Abstract
Introduction
Rewetting is an option to decrease greenhouse gas emissions of drained peatlands. With continued cultivation of wetland plants (paludiculture), it is possible to provide renewable raw materials. In Finland, peat has been used as a growing media and animal bedding. Since peat availability is decreasing, new materials are needed.
Methods
The emissions of the paludiculture system were compared to emissions of the current system based on use of peat. We assumed that abandoned organic croplands were under paludicrop cultivation. Paludicrops were used instead of peat as animal bedding material and growing media. The assessment included an analysis of uncertainties related to the key parameters.
Results
Paludiculture would generate emissions savings of 352,000 tons of CO2 eq in 2050 compared to the peat use system. The emissions savings are mostly generated by land-use emissions reductions. Emissions of peat decay represent 22% of the emissions of the peat use system, whereas emissions of peat extraction are less significant. Emissions of the paludiculture system are mainly caused by paludicrop cultivation, with 300,000 tons of CO2 eq in 2050. Paludiculture mitigates climate change by increasing biogenic carbon sink: 48,000 tons of CO2 eq in 2050.
Discussion
It is highly unlikely that the paludiculture system would generate more emissions than the peat use system. However, peat substitution does not offset emissions of abandoned organic croplands, even under paludicrop cultivation. Therefore, other land-use options, such as afforestation or restoration, could provide more emissions savings even though they do not provide raw materials.
Introduction
About 15% of peatlands worldwide have been drained to accommodate agriculture, peat extraction, forestry and urbanization [Citation1]. Natural peatlands form carbon sinks, in which the carbon intake into layers of organic matter exceeds the climate impact of the emission of methane (CH4) and carbon dioxide (CO2). Peatland draining has enhanced aerobic decomposition of organic matter and increased greenhouse gas (GHG) emissions, turning peatlands from sinks to sources of carbon. For instance, in Finland, the GHG emissions from cultivated organic croplands (histosols) are significant: 8.7 million tons CO2-eq annually, approximately 10% of all national emissions (including the Land Use, Land-Use Change and Forestry (LULUCF) sector). Furthermore, the decomposition of drained peat continues after cultivation activities have ended, resulting in considerable emissions from abandoned organic croplands [Citation2]. In order to decrease these emissions, alternative uses for organic croplands are needed.
One promising option to decrease GHG emissions of organic croplands is rewetting: Peat mineralization and consequent CO2 emissions are reduced through rewetting because peat decay decreases and N2O emissions remain at a negligible level due to the lower availability of mineral nitrogen [Citation2]. On the other hand, CH4 emissions increase because of anaerobic respiration [Citation1]. Considering the time-dependency of climatic effects, however, CH4 radiative forcing does not undermine the climate mitigation impact of rewetting [Citation3]. Vegetation can also mitigate CH4 emissions [Citation4]. The benefits of rewetting on GHG emissions have been addressed in several studies [Citation5].
Paludiculture is the cultivation of crops on rewetted peatlands, providing not only climate change mitigation but also agricultural business options. Paludicultural crops, such as Typha, reeds and Phalaris arundinacea, among others, are suitable raw materials for, e.g. construction, growing media, biogas and energy pellets [Citation6–8]. Through paludiculture, it is possible to decrease GHG emissions arising from organic croplands but also reduce emissions by replacing more emission-intensive products on the market. Lahtinen et al. [Citation7] evaluated GHG emissions of two paludiculture product systems, i.e. Typha construction board and reed growing media, with life cycle assessment (LCA), concluding that both product systems result in much lower emissions than current agricultural land use and may be net greenhouse gas sinks (average −6.0 tCO2eq ha−1 for construction board; −3.0 tCO2eq ha−1 for reed growing media). In Finland, peat is one of the raw materials that must be replaced in the near future because of changing regulations concerning peat extraction.
In 2014, a total of 30,000 ha was under peat extraction in Finland [Citation9]. Annual production of horticultural and environmental peat in Finland was 2 million m3 [Citation10]. Peat energy use in 2017 generated GHG emissions of 5.8 Mt CO2-eq, representing 10% of the annual GHG emissions in Finland (LULUCF excluded), making it a major source of national GHG emissions [Citation11]. Finland aims to achieve GHG emission reduction by increasing carbon sinks and decreasing GHG emissions. One of the options to decrease emissions is to replace peat with renewable fuels. In 2030, the use of energy peat should be half of the current level. Peat extracted for animal bedding and growing media uses is a side stream of energy peat. Thus, a decrease in energy peat harvesting will also reduce the availability of peat for other uses [Citation12]. There is a growing demand to find new raw materials for animal bedding and growing media uses. Using paludicrops, such as reeds and Sphagnum moss, for animal bedding and growing media production has been suggested [Citation7,Citation12,Citation13]. The benefits of paludiculture for the climate could therefore be twofold: substituting for emission-intensive peat products and mitigating GHG emissions from cultivated peatlands [Citation14,Citation15].
The aim of this study is to assess the GHG emission-reduction potential of paludiculture in abandoned agricultural croplands in Finland between 2020 and 2050. Emission reduction through the conversion of abandoned organic croplands into paludiculture cultivation sites, and substituting peat as animal bedding and growing media with paludicultural crops, were considered. The biogenic carbon intake of paludicultural crops and peat decay were estimated. As most of the relevant parameters include substantial uncertainties, a Monte Carlo simulation approach was included to assess the sensitivity of the results.
Material and methods
In this study, we evaluated the total effect of shifting to paludiculture through a combination of two scenarios (). The peat use system scenario describes the continuation of current practice. In this alternative, we assumed that abandoned organic croplands would remain unmanaged and not converted into paludicrop cultivation sites, and peat would be used as a raw material for animal bedding and growing media. In the second, paludiculture system scenario, we assumed that abandoned organic croplands would be used for paludicrop cultivation, and instead of peat, paludicrops would be used as a raw material for animal bedding and growing media. In this scenario, peat extraction sites would remain unaltered. The total emissions savings of the paludiculture system are estimated by subtracting the emissions of the paludiculture system from the emissions of the peat use system (i.e. the difference compared to the continuation of current practices).
Figure 1. Flowchart of the peat use system and paludiculture system, and sources of greenhouse gas emissions and carbon intake.
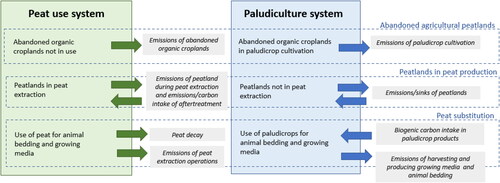
In Finland, there are ca. 30,000 ha abandoned organic croplands that no longer produce food or feed [Citation16]. We assumed that these croplands would be converted into paludicrop cultivation sites by raising the ground water table. It was assumed that the change would take place gradually, beginning in 2020, and that all 30,000 ha of abandoned organic croplands would be used for paludicrop cultivation by 2050.
A substantial number of potential crops exist for paludiculture. In this study, we selected five plants that are suitable for Finnish growing conditions [Citation17] and are appropriate for the production of growing media and/or animal bedding [Citation7,Citation12]. These five plants are: Phragmites, Salix, Typha, Sphagnum and Phalaris arundinacea. We assumed that by 2050, each plant would be cultivated in an area of 6000 ha. Salix would only be used to replace peat as animal bedding material, and the rest would be used as animal bedding material and growing media, with equal amounts for both uses.
Emissions of abandoned agricultural peatlands
Although abandoned organic croplands are typically regarded as sources of GHGs, some estimates suggest that they may act as minor carbon sinks [Citation2]. An average value of GHG emissions of abandoned organic cropland was determined based on data on drained boreal grasslands, with an average value of 20.9 t CO2 eq ha/year [Citation18].
Paludiculture cultivation and production emissions
The cultivation emissions of paludicrops were estimated using emission factors from a review by Bianchi et al. [Citation19]. The GHG emission factors include carbon intake in photosynthesis and carbon release in plant and soil respiration, as well as emissions of CH4 and N2O. Global warming potentials of 27 and 273 were used for CH4 and N2O, respectively, to convert the results to CO2 equivalents. Bianchi et al. [Citation19] assessed GHG emissions for so-called emergent plants (Typha, Phalaris arundinacea, Salix and Phragmites) and Sphagnum. Emissions of emergent plants were, on average, 17.70 t CO2 eq/ha/year, which is close to the total of the emission factors for shallow-drained grasslands according to the Intergovernmental Panel on Climate Change (IPCC) [Citation18]. This value is moderately high as the sites included in a review were recently established and consequent CH4 emissions were still at a high level [Citation20]. In this study, we assumed that the emissions of paludiculture would linearly decrease after establishment of a paludiculture site. An average estimate of 10.00 t CO2eq/ha/year [Citation21,Citation22] after 30 years of establishment of a paludiculture site was applied, e.g. a farming site established in 2020 would generate 44% less emissions in 2050 than in 2020. The same values were used for Phalaris arundinacea, Typha, Salix and Phragmites. An average emission for Sphagnum cultivation was set as 12.74 t CO2 eq/ha/year. This estimate is based on the review by Bianchi et al. [Citation19], who found that emissions from Sphagnum farming were −2.8 t CO2 eq/ha/year, including the assumption that no harvesting would occur. We extracted the carbon intake of Sphagnum from the emissions factor, assuming that the water content of harvested Sphagnum is 84% and half of the dry matter is carbon [Citation12]. A linear decrease for Sphagnum cultivation emissions was also assumed. Thus, emissions would be, on average, 7.20 CO2 eq/ha/year 30 years after the establishment of a Sphagnum farming site. The carbon intake and release of harvested paludicrops were included as a separate parameter (see the section ‘Biogenic carbon intake’).
In order to control eutrophication, CH4 emission and dead organic matter loss, topsoil removal is strongly recommended for Sphagnum farming [Citation23]. Following this recommendation, our study assumed the removal of a 30 cm layer of topsoil, which was then piled in the vicinity of the harvesting site. We also assumed the emission factors of grasslands on boreal peatland for piled topsoil [Citation18]. Sphagnum cultivation sites were assumed to be established following the moss layer transfer technique [Citation24]. Data on the establishment of Typha and Phragmites cultivation site were obtained from Lahtinen et al. [Citation7]. The same values were used for Phalaris arundinacea, as we assumed that the establishment of a cultivation site for this plant was similar to the establishment of a cultivation site for Typha or Phragmites. The assumption was that emissions of these operations would be minimal and use of data on other paludicrops would not influence the results. For Salix cultivation, all operations related to its establishment and maintenance were adopted from Murphy et al. [Citation25]. It was assumed that after harvesting re-planting takes place.
All paludicrops, excluding Sphagnum, were assumed to be fertilized. Salix requires the addition of nitrogen, around 70 kg N/ha/year, during the first cutting cycle, applied especially during the third and fourth years [Citation26]. The addition of phosphorus (10 kg ha/year) and potassium (35 kg ha/year) is also recommended [Citation27]. We assumed that Salix was cultivated in cycles of 10 years, with an average annual yield of 6.95 t ha/year. Phragmites requires the addition of nitrogen fertilizers (60 kg/ha/year) [Citation7], and the annual yield of Phragmites was assumed to be 8.0 t/ha [Citation7]. Typha was assumed to be fertilized with 150 kg/ha N in the form of coated urea and 150 kg/ha/year K as coated potassium nitrate [Citation28]. The annual yield of Typha was assumed to be 8.96 t/ha/year [Citation7]. Typha paludiculture requires fertilization with nitrogen and potassium nitrate (150 kg/ha for both) [Citation7]. Phalaris arundinacea requires fertilization in the year of establishment with 40 kg/ha nitrogen fertilizer, 40 kg/ha phosphorus fertilizer and 80 kg/ha potassium. For the following two years, the assumed fertilizer requirements were 60 kg ha/year nitrogen fertilizer, 30 kg/ha/year phosphorus fertilizer and 70 kg ha/year potassium. Three years after establishment, the fertilizer requirement was assumed to be 55 kg/ha/year nitrogen, 20 kg/ha/year phosphorus and 70 kg/ha/year potassium [Citation29].
Values for emissions caused by the production of fertilizers were adopted from the Ecoinvent database. Fertilizing paludicrops with nitrogen fertilizers generates some N2O emissions. These emissions were estimated by Kandel et al. [Citation30], assuming that 1.6% of nitrogen is released as N2O-N.
Typha, Phragmites and Phalaris arundinacea were assumed to be harvested and chipped with a shredder, using estimates by Lehtoranta et al. [Citation12] on fuel consumption. Emissions of transportation were adopted from Lehtoranta et al. [Citation12]. It was assumed that the harvesting of Sphagnum was actualized with a front loader and semi-trailer, with fuel consumption of 2 litres/m3 harvested Sphagnum [Citation12]. Emissions of Salix harvesting were adopted from Murphy et al. [Citation25].
Typha, Phragmites, Phalaris arundinacea and Salix chips can be used as animal bedding material without any processing, although it is also possible to pelletize them. Sphagnum is also suitable for litter use without any processing but requires air-drying to reach an appropriate moisture content (50%).
Growing media production using Typha, Phragmites and Phalaris arundinacea was considered to be similar regardless of the raw material. Growing media production requires vermicomposting of paludiculture crops (5 months) and a dolomite addition. Data on growing media production processes and emissions were adopted from Lahtinen et al. [Citation7].
Biogenic carbon intake
Biogenic carbon is stored in products with a long lifespan when paludiculture crops are used [Citation7,Citation31]. Although it was assumed that the use-phase of both growing media and animal bedding is only 1 year, most of the carbon stored in these products is not instantly released into the atmosphere. We included a temporal carbon storage in the paludiculture system as negative GHG emissions. It was assumed that both discarded growing media and animal bedding were used for soil improvement on agricultural lands, where carbon is slowly released into the atmosphere as CO2. We assumed that during the first 5 years, 80% of biogenic carbon in these products would decay, and after the first 5 years, the remaining carbon would decay at 1% as an annual basis [Citation32]. The carbon intake in biogenic carbon storage accumulates as production volumes increase; thus, biogenic carbon storage also increases. At the same time, however, biogenic carbon decays, decreasing the biogenic carbon storage. The biogenic carbon intake occurs between 2020 and 2050. However, a substantial amount of biogenic carbon in paludicrops decays after 2050.
GHG emissions of peat harvesting
Peat extraction has an impact on the GHG emissions and carbon sinks of peatlands. In order to produce a tonne of peat, an area of 2.9 m2 over a period of 20 years is required [Citation12]. In this study, we assumed that peat is targeted at drained peatlands, as in Sweden, where peat is harvested only in peatlands with previous human alterations [Citation11]. The GHG emissions from peat harvesting consist of three phases: reference state, initial state and aftertreatment.
The reference state describes the state of the peat extraction area before peat extraction takes place. The reference state in this study is a forestry drained peatland. The average GHG emissions of peat harvesting sites were determined based on the review by Pohjala [Citation33]. The average GHG emissions were 200 g CO2/m2/year, 0.94 g CH4/m2/year and 0.04 g N2O/m2/year. When peat extraction begins, GHG emissions of the peat extraction area change radically. In the review by Pohjala [Citation33], the average value of peat harvesting GHG emissions was 13.90 t CO2/ha/year, 2.6 g/CH4/m2 year and 0.01 g N2O/m2/year. It was assumed that the extraction phase would last for 20 years. After the peat harvesting phase, the harvested peat extraction areas are either afforested or restored. For restoration, the average GHG emissions were set as −125 g CO2/m2/year and 25 g CH4/m2/year; N2O emissions were excluded, as they are assumed to be negligible [Citation33]. The annual GHG emissions from afforested areas were adopted from Grönroos et al. [Citation34]. In this study, we assumed that afforestation was the treatment option for 75% and restoration for 25% of extracted peatlands.
The paludiculture system does not need as many new peat extraction sites as the peat use system does. Annual GHG emissions from these unexploited areas were assumed to be the same as those of the reference state for the peat use system.
Peat decay
The majority of GHG emissions from peat energy use are caused by carbon decay [Citation34]. In peat energy use, carbon is instantly released into the atmosphere.. However, discarded growing media and a mixture of manure and discarded bedding material are typically used as a soil improver. In this case, carbon release is slow. We applied estimations by Karhu et al. [Citation35] to assess the timing of the carbon release of peat decay. In their study, it was assumed that 97% of carbon remains after one year, and half of the carbon is released after 30 years. After 100 years, 14% of the carbon remains. The GHG emissions caused by peat decay were inserted within the time frame 2020–2100.
Uncertainty assessments
As the majority of the parameters used in this study include substantial uncertainties, a Monte Carlo approach was applied. Because the number of parameters used in this study is substantial, we included only 16 parameters that were considered the most influential. Monte Carlo simulates uncertainty by considering the uncertainty distributions for each variable. The values and distributions of the key parameters were determined based on scientific articles (). A Monte Carlo simulation (Simulacion 4.0 Microsoft Excel add-in, with 2000 iterations) was applied to assess the uncertainties related to the key parameters.
Table 1. List of key parameters and uncertainty assumptions.
The yield data for paludicrops were adopted from the literature (). GHG emissions from paludicrop cultivation are influenced by several factors, such as ground water table level, vegetation composition, time since rewetting and land use history [Citation5]. To assess uncertainties related to paludicrop cultivation GHG emissions, the range of emission factors in Bianchi et al. [Citation19] were applied. For these parameters, a normal distribution was assumed. The ranges for the GHG emissions of the peat extraction sites were obtained from a literature review by Pohjala [Citation33]. For afforestation emissions, however, no suitable datasets were available to support uncertainty assessments. Also, no uncertainty assessment was included for N2O and CH4 emissions because they were not considered as relevant as CO2 emissions and insufficient datasets were available.
Peat has proven to be a suitable raw material for both animal litter and growing media. Alternative materials should meet the same quality requirements. Currently, there is no in-depth information available on the functional equivalency of paludicrops and peat. In other words, it is unclear how many tonnes of paludicrops are needed to replace a tonne of peat. Previously, it has been estimated that one tonne of chipped Phalaris arundinacea or Phragmites replaces a tonne of peat when used as chicken bedding [Citation12]. On the other hand, when used as material for horse bedding, one tonne of pelletized Phalaris arundinaceareplaced one tonne of peat [Citation12].
As these substitution assumptions were considered an influential, yet poorly understood, factor in the assessment of the net climate impacts of the paludiculture system, a wider range of uncertainty was included. We set the substitution ratio assuming that both mass-based and volume-based substitution is possible, and set a range to include both assumptions. The range was further widened to include a 20% increase or decrease in minimum and maximum substitution ratios. A uniform distribution was assumed for the substitution ratio parameters.
Time horizon
The dynamics of GHG emissions and carbon sinks influence the climate impacts of the paludiculture and peat use systems. In this study, GHG emissions and biogenic carbon intake were considered between 2020 and 2050. Peat decay, however, is a slow process, and a large share of peat will decay after 2050. Therefore, peat decay was considered over a longer period (2020–2100). Paludicrop decay is faster than peat decay. Nevertheless, some biogenic carbon in paludicrops will decay after 2050. As paludicrop carbon is of biogenic origin, paludicrop decay was not considered to be an emissions source, although biogenic carbon decay decreases the biogenic carbon storage.
We used the approach by Helin et al. [Citation38] to estimate the impacts of the timing of GHG emissions and carbon sinks. In this approach, GHG emissions and carbon intake today are given more weight than those occurring in the future. The reason for this is that when using the global warming potential factors evaluated over 100 years (GWP100), which is the most common case, we estimate climate impacts from the present to 100 years into the future. However, if the same method is applied to assess future GHG emissions (or sinks) as is used for emissions occurring today, the climate impact of future emissions becomes disproportionately high due to their effects extending beyond the 100-year time frame. This is corrected by using weighting factors that adjust the impacts of future emissions to match the time frame of impact assessment. For instance, according to Helin et al. [Citation38] GHG emissions occurring in 2020 are multiplied by a weighting factor of 1.0, whereas emissions in 2040 are given a weight of 0.82. Temporary carbon intake is weighted following a similar approach.
Results
Based on the results of this study, net GHG emissions in Finland can be decreased through paludiculture in abandoned organic croplands compared to the current situation based on peat use without paludiculture. Emissions of the peat use system were ca. 645,000 tonnes CO2 eq. The emissions of the paludiculture system in 2050 were 293,000 tonnes CO2 eq, generating emissions savings of 352,000 tonnes CO2 eq (). Compared to the peat use system, the paludiculture system was not an emissions source in any of the simulation rounds. It is therefore highly unlikely that the paludiculture system would generate more emissions than the peat use system.
Table 2. Annual greenhouse gas (GHG) emissions of peat use system and paludiculture system, and emissions savings, 2030–2050 (as tonnes of CO2 eq).
Through paludiculture, GHG emissions savings can be generated by decreasing emissions from abandoned organic croplands and peat substitution (Figure 3). Most of the emissions (ca. 70%) in the peat use system originate from abandoned organic croplands, and these emissions are thus fundamental when determining the potential GHG emissions savings. In 2050, these emissions in the paludiculture system are ca. 30% lower than in the peat use system because average emissions of paludicrop cultivation are lower than emissions of abandoned organic croplands. This makes paludiculture a less emissions-intensive land-use option. Although paludiculture decreased emissions of abandoned organic croplands, emissions of paludicrop cultivation were substantial, with an annual average of 300,000 tons of CO2 eq in 2050. Emissions of paludicrop cultivation are much higher (ca. 30%) than emissions from peat use (including peat decay and emissions of extraction sites and operations). Thus, peat substitution does not offset emissions of paludicrop cultivation. GHG emissions caused by the establishment and maintenance of a paludicrop cultivation site, as well as emissions of harvesting and manufacturing, constitute only ca. 10% of the paludiculture cultivation emissions, and peat extraction site emissions contribute even less (Figure 3).
Although land-use emissions of abandoned agricultural croplands dominate the results of this study, the paludiculture system provides some GHG emissions savings via peat substitution. The majority of GHG emissions of the peat use system are caused by abandoned organic croplands (). In this system, 23% of all GHG emissions in 2050 are caused by peat decay. When emissions of abandoned agricultural croplands are excluded, peat decay generates ca. 75% of the peat use system emissions. Carbon is released immediately in peat energy use, whereas it is released slowly and over a longer time frame in bedding and growing media use. As the time frame considered in this study was only 30 years, a substantial part of peat will decay after this time frame, making peat use appear less emission intensive. By 2100, cumulative GHG emissions of peat decay will be ca. 2 M tons of CO2 ().
Figure 2. Greenhouse gas emissions of avoided peat use system in 2050. Emissions related to land use from the peatland extraction sites are so small (less than 1% of the total emissions) that they cannot be distinguished.
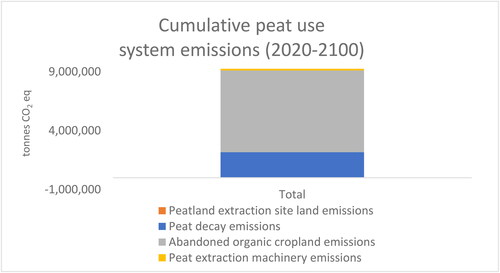
Table 3. Annual greenhouse gas emissions and carbon intake (as tons of CO2 eq) of the peat use system and the paludiculture system.
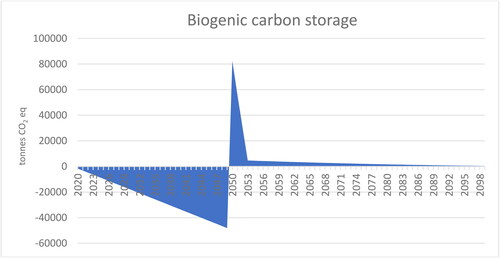
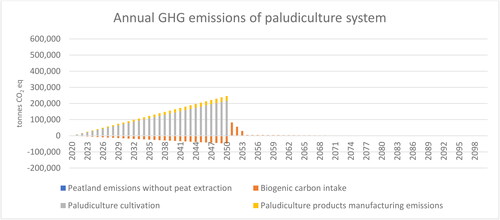
In this study, the biogenic carbon intake of paludicrops was estimated by assessing the intake of carbon into the system and outflows from the system. It was assumed that discarded paludiculture animal bedding manure mix and growing media would be used for soil improvement. The decay rate of discarded paludicrops is faster than the decay rate of peat. Biogenic carbon intake in paludicrops was, on average, 48,000 tons CO2 eq in 2050, and this offsets ca. 16% of the total GHG emissions of the paludiculture system in 2050 (). As biogenic carbon decays faster than peat, the biogenic carbon starts to sharply decrease after 2050 (). Thus, the biogenic carbon intake of paludicrops does not provide climate mitigation after 2050.
Figure 3. Greenhouse gas emissions and carbon intake of the paludiculture system in 2050. Emissions related to land use from the peatland extraction sites are so small (less than 2% of the total emissions) that they cannot be distinguished.
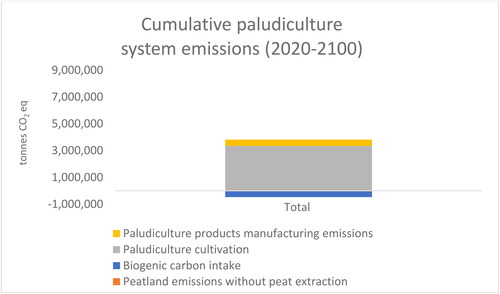
Figure 4. Biogenic carbon net flows of paludiculture crops cultivated in the period 2020–2050. A negative value indicates a net increase in biogenic carbon storage, whereas a positive value indicates a net decrease. The cessation of carbon intake is attributed to the assumption that paludiculture is projected to conclude by the year 2050, marking the end of its carbon sequestration. The peak in biogenic carbon outtake signifies the point at which biogenic carbon, originating from previously harvested biomass, continues to be released into the atmosphere, while carbon intake from paludicrops has ceased.
Peat extraction temporarily increases the GHG emissions of a peat extraction site. This effect is insignificant considering the total emissions of the peat use system. Peat extraction site emissions are less than 4% of the total peat use system emissions. Furthermore, GHG emissions of peat extraction sites decrease when peat extraction ends, as emissions of both afforestation and restoration are lower than emissions of a peat extraction phase. In the paludiculture system, because the peat extraction sites were assumed to remain unaltered, the emissions of these sites were assumed to remain at the same level. Emissions of peat extraction sites without peat extraction generate only 2% of the total emissions of the paludiculture system. Thus, emissions of peat harvesting sites are not significant in either system.
The cumulative GHG emissions (2020–2100) of the peat use system are nearly 3 times higher than the emissions of the paludiculture system (). The cumulative GHG emissions of the peat use system are 9.3 million tons CO2 eq (), whereas cumulate emissions of the paludiculture system are 3.3 million tons CO2 eq (). When cumulative emissions of the peat use system are considered, peat decay generates 23% of the emissions. Thus, although the emissions caused by peat decay generate the larger share of the total peat use system emissions when a longer time frame is considered, they remain a much less prominent emissions source than abandoned organic croplands, which generate ca. 75% of the total emissions of the peat use system.
Figure 5. Annual greenhouse gas emissions of peat use system between 2020 and 2100. Peat decay from 2050 onward is caused by peat used between 2020 and 2050. Emissions related to land use from the peatland extraction sites are so small (less than 1% of the total emissions) that they cannot be distinguished.
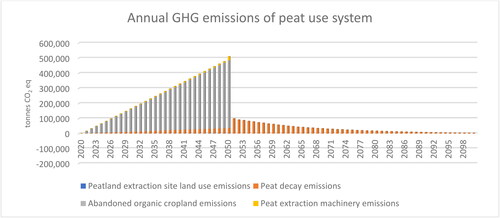
Figure 6. Annual greenhouse gas emissions and carbon intake of paludiculture system between 2020 and 2100. A positive value represents emissions, whereas a negative value indicates carbon intake. Emissions related to land use from the peatland extraction sites are so small (less than 2% of the total emissions) that they cannot be distinguished.
Discussion
In this study, we assessed the GHG emission reduction potential of paludiculture in abandoned organic croplands in Finland, considering both land-use emissions and peat substitution. Emissions savings were particularly achieved when the land-use emissions from abandoned organic croplands decreased after their transition to the paludiculture system. Still, paludicrop cultivation was a major source of emissions in the paludiculture system. Paludiculture should be carbon-neutral or negative in the long term [Citation39]. For instance, it has been estimated that peat extraction sites will become net carbon sinks within 30 years of rewetting [Citation20]. As climate change mitigation must take place in the near future, shorter time frames should be considered when assessing the climate mitigation potential of paludiculture. However, recently rewetted sites that are still undergoing transitional changes – for example, the decomposition of a residual biomass pool – may act as emissions sources [Citation5]. In this study, we focused solely on a 30-year time frame to assess the land-use emissions associated with paludicrop cultivation, assuming some reduction in cultivation emissions during that period. The benefit of paludiculture is that it not only generates land-use GHG emissions savings but also provides renewable raw material. As Finland has set ambitious targets to decrease peat use, new raw materials to replace peat are urgently needed. In this study we assumed that paludicrops would be harvested and used as a raw material for growing media and animal bedding. This would generate so-called substitution benefits when emissions caused by the use of peat are avoided. As discussed in previous chapters, however, peat substitution is not sufficient to offset GHG emissions of paludicrop cultivation. To decrease the net GHG emissions of the paludiculture system, it is crucial to decrease cultivation emissions and increase carbon intake. For instance, Sphagnum farming is considered a carbon sink, when biomass is not harvested [Citation19]. Other paludicrops considered in this study can also become carbon sinks; however, they can also promote increased CH4 emissions [Citation7,Citation12,Citation19]. One option for reducing GHG emissions is to remove organic croplands from agricultural production. Afforesting former organic croplands can also generate emissions savings. Lohila et al. [Citation40] found that peat decay exceeded the carbon intake in forested organic croplands, making them small sources of emissions with an annual average of 50 g CO2 eq m2. However, evidence on the long-term climate benefits of afforesting drained peatlands is still lacking, and without restoring their hydrology, the ecosystem functions are not fully restored [Citation41]. Afforestation makes possible the generation of renewable biomass. However, harvesting can occur in the future, whereas the biomass in paludiculture can be harvested a couple of years after the establishment of a paludicrop cultivation site. Restoration, i.e. rewetting, should turn organic croplands into net CO2 sinks [Citation5], but a drawback is that no renewable raw material is produced simultaneously with the rewetting. Nonetheless, based on the results of this study, peat substitution does not offset emissions of paludicrop cultivation. Thus, when assuming the GHG emissions used in this study, transforming former organic croplands into carbon sinks in a short time period would generate more emissions savings than paludiculture, even without peat substitution. As discussed earlier, it is possible that the cultivation emissions of paludiculture are overestimated in this study. As uncertainties related to GHG emissions of recently established paludiculture cultivation sites are substantial, it is challenging to make robust assumptions about the most beneficial land-use option for abandoned organic croplands. It is, however, evident that existing abandoned agricultural croplands are a major source of GHG emissions, and paludiculture, as well as other treatment options, could substantially decrease these emissions. It is likely that global warming could affect various key parameters of this study. Warmer temperatures could increase the rate of organic material decay, and this may result in the release of the carbon stored. Thus, the anticipated emissions of peatlands could increase in the future because of warming climate. Global warming can also accelerate the rate of decay of peat and discarded paludicrop products.
In this study, we assumed that all abandoned organic croplands in Finland would be suitable for paludiculture. However, this is not likely because of technical and economic restrictions. For instance, small or scattered areas may be unsuitable for paludiculture as there is no incentive or need for the associated machinery and infrastructure [Citation42]. Although paludicrops considered in this study are moderately adaptable to various growing conditions, Sphagnum farming has some special requirements related to e.g. sufficient water sources, pH and nutrient availability [Citation43]. Organic croplands taken into paludiculture need to have suitable hydrological conditions, and how draining would affect neighboring areas should also be considered [Citation44]. Overall, controlling the water table level is a key element in the climate change mitigation potential of paludiculture [Citation45,Citation46].
The focus of this study was climate change mitigation; however, paludiculture also has beneficial impacts for biodiversity [Citation21,Citation42] and water quality [Citation47]. In this study, the economic feasibility of paludiculture was not assessed. From previous studies, paludiculture does not appear to be an economically viable option, mainly due to its high cultivation costs and low revenues [Citation6,Citation31]. In Germany, under favorable conditions, paludiculture can be economically viable, but costs and revenues vary considerably [Citation21]. The economic feasibility should be assessed case by case, as the local circumstances have a substantial impact on the economics of paludiculture. Its economic viability could be further improved by introducing, for example, carbon credits and financial and policy support [Citation31].
Conclusions
Establishing paludiculture in abandoned organic croplands and substituting peat as bedding material and growing media with products based on paludicrops appear to provide GHG mitigation compared to the current peat use system in Finland. The majority of the emissions savings are caused by decreasing emissions of abandoned organic croplands, but peat substitution also generates some emissions savings. However, peat substitution does not offset the cultivation emissions of paludicrops. Although paludiculture appears to generate emissions savings compared to the current peat use system, other land-use options, such as afforestation or restoration, could also generate emissions savings. As the means to decrease emissions should be efficient over short time frames, it is crucial to consider land-use options that can rapidly turn abandoned organic croplands into carbon sinks or minor emissions sources.
Data availability statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
Disclosure statement
The authors do not have conflicts of interest to disclose.
Additional information
Funding
References
- Joosten H, Sirin A, Couwenberg J, et al. The role of peatlands in climate regulation. In: Bonn A, Allott T, Evans M, Joosten H, Stoneman R, editors. Peatland restoration and ecosystem services. Cambridge (UK): Cambridge University Press; 2016; pp. 63–76.
- Maljanen M, Hytönen J, Mäkiranta P, et al. Greenhouse gas emissions from cultivated and abandoned organic croplands in Finland. Boreal Environ Res. 2007;12:133–140.
- Günther A, Barthelmes A, Huth V, et al. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat Commun. 2020;11(1):1644. doi: 10.1038/s41467-020-15499-z.
- Vroom RJE, Xie F, Geurts JJM, et al. Typha latifolia paludiculture effectively improves water quality and reduces greenhouse gas emissions in rewetted peatlands. Ecol Eng. 2018;124:88–98. doi: 10.1016/j.ecoleng.2018.09.008.
- Wilson D, Blain D, Couwenberg J, et al. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Peat. 2016;17:1–28.
- Wichmann S. Commercial viability of paludiculture: a comparison of harvesting reeds for biogas production, direct combustion, and thatching. Ecol Eng. 2017;103:497–505. doi: 10.1016/j.ecoleng.2016.03.018.
- Lahtinen L, Mattila T, Myllyviita T, et al. Effects of paludiculture products on reducing greenhouse gas emissions from agricultural peatlands. Ecol Eng. 2022;175:106502. doi: 10.1016/j.ecoleng.2021.106502.
- Hartung C, Andrade D, Dandikas V, et al. Suitability of paludiculture biomass as biogas substrate – biogas yield and long-term effects on anaerobic digestion. Renew Energy. 2020;159:64–71. doi: 10.1016/j.renene.2020.05.156.
- Bionenergia ry. Peat information databank (In Finnish). 2023. Available from: https://www.bioenergia.fi/tietopankki/turve/
- Natural resources institute Finland 2021. Production, consumption and foreign trade of peat 1970-2021 by year and use Table: Production, consumption and foreign trade of peat 1970-2021. Available from: https://statdb.luke.fi/PxWeb/pxweb/en/LUKE/LUKE__04%20Metsa__08%20Muut__Energia/11.00_Turpeen_tuotanto_kulutus_ja_ulkomaank.px/table/tableViewLayout2/
- Soimakallio S, Sankelo P, Kopsakangas-Savolainen M, et al. Turpeen rooli ja sen käytöstä luopumisen vaikutukset Suomessa. Sitra; 2019. Available from: https://www.sitra.fi/julkaisut/turpeen-rooli-ja-sen-kaytosta-luopumisen-vaikutukset-suomessa
- Lehtoranta S, Johansson A, Myllyviita T, et al. Turvetta korvaavien kuivikemateriaalien ilmastovaikutukset. Finnish Environment Institute; 2021. Available from: https://helda.helsinki.fi/items/3ec407d1-b888-4c48-a88a-361f7afcad4c
- Gaudig G, Krebs M, Joosten H. Sphagnum growth under N‐saturation: interactive effects of water level and P or K fertilisation. Plant Biol J. 2020;22(3):394–403. doi: 10.1111/plb.13092.
- Martens M, Karlsson NPE, Ehde PM, et al. The greenhouse gas emission effects of rewetting drained peatlands and growing wetland plants for biogas fuel production. J Environ Manage. 2021;277:111391. doi: 10.1016/j.jenvman.2020.111391.
- Ziegler R, Wichtmann W, Abel S, et al. Wet peatland utilisation for climate protection: an international survey of paludiculture innovation. Clean Eng Technol. 2021;5:100305. doi: 10.1016/j.clet.2021.100305.
- Kekkonen H, Ojanen H, Haakana M, et al. Mapping of cultivated organic soils for targeting greenhouse gas mitigation. Carbon Manage. 2019;10(2):115–126. doi: 10.1080/17583004.2018.1557990.
- Naukkarinen V. Kosteikkoviljelyn kasviopas. 2021. Available from: https://carbonaction.org/wp-content/uploads/2021/02/Kosteikkoviljelyn_kasviopas_2021.pdf
- IPCC. 2013 supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: wetlands (Hiraishi T, Krug T, Tanabe K, Srivastava N, Baasansuren J, Fukuda M, Troxler TG, editors.). Geneva (Switzerland): IPCC; 2014.
- Bianchi A, Larmola T, Kekkonen H, et al. Review of greenhouse gas emissions from rewetted agricultural soils. Wetlands. 2021;41(8):108. doi: 10.1007/s13157-021-01507-5.
- Beyer C, Höper H. Greenhouse gas exchange of rewetted bog peat extraction sites and a Sphagnum cultivation site in Northwest Germany. Biogeosciences. 2015;12(7):2101–2117. doi: 10.5194/bg-12-2101-2015.
- Tanneberger F, Appulo L, Ewert S, et al. The power of nature-based solutions: how peatlands can help us to achieve key EU sustainability objectives. Adv Sustain Syst. 2021;5(1):2000146. doi: 10.1002/adsu.202000146.
- Tanneberger F, Birr F, Couwenberg J, et al. Saving soil carbon, greenhouse gas emissions, biodiversity and the economy: paludiculture as sustainable land use option in German fen peatlands. Reg Environ Change. 2022;22(2):69. doi: 10.1007/s10113-022-01900-8.
- Harpenslager SF, van den Elzen E, Kox MAR, et al. Rewetting former agricultural peatlands: topsoil removal as a prerequisite to avoid strong nutrient and greenhouse gas emissions. Ecol Eng. 2015;84:159–168. doi: 10.1016/j.ecoleng.2015.08.002.
- Quinty F, Rochefort L. Peatland restoration guide. 2nd ed. Québec (Canada): Canadian Sphagnum Peat Moss Association and New Brunswick Department of Natural Resources and Energy; 2003.
- Murphy F, Devlin G, McDonnell K. Energy requirements and environmental impacts associated with the production of short rotation willow (Salix sp.) chip in Ireland. GCB Bioenergy. 2014;6(6):727–739. doi: 10.1111/gcbb.12111.
- Nordh NE. Long term changes in stand structure and biomass production in short rotation willow coppice [doctoral thesis no. 2005, 120]. Uppsala (Sweden): Faculty of Natural Resources and Agricultural Sciences (SLU); 2000.
- Boyd J, Christersson L, Dinkelbach L. Energy from Willow. Edinburgh (UK): The Scottish Agricultural College; 2000. Available from: https://www.ecologieforum.eu/download/Willow_s.pdf
- Pijlman J, Guerts J, Vroom R, et al. The effects of harvest date and frequency on the yield, nutritional value and mineral content of the paludiculture crop cattail (Typha latifolia L.) in the first year after planting. Mire Peat. 2019;251:1–19.
- Pahkala K, Partala A, Suokangas A, et al. Ruokohelven viljely ja korjuu energian tuotantoa varten. 2002. Available from: http://www.mtt.fi/met/html/met1.htm
- Kandel TP, Karki S, Elsgaard L, et al. Fertilizer-induced fluxes dominate annual N2O emissions from a nitrogen-rich temperate fen rewetted for paludiculture. Nutr Cycl Agroecosyst. 2019;115(1):57–67. doi: 10.1007/s10705-019-10012-5.
- de Jong M, van Hal O, Pijlman J, et al. Paludiculture as paludifuture on Dutch peatlands: an environmental and economic analysis of Typha cultivation and insulation production. Sci Total Environ. 2021;792:148161. doi: 10.1016/j.scitotenv.2021.148161.
- Heinonsalo J, editor. Hiiliopas. Katsaus maaperän hiileen ja hiiliviljelyn perusteisiin. 2020. Available from: https://carbonaction.org/wp-content/uploads/2020/01/BSAG-hiiliopas-1.-painos-2020.pdf
- Pohjala M. Mikä on energia-ja kasvuturpeen elinkaaren ilmastovaikutus? [master thesis]; 2014. Available from: https://helda.helsinki.fi/bitstream/handle/10138/136417/MariaBPohjala_ProG_9_11_2014.pdf?sequence=1&isAllowed=year
- Grönroos J, Seppälä J, Koskela S, et al. Life-cycle climate impacts of peat fuel: calculation methods and methodological challenges. Int J Life Cycle Assess. 2013;18(3):567–576. doi: 10.1007/s11367-012-0512-x.
- Karhu K, Gärdenäs AI, Heikkinen J, et al. Impacts of organic amendments on carbon stocks of an agricultural soil – comparison of model-simulations to measurements. Geoderma. 2012;189-190:606–616. doi: 10.1016/j.geoderma.2012.06.007.
- Mola-Yudego B. Regional potential yields of short rotation willow plantations on agricultural land in Northern Europe. Silva Fenn. 2010;44(1):63–76. doi: 10.14214/sf.163.
- Heikkilä M. Uusiutuva sammal korvaamaan kasvuturvetta. Maatilan Pellervo; 2020. Available from: https://maatilanpellervo.fi/2020/08/13/uusiutuva-sammal-korvaamaan-kasvuturvetta/
- Helin T, Salminen H, Hynynen J, et al. Global warming potentials of stemwood used for energy and materials in Southern Finland: differentiation of impacts based on type of harvest and product lifetime. GCB Bioenergy. 2016;8(2):334–345. doi: 10.1111/gcbb.12244.
- Zu Dienle T, Lupascu M, Wijedasa LS. Paludiculture as a sustainable land use alternative for tropical peatlands: a review. Sci Total Environ. 2021;753:142111. doi: 10.1016/j.scitotenv.2020.142111.
- Lohila A, Laurila T, Aro L, et al. Carbon dioxide exchange above a 30-year-old Scots pine plantation established on organic-soil cropland. Boreal Environ Res. 2007;12:141–157.
- Jurasinski G, Barthelmes A, Byrne KA, et al. Active afforestation of drained peatlands is not a viable option under the EU Nature Restoration Law. Ambio. 2024;53(7):970–983. doi: 10.1007/s13280-024-02016-5.
- Mulholland B, Abdel-Aziz I, Lindsay R, et al. An assessment of the potential for paludiculture in England and Wales. Report to Defra for Project SP1218; 2020. p. 98.
- Temmink RJM, Fritz C, van Dijk G, et al. Sphagnum farming in a eutrophic world: the importance of optimal nutrient stoichiometry. Ecol Eng. 2017;98:196–205. doi: 10.1016/j.ecoleng.2016.10.069.
- Niemi J. Monitavoitteinen päätösanalyysi turvepeltojen käyttömuotojen vertailussa [master’s thesis]. Available from: https://helda.helsinki.fi/server/api/core/bitstreams/27ce2f63-0fbf-4953-ad71-85d56c085d94/content
- Ojanen P, Minkkinen K. The dependence of net soil CO2 emissions on water table depth in boreal peatlands drained for forestry. Mire Peat. 2019;24:1–8.
- Koch J, Elsgaard L, Greve MH, et al. Water-table-driven greenhouse gas emission estimates guide peatland restoration at national scale. Biogeosciences. 2023;20(12):2387–2403. doi: 10.5194/bg-20-2387-2023.
- Geurts JJM, Oehmke C, Lambertini C, et al. Nutrient removal potential and biomass production by Phragmites australis and Typha latifolia on European rewetted peat and mineral soils. Sci Total Environ. 2020;747:141102. doi: 10.1016/j.scitotenv.2020.141102.
