ABSTRACT
Background: Combining statins with ezetimibe synergistically enhances lipid lowering, thereby reducing the need to prescribe maximal statin doses to achieve low-density lipoprotein cholesterol (LDL-C) goals. Real-world data on concurrent ezetimibe + statin therapy in Asians are sparse. Therefore, we evaluated the effectiveness of a single combined tablet of ezetimibe + simvastatin 10.0 + 20.0 mg (EZE + SIM) in Taiwanese patients with hypercholesterolaemia. Methods: We analysed retrospective data from patients who received EZE + SIM at a community hospital in New Taipei City, Taiwan, took EZE + SIM continuously for ≥24 weeks, and had before and after lipid data. Outcomes including lipid lowering, LDL-C goal attainment and safety (non-lipid serum biochemistry), were compared between diabetic versus non-diabetic patients and subgroups prescribed different EZE + SIM doses. Results: Among 157 EZE + SIM users, more than half had diabetes (64.3%) and/or hypertension (52%) and 24.1% had coronary artery disease. A mean EZE + SIM dose of 6.5 + 13.0 (median 5.0 + 10.0) mg/day for a mean of 51.6 weeks, significantly reduced total cholesterol (−30.4%), LDL-C (−36.2%) and triglycerides (−14.5%); consequently, attainment rates of LDL-C ≤ 100 mg/dl and ≤70 mg/dl goals were significantly higher after EZE + SIM treatment. There were no clinically significant changes in biomarkers of hepatic or renal function. Consistent with other reports, we observed indications of greater lipid-lowering efficacy and LDL-C goal attainment among patients with diabetes versus those without, at equivalent EZE + SIM doses. Conclusions: Our findings affirm the lipid-lowering efficacy of single-tablet fixed-dose EZE + SIM in real-world Taiwanese-Chinese patients with hypercholesterolaemia, especially at the recommended dose. Trends towards greater efficacy in diabetic than non-diabetic patients suggest that EZE + SIM may be a rational choice for treating patients with hypercholesterolaemia and diabetes.
Introduction
Hypercholesterolaemia is an important risk factor for cardiovascular (CV) disease, and medications that lower serum lipid levels reduce the risk of adverse CV sequelae [Citation1–Citation3]. Accordingly, international guidelines recommend prescribing lipid-lowering agents to patients at risk for CV disease, to reduce morbidity and mortality from outcomes such as myocardial infarction, stroke and peripheral arterial disease [Citation3,4]. The European Society of Cardiology (ESC) and European Atherosclerosis Society 2011 guideline sets low-density lipoprotein cholesterol (LDL-C) treatment targets of ≤100 mg/dl for patients at high risk and ≤70 mg/dl and/or ≥50% LDL-C reduction for those in the very high-risk category, which includes patients with diabetes [Citation3]. Based on positive results from large-scale clinical trials, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), which inhibit cholesterol biosynthesis, are the current drugs of choice for reducing serum cholesterol [Citation1,3]. Notwithstanding undoubted lipid-lowering efficacy, statin therapy carries some risk of muscle and liver toxicity, especially at higher doses [Citation3,5,6], and has also been associated with an increased incidence of new onset type 2 diabetes mellitus, also dose dependently [Citation7]. Consequently, in actual clinical practice, some physicians may be reluctant to up-titrate statins to doses sufficient to attain guideline-recommended LDL-C goals, or to prescribe intensive lipid-lowering therapy for patients with certain risk profiles [Citation8,9].
Ezetimibe, which decreases intestinal cholesterol absorption by inhibiting the Niemann–Pick C1-like 1 (NPC1L1) cholesterol transporter [Citation10], is an alternative for patients who cannot tolerate statins. However, it is most commonly co-administered as an adjunctive lipid-lowering agent for patients still not at LDL-C goal despite statin monotherapy [Citation3,6,11]. Numerous studies have affirmed that co-administering statins with ezetimibe has greater cholesterol-lowering potency than statin (or ezetimibe) monotherapy, due to the synergistic effect of simultaneously inhibiting both cholesterol synthesis and absorption [Citation1,2,4,5,12–14]; for example, pooled analysis of patients with primary hypercholesterolaemia showed that ezetimibe plus the lowest dose of statin lowered LDL-C as efficaciously as the highest dose of statin monotherapy [Citation12]. Early initiation of concomitant ezetimibe plus statin is therefore a rational strategy to achieve target LDL-C levels in patients with hypercholesterolaemia, without having to resort to maximal statin doses [Citation1,4–6,12,14].
Evident discrepancy between the success of statin therapy in clinical trials versus actual clinical practice stems in part from real-world drug adherence issues. In this context, a combined single-pill treatment may improve patient adherence and thereby result in more consistent outcomes [Citation15]. A single-pill combination of ezetimibe + simvastatin (EZE + SIM), which is typically prescribed at a starting dose of EZE 10.0 mg and SIM 20.0 mg (Vytorin®; Merck & Co., Inc., Whitehouse Station, NJ, USA) [Citation16], has proven safe and effective in clinical trials and is in widespread use worldwide [Citation1,4–6]. Although landmark statin trials involved huge numbers of subjects, most were from populations with predominantly European ancestry; Asians might respond differently [Citation17]. However, clinical studies in Asian populations have mostly been limited to Japan, and real-world efficacy and safety data are lacking from most other Asian countries.
In this context, we conducted a retrospective chart review to determine the effectiveness of single-pill EZE + SIM 10.0 + 20.0 mg in the real-life treatment of patients at our community-based hospital in New Taipei City, Taiwan. Almost all of the participants were Taiwanese Chinese and taking lipid-lowering therapy for hypercholesterolaemia.
Methods
Study design and subjects
This was a retrospective, non-interventional, chart review analysis of patients who received EZE + SIM at a community hospital in New Taipei City, Taiwan. We retrieved electronic hospital records of patients who had attended the clinic for follow-up consultations from 1 January to 31 March 2013, and were dispensed EZE + SIM from the pharmacy. They had all been prescribed EZE + SIM at the fixed dose of 10.0 + 20.0 mg, but with various dosing regimens, specifically: half a pill twice-weekly; half a pill every other day; two pills per week; three per week; one every other day; or once daily (7/week), which is the label recommended dose [Citation16].
Patient records were screened against inclusion criteria: (1) had taken EZE + SIM continuously for ≥24 weeks and (2) had before and after treatment serum lipid data available. We assumed that the lipid-lowering effect of regular EZE + SIM treatment for more than 24 weeks would plateau, such that the lipid profile change from baseline would indicate the efficacy of this treatment.
Data that had been recorded from eligible patients before and after ≥24-weeks EZE + SIM treatment, were analysed; this information included demographics, comorbidities, risk factors, the EZE + SIM regimen and blood test results. The study variables included total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), serum creatinine, liver transaminases, uric acid and glycated haemoglobin (HbA1c). Diagnostic criteria for comorbidities besides hypercholesterolaemia were: hypertension = systolic/diastolic blood pressure of 140/90 mm Hg; diabetes = HbA1c ≥6.5% and/or using medication(s) or dietary modifications to control diabetes; and renal insufficiency = serum creatinine >1.24 mg/dl.
Statistical analysis
Statistical analyses of before versus after treatment data included comparisons between diabetic and non-diabetic patients, as well as subgroups who had taken either ≤2, 3 or 4 or 7 EZE + SIM pills/week. Continuous data are presented as mean ± standard deviation or medians with interquartile ranges, as appropriate to the data distribution; categorical variables are described by frequency in the entire cohort or patients with available data (percentage). Subjects with missing data values were excluded from before and after EZE + SIM treatment comparisons, with no statistical adjustment. The Pearson chi-squared test or Fisher’s exact test were used as appropriate to examine the significance of differences between sets of categorical variables. The Mann–Whitney U-test was used to analyse the statistical significance of absolute differences and percentage changes in continuous variables between diabetic and non-diabetic patient subgroups. The Wilcoxon signed ranks test was applied to analyse the statistical significance of differences between groups of dependent paired data values obtained before/after EZE + SIM treatment. The significance of changes from baseline in ESC LDL-C goal attainment after EZE + SIM treatment in the entire study cohort, patients with and without diabetes, and different dosing subgroups was determined using the McNemar test. Two-tailed p-values <0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics for Windows Version 21 (IBM Corp., Armonk, NY, USA).
Ethics
This protocol was exempt from Institutional Review Board (IRB) approval according to Article 5 of the Human Subjects Research Act (28 December 2011), Ministry of Health and Welfare, Republic of China (Taiwan). Specifically, according to the Taiwan Food & Drug Administration Announcement No. 1010265075, the participants did not meet the specified criteria for subjects mandating such review, were subjected to no medical interactions or interventions other than usual ongoing care and are not identifiable individually from the data published.
Results
Between 1 January and 31 March 2013, 414 patients at New Taipei City Hospital San-Chong Branch were dispensed a prescription for EZE + SIM, of whom 157 had taken EZE + SIM for ≥24 weeks continuously and had before and after lipid data available; 257 did not meet these inclusion criteria.
Patient characteristics
The study sample comprised similar proportions of males and females, average age 56.9 years, and predominantly never-smokers (); however, the actual proportion of smokers was uncertain because 29/157 had unknown smoking status. More than half of those for whom data were available had diabetes mellitus (64.3%) and/or hypertension (52.0%), more than one-quarter (27.4%) had macrovascular complications (), predominantly coronary artery disease (24.1%) and 11.0% had renal insufficiency; however, macrovascular complications besides coronary artery disease were less prevalent and none had occlusive peripheral artery disease. Excluding diabetes, 55.2% of patients had at least one of the aforementioned comorbid conditions.
Table 1. Patient demographics and characteristics.
Table 2. Patient comorbidities at baseline.
Statin use and dosing
Most patients prescribed EZE + SIM were new statin users, with only 19.1% having received another statin as monotherapy within 1 year before starting EZE + SIM. More than 80% were prescribed EZE + SIM at doses equivalent to ≥3 pills/week, with 40.1% taking the recommended once-daily dose [Citation16]; the mean dose was 6.5 mg ezetimibe and 13.0 mg simvastatin per day (median 5.0 + 10.0 mg/day), for a mean treatment duration of approximately 1 year (EZE + SIM start date till date of blood sampling for the lipid profile after ≥24 weeks of treatment). Since the study population attended the hospital dispensary regularly to refill their EZE + SIM prescriptions, the exact numbers of pills each had taken were recorded: according to this index, non-adherence was not an issue.
Diabetic vs. non-diabetic subgroups
Compared with non-diabetic patients, those with diabetes were approximately 10 years older, significantly more likely to have previously taken statins, and took EZE + SIM at significantly higher mean daily doses (); almost half took the standard once-daily dose, compared with only a quarter of non-diabetics. A significantly higher proportion among the diabetes cohort had renal insufficiency (), with higher creatinine levels pre- and post-treatment (). Although there were no significant differences between the groups in the prevalence of other non-diabetes comorbidities, patients with diabetes did tend to have more non-diabetes comorbidities than non-diabetics (60.6 vs. 45.5%) (); however, this was not quite statistically significant (p = 0.056).
Table 3. Non-lipid serum biochemistry before and after EZE + SIM treatment.
Serum lipid changes and goal attainment
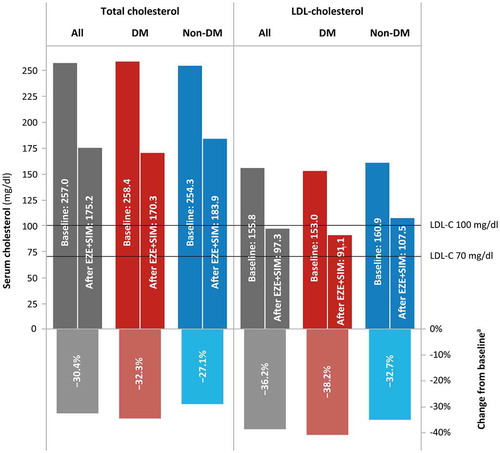
Mean total cholesterol before patients commenced their qualifying EZE + SIM treatment period was 257 mg/dl (), with mean baseline LDL-C > 150 mg/dl and median TG level of 179 mg/dl (range 39–4985 mg/dl). As HDL-C data were available for only 17 patients at baseline and 14 after EZE + SIM treatment, only 7 of whom had baseline data, HDL-C and non-HDL-C levels were not analysed.
Table 4. Serum lipid profile before and after EZE + SIM treatment.
EZE + SIM treatment significantly reduced levels of TC, LDL-C and TG from baseline (all p ≤ 0.001), with a trend for greater reductions in TC (p = 0.017) and LDL-C (p = 0.076) levels among patients with versus without diabetes from comparable baseline levels in these groups (, ).
LDL-C goal attainment
Attainment of ESC LDL-C goals reflected the significantly improved lipid profiles. At baseline, only seven patients (four prescribed statins for fatty liver and three with coronary artery disease) had baseline LDL-C below the ESC cut-offs of ≤100 mg/dl (5.3%) or ≤70 mg/dl (2.3%), compared with 59.0% and 17.4% (p < 0.001), respectively, who attained these goals after EZE + SIM therapy (, ). Higher proportions of patients with versus without diabetes, achieved either LDL-C goal, which was statistically significant for LDL-C ≤70 mg/dl (p = 0.039).
Figure 2. ESC LDL-C goal attainment in patients with vs. without diabetes.
Notes: ESC, European Society for Cardiology; LDL-C, low-density lipoprotein cholesterol; DM, diabetes mellitus; EZE+SIM ezetimibe + simvastatin 10.0 + 20.0 mg.
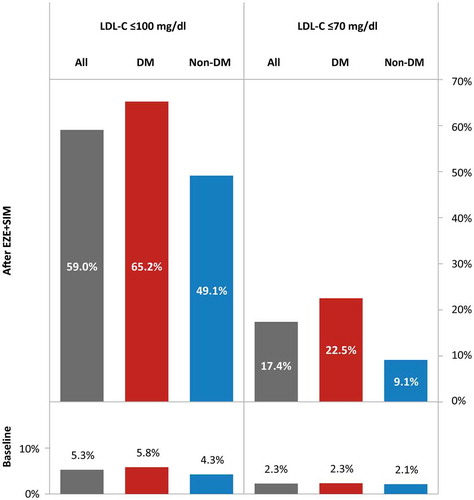
Table 5. LDL-C goal attainment in patient groups on different EZE + SIM regimens.
Changes in non-lipid serum biomarkers
At follow-up after EZE + SIM therapy, serum creatinine remained similar to its baseline level; decreases in liver transaminases and uric acid were not clinically significant ().
During the observation period, the median HbA1c level in diabetic patients, who were all using medication(s) and/or or diet modification to control diabetes concurrent with lipid-lowering therapy, decreased significantly, by 0.3 from 7.7% at baseline (). Despite a statistically significant increase from 5.6 to 5.9% in median HbA1c among non-diabetic patients, no cases of new diabetes onset occurred in this group at follow-up.
Subgroup analysis by EZE + SIM regimen
Since some patients were prescribed less than the recommended daily dose of EZE + SIM, we analysed LDL-C lowering and ESC goal attainment in subgroups of patients who had taken ≤2, 3 or 4, or 7 EZE + SIM pills once daily per week. The number of EZE + SIM pills prescribed per week was positively correlated with baseline TC, such that patients with the highest cholesterol levels were most likely to be prescribed EZE + SIM once-daily (). Over each of the three dosing ranges, diabetic patients consistently had greater percentage LDL-C reductions from baseline than non-diabetic patients who took equivalent doses (, ); however, with no statistically significant differences. Among patients who had taken the recommended EZE + SIM dose, 70.2% overall achieved LDL-C ≤ 100 mg/dl (p < 0.001) and >25% achieved LDL-C ≤ 70 mg/dl (p = 0.003) (); although patients with diabetes were more likely than those without to attain LDL-C ≤ 100 mg/dl (76.7 vs. 50.0%) or ≤70 mg/dl (27.9 vs. 21.4%), these differences were not statistically significant.
Figure 3. LDL-C reduction and ESC goal attainment after ≥24 weeks treatment with differing EZE + SIM dosing regimens.
Notes: LDL-C, low-density lipoprotein cholesterol; ESC, European Society for Cardiology; EZE+SIM, ezetimibe + simvastatin 10.0 + 20.0 mg; TC, total cholesterol; DM, diabetes mellitus.
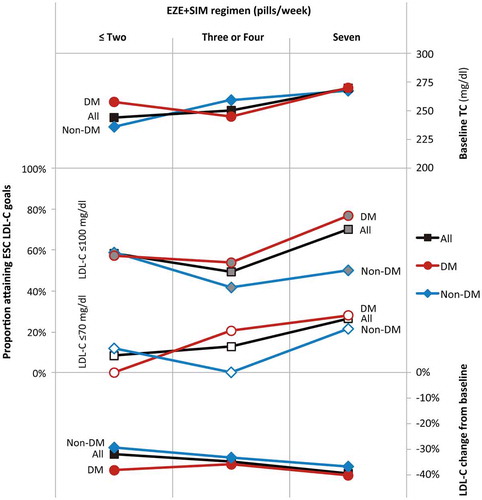
Table 6. Baseline cholesterol and LDL-C lowering in patient groups on different EZE + SIM regimens.
Discussion
This study provides rare insights into the prescription and efficacy of fixed-dose EZE + SIM in a real-life cohort of Taiwanese-Chinese patients, many of whom had high risk of CV disease. Consistent with other studies, we observed trends towards greater lipid-lowering efficacy and LDL-C goal attainment in patients with diabetes than without, although further confirmatory data are needed. No particular treatment-related safety issues were evident.
Our results corroborate the substantial lipid-lowering efficacy of EZE + SIM in real-life treatment of Asian patients with hypercholesterolaemia; this is especially relevant given the relatively low EZE + SIM mean daily dose of only 6.5 + 13.0 mg (median 5.0 + 10.0 mg) compared with the label dose of 10.0 + 20.0 mg. Furthermore, the fact that 19.1% of the patients had already received various statin treatments within 1 year before starting EZE + SIM suggests that they constituted a population in whom achieving treatment goals was particularly challenging. Besides hypercholesterolaemia, some patients had very high TG levels – 15 with TG > 500 mg/dl at baseline, 11 of whom were diabetic (3 had HbA1c > 9.0%), and 8 after EZE + SIM treatment; these patients were not prescribed concurrent fibrate because Taiwan National Health insurance does not cover statin plus fibrate therapies.
Although intolerance to statin therapy and non-adherence are common barriers to successful lipid-lowering therapy, especially among the highest risk patients [Citation4,5,14,18], neither was an issue in this patient cohort. Adding ezetimibe to statins, lowers LDL-C by up to 25% over and above statin monotherapy [Citation2,4,6,19] thereby enabling more patients to attain their lipid goals without using very high statin doses that have been associated with muscle and liver-related adverse effects [Citation5]. Specifically, statins may incur a dose-dependent risk of liver toxicity, causing serum aminotransferase elevations and very rarely, acute, even fulminant, hepatitis [Citation20]. However, at the EZE + SIM doses used in this study, there were no clinically significant changes in serum creatinine, aminotransferases, or uric acid, indicating that there was no detrimental treatment-related impact on hepatic or renal function. This, together with the convenience of once-daily fixed-dose administration, may explain why medication monitoring in this study cohort showed excellent adherence to the prescribed doses. Rather, underdosing reflects real-world clinical practice in Taiwan: although National Health Insurance reimburses statin therapy for patients with hypercholesterolaemia who are at high CV risk due to diabetes or other factors, the LDL-C goal for such high-risk patients is set at only ≤100 mg/dl, rather than the more stringent 70 mg/dl; consequently, physicians in Taiwan tend to use the lowest possible effective dose. For example, a diabetic patient with baseline LDL-C of 112 mg/dl may be prescribed EZE + SIM only twice weekly, whereas one with LCL-C 200 mg/dl would get a once-daily prescription. Occasionally, EZE + SIM may also be prescribed to patients with LDL-C < 100 mg/dl; for example, those with fatty liver disease, based on evidence that ezetimibe may improve non-alcoholic steatohepatitis [Citation21].
Nonetheless, reductions of 30.4% in TC and 36.2% in LDL-C from baseline were greater than those in another study of EZE + SIM 10.0 + 20.0 mg for 6 weeks in high-risk hypercholesterolaemic patients: TC −17.5%; LDL-C −27.7% [Citation5]. Unsurprisingly, maximal lipid-lowering and LDL-C goal attainment were seen in patients who took EZE + SIM once daily, with LDL-C reduced by −39.6%; 70.2% achieved LDL-C ≤ 100 mg/ml. On the other hand, despite substantially higher LDL-C goal attainment after EZE + SIM treatment, 40% of patients still had LDL-C > 100 mg/dl and 82% LDL-C > 70 mg/dl. Even better results perhaps comparable to those reported in non-Asian populations, could be expected if more patients were prescribed the labelled dose.
Indeed, in both clinical study subjects and real-world cohorts, EZE + SIM (at any dose) lowered LDL-C by approximately 40–60% [Citation1,4,12]; for example, in a 1528-subject clinical trial, Bays et al. reported a 52% reduction in LDL-C among hypercholesterolaemic subjects who took EZE + SIM (10.0 + 20.0 mg) for 12 weeks [Citation1]; comparatively higher efficacy in this context than our patients probably reflects closer monitoring and enforcement of the prescribed regimen than in real-world settings, as well as selection bias towards patients more likely to benefit [Citation4]. On the other hand, efficacy rates of >40% in other real-world populations probably reflect the use of higher statin dose equivalents than SIM 20.0 mg in a proportion of subjects [Citation4].
Recent studies have highlighted the importance of maximally effective lipid lowering therapy, not only in terms of achieving more stringent LDL-C goals but, more importantly, in reducing CV morbidity and mortality; in The Study of Heart and Renal Protection (SHARP) study, EZE + SIM 10.0 + 20.0 mg reduced the incidence of major atherosclerotic events by 17% versus placebo [Citation6]. Likewise, acute coronary syndrome patients in the IMPROVE-IT trial who received SIM 40.0 (or 80.0) mg, were significantly less likely to suffer myocardial infarction or ischemic stroke [Citation2,6]; moreover, IMPROVE-IT demonstrated that LDL-C lowering improves CV outcomes, with a hazard ratio of 0.8 for clinical benefit per 1.0 mmol/l LDL-C decrement [Citation2]. Therefore, there is a strong evidence-based rationale for treating all patients, even those with LDL-C a little over 100 mg/dl, with the recommended EZE + SIM dose of 10.0 + 20.0 mg, or even higher, and for endeavouring to achieve the more stringent LDL-C goal of ≤70 mg/dl in high-risk patients.
In general, the differences observed between hypercholesterolaemic patients in this cohort with and without diabetes were unsurprising. Subjects with diabetes were older and tended to have more comorbidities, most significantly renal insufficiency, which is to be expected given the causal link between diabetes and nephropathy. Rising HbA1c levels during follow-up of nearly 1 year among the cohort who were not diabetic or receiving diabetes control treatment(s) at baseline, is consistent with a proportion of them having pre-diabetic status; indeed, 25% at baseline and 40% at follow-up had an HbA1c level of ≥6.0%. However, we did also notice a trend towards greater lipid-lowering efficacy and LDL-C goal attainment in patients with diabetes compared with those without. Presumably, proportionally greater TC and LDL-C reductions in diabetic subjects than non-diabetics were largely due to the former having received a higher average EZE + SIM dose. However, the same trend was evident even when we compared patients with versus without diabetes who had taken similar EZE + SIM doses (, ).
NPC1L1 receptors in the intestinal mucosa and hepatocyte membranes, which absorb dietary and bile acid cholesterol, positively influence insulin sensitivity through complex mechanisms [Citation22,23]. Ezetimibe improved glucose tolerance in a hamster model of insulin resistance [Citation24] and ameliorated insulin sensitivity in 75 Japanese subjects with hypercholesterolaemia [Citation25], all of whom had two or more CV risk factors, including 28 with diabetes, and were overweight (mean body mass index 24.0 kg/m2) according to World Health Organisation Western Pacific Region criteria [Citation26]. Mechanistically, the additional benefit of this positive side effect may offset the glucose tolerance issue with statins, which have been reported to slightly increase the incidence of new-onset diabetes in a dose-dependent manner [Citation7].
In the IN-CROSS study, greater LDL-C lowering efficacy of EZE + SIM 10.0 + 20.0 mg versus rosuvastatin 10.0 mg was more pronounced among patients with diabetes than without [Citation5]. Similarly, a meta-analysis of 27 clinical trials showed that EZE + SIM provided comparatively greater reductions in TC, LDL-C, and non-HDL-C in subjects with diabetes [Citation19]. Japanese patients with diabetes who were treated with EZE plus statin due to poor statin control, also had improved atherogenic lipoprotein profiles [Citation14]. Furthermore, the IMPROVE-IT study found the greatest CV benefits of EZE + SIM therapy among patients with diabetes, with 24% reduction in myocardial infarction and 39% reduction in ischemic stroke [Citation2]. Our observations in a real-world clinical setting in Taiwan are consistent with these findings.
NPC1L1 expression is elevated in patients with diabetes; high glucose levels directly increase NPC1L1 expression in intestinal epithelial cells but the underlying mechanism is unclear [Citation27]. It is likely that ezetimibe not only improves insulin sensitivity in patients with diabetes but also targets the NPC1L1 overexpression, resulting in greater lipid lowering. In this context, especially given the importance of glycaemic control in reducing CV risk in diabetes, these results suggest that fixed-dose EZE + SIM appears to be a rational component of the treatment regimen for patients with both diabetes and hypercholesterolaemia.
Study limitations
The retrospective design and Chinese ethnicity of study subjects limits the generalisation of our findings. As in other real-world studies [Citation4], there was no untreated control group and a minority of eligible patients fulfilled the inclusion criteria of having before and after treatment lipid profile data. Furthermore, most patients had missing data; for example, the smoking status of 18.5% of the patients was unknown, because the study clinic records this routinely only for patients with coronary arterial disease or its contributory risk factors, for example, hypertension. A lack of serum HDL-C data from most patients precluded analysis of non-HDL-C, which may be a better indicator of CV risk than LDL-C [Citation28]. For these reasons, extrapolation of the trends we observed to the general population would require large-scale, prospective, controlled trials.
Conclusions
This analysis of actual patients in a community clinical setting contributes to the larger picture of lipid-lowering treatment in Taiwan; in particular, this study affirms the effectiveness of fixed-dose EZE + SIM for treating patients with hypercholesterolaemia, especially when administered at the label recommended dose. Our findings are consistent with other studies showing that EZE + SIM appears to be more efficacious in diabetic patients than non-diabetics, and may therefore be a good choice for treating patients with hypercholesterolaemia and diabetes. Further research is warranted to test this hypothesis.
Disclosure statement
Huan-Sheng Lin has received speaker fees from AstraZeneca, Merck Sharp & Dohme, Novartis, and Novo Nordisk. Yi-Chung Shih reports no conflicts of interest. Dr. David Neil (PhD), of Content Ed Net (Taiwan) provided medical writing services and Ms. Cathy Kuo of Content Ed Net (Taiwan) provided statistics support, which were remunerated by Merck Sharp & Dohme (I.A.) Corp, Taiwan.
References
- Bays HE, Ose L, Fraser N, et al. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26:1758–1773.10.1016/j.clinthera.2004.11.016
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.10.1056/NEJMoa1410489
- Reiner Z, Catapano AL, Backer G, et al. ESC/EAS guidelines for the management of dyslipidemias. Eur Heart J. 2011;32:1769–1818.
- Friedman HS, Rajagopalan S, Barnes JP, et al. Combination therapy with ezetimibe/simvastatin versus statin monotherapy for low-density lipoprotein cholesterol reduction and goal attainment in a real-world clinical setting. Clin Ther. 2011;33:212–224.10.1016/j.clinthera.2011.02.011
- Farnier M, Averna M, Missault L, et al. Lipid-altering efficacy of ezetimibe⁄simvastatin 10/20 mg compared with rosuvastatin 10 mg in high-risk hypercholesterolaemic patients inadequately controlled with prior statin monotherapy – the IN-CROSS study. Int J Clin Pract. 2009;63:547–559.10.1111/ijcp.2009.63.issue-4
- Gryn SE, Hegele R. Ezetimibe plus simvastatin for the treatment of hypercholesterolemia. Expert Opin Pharmacother. 2015;16:1255–1262.
- Preiss D, Sattar N. Statins and the risk of new-onset diabetes: a review of recent evidence. Curr Opin Lipidol. 2011;22:460–466.10.1097/MOL.0b013e32834b4994
- Branch WT, Higgins S. Clinical inertia: hard to move it forward. Rev Esp Cardiol. 2010;63:1399–1401.10.1016/S0300-8932(10)70265-7
- Vijayakrishnan R, Kalyatanda G, Srinivasan I, et al. Compliance with the adult treatment panel III guidelines for hyperlipidemia in a resident-run ambulatory clinic: a retrospective data analysis. J Clin Lipidol. 2013;7:43–47.10.1016/j.jacl.2012.06.004
- Altmann SW, David HR Jr., Zhu LJ, et al. Niemann-pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204.10.1126/science.1093131
- Gazi IF, Daskalopoulou SS, Nair DR, et al. Effect of ezetimibe in patients who cannot tolerate statins or cannot get to the low density lipoprotein cholesterol target despite taking a statin. Curr Med Res Opin. 2007;23:2183–2192.10.1185/030079907X226267
- Davidson MH, Ballantyne CM, Kerzner B, et al. Efficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract. 2004;58:746–755.10.1111/ijcp.2004.58.issue-8
- Feldman T, Koren M, Insull W Jr., et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain national cholesterol education program adult treatment panel III low-density lipoprotein cholesterol goals. Am J Cardiol. 2004;93:1481–1486.10.1016/j.amjcard.2004.02.059
- Sakamoto K, Kawamura M, Kohro T, et al. Effect of ezetimibe on LDL-C lowering and atherogenic lipoprotein profiles in type 2 diabetic patients poorly controlled by statins. PLoS One. 2015;10:e0138332.10.1371/journal.pone.0138332
- Bangalore S, Shahane A, Parkar S, et al. Compliance and fixed-dose combination therapy. Curr Hypertens Rep. 2007;9:184–189.10.1007/s11906-007-0033-3
- Merck Sharp and Dohme. VYTORIN® (ezetimibe and simvastatin) tablets | full prescribing information. Whitehouse Station (NJ): MSD International GmbH, a subsidiary of Merck. © 2004–2015: Revised 03/2015. [cited 2017 Feb 20] Available from: https://www.merck.com/product/usa/pi_circulars/v/vytorin/vytorin_pi.pdf
- Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410–414.10.1016/j.amjcard.2006.08.051
- Kim HS, Wu Y, Lin SJ, et al. Current status of cholesterol goal attainment after statin therapy among patients with hypercholesterolemia in Asian countries and region: the return on expenditure achieved for lipid therapy in Asia (REALITY-Asia) study. Curr Med Res Opin. 2008;24:1951–1963.10.1185/03007990802138731
- Leiter LA, Betteridge DJ, Farnier M, et al. Lipid-altering efficacy and safety profile of combination therapy with ezetimibe/statin vs. statin monotherapy in patients with and without diabetes: an analysis of pooled data from 27 clinical trials. Diabetes Obes Metab. 2011;13:615–628.10.1111/j.1463-1326.2011.01383.x
- de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2012;24:584–591.
- Yoneda M, Fujita K, Nozaki Y, et al. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: an open-label, pilot study. Hepatol Res. 2010;40:566–573.10.1111/j.1872-034X.2010.00644.x
- Sarigianni M, Katsiki N, Mikhailidis DP. Ezetimibe in diabetes: more than cholesterol lowering? Curr Med Res Opin. 2010;26:2517–2520.10.1185/03007995.2010.518519
- Chang TY, Chang C. Ezetimibe blocks internalization of the NPC1L1/Cholesterol complex. Cell Metab. 2008;7:469–471.10.1016/j.cmet.2008.05.001
- Naples M, Baker C, Lino M, et al. Ezetimibe ameliorates intestinal chylomicron overproduction and improves glucose tolerance in a diet-induced hamster model of insulin resistance. AJP Gastrointest Liver Physiol. 2012;302:G1043–G1052.10.1152/ajpgi.00250.2011
- Tamaki N, Ueno H, Morinaga Y, et al. Ezetimibe ameliorates atherosclerotic and inflammatory markers, atherogenic lipid profile, insulin sensitivity, and liver dysfunction in Japanese patients with hypercholesterolemia. J Atheroscler Thromb. 2012;19:532–538.10.5551/jat.10835
- World Health Organization Regional Office for the Western Pacific, International diabetes institute, international association for the study of obesity, international obesity task force. The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne: Health Communications Australia; 2000 [cited 2017 Jun 23]. Available from: http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf
- Malhotra P, Boddy CS, Soni V, et al. D-Glucose modulates intestinal Niemann-Pick C1-like 1 (NPC1L1) gene expression via transcriptional regulation. AJP Gastrointest Liver Physiol. 2013;304:G203–G210.10.1152/ajpgi.00288.2012
- Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307:1302–1309.10.1001/jama.2012.366