Abstract
Aim: To investigate the association of time interval between neoadjuvant RT and surgery (RS-interval) with pathological complete response (pCR) and overall survival (OS) of rectal cancer patients. Methods: We used the National Cancer Database and reported Odds ratios (OR) and Hazard ratios. Results: Patients with an RS-interval of 5–24 weeks were more likely to achieve pCR compared with ≤4 weeks. RS-interval of 13–24 weeks was associated with a reduced OS compared with ≤4 weeks only in patients who did not achieve pCR. Conclusion: RS-interval of 5–8, 9–12, 13–16, 17–20, or 21–24 weeks was associated with a higher likelihood of achieving pCR compared with ≤4 weeks. In patients without pCR, delaying surgery >12 weeks was associated with reduced OS.
The optimal time between the completion of neoadjuvant radiation therapy (RT) and surgery in patients diagnosed with locally advanced rectal cancer is controversial and there is no study that has investigated this association by the pathological completed response (pCR) status.
Patients who received surgery between 5 and 8 weeks, 9 and 12 weeks, 13 and 16 weeks, 17 and 20 weeks, or 21 and 24 weeks after the completion of RT were more likely to achieve pCR compared with ≤4 weeks.
Patients who achieved pCR had comparable overall survival (OS) regardless of when the surgery took place within 24 weeks after RT completion.
Among patients who did not achieve pCR, OS was only similar if surgery was received between 5 and 8 or 9 and 12 weeks after RT compared with ≤4 weeks.
Among patients who did not achieve pCR, Surgery between 13 and 16, 71 and 20 or 21 and 24 weeks after RT was associated with worse OS compared with ≤4 weeks.
1. Background
In 2020, more than 43,000 patients were diagnosed with rectal cancer in the USA [Citation1]. The multimodal treatment approach, which includes neoadjuvant chemoradiation followed by total mesorectal excision (TME), has become a standard of care treatment for locally advanced rectal cancer [Citation2,Citation3]. Neoadjuvant chemoradiation therapy is associated with improved local recurrence (LR) rate and disease-free survival (DFS) compared with adjuvant chemoradiation therapy [Citation2,Citation4,Citation5].
Two radiation therapy regimens exist for neoadjuvant therapy prior to resection, short-course radiotherapy (SCRT) and long-course chemoradiation therapy (LCCRT) [Citation6]. SCRT encompasses 25 Gray (Gy) delivered over five fractions and is often followed by immediate surgery within 7 days [Citation6,Citation7]. LCCRT involves administering 50 to 50.4 Gy radiation over 25–28 fractions in combination with concurrent intravenous 5-Fluorouracil or oral capecitabine, followed by surgery 6–8 weeks later [Citation2,Citation5–9]. SCRT is common in Europe, while LCCRT is the favored approach in the USA. Studies have indicated that LCCRT with an 8-week delayed surgery is associated with a higher pathological complete response (pCR) rate when compared with SCRT with a 1-week immediate surgery. Pathological complete response is associated with better local control, recurrence rate, improved progression-free survival (PFS), and overall survival (OS) [Citation10–13].
Due to the clinical importance of pCR, there is a great interest in identifying factors associated with achieving pCR. In addition, the time interval between the end of neoadjuvant RT and performing surgery is of research interest when it relates to the tumor response. The Stockholm III trial demonstrated that an interval of 4–8 weeks after SCRT is associated with a higher pCR than an interval of <1 week (11.8 vs. 1.7%) [Citation14]. Some studies reported that an interval >8 vs. ≤8 [Citation15–18] weeks or >7 vs. ≤7 weeks [Citation19,Citation20] or 9–11 vs. <9 weeks after LCCRT were associated with better pCR [Citation21]. Other studies reported an increased pCR for 6–8 vs. 2 weeks, 12 versus 6 weeks and 10 weeks [Citation22–24]. Some studies did not report any association between pCR and longer intervals between RT and surgery [Citation25–27]. The optimal time of surgery after neoadjuvant RT is controversial, and there is no international consensus. Historically the 6–8 weeks interval has been used [Citation23,Citation28]. However, whether a longer interval between RT and surgery is associated with improved pCR and better OS is unclear. It is also unclear how much delay between the completion of RT and receipt of surgery is acceptable.
The primary goal of this study is to explore the optimal time interval between neoadjuvant RT and surgery that is associated with the highest rate of pCR and evaluate its impact on overall survival in patients diagnosed with stage II and III rectal cancer.
2. Methods
2.1. Data source
The data was extracted from the National Cancer Database (NCDB), a nationwide oncology outcomes database for more than 1500 Commission-accredited cancer programs in the USA. It captures >70% of the cancer cases diagnosed annually in the US. The University of Nebraska Medical Center is a member of the Commission on cancer-accredited facilities. The data in the NCDB is collected from more than 1500 such facilities. Access to the NCDB data was provided by the American College of Surgeons after a thorough review of our research proposal about the current hypothesis. The data was de-identified, so this study was exempt from the Institutional Review Board (IRB) review. Informed consent was also not required. During the patient visit to the treatment facility, informed consent is obtained by explaining that the facility is a member of the American College of Surgeons and that de-identified data will be used for research purposes.
2.2. Study population
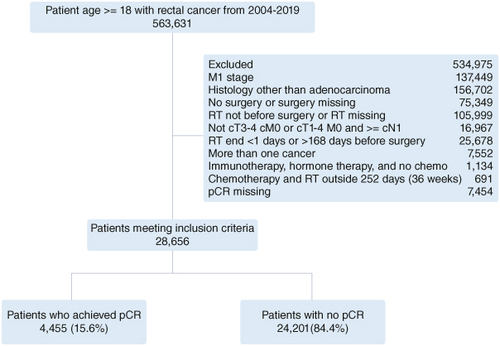
Patients diagnosed with cT3-4 cM0 or cT1-4 M0 and ≥cN1 adenocarcinoma of the anus, rectum, or rectosigmoid between 2004–2019 who were ≥18 years old and had only one cancer at the time of diagnosis were included in the study. Patients who did not receive surgery or received surgery before RT were excluded. Patients who finished RT >24 weeks before surgery, those who finished RT on the day of surgery, and those who received RT and chemotherapy >36 weeks from each other were also excluded ().
2.3. Outcome & covariates
The primary outcome of the current study was to explore the association of time intervals between neoadjuvant RT and surgery with pCR and OS. We also reported the predictors of achieving pCR. The primary variables of interest were pCR and the time interval from the end of RT to surgery.
2.4. Statistical analysis
Descriptive statistics by pCR and no pCR were calculated as mean and standard deviation (SD) for continuous variables and frequency and percent for categorical variables. Multivariable logistic regression analysis was used to determine factors associated with achieving pCR. Odds ratios (OR) were reported to measure the association between the covariates of interest and the outcome of achieving pCR. The Kaplan-Meier method was used to report the median overall survival time, while the log-rank test was used to compare the OS of different groups. Survival time was calculated from the time of diagnosis to the time of death or last contact. Those alive or lost to follow-up were censored. Cox regression analysis was conducted to estimate hazard ratios (HR) and their associated 95% confidence intervals. All statistical analyses were performed with SAS version 9.4 (SAS Institute). Statistical significance was set at p < 0.05, and all tests were 2-tailed.
3. Results
3.1. Patient characteristics
Among the 28,656 patients included in the current study, 25,759 (89.9%) finished RT ≤12 weeks before surgery and 2897 (10.1%) finished RT >12 weeks before surgery. There were 4455 (15.6%) who achieved pCR and 24,201 (84.4%) who did not achieve pCR ().
Table 1. Baseline characteristics and factors associated with the probability of achieving pathological complete response of patients diagnosed with rectal and rectosigmoid cancer between 2004–2019 (n = 28,656) and reported to the National Cancer Database.
3.2. Predictors of pCR
In the multivariable logistic regression analysis, patients who received surgery between 5–8 weeks, 9–12 weeks, 13–16 weeks, 17–20 weeks, or 21–24 weeks after the completion of RT were more likely to achieve pCR compared with ≤4 weeks (OR: 1.63, CI: 1.24–2.14, p < 0.001; OR: 2.16, CI: 1.64–2.84, p < 0.001; OR: 1.82, CI: 1.35–2.46, p < 0.001; OR: 1.73, CI: 1.19–2.52, p < 0.001 and OR: 1.75, CI: 1.08–2.82, p < 0.001) (). In the subgroup analysis of the 9–12 weeks interval, the OR of achieving pCR compared with ≤4 weeks was 1.95 (CI: 1.47–2.59, p < 0.001) for week 9, 2.17 (CI: 1.63–2.88, p < 0.001) for week 10, 2.58 (CI: 1.93–3.45; p < 0.001) for week 11 and 2.28 (CI: 1.68–3.10, p < 0.001) for week 12. When the study participants were divided into two groups ≤12 weeks vs. >12 weeks, ≤11 weeks vs. >11 weeks, ≤10 weeks vs. >10 weeks, or ≤9 weeks vs. >9 weeks, there was no difference in the OR of achieving pCR between ≤12 weeks vs. >12 weeks and ≤11 weeks vs. >11 weeks, while the OR of achieving pCR was smaller than one for ≤10 weeks vs. >10 weeks, or ≤9 weeks vs. >9 weeks. The OR for other factors associated with pCR is reported in .
The proportion of patients who achieved pCR increased from 9% for those who received surgery ≤4 weeks after RT to 14, 18, 16 and 15% for those who received surgery between 5 and 8 weeks, 9 and 12 weeks, 13 and 16 weeks and 17 and 24 weeks after RT, respectively (Supplementary Figure S1).
3.3. Survival outcome
The median follow-up time for the entire cohort was 70 months, with a range of 2.8 and 183 months. Based on the Kaplan-Meier curve, patients who achieved pCR had a higher OS compared with patients who did not achieve pCR (Supplementary Figure S2). Patients who had surgery ≤12 weeks after RT had a higher OS compared with those who had surgery >12 weeks after RT (Supplementary Figure S3).
In the multivariable Cox regression analysis, there was no difference in the OS of patients who received surgery between 5–8 weeks or 9–12 weeks after the completion of RT and patients who received surgery ≤4 weeks after completing RT (HR: 0.90, CI: 0.78–1.04; p = 0.16 and HR: 1.01, CI: 0.87–1.17; p = 0.90). However, patients who received surgery between 13–16 weeks, 17–20 weeks, or 21–24 weeks after the completion of RT had reduced OS compared with patients who received surgery ≤4 weeks after completing RT (HR: 1.27, CI: 1.09–1.50; p = 0.007, HR: 1.63, CI: 1.31–2.02; p < 0.001 and HR: 1.73, CI: 1.31–2.79; p < 0.001) (). Patients with no pCR had reduced OS compared with patients who achieved pCR (HR: 2.09, CI: 1.89–2.31; p < 0.001) ().
Table 2. Factors associated with overall survival of patients diagnosed with rectal and rectosigmoid cancer between 2004 and 2019 and reported to the National Cancer Database.
In the analysis stratified by the time interval between completing RT and receiving surgery, no pCR was associated with reduced OS compared with pCR among patients who received surgery between 5 and 8 weeks, 9 and 12 weeks, 13 and 16 weeks, 17 and 20 weeks and 21 and 24 weeks after finishing RT. There was no difference in the OS of patients who did not achieve pCR and patients who achieved pCR among patients who received surgery within ≤4 weeks after the completion of RT (). Patients who received surgery ≤12 weeks after completing RT had a higher OS compared with patients who received surgery >12 weeks after completing RT (HR: 0.69, CI: 0.64–0.75; p < 0.001).
3.4. Survival outcome by pCR status
In the multivariable Cox regression analysis, patients who achieved pCR had comparable OS regardless of when the surgery took place between ≤4 to 24 weeks after completing RT. The HR was (HR: 0.71, CI: 0.37–1.36; p = 0.30) for those who received surgery within 5–8 weeks after RT, (HR: 0.75, CI: 0.39–1.43; p = 0.39) for 9–12 weeks, (HR: 0.70, CI: 0.34–1.45; p = 0.33) for 13–16 weeks, (HR: 1.05, CI: 0.44–2.56; p = 0.91) for 17–20 weeks and (HR: 1.06; CI: O.33–3.44; p = 0.92) for 21–24 weeks after RT compared with ≤4 weeks after RT. Interestingly, as we moved from 5–8 to 21–24 weeks, the HR became closer to the null ().
Table 3. Overall survival of patients diagnosed with rectal and rectosigmoid cancer stratified by pathological complete response status.
Among patients who did not achieve pCR, OS was only similar if surgery was received between 5 and 8 weeks or 9 and 12 weeks after RT compared with ≤4 weeks after RT (HR: 0.90, CI: 0.78–1.05; p = 0.19 and HR: 1.02, CI: 0.88–1.19; p = 0.78). However, surgery between 13 and 16 weeks after RT, 71 and 20 weeks or 21 and 24 weeks after RT was associated with worse OS compared with ≤4 weeks after RT (HR: 1.30, CI: 1.10–1.57; p = 0.002, HR: 1.67, CI: 1.33–2.08; p < 0.001 and HR: 1.79, CI: 1.34–2.38; p < 0.001). The HR increased when the time interval increased from 5–8 to 21–24 weeks ().
4. Discussion
The current study is the most comprehensive and largest study that has investigated the association of the time interval between the completion of neoadjuvant RT and receipt of surgery with achieving pCR and the OS. It is the first study that has reported the association stratified by pCR status. The time interval between the completion of neoadjuvant RT and receipt of surgery was not associated with OS among patients who achieved pCR, while delaying surgery >12 weeks after the completion of RT was associated with reduced OS among patients who did not achieve pCR (). The association between the interval time and pCR was the highest at 9–12 weeks ().
Receiving surgery at week 11 or 12 after the completion of RT may be the optimal cutoff point. This is because, during weeks 11–12, the OR of achieving a pCR is higher than during weeks 9–10, when compared with intervals of ≤4 weeks. It's important to note that there is no significant difference in survival when the RT-to-surgery interval is within 12 weeks.
These findings are clinically important as surgery can be delayed to a certain extent to get optimal tumor regression in patients who are more likely to achieve pCR without compromising their OS. A prolonged interval between RT and surgery is associated with reduced post-surgery complications and improved treatment response [Citation29,Citation30]. Patients who do not respond well to RT should undergo surgery without delay, as delaying surgery may be associated with poorer survival outcomes in these patients [Citation31]. Only 258 (0.9%) of the study population received surgery more than 20 weeks after the completion of neoadjuvant RT. Although a small proportion but an indication that in addition to that, in a real-world setting, the guidelines may not be followed due to various reasons. These patients may have poor performance status or other reasons for an over 20-week delayed surgery. In addition, radicality between patients who received surgery >20 weeks after RT completion and those who received surgery ≤12 weeks after RT completion is the same as all patients in the current study who received definitive radical cancer surgery of the tumor site. The 30-day postoperative mortality in both groups was comparable. Approximately 5/256 (1.9%) of those who received surgery more than 20 weeks after the completion of neoadjuvant RT died within 30 days of surgery and 134/25,690 (0.5%) died within 30 days in patients who received surgery ≤12 weeks after surgery. We should also note that the number of patients who died within 30 days of surgery in the >20 weeks group is small (n = 5), which cannot be used to make the decision. The worse OS of those who received surgery >20 weeks after RT compared with those who received surgery ≤12 weeks may not be due to postoperative complications and radicality.
No difference in the OS of patients who achieved pCR and those who did not achieve pCR among patients undergoing surgery ≤4 weeks after RT indicates that the benefit of achieving pCR could not be translated to benefit in OS if surgery is performed soon after RT (). The survival advantage of patients who achieved pCR compared with those who did not achieve pCR widened as we moved from the 5–8 weeks interval between RT and surgery to 21–24 weeks (), indicating that patients with no pCR do worse as the time between RT and surgery becomes longer which may be secondary to the higher chance of regrowth of residual disease and metastases, leading to a worse OS.
The benefits of a longer interval between the completion of neoadjuvant RT and receipt of surgery should be weighed against the consequence of delaying surgery, which in some cases, would lead to more complicated surgery [Citation32]. Alternatively, surgery could be delayed to achieve pCR, which could help the watch-and-wait approach or even omit surgical resection if needed [Citation26]. There is debate as to whether a longer interval between RT and surgery is associated with a higher pCR rate and how much delay in surgery is acceptable not to jeopardize the OS. Some benefits and risks are associated with the length of time between RT and surgery [Citation26,Citation33]. The longer interval could increase tumor shrinkage but could also lead to tumor and disease progression and decreased survival [Citation26,Citation33].
To our knowledge, the current study is the first to report results of stratified analyses by pCR and the largest to investigate the association of various time intervals between RT completion and surgery with pCR and its impact on the OS of rectal cancer patients. Although an interval of ≤12 weeks between the completion of RT and undergoing surgery was not associated with achieving a higher pCR, it was associated with improved OS compared with >12 weeks. Our findings about the no difference in achieving pCR between the two groups agree with the findings of the only two previously published studies that compared ≤12 weeks to >12 weeks [Citation26,Citation34]. However, our survival analysis results are different from these two studies, which could be due to the small sample size in these two studies [Citation26,Citation34]. One study included 124 patients, among whom only 16 had pCR, and found no difference in 30-day mortality between the two intervals [Citation34]. The other study was based on a single institution and included 250 patients, among whom only 23 patients had pCR. This study also found no difference in the OS of patients between intervals of ≤12 weeks and >12 weeks [Citation26]. Multivariable survival analyses were not performed in these studies [Citation26,Citation34]. pCR status, T stage, N stage, dose of RT, and comorbidity score are some important confounding factors that are adjusted in our study but were not adjusted for in the two previous studies.
Some other studies have used a different cut-off point for long vs. short intervals between the completion of RT and undergoing surgery [Citation15–27]. A few studies have reported increased OR of achieving pCR for >8 weeks vs. ≤8 weeks intervals after the completion of RT and receiving surgery [Citation19–22]. Some studies have also reported an increased rate of pCR for >7 weeks vs. ≤7 weeks intervals [Citation19,Citation20]. Most of these studies reported no difference in OS or better survival for >8 vs. ≤8 weeks or >7 vs. ≤7 weeks intervals [Citation15–20]. In our study, we also found that receiving surgery >8 vs. ≤8 weeks or >7 vs. ≤7 weeks after the completion of RT was associated with a higher OR of achieving pCR. However, in our study, shorter intervals were associated with improved OS compared with longer intervals. The difference in survival could be that most of these studies did not perform multivariable survival analyses and had a small number of patients. The two meta-analyses that compared OS for an interval time >8 weeks vs. ≤8 weeks had 1,476 (deaths = 327) [Citation17] and 579 patients [Citation15], while our study included 28,619 patients (deaths = 6,518), among whom 4,445 had pCR (deaths = 530). Another reason could be the stratification by pCR status. When we stratified by pCR status, the survival benefit associated with ≤8 vs. >8 weeks and ≤7 vs. >7 weeks was only seen in patients who did not achieve pCR. A subset analysis from a pooled analysis of seven randomized trials, which included 3,085 patients (14% had pCR), showed no difference in OS between an interval time of ≤6 vs. >6 weeks, which is consistent with our findings for comparing the same time interval [Citation24].
The proportion of patients who received surgery ≤12 weeks after RT decreased over time, an indication that surgeons are increasingly delaying surgery beyond 12 weeks (Supplementary Figure S4). Given that our study found such a delay to be associated with reduced OS, this should be of some concern, especially for patients who do not achieve pCR. It is possible that some patients may have received total neoadjuvant therapy (TNT), a novel therapeutic approach used increasingly in recent years. However, our data is before 2020 with only a few patients from 2018 and 2019. Also, only 571 (2%) of the participants in our study started chemotherapy >14 weeks before starting RT, indicating that most patients did not receive TNT with induction chemotherapy. In addition, only 471 (1.6%) patients completed RT >18 weeks before surgery, which indicates that our findings are not due to the use of TNT with consolidation chemotherapy. The overall proportion of patients who achieved pCR in the current study was 16%, similar to those previously reported [Citation19,Citation20,Citation35,Citation36]. In the current study, 2,269/28,656 (7.9%) of the patients had rectosigmoid cancer who received neoadjuvant RT, which is an indication that in USA, in real-world setting, patients with high-rectosigmoid cancer have been receiving neoadjuvant radiation therapy although recent results from PROSPECT trial have shown that neoadjuvant chemotherapy alone was noninferior to chemoradiotherapy for disease-free survival in high-rectosigmoid cancer patients [Citation37].
Despite being the largest study of its nature, it is not without limitations. Lack of information about the exact chemotherapy agents, treatment breaks, surgical complications, and treatment-related toxicity are some of the limitations. Other limitations include the retrospective nature of the study and the lack of information about the cause of death. Finally, given that there is only a start date of the first course of chemotherapy available when a patient started chemotherapy before surgery, we will not be able to tell if this patient also received chemotherapy after the surgery.
5. Conclusion
In this comprehensive analysis, we found that patients who received surgery between 5 and 8 weeks, 9 and 12 weeks, 13 and 16 weeks, 17 and 20 weeks or 21 and 24 weeks after the completion of RT were more likely to achieve pCR compared with ≤4 weeks. Among patients who achieve pCR, the time interval between the completion of RT and receipt of surgery was not associated with OS, while among patients who do not achieve pCR, delaying surgery >12 weeks was associated with reduced OS. An interval of 9–12 weeks was identified as the optimal time interval as it was associated with the highest pCR rate, and a superior OS compared with other intervals.
Author contributions
Amin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: SA Amin, M Patel, C Lin. Acquisition, analysis, or interpretation of data: SA Amin, M Patel, C Lin. Drafting of the manuscript: SA Amin, M Patel, C Lin. Critical revision of the manuscript for important intellectual content: SA Amin, M Patel, C Lin. Statistical analysis: SA Amin. Administrative, technical, or material support: C Lin. Supervision: SA Amin, C Lin.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
University of Nebraska Medical Center is a member of the Commission on cancer-accredited facilities. The data in the NCDB is collected from more than 1500 such facilities. Access to the NCDB data was provided by the American College of Surgeons after a thorough review of our research proposal about the current hypothesis. The data was de-identified, so this study was exempt from the Institutional Review Board (IRB) review. Informed consent was also not required. During the patient visit to the treatment facility, informed consent is obtained by explaining that the facility is a member of the American College of Surgeons and that de-identified data will be used for research purposes.
Supplementary Figures S1-S4
Download Zip (102.6 KB)Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/1758194X.2024.2354650
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi:10.1056/NEJMoa040694
- Fleming FJ, Påhlman L, Monson JR. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum. 2011;54(7):901–912. doi:10.1007/DCR.0b013e31820eeb37
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27(31):5124–5130. doi:10.1200/JCO.2009.22.0467
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized Phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi:10.1200/JCO.2011.40.1836
- Cambray M, Gonzalez-Viguera J, Berenguer MA, et al. Short-course radiotherapy in locally advanced rectal cancer. Clin Transl Gastroenterol. 2020;11(6):e00162. doi:10.14309/ctg.0000000000000162
- Bujko K, Nowacki MP, Kępka L et al. Postoperative complications in patients irradiated pre-operatively for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs chemoradiation. Colorectal Dis. 2005;7(4):410–416. doi:10.1111/j.1463-1318.2005.00796.x
- Allegra CJ, Yothers G, O'Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a Phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11):djv248. doi:10.1093/jnci/djv248
- Hofheinz R-D, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–588. doi:10.1016/S1470-2045(12)70116-X
- Diaz-Gonzalez JA, Calvo FA, Felipe A, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys. 2006;64(4):1122–1128. doi:10.1016/j.ijrobp.2005.09.020
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi:10.1016/S1470-2045(10)70172-8
- Martin S, Heneghan H, Winter D. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99(7):918–928. doi:10.1002/bjs.8702
- Vecchio FM, Valentini V, Minsky BD, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62(3):752–760. doi:10.1016/j.ijrobp.2004.11.017
- Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18(3):336–346. doi:10.1016/S1470-2045(17)30086-4
- Du D, Su Z, Wang D, et al. Optimal interval to surgery after neoadjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2018;17(1):13–24. doi:10.1016/j.clcc.2017.10.012
- Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582–589. doi:10.1097/SLA.0b013e3181b91e63
- Petrelli F, Sgroi G, Sarti E, et al. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer. Ann Surg. 2016;263(3):458–464. doi:10.1097/SLA.0000000000000368
- Probst CP, Becerra AZ, Aquina CT, et al. Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg. 2015;221(2):430–440. doi:10.1016/j.jamcollsurg.2015.04.010
- Tulchinsky H, Shmueli E, Figer A, et al. An interval >7weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661–2667. doi:10.1245/s10434-008-9892-3
- Wolthuis AM, Penninckx F, Haustermans K, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19:2833–2841. doi:10.1245/s10434-012-2327-1
- Kim MJ, Cho JS, Kim EM, et al. Optimal time interval for surgery after neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: analysis of health insurance review and assessment service data. Ann Coloproctol. 2018;34(5):241. doi:10.3393/ac.2018.01.01
- Evans J, Bhoday J, Sizer B, et al. Results of a prospective randomised control 6 vs 12 trial: is greater tumour downstaging observed on post treatment MRI if surgery is delayed to 12-weeks versus 6-weeks after completion of neoadjuvant chemoradiotherapy?. Ann Oncol. 2016;27:vi149. doi:10.1093/annonc/mdw370.01
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396. doi:10.1200/JCO.1999.17.8.2396
- Gambacorta MA, Masciocchi C, Chiloiro G, et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol. 2021;154:154–160. doi:10.1016/j.radonc.2020.09.026
- Dolinsky CM, Mahmoud NN, Mick R, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96(3):207–212. doi:10.1002/jso.20815
- Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome?. Int J Radiat Oncol Biol Phys. 2008;71(4):1181–1188. doi:10.1016/j.ijrobp.2007.11.035
- Tran C-L, Udani S, Holt A, et al. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg. 2006;192(6):873–877. doi:10.1016/j.amjsurg.2006.08.061
- Schmiegel W, Buchberger B, Follmann M, et al. S3-guideline-colorectal cancer. Z Gastroenterol. 2017;55(12):1344–1498.
- Dhadda A, Zaitoun A, Bessell E. Regression of rectal cancer with radiotherapy with or without concurrent capecitabine-optimising the timing of surgical resection. Clin Oncol. 2009;21(1):23–31. doi:10.1016/j.clon.2008.10.011
- Feeney G, Sehgal R, Sheehan M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25(33):4850. doi:10.3748/wjg.v25.i33.4850
- Deidda S, Elmore U, Rosati R, et al. Association of delayed surgery with oncologic long-term outcomes in patients with locally advanced rectal cancer not responding to preoperative chemoradiation. JAMA Surg. 2021;156(12):1141–1149. doi:10.1001/jamasurg.2021.4566
- Qwaider YZ, Sell NM, Stafford CE, et al. The time interval between the end of radiotherapy and surgery does not affect outcomes in rectal cancer. J Am Col Surg. 2021;89(4):00031348211047215.
- Foster JD, Jones EL, Falk S, et al. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56(7):921–930. doi:10.1097/DCR.0b013e31828aedcb
- Figueiredo N, Panteleimonitis S, Popeskou S, et al. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol. 2018;44(4):484–489. doi:10.1016/j.ejso.2018.01.088
- de Campos-Lobato LF, Geisler DP, da Luz Moreira A, et al. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg. 2011;15:444–450. doi:10.1007/s11605-010-1197-8
- Kerr S, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg. 2008;95(12):1534–1540. doi:10.1002/bjs.6377
- Schrag D, Shi Q, Weiser MR, et al. PROSPECT: a randomized Phase III trial of neoadjuvant chemoradiation versus neoadjuvant FOLFOX chemotherapy with selective use of chemoradiation, followed by total mesorectal excision (TME) for treatment of locally advanced rectal cancer (LARC)(Alliance N1048). J Clin Oncol. 2023;41(Suppl. 17):LBA2–LBA2. doi:10.1200/JCO.2023.41.17_suppl.LBA2

![Figure 2. Hazard ratio [no pCR vs. pCR] as a function of the time interval between RT completion and surgery.CI: Confidence interval; pCR: Pathological complete response; RT: Radiation therapy.](/cms/asset/e63c39f2-19fb-432b-85b4-864b820e0562/icrc_a_2354650_f0002_c.jpg)