 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Carbon storage is one of the most important ecosystem services provided by kelp beds. Laminarialean kelps are widely harvested along the Warm Temperate South-eastern Pacific coast, a marine province shared by Chile and Peru. Carbon storage assessments of kelps in Peru are lacking. From a blue economy and sustainable management perspectives, information on the carbon storage of kelps is important. We conduct the first carbon storage assessment of Lessonia trabeculata in Peru and contribute to the development of biomass estimation models in order to monitor kelps using the least destructive methodologies. We chose three commonly harvested sites in San Juan de Marcona to haphazardly extract Lessonia trabeculata sporophytes using transects. Sporophyte height (m), wet biomass (kg), maximum (D) and minimum (d) holdfast disk widths (cm) were measured in the field. In the laboratory, C content was measured to calculate the best-fitting coefficients for future estimations by using allometric equations. Individual biomass was best estimated from sporophyte height through a rational model, while holdfast area (ellipse in cm2) was a good proxy of biomass with a sinusoidal model. The southernmost, least accessible, and most exposed site (Elefante) had significantly higher values of stored carbon. We estimated a carbon standing stock (from sporophytes only) of 2044 t C in these kelp beds. Nevertheless, additional and more detailed measurements will likely produce more accurate estimates both in time and space. We provide allometric equations for future carbon assessments. Our results highlight the importance carbon assessment for kelp management and blue carbon estimates and the need to develop science-based marine planning strategies.
Introduction
Under the scope of the Sustainable Development Goal (SDG) 13 to mitigate climate change, developing carbon neutral economies have raised the attention on carbon storage processes within ecosystems. In this regard, natural carbon sinks are vital as they absorb large amounts of carbon, being referred to as negative emitters of carbon dioxide (CO2) [Citation1]. The destination of this carbon will vary according to the organism in question and its carbon incorporation process, which can be a) immobilization (i.e. time-limited annual net accumulation of carbon in structures) or b) sequestration (i.e. when carbon has avoided decomposition by being buried for many years) [Citation2].
Vegetated coastal habitats act as massive natural carbon sinks (blue carbon), absorbing a significant share of anthropogenic CO2 from the atmosphere in the substrate they settle on and in the living biomass (standing stock) [Citation3,Citation4]. Macroalgal communities contribute to human well-being by providing ecosystem services, which are yet underestimated [Citation5], but are responsible for the sequestration of 173 Tg C yr−1 globally (with a range of 61–268 Tg C yr−1) [Citation6]. This is partly due to their capacity to store vast quantities of carbon in living biomass [Citation5], making it different to other coastal vegetated communities. On the other hand, because they prevail growing in hard substrata where sediment accretion does not occur [Citation7], they have limited capacity for long-term carbon capture (immobilized) within the kelp forest habitat per se, accelerating the donation or transmission to other levels in the food chain [Citation5]. This has promoted disagreement as to whether macroalgae should be considered within the blue carbon concept [Citation8]. However, the direct assimilation, the temporal storage in living biomass, and the indirect C input through export and deposition of macroalgal detritus (detrital carbon flux) into allochthonous habitats where it can be further captured effectively [Citation9,Citation10] are strong reasons to incorporate macroalgae into blue carbon accounting, particularly under the climate change mitigation approach [Citation7].
Despite being a research priority, carbon storage estimations in macroalgal communities have not been carried out in Peru [Citation11]. Located in the Humboldtian Ecoregion within the Warm Temperate Southeastern Pacific Marine Province (WTSP, sensu Spalding et al. [Citation12]), the southern Peruvian coast is characterized by strong winds, exposed areas and cliffy fringes which is home to the highest density of kelp beds in the country [Citation13]. In San Juan de Marcona (15°S), kelp harvesting activity targets two canopy-forming laminarialean species: Lessonia trabeculata and Macrocystis pyrifera. These kelps are extracted as raw material for the cosmetic, food, and pharmaceutical industries [Citation14]. This activity represents the second most important income for the Marcona fisher community and represents a fundamental part of their livelihoods (COPMAR, Comunidad Pesquera de Marcona) [Citation14]. Both species are passively harvested by traditional beach-cast recollection, which is modulated, inter alia, by wave intensity and tidal force. However, L. trabeculata landings are reported from managed active harvests which are conducted by surface-supplied divers using a modified ax barreta (sensu Vega et al. [Citation15]) to detach the holdfast from the rocky substrate. This tool is essential in kelp beds found in high-energy conditions, because of more robust holdfasts which increase wave resistance and attachment efficiency [Citation16]. While L. trabeculata is subject to extraction pressures, local research initiatives focused on this species has been continuing to developed.
Estimations of carbon storage in macroalgal communities pose methodological challenges. One main challenge is that methods are highly destructive [Citation17,Citation18]. This is problematic considering the potential cumulative impacts on kelp communities that 1) are already subject to natural stressors acting at local and regional scales (e.g. El Niño Southern Oscillation) [Citation18,Citation19], 2) tend to have inherent spatiotemporal variability [Citation18, Citation20], and 3) recover slowly after an extraction event [Citation21]. Thus, nondestructive methodologies are highly desirable, such as the use of allometric equations relating biometric variables (e.g. sporophyte height, frond, and holdfast size) with kelp biomass. Accurate carbon estimation is central to understanding and monitoring kelp’s net contribution in providing ecosystem services [Citation18]. Additionally, nondestructive sampling methods do not require laboratory work, thus simplifying data processing and reducing monitoring costs [Citation18].
We estimated the carbon storage, as standing stock, of the laminarialean kelp Lessonia trabeculata in three sites of San Juan de Marcona at a given point in time. Furthermore, we derived a set of species-specific allometric equations with the goal of developing nondestructive methodologies for estimating carbon in kelp communities. Finally, we aim to strengthen the knowledge on carbon flux of macroalgal communities in Peru and provide recommendations for further studies.
Aim
Quantify the amount of carbon stored in Lessonia trabeculata kelp beds in San Juan de Marcona, Peru.
Provide allometric equations for estimating carbon storage in Lessonia trabeculata kelp forests in Peru.
Methods
At sampling sites
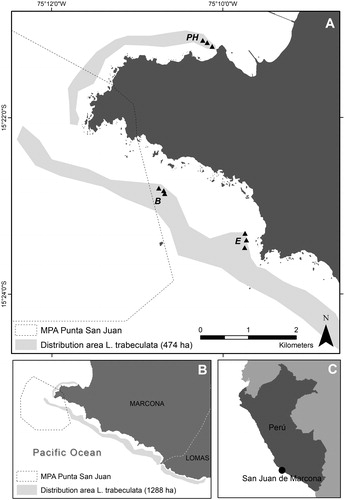
Three sampling sites were evaluated in areas comprising of polygons representing both passive beach-cast recollection and active extraction (harvest) of L. trabeculata. Sites were based on areas designated by the Programa Piloto Demostrativo (PPD, Demonstrative Pilot Program) administrated by COPMAR. The selected sites were a) Playa Hermosa, b) Basural, and c) Elefante (). At each site, 20 − 27 plots were sampled. Considering a depth range between 10 to 20 m, three 10 m transects were deployed randomly through a determined isobath to ensure a constant sampling depth. Replicate transects within a sampling site were >20 m in spacing. Three random numbers (0 ≤ ≥ 10) were generated for each transect. By using a negatively buoyant gas hose to form a circumference as a sampling unit, all sporophytes found within were carefully collected. The area of the sampling unit (ø = 1 m, A = 0.785 m2) was established to avoid overlap of consecutive random points within transects (i.e. marked at 1 m intervals). Depending on holdfast complexity and robustness, these were rather manually detached from the bottom (feasible in sheltered areas such as Playa Hermosa), or cut off with the modified ax procuring to extract the whole disk [Citation22]. Once all sporophytes were detached from each transect-point, these were tethered to a rope and pulled up to the surface. These could be either single individuals or sporophytes with coalescent holdfasts [Citation23]. At the surface, wet biomass from sampling units were weighed (kg m−2). Subsequently, the maximum height (m) of the sporophytes were recorded and the maximum (D) and minimum (d) disk widths were measured in order to estimate the holdfast area following the geometry of an ellipse (A=(D/2). (d/2). π). To verify if the sampling effort was adequate, a performance curve was calculated using wet biomass per plot (10 m x 2 m) for each sampling point [Citation24]. In order to estimate the carbon content as a function of dry biomass, four portions of the sporophytes were separately collected to measure dry biomass percentage and for a carbon analysis using the Walkley & Black technique [Citation25]),
Figure 1. Location of the transects (?) in each sampling site within San Juan de Marcona (A), Southern Peruvian coast (B, C). PH: Playa Hermosa, B: Basural and E: Elefante. Shaded light-grey belts indicate the distribution area of L. trabeculata from the study area (A, 474 ha) and the wider Marcona and Lomas southern regions (B, 1 288 ha). The estimated standing stock of 2 044 t C were calculated from the distribution area on (A) only.
Comparison between sampling sites
To compare biomass between areas, wet biomass was used as a proxy of the variability in the areas. Wet biomass was not normally distributed (p < 0.05; Shapiro-Wilk test for Basural and Playa Hermosa sites). Thus, a non-parametric Kruskal-Wallis test followed by a Mann-Whitney test were used to test for wet biomass differences between sites.
Carbon storage estimation
Once the average biomass per unit area (B, kg m−2) was obtained from each site, it was multiplied by the mean percentage of dry biomass values (%DW) and by the carbon content percentage (%C) in order to calculate carbon amount per unit area (C), with the following equation:
The averaged standing stock from the three sites was used to extrapolate carbon storage to the distribution area of L. trabeculata in San Juan de Marcona (, ).
Table 1. Summarized results computed from the laboratory analysis considering dry biomass (%DW 12.99 ± 0.65) and carbon content (%C 28.84 ± 4.59) as percentages of wet biomass measured on-site. C storage and the equivalent CO2 captured estimates for the study area (Punta San Juan de Marcona) are presented below.
Selecting the best-fitting allometric equation
In order to construct equations to predict the kelp biomass based on sporophyte height and holdfast area (biometrics), linear regressions were used following a model selection process in the Curve Expert Pro software [Citation26]. To this end, data of these three variables for the three sampling sites passed through a regression process considering all linear and non-linear models (except for linear polynomial models). The Akaike Information Criteria (AIC) and the coefficient of determination (R2) were used for model selection. The former is a measure of the relative quality between models and R2 is a measure of model fit. Therefore, best-fitting models were attributed when the lowest AIC and the highest R2 were observed [Citation27].
Results
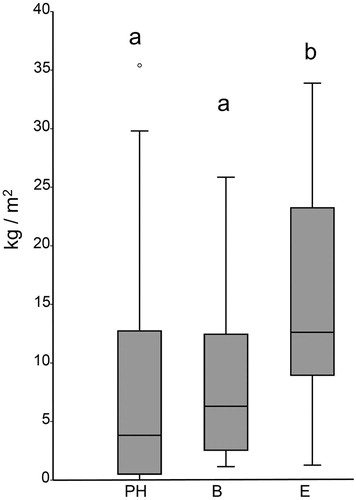
Wet biomass performance curves stabilized around plot 15 and 20, indicating that the number of samples taken per site (20 − 27) was adequate. The average wet biomass found in Playa Hermosa (PH) and Basural (B) were similar: 8.75 ± 1.79 kg m−2 and 8.33 ± 1.14 kg m−2, respectively. But biomass was higher in Elefante (E) (15.24 ± 9.08 kg m−2; Kruskal-Wallis and Mann-Whitney U tests p < 0.05; ).
Figure 2. Boxplot chart of the wet biomass (kg/m2) found in Playa Hermosa (PH), Basural (B) and Elefante (E). PH and B were grouped together (a) and higher median wet biomass was found in E (b) (p < 0.05, Mann-Whitney test).
The overall average was %DW= 12.99 ± 0.65, %C = 28.84 ± 4.59, resulting in a carbon standing stock estimate of PH= 3.51 t C ha−1, B = 3.32 t C ha−1 and E = 6.11 t C ha−1 which is equivalent into a transformed amount of CO2 of PH= 12.84 t CO2 ha−1, B = 12.14 t CO2 ha−1 and E = 22.35 t CO2 ha−1, respectively. The carbon storage estimation for the study area (474 ha, ) was 2 044 tC ().
Regarding the allometric equations, we focused on two statistical models, both estimating biomass but using two different parameters as proxies (i.e. height and holdfast elliptic area).
The best-fitting model to predict the wet biomass from height was the rational model:
where: x = sporophyte height; y = wet biomass; a = 1.70; b = 1.88; c=-6.04; d = 1.22 (AIC=-115.78; R2=0.78).
The best statistical model of biomass from holdfast area ((D/2). (d/2). π) was sinusoidal:
where x = holdfast area; y = wet biomass; a=-5.37; b = 28.69; c = 5.42 × 10−4, and d= −1.39 (AIC = 195.19; R2=0.74).
Discussion
Carbon stocks are commonly expressed in biomass of C per unit area, where 1 kg C m−2 = 10 t C ha−1 [Citation9,Citation10]. We compared our results with reported data on marine and terrestrial species. Our results on carbon standing stocks (4.31 ± 1.56 t C ha−1) were higher than those reported for the aquatic plants Limnobium laevigatum, Nymphoides rugdeana and Sarcocornia neei in wetland communities [Citation28–30] but lower than Polylepis forests [Citation31], Pinus sylvestris Scots pines [Citation32] and for Nymphoides indica; [Citation33–36]. Our estimates were similar to those reported for the Northeast Atlantic kelp Laminaria hyperborea in warmer regions (3.09 t C ha−1), but lower compared to the same species in colder regions (9.72 t C ha−1; 10° latitude of difference) [Citation9]. These differences provide insight into the importance of environmental variables influencing carbon storage of kelp beds. Carbon estimates across the latitudinal range of L. trabeculata would be useful to explore differences between the Humboldtian, Central Chile, and Araucanian ecoregions.
The northernmost distribution of Lessonia trabeculata in the southeastern Pacific is 14° S and it spans southward along the Peruvian littoral all the way to the Chilean border at 18°S (). Hard substrate occurrence, bathymetry, and slope are three of the strongest physical predictors of the distribution of laminarialean kelps [Citation37]. Considering these variables, the distribution area within Peruvian waters has been estimated to ca. 10,301 ha [Citation13]. Kelp wet biomass and the resulting carbon assimilation are highly variable both inter- and intra-specifically ([Citation9] and this study) and it may vary even within a regional scale. Therefore, we avoided extrapolating potential storage to the total distribution area in the Marcona region (), as more measurements are needed to account for potential variability. However, compared to the local study area, the twentyfold potential extension of the kelp beds within Peruvian waters is an important justification for additional research. Our carbon estimates correspond to the standing stock that exists in the kelp beds at a given time, but not to the carbon flux, which would be obtained with photosynthetic and growth rates data not yet available for the species making up Peruvian kelp ecosystems. Therefore, more research is needed to articulate the role that these ecosystems play in the carbon cycle of coastal-marine system and how it varies over time.
Allometric equations are used to convert field inventory data to biomass estimates [Citation17, Citation38,Citation39], to evaluate the relative amount of biomass [Citation17,Citation18, Citation40] and to adjust harvesting pressure according to biomass estimates at a given time [Citation18]. In the case of algae, these equations have been developed mainly for red and brown algae [Citation18, Citation41,Citation42]. For example, Scrosati and DeWreede [Citation43] have successfully applied nondestructive methods to estimate biomass of the red algae Mazzella cornucopiae based on the fronds and not on the individuals. Additionally, Stagnol et al. [Citation18] developed this type of equation for eight intertidal algae species finding that power equations (i.e. variable base raised to a fixed power) were the most explanatory for biomass prediction. It is challenging to construct allometric equations for biomass estimations in brown algae (as L. trabeculata) because these constitute a polyphyletic group, displaying different life cycles and morphologically diverse thalli, making estimates difficult to standardize [Citation18]. However, research suggests that allometric equations used for estimating biomass in terrestrial woody plants can be applied for kelps considering some characteristics (i.e. photosynthetic surface area and dry biomass) [Citation44].
Taking into consideration the spatiotemporal scale at which this research was conducted, our allometric equations had R2 values higher than 0.74 (R2model 1=0.78, and R2model 2=0.74). These results are comparable to the models developed by [Citation18] for brown algae (0.77 < > 0.92) and by [Citation44] for subtidal and intertidal kelp species (0.67 < > 0.76).
Growth of L. trabeculata is explained by morphological characteristics such as holdfast size and total length [Citation37] and the degree of confidence estimated for both models are within previously estimated ranges for other kelp species. We recommend the validation of our models across greater spatiotemporal scales since they were used to estimate biomass and carbon storage for a single species in a single location: Lessonia trabeculata in San Juan de Marcona. If validated, our results could be useful for subsequent monitoring in neighboring areas using nondestructive methods, and thereby reducing the impact of monitoring efforts within Marine Protected Areas (e.g. Punta San Juan; see ) as areas like these may represent potential locations of significant carbon sink [Citation8].
Interest in the role of kelp beds for carbon assimilation and storage processes is increasing [Citation45]. However, debate continues on their direct contribution to blue carbon accounting as standing stocks, and their potential inclusion into climate mitigation policies and national GHGs inventories [Citation8]. Nevertheless, there is increasing evidence that kelp beds contribute indirectly via detrital flux to be deposited and further immobilised in allochthonous blue carbon habitats or deeper soft-bottom environments [Citation6,Citation7, Citation46]. Kelp beds also provide important ecosystem services, such as habitat, nurseries, and natural coastal buffers and defenses [Citation20, Citation47]. Artisanal divers in San Juan de Marcona use L. trabeculata beds as habitats to harvest invertebrates including Loxechinus albus (red sea urchin) and molluscs Octopus mimus (Gould octopus), as well as multiple species of rocky reef fish: Cheilodactylus variegatus (Peruvian morwong ‘pintadilla’), Paralabrax humeralis (Peruvian rock seabass ‘cabrilla’) and Anisotremus scapularis (Peruvian grunt ‘chita’) (see , Supplementary Materials to see an expanded list of bioresources).
Our results highlight the carbon storage potential of L. trabeculata in southern Peru and suggest additional research is important to characterize the carbon dynamics of kelp ecosystems in the Humboldtian Ecoregion. More research is needed, both regionally and nationally, to inform holistic management of these ecosystems, along with the consideration of kelp playing a role in mitigation and adaptation strategies to confront climate change. Finally, we recommend more collaboration between marine policy stakeholders, local fishers, NGOs, and academia to promote marine planning efforts that include carbon-source habitats into account [Citation48] in order to manage Peruvian kelp ecosystems.
tcmt_a_1808765_sm0972.docx
Download MS Word (14.6 KB)Acknowledgements
This work was part of the project “Inmovilización de carbono azul por bosques de macroalgas en el litoral sur del Perú (Fondo Semilla 2018)”, funded by the Universidad Científica del Sur. We thank the Comunidad Pesquera de Marcona (COPMAR), Pether Mogrovejo Cerna (“Tiernito”), Alfredo Campos Cerna and Luis Carlos Flores Hechajalla (“Valentín”) for their assistance during the field work and data acquisition. The authors are grateful to Josh Donlan and Madeleine Wild for revising and improving the manuscript with constructive comments.
The author´s responsibilities were as follows – OAR, BM and HA: designed and conducted the research, wrote the manuscript and analysed the data; OAR had primarily responsibility for the final content of the manuscript; and all authors: edited, read and approved the final manuscript.
Disclosure statement
None of the authors had a conflict of interest.
References
- Bellassen V, Luyssaert S. Carbon sequestration: managing forests in uncertain times. Nature. 2014;506(7487):153–155. doi:10.1038/506153a.
- Barnes DKA, Ireland L, Hogg OT, et al. Why is the South Orkney Island shelf (the world's first high seas marine protected area) a carbon immobilization hotspot? Glob Chang Biol. 2016;22(3):1110–1120. doi:10.1111/gcb.13157.
- Canu D, Ghermandi A, Nunes PAL, et al. Estimating the value of carbon sequestration ecosystem services in the Mediterranean Sea: An ecological economics approach. Glob Environ Change. 2015; 32:87–95. doi:10.1016/j.gloenvcha.2015.02.008.
- Duarte CM, Losada IJ, Hendriks I, et al. The role of coastal plant communities for climate change mitigation and adaptation. Nature Clim Change. 2013;3(11):961–968. 12/17/2019;961-968. doi:10.1038/nclimate1970.
- Hill R, Bellgrove A, Macreadie PI, et al. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol Oceanogr. 2015;60:1689–1706. doi:10.1002/lno.10128.
- Krause-Jensen D, Duarte CM. Substantial role of macroalgae in marine carbon sequestration. Nature Geosci. 2016;9(10):737–742. doi:10.1038/ngeo2790.
- Krause-Jensen D, Lavery P, Serrano O, et al. Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biol Lett. 2018;14(6):20180236. doi:10.1098/rsbl.2018.0236.
- Howard J, Sutton-Grier A, Herr D, et al. Clarifying the role of coastal and marine systems in climate mitigation. Front Ecol Environ. 2017;15(1):42–50. doi:10.1002/fee.1451.
- Pessarrodona A, Moore PJ, Sayer MJD, et al. Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob Chang Biol. 2018;24(9):4386–4398. doi:10.1111/gcb.14303.
- Trevathan-Tackett SM, Kelleway J, Macreadie PI, et al. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology. 2015; 96(11):3043–3057. doi:10.1890/15-0149.1.
- McKinley E, Aller-Rojas O, Hattam C, et al. Charting the course for a blue economy in Peru: a research agenda. Environ Dev Sustain. 2019;21(5):2253–2275. doi:10.1007/s10668-018-0133-z.
- Spalding MD, Fox HE, Allen GR, et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience. 2007;57(7):573–583. doi:10.1641/B570707.
- Zavala J, Flores D, Donayre S, et al. Population assessment of Lessonia trabeculata (Villouta and Santelices, 1986) in San Juan de Marcona, March 2010. Inf Inst Mar Perú. 2015;42(4).
- Ministerio de la Producción (PRODUCE). Perú, País Rico y Diverso En Macroalgas: Estudio Económico de La Cadena Productiva Del Recurso Macro Algas Marinas.; 2016.
- Vega JA, Broitman BR, Vásquez JA. Monitoring the sustainability of Lessonia nigrescens (Laminariales, Phaeophyceae) in northern Chile under strong harvest pressure. J Appl Phycol. 2014;26(2):791–801. doi:10.1007/s10811-013-0167-4.
- Qin Y. Seaweed Bioresources. Bioactive seaweeds for food applications. Cambridge, MA: Academic Press, 2018. p. 3–24.
- Scrosati R. Length – biomass allometry in primary producers: predominantly bidimensional seaweeds differ from the ‘universal’ interspecific trend defined by microalgae and vascular plants. Can J Bot. 2006;84(7):1159–1166. 2006 doi:10.1139/B06-077Scrosati,.
- Stagnol D, Macé M, Destombe C, et al. Allometric relationships for intertidal macroalgae species of commercial interest. J Appl Phycol. 2016;28(6):3407–3411. doi:10.1007/s10811-016-0860-1.
- Krumhansl KA, Okamoto DK, Rassweiler A, et al. Global patterns of kelp forest change over the past half-century. Proc Natl Acad Sci USA. 2016;113(48):13785–13790. doi:10.1073/pnas.1606102113.
- Smale DA, Burrows MT, Moore P, et al. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol. 2013;3(11):4016–4038. doi:10.1002/ece3.774.
- Gorman D, Connell SD. Recovering subtidal forests in human-dominated landscapes. J Appl Ecol. 2009;46(6):1258–1265. doi:10.1111/j.1365-2664.2009.01711.x.
- Manual extraction of Lessonia trabeculata holdfast. Accessed 16 December 2019. https://www.youtube.com/watch?v=Prl0yYBaD8w.
- González AV, Beltrán J, Flores V, et al. Morphological convergence in the inter-holdfast coalescence process among kelp and kelp-like seaweeds (Lessonia, Macrocystis, Durvillaea). Phycologia. 2015;54(3):283–291. doi:10.2216/14-105.1.
- Elzinga CL, Salzer DW, Willoughby JW. Measuring & Monitoring Plant Populations; 1998. U.S. Bureau of Land Management Papers. 17. https://digitalcommons.unl.edu/usblmpub/17.
- Walkey AJ, Black IA. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934; 37:29–38.
- Hyams D. Curve Expert 1.3. A comprehensive curve fitting system for Windows© USA; 2003.
- Kebede B, Soromessa T. Allometric equations for aboveground biomass estimation of Olea europaea L. subsp. cuspidata in Mana Angetu Forest. Ecosyst Health Sustain. 2018; 4(1):1–12. doi:10.1080/20964129.2018.1433951.
- Palomino-Contreras D, Cabrera-Carranza C. Estimación del servicio ambiental de captura del CO2 en la flora de los humedales de Puerto Viejo. Rev del Inst Investig la Fac Ing Geológica, Minera, Met y Geográfica. 2012; 10:49–59.
- Aponte H. Productividad de Limnobium laevigatum (Hydrocharitaceae) bajo condiciones de laboratorio. Polibotanica. 2017;(44):157–166. doi:10.18387/polibotanica.44.12.
- Camargo A, Florentino E. Population dynamics and net primary production of the aquatic macrophyte Nymphaea rudgeana C. F. Mey in a lotic environment of the Itanhaém River basin (SP, Brazil) ). Rev Bras Biol. 2000;60(1):83–92. doi:10.1590/s0034-71082000000100011.
- Vásquez E, Ladd B, Borchard N. Carbon storage in a high-altitude Polylepis woodland in the Peruvian Andes. Alp Bot. 2014;124(1):71–75. doi:10.1007/s00035-014-0126-y.
- Lee J, Tolunay D, Makineci E, et al. Estimating the age-dependent changes in carbon stocks of Scots pine (Pinus sylvestris L.) stands in Turkey. Ann For Sci. 2016;73(2):523–531., doi:10.1007/s13595-016-0546-5.
- Martel C, Cairampoma L. Cuantificación del carbono almacenado en formaciones vegetales amazónicas en ¨CICRÄ, Madre de Dios (Perú). Ecol Apl. 2012;11(2):59–65.
- Menezes C. Biomassa e producao primária de tres espécies de macrófitas aquáticas da Represa do Lobo (Broa). Dissertacao (Maestrado). Universidade Federal de Sao Carlos, Brasil; 1984. p. 207
- Penha J. Ecologia populational de Pontederia lanceolata (Nutal) em una área alágavel do pantanal Matogrossense, MT. Dissertacao (Maestrado). Universidade Federal de Sao Carlos, Brasil; 1994. p. 92.
- Roa-García MC, Brown S. Caracterización de la acumulación de carbono en pequeños humedales andinos en la cuenca alta del río Barbas (Quindío, Colombia). Caldasia. 2016;38(1):117–135. doi:10.15446/caldasia.v38n1.57833.
- Vásquez JA. Estudio de investigación de poblaciones y de las condiciones de viabilidad ecológica de las actividades extractivas de algas pardas e invertebrados en la zona costera sur, en apoyo a la investigación y desarrollo del Instituto del Mar del Perú (IMARPE). Report elaborated by ICON-INSTITUT GmbH Private Sector. 2009
- Chave J, Andalo C, Brown S, et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia. 2005;145(1):87–99. doi:10.1007/s00442-005-0100-x.
- Paul KI, Roxburgh SH, England JR, et al. Development and testing of allometric equations for estimating above-ground biomass of mixed-species environmental plantings. For Ecol Manag. 2013;310:483–494. doi:10.1016/j.foreco.2013.08.054.
- Niklas KJ, Enquist BJ. On the vegetative biomass partitioning of seed plant leaves, stems, and roots. Am Nat. 2002;159(5):482–497. doi:10.1086/339459.
- Lindgreen A, Bouza N, Åberg P, et al. Spatial and temporal variation in distribution of Gelidium canariensis (Rhodophyta) from natural populations of the Canary Islands. J Appl Phycol. 1998; 10:273–278.
- Engel C, Åberg P, Gaggiotti OE, et al. Population dynamics and stage structure in a haploid- diploid red seaweed, Gracilaria gracilis. J Ecol. 2001;89(3):436–450. doi:10.1046/j.1365-2745.2001.00567.x.
- Scrosati R, DeWreede RE. Dynamics of the biomass-density relationship and frond biomass inequality for Mazzella cornucopiae (Gigartinaceae, Rhodophyta): implications for the understanding of frond interactions. Phycologia. 1997;36(6):506–516. doi:10.2216/i0031-8884-36-6-506.1.
- Starko S, Martone PT. An empirical test of 'universal' biomass scaling relationships in kelps: evidence of convergence with seed plants. New Phytol. 2016;212(3):719–729. doi:10.1111/nph.14120.
- Chung IK, Beardall J, Mehta S, et al. Using marine macroalgae for carbon sequestration: A critical appraisal. J Appl Phycol. 2011;23(5):877–886. doi:10.1007/s10811-010-9604-9.
- Burrows MT, Kamenos NA, Hughes DJ, et al. Assessment of carbon budgets and potential blue carbon stores in Scotland’s coastal and marine environment. Scottish Natural Heritage Commissioned Report. 2014. p. 761.
- Steneck RS, Graham MH, Bourque BJ, et al. Kelp forest ecosystems: biodiversity, stability, resilience and future. Envir Conserv. 2002;29(4):436–459. doi:10.1017/S0376892902000322.
- Smale DA, Moore PJ, Queirós AM, et al. Appreciating interconnectivity between habitats is key to blue carbon management. Front Ecol Environ. 2018;16(2):71–73. doi:10.1002/fee.1765.
