Abstract
Injection of pig slurry (PS) into soils under no-tillage system (NTS) is more efficient for improving soil chemical and physical attributes, and reducing C and N emissions, than surface applications. This study evaluated the effect of using injection and surface application of PS, compared to NPK and control treatments, on the soil aggregate, C and N contents, and isotopes 13C and 15N. The NTS consisted of rotations of summer (maize) and winter (black oat and wheat) grasses from 2011 to 2015. The treatments were PS injected into the soil (PSI), PS applied on the soil surface (PSS), chemical fertilization (NPK), and control (CTRL). The following soil properties were evaluated in the 0–5, 5–10, and 10–20 cm layers: aggregate stability (geometric mean diameter – GMD; aggregate mass distribution); total organic carbon (TOC) and total nitrogen (TN) in macroaggregates (8.0–0.25 mm), microaggregates (<0.25 mm), and bulk soil (<2.0 mm); and isotopes 13C and 15N in macro and microaggregates. The application of PSI improved the soil physical attributes, presenting higher GMD (0–5 cm) than the PSS, NPK, and CTRL treatments. In the 5–10 cm layer, the PSI treatments were more efficient in increasing the GMD and macroaggregate mass than the NPK. PSI also was more efficient in increasing TOC and TN when compared to PSS, and generated a higher GMD, which are protectors of these elements in the soil. The natural abundance of 15N denoted the lower soil organic matter decomposition in the PSI treatment when compared to the PSS. The natural abundance of 13C showed less-negative values in macroaggregates than in microaggregates, denoting that the soil management practices and crops used (grasses) affected positively the abundance of 13C. After seven applications of PS in maize-oat-wheat rotation in NTS, the application of PSI was more efficient in improving the soil physical and chemical attributes than the application of PSS.
Introduction
Successive applications of pig slurry (PS) to the surface of soils without incorporation, as made in no-tillage system (NTS) can result in significant losses of ammoniacal nitrogen (N) by volatilization of ammonia and N2O emission to the atmosphere [Citation1,Citation2]. These losses occur because a significant part of the N contained in the PS is in the ammoniacal form at time of application. This form is readily available to be absorbed by plants, but it is also prone to environmental losses through several pathways. The pH of PS is usually alkaline, facilitating ammonia volatilization when exposed to the atmosphere [Citation3,Citation4]. The PS nitrogen can also be nitrified to nitrate, and either leached to ground and surface waters, or denitrified with associated production of nitrous oxide, a potent greenhouse gas [Citation5]. However, the agricultural use of PS can add soluble carbon (C), water, and mineral N to the soil, which can affect the process of formation and stability of soil aggregates and also the C and N stocks [Citation6,Citation7], and the stable isotopes of C (δ13C) and N (δ15N) [Citation6].
The isotope signatures of stable C and N of plants and soil can serve as valuable natural markers and non-destructive integrators that show how plants today and in the past integrated and responded to biotic and abiotic environments [Citation8–10]. The use of stable N isotopes can show differences between areas with different soil managements or fertilizations. Loss et al. [Citation11] made a long-term evaluation of the natural abundance of 15N in macro and microaggregates in areas with forest and crops in NTS and conventional soil preparation system (CPS) and found that destruction of soil aggregates by the plowing and harrowing practices in CPS increases the decomposition and mineralization rates of the soil organic matter (SOM) that was protected by the aggregates; this affected the 15N values, which were higher than those found in the NTS and forest areas. Regarding the use of stable C isotopes, this can show differences between the carbon content incorporated in the different sizes of soil aggregates. The more negative 13C values in microaggregates (0.25–0.105 mm) indicate greater protection of C, while less negative values in macroaggregates (8–2 mm) indicate C recently incorporated [Citation11]. The most negative 13C results in the microaggregates and the least negative 13C in the macroaggregates demonstrate that the C is more susceptible to oxidation in the macroaggregates (preferably), but more protected in the microaggregates [Citation12,Citation13].
The aggregate stability is an important indicator of the soil susceptibility to erosion processes; it is associated with the soil porous structure and to the movement of water through these pores [Citation14]. However, studies on the effects of combining organic fertilization and minimal soil tillage on soil aggregation and stability of soil aggregates are incipient [Citation6,Citation15–18]. A study conducted in France by [Citation15] showed that minimal soil tillage combined with organic fertilization can contribute to the improvement of soil aggregate stability. In Brazil, an evaluation of soils in NTS with the application of PS for 10 years showed higher total C and N contents and higher formation of biogenic aggregates over physicogenic ones for areas with PS applications [Citation6].
Moreover, it is known that PS applications can alter the soil chemical [Citation19–21], physical [Citation22,Citation23], and biological [Citation24,Citation25] attributes. Some studies showed that PS applications to the soil surface can change some soil attributes, including total organic carbon (TOC) contents [Citation19,Citation23,Citation26,Citation27] and total N contents [Citation19,Citation25,Citation28], and can improve soil aggregation [Citation6,Citation7,Citation23]. However, few studies evaluate aggregation dynamics and C and N accumulation in aggregates after PS applications to soils under subtropical climate as in Brazil. These evaluations are even more scarce when considering PS distribution on soils by using alternative applications to the traditional surface applications, such as subsurface (10 cm depth) injection of PS [Citation29].
Despite de advances achieved by researches on subsurface application of PS into soils [Citation30,Citation31], the results are incipient and little conclusive, missing information regarding the evaluation of attributes related to soil aggregation and SOM fractions.
The interest in finding management practices for PS compatible with NTS and with less impact on the environment is increasing in Brazil and other countries. Thus, understanding the effect of using PS—applied to the soil surface and injected into the soil—on the SOM fractions, aggregate stability, and nutrient supply is important to define management practices that maintain or improve the soil quality. In this context, the hypothesis of the present work is that the injection of PS into the soil in NTS is more efficient to improve soil chemical and physical attributes than surface application. We also tested whether injection of PS can improve edaphic attributes compared to NPK and control. Thus, the objective was to evaluate the effect of using injection and surface application of PS, compared to NPK and control treatments, on the soil aggregate stability, C and N contents, and composition of 13C and 15N to define management strategies of PS that maintain or improve the soil quality.
Material and methods
Study site and treatments
The experiment was conducted at the Santa Maria Federal University (UFSM), in Santa Maria, state of Rio Grande do Sul, South region of Brasil (29°43′S, 53°43'W, and 105 m of altitude). The soil of the area was a Typic Hapludult (Argissolo Vermelho Alumínico úmbrico − Citation32] of loamy texture in the 0–10 cm layer (192 g kg−1 clay, 443 g kg−1 sandy, and 365 g kg−1 silt) (Pipette method; Citation33]. The soil chemical characteristics in the 0–10 cm layer at the beginning of the experiment was: pH (H2O) of 5.9; 20.5 g kg−1 of TOC; 1.6 g kg−1 of TN; 6.7 mg dm−3 of available P; 39.0 mg dm−3 of available K (Mehlich 1); 9.8 cmolc dm−3 of exchangeable Ca; 3.1 cmolc dm−3 of exchangeable Mg; and 0.0 cmolc dm−3 of exchangeable Al (extracted by KCl 1 mol L−1). The climate of the region is Cfa2, humid subtropical, according to the Koppen classification.
The area was fallow before the experiment, with vegetation consisting predominantly of Eragrostis plana Nees up to May 2010, when dolomitic lime was applied (4 Mg ha−1) and incorporated by plowing and harrowing. Then, the crop rotation in NTS was implemented—black oat (May to October 2010), maize (November 2010 to April 2011), and wheat (June to November 2011). The treatments were applied first for the maize crop on December 01, 2011 and, then, for a rotation system established with summer (maize) and winter (black oat or wheat) grasses up to December 2015 ().
Table 1. Cultural practices during the experiment period.
The treatments were applied in 16 plots of 31.5 m2 (6 m × 5.25 m), with four replications, using a randomized block experimental design. The treatments used were: pig slurry injected into the soil (PSI), PS applied to the soil surface (PSS), chemical fertilization (NPK), and a control without fertilization (CTRL). The applications were always in the same plots at 1 to 4 days before sowing.
The PS and NPK rates used were established based on the recommendations for organic and mineral soil fertilization of the Soil Chemistry and Fertilization Commission [Citation35,Citation36]. The PS rate was determined to supply approximately 150 (149 to 162) kg of TN ha−1 for the maize crop, 130 (133) kg of TN ha−1 for the wheat crop, and 150 (156) kg of TN ha−1 for the black oat crop at pre-sowing. The plots treated with NPK had applications of P and K at pre sowing, and N (urea) divided into pre-sowing fertilization (1/3) and topdressing fertilization (2/3).
The plots treated with PSS had manual applications using 10-liter watering can, and those treated with PSI had subsurface applications using a commercial mechanical injector device (Model DAOL-i 4000, Mepel, Estação, Brazil). This implement consisted of a 4000-liter metal tank, with injection started by turning on a hydraulic piston responsible to insert 8 injectors into the soil using discs and furrowers at the rear of the implement. The tractor with the injector implement was set to apply the PS rate at a work speed of the 3.7 km h−1, with the power takeoff at 540 rotations per minute. The furrowers were 2.0 cm thick and had a replaceable tip spaced 35 cm apart; the application depth varied from 8 to11 cm.
The PS used was collected in an anaerobic pond; it was from animals at the finishing stage and consisted of feces, urine, and feed and water leftovers. Its main characteristics and the rates of dry matter, C, and N added to the soil using the PS, and NPK in the mineral fertilization treatment are presented in .
Table 2. Main characteristics of the pig slurry (PS) (data expressed in wet base), PS rate applied, and N, P and K rates applied using PS and mineral fertilizer in each crop.
The mean data found for grain yield of maize and wheat, and dry matter yield of maize, wheat, and oat is shown in and .
Table 3. Yield of grain and dry matter (DM) of maize after surface and injected applications of pig slurry to soils under no-tillage system.
Table 4. Yields of grain and dry matter (DM) of black oat and wheat after surface and injected applications of pig slurry to soils under no-tillage system.
Maize (Zea mays L.) seeds were sowed manually in rows spaced 70 cm apart, for a population density of 75.000 plants ha−1. In situations of water deficit, the crops were irrigated using a sprinkler system. Wheat and black oat seeds were sowed mechanically, using a seeder (Model SHM 1517, Semeato, Passo Fundo, Brazil), in rows spaced 17 cm apart, for a density of approximately 350 plants m2.
Soil sampling and analyses
Deformed and undeformed soil samples were collected in May 2016 for replications of the four treatments. Trenches were opened, using a mattock and a spade, to collect the soil samples from the 0–5, 5–10 and 10–20 cm layers. The samples were placed in plastic bags. The undeformed samples were manually disaggregated following cracks or weak points, and sieved in 8.00-mm and 4.00-mm mesh sieves to obtain the soil aggregates [Citation33]. Deformed samples were sieved in a 2.00-mm mesh sieve to obtain the air-dried fine earth (bulk soil). The following analyses were performed on soil aggregates and bulk soil.
Physical analyses
Aggregate stability
A 25-gram sample of the aggregates retained in the 4.00-mm mesh sieve was sieved in a set of sieves with decreasing mesh diameter (2.00, 1.00, 0.50, 0.25, 0.105, and 0.053 mm) [Citation33]. The aggregates were placed in the 2.00-mm mesh sieve, moistened with water spray, and subjected to vertical wet sieving for 15 min in a Yoder device [Citation37]. Subsequently, the material retained in each sieve was removed, separated by water jet, placed in identified and weighed aluminum crucibles, and taken to an oven until constant weight to obtain its dry weight.
The geometric mean diameter (GMD) of the aggregates was calculated using the dry weight of the aggregates [Citation33], which was also used to evaluate their distribution in the following mean diameter classes: 8.00>Ø ≥ 2.0 mm (macroaggregates), 2.0>Ø ≥ 0.25 mm (mesoaggregates), and Ø < 0.25 mm (microaggregates) [Citation38].
Chemical analyses
The dry weights of macro and mesoaggregates were included for the chemical analysis to obtain a larger quantity of material. Therefore, the chemical parameters were evaluated considering the macroaggregates (8.00>Ø ≥ 0.25 mm), microaggregates (Ø < 0.25 mm), and bulk soil (Ø < 2.0 mm). These materials were macerated in a mortar, homogenized, and used in the following analyses:
Total organic carbon (TOC) and total nitrogen (TN)
TOC and TN contents in the bulk soil, macroaggregates, and microaggregates were determined in an elemental dry combustion analyzer (FlashEA 1112 Thermo Finnigan) at the Laboratory of Research in Biotransformation of Carbon and Nitrogen (LABCEN), in Santa Maria, RS, Brazil.
Natural abundance of 13C and 15N
The isotopic abundance of δ15N and δ13C was determined in aliquots of approximately 300 mg of each sample of bulk soil, macroaggregates, and microaggregates (milled and passed through a 100-µm mesh sieve), with precision of the 0.0001. The isotopic abundance of δ15N and δ13C was also determined in urea and pig slurry samples. The samples were, then, packed in tin capsules and evaluated in a continuous flow isotopic mass spectrometer coupled to a total C and N analyzer (DeltaPlus; Carlo Erba EA 1108, Finnigan MAT, Bremen, Alemanha) at the LABCEN. The isotopic variation of carbon was expressed as δ13C (‰) in relation to the PDB (Pee Dee Belemnite) international standard, and nitrogen as δ15N (‰) in relation to atmospheric air (0.3663%).
Statistical analyses
The results were analyzed for normality and homogeneity of the data by the Lilliefors [Citation39] and Bartlet [Citation40] tests, respectively. The data were subjected to analysis of variance (F test) and, when the effects were significant, the means were compared by the Tukey's test at 5% probability, using the Sisvar 5.6 program. Statistical analyses were performed for the four treatments, soil aggregates (macro and micro), and bulk soil. Subsequently, the data of TOC, TN, 13C and 15N of each treatment were subjected to statistical analysis independently, and the results of the soil aggregates and bulk soil were compared by the Tukey's test at 5%.
Results and discussion
Soil aggregate stability
Higher geometric mean diameters (GMD) in the 0–5 cm soil layer were found for the PSI treatment when compared to the other treatments. And in the 5–10 cm layer, PSI showed higher GMD values when compared to NPK treatment, but without differing from PSS and CTRL treatments. The highest GMD in the 10–20 cm layer was found for the PSS treatment and the lowest for the NPK, but without differing from PSI and CTRL treatments ().
Figure 1. Geometric mean diameter (GMD, mm) of soil aggregates in areas fertilized with pig slurry and mineral fertilizer. PSI = Pig slurry injected into the soil subsurface; PSS = pig slurry applied to the soil surface; NPK = Chemical fertilization; CTRL = control (without fertilization). Bars with the same letter in each soil layer are similar by the Tukey's test at 5% probability.
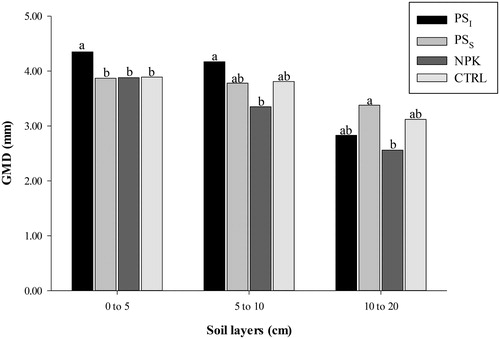
The lowest GMD in the NPK treatment when compared to the other treatments with pig slurry can be due to the SOM rapid mineralization in the NPK treatment, as also found by [Citation38,Citation39] and [Citation43]. Mineral N fertilization can reduce SOM contents, i.e. TOC, since mineral N becomes a nutrient source for microorganisms that decompose SOM, resulting in a faster SOM decomposition [Citation38].
Higher GMD in areas fertilized with swine manure was also found by [Citation43] when compared to that in areas fertilized with mineral fertilizer (NPK) in a Typic Hapludult (Argissolo Vermelho-Amarelo [Citation32]). The authors found higher GMD for the organic fertilizer treatment (3.84 and 3.20 mm, respectively to 0–5 and 5–10 cm) when compared to the mineral fertilizer (3.05 and 2.30 mm respectively to 0–5 and 5–10 cm).
[Citation43] found higher GMD in treatments with organic fertilizer compared to treatments with mineral fertilizer; they attributed this result to the greater amount of aggregates greater than 2 mm, and related this higher aggregation to the greater SOM accumulation generated by the treatments without mineral fertilizer. In addition, [Citation43] also reported that the addition of swine manure increased the production of dry matter in cover crops (black oats).
The higher GMD of treatments with pig slurry may be also related to the higher carbon input via organic fertilizer when compared to the mineral fertilizer (NPK) (). The greater the amount of carbon in the soil, the greater the aggregate stability, since the SOM favors the formation of more stable soil aggregates [Citation44]. A similar trend was found in the present study; the distribution of aggregates according to their diameter indicates a predominance of macroaggregates, whose amount were, in general, greater in treatments with pig slurry when compared to the treatments with NPK. In the 5–10 cm layer, PSI showed higher macroaggregates values compared to NPK ().
Figure 2. Distribution of aggregate classes in different soil layers of areas fertilized with pig slurry and mineral fertilizer. PSI = Pig slurry injected into the soil subsurface; PSS = pig slurry applied to the soil surface; NPK = Chemical fertilization; CTRL = control (without fertilization). Bars with the same letter in each soil layer are similar by the Tukey's test at 5% probability.
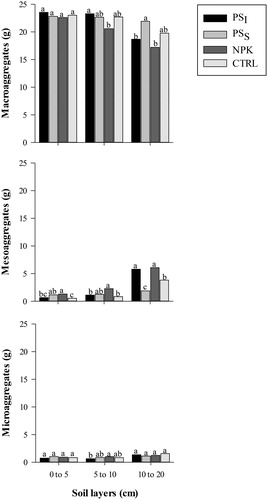
The amount of macroaggregates was higher than that of other classes of aggregates stable in water in the three evaluated layers (). This can be due to the use of grass species in the crop rotation, because their root system present large amounts of thin roots and has activity dependent on the biomass and length of hyphae of fungi that involve smaller aggregates forming macroaggregates; this factor associate with the absence of soil turning contributes effectively to formation of stable macroaggregates [Citation41,Citation42].
Formation of macroaggregates is important to the development of stable microaggregates, since macroaggregates provide an environment for accumulation of polysaccharides and humic substances that stabilize microaggregates within the macroaggregates [Citation43].
The areas fertilized with PSI and PSS, in general, presented larger amounts of macroaggregates in the 5–10 and 10–20 cm layers, respectively, than those fertilized with NPK. The application of animal manure to soils can improve physical attributes, lowering the soil density and increasing aggregation [Citation44]. The control treatment presented no differences regarding the use of pig slurry, denoting that the NTS is efficient in improving soil aggregation, as found by [Citation23].
The treatments presented differences in amount of mesoaggregates in all soil layers, with the treatment NPK having the highest values in all layers; with the exception of PSS for 0–10 cm layer, and PSI for 10–20 cm layer, which did not differ from NPK. In general, the results showed an increasing trend for amount of mesoaggregates in all treatments as the soil depth increases ().
The treatments had different amounts of microaggregates in the 5–10 cm layer; the treatment PSI presented the lowest value, and the treatment NPK the highest. These differences are in accordance with the highest GMD found () in PSI and PSS, since high amounts of stable macroaggregates generate higher GMD. The treatment NPK presented the lowest amount of macroaggregates and highest amount of microaggregates, corroborating its lower GMD ().
The agents responsible for the genetic process of formation of microaggregates are different than those responsible for the formation of larger aggregates, for which the SOM is more important [Citation71]. The results found showed that the main agent responsible for the formation of aggregates is the SOM, hence the higher amount of macroaggregates over meso and microaggregates.
Among the fractions of SOM that stand out for the formation of stable macroaggregates, there is particulate organic matter (POM) [Citation45,Citation46]. And, in the same areas of this study, Francisco [Citation29] found higher levels of POM in treatments with swine manure when compared with NPK treatment. PSI and PSS showed 11.76 and 8.55 g kg−1 compared to NPK, which presented 6.58 in the 0–5 cm layer depth.
The application of swine manure also increases soil fertility [Citation51,Citation52], which results in increased crop productivity and DM of cover plants [Citation31,Citation49]. According to the data of dry mass and grain yield of corn () and black oats (), it appears that the treatment with PSI showed values proportionally higher than the NPK. In general, in the PSI treatment there were 2 to 3 Mg ha−1 more than DM and corn yield (), and 0.6 Mg ha−1 of oat DM (). Possibly the injection of pig slurry should have a positive effect on the rooting of corn and oats ( and ). And plant roots are considered temporary binding agents, and in several studies it has been highlighted that the stability of macroaggregates is improved in the presence of roots [Citation40, Citation49, Citation55].
Assessing the carbon content of POM in experiment after 12 years of treatments with pig deep litter (DL), cattle slurry (CS), pig slurry (PS), NPK and control; Rodrigues et al. [Citation56] found higher levels of MOP in the 0–4 and 4–10 cm layer in treatments with animal manure (DL, CS and PS) compared to NPK and control. The higher amounts of macroagregates and GMD indexes in PS treatments can also be related to the increase of the POM content.
TOC and TN in bulk soil (Ø < 2.0 mm), macroaggregates (8.00>Ø ≥ 0.25 mm), and microaggregates (Ø < 0.25 mm)
The highest TOC in the 0–5 cm layer in the bulk soil and microaggregates were found in the treatment PSI, except for the control in the microaggregates, which did not differ in TOC from PSI. The treatments presented no differences in TOC in macroaggregates in the 0–5 cm layer. In the 5–10 and 10–20 cm layers, the treatments differed in TOC in the bulk soil and macroaggregates; the treatment PSI presented higher TOC than the other treatments, except for the treatment PSS, which presented no difference in TOC in the 5–10 cm layer in bulk soil when compared to PSI ().
Table 5. Total organic carbon (TOC) and total nitrogen (TN) contents in the bulk soil, macroaggregates and microaggregates.
The TN contents present similar trend to the TOC, higher in the treatment PSI when compared to the treatments NPK and control in the bulk soil in the 5–10 and 10–20 cm layers. The treatment PSI had higher TN in macroaggregates than the NPK (0–5 and 5–10 cm), and higher TN in macroaggregates than all treatments in the 10–20 cm soil layer. Differences in TN in the microaggregates were found only in the 0–5 cm soil layer, with higher TN in the treatment PSI when compared to the treatments NPK and PSS ().
The TOC and TN contents in the aggregate classes and in the bulk soil presented similar trend in all treatments. Higher TOC and TN in the 0–5 cm layer were found in the bulk soil, followed by the macroaggregates and microaggregates. Bulk soil and macroaggregates presented higher TOC and TN than microaggregates in the other soil layers ().
Soil C and N contents are dependent on the amount of animal manure or plant residues applied, their conversion rates into SOM, and the SOM mineralization rates [Citation51]. Therefore, the injection of pig slurry into the soil (PSI) presented higher TOC in the bulk soil (0–5 and 10–20 cm), macroaggregates (5–10 and 10–20 cm), and microaggregates (0–5 cm); as well as TN in the macro (10–20 cm) and microaggregates (0–5 cm) when compared to the treatment with surface application of pig slurry (PSS). Moreover, PSI was more efficient than the PSS in increasing soil TOC and TN contents when compared to the treatments NPK and CTRL.
Pig slurry adds nutrients (N, P, and K) and C to the soil (). Thus, the higher C and N contents found in the treatment PSI is probably related to the lower mineralization rate in this treatment; it generated higher maize and oat grain yields when compared to the other treatments ( and ). The treatment PSI had higher addition of plant residues to the soil due to the NTS, which increased TOC and TN contents. The highest TN in the treatment PSI when compared to the treatments PSS and NPK denotes the lower losses of N by volatilization because of the injection of pig slurry. Nicoloso et al. (2013) found similar results, with the injection of PS into the soil increasing the agronomical efficiency of the PS and promoting the accumulation of N. The injection of PS into the soil is strategies that improve the use of N in the PS by the plants [Citation52].
Aita et al. [Citation5] evaluated the potential of shallow injection of PS to abate gaseous ammonia and nitrous oxide emissions in winter crops in subtropical soils, Brazil. Injection was compared with surface broadcasting of PS, and its reduced ammonia volatilization compared with surface application. In the same area of the present study, Gonzatto et al. [Citation31] found that the injection of PS improved the yield of the crops and the N use efficiency by grain crops grown in NTS.
The macroaggregates presented higher TOC and TN contents in all soil layers and treatments than the microaggregates, denoting the higher capacity of protection against the decomposition of these elements associated with the NTS, since the trend was similar for all treatments. Thus, the higher the size of soil aggregates, the higher the TOC and TN contents in these aggregates.
Assis et al. [Citation53] found decreasing C and N contents as the aggregate size was decreased, denoting that the soil management and use systems change C and N contents in the different aggregate size classes. Intensive managements with soil turning increase losses of macroaggregates rich in C and increase microaggregates, with losses of C [Citation54].
Macroaggregates and microaggregates had higher TOC and TN contents in the soil surface layer (0–5 cm) when compared to the those in the subsurface layers (5–10 and 10–20 cm). This denotes that the accumulation of crop residues on the soil surface resulting from the NTS probably contributed to the TOC and TN found. Costa Júnior et al. [Citation55] found similar results when evaluating TOC contents in aggregates of a Hapludox under different uses and managements in Rio Verde, GO, Brazil. Loss et al. [Citation56] found higher TOC and of TN contents in aggregates in the 0–5 cm layer in soils under NTS when compared to those in deeper soil layers. Du et al. [Citation57] found that the adoption of a conservationist soil management system, especially NTS, can increase soil macroaggregation and C accumulation in macroaggregates.
In general, when we compared the results of TOC and TN between the treatments NPK, Control and PSS, both in bulk soil and soil aggregates, there are practically no differences (). The use of swine liquid manure (pig slurry) does not always result in an increase in the levels and stocks of C and N in the soil, and this effect has been attributed, mainly, to the addition of easily mineralized C and N compounds, and by the decomposition of native organic matter to the soil due to the priming effect [Citation58].
Some studies have demonstrated an increase in the content of C and N in the soil when applied solid swine manure compared to liquid manure [Citation23,Citation59,Citation51]. This is possibly due to the differences in the quality and quantity of C and N added between the two sources of pig wastes. Solid manure, for example a pig deep litter (PDL), has a higher C/N ratio than PS, due to the material commonly used in PDL, which is sawdust or rice husk. In addition, in PDL most of C and N are in organic form, while in PS in soluble forms (GIACOMINI; AITA, [Citation28]).
Natural abundance of carbon (δ13C) and nitrogen (δ15N) in macroaggregates (8.00>Ø ≥ 0.25 mm) and microaggregates (Ø < 0.25 mm)
The treatments presented differences in abundance of 13C only in the soil surface layer; the treatments PSS and NPK presented less-negative values for macro and microaggregates than the CTRL. The results for macro and microaggregates in each treatment showed little variation in 13C, with less-negative values for macroaggregates (). The 13C found resulted, in general, in the presence of plants of C4 photosynthetic cycle, since these plants present 13C of −6 to −19‰, whereas C3 plants present −24 to −34‰ [Citation60].
Table 6. Natural abundance of 13C and 15N in macroaggregates and microaggregates of soils fertilized with pig slurry and mineral fertilizer, and 13C and 15N in NPK and pig slurry samples.
The results found for 13C varied from −14.12 to −16.89‰ and were due to longer use of maize crops in a rotation system with oat (C3)/maize (C4)/wheat (C3) crops and due to the presence of Eragrostis plana plants, which covered the area before the implementation of the experiment. E. plana is a grass species of C4 photosynthetic cycle, thus, 13C presented less-negative values in the 10–20 cm layer than in the other layers.
This trend of less-negative 13C values in macroaggregates in all soil layers denotes the incorporation of new carbon from the present vegetation, probably from the maize or weed (E. plana) residues. The analysis of variation of the natural abundance of 13C serves as a tool to confirm the history of crops in the study area, and shows how much the plant residues of the sowed crops are contributing to the formation of SOM in an environment [Citation61].
The natural abundance of 15N presented greater variation between treatments and aggregate classes than 13C. The treatment PSS presented the highest natural abundance of 15N both macro and microaggregates of the 0–5 cm layer while the treatments NPK and CTRL presented the lowest; these treatments presented the same results for the microaggregates in 5–10 cm layer, with the treatment PSS presenting the highest 15N and NPK and CTRL presenting the lowest. Considering the 10–20 cm layer, the treatment PSS presented the highest 15N in macroaggregates while the CTRL presented the lowest; the treatment NPK had the highest 15N, and PSI the lowest in microaggregates ().
Högberg [Citation62] found increases in 15N of the SOM as the soil depth increased. Costa Júnior et al. [Citation63] found similar results when evaluating 15N associated with soil aggregates, with 15N presenting the same trend, with significantly lower values in surface layers and higher in deeper layers.
PSs presented higher 15N in macroaggregates of the 0–5 and 10–20 cm soil layers than the other treatments, as also found in microaggregates of the 0–5 and 5–10 cm layers. These results may be due to the pig slurry composition, which is rich in 15N due to isotopic fractioning processes—such as volatilization of NH3 and denitrification—occurring during the storage of animal manure or composting of organic residues [Citation64] and due losses of N poor in 15N (negative values of 15N) [Citation65].
Microaggregates presented the highest 15N value (24.09‰) in the 10–20 cm layer compared to the macroaggregates; this result can be connected to the transformation of N into its ionic form, involving organic matter decomposition and release of N [Citation72].
Costa Júnior et al. [Citation69] also found higher 15N values in microaggregates than in macroaggregates, indicating that the formation of large-size aggregates occurs by association of the organic matter added (decomposing plant residues) to the macroaggregates with subsequent transference and cementation of the SOM in high mineralization degree (high 15N) in the microaggregates.
The increase in natural abundance of 15N in soils indicates, mainly, the intensification of the nitrification process [Citation73], causing a higher nitrification in microaggregates than in macroaggregates. Considering the treatments PSI and PSS in all soil layers and the macro and microaggregates, PSI presented lower 15N values, indicating a slower SOM decomposition in this treatment when compared to the PSs.
Conclusion
The results of seven applications of pig slurry (PS) to soils under no-tillage system (NTS) with maize-oat-wheat crop rotation showed that the treatment with injection of PS into the soil (PSI) result in better soil physical attributes, especially the geometric mean diameter (GMD) of aggregates in the 0–5 cm soil layer than the treatments with surface application of PS (PSS), chemical fertilizer (NPK), and control without fertilization (CTRL). PSI was also more efficient than NPK in increasing GMD and amount of macroaggregates in the 5–10 cm soil layer. This increase occurred partly because of the use of NTS in the experiment.
The use of PSI resulted in higher soil TOC and TN contents when compared to PSS, denoting lower losses of N and C by volatilization and emissions to the atmosphere when PS is injected into the soil, resulting in a higher aggregate stability index and amount of macroaggregates, which are protectors of these elements in the soil.
The natural abundance of 15N showed a lower SOM decomposition in the treatment PSI when compared to the PSS. However, the natural abundance of 13C showed less-negative values in macroaggregates than in microaggregates, denoting that the soil management practices adopted and the crop species used (grasses) in the experiment affected positively the abundance of 13C.
Acknowledgment
The authors are grateful for the financial support of the Fundação Agrisus (PA 2494/18).
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ndegwa PM, Hristov AN, Arogo J, et al. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst Eng San Diego. 2008;100(4):453–469. doi:10.1016/j.biosystemseng.2008.05.010.
- Zaman M, Saggar S, Blennerhassett JD, et al. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol Biochem. 2009;41(6):1270–1280. doi:10.1016/j.soilbio.2009.03.011.
- Chantigny MH, Rochette P, Angers DA, et al. Ammonia volatilization and selected soil characteristics following application of anaerobically digested pig slurry. Soil Sci Soc Am J. 2004;68(1):306–312. doi:10.2136/sssaj2004.3060.
- Rochette P, Chantigny MH, Angers DA, et al. Ammonia volatilization and soil nitrogen dynamics following fall application of pig slurry on canola crop residues. Can J Soil Sci. 2001;81(4):515–523. doi:10.4141/S00-044.
- Aita C, Chantigny MH, Gonzatto R, et al. Winter-season gaseous nitrogen emissions in subtropical climate: impacts of pig slurry injection and nitrification inhibitor. J Environ Qual. 2019;48(5):1414. doi:10.2134/jeq2018.04.0137.
- Loss A, Lourenzi CR, Mergen Júnior CA, et al. Carbon, nitrogen and natural abundance of 13C and 15N in biogenic and physicogenic aggregates in a soil with 10 years of pig manure application. Soil Tillage Res. 2017;166:52–58. doi:10.1016/j.still.2016.10.007.
- Yagüe MR, Bosch-Serra AD, Antúnez M, et al. Pig slurry and mineral fertilization strategies effects on soil quality: macroaggregate stability and organic matter fractions. Sci. Total Environ. 2012;438:218–224. doi:10.1016/j.scitotenv.2012.08.063.
- Dawson TE, Mambelli S, Plamboeck AH, et al. Stable isotopes in plant ecology. Annu Rev Ecol Syst. 2002;33(1):507–559. doi:10.1146/annurev.ecolsys.33.020602.095451.
- Swap RJ, Aranibar JN, Dowty PR, et al. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Global Change Biol. 2004;10(3):350–358. doi:10.1111/j.1365-2486.2003.00702.x.
- Wittmer MHOM, Auerswald K, Bai YF, et al. Changes in the abundance of C3/C4 species of Inner Mongolia grassland: evidence from isotopic composition of soil and vegetation. Global Change Biol. 2010;16(2):605–616. doi:10.1111/j.1365-2486.2009.02033.x.
- Loss A, Pereira MG, Costa EM, et al. Carbon, nitrogen and the natural abundance of 13C and 15N in macro and microaggregates. Idesia. 2014;32(4):15–21. doi:10.4067/S0718-34292014000400003.
- Del Galdo I, Six J, Peressotti A, et al. Assessing the impact of land-use change on soil C sequestration in agricultural soils by means of organic matter fractionation and stable isotopes. Global Change Biol. 2003;9(8):1204–1213. doi:10.1046/j.1365-2486.2003.00657.x.
- Six J, Feller C, Denef K, et al. Soil carbon matter, biota and aggregation in temperate and tropical soils: effects of no-tillage. Agronomie. 2002;22(7-8):755–775. doi:10.1051/agro:2002043.
- Barthès B, Roose E. Aggregate stability as an indicator of soil susceptibility to runoff and erosion: validation at several levels. Catena. 2002;47(2):133–149. doi:10.1016/S0341-8162(01)00180-1.
- Bottinelli N, Angers DA, Hallaire V, et al. Tillage and fertilization practices affect soil aggregate stability in a Humic Cambisol of Northwest France. Soil Tillage Res. 2017;170:14–17. doi:10.1016/j.still.2017.02.008.
- Jiao Y, Whalen JK, Hendershot WH. No-tillage and manure applications increase aggregation and improve nutrient retention in a sandy-loam soil. Geoderma. 2006;134(1-2):24–33. doi:10.1016/j.geoderma.2005.08.012.
- Mikha MM, Rice CW. Tillage and manure effects on soil and aggregate-associated carbon and nitrogen. Soil Sci Soc Am J. 2004;68(3):809–816. doi:10.2136/sssaj2004.8090.
- Whalen JK, Hu Q, Liu A. Manure applications improve aggregate stability in conventional and no-tillage systems. Soil Sci Soc Am J. 2003;67(6):1842–1847. doi:10.2136/sssaj2003.1842.
- He YT, Zhang WJ, Xu MG, et al. Long-term combined chemical and manure fertilizations increase soil organic carbon and total nitrogen in aggregate fractions at three typical cropland soils in China. Sci Total Environ. 2015;532:635–644. doi:10.1016/j.scitotenv.2015.06.011.
- Lourenzi CR, Ceretta CA, Silva LSD, et al. Nutrients in soil layers under no tillage after successive pig slurry applications. Rev Bras Ciênc Solo. 2013;37(1):157–167. doi:10.1590/S0100-06832013000100016.
- Mergen Junior CA, Loss A, Santos Junior E, et al. Atributos químicos em agregados biogênicos e fisiogênicos de solo submetido à aplicação com dejetos suínos. Agraria. 2019;14(1):1–10. doi:10.5039/agraria.v14i1a5620.
- Andrade AP, Rauber LP, Mafra AL, et al. Changes in physical properties and organic carbono of a Kandiudox fertilized with manure. Cienc Rural. 2016;46(5):809–814. doi:10.1590/0103-8478cr20150540.
- Comin JJ, Loss A, Veiga M, et al. Physical properties and organic carbon content of a Typic Hapludult soil fertilised with pig slurry and pig litter in a no-tillage system. Soil Res. 2013;51(5):459–470. doi:10.1071/SR13130.
- Couto RR, Comin JJ, Soares CRFS, et al. Microbiological and chemical attributes of a Hapludalf soil with swine manure fertilization. Pesq Agropec Bras. 2013;48(7):774–782. doi:10.1590/S0100-204X2013000700010.
- Giacomini SJ, Aita C, Pujol SB, et al. Transformações do nitrogênio no solo após adição de dejeto líquido e cama sobreposta de suínos. Pesq Agropec Bras. 2013;48(2):211–219. doi:10.1590/S0100-204X2013000200012.
- Brunetto G, Comin JJ, Schmitt DE, et al. Changes in soil acidity and organic carbon in a sandy Typic Hapludalf after medium-term pig-slurry and deep-litter application. Rev Bras Ciênc Solo. 2012;36(5):1620–1628. doi:10.1590/S0100-06832012000500026.
- Mafra MSH, Cassol PC, Albuquerque JA, et al. Acúmulo de carbono em Latossolo adubado com dejeto líquido de suínos e cultivado em plantio direto. Pesq Agropec Bras. 2014;49(8):630–638. doi:10.1590/S0100-204X2014000800007.
- Giacomini SJ, Aita C. Cama sobreposta e dejetos líquidos de suínos como fonte de nitrogênio ao milho. Rev Bras Ciênc Solo. 2008;32(1):195–205. doi:10.1590/S0100-06832008000100019.
- Francisco CAL. Matéria orgânica e agregação do solo em áreas adubadas com dejeto líquido de suínos Dissertação (mestrado) – Universidade Federal de Santa Catarina, Centro de Ciências Agrárias, Programa de Pós-Graduação em Agroecossistemas, Florianópolis; 2019. p. 67. https://repositorio.ufsc.br/bitstream/handle/123456789/211639/PAGR0429-D.pdf?sequence=-1&isAllowed=y.
- Aita C, Tonetto F, Gonzatto R, et al. Nitrous oxide emissions in a wheat/corn succession combining dairy slurry and urea as nitrogen sources. Rev Bras Ci Sol. 2018;42:1–14.
- Gonzatto R, Aita C, Bélanger G, et al. Response of no-till grain crops to pig slurry application methods and a nitrification inhibitor. Agron J. 2017;109(4):1687–1610. doi:10.2134/agronj2016.09.0547.
- Embrapa. Sistema Brasileiro de Classificação de Solos. 3rd ed. Brasília (Brazil): Embrapa Produção de informação; Rio de Janeiro: Embrapa Solos; 2013. 312 p.
- Embrapa. Manual de métodos de análises de solo. 2nd ed. ver. E atual. Rio de Janeiro (Brazil): Ministério da Agricultura e do Abastecimento; 1997. 212 p.
- Gonzatto R. Eficiência de uso do nitrogênio por gramíneas em função do modo de aplicação de dejetos suínos no solo e do uso de inibidor de nitrificação. Tese de Doutorado. Universidade Federal de Santa Maria. 2016. http://repositorio.ufsm.br/handle/1/3374
- Comissão de Química e Fertilidade do Solo – RS/SC. Manual de adubação e calagem para os Estados do Rio Grande do Sul e de Santa Catarina. 10th ed. Porto Alegre (Brazil): Sociedade Brasileira de Ciência do Solo. Núcleo Regional Sul; 2004. 400 p.
- Comissão de Química e Fertilidade do Solo – RS/SC. Manual de adubação e calagem para os Estados do Rio Grande do Sul e de Santa Catarina. 11th ed. Porto Alegre (Brazil): SBCS - Núcleo Re-gional Sul/UFRGS; 2016.
- Yoder REA. Direct method of aggregate analysis of soils and a study of the physical nature of erosion losses. Journal of the American Society of Agronomy, 1936;28(5):337–351. https://doi.org/10.2136/sssaj1936.036159950B1720010046x
- Costa Junior C, Piccolo MC, Siqueira Neto M, et al. Carbono em agregados do solo sob vegetação nativa, pastagem e sistemas agrícolas no bioma Cerrado. Revista Brasileira de Ciência do Solo, 2012;36(4):1311–1322. https://doi.org/10.1590/S0100-06832012000400025
- Lilliefors HW. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;62(318):399–402. doi:10.1080/01621459.1967.10482916.
- Bartlett MS. Properties of sufficiency and statistical tests. Proc R Soc Lond Ser A. 1937;160:268–282.
- Fonte SJ, Yeboah E, Ofori P, et al. Fertilizer and residue quality effects on organic matter stabilization in soil aggregates. Soil Biol. Bioc. 2009;73:961–966.
- Schmitz D, Loss A, Lourenzi CR, et al. Atributos físicos de Cambissolo Húmico submetido a fontes de nitrogênio em pomar de macieira. Com Sci. 2018;8(2):316–325. doi:10.14295/cs.v8i2.1757.
- Ferreira GW, Benedet L, Trapp T, et al. Soil aggregation indexes and chemical and physical attributes of aggregates in a Typic Hapludult fertilized with swine manure and mineral fertilizer. International Journal of Recycling of Organic Waste in Agric. 2021;10:1896960.1051. doi:10.30486/IJROWA.2021.1896960.1051
- Six J, Bossuyt H, Degryze S, et al. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004;1(7–31).
- Haynes RJ, Beare MH. Influence of six crop species on aggregate stability and some labile organic matter fractions. Soil Biol Biochem. 1997;29(11-12):1647–1653. doi:10.1016/S0038-0717(97)00078-3.
- Salton JC, Mielniczuk J, Bayer C, et al. Agregação e estabilidade de agregados do solo em sistemas agropecuários em Mato Grosso do Sul. Rev Bras Ciênc Solo. 2008;32(1):11–21. doi:10.1590/S0100-06832008000100002.
- Oades JM. Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil. 1984;76(1-3):319–337. doi:10.1007/BF02205590.
- Oliveira JGR, Tavares Filho J, Barbosa GMC. Alterações na física do solo com a aplicação de dejetos animais. Geogr Opportuno Tempore. 2016;2(2):66–80.
- Ferreira LB, Loss A, Giumbelli LD, et al. Organic carbon and nitrogen contents and their fractions in soils with onion crops in different management systems. Soil Res. 2018;56(8):846–855. doi:10.1071/SR18167.
- Tivet F, Sá JCM, Lal R, et al. Aggregate C depletion by plowing and its restoration by diverse biomass-C inputs under no-till in sub-tropical and tropical regions of Brazil. Soil Tillage Res. 2013;126:203–218. doi:10.1016/j.still.2012.09.004.
- Loss A, Couto RR, Brunetto G, et al. Animal Manure As Fertilizer: Changes In Soil Attributes, Productivity And Food Composition. International Journal of Research – Granthaalayah, 2019;7(307–331). doi: 10.5281/zenodo.347556
- Lourenzi CR, Ciancio NHR, Tiecher TL, et al. Forms of N and P transfer by runoff in soil under no-tillage with successive organic waste and mineral fertilizers applications. Agric Water Manage. 2021;248:106779. doi:10.1016/j.agwat.2021.106779.
- Bacca A, Ceretta CA, Ferreira PAA, et al. Residual and immediate effect after 16 applications of organic sources on yield and nitrogen use efficiency in black oat and corn. Rev Brasil Ciência Solo. 2020;44:1–15. doi:10.36783/18069657rbcs20190013.
- Comin JJ, Ferreira LB, Santos LH, et al. Carbon and nitrogen contents and aggregation index of soil cultivated with onion for seven years using crop successions and rotations. Soil Tillage Res. 2018;184:195–202. doi:10.1016/j.still.2018.08.002.
- Loss A, Pereira MG, Perin A, et al. Particulate organic matter in soil under different management systems in the Brazilian Cerrado. Soil Res. 2012;50(8): 685–693.doi.org/10.1071/SR12196
- Rodrigues LAT, Giacomini SJ, Aita C, et al. Short-and long-term effects of animal manures and mineral fertilizer on carbon stocks in subtropical soil under no-tillage. Geoderma. 2021;386:e114913. doi:10.1016/j.geoderma.2020.114913.
- Dortzbach D, Araujo IS, Pandolfo CM, et al. Carbono e nitrogênio no solo e na biomassa microbiana em glebas com diferentes usos e períodos de aplicação de dejetos líquidos de suínos. Rev Agropecuária Catarinense. 2013;26(2):69–73.
- Pellegrin MABP, Muraro DS, Basso CJ, et al. Estratégias de manejo do dejeto líquido de suínos associado com inibidores de nitrificação na produção de massa seca do trigo. XI Reunião Sul-Brasileira de Ciência do Solo. Frederico Westphalen, RS, 2016.
- Assis CP, Jucksch I, Mendonça ES, et al. Carbon and nitrogen in aggregates of an Oxisol submitted to different use and management systems. Pesq Agropec Bras. 2006;41(10):1541–1550. doi:10.1590/S0100-204X2006001000012.
- Six J, Paustian K, Elliott ET, et al. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate associated carbon. Soil Sci Soc Am J. 2000;64(2):681–689. doi: 10.2136/sssaj2000.642681x.
- Costa Júnior C, Píccolo MDC, Siqueira Neto M, et al. Carbono em agregados do solo sob vegetação nativa, pastagem e sistemas agrícolas no bioma Cerrado. Rev Bras Ciênc Solo. 2012;36(4):1311–1322. doi:10.1590/S0100-06832012000400025.
- Loss A, Pereira MG, Giácomo SG, et al. Agregação, carbono e nitrogênio em agregados do solo sob plantio direto com integração lavoura-pecuária. Pesq Agropec Bras. 2011;46(10):1269–1276. doi:10.1590/S0100-204X2011001000022.
- Du Z, L.; Ren T-S, Hu C-S, Zhang, et al. Soil aggregate stability and aggregate-associated carbon under different tillage systems in the north China plain. J Integr Agric. 2013;12(11):2114–2123. doi:10.1016/S2095-3119(13)60428-1.
- Angers DA, Chantigny MH, Macdonald JD, et al. Differential retention of carbon, nitrogen and phosphorus in grassland soil profiles with long-term manure application. Nutr Cycl Agroecosyst. 2010;86(2):225–229. doi:10.1007/s10705-009-9286-3.
- Aguilera E, Lassaletta L, Gattinger A, et al. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: a meta-analysis. Agric Ecosyst Environ. 2013;168:25–36. doi:10.1016/j.agee.2013.02.003.
- Smith BN, Epstein S. Two categories of 13C/12C ratios for higher plants. Plant Physiol. 1971;47(3):380–384. doi:10.1104/pp.47.3.380.
- Guareschi RF, Pereira MG, Perin A. Deposição de resíduos vegetais, matéria orgânica leve, estoques de carbono e nitrogênio e fósforo remanescente sob diferentes sistemas de manejo no cerrado goiano. Rev Bras Ciênc Solo. 2012;36(3):909–920. doi:10.1590/S0100-06832012000300021.
- Högberg P. 15N natural abundance in soil-plant systems. New Phytol. 1997;137(2):179–203. doi:10.1046/j.1469-8137.1997.00808.x.
- Costa Júnior C, Piccolo MDC, De Camargo PB, et al. Nitrogênio e abundância natural de 15N em agregados do solo no bioma Cerrado. Rev Educ. 2011;15(2):47–66.
- Chalk PM, Inácio CT, A MT. From fertilizer to food: tracing nitrogen dynamics in conventional and organic farming systems using 15N natural abundance. In: Heng LK, Sakadevan K, Dercon G, Nguyen ML. editors. Proceedings – International Symposium on Managing Soils for Food Security and Climate Change Adaptation and Mitigation. Rome, Food and Agriculture Organization of United Nations; 2014. p. 339–349.
- Inácio CT. Uso da Abundância Natural de 15N em Estudos com Fertilizantes Orgânicos Tese de doutorado. Universidade Federal Rural do Rio de Janeiro. 2015.
- Guareschi RF, Pereira MG, Perin A. Carbono, nitrogênio e abundância natura de δ13C e δ15N em uma cromossequência de agricultura sob plantio direto no cerrado goiano. Rev Bras Ciênc Solo. 2014;38(4):1135–1142. doi:10.1590/S0100-06832014000400009.
- Pegoraro RF, Silva IRD, Novais RFD, et al. Abundância natural de 15N e formas de nitrogênio em Argissolo cultivado com eucalipto e acácia. Ci Fl. 2016;26(1): 295–305. doi: 10.5902/1980509821121.
