. These interventions include, but are not limited to, sweet solutions like sucrose [Citation8], non-nutritive suckling, swaddling, facilitated tucking, touch massage, light reduction and multisensory bundles [Citation7].
The pain scores used in the included studies were variable. While the Neonatal Infant Pain Scale (NIPS) was used in three studies, two used the Premature Infant Pain Profile (PIPP) and one used the Premature Infant Pain Profile-Revised (PIPP-R). Although several pain scales were validated in neonates, NIPS, PIPP and PIPP-R scales were among the most suited in the context of this meta-analysis. These scores were specifically designed to assess procedural pain, were well studied in both term and preterm neonates and are, by far, the most commonly used ones [Citation28,Citation29]. However, it must be noted that the pain scores used in neonatal settings are not completely objective and they indirectly assess pain based on physiological and behavioural responses.
4.1. Strengths & limitations
The strengths of this meta-analysis include a comprehensive search strategy and compliance with Cochrane Handbook recommendations. Sensitivity analysis after excluding studies at a high risk of bias showed that automatic lancing devices reduce pain during heel prick.
The limitation is a limited number of studies with few participants. Two of the included studies were at a high risk of bias [Citation17,Citation20]. However, the analgesic benefit persisted in sensitivity analysis after excluding these two studies. There was significant heterogeneity for the primary outcome (pain during heel prick), which could not be explained in the subgroup analysis. The possible reasons for heterogeneity include varied reasons for sample collection (blood glucose estimation, capillary blood gas, dried blood spot for newborn screening and bilirubin estimation), differences in population characteristics (gestational age and day of life) and differences in the heel prick procedure (expertise, technique and co-interventions used to reduce pain).
Although heterogeneity was statistically significant for some outcomes, the findings of decreased pain, bruising and repeated punctures were noted consistently across the studies on visual inspection of the forest plots. The imprecision in the results is not substantial. These observations indicate that including the results of further well-designed randomized trials in the meta-analysis will likely improve the certainty of evidence.
5. Conclusion
Despite the limited certainty of evidence ranging from very low to moderate, this meta-analysis implies that using an automatic lancing device for heel prick in neonates results in lesser pain (during and after the procedure), shorter sampling time and lesser probability of bruising and re-puncture. Hence, an automatic lancing device can be preferred over a manual lancet or a hypodermic needle for heel pricks in neonates.
6. Future perspective
Further, well-designed trials are required to assess the benefit of the automatic lancing device for different purposes of sample collection, including blood glucose assessment, bilirubin and other biochemical tests, capillary blood gas assessment and the dried blood spot.
Author contributions
RPA and EAR conceptualized the study, did a literature search, title and abstract careening, full-text assessment, data extraction and quality assessment. RPA did the analysis and provided the first draft of the manuscript. Both authors approve the final version of the article.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Supplementary Materials
Download MS Word (2.3 MB)Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/17581869.2024.2368451
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Hug L, Alexander M, You D, et al. National, regional and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Global Health. 2019;7:e710–e720. doi:10.1016/S2214-109X(19)30163-9
- Soll RF, McGuire W. Evidence-based practice: improving the quality of perinatal care. Neonatology. 2019;116:193–198. doi:10.1159/000496214
- Altimier L, Phillips R. The neonatal integrative developmental care model: advanced clinical applications of the seven core measures for neuroprotective family-centered developmental care. Newborn Infant Nurs Rev. 2016;16:230–244. doi:10.1053/j.nainr.2016.09.030
- Allegaert K, van den Anker JN. Neonatal pain management: still in search of the Holy Grail. Int J Clin Pharmacol Ther. 2016;54:514–523. doi:10.5414/CP202561
- Walker SM. Long-term effects of neonatal pain. Semin Fetal Neonatal Med. 2019;24:101005. doi:10.1016/j.siny.2019.04.005
- Carbajal R. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60. doi:10.1001/jama.300.1.60
- Pillai Riddell RR, Bucsea O, Shiff I, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2023;6:CD006275. doi:10.1002/14651858.CD006275.pub4
- Yamada J, Bueno M, Santos L, et al. Sucrose analgesia for heel-lance procedures in neonates. Cochrane Database Syst Rev. 2023;8:CD014806. doi:10.1002/14651858.CD014806
- Shah V, Taddio A, Kulasekaran K, et al. Evaluation of a new lancet device (BD QuikHeel) on pain response and success of procedure in term neonates. Arch Pediatr Adolesc Med. 2003;157:1075–1078. doi:10.1001/archpedi.157.11.1075
- Goto T, Inoue T, Kamiya C, et al. Neonatal pain response to automatic lancet versus needle heel-prick blood sampling: a prospective randomized controlled clinical trial. Pediatr Int. 2020;62:357–362. doi:10.1111/ped.14142
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi:10.1186/1471-2288-14-135
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi:10.1002/sim.1186
- Dettori JR, Norvell DC, Chapman JR. Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Global Spine J. 2022;12:1624–1626. doi:10.1177/21925682221110527
- Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth. 2019;123:554–559. doi:10.1016/j.bja.2019.08.015
- Devi R, Priyadarshi M, Singh P, et al. Neonatal pain response to various heel prick devices: a randomized controlled trial. Indian Pediatr. 2023;60:893–898. doi:10.1007/s13312-023-3032-9
- Dur Ş, Balcı S. Assessing neonatal pain, duration of crying and procedure time following use of automatic or manual heel lances: a randomized controlled study. J Trop Pediatr. 2018;64:488–494. doi:10.1093/tropej/fmx100
- Hwang M-J, Seol GH. Cerebral oxygenation and pain of heel blood sampling using manual and automatic lancets in premature infants. J Perinat Neonatal Nurs. 2015;29:356–362. doi:10.1097/JPN.0000000000000138
- Lim HB, Rhu MJ, Jung JM, et al. A comparative study of two different heel lancet devices for blood collection in preterm infants. J Korean Soc Neonatol. 2010;17:239. doi:10.5385/jksn.2010.17.2.239
- Merter OS, Bolişik ZB. The effects of manual and automatic lancets on neonatal capillary heel blood sampling pain: a prospective randomized controlled trial. J Pediatr Nurs. 2021;58:e8–e12. doi:10.1016/j.pedn.2020.11.015
- Hoffman MSF, McKeage JW, Xu J, et al. Minimally invasive capillary blood sampling methods. Expert Rev Med Devices. 2023;20:5–16. doi:10.1080/17434440.2023.2170783
- Shah V, Taddio A, Kulasekaran K, et al. Evaluation of a new lancet device (BD QuikHeel) on pain response and success of procedure in term neonates. Arch Pediatr Adolesc Med. 2003;157:1075–1078. doi:10.1001/archpedi.157.11.1075
- Sorrentino G, Fumagalli M, Milani S, et al. The impact of automatic devices for capillary blood collection on efficiency and pain response in newborns: a randomized controlled trial. Int J Nurs Stud. 2017;72:24–29. doi:10.1016/j.ijnurstu.2017.04.001
- Hammermeister M, Baskin L, Lemaire C, et al. Comparison of two infant lancet devices on ease of use and post lance bleeding times. J Neonatal Nurs. 2013;19:71–75. doi:10.1016/j.jnn.2012.10.003
- Ballardini G, Spruzzola A, Boneschi L, et al. [To reduce the pain of heel prick in the newborn: comparison of six types of lancets]. Pediatr Med Chir. 2012;34:182–185. doi:10.4081/pmc.2012.71
- Shepherd AJ, Glenesk A, Niven CA, et al. A Scottish study of heel-prick blood sampling in newborn babies. Midwifery. 2006;22:158–168. doi:10.1016/j.midw.2005.07.002
- Anne RP, Deshabhotla S, Ahmed SW, et al. A quality improvement initiative to improve management of procedural pain in preterm neonates. Paediatr Anaesth. 2021;31:221–229. doi:10.1111/pan.14075
- Llerena A, Tran K, Choudhary D, et al. Neonatal pain assessment: do we have the right tools? Front Pediatr. 2023;10:1022751. doi:10.3389/fped.2022.1022751
- Olsson E, Ahl H, Bengtsson K, et al. The use and reporting of neonatal pain scales: a systematic review of randomized trials. Pain. 2021;162:353–360. doi:10.1097/j.pain.0000000000002046