Abstract
Soil amendments are a broad class of materials that enhance physical, chemical or biological characteristics in croplands, pastures, or rangelands. While organic soil amendments such as manure, mulch and seaweed have well established agronomic benefits, there has been renewed private and governmental interest in quantifying and incentivizing their role in mitigating climate change. Likewise, biostimulants and biopesticides, which are intended to target specific plant or microbial processes, are emerging with claims of improved soil health, crop yields, soil organic carbon sequestration, and greenhouse gas emission reductions. We conducted a literature review to address the climate mitigation potential of organic soil amendments, including biostimulants and biopesticides. In doing so, we identify three elements of climate mitigation through the use of soil amendments: soil organic carbon sequestration, soil greenhouse gas emission reductions, and life cycle emission reductions. We review common soil amendment classes in detail, addressing the empirical evidence (or lack thereof) in which they meet these three elements of climate mitigation. We conclude by suggesting priorities for government and private investment.
Introduction
Agricultural soils globally are highly degraded, amounting to a global soil organic carbon (SOC) debt of 116 Pg C [Citation1]. SOC loss is both a response to and a contributor to climate change, and there is increasing interest in regenerative practices to enhance SOC or slow SOC losses [Citation2, Citation3]. Applying soil amendments is one way to promote soil structure, soil organic matter (SOM), and soil fertility that has been depleted through planting, tillage, grazing and harvest operations. Over the past decade, there has been a growing interest in soil amendments for other environmental benefits–in particular, climate mitigation through SOC sequestration, or avoiding emissions of the three major biogenic greenhouse gases (Box 1). These benefits are highly dependent on geography, soil biology, and life cycle characteristics. This context dependency currently limits our ability to generalize the role of amendments for explicit climate mitigation benefits, or to include soil amendments in national greenhouse gas inventories.
Box 1 A common language for climate mitigation
Soil amendments are thought to enhance microbial habitat and increase SOM, the portion of soils composed of plant, animal, or microbial tissue in various stages of decay. Soil amendments can also slow erosion (as seen with mulch), increase aggregate stability (as seen with biosolids), or enhance soil fertility through addition of slow release fertilizers (such as manure). While these soil health and yield benefits of soil amendments are well understood [Citation4], the role of soil amendments in net SOC drawdown and storage is more elusive [Citation5]. For instance, compost amendments increase SOC (which comprises 58–60% of SOM) relative to unamended soils, but this does not confer a net climate benefit on its own. For compost to result in climate mitigation, it would need to: mineralize (decompose) more slowly when applied to soils than not applied to soils; slow decomposition of existing SOC within soil, increase fertility and plant growth so that the quantity or quality of SOC results in a net gain of C relative to losses; or result in avoided emissions off site, such as reduced inorganic fertilizer production. For a detailed explanation of the mechanisms of SOC storage and loss in soils, we refer to Box 2.
Box 2 Mechanisms of SOC storage and loss
Beyond SOC, the net climate benefit of soil amendments requires accounting for indirect greenhouse gas emissions due to fuel and electricity use throughout harvest, manufacturing, processing, transportation and application, known as life cycle emissions. Life cycle emissions are a major component of the net greenhouse gas balance of soil amendments, but these calculations depend on how system boundaries are defined, and whether accurate definitions of baseline (or “business as usual”) scenarios are used for comparison. While life cycle emissions are critical to net climate mitigation, they are also highly subjective and are not often reported in the soil amendment empirical literature, or in the major process-based biogeochemical models used to infer net climate mitigation. For example, COMET-Farm, a carbon and greenhouse gas emissions planning tool commonly used by the USDA, predicts SOC sequestration for adding mulch or compost to soil but it does not factor in life cycle emissions from transportation and application to soils (COMET-Farm clearly states this caveat as a footnote). Due to these limitations, soil amendments are not often included as eligible practices in greenhouse gas inventories or SOC markets. One notable exception is biochar, which has several active protocols and online marketplaces (e.g. Verra, Puro.Earth, Climate Action Reserve and Carbonfuture) for buying and selling biochar carbon credits as of December 2021.
Within the voluntary SOC market space, quantifying net climate mitigation is particularly challenging [Citation3]. Compared to forest carbon markets, which rely on standing woody biomass as the verifiable metric for “permanent” carbon storage, carbon stocks in agricultural soils are highly dynamic [Citation6]. On the measurement side, high microsite variation may require sampling at a high density (at least 25 samples per hectare) in order to detect realistic increases in SOC stocks [Citation2, Citation3]. As a result, voluntary SOC markets are shifting to process-based models in lieu of sampling, or hybrid approaches involving a combination of modeling and sampling. These models are currently calibrated for eligible practices such as cover crop adoption and no-till agriculture, but do not yet include soil amendments, either because there is a lack of data on soil emissions or SOC sequestration, or because the potential life cycle emissions might cancel out the climate benefit of adding the amendment to soil. For the time being, accounting for climate mitigation from soil amendments relies on cobbling together multiple data sources, including direct measurement of SOC stocks over time, calculated, modeled or measured emissions of carbon dioxide (CO2), nitrous oxide (N2O) and methane (CH4) from soils, and the use of life cycle analysis (LCA) tools borrowed from the bioenergy sector.
In addition to quantification challenges, inconsistent terminology presents a communication barrier to mitigating climate change [Citation7–9]. To use bioenergy as an example, a “carbon neutral” label was given to the burning of woody biomass under the 1997 Kyoto Protocol, and then legacied into the Paris Climate Agreement, but has been widely characterized as doing more harm than good. In the UK and EU, biomass loopholes continue to promote deforestation for wood pellet combustion under the carbon neutral label, at the expense of habitat and biodiversity loss [Citation10]. As recently as 2018, an EPA administrator announced that the same “carbon neutral” loophole for biomass combustion should be created in the US [Citation11]. This issue is perpetuated in biochar carbon markets today, because pyrolysis incurs upfront combustion emissions, while the avoided future emissions from future wood decomposition, or from a hypothetical alternative disposal pathway (e.g. open combustion in a slash pile) are counted as carbon credits. To reach consensus across sectors, it is critical to define the climate mitigation elements for soil amendments, identify how these pathways could be accurately measured or accounted for, and to chart a path forward to ensure that the use of soil amendments will benefit rather than harm the climate.
Here we review the applicability of soil amendments and biostimulants for climate mitigation. The purpose of this review is to (1) define the main elements of climate mitigation from soil amendments, (2) provide historical and current context on soil amendments, including classic soil amendments (e.g. manure and mulch), as well as biostimulants and biopesticides (e.g. microbial inoculants), and (3) address scalability and feasibility considerations to guide evaluation of the practicality of soil amendments in today’s economy. While a number of synthetic (plastic and rubber mulches) and non-organic soil amendments (ground rock dust or clays) have been proposed to mitigate erosion [Citation12], directly drawdown CO2 via mineral weathering reactions [Citation13], or to preserve SOC through adsorption [Citation14], we constrain this review to organic soil amendments due to environmental toxicity concerns (plastics) or lack of available literature (enhanced mineral weathering or rock dust). While there are multiple natural and synthetic greenhouse gases that contribute to climate change, we also constrain our scope to the three major biogenic greenhouse gases (N2O, CH4, CO2) that are most relevant to soil amendments. We expect that this review can be used by various stakeholders including industry, investors, academics, and policy makers to improve the collective understanding of soil amendments.
Section 1: Elements of climate mitigation, and how they are quantified
There are a number of proposed mechanisms and terms used within industry to describe how soil amendments could cool the planet; including “carbon stabilization”, “carbon capture”, “carbon drawdown” and “carbon dioxide removal”. This terminology can misrepresent the real meaning of climate mitigation: reducing emissions and removing greenhouse gases from the atmosphere [Citation7]. To provide clarity we conducted a literature review and identified three mechanisms for climate mitigation across all soil amendments, given the available evidence and quantification methods. Three major elements of climate mitigation emerged: 1) reduced soil greenhouse gas emissions (e.g. woodchips reduce soil N2O emissions through microbial N immobilization), 2) SOC sequestration (e.g. dissolved organic C from manure leaches deep in the soil profile and evades decomposition), and 3) reduced life cycle emissions (e.g. the use of an organic N amendment results in avoided N2O production ). This quantification framework does not include initial “carbon capture” by plants or photosynthetic microorganisms as a valid climate mitigation method, since this recently fixed biogenic carbon resides in short-lived pools and would already be accounted for as part of the SOC stock quantification.
Figure 1. The three elements that should be included in accounting frameworks for evaluating the role of soil amendments in climate mitigation. Reduced soil emissions should be quantified using direct measurement or models calibrated for the soil type. SOC stock changes are a major purported element for climate mitigation through the use of soil amendments and should be quantified when SOC gains are being claimed. Finally, full life cycle emissions should always be considered to give confidence that climate mitigation benefits are real. This figure style was adapted from EPA 2011 [Citation21].
![Figure 1. The three elements that should be included in accounting frameworks for evaluating the role of soil amendments in climate mitigation. Reduced soil emissions should be quantified using direct measurement or models calibrated for the soil type. SOC stock changes are a major purported element for climate mitigation through the use of soil amendments and should be quantified when SOC gains are being claimed. Finally, full life cycle emissions should always be considered to give confidence that climate mitigation benefits are real. This figure style was adapted from EPA 2011 [Citation21].](/cms/asset/113bef17-baab-4af0-9ae3-bc591ebc0949/tcmt_a_2217785_f0001_c.jpg)
Reducing soil greenhouse gas emissions
Measuring the net exchange of the major biogenic greenhouse gas emissions (namely CO2, CH4, and N2O) between the soil and atmosphere in amended and unamended control field plots or mesocosms is the most rigorous method for quantifying climate mitigation, but requires expensive equipment and high user expertise [Citation22]. Some carbon amendments, including biochar, are capable of slowing SOM decomposition, inhibiting methanogenesis (anaerobic respiration that generates CH4), increasing methanotrophy (oxidative consumption of CH4 as an energy source), or promoting complete denitrification (the two step reduction of nitrate to dinitrogen gas, which has no global warming potential), all of which can suppress the net flux of soil GHGs to the atmosphere from soils. These three gases can be measured through a combination of static flux chamber or eddy covariance methods and expressed as CO2e. Regular measurements over multiple seasons and years can provide an accurate picture of net climate mitigation for a given project. These high quality datasets are also used to inform models and emissions factors (calculations) when direct flux measurements are not practical or feasible for accounting projects.
Soil organic carbon sequestration
SOC sequestration is the net change in SOC stocks in response to amendment application, after accounting for the new C that was contained within the amendment. Accounting for C in the original amendment is essential for determining whether the SOC increase over time is additional, and whether positive or negative priming effects are occurring (the activation or suppression of decomposers in response to amendment addition [Citation23, Citation24].
In practice, SOC sequestration is referred to as an increase in SOC stocks after an intervention (C concentration × soil bulk density at end of intervention - C concentration × soil bulk density at beginning of intervention - total C that was added through amendment application) or as the difference between treatment and control plots after a number of years. Sequestration rates typically peak 5–10 years after a management intervention before slowing to zero as SOC reaches a steady-state in 20–50 years [Citation25]. However, for C-based additions, as long as the amendment is continually supplied, there can be sustained increases in SOC for over a century [Citation26]. Since SOC sequestration is expressed in metric tons per hectare to a fixed depth (typically 30 cm), several pieces of information are needed, including C concentration, bulk density and stone content, to calculate stocks ideally on an equivalent mass basis to remove bias from soil compaction or aeration [Citation27, Citation28].
Instead of evaluating C stocks over time, using a “static” baseline scenario (SOC concentration at time = 0), a dynamic baseline scenario could be used [Citation29]. This approach is favored by most SOC accounting protocols because it accounts for C preservation when the landscape is actively losing C due to climate or weather. For instance, if wood mulch protects soils from erosion but does not result in a net gain of SOC over time, the avoided loss of SOC can still be counted as SOC sequestration.
While direct SOC measurements are one of the most robust means to measure climate mitigation, this index notably does not include nitrogen losses (N2O emissions in particular). For this reason, quantifying SOC sequestration from amendment application alone is inadequate without complementary modeling or calculation of N2O losses [Citation30], and life cycle emissions from harvest, transport and application.
Reduced life cycle emissions
Life cycle emissions are the “cradle to grave” impact of a product, including each stage of a product’s production and use. Life cycle emissions calculations can be subjective, depending on how system boundaries are defined and which “business as usual” scenarios are chosen as baseline scenarios, yet are critical for determining the net atmospheric benefit of a practice. Life cycle emissions are the least well-established climate mitigation element in the literature, but underlie the logic of climate mitigation potential for many soil amendments including biochar and compost. For biochar, the theory goes that pyrolysis stabilizes carbon that would have ordinarily been lost to the atmosphere through decomposition or combustion at climate relevant timescales (<100 years). The offset value is determined through the measured carbon content in biochar, with an uncertainty adjustment applied based on the biochar C composition (e.g. H:C or O:C ratios). For compost, the premise is that food waste or manure would have otherwise been left to decompose anaerobically, and anaerobic decomposition produces more potent greenhouse gases (CH4, and N2O) than aerobic decomposition (which primarily produces CO2). This climate offset is typically determined from models rather than measurements (e.g. the GREET model [Citation31]) to estimate avoided CH4 from anaerobic decomposition in landfills.
While this makes sense in theory, applying LCA’s is highly subjective, starting with the initial branding of all biomass feedstocks, including agricultural and agroforestry residues, as “waste” products. The US EPA defines waste products broadly as “any garbage or refuse, sludge from a wastewater treatment plant, water supply treatment plant, or air pollution control facility and other discarded material, resulting from industrial, commercial, mining, and agricultural operations, and from community activities” [Citation32]. Waste products can either be assumed to be “burden free” (it carries zero baseline emissions), or to carry an emissions burden (e.g. an all-combustion or all-decomposition baseline), which impacts the net climate benefit determination. Are agricultural residues, such as corn stalks, a waste product destined to be hauled off site and combusted, or a valuable component of the agro-ecosystem that should be left to decompose and recharge SOC [Citation33]? If soil amendments are to be used for the purpose of climate mitigation, it is clear that carbon markets will need to syncronize their definition with EPA, and that EPA’s original definition may require additional refinement [Citation34].
The subjectivity in defining baseline emissions is apparent in biochar LCA’s, which often assume that feedstocks (typically wood waste) would have been immediately combusted in a high oxygen environment if not for the biochar project. Even if this assumption were true, biochar production still incurs an upfront emissions cost through pyrolysis (superheating under low O2 conditions), which releases 50% or more of the original biomass carbon contained in the wood, and pyrolysis emissions are not often quantified. In contrast, allowing the wood to decompose naturally would take 25–50+ years [Citation35]. Finally, even if we accept that woody biomass is a waste product, there are other options for disposing this waste other than full combustion, such as, mulching and appying the mulch to soil.
Another common life cycle mechanism that is sometimes claimed through the use of biostimulants is indirect reductions in fossil energy usage. For example, replacing inorganic fertilizers with nitrogen fixing organisms can decrease fertilizer requirements up to 30% without compromising yield [Citation36]. Avoided energy use, and its equivalent CO2 emissions, can be calculated as the difference in manufacturing emissions between an all-inorganic fertilizer scenario compared to a scenario with partial or full replacement with biological N fixation, as well as any savings in fuel usage through the use of a microbial seed coat in lieu of spraying fertilizers. In addition, energy usage for producing the microbial seed coat in terms of CO2e emissions will need to be deducted from the net climate benefit. We define energy broadly here, including renewable and nonrenewable energy, in recognition that as energy sources are replaced with non-combustion alternatives (e.g. wind and solar), baseline emissions would need to be lowered accordingly.
In light of these quantification challenges, soil amendments should be carefully evaluated for all three mitigation elements at the same time, including soil greenhouse gas fluxes, SOC sequestration, and life cycle emissions. Due to the high subjectivity involved in life cycle analysis, LCA should not be relied on alone when inferring a net climate benefit. Furthermore, direct measurements of soil greenhouse gas emissions or SOC sequestration should be well established, either at the project level or in similar studies across the literature, before considering the more uncertain elements of avoided decomposition/combustion or avoided energy use. Because models are not yet calibrated for soil amendments, any C offsets derived from soil amendments should utilize a primarily measurement-based approach but can be modified as knowledge and tools progress (Box 3).
Box 3 State of modeling alternatives for soil amendments
Section 2: Summary and qualitative assessment of soil amendments
We reviewed the historical and current use of soil amendments, their agronomic benefits, and the current science on their technical potential to mitigate climate change, which can be found as supplemental information in Appendix 1: Soil Amendment Guide. In this literature review we comment on the historical uses of soil amendments, how they might mitigate climate change, under which circumstances they are most effective, and provide justification for the technical potential. reflects a high-level summary of this information, including their classification, examples, mitigation elements involved for each amendment, and a qualitative assessment of magnitude of the climate mitigation effect and confidence in the magnitude of effect.
Table 1. Summary of commonly used soil amendments as well as newer soil amendments that are proliferating in agribusiness for the purpose climate mitigation.
To provide a magnitude and confidence assessment for each soil amendment, we conducted a literature search using Web of Science Title and Abstract search using a set of search terms that best characterize the three major elements of climate mitigation for soil amendments. Because the scope of this topic was too broad for a systematic review of all literature on soil amendments, we provide a bird’s-eye view on the different approaches addressed in the literature for climate mitigation, and identify key data gaps.
The most well-characterized soil amendment for climate mitigation is manure, followed by compost and biochar (). While there is high confidence that regular manure additions increase SOC levels [Citation39], there is also strong evidence that emissions of other greenhouse gases (CH4 and N2O) increase [Citation40, Citation41]. Composting manure and other waste streams generally mitigates against increased GHG emissions while maintaining a SOC sequestration benefit [Citation42]; however, the largest climate mitigation benefit from compost is derived from the potential alternative fate of the waste residue (anaerobic decomposition in a landfill) [Citation43] which requires a full life cycle analysis.
Figure 2. Web of Science Title and Abstract results displaying the state of knowledge of common soil amendments as they pertain to the three elements of climate mitigation (soil emissions, SOC sequestration, and life cycle analysis). This figure indicates that manure is the most well-studied soil amendment for climate mitigation, whereas less is known about biostimulants. Within each category, life cycle analyses are severely lacking. The Web of Science search was conducted on December 20, 2021, and a table with the specific search terms is provided in the supplementary material.
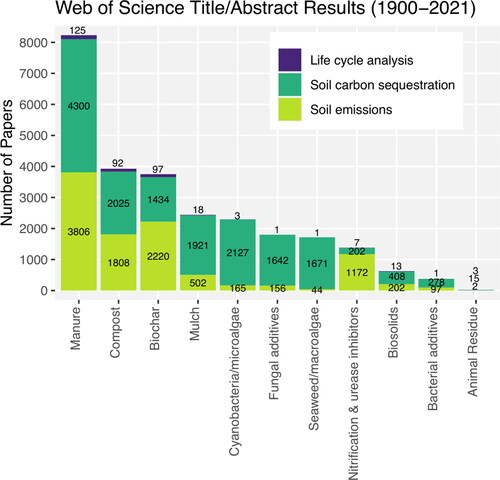
Biostimulants are far less characterized for climate mitigation and as such have received low confidence scores in . Our review suggests that cyanobacteria, when applied to systems favorable to their growth and persistence (e.g. rice farms), have potential for increased C sequestration, reduced N2O emissions, and reduced life cycle emissions but field evidence is still lacking. Fungal additives, rhizobacteria, and seaweeds all received a ± rating due to lack of evidence of climate benefits, and in the case of fungal additives, lack of evidence that the amendments remain viable when applied to soil. Seaweeds and seaweed extracts should theoretically operate much like other organic amendments in stimulating plant productivity and thus indirectly increasing SOC stocks, but field studies are still lacking. There is evidence that nitrification inhibitors decrease N2O emissions [Citation44] but there may be some unintended changes to the extant soil bacterial diversity [Citation45, Citation46] which warrants further study. For most additives, there is a non-insignificant amount of life cycle emissions associated with the production, transport, and application of the product but we were not able to find any published life cycle analyses ranging from production to soil application for any of these products.
Section 3: Scalability and economic feasibility considerations
Regardless of an amendment’s technical potential for SOC or soil health, a number of factors can reduce the achievable potential of an amendment intervention. Scalability is determined by the quantity of available feedstocks, the area of eligible croplands or rangelands to apply the amendment to, the economic feasibility of introducing the amendment at large enough geographic scales to contribute to climate change mitigation, the cost compared to existing alternatives, the cost of measurement, the societal acceptability of the amendment and the assurance that practices are being followed as intended by farmers or practitioners.
The main scalability concerns for most organic amendments are limited feedstock quantity or limited land by which to apply the amendment to. Some soil amendments can be applied to all croplands whereas other soil amendments such as raw manure and biosolids can only be applied to a portion of these croplands. In the US, 290 million tons of human feces are produced per year, but only 4.7 million tons of biosolids are produced from this waste [Citation47]. Yet production of biosolids still exceeds the available land to apply it to due to federal and state restrictions to limit human health impacts from contaminant exposure. Other amendments such as seaweed have the opposite issue: they cannot be produced in large enough quantities to meet the amount of available lands to apply it to.
Another issue is the economic feasibility of introducing the amendment at a large enough scale to mitigate climate change. The American Carbon Registry and Environmental Defense Fund co-developed a methodology for quantifying carbon sequestration following amendment application of manure and vegetable compost, but the methodology has yet to be adopted for a compliance offset framework due to economic infeasibility. The California Compliance Taskforce [Citation48] ruled that the breakeven cost would require payments of over $900 per metric ton of applied compost (assuming a 0.25 inch application rate, transportation distance between 28 miles and 84 miles, and compost cost of $34 per metric ton). No matter the benefits of soil amendment for carbon sequestration, the low price that carbon offsets currently command on the voluntary market hampers the ability for manure and compost-based amendments to compete with other types of offsets.
Soil amendments are also expensive, so there often isn’t a strong business case that the agronomic benefit justifies the purchase (Box 4). While government incentive programs such as the Environmental Quality Incentives Program and Conservation Stewardship Program can help de-risk these investments for farmers, they are only available for amendments with proven environmental benefits and these programs have limited funds. If the cost of doing business-as-usual practices changes, markets may shift in favor of soil amendments. For example, chemical fertilizers have historically been cheaper than manure, but fertilizer shortages during COVID-19 pandemic increased the cost of chemical fertilizers, leading to increased demand for manure [Citation49]. Historically, manure was only shipped within a few miles of its source, but manure hauling and brokerage businesses offer a new means to move manure around. Thus, economic feasibility for substituting an improved amendment for baseline conditions could be achieved by increasing the cost of the business-as-usual method (inorganic fertilizers) through a carbon tax, or reducing the cost of the alternative (manure).
Box 4 Economic scalability case study: biochar
Another consideration is farmer perspectives on soil health practices. Approximately 40% of farmlands in the US are rented, and these farmers may not have strong incentives to make long-term investments in soil health or SOC. Adoption rates of soil health practices varies widely by region [Citation50], and it is unknown whether carbon credits will be sufficient to overcome barriers to adoption. Continued investment in social and technical assistance could prove to be a useful companionate effort along with financial assistance for soil health practices.
Ways forward
Despite recent policy interest in climate mitigation, and a continuously growing body of research on soil amendments, progress has been slow to incorporate amendments in incentive programs and carbon markets. For some amendments such as biochar and compost, the technical potential to mitigate climate change is well established, but adoption cost and labor greatly limit the scalability of the intervention. For other amendments, such as biofertilizers, the technical potential to mitigate climate change is not well understood and there are ongoing concerns that both agronomic and climate benefits are being overstated by these nascent industries [Citation51].
The US government has offered incentives for conservation practices since 1985, with more funding provided through the Environmental Quality Incentives Program, via the Infrastructure Investment and Jobs Act (Public Law 117 − 58). In addition to these efforts, the government can build a compliance market to ignite these markets. In Canada, a heavy carbon tax for emitters, increasing to $170 per ton of CO2e by 2030 (Greenhouse Gas Pollution Pricing Act of 2018) has created a high demand for carbon offsets. In the US, the Inflation Reduction Act (Public Law 117–169) will continue to provide soil conservation incentives through USDA NRCS voluntary programs, as well as $300 Million towards measuring and monitoring of climate benefits, but carbon taxes have not gained much traction. Continued investment in private-public partnerships may help to overcome scalability considerations and get us closer to achieving the potential climate benefits of soil amendments.
Several key research priorities emerged from this review. A major priority is to refine our mechanistic understanding of N2O production and how this is influenced by soil amendments. In the case of compost management, the ratio of N2 to N2O produced is highly dependent on management (timing of compost turning) and environmental conditions (wet-dry cycles). In other cases, physical properties of the soil amendment may directly suppress N2O under a range of conditions. For example, biochar appears to facilitate the last step of denitrification, by shuttling electrons to denitrifiers to reduce the ratio of N2O to N2+N2O produced [Citation52]. In this example, C can be added without the added emissions cost, since N2 has zero global warming potential. This mechanism, along with biological nitrification inhibition (e.g. through plant breeding [Citation53]) stand out as high research priorities that are relatively underexplored.
In addition to the need for mechanistic studies, this review revealed that a large portion of empirical studies that are published within the topical research area of climate change would not meet minimum criteria to be used in modeling or decision support tools for climate change accounting purposes. For biochar for instance, direct measurements of soil emissions of CO2, CH4, and N2O are well documented in field and laboratory studies [Citation54–57]. However, a closer look reveals that very few empirical studies measured CO2, CH4, and N2O at the same time, which is needed to report CO2e. Measuring all three of the major greenhouse gases together will help to build out the knowledge base that is needed so that model estimates can more accurately reflect reality, under a wide range of geographical contexts and conditions.
For emerging biostimulants, improving transparency of branding and marketing claims would help to lend credibility to products that are indeed effective for climate mitigation. One study [Citation58] demonstrated that 85% of commercial AMF products did not contain viable propagules. They suggest clear guidelines for labeling and reporting the number of viable propagules and greenhouse pilot studies demonstrating successful colonization of plant roots for each inoculant product. Implementable quality standards for rhizosphere bacteria, fungal endophytes, and AMF would increase confidence in products. If claims of climate mitigation are also being made, products should be accompanied with transparent data, drawn from lab, mesocosm and field trials clearly demonstrating SOC accrual or avoided emissions over time using one or multiple quantifiable elements outlined in Section 1.
Lastly, SOC builds slowly but disappears quickly during disturbance and extreme weather events, referred to as “reversals” in carbon accounting. Soil amendments have a clear role in protecting crops from climate events such as dust storms, floods, droughts and heat waves [Citation59]. For instance, a biostimulant could contribute little in the way of immediate carbon gains, but could contribute significantly towards preserving existing SOC, or preserving crop yields that would have been lost due to drought and dust storms. Closing the yield gap (unforeseen losses in crop yields due to climate, pests or disease) and continued investment in SOC preservation against these large-scale “reversals” on working lands (e.g. the great European heat wave of 2003 [Citation60]), are important considerations for mitigating the impacts climate change.
Conclusions
We reviewed the available body of literature on soil amendments and climate mitigation and determined that very few soil amendments are backed with substantial evidence on the climate mitigation elements of reduced soil emissions, increased soil carbon stocks, or reduced life cycle emissions. Among this available literature, the soil amendments that are most likely to contribute to climate change mitigation are manure, compost and biochar, since they likely achieve multiple climate mitigation elements: increasing SOC sequestration, reducing greenhouse gas emissions, and avoiding future decomposition. Cyanobacteria also have high potential to contribute to SOC sequestration while avoiding fossil emissions from fertilizer production, but field data from these products remains limited. Continued work to increase the energy efficiency of algal growth systems will improve the sustainability and scalability of these systems. For other biostimulants, there is a near complete lack of peer-reviewed evidence for their climate mitigation potential. Priorities for government investment could include a carbon tax for the use of conventional approaches that emit greenhouse gases, financial incentives for the use of soil amendments that are known to reduce greenhouse gases, increased research support directed at documenting net climate mitigation and key mechanisms involved, and oversight to ensure rigorous quantification is at the heart of climate mitigation accounting.
Supplemental Material
Download MS Word (15.7 KB)Acknowledgments
This work was funded through a collaborative grant from Breakthrough Energy Ventures and through Environmental Defense Fund with awards from the Earth Fund, King Philanthropies, and Arcadia, a charitable fund of Lisbet Rausing and Peter Baldwin. This paper is a product of literature review and round-table discussions between Woodwell Climate Research Center, Breakthrough Energy Ventures, and Environmental Defense Fund, towards the goal of articulating elements of scientific rigor & integrity in soil carbon crediting frameworks and emerging biotechnologies. We thank Anders Claasens for insightful review of the fungal amendments section.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Sanderman J, Hengl T, Fiske GJ. Soil carbon debt of 12,000 years of human land use. Proc. Natl Acad Sci USA. 2017;114(36):9575–9580. doi: 10.1073/pnas.1706103114.
- Amelung W, Bossio D, de Vries W, et al. Towards a global-scale soil climate mitigation strategy. Nat Commun. 2020;11:5427.
- Oldfield E, Eagle A, Rubin RL, et al. Crediting agricultural soil carbon sequestration. Science. 2022;375(6586):1222–1225. doi: 10.1126/science.abl7991.
- Abbott LK, Macdonald LM, Wong MTF, et al. Potential roles of biological amendments for profitable grain production – a review. Agric Ecosys Environ. 2018;256:34–50. doi: 10.1016/j.agee.2017.12.021.
- Amundson R, Biardeau L. Opinion: soil carbon sequestration is an elusive climate mitigation tool. Proc Natl Acad Sci U S A. 2018;115(46):11652–11656. doi: 10.1073/pnas.1815901115.
- Dynarski KA, Bossio DA, Scow KM. Dynamic stability of soil carbon: reassessing the “permanence” of soil carbon sequestration. Front Environ Sci. 2020;8:514701. doi: 10.3389/fenvs.2020.514701.
- Watkins J, Durning B. Carbon definitions and typologies in environmental impact assessment: greenhouse gas confusion? Impact Ass Proj App. 2012;30:4.
- Hsu A, Hohne N, Kuramochi T, et al. A research roadmap for quantifying non-state and subnational climate mitigation action. Nat Clim Change. 2019;9(1):11–17. doi: 10.1038/s41558-018-0338-z.
- de Bruin WB, Rabinovich L, Weber K, et al. Public understanding of climate change terminology. Climate Change. 2021;167:37.
- Moomaw W. 2017. To curb climate change, we need to protect and expand US forests (https://theconversation.com/to-curb-climate-change-we-need-to-protect-and-expand-us-forests-76380).
- US EPA. EPA’s treatment of biogenic carbon dioxide (CO2) emissions from stationary sources that use Forest biomass for energy production. (Washington, DC: the United States Environmental Protection Agency); 2018.
- Sintim HY, Flury M. Is biodegradable plastic mulch the solution to agriculture’s plastic problem? Environ Sci Technol. 2017;51(3):1068–1069. doi: 10.1021/acs.est.6b06042.
- Goll DS, Ciais P, Amann T, et al. Potential CO2 removal from enhanced weathering by ecosystem responses to powdered rock. Nat Geosci. 2021;14(8):545–549. doi: 10.1038/s41561-021-00798-x.
- Ye L, Camps-Abserstain M, Shen Q, et al. Biochar effects on crop yields with and without fertilizer: a meta-analysis of field studies using separate controls. Soil Use Manage. 2020;36(1):2–18. doi: 10.1111/sum.12546.
- Forster PT, Storelvmo K, Armour W, et al. The earth’s energy budget, climate feedbacks, and climate sensitivity. In Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 923–1054; 2021. doi: 10.1017/9781009157896.009.
- EPA.gov. Greenhouse gas equivalancies calculator. April 2022. https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator.
- Keiluweit M, Bougoure JJ, Nico PS, et al. Mineral protection of soil carbon counteracted by root exudates. Nat Clim Change. 2015;5(6):588–595. doi: 10.1038/nclimate2580.
- Finley BK, Dijkstra P, Rasmussen C, et al. Soil mineral assemblage and substrate quality effects on microbial priming. Geoderma. 2018;322:38–47. doi: 10.1016/j.geoderma.2018.01.039.
- Bailey VL, Hicks Pries C, Lajtha K. What do we know about soil carbon destabilization? Environ Res Lett. 2019;14(8):083004. doi: 10.1088/1748-9326/ab2c11.
- Dijkstra FA, Carrillo Y, Pendall E, et al. Rhizosphere priming: a nutrient perspective. Front Microbiol. 2013;4:1–8. doi: 10.3389/fmicb.2013.00216.
- EPA. Accounting framework for biogenic CO2 emissions from stationary sources. Office of atmospheric programs. 2011. (https://yosemite.epa.gov/sab/sabproduct.nsf/0/2F9B572C712AC52E8525783100704886/$File/Biogenic_CO2_Accounting_Framework_Report_LATEST.pdf).
- Novick K, Metzger S, Anderegg WRL, et al. Informing nature-based climate solutions for the United States with the best-available science. Glob Chang Biol. 2022;28(12):3778–3794. doi: 10.1111/gcb.16156.
- Kuzyakov Y, Domanski G. Carbon input by plants into the soil. Rev. J Plant Nutr Soil Sci. 2000;163(4):421–431. doi: 10.1002/1522-2624(200008)163:4<421::AID-JPLN421>3.0.CO;2-R.
- Jackson O, Quilliam RS, Stott A, et al. Rhizosphere carbon supply accelerates soil organic matter decomposition in the presence of fresh organic substrates. Plant Soil. 2019;440(1-2):473–490. doi: 10.1007/s11104-019-04072-3.
- West TO, Marland G, King AQ, et al. Carbon management response curves: estimates of temporal soil carbon dynamics. Environ Manage. 2004;33(4):507–518. doi: 10.1007/s00267-003-9108-3.
- Jenkinson DS, Johnston AE. 1977 Soil organic matter in the hoosfield continuous barley experiment. Rothamsted Experimental Station Report for. 1976;Part 2:87–101.
- Gifford RM, Roderick ML. Soil carbon stocks and bulk density: spatial or cumulative mass coordinates as a basis of expression? Glob Change Biol. 2003;9(11):1507–1514. doi: 10.1046/j.1365-2486.2003.00677.x.
- von Haden AC, Yang WH, DeLucia EH. Soil’s dirty little secret: depth based comparisons can be inadequate for quantifying changes in soil organic carbon and other mineral soil properties. Glob Chang Biol. 2020;26(7):3759–3770. doi: 10.1111/gcb.15124.
- Sanderman J, Baldock JA. Accounting for soil carbon sequestration in national inventories: a soil scientist’s perspective. Environ Res Lett. 2010;5(3):034003. doi: 10.1088/1748-9326/5/3/034003.
- van Groenigen JW, van Kessel C, Hungate BA, et al. Sequestering soil organic carbon: a nitrogen dilemma. Environ Sci Technol. 2017;51(9):4738–4739. doi: 10.1021/acs.est.7b01427.
- California Air Resources Board. 2019. CA-GREET3.0 model and tier 1 simplified carbon intensity calculators. https://ww2.arb.ca.gov/resources/documents/lcfs-life-cycle-analysis-models-and-documentation. Accessed Jan 20, 2022
- RCRA. 1976. Criteria for the definition of solid waste and solid and hazardous waste exclusions. https://www.epa.gov/hw/criteria-definition-solid-waste-and-solid-and-hazardous-waste-exclusions. Accessed 7/22/2022.
- Li Y, Li Z, Chang SX, et al. Residue retention promotes soil carbon accumulation in minimum tillage systems: implications for conservation agriculture. Sci Total Environ. 2020;740:140147. doi: 10.1016/j.scitotenv.2020.140147.
- Titus BD, Brown K, Helmisaari HS, et al. Sustainable Forest biomass: a review of currency residue harvesting guidelines. Energ Sustain Soc. 2021;11(1):10. doi: 10.1186/s13705-021-00281-w.
- Woolf D, Lehmann J, Ogle S, et al. Greenhouse gas inventory model for biochar additions to soil. Environ Sci Technol. 2021;55(21):14795–14805. doi: 10.1021/acs.est.1c02425.
- Adesemoye AO, Torbert HA, Kloepper JW. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb Ecol. 2009;58(4):921–929. doi: 10.1007/s00248-009-9531-y.
- Mondini C, Cayuela MC, Sinicco T. Soil C potential of exogenous organic matter at regional level under climate change simulated by RothC model modified for amended soils. Front Environ Sci. 2018;29:1–17.
- Levavasseur F, Mary B, Christensen B, et al. The simple AMG model accurately simulates organic carbon storage in soils after repeated application of exogenous soil organic matter. Nutr Cycl Agroecosyst. 2020;117(2):1–15.
- Liu E, Yan C, Mei X, et al. Long-term effect of manure and fertilizer on soil organic carbon pools in dryland farming in northwest China. PLoS One. 2013;8(2):e56536. doi: 10.1371/journal.pone.0056536.
- Jeong ST, Rae Cho S, Lee JG, et al. Composting and compost application: trade-off between greenhouse gas emission and soil carbon sequestration in whole rice cropping system. J Clean Prod. 2019;212:1132–1142. doi: 10.1016/j.jclepro.2018.12.011.
- Zhou M, Zhu B, Wang S, et al. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: a global meta-analysis. Glob Change Biol. 2017;23(10):4068–4083. doi: 10.1111/gcb.13648.
- Tiefenbacher A, Sandén T, Haslmayr H-P, et al. Optimizing carbon sequestration in croplands: a synthesis. Agronomy. 2021;11(5):882. doi: 10.3390/agronomy11050882.
- DeLonge MS, Ryals R, Silver WL. A lifecycle model to evaluate carbon sequestration potential and greenhouse gas dynamics of managed grasslands. Ecosystems. 2013;16(6):962–979. doi: 10.1007/s10021-013-9660-5.
- Qiao C, Liu L, Hu S, et al. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob Change Biol. 2015;21(3):1249–1257. doi: 10.1111/gcb.12802.
- Deng S, Wipf HML, Pierroz G, et al. Microbial soil amendment dynamically alters the strawberry root bacterial microbiome. Sci Rep. 2019;9(1):17677. doi: 10.1038/s41598-019-53623-2.
- Corrochano-Monsalve M, Gonzalez-Murua C, Estavillo J, et al. Impact of dimethypyrazole-based nitrification inhibitors on soil-borne bacteria. Sci Total Environ. 2021;792:1–12.
- EPA. Biosolids Technology Factsheet. Land Application of Biosolids. EPA Office of Water. EPA. 2000. 832-F-00-064.
- CABR working group. Compliance Offsets Protocol Task Force Draft Final Recommendations. February 8, 2021 https://ww2.arb.ca.gov/sites/default/files/2021-02/offsets_task_force_draft_final_report_020821.pdf.
- Fertilizer costs make manure look better. Ohio State University, 2021. Accessed 6/07/23 from: https://wayne.osu.edu/news/fertilizer-costs-make-manure-look-better.
- Wade T, Classen R, Wallander S. Conservation-Practice adoption rates vary widely by crop and region. 2015. Washington, DC: Economic Research Service.
- Quilty JR, Cattle SR. Use and understanding of organic amendments in Australian agriculture: a review. Soil Res. 2011;49(1):1–26. doi: 10.1071/SR10059.
- Cayuela ML, Sanchez-Monedero MA, Roig A, et al. Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Sci Rep. 2013;3:1732. doi: 10.1038/srep01732.
- Jochum MD, McWilliams KL, Pierson EA, et al. Host mediated microbiome engineering (HMME of drought tolerance in the rhizosphere). PLoS One. 2019;14(12):e0225933. doi: 10.1371/journal.pone.0225933.
- Feng Y, Xu Y, Yu Y, et al. Mechanisms of biochar decreasing methane emission from chinese paddy soils. Soil Biol Biochem. 2012;46:80–88. doi: 10.1016/j.soilbio.2011.11.016.
- Jeffery S, Verheijen FGA, Kamman C, et al. Biochar effects on methane emissions from soils: a meta-analysis. Soil Biol Biochem. 2016;101:251–258. doi: 10.1016/j.soilbio.2016.07.021.
- Liu Q, Zhang Y, Liu B, et al. How does biochar influence soil N cycle? A meta-analysis. Plant Soil. 2018;426(1-2):211–225. doi: 10.1007/s11104-018-3619-4.
- Verhoeven E, Pereira E, Decock C, et al. Toward a better assessment of biochar-nitrous oxide mitigation potential at the field scale. J Environ Qual. 2017;46(2):237–246. doi: 10.2134/jeq2016.10.0396.
- Salomon MJ, Demarmels R, Watts-Williams SJ, et al. Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl Soil Ecol. 2022;169:104225. doi: 10.1016/j.apsoil.2021.104225.
- Navarro-Pedreno J, Almendro-Candel MB, Zorpas AA. The increase of soil organic matter reduces global warming, myth or reality? Science 2021;3(1):18. doi: 10.3390/sci3010018.
- Ciais P, Reichstein M, Viovy N, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437(7058):529–533. doi: 10.1038/nature03972.
- Franke-Whittle IH, Insam H. Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: a review. Crit Rev Microbiol. 2013;39(2):139–151. doi: 10.3109/1040841X.2012.694410.
- Savin M, Alexander J, Bierbaum G, et al. Antibiotic-resistant bacteria, antibiotic resistance genes, and antibiotic residues in wastewater from a poultry slaughterhouse after conventional and advanced treatments. Sci Rep. 2021;11(1):16622. doi: 10.1038/s41598-021-96169-y.
- Thomas BW, Luo Y, Li C, et al. Utilizing composted beef cattle manure and slaughterhouse wasted as nitrogen and phosphorus fertilizers for calcareous soil. Compost Sci Util. 2017;25(2):102–111. doi: 10.1080/1065657X.2016.1219681.
- Cayuela ML, Sinicco T, Mondini C. Mineralization dynamics and biochemical properties during initial decomposition of plant and animal residues in soil. Appl Soil Ecol. 2009;41(1):118–127. doi: 10.1016/j.apsoil.2008.10.001.
- Olayemi OP, Kallenbach CM, Scheekloth JP, et al. From factory to field: effects of a novel soil amendment derived from cheese production on wheat and corn production. Front Sustain Food Syst. 2020;3:127.
- Mona S, Malyan SK, Saini N, et al. Towards a sustainable agriculture with carbon sequestration, and greenhouse gas mitigation using algal biochar. Chemosphere. 2021;275:129856. doi: 10.1016/j.chemosphere.2021.129856.
- Zhao L, Cao X, Masek O, et al. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J Hazard Mater. 2013;1:256–257. doi: 10.1016/j.jhazmat.2013.04.015.
- Farhangi-Abriz S, Torabian S, Qin R, et al. Biochar effects on yield of cereal and legume crops using meta-analysis. Sci Tot Environ. 2021;775:145869. doi: 10.1016/j.scitotenv.2021.145869.
- Chintala R, Mollinedo J, Schumacher TE, et al. Effect of biochar on chemical properties of acidic soil. Arch Agron Soil Sci. 2014;60(3):393–404. doi: 10.1080/03650340.2013.789870.
- Kammann C, Ippolito J, Hagemann N, et al. A tool to reduce the agricultural greenhouse gas burden- knowns, unknowns and future research needs. J Environ Eng Landsc Manage. 2017;25(2):114–139. doi: 10.3846/16486897.2017.1319375.
- Hale L, Luth M, Crowley D. Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biol Biochem. 2015;81:228–235. doi: 10.1016/j.soilbio.2014.11.023.
- Sikder S, Joardar JC. Biochar production from poultry litter as a management approach and effects on plant growth. Int J Recycl Org Waste Agricult. 2019;8(1):47–58. doi: 10.1007/s40093-018-0227-5.
- Schmidt HP, Kamman C, Hagemann N, et al. Biochar in agriculture - a systematic review of 26 global meta-analyses. GCB-Bioenergy. 2021;13(11):1708–1730. doi: 10.1111/gcbb.12889.
- Paustian K, Lehmann J, Ogle S, et al. Climate smart soils. Nature. 2016;532(7597):49–57. doi: 10.1038/nature17174.
- Roe S, Streck C, Beach R, et al. Land-based measures to mitigate climate change: potential and feasibility by country. Glob Chang Biol. 2021;27(23):6025–6058. doi: 10.1111/gcb.15873.
- Woolf D, Amonette JE, Street-Perrot FA, et al. Sustainable Biochar to Mitigate Global Climate Change. Nature. 2010;1:56.
- Lehmann J, Cowie A, Masiello CA, et al. Biochar in climate change mitigation. Nat Geosci. 2021;14(12):883–892. doi: 10.1038/s41561-021-00852-8.
- Blanco-Canqui H, Laird DA, Heaton EA, et al. Soil carbon increased by twice the amount of biochar carbon applied after 6 years: field evidence of negative priming. Bioenergy. 2019;12:240–251.
- Kuzyakov Y, Subbotina I, Chen H, et al. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009;41(2):210–219. doi: 10.1016/j.soilbio.2008.10.016.
- Singh BP, Cowie AL. Long-term influence of biochar on native organic carbon mineralization in a low-carbon clayey soil. Sci Rep. 2014;4:3687. doi: 10.1038/srep03687.
- Fatima S, Riaz M, Al-Wabel MI, et al. Higher biochar rate strongly reduced decomposition of soil organic matter to enhance C and N sequestration in nutrient-poor alkaline calcareous soil. J Soils Sedim. 2021;21(1):148–162. doi: 10.1007/s11368-020-02753-6.
- Khan M, Huang J, Shah A, et al. Mitigation of greenhouse gas emissions from a red acidic soil by using magnesium-modified wheat straw biochar. Environ Res. 2021;203:11879.
- Roberts KG, Gloy B, Joseph S, et al. Life cycle assessment of biochar systems: estimating the energetic, economic and climate change potential. Environ Sci Technol. 2010;44(2):827–833. doi: 10.1021/es902266r.
- Matuštik Hnakova T, Koci V. Life cycle assessment of biochar-to-soil systems: a review. J Clean Prod. 2020;259:120998.
- Xiao X, Chen Z, Chen B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci Rep. 2016;6:22644. doi: 10.1038/srep22644.
- Cornelissen G, Pandit N, Taylor P, et al. Emissions and char quality of Flame-Curtain "kon tiki" kilns for Farmer-Scale charcoal/biochar production. PLoS One. 2016;11(5):e0154617. doi: 10.1371/journal.pone.0154617.
- IPCC. 2019). Appendix 4 Method for Estimating the Change in Mineral Soil Organic Carbon Stocks from Biochar Amendments: basis for Future Methodological Development. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Volume 4: agriculture, Forestry and Other Land Use.
- Verra. 2021. Methodology for biochar utilization in soil and non-soil applications. Consortium: FORLIANCE, South Pole, and Biochar Works. In joint collaboration with Delaney Forestry Services. Accessed 7/2022 from https://verra.org/wp-content/uploads/2021/08/210803_VCS-Biochar-Methodology-v1.0-.pdf.
- Lu Q, He ZL, Stoffella PJ. Land application of biosolids in the USA: a review. Appl Environ Soil Sci. 2012;2012:1–11. doi: 10.1155/2012/201462.
- Leonard E, Bodas J, Brown S, et al. Carbon balance for biosolids use in commercial douglas fir plantations in the pacific northwest. J Environ Manage. 2021;295:113115. doi: 10.1016/j.jenvman.2021.113115.
- McIvor K, Cogger C, Brown S. Effect of biosolids based soil properties on soil chemical and physical properties in urban gardens. Compost Sci Util. 2012;10:199–206. 2013
- Brown S, Kurtz K, Bary A, et al. Quantifying benefits associated with land application of organic residuals in Washington state. Environ Sci Technol. 2011;45(17):7451–7458. doi: 10.1021/es2010418.
- Villa YB, Ryals R. Soil carbon response to long-term biosolids application. J Environ Qual. 2021;50(5):1084–1096. doi: 10.1002/jeq2.20270.
- Wallace BM, Krzic M, Forge TA, et al. Biosolids increase soil aggregation and protection of soil carbon five years after application on a crested wheatgrass pasture. J Environ Qual. 2009;38(1):291–298. doi: 10.2134/jeq2007.0608.
- Tian G, Granato TC, Cox AE, et al. Soil carbon sequestration resulting from long-term application of biosolids for land reclamation. J Environ Qual. 2009;38(1):61–74. doi: 10.2134/jeq2007.0471.
- Williams DE, Vlamis J, Pukite A, et al. Trace element accumulation, movement and distribution in the soil profile from massive applications of sewage sludge. Soil Sci. 1980;129(2):119. doi: 10.1097/00010694-198002000-00007.
- Ryals R, Silver W. Effects of organic matter amendments on net primary productivity and greenhouse gas emissions in annual grasslands. Ecol Appl. 2013;23(1):46–59. doi: 10.1890/12-0620.1.
- Blumenthal DM, LeCain DR, Augustine DJ. Composted manure application promotes long-term invasion of semi-arid rangeland by bromus tectorum. Ecosphere. 2017;8(10):e01960. doi: 10.1002/ecs2.1960.
- Tautges NE, Chiartas JL, Gaudin ACM, et al. Deep soil inventories reveal that impacts of cover crops and compost on soil carbon sequestration differ in surface and subsurface soils. Glob Change Biol. 2019;25:3754–3766.
- Leip A, Busto M, Winiwarter W. Developing spatially stratified N2O emission factors for Europe. Environ Poll. 2011;159(11):3223–3232. doi: 10.1016/j.envpol.2010.11.024.
- Liu M, Qiao N, Xu X, et al. C:N stoichiometry of stable and labile organic compounds determine priming patterns. Geoderma. 2021;362:11422.
- Sánchez A, Artola A, Font X, et al. Greenhouse gas emissions from organic waste composting. Environ Chem Lett. 2015;13(3):223–238. doi: 10.1007/s10311-015-0507-5.
- Bogaard A, Fraser R, Heaton THE, et al. Crop manuring and intensive land management by europe’s first farmers. Proc Natl Acad Sci USA. 2013;110(31):12589–12594. doi: 10.1073/pnas.1305918110.
- Li XR, Zhang P, Su YG, et al. Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: a four year field study. Catena. 2012;97:119–126. doi: 10.1016/j.catena.2012.05.009.
- Insam H, Gómez-Brandón M, Ascher J. Manure-based biogas fermentation residues – friend or foe of soil fertility? Soil Biol Biochem. 2015;84:1–14. doi: 10.1016/j.soilbio.2015.02.006.
- Pires AFA, Millner PD, Baron J. Assessment of current practices of organic farmers regarding biological soil amendments of animal origin in a multi-regional US study. Food Prod Trends. 2018;38:347–362.
- Maillard É, Angers DA. Animal manure application and soil organic carbon stocks: a meta-analysis. Glob Chang Biol. 2014;20(2):666–679. doi: 10.1111/gcb.12438.
- Gross A, Glaser B. Meta-analysis on how manure application changes soil organic carbon storage. Sci Rep. 2021;11(1):5516. doi: 10.1038/s41598-021-82739-7.
- Poulton P, Johnston J, Macdonald A, et al. Major limitations to achieving “4 per 1000” increases in soil organic carbon stock in temperate regions: evidence from long-term experiments at rothamsted research, United Kingdom. Glob Chang Biol. 2018;24(6):2563–2584. doi: 10.1111/gcb.14066.
- Guenet B, Gabrielle B, Chenu C, et al. Can N2O emissions offset the benefits from soil organic carbon storage? Glob Change Biol. 2021;27(2):237–256. doi: 10.1111/gcb.15342.
- Owen JJ, Parton WJ, Silver WL. Long-term impacts of manure amendments on carbon and greenhouse gas dynamics of rangelands. Glob Change Biol. 2015;21(12):4533–4547. doi: 10.1111/gcb.13044.
- Schlesinger WH. Carbon sequestration in soils: some cautions amidst optimism. Agric Ecosyst Environ. 2000;82(1-3):121–127. doi: 10.1016/S0167-8809(00)00221-8.
- Zhang B, Tian H, Lu C, et al. Global manure nitrogen production and application in cropland during 1860-2014: a 4 arcmin gridded global dataset for earth system modeling. Earth Syst Sci Data. 2017;9(2):667–678. doi: 10.5194/essd-9-667-2017.
- Key N, Sneeringer S. Carbon emissions, renewable electricity, and profits: comparing policies to promote anaerobic digestion on dairies. Agric Resour Econ Rev. 2012;41(2):139–157. doi: 10.1017/S1068280500003312.
- Iqbal R, Raza MAS, Valipour M, et al. Potential agricultural and environmental benefits of mulches – a review. Bull Nat Res Centre. 2020;44:75.
- Akhtar K, Wang W, Khan A, et al. Wheat straw mulching offset soil moisture deficiency for improving phyiological and growth performance of summer soybean. Agric Water Manage. 2019;211:16–25. doi: 10.1016/j.agwat.2018.09.031.
- Cotrufo MF, Wallenstein MD, Boot CM, et al. The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol. 2013;19(4):988–995. doi: 10.1111/gcb.12113.
- Wu L, Zhang W, Wei W, et al. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol Biochem. 2019;135:383–391. doi: 10.1016/j.soilbio.2019.06.003.
- Fontaine S, Mariotti A, Abbadie L. The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem. 2003;35(6):837–843. doi: 10.1016/S0038-0717(03)00123-8.
- Liu C, Lu M, Cui J, et al. Effect of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Glob Chang Biol. 2014;20(5):1366–1381. doi: 10.1111/gcb.12517.
- Bamber N, Jones M, Nelson L, et al. Life cycle assessment of mulch use on okanagan apple orchards: part 1 - Attributional. J Clean Prod. 2020;267:121960. doi: 10.1016/j.jclepro.2020.121960.
- Chamizo S, Mugnai G, Rossi F, et al. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: gaining insights for applicability in soil restoration. Front Environ Sci. 2018;6:1–14. doi: 10.3389/fenvs.2018.00049.
- Khoja TM. Heterotrophic growth of blue-green algae Doctoral thesis, Durham University. 1973 http://etheses.dur.ac.uk/1315/.
- Meeks JC, Elhai J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol Mol Biol Rev. 2002;66(1):94–121; table of contents. doi: 10.1128/MMBR.66.1.94-121.2002.
- Ronga D, Biazzi E, Parati K, et al. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019;9(4):192. doi: 10.3390/agronomy9040192.
- Goncalves AL. The use of microalgae and cyanobacteria in the improvement of agricultural practices: a review on their biofertilising, biostimulating and biopesticide roles. Appl Sci. 2021;11(2):871. doi: 10.3390/app11020871.
- De PK. The role of blue-green algae in nitrogen fixation in rice-fields. Proc R Soc B. 1939;127:121–139.
- Kollah B, Patra AK, Mohanty SR. Aquatic microphylla azolla: a perspective paradigm for sustainable agriculture, environment and global climate change. Environ Sci Pollut Res. 2016;23(5):4358–4369. doi: 10.1007/s11356-015-5857-9.
- Nascimento MD, Battaglia ME, Rizza LS, et al. Prospects of using biomass N2-fixing cyanobacteria as an organic fertilizer and soil conditioner. Algal Res. 2019;43:101652. doi: 10.1016/j.algal.2019.101652.
- Alvarez AL, Weyers SL, Goemann HM, et al. Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Res. 2021;54:2211–9264.
- Li G, Xiao W, Yang T, et al. Optimization and process effect for microalgae carbon dioxide fixation technology applications based on carbon capture: a comprehensive review. C J Carbon Res. 2023;9(1):35. doi: 10.3390/c9010035.
- Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294–306. doi: 10.1016/j.biotechadv.2007.02.001.
- Das NP, Kumar A, Singh PK. Cyanobacteria, pesticides and rice interaction. Biodivers Conserv. 2015;24(4):995–1005. doi: 10.1007/s10531-015-0886-8.
- Song X, Zhang J, Peng C, et al. Replacing nitrogen fertilizer with nitrogen-fixing cyanobacteria reduced nitrogen leaching in red soil paddy fields. Agric Ecosys Environ. 2021;312:107320. doi: 10.1016/j.agee.2021.107320.
- Williams W, Budel B, Williams S. Wet season cyanobacterial N enrichment highly correlated with species richness and nostoc in the Northern Australian savannah. Biogeosciences. 2018;15(7):2149–2159. doi: 10.5194/bg-15-2149-2018.
- Russow R, Veste M, Böhme F. A natural N-15 approach to determine biological fixation of atmospheric nitrogen by biological soil crusts of the negev desert. Rapid Commun Mass Spectrom. 2005;19(23):3451–3456. doi: 10.1002/rcm.2214.
- Booshan N, Pabbi S, Singh A. Impact of blue green algae (BGA) technology: an empirical evidence from northwestern Indo-Gangetic plains. Biotechnology. 2018;8:324.
- Osman MEH, El-Sheekh MM, Naggar AH, et al. Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth and yield of pea plant. Biol Fertil Soils. 2010;46(8):861–875. doi: 10.1007/s00374-010-0491-7.
- Nascimento MD, Rizza L, Di Palma AA, et al. Cyanobacterial biological nitrogen fixation as a sustainable nitrogen fertilizer for the production of microalgal oil. Algal Res. 2015;12:142–148.
- Dojani S, Büdel B, Deutschewitz K, et al. Rapid succession of biological soil crusts after experimental disturbance in the succulent karoo, South Africa. Appl Soil Ecol. 2011;48(3):263–269. doi: 10.1016/j.apsoil.2011.04.013.
- Razon LF. Life cycle energy and greenhouse gas profile of a process for the production of ammonium sulfate from nitrogen-fixing cyanobacteria. Bioresour Technol. 2012;107:339–346. doi: 10.1016/j.biortech.2011.12.075.
- Bauer L, Ranglová K, Masojídek J, et al. Digestate as a sustainable nutrient source for microalgae-challenges and prospects. Appl Sci. 2021;11(3):1056. doi: 10.3390/app11031056.
- Solovchenko A, Verschoor AM, Jablanoski ND. Phosphorus from wastewater to crops: an alternative path involving microalgae. Biotech Adv. 2015;:07012.
- Pereira L, Cotas J. Historical use of seaweed as an agricultural fertilizer in the European Atlantic Area. In: seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder. In Seaweeds as plant fertilizer, agricultural biostimulants and animal fodder. Chapter 1. Boca Raton, FL: CRC Press; 2019.
- Duarte CM, Wu J, Xiao X, et al. Can seaweed farming play a role in climate change mitigation and adaptation? Front Mar Sci. 2017;4:1–8. doi: 10.3389/fmars.2017.00100.
- EL Boukhari MEM, Barakate M, Bouhia Y, et al. Trends in seaweed extract based biostimulants: manufacturing process and beneficial effect on soil-plant systems. Plants. 2020;9(3):359. doi: 10.3390/plants9030359.
- Sible CN, Seebauer JR, Below FE. Plant biostimulants: a categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy. 2021;11(7):1297. doi: 10.3390/agronomy11071297.
- Khan W, Rayirath UP, Subramanian S, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul. 2009;28(4):386–399. doi: 10.1007/s00344-009-9103-x.
- Filbee-Dexter K, Feehan C, Smale D, et al. Ocean temperature controls kelp decomposition and carbon sink potential. 2020 Preprint (Research Square).
- Santaniello A, Scartazza A, Gresta F, et al. Ascophyllum nodosum seaweed extract alleviates drought stress in arabidopsis by affecting photosynthetic performance and related gene expression. Front Plant Sci. 2017;8:1362. doi: 10.3389/fpls.2017.01362.
- Suh S, Johnson JA, Tambjerg L, et al. Closing yield gap is crucial to avoid potential surge in global carbon emissions. Glob Environ Change. 2020;63:102100. doi: 10.1016/j.gloenvcha.2020.102100.
- Shukla PS, Mantin EG, Adil M, et al. Ascophyllum nodosim-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth- stress tolerance and disease management. Front Plant Sci. 2019;20:665.
- Roberts DA, Paul NA, Dworjanyn SA, et al. Biochar from commercially cultivated seaweed for soil amelioration. Sci Rep. 2015;5:9665. doi: 10.1038/srep09665.
- Fesel P, Zuccaro A. Dissecting endophytic lifestyle along the parasitism/mutualism continuum in arabidopsis. Curr Opin Microbiol. 2016;32:103–112. doi: 10.1016/j.mib.2016.05.008.
- Hardoim PR, van Overbeek LS, Berg G, et al. The hidden world within plants: ecological and evolutionary consideriona for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 2015;79(3):293–320. doi: 10.1128/MMBR.00050-14.
- Smith SE, Read DJ. The mycorrhizal symbiosis. Academic Press, San Diego, CA. 2008.
- Witzgall K, Vidal A, Schubert D, et al. 2021 (preprint). Soil organic carbon under lockdown: fresh plant litter as the nucleus for persistent carbon.
- Li N, Xu Y, Han X, et al. Fungi contribute more than bacteria to soil organic matter through necromass accumulation under different agricultural practices. Eur J Biol. 2015;67:51–58.
- Shahzad R, Khan AL, Bilal S, et al. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front Plant Sci. 2018;9:24. doi: 10.3389/fpls.2018.00024.
- Geisen S, Kostenko O, Cnossen MC, et al. Seed and root endophytic fungi in a range expanding and a related plant species. Front Microb. 2017;8:1–11.
- Mack KML, Rudgers JA. Balancing multiple mutualists: asymmetric interactions among plants, arbuscular mycorrhizal fungi, and fungal endophytes. Environ Sci Ecol. 2008;117:310–320.
- Newsham KK. A meta-analysis of plant response to dark septate root endophytes. New Phytol. 2011;190(3):783–793. doi: 10.1111/j.1469-8137.2010.03611.x.
- Della Monica IF, Saparrat MCN, Godeas AM, et al. The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecol. 2015;17:10–17. doi: 10.1016/j.funeco.2015.04.004.
- Berthelot C, Chalot M, Leyval C, et al. From darkness to light: emergence of the mysterious dark septate endophytes in plant growth promotion and stress alleviation. In T. R. Hodkinson, F. M. Doohan, M. J. Saunders, & B. R. Murphy (Eds.), Endophytes for a growing world 2019. (pp. 143–164. Cambridge: Cambridge University Press.
- Thapa S, Rai N, Limbu A, et al. Impact of trichoderma sp. in agriculture: a mini review. J Biol Todays World. 2020;9:7227.
- Harman GE. Overview of mechanisms and uses of trichoderma spp. Phytopathology. 2006;96(2):190–194. doi: 10.1094/PHYTO-96-0190.
- Elnahal ASM, El-Saadony MT, Saad AM, et al. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol. 2022;162(4):1007–1007. Springer Netherlands. doi: 10.1007/s10658-022-02472-3.
- Rillig MC, Aguilar-Trigueros CA, Camenzind T, et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019;222(3):1171–1175. doi: 10.1111/nph.15602.
- Ryan MH, Graham JH, Morton JB, et al. Research must use a systems agronomy approach if management of the arbuscular mycorrhizal symbiosis is to contribute to sustainable intensification. New Phytol. 2019;222(13):1–3.
- Säle V, Palenzuela J, Azcón-Aguilar C, et al. Ancient lineages of arbuscular mycorrhizal fungi provide little plant benefit. Mycorrhiza. 2021;31(5):559–576. doi: 10.1007/s00572-021-01042-5.
- Sepp SK, Davison J, Jairus T, et al. Non-random association patterns in a plant–mycorrhizal fungal network reveal host–symbiont specificity. Mol Ecol. 2019;28(2):365–378. doi: 10.1111/mec.14924.
- Boddington CL, Dodd JC. The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. Studies in experimental microcosms. Plant Soil. 2000;218:145–157.
- Schwartz MW, Hoeksema JD, Gehring CA, et al. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol Lett. 2006;9(5):501–515. doi: 10.1111/j.1461-0248.2006.00910.x.
- Ryan MH, Graham JH. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018;220(4):1092–1107. doi: 10.1111/nph.15308.
- Hart MM, Antunes PM, Chaudhary VB, et al. Fungal inoculants in the field: is the reward greater than the risk? Funct Ecol. 2018;32(1):126–135. doi: 10.1111/1365-2435.12976.
- Verzeaux J, Hirel B, Dubois F, et al. Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae: basic and agronomic impacts. Plant Sci. 2017;264:48–56. doi: 10.1016/j.plantsci.2017.08.004.
- Dietrich P, Roscher C, Clark AT, et al. Diverse plant mixtures sustain a greater arbuscular mycorrhizal spore viability than monocultures after 12 years. J Plant Ecol. 2020;13(4):478–488. doi: 10.1093/jpe/rtaa037.
- Contos P, Wood JL, Murphy NP, et al. Rewilding with invertebrates and microbes to restore ecosystems: present trends and future directions. Ecol Evol. 2021;11(12):7187–7200. doi: 10.1002/ece3.7597.
- Wubs ERJ, Putten WH, Mortimer SR, et al. Single introductions of soil biota and plants generate long-term legacies in plant community assembly. Ecol Lett. 2019;22(7):1145–1151. doi: 10.1111/ele.13271.
- Bago B, Pfeffer PE, Shachar-Hill Y. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 2000;124(3):949–958. doi: 10.1104/pp.124.3.949.
- Gavito ME, Jakobsen I, Mikkelsen TN, et al. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induces carbon sink strength. New Phytol. 2019;223(2):896–907. doi: 10.1111/nph.15806.
- Talbot JM, Allison SD, Treseder KK. Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol. 2008;22(6):955–963. doi: 10.1111/j.1365-2435.2008.01402.x.
- Treseder KK, Holden SR. Fungal carbon sequestration. Science. 2013;338:6127.
- Wilson GWT, Rice CH, Rillig MC, et al. Soil aggregation and carbon sequestration are tightly correlated with the abundance of mycorrhizal fungi: results from long-term field experiments. Ecol Lett. 2009;12(5):452–461. doi: 10.1111/j.1461-0248.2009.01303.x.
- Cheng L, Booker FL, Tu C, et al. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science. 2012;337(6098):1084–1087. doi: 10.1126/science.1224304.
- Verbruggen E, Veresoglou SD, Anderson IC, et al. Arbuscular mycorrhial fungi: short term liability but long term benefits for soil carbon storage? New Phytol. 2013;197(2):366–368. doi: 10.1111/nph.12079.
- Agnihotri R, Sahni S, Sharma MP, et al. Facets of AM fungi in sequestering soil carbon and improving soil health. In Fungal diversity, ecology and control management. Springer, Singapore. 2022;327–344.
- Franzluebbers AJ, Nazih N, Stuedemann JA, et al. Soil carbon and nitrogen pools under low-and high-endophyte infected tall fescue. Soil Sci Soc Am J. 1999;63(6):1687–1694. doi: 10.2136/sssaj1999.6361687x.
- He C, Wang W, Hou J. Plant growth and soil microbial impact of enhancing licorice with inoculating dark septate endophytes under drought stress. Front Microbiol. 2019;10:2277. doi: 10.3389/fmicb.2019.02277.
- Iqbal J, Siegrist JA, Nelson JA, et al. Fungal endophyte infection increases soil carbon sequestration potential of southeastern USA tall fescue stands. Soil Biol Biochem. 2012;44(1):81–92. doi: 10.1016/j.soilbio.2011.09.010.
- Franzleubbers AJ. Short-term responses of soil C and N fractions to tall fescue endophyte infection. Plant Soil. 2006;383:153–164.
- Jenkins MB, Franzluebbers AJ, Humayoun SB. Assessing short-term responses of prokaryotic communities in bulk and rhizosphere soils to tall fescue endophyte infection. Plant Soil. 2006;289(1–2):309–320. doi: 10.1007/s11104-006-9141-0.
- Omacini M, Semmartin M, Pérez LI, et al. Grass-endophyte symbiosis: a neglected aboveground interaction with multiple belowground consequences. Appl Soil Ecol. 2012;61:273–279. doi: 10.1016/j.apsoil.2011.10.012.
- Kane KH. Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the mediterranean region. Environ Exp Bot. 2011;71:337–344. doi: 10.1016/j.envexpbot.2011.01.002.
- Kivlin SN, Emery SM, Rudgers JA. Fungal symbionts alter plant responses to global change. Am J Bot. 2013;100(7):1445–1457. doi: 10.3732/ajb.1200558.
- Butler JL, Bottomley PJ, Griffith SM, et al. Distribution and turnover of recently fixed photosynthate in ryegrass rhizospheres. Soil Biol Biochem. 2004;36(2):371–382. doi: 10.1016/j.soilbio.2003.10.011.
- Nguyen C. Rhizodeposition of organic C by plant: mechanisms and controls. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C. (eds) Sustainable Agriculture. Springer, Dordrecht, pp. 97–123; 2009. doi: 10.1007/978-90-481-2666-8_9.
- Swinnen J, Van Veen JA, Merckx R. Carbon fluxes in the rhizosphere of winter wheat and spring barley with conventional vs integrated farming. Soil Biol Biochem. 1995;27(6):811–820. doi: 10.1016/0038-0717(94)00230-X.
- Fan K, Weisenhorn P, Gilbert J, et al. Soil pH correlates with the co-occurence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil Biol Biochem. 2018;121:185–192. doi: 10.1016/j.soilbio.2018.03.017.
- Gahan J, Schmalenberger A. The role of bacteria and mycorrhiza in plant sulfur supply. Front Plant Sci. 2014;5:723. doi: 10.3389/fpls.2014.00723.
- Timmusk S, Timmusk K, Behers L. Rhizobacterial plant drought stress tolerance enhancement: towards sustainable water resource management and food security. J Food Sec. 2013;1:6–9.
- Rubin RL, van Groenigen KJ, Hungate BA. Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant Soil. 2017;416(1–2):309–323. doi: 10.1007/s11104-017-3199-8.
- Vejan P, Abdullah R, Khadiran T, et al. Role of plant growth promoting rhizobacteria in agricultural sustainability: a review. Molecules. 2016;21(5):573. doi: 10.3390/molecules21050573.
- Harman GE, Doni F, Khadka RB, et al. Endophytic strains of trichoderma increase plants’ photosynthetic capability. J Appl Microbiol. 2021;130(2):529–546. doi: 10.1111/jam.14368.
- Berhongaray G, Cotrufo FM, Janssens IA, et al. Below-ground carbon inputs contribute more than above-ground inputs to soil carbon accrual in a bioenergy poplar plantation. Plant Soil. 2019;434(1-2):363–378. doi: 10.1007/s11104-018-3850-z.
- Rubin RL, Jones AN, Hayer MH, et al. Opposing effects of bacterial endophytes on biomass allocation of a wild donor and agricultural recipient. FEMS Microb Ecol. 2020;96:fia012.
- Nie M, Bell C, Wallenstein MD, et al. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci Rep. 2015;5:9212. doi: 10.1038/srep09212.
- Sethi S, Gupta S. Impact of pesticides and biopesticides on soil microbial biomass carbon. Univ J Sci Res Technol. 2013;3(2):326–330.
- Calvo P, Watts DB, Ames RN, et al. Microbial-based inoculants impact nitrous oxide emissions from an incubated soil medium containing urea fertilizers. J Environ Qual. 2013;42(3):704–712. doi: 10.2134/jeq2012.0300.
- Souza EFC, Rosen CJ, Venterea RT. Contrasting effects of inhibitors and biostimulants on agronomic performance and reactive nitrogen losses during irrigated potato production. Field Crops Res. 2019;240:143–153. doi: 10.1016/j.fcr.2019.05.001.
- Tennakoon PLK, Rajapaksha RMCP, Hettiarachchi LSK. Tea yield maintained in PGPR inoculated field plants despite significant reduction in fertilizer application. Rhizosphere. 2019;10:100146. doi: 10.1016/j.rhisph.2019.100146.
- Berninger T, González López Ó, Bejarano A, et al. Maintenance and assessment of cell viability in formulation of non-sporulating inoculants. Microb Biotechnol. 2018;11(2):277–301. doi: 10.1111/1751-7915.12880.
- Yang M, Fang Y, Sun D, et al. Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: a meta-analysis. Sci Rep. 2016;6(1):10. doi: 10.1038/srep22075.
- Guardia G, Marsden KA, Vallejo A, et al. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci Total Environ. 2018;624:1202–1212. doi: 10.1016/j.scitotenv.2017.12.250.
- Dalal RC, Wang W, Robertson GP, et al. Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian journal of. Soil Res. 2003;41(2):165–195. doi: 10.1071/SR02064.
- Subbarao GV, Searchinger TD. A “more ammonium solution” to mitigate nitrogen pollution and boost crop yields. PNAS. 2021;118(22):e2107576118.
- Lam SK, Suter H, Mosier AR, et al. Using nitrification inhibitors to mitigate agricultural N2O emission: a double-edged sword? Glob Change Biol. 2017;23(2):485–489. doi: 10.1111/gcb.13338.
Appendix 1
Soil amendment guide
The goal this guide is to provide information on the possible modes of action for climate mitigation, and important considerations and caveats for each type of amendment. A summary of all amendments is provided in in the main text of the document.
Animal residues
Characterization
Animal products and residues are inedible byproducts from the beef, poultry, swine and dairy industries, and can comprise up to 45% of the slaughtered animal [Citation61]. Slaughterhouse waste can be disposed of in a variety of ways, including composting, anaerobic digestion, alkaline hydrolysis, rendering, incineration, and pyrolysis. Animal residues that are sold as soil amendments are collected at the slaughterhouse (e.g. blood meal), rendering plants (e.g. bone meal), aerobically composted in vessels or windrows, or anaerobically digested in mesophilic or thermophilic conditions. Through anaerobic digestion, biogas can also be used for bioenergy, and the leftover sludge can be dried into biosolids for agricultural amendments. For both methods, one of the concerns with these methods is the survival and spread of thermophilic/thermotolerant animal, human and plant pathogens including endospore forming bacteria (e.g. Bacillus anthracis, Clostridium botulinum, Clostridium tetani) so the end product (digestate) needs to be pasteurized.
Climate mitigation
Animal agriculture is the second largest contributor to human-made greenhouse gas emissions after fossil fuels, and a leading cause of deforestation, water, and air pollution. Reducing meat production and changing practices of meat production is the most direct way to mitigate climate change. If we assume that some level of animal agriculture will continue, recycling slaughterhouse waste, estimated to be in excess of 200 million tons annually, is one way to return nutrients back to soils, assuming that antibiotics and antibiotic resistant bacteria can be safely removed [Citation62]. Similar to other organic amendment classes, the carbon in the residues represents a direct addition to the soil with persistence based on the decomposability of the amendment; the nutrients may stimulate additional productivity leading to new carbon sequestration in the soil, as well as avoided emissions of CH4 in particular.
Despite growing interest in recycling the nutrients from slaughterhouse waste and emerging work on the agronomic implications [Citation63], few studies have characterized soil responses to animal residues and none have estimated their climate mitigation potential. It has been shown that animal residues may actually speed up C metabolism: bone meal and blood meal increased beta-glucosidase production, a C metabolizing enzyme produced by fungal species [Citation64]. A comparison between plant and animal residues found that animal residues increased respiration as a function of C added, with bone meal having the largest cumulative CO2 evolution (13.4% of the added C) after 28 days, followed by blood meal, wheat straw and cotton cardings. CO2 losses peaked within 38–42 h after application, suggesting a short term decomposition pulse from residue addition. The study also noted diminishing returns (inhibition of microbial biomass growth) at high application rates due to ammonium toxicity. This is one of the only studies of its kind, and further monitoring would be needed to determine whether the amount of C respired continues to stay under the amount of C added, which would indicate C sequestration, or exceeds the amount added, which would indicate priming (net loss of C).
Lactobionate, a waste product from cheesemaking, was also recently evaluated as a novel soil amendment in corn and winter wheat fields [Citation65]. Lactobionate increased soil health characteristics, including microbial biomass C, and also increased yield and %C at the site of application, after only 5 weeks, though results were not statistically significant.As the authors noted, keeping lactobionate out of the landfill might added benefit of avoided CH4 emissions (as discussed for compost). However, given the N-rich nature of lactobionate, there is potential for increased N2O emissions with field application and more work is needed to evaluate the net climate impact of this product.
Biochar
Characterization
Biochar is an organic, charcoal-like substance produced through thermal conversion of biomass with insufficient oxygen for complete combustion, known as pyrolysis. Biochar can be produced from any organic material, such as wood, rice straw, corn cobs, manure, algae, or even animal carcasses [Citation66], with variation in physicochemical properties differing with feedstock types [Citation14], and pyrolysis temperature [Citation67]. Biochars have shown strong benefits for plant productivity, plant nitrogen use efficiency, microbial biomass, soil structure and water holding capacity [Citation68]. Biochar also consistently has a liming effect on soil, ameliorating acidity, improving yield, and precipitating toxins [Citation69]. Due to its high surface area and adsorption/desorption capacity, biochar is effective at filtering nutrients and trace contaminants. Biochars can be applied with inorganic or organic fertilizers [Citation70] or microbial inoculants [Citation71], or used as a fertilizer on its own in the case of animal manure biochar [Citation72]. A recent synthesis evaluated 26 meta-analyses on the effects biochar for agronomic performance and concluded that biochar has compelling evidence for improving several agronomic parameters [Citation73].
Climate mitigation
Biochar’s climate mitigation potential is well studied, with average per-hectare sequestration rates of 0.2 − 1.0 Mg CO2 ha-1 yr-1 and global technical potentials of 1.0–2.2 Pg CO2 yr-1 [Citation74–76]. Biochar climate mitigation encompasses multiple elements, including SOC sequestration, emission reductions, and avoided decomposition, as reviewed recently [Citation77]. There have been over 5,000 studies and meta-analyses reporting broadly on the role of biochar in climate mitigation (), including directly increasing SOC stocks. For example, applying 3 tons/acre of biochar for six years led to twice the amount of SOC than what was originally added in the biochar C indicating newly fixed C by the plants were being sequestered along with the C in the biochar itself [Citation78]. Similar results were found using [Citation14] C labeled biochar [Citation79]. However, positive priming, where native SOC is lost, has also been documented across four different feedstocks [Citation80]. Other work suggests that biochar carbon sequestration is higher at higher application rates, suppressing microbial metabolic quotients (qCO2) [Citation81].
Soil emission reductions of CO2, CH4, and N2O from biochar are also well documented [Citation54–57]. One study estimates that 20% of the maximum sustainable technical climate mitigation potential of biochar comes from reductions in soil N2O and CH4 emissions [Citation76]. However, a closer look reveals that very few empirical studies measured CO2, CH4, and N2O at the same time. In a web of science search, 2,697 studies appeared using the keywords biochar AND CO2 OR CH4 OR N2O, only 111 studies reported simultaneous emissions of CO2, CH4, and N2O (biochar AND CO2 AND CH4 AND N2O). While measuring individual GHGs (such as CO2) can reveal trends, these studies cannot be used for accounting purposes, since decreases in the emissions of one gas are often accompanied by increased emissions of other gases [Citation82].
The third mitigation element for biochar, life cycle emission reductions, which is most commonly invoked in carbon markets, signifies avoided future decomposition or combustion of biochar feedstocks. A common assumption is that feedstocks (e.g. woody residues) are sustainably sourced waste products that would have otherwise been left to decompose or combust in an uncontrolled setting, and that their production and harvest did not incur any land use change [Citation83]. Under this assumption, biochar climate mitigation is conceptualized as the amount of C stabilized through pyrolysis, after accounting for equivalent emissions from biochar production and soil application, calculated using life cycle assessment tools [Citation83, Citation84]. Next, a permanence adjustment factor is applied depending on the stability of biochar, typically the hydrogen to organic C ratio (H:Corg) a relative indicator of carbonization [Citation35, Citation77, Citation85]. Typically, 50 – 70% of the carbon remaining in the biochar after pyrolysis is considered stable for at least 100 years depending on feedstock, technology and pyrolysis conditions [Citation86, Citation87]. Given this variability, registries may use permanence thresholds to determine a margin of safety buffer. For example, the Verra methodology for biochar utilization in soil and non-soil applications deducts 26% of the original C credit calculation if the H:Corg is below 0.4 (assuming higher permanence) and deducts 46% of the original C credit calculation if the H:Corg is above 0.4 (assuming lower permanence) [Citation88].
Biosolids
Characterization
Biosolids are a term adopted by the EPA to distinguish raw sewage sludge from the anaerobically digested end product. Biosolid production is a waste diversion and recycling system that diverts human waste from landfill burial or incineration. Following screening and settling, water is treated and discharged, and the remaining dissolved biological matter is converted into a solid mass by anaerobic bacteria. About 6.5 million dry metric tons of biosolids are produced annually in the US, and 60% of this total amount are applied to topsoils [Citation89]. On farms, biosolids are incorporated into the soil using manure spreaders, or combined with a bulking agent such as woodchips and further composted aerobically in windrows. While some amendments are limited by feedstock supply (e.g. biochar and compost), there is a nearly unlimited supply of biosolids, and not enough land to apply them to. Ongoing work is evaluating new applications, such as timber farms [Citation90] and flower gardens in industrial areas, where heavy metal concentrations in the biosolids may be lower than the heavy metal concentrations in the soil it is applied to [Citation91].
Climate mitigation
Our review of biosolids field studies showed a technical carbon sequestration potential that is highly variable, ranging from 0.014 Mg C per Mg dry biosolids to as high as to as high as 0.5 Mg C per Mg dry biosolids [Citation92, Citation93]. A study on crested wheatgrass pasture found that 0.075 Mg C were sequestered for every Mg of biosolids applied [Citation94]. In Illinois, biosolids additions of 455–1654 Mg ha-1 for 8 to 23 years led to a mean net sequestration of 1.73 Mg C ha yr-1, whereas control plots that received inorganic fertilizer lost up to 0.07 Mg C ha yr-1 [Citation95]. Encouraging results were found in California, with sequestration rates varying from 0.2 to 0.5 Mg C ha-1 y-1 across three study sites [Citation93] but results depended on soil type.
EPA’s Part 503 rule establishes requirements for the final use or disposal of sewage sludge when biosolids are applied to fertilize crops. In addition, states have their own rulings regulating the use of biosolids, such as maximum application rates or allowable thresholds of heavy metals and contaminants of emerging concern such as per- and polyfluoroalkyl substances (PFAS). Some countries (e.g. Australia) and states (e.g. California) have criteria on conditions of use, whereby biosolids can only be applied to crops grown as animal and not to crops for human consumption. Most states allow the use of biosolids on rangelands, forests or disturbed reclamation areas, but the effects of applying large quantities of composted human waste on biodiversity and water quality should be evaluated [Citation96].
Compost (manure and vegetable)
Characterization
Composting is the aerobic decomposition of organic waste, such as manure or food waste. The composting process increases the C:N ratio and creates more stable organic matter by incorporating labile molecules into hydrophobic domains. When performed correctly, aerobic composting promotes complete denitrification of nitrate to harmless dinitrogen gas, reduces nitrate ammonia leaching, and reduces nitrous oxide and methane emissions compared to a baseline of raw manure application. The agronomic benefits of compost are increased plant growth and establishment, increased soil fertility, stimulation of microbial activity and erosion protection. On rangelands, plant productivity has been shown to increase with compost application [Citation97], though some have noted that compost enhances invasion by annual grasses [Citation98], which can threaten native biodiversity.
Climate mitigation
Application of anaerobically decomposed or composted manure typically results in an increase in SOC stocks in the upper profile [Citation99]. In theory, the other benefit of using composted manure over raw manure is that materials have already undergone partial decomposition before application, limiting emissions at the site of application. In one study, soil N2O emissions from composted manure were 40% lower than emissions from raw manure application, attributed to slowed rates of N mineralization [Citation100]. Since SOM priming is highest in soils with high C:N ratios and substrates with low C:N ratios [Citation101], increasing the substrate C:N ratio through composting should also limit priming. In a recent synthesis it was found that average sequestration rates for composted plant-based materials was 0.7 Mg C ha-1 yr-1 in experimental trials, but varied greatly depending on application rate with rates < 0.1 Mg C ha-1 yr-1 for recommended application rates [Citation42].
The Marin Carbon Project is a notable field trial using compost, perennial grassland plantings, and managed grazing to increase SOC. They added ½ inch of compost (31 tons per acre) and tracked it for four years. Control plots lost carbon but amended plots had increased forage production and belowground carbon [Citation97]. A life cycle analysis revealed that while fresh manure resulted in high greenhouse gas emissions, composted manure and plant waste led to offsets that exceeded emissions, saving 23 Mg CO2e ha-1 over three years [Citation43] with the majority of reductions attributed to calculated avoided CH4 emissions (emissions factors) from anaerobic decomposition. SOC sequestration and ANPP, as well as reduced need for supplemental feed (hay) for cattle were revealed to be marginal co-benefits. While these results are encouraging, there are two key caveats. Similar to the accounting issues associated with manure, it is difficult to know what the alternative disposal pathway for compost would have been if not for its use as a soil amendment. Furthermore, composting is not a solely aerobic process and often has incidental or unintended CH4 and N2O emissions even when managed well. This raises concerns that reliance on IPCC emissions factors, which are based on following best practices, can overestimate these avoided emissions [Citation102].
Manure
Characterization
Manure is the original fertilizer, and early farmers intuitively applied manure to their fields as long as 8,000 years ago [Citation103]. It is also the most thoroughly researched for the purpose of climate mitigation (). Manure provides phosphorus, nitrogen, potassium and micronutrients, while also improving soil aggregation, water holding capacity, porosity, microbial activity and erosion resistance [Citation104, Citation105]. The nutrient and microbiological composition of raw manure depends on animal source, animal diet and bedding material. A survey found that 58% of organic growers apply raw manure to their fields [Citation106], generally as forage crops used for livestock (62% of respondents). The same survey found that 24.7% of respondents used fresh manure for fresh produce. NRCS currently rewards farmers through conservation practice standard 590 (Nutrient Management) for replacing synthetic N fertilizer by 4% per year for 5 years, through the use of chicken manure, beef feedlot manure, dairy manure, swine or sheep manure. The USDA National Organic program sets criteria and wait times (e.g. applied more than 90 days prior to crop harvest) and the FDA is responsible for performing risk assessment for pathogens.
Climate mitigation
Manure recharges soil organic C stocks at the site of application. Some of the applied manure is rapidly decomposed by microbes, and the rest is incorporated into aggregates, physically protected by minerals or lost to leaching. If the residence time of manure carbon is extended when buried into soil as opposed to decomposition in a manure pile, this could amount to net carbon storage. Multiple articles demonstrate an increase in C stock in manured plots compared to non-manured plots [Citation107, Citation108]. The longest running empirical study testing fertilization on yields and other parameters are the Hoosfield Barley and Broadbalk Wheat experiments, which have been running since 1851 (Hoosfield) and 1843 (Broadbalk). These studies suggest a long-term integrated annual accumulation rate of 0.13 ± 0.081 Mg C ha-1 yr-1 with sequestration rates > 0.40 Mg C ha-1 yr-1 for the first 40 years, at an application rate of 25.1 t ha every fourth year [Citation109], which is about twice the application rate that is typical on working farms.
While manure additions increase SOC, nitrous oxide emissions also increase with manuring. A global meta-analysis estimated that manure application increases N2O emissions by an average of 32.7% compared to application of synthetic N fertilizer [Citation41]. Another study estimated emissions of 419.2 CO2e per year, offsetting 37% of the original SOC gains from manure [Citation110]. A long term (60 year) empirical and modeling study (using DAYCENT) evaluating long term impacts of manuring in California rangelands yielded 19 Mg C ha-1 over 60 years or 0.32 Mg ha-1 yr-1, but accounting for concomitant increases in nitrous oxide emissions (2 kg N2O ha-1 yr-1) meant that manured fields were neither C sinks nor sources (0.74 ± 0.73 Mg CO2e ha-1 yr-1) [Citation111]. In rice fields, manure increased net GHG emissions by 80% over conventional practices (optimal inorganic fertilizer use) due to increases in CH4 and N2O emissions and priming loss of SOC [Citation40].
Whether SOC change from manure addition constitutes additional carbon removed from the atmosphere depends on the system boundaries. Some have argued that manure application is not additional [Citation112], since applying it to one field represents a missed C sink opportunity in another field. Indeed, manure is often in limited supply and a farmer may need to forgo application on one field in order to apply manure to another field. However, globally, manure application to cropland accounts for less than one fifth of the total manure production, suggesting that this is not a supply issue [Citation113], but rather a supply chain issue.
In addition to the system boundary issues discussed above, an accurate assessment of business as usual or baseline conditions is key to understanding the climate benefit of manure application. For instance, capturing methane through a controlled process such as anaerobic digestion and reusing the biogas (CH4) would lower the carbon footprint of the baseline conditions [Citation114], while still providing a leftover product (biosolids) that can also be applied as a soil amendment. The same alternative disposal pathway also applies for human waste, discussed in the biosolids section.
Mulch
Characterization
Under the USDA NRCS definition, mulch is defined as plant residues or other suitable materials that are produced off site and applied to the cropping surface. The definition differs from “green mulch” or crop residues that are mowed (or rolled down in the direction of travel) and left on site to decompose. Mulching has chemical (e.g. N immobilization), physical (erosion prevention), and biological (weed suppression) effects, which differ based on mulch feedstocks. In general, mulching is a soil health practice that limits soil erosion, increases water retention, increases native SOC accrual through decomposition, and provides micronutrients and energy towards the formation of microbial biomass [Citation115]. In many cases, mulch also increases crop yields, particularly in rain-fed agriculture [Citation116].
Climate mitigation
Mechanisms for SOC storage from litter addition include increased energy for microbial growth and incorporation into microbial biomass, incorporation of particulate C in soil aggregates or stabilization of leached C onto mineral surfaces [Citation117]. Effects of mulching on SOC storage are nitrogen dependent, with fertilization increasing mulch-derived microbial biomass C and mulch-derived SOC [Citation118]. Mulching has also been posited to increase competition for energy and nutrients between the microorganisms specialized in fresh organic matter decomposition [Citation119]. Mulching effects are ecosystem-dependent; for example, straw addition is often a C sink in upland soils but increases C emissions in paddy soils due to CH4 priming [Citation120]. The issue with mulch accounting is that most studies and models do not include life cycle emissions, thus overestimating the climate mitigation effect of mulches. In one of the only studies to conduct a full LCA [Citation121], life cycle emissions from mulch application in apple orchards led to 25% higher CO2e emissions than the alternative scenario of no mulch, mostly attributed to mastication and transportation emissions.
Cyanobacteria
Characterization
Cyanobacteria, also known as blue-green algae (e.g. Nostoc, Anabaena), are a diverse group of photosynthetic prokaryotes ubiquitous in aquatic, marine and terrestrial ecosystems. In deserts, they are early colonizers on bare soil, produce exopolysaccharides that anchor biological soil crusts, improve water holding capacity, and prevent soil erosion [Citation122]. Cyanobacteria are metabolically diverse: they contain opportunists that cause algal blooms in eutrophic waters, oligotrophs capable of simultaneous C and N fixation, and facultative autotrophs that csn thrive on either organic or inorganic C sources [Citation123, Citation124]. In water and soil, cyanobacterial C and N fixation is coordinated by differentiated cell types: under N limitation, specialized cells called heterocysts form from vegetative cells, which create the anaerobic environment needed for N fixation.
Cyanobacteria are common in agricultural soils both as free living organisms and root colonizers. Microalgae produce a variety of plant growth promoters including auxins, cytokinins, betaines, amino acids, vitamins, polysaccharides, and gibberelins [Citation125, Citation126]. An early study showed that rice could be grown without manure requirements, inferring that cyanobacteria are the likely main source of biogenic N fixation in rice [Citation127]. Several studies have demonstrated the agronomic benefits of cyanobacteria, including growth and yield [Citation128], and as fertilizer substitutes. Cyanobacteria have also been demonstrated to improve nutrient retention, root length, and total plant mass in wheat fields compared to non-inoculated control plots [Citation129]. Some microalgae have biopesticide capabilities, by producing phenolic compounds, exopolysaccharides, terpenoids, and free fatty acids [Citation130].
Climate mitigation
Cyanobacteria can theoretically mitigate climate change through SOC sequestration, reduced soil greenhouse gas emissions, and avoided energy consumption. Cyanobacteria can be extremely productive in culture; for instance, cyanobacteria can fix 0.1–2 grams of CO2 per liter per day in a photobioreactor [Citation131]. Since microalgae cells contain 50% C, 1.83 kg CO2 is fixed for each kg of algal biomass [Citation132]. On fields, any increases in C, at least in the surface soils, would be reflected in the difference in SOC stocks between treated and control soils following cyanobacterial application, after subtracting the C that was added from the inoculant (since recently fixed C in algal biomass itself is ephemeral). This method would also capture any additional growth that occurs after application; estimates from paddy mesocosms show an increase from 10.13 grams to 31.3 grams C in pots, an equivalent increase of 20 kg C ha-1 100 days-1 in the field [Citation133]. Song et al. (2021) [Citation134] also observed a 6.65-fold increase in cyanobacterial biomass on the soil surface after only 10 days of growth in rice paddies. Given the dearth of studies in real agricultural systems, we can turn to biocrust literature for an estimate of terrestrial cyanobacterial growth. Mature, 50 year old cyanobacteria-dominated biocrusts can fix 113.6 kg C ha-1 yr-1 [Citation104]. In croplands, it is unknown how disturbance, flooding, irrigation and tillage may influence C fixation of added inoculants, and this is an area that warrants future investigation.
In addition to fixing C, some cyanobacteria can fix ammonia under N limitation. Therefore, cyanobacteria could mitigate climate change if N fixation rates in rice fields are high enough to reduce the need for synthetic fertilizers and if greenhouse gas emissions from cyanobacterial production are lower than greenhouse gas emissions from fertilizer production. In culture, N fixation ranges from 3–20 mg of N per 100 cubic cm of nutrient free medium over 60 days (Anabaena variabilis). Again, there is little to no field data for croplands, but there are estimates from desert biocrusts, which range from 5.2 kg N ha-1 yr-1 (Nostoc in savannasCitation135], to 10–41 kg N ha-1 yr -1 [Citation136]. A consortium of Anabaena, Nostoc, Aulosira and Tolypothrix decreased urea application needs by 25%, with a 3.8 increase in rice yield [Citation137]. Other estimates are as high as a 50% reduction in ammonium sulfate [Citation138] and urea [Citation134], also from rice paddies. These studies applied equal quantities of N in each treatment, so the most likely explanation for maintaining yields was that the inoculant provided slow release N close to the rooting surface, slowing leaching and increasing plant N use efficiency.
Key considerations for cyanobacteria are the temperature and moisture regime of the recipient field, which determines viability, and whether the product is applied live or dead. Many terrestrial and aquatic cyanobacterial species can be grown in photobioreactors, but biomass growth is fastest and more efficient when growing aquatic cyanobacteria. Perhaps, applying dead cyanobacteria for its ammonia content alone could outweigh the benefits of using a hardier but slower growing terrestrial cyanobacteria inoculant. Other sources suggest that dead cyanobacteria or cyanobacterial extracts could contribute metabolites that act as prebiotics for the rest of the soil microbiome [Citation139]. Another key consideration is their sensitivity to disturbance; while natural biocrust cyanobacteria can recover after following disturbance to their original population sizes after only 6 months [Citation140], annual effects of seeding, herbicides, tillage, harvest operations will reduce populations on farms [Citation133].
Life cycle analysis for cyanobacterial soil amendments is lacking, so while there is solid theoretical support for in-field carbon sequestration and the potential for N2O emission reductions, it is unknown whether cyanobacterial applications will mitigate climate change. Algal cultivation can be energy intensive, especially during the flocculation, filtering, and drying states. Individual components of the cyanobacterial life cycle have been compiled. Razon (2012) [Citation141] compared the production of ammonium sulfate crystals through the Haber Bosch process versus through production of Anabaena sp., and concluded that ammonia production using cyanobacteria releases 384–758 less kg CO2e per 1000 kg of ammonia produced, as compared to the Haber Bosch process. Life cycle emissions also vary with production method (e.g. tubular photobioreactors, raceways, bubble columns and flat panel photobioreactors), which also differ at scale.
There are potential environmental downsides to cyanobacterial production that should be considered, including its high phosphorus requirements, its high spatial footprint (cyanobacterial photobioreactors need a high surface area to volume ratio), and chemical pest control needs (especially for raceway systems). Recent developments to address this include the use of biogas digestate or wastewater as an alternative phosphorus source for growing microalgae [Citation142, Citation143].
Seaweeds and seaweed extracts
Characterization
Seaweeds are a broad category of marine macroalgae, including red, brown and green algae. The earliest record of using seaweed extracts in agriculture dates back to the first century where seaweeds were used to enrich sandy coastal soils in the European Atlantic coast [Citation144]. Seaweed farming is the fastest growing aquaculture sector, supplying industrial products such as agar & carrageenan, and food for human or animal consumption. Natural seaweed production is responsible for an estimated uptake of 173 Tg C per year, while the technical potential (upper limit) for seaweed aquaculture can capture as high as 0.68 Tg CO2 per year globally [Citation145]. With respect to agriculture, seaweeds also comprise 33% of the foliar, root and soil biostimulant market [Citation146]. The most common species in commercial products are Ascophyllum nodosum, Laminaria spp and Durvillea spp. They are prized for their antioxidant effects in plants, reducing cell damage from reactive oxygen species during times of drought [Citation147]. Likewise, they produce plant hormones or plant hormone analogues such as cytokinins (cell elongation), and auxins (root growth), which can mediate crop stress response [Citation146]. Furthermore, seaweeds can directly supply micronutrients accumulated through their growth, such as iodine, B12 and cobalt. As a soil amendment, seaweeds also produce co-benefits of improved phosphorus efficiency and antioxidants that lend drought resistance to plants. They can be applied in dried forms, added to irrigation systems or applied as a foliar spray. Alginates, polysaccharides that give brown seaweeds their gelatinous nature, allow for the formation of micro-colloids over the surface of plants and will act as a feed and nutrient source for beneficial microbes [Citation148].
Climate mitigation
While seaweed application has clear agronomic benefits, net climate mitigation is more uncertain, evidenced by the dearth of studies on this topic (). As an oceanic carbon drawdown strategy, seaweed is grown in the photic zone where it rapidly captures CO2, then the plant biomass is sunk to the sea floor where decomposition is impeded. Decomposition rates in the ocean are very likely slower than in soil, where the seaweed is oxidized [Citation149], so the argument for increased residence time in soil cannot be made here. However, avoided fossil emissions (from the Haber Bosch process) could be calculated based on the replacement rate between inorganic fertilizers and seaweeds, after subtracting life cycle emissions.
Likewise, and similar to other organic amendments, a SOC sequestration benefit could be inferred if seaweeds indirectly increase SOC stocks through plant growth enhancement; however, field trials are currently lacking. Seaweeds possess antioxidants that protect plants from extreme events, such as drought and heat waves leading to greater productivity especially in tough weather years relative to unamended fields [Citation150]. At a systems level, if an amendment like seaweed can effectively close yield gaps, the avoided agricultural expansion, and associated emissions, that would be necessary to meet growing food demand could be counted as a climate mitigation benefit [Citation151].
In order for seaweed amendments to be credited as a climate mitigation strategy, further refinements of life cycle emissions of GHGs and other pollutants are also needed. Emissions and other pollutants vary greatly depending on the method used to extract and purify seaweeds, which include ultrasound-assisted extraction, enzyme-assisted extraction, supercritical fluid extraction, microwave-assisted extraction, and pressurized liquid extraction (methods are discussed in detail in Shukla et al. 2019 [Citation152]. Other emerging approaches include converting seaweed into biochar [Citation153], or blending seaweeds with animal feed to reduce enteric CH4 emissions [Citation4].
Fungal additives
Characterization
Plant-fungal relationships can be commensal, parasitic or mutualistic [Citation154, Citation155]. Plants form associations with symbiotic fungi that interface between roots and soil, endophytic fungi that inhabit above- or belowground plant organs, and saprotrophic fungi that occupy rhizospheric or bulk soil. As with bacteria, a fungal species can occupy multiple niches of the plant-soil interface at the same time. Among rhizosphere fungi, the most well characterized in managed grasslands and croplands are arbuscular mycorrhizal fungi (AMF), which are associated with 85% of plant species, including major crops [Citation156]. AMF form structures (arbuscules) inside roots that support exchange of carbon (photosynthate) and nutrients, and extraradical hyphae that mineralize N and C, and transport water, N and soluble P to the plant, and export carbon away from the plant [Citation157]. Hyphae (mycelium) can grow >100 kg ha-1 yr-1, and fungal necromass contributes significantly to total SOM [Citation158].
Endophytes reside wholly within plant cells (leaves, stems or roots), rather than residing either external to the root (EMF) or externally and internally (AMF). Endophyte is a lifestyle term rather than a taxonomic term, and many “endophytes” survive saprophytically in soil, where they wait for a suitable plant root to arrive. Endophytes are transmitted vertically through a parental seed coat [Citation159], as well as horizontally, where roots become colonized from the soil environment [Citation160]. In roots, endophytes, compete for space and resources [Citation161] with other bacteria, fungi and soil animals, and may produce benefits to plants similar to that of rhizobacteria; meta-analyses report an increase in tissue N and P by 44–116% with dark septate endophyte inoculation as compared to uninoculated or sterilized controls [Citation162]. Additionally, dark septate endophytes may enhance the symbiotic functioning of AMF resulting in improved plant performance [Citation163].Other studies report enhanced biotic and abiotic stress tolerance [Citation164]. One of the most well-known organisms known to have an endophytic habit is Trichoderma, famous for its voracious appetite for plant pathogenic fungi and nematodes, including Pythium, Phytopthera, Fusarium, and Rhizoctonia [Citation165]. Trichoderma spp have been known to act as biocontrol agents since at least the 1920s, but uncovering their diverse modes of action is an active area of research [Citation166].
Microbial inoculants present an opportunity to improve the productive capacity and sustainability of crops using biological approaches [Citation167]. AMF and Trichoderma spp. were the first class of organisms to be commercialized; however, the application of arbuscular mycorrhizal fungi for soil health and crop yield, let alone C sequestration, remains contested [Citation168, Citation169]. One of the reasons for this is that high expectations for AMF and a lack of regulation has led to a number of commercial products (typically dried spores or root fragments) that are not viable or not effective. In a global evaluation of commercial arbuscular mycorrhizal inoculants, inoculation only increased plant biomass in one out of 25 treatments and 85% of products did not contain viable propagules [Citation58]. There is also a growing body of evidence suggesting that fungal host compatibility, and therefore the benefit of the added inoculant to the plant and the amount of carbon added to soil is flexible and nuanced, tightly controlled by genetic factors [Citation170, Citation171] as well as environmental factors (e.g. N, P and soil moisture).
Therefore, most researchers have reached a consensus that a practice-based approach should preside for managing AMF (e.g. by planting mycorrhizal cover crops) rather than introducing inoculum itself [Citation172–177]. Likewise, site specific tailored inoculants that are sourced from reference systems will continue to be a useful tool for managing perennial grasslands and rangelands, restoring highly degraded or contaminated soils, and steering ecological trajectories [Citation178, Citation179].
Climate mitigation
In plant-mycorrhizal partnerships, between 20–30% of photosynthetically-fixed carbon may be transferred to the fungal partner [Citation180]. Hyphae transport carbon away from the plant and into the soil matrix, leading to the increased C sink strength in mycorrhizal soils [Citation181]. In order for AMF or endophytes to lead to net SOC storage, the amount of belowground rhizodeposits and residence time of those rhizodeposits as a result of the added inoculant would need to outweigh priming effects as well as decomposition of the added inoculant [Citation182, Citation183]. Much of the interest in AMF for carbon sequestration stemmed from research showing that AMF produce a sticky glycoprotein which aids in soil aggregation and thus should lead to greater carbon stabilization and persistence [Citation184]. However, there is also evidence that AMF prime the decomposition of stable SOM [Citation185], leading Verbruggen et al. (2013) [Citation186] to ask the question, are AMF a short-term liability but long-term benefit for SOC? Despite significant research into this topic [Citation187], controlled field trials are still generally lacking primarily because of the difficulty in manipulating AMF abundance within a particular cropping system.
The available literature on manipulating the endophyte microbiome for SOC or climate benefits is scarce. Only 34 studies correspond to the keywords endophytes AND “SOC” on a Web of Science search (conducted December 10th, 2021), and most are studies on foliar endophytes rather than root endophytes. In a comparison of naturally high and low foliar endophyte infection in tall fescue, it was found that tall fescue plants that had high endophyte infection had 13% higher soil organic C than plants with low endophyte infection [Citation188]. Other work has shown decreased SOC or no change in SOC concentration in response to endophyte infection [Citation189, Citation190]. Experimentally adding endophytes in a mesocosm study did not increase SOC in the short term either [Citation191], but it did increase active carbon, suggested to be caused by suppressed growth of archaea and gram positive bacteria [Citation192]. An early review suggested that endophytes reduce root biomass and mycorrhizal colonization, stimulate root exudates, and have no effect on respiration [Citation193]. Other studies have shown improvement in plant heat and drought tolerance [Citation194, Citation195]. At the moment, there is little evidence that endophytes can be successfully introduced across a range of crop hosts or even specific crop hosts in order to enhance SOC stocks.
Rhizobacteria
Characterization
The rhizosphere has hundreds of species of bacteria and archaea that live inside roots (endosphere), on the root surface (rhizoplane) or in the area of soil under the influence of roots along with 200 different carbon compounds [Citation23]. Between 5 and 20%, but up to 40% under stressed conditions, of plant derived photosynthate transported to roots is lost into the rhizosphere [Citation196–198], creating a hot spot of microbial activity, and a microbial community that is unique from bulk soil [Citation199]. Rhizobacteria perform key nutrient transformations including nitrogen fixation, nitrogen mineralization, denitrification, iron chelation, phosphorus solubilization and transformation of other micronutrients [Citation200]. They produce plant hormones that regulate root growth and cell elongation (cytokinins, gibberellins), and enzymes that regulate stress response through delaying wilting [Citation201]. Rhizobacteria can also mediate impacts of biotic stressors; Bacillus thuringiensis is one of the most well-known biopesticides, which produces a crystalline protein that is toxic to insect larvae. Rhizobacteria have a well-established effect on increasing yields in controlled studies, and the effect is even more pronounced under drought conditions, suggesting they could help to close yield gaps from drought on marginal lands [Citation202, Citation203].
Climate mitigation
Rhizobacterial inoculants clearly influence plant health and resilience, and their ability to mitigate climate change through increased SOC or avoided emissions is theoretically possible. If plants and their microbiomes can be dually engineered to increase belowground inputs or improved carbon use efficiency (the ratio of carbon stored versus respired), for example, then a net climate benefit could be achieved. Rhizobacteria can indirectly increase SOC stocks by upregulating photosynthesis and plant health (through ROS accumulation, proline and sugars) [Citation204], or by increasing the root to shoot ratio, which maximizes belowground carbon inputs once the plant dies [Citation205, Citation206]. Nie et al. (2015) [Citation207] tested whether inoculation by Pseudomonas flourescens could alter SOC use efficiency, by using chambers containing 13 C depleted CO2 and a common rangeland grass, Bouteloua gracilis. They found that the inoculant increased total root length, and the plant stored more biomass C per unit of N under inoculation. In a study evaluating the biopesticides Paeciliomyces lilacinus, Bacillus subtilis, Pseudomonas florescens and Beauveria bassiana on microbial biomass carbon, microbial biomass carbon was higher in all five biopesticide treatments as compared to an uninoculated control [Citation208], which may indicate short term evidence that the system is accumulating carbon. At this time, there are no published papers to our knowledge that evaluate the effects of rhizobacterial inoculants on SOC stocks over time, in side by side inoculated and uninoculated control plots, under field conditions.
Along with C sequestration, the effect of rhizobacteria on N use efficiency (the ratio of N taken up by plants to the amount of N applied) should also be considered. Calvo et al. (2013) [Citation209] found that the microbial based inoculum SoilBuilder (Bacillus licheniformis) reduced N2O emissions compared to uninoculated controls, but reductions in N2O emissions were accompanied by increased CO2 emissions. N-fixing rhizobacteria co-locate ammonia next to the plant root, maximizing the N that gets into plant tissue rather than being lost through denitrification, ammonia volatilization, or leaching. As a result, less fertilizer is wasted. However, Souza et al. (2019) [Citation210] found that rhizobacteria alone led to large increases in N2O emissions (32–56%) while when added in combination with nitrification inhibitors, emissions decreased by 42–75% compared to urea alone. Adesomoye et al. (2009) [Citation36] found that the N fixing rhizobacteria Bacillus amyloquefaciens allowed for a 25% reduction in NPK fertilizer without compromising yield in tomatoes. Another study found that Camellia sinensis (tea) could be grown at 2/3 the nitrogen and ½ the phosphorus application recommendations in field trials through the addition of N fixing and P solubilizing bacteria [Citation211]. Another possible mechanism is an alteration of the balance between inoculation-increased root exudation and plant/microbial competition for nitrate. That is, increased root inputs (from increased plant size due to inoculation) in C limited soils would lead to plant and microbe competition for nitrate, suppressing N2O emissions.
There are several life cycle emissions considerations unique to rhizobacteria. First are the emissions from cultivation under a sterile environment, including energy and materials for media preparation, incubation, and autoclaving. Second are the emissions from packaging, storing, and preserving inoculants. Third are the emissions for distributing and applying inoculants. While endospore forming bacteria can remain dormant for several years, and progress is being made to extend the shelf life of these products, non-sporulating bacteria have a limited shelf life, from 4 to 12 months [Citation212], and require carriers (e.g. talc, biochar), preservatives (e.g. guar gum), which also have their own life cycle emissions. To our knowledge, there are no published studies yet evaluating the life cycle emissions of rhizobacteria intended for use in agroecosystems.
Nitrification and urease inhibitors
Characterization
Nitrification and urease inhibitors are products that can either be applied as a coating on granular fertilizers or sprayed on the soil surface to improve nitrogen use efficiency even at high N application rates. Nitrification inhibitors reduce populations of bacteria that convert ammonia to nitrite (Nitrosomonas) and nitrite to nitrate (Nitrobacter), and urease inhibitors slow the hydrolysis of urea to dissolved ammonium ions. In theory, greater N remains available to the crop resulting in greater yield and plant N uptake, and meta-analyses of trial data show improved agronomic performance and farm income [Citation44]. However, there isn’t always a clear agronomic benefit with different inhibitors showing highly varying results [Citation213, Citation214].
Climate mitigation
Climate mitigation is as much a nitrogen concern as a carbon concern [Citation30]. Agriculture is the largest source of anthropogenic N2O emissions (60–80% on a global scale [Citation215], from animal waste and overapplication of nitrogen fertilizers. Crops take up only 42 to 47% of the total applied N; the other half is lost to the environment as surface runoff, denitrification, ammonia volatilization and microbial consumption [Citation216].
Nitrification inhibitors clearly decrease the N2O emissions and urease inhibitors decrease nitrate leaching. Meta-analyses suggest 39–48% reductions in N2O emissions and 38–56% reductions in NO3 leaching [Citation44, Citation213]. However, some studies show an increase in ammonia volatilization, and subsequent indirect N2O emissions, suggesting that one form of nitrogen is being swapped for another. Lam et al. (2017) [Citation217] found that nitrification inhibitors decreased direct N2O emissions by 0.2–4.5 kg N2O-N ha−1 while increasing ammonia volatilization by 0.2–18.7 kg NH3-N ha−1. If inhibitors are applied as part of a nutrient management strategy, it is likely that real reductions in N2O emissions will be seen, but it is unclear how much the overall life cycle emissions will offset the N2O emission reduction benefit. Given the patchiness of N2O production and difficulty in monitoring N2O, direct quantification of the climate benefits will remain expensive.
