ABSTRACT
Background: Worldwide, the prevalence of the metabolic syndrome (MetS) is estimated to be 70% among those with type 2 diabetes mellitus (T2DM). T2DM and MetS are associated with abnormal liver enzyme levels, which can be the result of non-alcoholic fatty liver disease, cirrhosis, hepatocellular carcinoma or acute liver failure. The present study investigated the association between transaminases and MetS in T2DM patients.
Methods: A descriptive cross-sectional study was conducted over the period of 6 months among 540 diabetic patients attending a tertiary care hospital in Nepal. The diagnosis of MetS was based on International Diabetes Federation (IDF), National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and Harmonized definition 2009. Association between metabolic components and liver enzymes was established by crude and adjusted logistic regression analysis.
Results: Overall, the prevalence of elevated enzyme levels was 58.9% for alanine aminotransferase (ALT), 42.2% for aspartate aminotransferase (AST) and 59.4% for gamma-glutamyl transferase (GGT). The presence of MetS was 23.3%, 36.1% and 51.9% according to NCEP ATP III, IDF and Harmonized criteria, respectively. In the binary logistic regression analysis, waist circumference > 102 cm (M) or > 88 cm (F) was only independently associated with all three elevated liver enzymes, odds ratio (OR) = 4.172 for ALT, OR = 2.795 for AST and OR = 0.245 for GGT. When all three criteria were entered for multivariate risk analysis, only the NCEP ATP III (+) was found to be associated independently with raised all three liver enzymes.
Conclusion: Central obesity and MetS following NCEPATP III criteria were independently associated with elevated ALT, AST and GGT in our diabetic population. Clinicians may consider hepatic complication as a negligible component in T2DM. The present findings may encourage more attention.
Background
Diabetes mellitus (DM), the most common chronic metabolic disorder, is characterised by insulin resistance (IR) and micro and macrovascular complications affecting various organs including the liver [Citation1–Citation3]. DM is constantly increasing worldwide due to the ageing population, urbanisation and obesity [Citation4]. According to the World Health Organization (WHO), the number of patients with DM will exceed 350 million by 2030 [Citation5]. Worldwide, the prevalence of the metabolic syndrome (MetS) is estimated to be 70% among those with type 2 diabetes mellitus (T2DM) [Citation4]. In addition, various studies have documented the association of elevated hepatic enzymes with the risk of T2DM and MetS which are the major causes of mortality [Citation6].
Worldwide, around 3.2 million people die per year from a complication of DM. Several features of the MetS are present in most T2DM patients [Citation7]. MetS consists of a cluster of metabolic abnormalities that confer increased risk of mortality and the development of chronic kidney disease (CKD), T2DM and cardiovascular disease (CVD) [Citation8]. Several criteria have been established to define MetS [Citation9]. The concordance and diagnostic accuracy of the WHO, National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and International Diabetes Federation (IDF) definitions in identifying cases of MetS vary [Citation10]. In 2009, a joint statement from the IDF, National Heart, Lung and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society and International Association for study for Obesity proposed a Harmonized definition, where a single set of cut-off value would be used for all the components except waist circumference (WC), which varies with population and country-specific definitions [Citation11]. Tan et al.[Citation12] suggested IDF criteria were applicable in an Asian population for risk assessment of MetS in T2DM patients, whereas Pokharel et al. [Citation10] suggested that the NCEP ATP III criteria are more suitable.
T2DM and MetS are associated with abnormal liver enzymes which can be the result of non-alcoholic fatty liver disease (NAFLD), cirrhosis, hepatocellular carcinoma or acute liver failure [Citation6,Citation13]. NAFLD has been considered as a hepatic component of MetS, and major risk factors of NAFLD are obesity, IR, T2DM and hypertriglyceridaemia [Citation14,Citation15]. The circulating concentrations of the liver transaminases – alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) – are commonly used as markers of NAFLD [Citation6].
To the best of our knowledge, only the prevalence of MetS [Citation10,Citation16] and liver enzymes in a diabetic population [Citation17] has been reported from Nepal. Several studies purposed that even with increased liver enzymes and disease progressing to hepatic failure diabetic patients can still be asymptomatic [Citation13]. The present study was undertaken to assess the association between elevated liver enzymes and MetS in T2DM patients.
Methods
This descriptive cross-sectional study was conducted during the period of 6 months (February 2017 to July 2017) in Manmohan Memorial Teaching Hospital (MMTH), Kathmandu, Nepal.
Inclusion and exclusion criteria
Already diagnosed patients with T2DM aged between 40 and 70 years attending the Department of Medicine and Endocrinology for their follow-up were consecutively selected for the study.
Patients with a history of CVD, hypothyroidism, jaundice and liver cirrhosis were excluded. Each individual was screened for hepatitis B surface antigen and hepatitis C antibody-positive patients were excluded. The population with a history of regular alcohol intake, considerable abuse of alcohol (> 20 g ethanol/day) in the past six months, history of steroid intake for >2 weeks in the past six months and any evidence of prescribed hepatotoxic drugs (e.g. methotrexate) were excluded. Further, patients on current psychiatric treatment, chronic smokers and pregnant women were also excluded.
All 540 patients fulfilled with above inclusion criteria were recorded with their socio-demographic data and diagnosis history, using a standard proforma. Blood pressure (BP), body weight, height and WC were measured and recorded.
Experimental protocol
Fasting (8–12 h) and postprandial venous blood samples were collected and serum was separated for biochemical analysis. Fasting blood sugar (FBS) and postprandial blood sugar and liver enzymes activity were estimated. The standard methods for the assays were based on the guidelines provided by the reagent manufacturer (Human GmBh, Wiesbaden, Germany).
Fasting blood samples were analysed for total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) as per the instructions provided by the reagent manufacturer (Human GmBh, Wiesbaden, Germany). All the parameters were analysed using a Statfax 3300 (Awareness Technology, Inc. Bellport, USA, semi-automated analyzer) in the Department of Biochemistry, MMTH. HbA1c was estimated by the ion exchange resin method as per the instructions provided by the reagent manufacturer (Human GmBh, Wiesbaden, Germany).
All the anthropometric, demographic and clinical baseline characteristics from each individual were recorded. Body mass index (BMI) was calculated (kg/m2), and the cut-off value was considered as 25 kg/m2; higher values were considered as overweight. The normal range for ALT and AST was considered, up to 42 IU/L in males and up to 32 IU/L in females and for GGT up to 30 IU/L in both genders as referenced by the manufacturer’s instructions. The diabetic population with a level of HbA1c< 7.0% was considered as good glycaemic control and ≥7.0% was considered as poor glycaemic control as defined by the IDF [Citation18]. Further, study population was categorised as diabetic patient with and without MetS based on the diagnostic criteria provided by the NCEP ATP III 2001, IDF 2005 and Harmonized definition 2009 [Citation7,Citation11,Citation18].
Statistical analysis
Data were analysed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA) and Microsoft Excel 2013. Independent sample t-test was used to analyse the differences in biochemical markers between male and female population and for mean comparison of liver enzymes between different groups. Likewise, risk estimate was obtained for the elevated liver enzymes. Univariate analysis was done using chi-square tests to obtain the association of risk factors for elevated liver enzymes. Further, the associated covariates were entered in stepwise multivariate risk analysis for adjusted odds ratio (OR) using binary logistic regression model.
The proposal was submitted and discussed under Institutional Review Committee (IRC) of Manmohan Memorial Institute of Health Sciences (MMIHS) and ethical approval was obtained. Written informed consent was obtained from each participant for the study.
Results
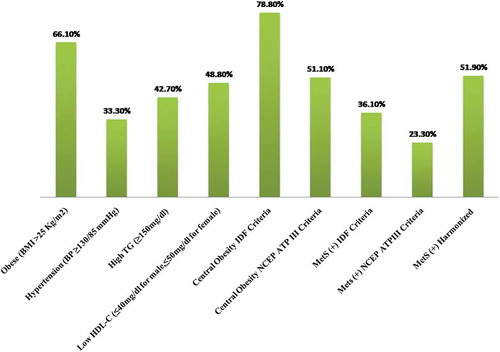
Total number of the study population was 540, among which 282 were male and 258 were female of different ethnicity. Around 50.5% of the diabetic population were from the Brahmin-Chettri community while 49.5% belonged to the Janjati community. The median age of the study population was 52 years. Elevated levels of ALT, AST and GGT were observed in various percentages (58.9%, 42.2% and 59.4%, respectively) of the study population. In addition, the frequency distribution of metabolic components was 78.8% with central obesity (IDF criteria) followed by central obesity (NCEP ATP III) (51.1%), low HDL-C (48.8%), high TG (42.7%) and hypertension with the least incidence (33.3%) (). Duration of T2DM, number of medications and lifestyle interventions (habit of exercise and following meal plan) were significantly associated with a poor glycaemic control in our diabetic population ().
Table 1. Socio-demographic and health risk factors.
All baseline characteristics are reported in . The average TC, TG, ALT and GGT activity was significantly higher in the population with poor glycaemic control compared with those with good glycaemic control. Significantly higher levels of liver enzymes were recorded in the obese population compared with the normal weight diabetic population, whereas ALT level alone was significantly elevated in patients with central obesity (p < 0.001). The significant elevation of ALT and GGT was associated with hypertensive patients compared with non-hypertensive patients (p < 0.001). In addition, the levels of both transaminases were significantly higher in patients with dyslipidaemia. AST and GGT were significantly elevated in patients with low HDL-C. No significant rise of liver enzymes was associated with duration of T2DM ().
Table 2. Baseline characteristics based on glycaemic status of the study population.
Table 3. Association of metabolic components with liver enzyme activity in the study population.
(univariate analysis) shows crude OR with 95% confidence interval (CI) of elevated liver enzymes across different variables. Higher age was associated with a higher OR (95% CI) = 0.262 (0.140–0.491) and OR (95% CI) = 0.359 (0.194–0.666) for elevated ALT and AST, respectively. Male gender was significantly associated with an increased AST OR (95% CI) = 4.022 (2.146–7.536) and GGT OR (95% CI) = 2.084 (1.115–3.896). In addition, patients with a higher WC (according to both NCEP ATP III and IDF criteria) had a higher tendency to have elevated liver enzymes. Higher TG was the risk factor OR (95% CI) = 0.243 (0.128–0.463), 0.341 (0.185–0.629) and 3.478 (1.786–6.771) for elevated ALT, AST and GGT, respectively. However, patients with all three MetS criteria [NCEP ATP III OR (95% CI) = 13.776 (4.064–46.701), IDF OR (95% CI) = 12.304 (2.825–53.586) and Harmonized OR (95% CI) = 0.168 (0.502–0.760)] were associated with high risk for elevated ALT activity.
Table 4. Metabolic syndrome and individual components associated with risk of elevated liver enzyme activity.
To identify the independent effect of each covariate and the most significant predictors of elevated liver enzymes, multiple regression analysis (adjusted OR) was carried out. WC > 102 cm (M) or >88 cm (F) was only independently associated with all elevated liver enzymes, OR = 4.172 (1.583–10.992) for ALT, OR = 2.795 (1.044–7.477) for AST and OR = 0.245 (0.097–0.621) for GGT. Further, MetS following NCEPATP III (+) was a significant factor associated with raised all three enzymes. Previously diagnosed (duration >10 years since diagnosed) differ qualitatively in multivariate analysis with a 2.874 times higher risk of having elevated ALT than those population with newly diagnosed DM (duration <10 years since diagnosed). High TG was the independent variable for high ALT and AST in the diabetic population with higher OR, whereas low HDL-C was independently associated with high ALT and GGT. In addition, a diabetic population with MetS (IDF +) had 0.195 times greater risk (0.041–0.932) of having a high ALT compared with those without MetS (IDF –). Similarly, the population with MetS (NCEP ATP III +) had 0.116 times higher risk (0.033–0.410) of having ALT than those without the MetS (NCEP ATP III –) ().
Discussion
Elevated liver enzymes are commonly associated with T2DM [Citation19]. The overall prevalence of elevated liver enzyme levels in our study population was 58.9% for ALT, 42.2% for AST and 59.4% for GGT. This prevalence was reported as relatively lower in developed countries [Citation20,Citation21] but higher in developing countries [Citation17,Citation22–Citation25]. For example, for low and middle economy countries abnormal liver profiles are reported in 50–70% of diabetic subjects [Citation26] but this value is 7.8–22.9% for Europe and the United States [Citation19]. The prevalence of elevated enzymes for males and females is not consistently reported in different studies. In the present study, males had a high prevalence of elevated GGT (36.6% vs. 22.2%) than females while females had a high prevalence of elevated AST than males (28.30% vs. 13.90%); ALT was almost similar between both genders. Compared with our study, Atiba et al.[Citation22] and Thanpari et al. [Citation17] reported a high prevalence of deranged liver enzymes in females, whereas Judi et al. [Citation21] reported a high incidence of elevated liver enzymes in males with T2DM.
The prevalence of MetS in our T2DM population was relatively lower than all other studies previously conducted in our country. The study showed the prevalence of MetS in T2DM patient to be 23.3%, 36.1% and 51.9% according to NCEP ATP III, IDF and Harmonized criteria, respectively, which was lower than the frequency reported by Pokharel et al.[Citation10], i.e. 73.9% by NCEP ATP III, 66.8% by IDF and 80.3% by Harmonized criteria. In addition, many studies had reported sex-specific, gender-specific and age-specific disparities in MetS prevalence. The reason behind such inconsistency could be due to differences in study population, lifestyles, genetic factors, socio-economic status and investigation methods used. The joint statement in 2009 [Citation11] attempted to unify criteria to define MetS; they proposed ethnic-based WC threshold for abdominal obesity and recommend cut-off values of ≥90 cm for males and ≥80 cm for females in our Asian population.
Our study showed central obesity as the most prevalent component according to IDF criteria, whereas decreased HDL-C level was the most prevalent component followed by overweight and central obesity according to the NCEPATP III criteria. The lower prevalence of central obesity as per NCEPATP III criteria is due to its relatively higher cut-off values for WC; this can underestimate the prevalence of MetS in our population [Citation16]. In addition, Pokharel et al. also reported that the NCEPATP III criteria might be more specific than the IDF criteria but is less applicable to the Nepalese population because of their smaller body size [Citation10]. On the other hand, in our study population, hypertension was found to be the least prevalent component. These findings are similar to earlier studies conducted in Nepal [Citation16,Citation27] but contradict some international studies [Citation28,Citation29].
MetS is related to obesity, inflammation and IR, and a higher prevalence is reported in association with the pre-disease states for CVD, CKD, NAFLD and DM [Citation11]. Since the prevalence of MetS in T2DM has been increasing in developing countries, its association with liver enzymes has drawn significant attention in recent years. There are no studies reported yet assessing the association between liver enzymes and MetS in T2DM in our nation. Finally, our study reveals identification of liver damage in patients with T2DM is due to IR. Association of raised liver enzymes with IR is a known characteristic of NAFLD [Citation20]. NAFLD has been defined as a hepatic component of MetS [Citation15,Citation30–Citation32]. In the present study, a significant rise of transaminases was associated with obesity (high BMI) and high TG in diabetic patients. One study reported similar results [Citation15], but Judi et al. [Citation16] reported that obesity (high BMI) was not significantly associated with increase transaminases but central obesity (high WC) was significantly associated. The possible explanation of elevated liver enzymes in IR is due to the presence of a large mass of adipose tissue in the obese. There increases non-esterified free fatty acid flux from visceral fat to the liver, which causes fatty liver and other consequences [Citation20,Citation21,Citation33]. Further, the presence of NAFLD in IR with all these metabolic components (dyslipidaemia, hypertriglyceridaemia and hypertension) is a risk factor for CVD [Citation10,Citation30,Citation34].
ALT is considered as a specific marker of liver pathology [Citation6]. In the present study, elevated ALT was independently associated with age, central obesity, low HDL-C and MetS defined by IDF and NCEP ATP III criteria. In support of our study, several studies demonstrated that central obesity and NCEPATP III MetS (+) are independent variables associated with high ALT in the diabetic population [Citation20,Citation21,Citation33]. Forlani et al.[Citation20] predicted high TG as an independent risk factor for elevated ALT but Judi et al. [Citation21], Esteghamati et al.[Citation33] and our report found high TG levels not to be an independent risk factor for elevated ALT. However, MetS NCEPATP III (+), central obesity, age, male gender and high TG levels were associated as an independent marker for elevated AST. Central obesity following IDF criteria was not found to be associated with elevated liver enzymes in our study population, probably because of consideration of lower cut-off value for WC. Even increased BMI (obesity) and increased WC (central obesity) both are metabolic components; however, only central obesity (NCEPATP III criteria) was strongly associated with elevated liver enzymes in multivariate risk analysis, which is also concluded by many other studies [Citation20,Citation33]. The biochemical basis is that rather than the high body weight central fat is associated with IR [Citation20]. IR in adipose tissue stimulates hormone-sensitive lipase and increases the release of free fatty acids, which is taken up by the liver. In addition, hyperinsulinaemia in hepatocytes also stimulates de novo synthesis of fatty acids [Citation8]. Finally, the influx of high fatty acids in the liver causes liver toxicity and additionally, inflammatory cytokines and tumour necrosis factor-6also play a major role in the development of hepatocellular injury causing NAFLD and fatty liver with mild to moderate increase of liver enzymes [Citation33].
MetS (NCEPATP III criteria) was independently associated with elevated AST and ALT in our population. One of the studies also suggested that even with mild stages of fatty liver (which is ultrasonographically undetectable) MetS is shown to be strongly associated with elevated transaminases in the diabetic population [Citation33]. We did not screen the liver by ultrasonography or liver biopsy for the presence of fatty liver and NAFLD which may further provide the clear picture to describe the association of IR, MetS and elevated liver enzymes.
All significant independent variables in univariate analysis were analysed stepwise for binary logistic regression analysis. WC > 102 cm (M) or >88cm (F) was only independently associated with all elevated liver enzymes, OR = 4.172 (1.583–10.992) for ALT, OR = 2.795 (1.044–7.477) for AST and OR = 0.245 (0.097–0.621) for GGT. Though, MetS defined by Harmonized criteria is more sensitive [Citation11], Pokharel et al. [Citation10] suggested that MetS defined by NCEP ATP III and IDF are more applicable in detecting MetS in our T2DM population. Hence, of all three criteria were assessed for risk analysis, only the NCEPATP III (+) was found to be an independent factor associated with raised all three enzymes. In multivariate analysis, duration of DM > 10 years (prolonged DM) showed a high risk of having elevated ALT 2.87 times and ALT 2.62 times than those with newly diagnosed DM. High TG was the independent variable for high ALT and AST in the diabetic population with higher OR, whereas low HDL-C was independently associated with high ALT and GGT. In addition, the diabetic population with MetS (IDF +) had a 19% greater risk of having high ALT compared with those population without MetS (IDF –).
GGT is considered as a marker of NAFLD and fatty liver is equally considered as a risk factor of CVD specifically in T2DM [Citation35]. Among all liver enzymes, GGT is a sensitive marker of liver damage but is less specific. Elevated GGT can occur even in low-grade hepatic inflammation or hepatocellular damage which is common in MetS and T2DM [Citation6]. In our diabetic population, levels of GGT were significantly higher in males than in females. Evidence of gender difference in GGT levels has been reported [Citation36]; however, this finding is inconsistent and a single reference range used in our study as per manufacturer’s instructions. Many studies confirmed that GGT remained significantly associated with MetS and DM, especially with males [Citation37,Citation38], and GGT are independent predictors of T2DM and MetS [Citation37]. We conclude that GGT was the independent marker of MetS in T2DM which was strongly associated with central adiposity and dyslipidaemia. GGT is associated with oxidative stress and hepatic steatosis with additive adiposity and its increased level reflects oxidative stress in hypertension, MetS and DM and is actively involved in further pathogenesis [Citation39]. In diabetic patients with dyslipidaemia, circulating LDL can get oxidised and show an increased atherogenic effect [Citation37]. Finally, we can predict elevated GGT is associated with all cases of CHD and mortality in diabetic patients with MetS, independent of alcoholism, hepatitis and liver cirrhosis.
Our analysis has some limitations. This time framed study cannot establish causality since it is a cross-sectional study and with a small sample size. Follow-up studies are required to establish liver enzymes as hepatic components of MetS in the diabetic population. Further, screening of liver for NAFLD and histopathological grading along with liver enzymes could establish a proper management strategy of hepatic complication in diabetic populations.
Conclusions
The prevalence of MetS was higher using the Harmonized criteria than the IDF criteria and was lowest using the NCEPATP III criteria in our patients with T2DM. Central obesity and MetS following NCEPATP III criteria were independently associated with elevated ALT, AST and GGT. Despite clinicians considering hepatic complications as a negligible component in T2DM, the present findings may encourage more attention. Better management towards lifestyle interventions [Citation40], routinely and timely monitoring of metabolic components along with liver enzymes in T2DM may help with early detection and treatment of progressive liver disease.
Ethical clearance
This research was approved by the Institutional Review Committee of Manmohan Memorial Institute of Health Sciences (IRC MMIHS), Kathmandu, Nepal (letter of approval Ref No: 107/MMIHS/2073). Informed and written consent was taken from the patients before participating in the study. Data regarding personal information were coded and kept confidential.
Availability of data and materials
All the data generated during this study are presented in this paper. The primary raw data will be made available to interested researchers by the corresponding author if requested.
Acknowledgements
The authors thank all the patients participating in this study. Special thanks go to all the laboratory staff, management and officials of Manmohan Memorial Teaching Hospital Kathmandu for providing the opportunity to carry out this research work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Bashu Dev Pardhe
BDP and OSK conceived the design of the study, reviewed literature, performed necessary interventions including laboratory investigations and analysed the data. JM, AB, JS, PS, MP, SP, NAK and PRK participated in hospital data collection, laboratory procedure and data analysis. BDP and JM prepared the manuscript. All authors contributed towards drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors read the final version of the manuscript and approved it.
References
- Ko SH, Baeg MK, Han KD, et al. Increased liver markers are associated with higher risk of type 2 diabetes. World J Gastroenterol. 2015;21:7478–7487.
- Coban E, Kucuktag S, Basyigit S. Platelet activation in subjects with impaired glucose tolerance. Platelets. 2007;18:591–594.
- Akinsegun A, Olusola DA, Sarah J-O, et al. Mean platelet volume and platelet counts in type 2 diabetes: mellitus on treatment and non-diabetic mellitus controls in Lagos, Nigeria. Pan Afr Med J. 2014;18(42):3651.
- Hu FB. Globalization of diabetes. Diabetes Care. 2011;34:1249–1257.
- Al-Jameil N, Khan FA, Arjumand S, et al. Associated liver enzymes with hyperlipidemic profile in type 2 diabetes patients. Int J ClinPathol. 2014;7:4345–4349.
- Music M, Dervisevic A, Pepic E, et al. Metabolic syndrome and serum liver enzymes level at patients with type 2 diabetes mellitus. Med Arch. 2015;69:251–255.
- Alberti G, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The Metabolic Syndrome: a New World Wide Definition. Lancet. 2005;366:1059–1062.
- Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9.
- Grundy S, Becker D, Clark L, et al. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Circulation. 2002;106:3143–3421.
- Pokharel DR, Khadka D, Sigdel M, et al. Prevalence of metabolic syndrome in Nepalese type 2 diabetic patients according to WHO, NCEP ATP III, IDF and harmonized criteria. J Diabetes MetabDisord. 2014;13:104.
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645.
- Tan C-E, Ma S, Wai D, et al. Can we apply the national cholesterol education program adult treatment panel definition of the metabolic syndrome to Asians? Diabetes Care. 2004;27:1182–1186.
- Fraser A, Harris R, Sattar N, et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British women’s heart and health study and meta-analysis. Diabetes Care. 2009;32:741–750.
- Neukam K, Bagwani S, Rodger A, et al. High prevalence of non-alcoholic fatty liver disease (NAFLD) among Gujarati Indians in North London: a population based study. ClinLipidol. 2017;12:33–39.
- Villegas R, Xiang Y-B, Elasy T, et al. Liver enzymes, type 2 diabetes, and metabolic syndrome in middle-aged, urban Chinese. men.MetabSyndrRelatDisord. 2011;9:305–311.
- Sharma SK, Ghimire A, Radhakrishnan J, et al. Prevalence of hypertension, obesity, diabetes, and metabolic syndrome in Nepal. Int J Hypertens. 2011;2011:821971.
- Thanpari C, Yadav N, Thakelmayum R. Status of antioxidant and liver function in type-2 diabetic patients attending Nepalgunj medical college. Bali Med J. 2013;2:1–4.
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237.
- Harris EH. Elevated liver function tests in type 2 diabetes. ClinDiabetes. 2005;23:115–119.
- Forlani G, Di Bonito P, Mannucci E, et al. Prevalence of elevated liver enzymes in Type 2 diabetes mellitus and its association with the metabolic syndrome. J Endocrinol Invest. 2008;31:146–152.
- Judi L, Toukan A, Khader Y, et al. Prevalence of elevated hepatic transaminases among Jordanian patients with type 2 diabetes mellitus. Ann Saudi Med. 2010;30:25–32.
- Atiba AS, Oparinde DP, Babatunde OA, et al. Liver enzymes and lipid profile among type 2 diabetic patients in Osogbo, Nigeria. Greener JMed Sci. 2013;3:174–178.
- Bora K, Borah M, Chutia H, et al. Presence of concurrent derangements of liver function tests in type 2 diabetes and their relationship with glycemic status: A retrospective observational study from Meghalaya. J Lab Physicians. 2016;8:30–35.
- Balogun W, Adeleye J, Akinlade K, et al. Frequent occurrence of high gamma-glutamyl transferase and alanine amino transferase among Nigerian patients with type 2 diabetes. Afr J Med Med Sci. 2008;37:177–183.
- Ni H, Soe HHK, Htet A. Determinants of abnormal liver function tests in diabetes patients in Myanmar. Int J Diabetes Res. 2012;1:36–41.
- Prabhudeva N, Pasha G, Mounika K. Hepatic dysfunction in diabetes mellitus: biochemical and ultrasonological study. J AcadInd Res. 2014;3:164–167.
- S KS, Sapkota S. Prevalence of metabolic syndrome in type 2 diabetes mellitus patients using NCEP/ATP III and IDF criteria in Nepal. Nepal J Med Sci. 2012;1:79–83.
- Tan MC NO T, Wong TW, Joseph A, et al. Prevalence of metabolic syndrome in type 2 diabetic patients: a comparative study using WHO, NCEP ATP III, IDF and Harmonized definitions. Health. 2013;5:1689–1696.
- de Simone G, Devereux RB, Chinali M, et al. Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the strong heart study. Diabetes Care. 2007;30:1851–1856.
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
- Rector RS, Thyfault JP, Wei Y, et al. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World JGastroenterol. 2008;14:185–192.
- Uchil D, Pipalia D, Chawla M, et al. Non-alcoholic fatty liver disease (NAFLD)-the hepatic component of metabolic syndrome. J Assoc Physicians India. 2009;57:201–204.
- Esteghamati A, Jamali A, Khalilzadeh O, et al. Metabolic syndrome is linked to a mild elevation in liver aminotransferases in diabetic patients with undetectable non-alcoholic fatty liver disease by ultrasound. DiabetolMetabSyndr. 2010;2:65.
- Chen S, Guo X, Yu S, et al. Metabolic syndrome and serum liver enzymes in the general chinese population. Int J Environ Res Public Health. 2016;13:368.
- Belkacemi L, Belalia M. Cross-sectional pilot study about the liver enzymes profile in type 2 diabetic patients from an Algerian west region: wilaya of Mostaganem. Diabetol MetabSyndrClin Res Rev. 2016;10:S147–S50.
- Nilssen O, Forde OH, Brenn T. Distribution of population determinants of gamma-glutamyl transferase. Am J Epidemiol. 1990;132:318–326.
- Nakanishi N, Suzuki K, Tatara K. Serum γ-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27:1427–1432.
- Wannamethee SG, Shaper AG, Lennon L, et al. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care. 2005;28:2913–2918.
- Onat A, Can G, Çiçek G, et al. Serum γ‐glutamyltransferase: independent predictor of risk of diabetes, hypertension, metabolic syndrome, and coronary disease. Obesity. 2012;20:842–848.
- Perez-Martinez P, Mikhailidis DP, Athyros VG, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75:307–326.