Abstract
Several common neuropsychiatric disorders (e.g., obsessive-compulsive disorder, Tourette syndrome (TS), autistic spectrum disorder) are associated with unpleasant bodily sensations that are perceived as an urge for action. Similarly, many of our everyday behaviors are also characterized by bodily sensations that we experience as urges for action. Where do these urges originate? In this paper, we consider the nature and the functional anatomy of “urges-for-action,” both in the context of everyday behaviors such as yawning, swallowing, and micturition, and in relation to clinical disorders in which the urge-for-action is considered pathological and substantially interferes with activities of daily living (e.g., TS). We review previous frameworks for thinking about behavioral urges and demonstrate that there is considerable overlap between the functional anatomy of urges associated with everyday behaviors such as swallowing, yawning, and micturition, and those urges associated with the generation of tics in TS. Specifically, we show that the limbic sensory and motor regions––insula and mid-cingulate cortex––are common to all of these behaviors, and we argue that this “motivation-for-action” network should be considered distinct from an “intentional action” network, associated with regions of premotor and parietal cortex, which may be responsible for the perception of “willed intention” during the execution of goal-directed actions.
Many of our everyday behaviors are characterized by bodily sensations that we experience either as an urge or a desire for action. For instance, we may experience a sensation that our bladder is full that is accompanied, to a greater or lesser extent, by an urge or desire to urinate (micturate). In extreme cases, this sense of fullness can be quite uncomfortable and the urge to urinate can be hard to suppress. Similarly, we may experience a tickle in our throat that is associated with an urge to cough or to swallow that can also be difficult to suppress voluntarily. However, not all urges for action are necessarily preceded by bodily sensations of which we are aware. For example, we may suddenly experience a strong urge to yawn, or even find ourselves yawning, without being aware of a sensory “trigger” for the action. In this paper, we consider the nature and the functional anatomy of these “urges-for-action,” both in the context of everyday behaviors such as yawning, swallowing, and urinating, and in relation to clinical disorders in which the urge-for-action is considered pathological and substantially interferes with activities of daily living (e.g., Tourette syndrome, obsessive-compulsive disorder, addiction).
A CONCEPTUAL MODEL OF THE URGE-FOR-ACTION
In common usage, the terms “urge” and “desire” are frequently encountered as both a verb (as in to “urge someone on”––i.e., to motivate, impel, or stimulate a person toward an action) and as a noun (as in “he felt an urge to shout”––i.e., a force, drive, or impulse that impels toward a goal). Furthermore, these terms are often used interchangeably with one another, and are frequently listed as synonyms.
Nevertheless, it has been suggested that there is an important distinction to be made between urges and desires (e.g., Cameron, Citation2002; Davenport, Citation2008). Thus, Cameron (Citation2002), when discussing interoception, and the relationship between conscious awareness and visceral events, makes a distinction between “detection,” which is an organism's reflexive response based solely upon afferent physiological information, and “perception,” which refers to an organism's response based upon all information available to the organism (which might include learned information and expectations that might be generated as a result of learning). Similarly, Davenport, when discussing mechanisms associated with the urge-to-cough, defines an urge as a physical need to respond to a sensory stimulus, and a desire as the translation of an urge into what he refers to as “a central neural targeted goal” (Davenport, Sapienza, & Bolser, 2002). Davenport's motivation-to-action model is presented in .
Figure 1. The motivation-for-action model proposed by Davenport to account for the urge to cough (adapted from Davenport, Sapienza, & Bolser, 2002).
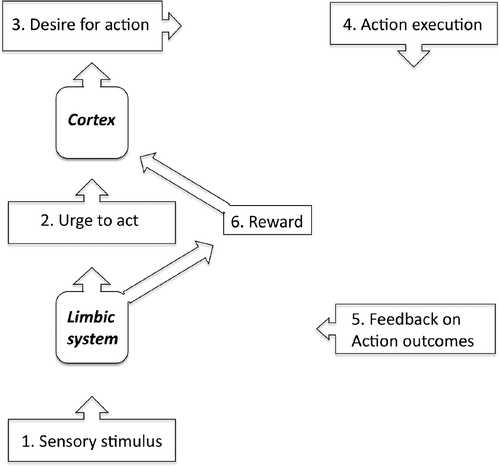
Combining these two ideas we might conclude that an urge––as in a drive for action––need not enter conscious awareness, but that we are always aware of our desires. An example might help make this distinction clearer. Imagine that you are driving your car and you suddenly become aware of an uncomfortable sensation that your bladder is full, which you experience as the urge to urinate. Given that you can in all likelihood control this urge, you may then form a desire to urinate in which you construct a goal or plan which includes a representation of the behaviors required to complete the action (e.g., remembering that you passed a filling station a mile back and knowing that filling stations usually have public toilets) and a representation of the likely outcomes of the action. Can an urge exist if we are not aware of it? What factors determine whether an urge enters awareness? How is an urge to act different from an intention to act? These issues are discussed below.
ARE URGES SIMPLY REFLEXES?
As outlined above, urges are often defined as the drives or impulses that impel us to act. Nevertheless, it is argued that actions can, and frequently do, occur in the absence of any awareness of such drivers, as in the case where one finds oneself yawning without previously being aware of either the desire to yawn or of any bodily sensation that might reasonably be identified as giving rise to the yawn. Instead, one simply finds oneself yawning. In this case, it might be argued that this is a reflexive behavior and not an urge-for-action.
One possible distinguishing feature of urges, as distinct from reflexes, may be that urges are chiefly associated with actions that cannot be realized immediately and must be held in check until an appropriate time when they might be released. For instance, when we become aware of having a full bladder, we experience an “urge-to-void” because we do not simply void our bladder, but instead employ a coordinated set of central, autonomic, and peripheral neural mechanisms to withhold micturition until we are in an appropriate behavioral context. Similarly, in the case of yawning, we might define the urge-to-yawn as arising in circumstances where we are forced to try to stifle the yawn rather than in the situation where we find that we are yawning.
HOW ARE URGES RELATED TO AWARENESS?
One factor that may determine whether an urge enters awareness is the intensity of the physiological afferent. It has been demonstrated in the context of the urge-to-cough that the perceived strength of the urge that is experienced is related to the intensity of stimulation. Specifically, when capsaicin is added to the breathing circuit, it results in a sensation that is perceived as an urge-to-cough. Furthermore, increasing capsaicin levels leads to a reliable increase in estimates of this urge (Davenport, Sapienza, & Bolser, 2002). Similarly, in our own unpublished studies of the effects of oropharyngeal stimulation on the urge-to-swallow, we have found that oropharyngeal stimulation using pulses of air produces both an urge-to-swallow and overt swallowing (for similar findings, see also Lowell et al., Citation2008). More importantly, we have found that increasing the intensity of oropharyngeal stimulation leads to an increase in the strength of the perceived urge-to-swallow. An important question therefore concerns the role of awareness, and by implication “desire,” in the initiation of urge-related actions.
According to the motivation-to-action model represented in , actions are initiated only after a desire to perform an action has been formed. However, we do not see this step as necessary and would amend this model, so as to allow for actions to be initiated directly, without the need to first construct an explicit “desire for action.” Two factors motivate this amendment. First, it strikes us that in many instances actions (e.g., yawning) can be triggered without our necessarily being aware of any explicit desire. Second, in many clinical conditions (e.g., Tourette syndrome), as we shall see below, unwanted actions or behaviors, which individuals struggle actively to suppress, are nevertheless preceded by strong premonitionary urges. In our view, it is difficult to reconcile the concept of a “desire,” which is often defined as “a longing or craving for something that brings satisfaction or enjoyment,” with unwanted actions, the execution of which is experienced as unpleasant and distressing.
If we accept the premise outlined above that urges-for-action are often accompanied by bodily sensations, then it strikes us that an important distinction can be drawn between being aware of a bodily sensation and being aware of an urge-for-action. This distinction can be best illustrated by considering the following examples. In the case of an itch, we may be aware of experiencing both an itch (bodily sensation) and an urge to scratch the itch (urge-for-action). By contrast, while we may become aware of an urge-to-yawn, it is not entirely clear that we are ever aware of the bodily sensation that gives rise to the urge to yawn. Furthermore, this becomes more important if we accept the argument outlined above that urges occur primarily in circumstances in which actions may need to be suppressed or their execution deferred. In such circumstances, we might distinguish between suppression of the action associated with the urge-for-action, or suppression of the bodily sensation giving rise to the urge. This distinction has important clinical implications (see below), and an interesting issue for future research will be to determine whether the suppression of bodily sensations and the suppression of urges-for-action differ in terms of their functional anatomy.
HOW IS AN URGE TO ACT DIFFERENT FROM AN INTENTION TO ACT?
The kinds of actions that we have considered as representative of urges-for-action are highly automatic, habitual responses that occur primarily in response to sensory stimulation. These might include brushing an insect off your arm, scratching an itch, yawning when tired, coughing in response to a tickle in your throat, etc. While such actions can, in some circumstances, be executed with little or no awareness of the sensory stimulation that triggered the action, as when one finds oneself yawning or coughing, we have argued that a key characteristic of urges-for-action is that they involve the suppression or deferment of an action. Such actions might therefore be contrasted to intentional, goal-directed, forms of action.
The circumstances in which the “willed intention” to execute an action can be shown to follow the brain processes involved in the preparation for action were famously studied by Benjamin Libet (Libet, Gleason, Wright, & Pearl, Citation1983), and more recently by Patrick Haggard and colleagues (e.g., Haggard, 2005; Haggard & Eimer, Citation1999; Sirigu et al., Citation2004). In Libet's task, participants fixated on a time-varying, rotating visual spot and were instructed to make a voluntary hand movement whenever they felt the “urge” to do so. Participants were asked to indicate the location occupied by the moving spot when they had first felt the urge to move their hand. Libet showed that this “W judgment” occurred some 200 ms prior to movement onset, but, more importantly, he also showed that the preparatory brain activity that precedes voluntary action, the so-called “readiness potential,” itself preceded the “W judgment” by several hundred milliseconds. Such readiness potentials arise in the premotor regions of cortex, including both the supplementary motor area (SMA) and the presupplementary motor area (pre-SMA), regions that have been linked to the planning and preparation of intentional, goal-directed, actions and sequences of actions (Deecke & Kornhuber, Citation1978).
Haggard has argued that conscious awareness of our intention to act arises during the preparatory processes that precede an action, and is linked to the joint activity of premotor and parietal brain areas (Haggard, 2005). In support of this view, he has shown that patients with damage to the parietal cortex show a specific impairment in reporting when they became aware of their intention to move (i.e., Libet's “W judgment”). The proposal that the parietal cortex may maintain a dynamically updated state estimate of the current postural configuration of the body (the body schema) is well supported by neuropsychological (e.g., Wolpert, Goodbody, & Husain, Citation1998) and recent fMRI studies (e.g., Parkinson, Condon, & Jackson, Citation2010; Pellijeff, Bonilha, Morgan, McKenzie, & Jackson, Citation2006).
While it is clearly the case that in such experiments individuals can report when they first perceived themselves to have formed an “intention” to move, it is another thing entirely to argue that such conscious “intentions” typically precede everyday actions. Thus, when I am sitting at my desk typing and I break off to reach for my coffee cup I am not aware of forming an intention prior to each keystroke that I make or of forming an intention to reach for my coffee. Instead, I am aware of the actions I am making. Similarly, while it is clearly demonstrated that our actions are preceded by neural activity, as discussed by Libet and by Haggard and colleagues, it is currently unclear how these activations relate to the phenomenology of intention.
Haggard and others (e.g., Blakemore, Wolpert, & Frith, Citation2002; Haggard, 2005) have argued that the sense-of-agency that typically accompanies the execution of voluntary movements arises as a result of internal forward sensory models that generate a prediction of the sensory consequences of an action that is then matched against afferent sensory signals. It has been proposed that in cases where the link between these sensory predictions and confirmatory sensory input is broken, neurological syndromes such as anosagnosia (lack of awareness of injury) or somatoparaphrenia (denial of limb ownership) may occur (Tsakiris, Citation2010). Interestingly, both of these disorders have been linked to damage of the anterior insular cortex of the right hemisphere (Baier & Karnath, Citation2008; Karnath, Baier, & Nagele, Citation2005). However, it is important to note that this sense of agency may in fact have a significant postdictive or reconstructive component (Moore, Lagnado, Deal, & Haggard, Citation2009; Wegner, Citation2002) and thus is not necessarily an unambiguous index of intentionality.
URGES-FOR-ACTION, TICS, AND TOURETTE SYNDROME
Tics are involuntary, repetitive, stereotyped behaviors that occur with a limited duration. Motor tics can be simple or complex in appearance, ranging from repetitive movements to coordinated action sequences (Leckman, Citation2002). Verbal tics can consist of repeating words or utterances (palilalia), producing inappropriate or obscene utterances (coprolalia), or the repetition of another's words (echolalia).
Tics occur in bouts, typically many times in a single day, and are the most common form of movement disorder in children, with a prevalence of 1–29% depending upon the precise characteristics of the study population, the diagnostic criteria used, and the study design and methods employed (Leckman, Citation2002). Tics include a continuum of disorders: transient tic disorder (TTD), chronic tic disorder (CTD), nonspecific tic disorder (NSTD), and Tourette syndrome (TS). The etiology of tics is poorly understood and probably involves a complex interaction between genetic and environmental factors that exert an influence over brain development.
TS is a developmental neuropsychiatric disorder that lies at the extreme of the tic disorder spectrum and is characterized by the presence of chronic vocal and motor tics (Leckman, Citation2002). The neurological basis of TS is unclear; however, it is agreed that the basal ganglia, including circuits that link the striatum to the frontal lobes, are dysfunctional (Albin & Mink, Citation2006). A specific model of basal ganglia dysregulation in TS has been proposed as follows. Subsets of striatal neurons (matrisomes) are thought to become abnormally active in inappropriate contexts, leading to the disinhibition of thalamocortical circuits that in turn lead to tics. Activity-dependent dopamine inappropriately reinforces such activity, leading to stereotyped repetition of behavior (Albin & Mink, Citation2006). Brain-imaging and postmortem studies provide general support for the view that cortical–striatal–thalamocortical pathways are dysfunctional in TS (Gerard & Peterson, Citation2003). Furthermore, deep-brain stimulation of the globus pallidus or the thalamus has been shown to be effective in suppressing tics in individuals with TS (e.g., Ackermans et al., Citation2011).
The occurrence of repetitive, stereotypical behaviors in TS has been linked to operation of the brain “reward” and “habit” systems, and tics have been likened to an inappropriate overextension of habit learning (Graybiel, 2008). Key characteristics of habitual behaviors are that they are largely learnt, occur repeatedly, are performed almost automatically, and often involve stereotypical, ordered, action sequences (Graybiel, 2008). In this context, it is important to note that many individuals with TS report that their tics are often preceded by “premonitory sensory phenomena” (PSPs), which are described as the presence of uncomfortable cognitive or bodily sensations (e.g., tension, pressure, tickle), that precede the execution of a tic, and are experienced as a strong urge for motor discharge (Banaschewki, Woerner, & Rothenberger, 2003). Furthermore, whereas individuals with TS perceive a relatively constant demand to suppress their tics in social situations, and while the voluntary suppression of tics is possible in many cases, individuals with TS nevertheless report that it can be uncomfortable and stressful to suppress tics, and that the urge to tic becomes uncontrollable after a period of suppression. It is therefore likely that some tics at least are learnt motor or vocal behaviors that function to alleviate or reduce uncomfortable bodily sensations.
Functional anatomy of tics in TS
Several attempts have been made to investigate the brain regions associated with the occurrence of tics in TS using human neuroimaging techniques. These studies have indicated that the neural mechanisms responsible for triggering of tics may in fact differ from those involved in voluntary movements (Bohlhalter et al. Citation2006). One notable study was that reported by Hallett and colleagues, which used functional magnetic resonance imaging (fMRI) to examine brain areas activated immediately preceding the spontaneous occurrence of motor and/or vocal tics, and thus likely to be associated with the urge to tic (Bohlhalter et al., Citation2006). This study identified a network of brain areas that were activated immediately prior to tic onset, and, most importantly, identified the insular cortex, the anterior cingulate cortex, and the parietal operculum, which includes the secondary somatosensory cortex (SII) (Eickhoff, Amunts, Mohlberg, & Zilles, Citation2006), as the most likely anatomic regions responsible for the uncomfortable feelings associated with premonitory urges to tic (Bohlhalter et al., Citation2006). Consistent with this proposal, electrical stimulation of the insular cortex or the parietal operculum can elicit unpleasant somatosensory or visceral sensations (Augustine, Citation1996; Ostrowsky et al., Citation2002; Penfield & Faulk, Citation1955). By contrast, electrical stimulation of the medial frontal lobes produces motor outputs in the face and upper limbs comparable to tics (Bancaud et al., Citation1976; Lim et al., Citation1994; Talairach et al., Citation1973).
The proposal that the insular and cingulate cortices are associated with the uncomfortable feelings associated with the urge to tic in TS is consistent with the putative role of these areas in the neural representation of bodily states more generally (interoception), and the initiation of behaviors associated with these bodily representations (for reviews, see Craig, Citation2002, 2009; Naqvi & Bechara, Citation2008). Thus, Craig suggests that these two regions are linked functionally and can be thought of as the limbic sensory and motor areas. He has proposed that these two areas form part of a functional brain system that is associated with the awareness of bodily states (particularly the right insular cortex) and the maintenance of homeostasis (Craig, Citation2009; cf. Damasio, Citation1999).
It is important to note that Craig's particular view of interoception includes a representation of all body states relevant to homeostasis, including pain, temperature, taste, visceral sensation, inflammation, itch, and many aspects of touch and proprioception that are often viewed as part of an “exteroceptive” somatosensory system (Craig, Citation2003). Consistent with this view, recent functional brain-imaging studies have demonstrated that punctate somatosensory stimulation of the upper limbs produces significant increases in brain activity––blood oxygen level-dependent (BOLD) response)––bilaterally within the insular cortex (e.g., Jackson, Parkinson, Pears, & Nam, Citation2011; Parkinson et al., Citation2011). However, neurophysiological studies suggest that the insular cortex may play a particularly important role in representing the emotional significance of somatosensory signals. Thus, it has been shown that a neural pathway, consisting of unmyelinated fibers, projects to the insula (Olausson et al., Citation2002; Vallbo, Olausson, & Wessberg, Citation1999), and that these fibers are associated with affective or sensual touch (e.g., pleasant touch sensation).
Brain-imaging and neurological studies also indicate that the posterior and mid-insular cortex may play an important role in body ownership and our sense of agency (control) over our body. Thus, a positron emission tomography (PET) imaging study reported by Tsakiris, Hesse, Boy, Haggard, and Fink (Citation2007) reported that body ownership, as indexed by the strength of the rubber hand illusion, was associated with activation increases within the right posterior insula and right frontal operculum. Similarly, Karnath and colleagues have shown that lesions involving the right insula impair body awareness, and the sense of limb ownership (e.g., Baier & Karnath, Citation2008; Karnath, Baier, & Nagele, Citation2005).
Arguably, the most direct neuropsychological evidence that the insular cortex is key to the experience of urges for action comes from a study that investigated the effect of insula lesions on the urge to smoke in those addicted to smoking (Naqvi, Rudrauf, Damasio, & Bechara, 2007). This study compared smokers who had sustained damage involving the insula with a group of smokers whose damage involved other brain areas, but spared the insula. The study investigated changes in smoking behavior post-stroke and demonstrated that smokers whose brain damage involved the insula were significantly more likely than smokers with lesions sparing the insula to exhibit a “disruption of smoking addiction.” Importantly, individuals described this disruption of addiction as like their body “forgetting the urge to smoke” (Naqvi et al., 2007).
LIMITATIONS OF INDIVIDUAL BRAIN-IMAGING STUDIES
Functional brain-imaging studies using fMRI have become central to cognitive neuroscience; however, it should be recognized that such studies have limitations. Many of these are well known and relate to the constraints imposed by hemodynamic signals (Logothetis, 2008). A discussion of these limitations is generally beyond the scope of this paper; however, below, we briefly outline some issues that specifically relate to difficulties in interpreting fMRI activations associated with the urge to tic in individuals with TS.
First, one obvious difficulty associated with interpreting the meaning of the patterns of BOLD activity reported in fMRI studies that have sought to identify brain regions associated with the occurrence of tics in TS, is that individuals with TS are instructed to remain still in the MRI scanner and to suppress their tics. Thus, the regions activated can reflect brain areas associated with the generation of tics or brain areas linked to their active suppression. Second, recent evidence suggests that individuals with neurodevelopmental disorders may follow unique developmental trajectories whereby they undergo compensatory, neuroplastic changes in brain structure and function that help them gain control over their symptoms (Jackson, Parkinson, Jung, et al., Citation2011). As a consequence, individuals with TS may exhibit differences in functional anatomy, compared to typically developing individuals, even when they are performing an identical behavioral task with comparable levels of task performance. Third, the functional anatomy of the urge to tic in TS may differ from the functional anatomy of other forms of the urge for action. In fact, a related limitation of fMRI studies is that it is very often difficult to carry out the range of control studies that might be conducted when using non-imaging experimental techniques. Finally, an important limitation of individual fMRI studies is that it is very often difficult to compare across studies. Thus, a comparison of individual fMRI studies of, for example, the urge to urinate and the urge to swallow, might reveal differences in functional anatomy because these behaviors have different underlying neural circuitry, or because of differences in the following factors: the behavioral paradigms used, the scanner hardware and imaging protocols adopted, analysis protocols and statistical thresholds, etc.
This last limitation can be overcome, however, through the use of quantitative meta-analytic studies. Such studies permit an estimation of the fMRI BOLD response associated with different behaviors by drawing upon the entire body of published studies within a particular behavioral domain. One method that has proven popular recently has been the activation likelihood estimation (ALE) method developed by Turkeltaub, Eden, Jones, and Zeffiro (Citation2002) and modified by Eickhoff and colleagues (Eickhoff et al., Citation2009).
A QUANTITATIVE META-ANALYTIC COMPARISON OF THE URGE TO MICTURATE AND THE URGE TO SWALLOW
Here we report the use of the ALE method to directly compare brain activity associated with the urge to urinate (micturition) and the urge to swallow.
Study selection criteria
Functional neuroimaging studies were retrieved via searches in the PubMed, ISI Web of Knowledge, and Scopus databases, as well as identified by reference tracing and through reviews. Experiments reported in these papers that corresponded to the core behavioral contrasts under consideration, such as contrast of urine withholding against a resting baseline, urine voiding against baseline, and volitional swallowing against baseline, were included in the meta-analyses if they fulfilled the following criteria: analyses must be computed across the whole brain and not restricted by partial coverage or regions of interest analyses; coordinates must be reported in an XYZ format, either in MNI or Talairach space; only experiments that investigated differences between stimulation conditions in healthy control population were included; and experiments focusing on between-group differences were excluded. No selection was made on the basis of the applied statistical threshold, as all studies were obtained from peer-reviewed journals. Additional methodological information is provided in detail in the online supplementary material, available via the ‘Supplementary’ tab on the article's online page (http://dx.doi.org/10.1080/17588928.2011.604717).
Experiments investigating the functional anatomy of swallowing were selected if they contrasted saliva swallowing, water swallowing, overt swallowing, or covert swallowing with a rest condition or other suitable control condition. Experiments investigating the functional anatomy of micturition were selected if they contrasted micturition, urine withholding, bladder filling, or the urge to void with a rest condition or other suitable control condition. See Supplementary Material for further details.
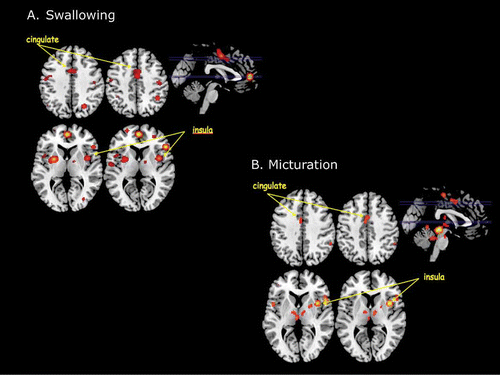
The quantitative comparison of ALEs across studies of swallowing revealed widespread clusters of activation that exceeded the conservative statistical threshold (A). The areas of cortex that exceeded threshold included primary motor cortex (BA 4), premotor cortex (BA 6), frontal cortex (BA 9, 10, 44, 45), parietal cortex (BA 7, 39, 40), cingulate cortex (BA 24, 31), and insular cortex (BA 13). Subcortical activations included the putamen, thalamus (ventral anterior nucleus), and claustrum. Quantitative comparison of ALEs across studies of micturition also revealed widespread clusters of activation in both cortex and subcortical brain areas (B). Areas of cortex that exceeded threshold included premotor cortex (BA 6), frontal cortex (BA 44), parietal cortex (BA 40), cingulate cortex (BA 24, 32), and insular cortex (BA 13). Subcortical activations included the cerebellum, putamen, thalamus (medial dorsal and ventral lateral nuclei), and claustrum.
Figure 2. (A) Main results of an ALE meta-analysis of neuroimaging studies of swallowing. This analysis revealed a number of activation foci that survived conservative statistical correction (p < .05 corrected for false discovery rate). Among these were activations within the insular cortex and the dorsal mid-cingulate cortex. (B) Main results of an ALE meta-analysis of neuroimaging studies of micturition. Again this analysis revealed statistically significant foci of activation within the insular cortex and the dorsal mid-cingulate cortex.
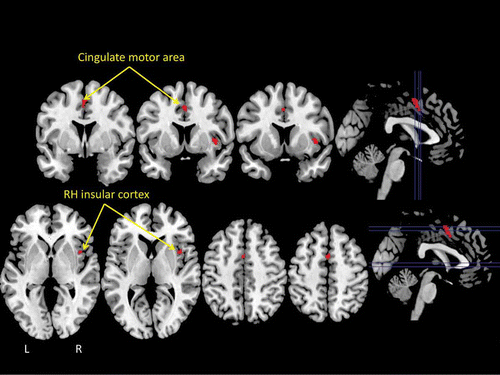
To determine whether areas of overlap exist between brain regions activated during swallowing, or when individuals have an urge to swallow, and regions activated when individuals urinate, or have an urge to urinate, we carried out a conjunction analysis between the swallowing and micturition ALE maps. This analysis confirmed that, of the widespread regions activated in both studies, only two areas survived statistical comparison (p < .05): the insular cortex of the right hemisphere and the mid-cingulate cortex bilaterally (). This finding is consistent with the view, outlined above, that the insular cortex, particularly the right insula, may play an important role in the awareness of bodily sensations, including behavioral urges. It is noteworthy that the two behaviors chosen for study here, swallowing and micturition, involve body areas (i.e., the mouth and oropharyngeal area, and the bladder and genitals) that are located far from one another, and have representations in primary sensorimotor cortex that are also quite distinct and spatially separate.
Figure 3. The results of a statistical “conjunction” analysis between the ALE meta-analyses of swallowing and micturition illustrated in . The conjunction analysis revealed only two brain areas that reached statistical threshold: the insular cortex of the right hemisphere (RH) and the mid-cingulate cortex (cingulate motor area) bilaterally.
An fMRI study of the urge to yawn
To further investigate this issue, we sought to investigate the functional anatomy of the urge to yawn. As there are insufficient neuroimaging studies currently published to permit an ALE meta-analysis, we carried out an fMRI study of the urge to yawn. Full methodological information is provided in detail in the online supplementary material.
While being scanned, participants observed a sequence of video clips, each approximately 12 s in length, which illustrated an actor yawning. These stimuli had been previously shown to induce high levels of spontaneous yawning in a previous fMRI study (Schüermann et al., Citation2005). In the current study, participants were instructed to suppress their yawns (which they were able to do successfully) but were required to report periods during which they experienced a strong urge to yawn.
fMRI and anatomic data analysis
Anatomic images were transformed into the Talairach coordinate system and co-registered with each fMRI data set. Regional activation maps were obtained with a single-subject GLM (general linear model) for each individual. We defined a single predictor that modeled the periods that participants reported experiencing the urge to yawn. All 10 individuals included in the analyses reported successfully suppressing their yawns, and inspection of the motion data confirmed this. Second-level analyses involved calculating three-dimensional statistical parametric maps with separate-subject predictors for the group, using a random effects GLM (RFX). The resulting fMRI activity maps were thresholded at a Z value of 3.29 corresponding to p < .001uncorrected with a minimum cluster threshold of at least 20 voxels. The results of this analysis revealed a number of statistically significant clusters of activation. Details are provided in .
TABLE 1 Coordinates for center-of-gravity and peak activations for statistically significant clusters of activation associated with the urge to yawn
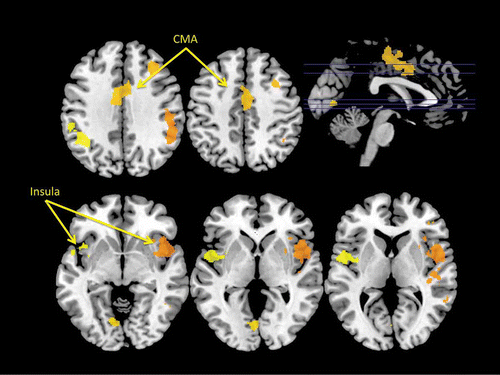
Details of statistically significant fMRI BOLD activations associated with the urge to yawn are presented in and . Importantly, the analyses of the fMRI BOLD response associated with participants' subjective reports of their urge to yawn revealed that both the cingulate motor area (CMA) and the insular cortex bilaterally were activated when individuals reported an urge to yawn.
Figure 4. Regions exhibiting a statistically significant increase in blood oxygen level-dependent (BOLD) signal corresponding to the self-reported urge to yawn in an fMRI study of yawning. Again this analysis revealed statistically significant foci of activation within the insular cortex and the dorsal mid-cingulate cortex. CMA: cingulate motor area.
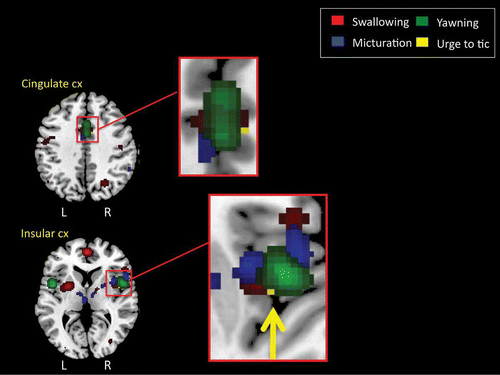
To examine further whether the coordinates of the yawning activations overlapped with the activation foci observed for the ALE analyses of swallowing and micturition reported above, we obtained ALEs for the peak activations of the yawning CMA and insula activations so that they might be compared directly with the results of the ALE results for swallowing and micturition. Furthermore, we also obtained ALEs for the peak activations associated with the urge to tic in individuals with TS reported by Bohlhalter et al. (Citation2006). These data are presented in , which illustrates that all four behavioral domains overlap in the region of the mid-cingulate cortex and the insular cortex of the right hemisphere.
Figure 5. Regions of overlap between ALE meta-analytic studies of swallowing and micturition and fMRI studies of the urge to tic in individuals with TS (Bohlhalter et al., Citation2006) and the urge to yawn in neurologically normal adults. Again these analyses reveal regions of overlap within the insular cortex (CX) of the right hemisphere and the mid-cingulate cortex bilaterally.
Picard and Strick (Citation2001) review the location and functional anatomy of the motor areas located on the medial surface of the human brain. In addition to identifying the supplementary motor area (SMA) and pre-SMA, these authors identify three separable areas within the human cingulate cortex: a caudal cingulate zone (CCZ) situated ventral to the SMA, and a rostral cingulate zone (RCZ) that is further subdivided into anterior and posterior subregions. It is noteworthy that the cingulate fMRI BOLD activations associated with the urge for action in three everyday behaviors (i.e., swallowing, micturition, and yawning), and with the urge to tic in TS, are each located in a region of cingulate cortex that corresponds closely to the CCZ. While the RCZ is associated with conflict detection, attention and arousal processes, and the selection of action, the CCZ is, by contrast, associated with the execution of simple movements and is also activated in response to bodily stimulation such as the delivery of painful cutaneous heat and cold stimuli (Picard & Strick, Citation2001). It should be noted that whereas the SMA has itself been linked to the conscious intention to move (see Desmurget & Sirigu, Citation2009), and while the CCZ and SMA may be co-activated during movement execution, the SMA and CCA should be considered functionally distinct from one another (Picard & Strick, Citation2001).
EFFECTIVE CONNECTIVITY BETWEEN INSULAR AND CINGULATE CORTICES
The insula and anterior cingulate cortex have been considered to be the input and output regions of a functional system that is engaged in cognitive, affective, and behavioral contexts (Craig, Citation2009; Medford & Critchley, Citation2010). Consistent with this proposal there is now considerable evidence that these regions are jointly active across a wide range of experimental conditions (for a recent review, see Medford & Critchley, Citation2010). In addition, functional connectivity analyses of resting state fMRI BOLD, using seed regions located within the insula, have shown that (1) the anterior insula is connected functionally with the anterior and mid-cingulate cortex; and (2) the mid- and posterior regions of the insula are connected only with the posterior region of the mid-cingulate cortex (Taylor, Seminowicz, & Davis, Citation2009).
To investigate the effective, or directional, connectivity of the anterior cingulate and insular cortices, we used the Granger causality mapping (GCM) technique. GCM implements a statistical concept of causality that is based on temporal prediction. Unlike some other methods used to examine effective connectivity, GCM makes no a priori assumptions concerning the connectivity of the seed region, but examines the association between the BOLD time course of the seed region and the time course of each voxel outside the seed region. In the current study, two reference or “seed” regions were defined from the BOLD activations associated with the urge-to-yawn versus rest RFX contrast referred to above. The first “seed” region was located within the mid-cingulate cortex, and the second within the insula cortex of the right hemisphere (see the online supplementary material for additional detail).
The GCM analyses revealed a number of brain areas with effective connectivity values that exceeded statistical threshold (p < .001). The Talairach coordinates of these areas are presented in . For the anterior cingulate cortex seed region, the GCM analysis identified four brain areas that likely exerted an influence over the “seed” region. These were the anterior portion of the insular cortex in the left and right hemisphere, and the anterior portion of the thalamus bilaterally. These regions are shown in blue in . The GCM analysis also identified four brain areas that were themselves influenced by the “seed” region. These were the mid/posterior region of the insular cortex in the left and right hemisphere, and the centromedial region of the thalamus bilaterally. These regions are shown in pink in .
TABLE 2 Results of GCM analysis of effective connectivity for mid-cingulate cortex and right hemisphere insula “seed” regions
Figure 6. (A) Results of the effective connectivity analyses, based upon Granger causality mapping (GCM), of the yawning fMRI study. In this case, the “seed” region for the GCM has been defined as the region of the mid-cingulate cortex significantly activated during the urge to yawn. The GCM analysis revealed that regions of the anterior insula bilaterally exert a significant influence over the seed region (blue), whereas regions of the mid-insula and inferior frontal lobe bilaterally are influenced by the seed area (pink). TRA: transverse; IFG: inferior frontal gyrus. (B) Further results of the GCM analysis based upon the mid-cingulate “seed” region. The analysis also revealed that regions of the centromedial thalamus bilaterally exert a significant influence over the seed region (blue), and bilateral regions of the anterior thalamus are influenced by the seed area (pink). CMA: cingulate motor area.
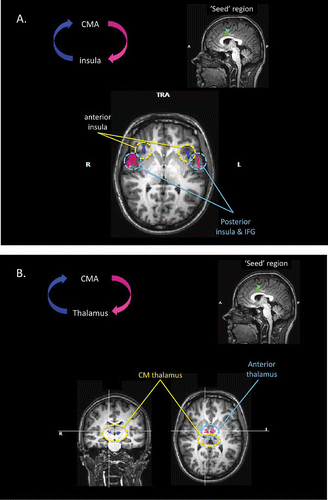
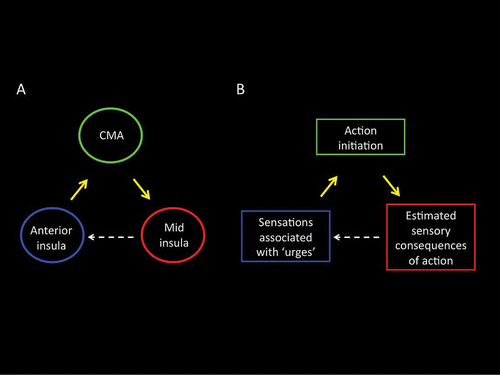
In addition to revealing patterns of effective connectivity between the thalamus and the cingulate motor areas, the GCM confirmed that the cingulate motor region is influenced by the anterior portion of the insular cortex (i.e., the BOLD response in the anterior insular cortex predicts the BOLD response in the cingulate motor region). This finding is entirely consistent with the view of Craig (Citation2002, 2009) that the insular and cingulate cortices form the input and output regions of a functional system, and can be characterized as the limbic sensory and motor areas. Importantly, the GCM analysis confirms that the cingulate motor region also exerts an influence over the activation of a different region of the insular cortex; specifically the mid-insular cortex. This can be clearly seen in A, which illustrates insular regions exerting an influence over the cingulate motor region (blue) and being influenced by the cingulate motor region (pink). Later in this paper, we speculate on the likely functional significance of this anterior insula → cingulate → mid-insular loop.
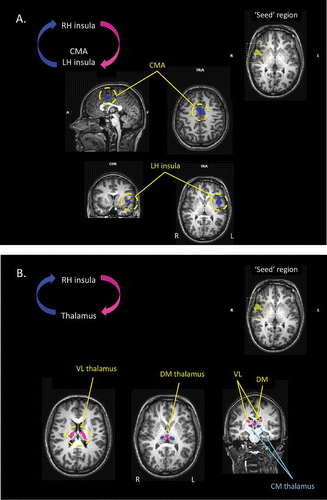
For the right hemisphere insular cortex seed region, the GCM analysis identified four brain areas that likely exerted an influence over the “seed” region (). These were the anterior portion of the insular cortex in the left hemisphere, the mid-portion of the cingulate cortex (cingulate motor area), and the centromedial portion of the thalamus bilaterally. These regions are shown in blue in . The GCM analysis also identified four regions of the thalamus that were themselves influenced by the “seed” region. These were the ventral lateral region of the thalamus bilaterally, and the medial dorsal region of the thalamus bilaterally. These regions are shown in pink in .
Figure 7. (A) Results of the effective connectivity analyses, based upon Granger causality mapping (GCM), of the yawning fMRI study. In this case, the “seed” region for the GCM has been defined as the region of the insular cortex of the right hemisphere that was significantly activated during the urge to yawn. The GCM analysis revealed that corresponding regions of the insular cortex within the left hemisphere, and the mid-cingulate cortex bilaterally, each exert a significant influence over the seed region (blue). CMA: cingulate motor area; TRA: transverse. (B) Further results of the GCM analysis based upon the right insula “seed” region. The analysis also revealed that regions of the centromedial thalamus bilaterally exert a significant influence over the seed region (blue), and that bilateral regions of the ventral-lateral and dorsomedial thalamus are influenced by the seed area (pink).
It is important to keep in mind that the patterns of effective connectivity revealed by the GCM analyses do not necessarily reflect anatomic connections. Nevertheless, the patterns of connectivity revealed by the GCM are consistent with known anatomy and function of specific thalamocortical connections. For instance, the anterior thalamic nuclei receive their primary input from the hypothalamus, project to the cingulate cortex, and are associated with visceral and emotional processing. In the current study, the GCM analysis confirms that the activity of the anterior thalamus exerts an influence over the cingulate cortex and thus provides a route by which emotional and visceral signals might influence the selection of motor responses.
The centromedian nuclei of the thalamus are a primary source of thalamostriatal projections and play a key role in motor function. In the current study, the GCM analyses confirm that the centromedian region of the thalamus is influenced by the activation of the cingulate cortex but in turn exerts an influence over the activity of the right insular cortex. This pattern of connectivity suggests that motor processes in the cingulate motor regions of cortex may gain influence over motor selection mechanisms within the striatum via their influence over the centromedial thalamus. Furthermore, the centromedial thalamus may also signal outcomes of the motor selection process to the insula. We speculate that the insular cortex may accumulate evidence on the outcomes of the action, determine whether the conditions giving rise to the urge for action have been resolved, and, if appropriate, generate a sense that the urge for action has been satisfied (see below). In the current context, it is of interest to note that deep-brain stimulation (DBS) of the centromedian region of the thalamus has been demonstrated to be an effective treatment for intractable TS and produces a substantial reduction in the occurrence of tics (Ackermans et al., Citation2011).
The GCM analysis also revealed that the right hemisphere insular cortex “seed” area is influenced by the BOLD activation within the ventral lateral and dorsal medial regions of the thalamus. The ventral lateral nuclei of the thalamus receive their input from the basal ganglia and cerebellum and send outputs to motor regions of cortex. As illustrated in , a key aspect of the motivation-for-action model outlined above is that information about the outcomes of motor events is relayed to the limbic system, where it might be used to determine whether the conditions giving rise to the urge for action have been resolved. The medial dorsal thalamic nuclei are known to receive their inputs primarily from prefrontal and limbic regions of cortex, and project to association areas of the frontal lobe. These nuclei have been associated with complex aspects of cognition, including attentional control and multitasking, goal-directed action planning, and learning and working memory.
A ROLE FOR INSULAR CORTEX IN SENSORIMOTOR PREDICTION?
Our everyday movements often involve interactions between our body and physical objects located within our environment, and engage multiple sensorimotor systems acting in concert. Action selection mechanisms must therefore take account of information about the current state of the motor apparatus (our body) and also of the behavior of objects within our immediate environment. Recent computational neuroscience approaches to the selection and control of movement (e.g., Wolpert, Citation1997; Wolpert & Ghahramani, Citation2000) and to reinforcement learning (e.g., Dayan & Niv, Citation2008) have emphasized the importance of prediction mechanisms to these processes.
It has been argued that efficient motor behavior relies to a large extent upon predictive mechanisms that provide accurate estimates, or “internal models,” of the changing state of our body and the objects with which we interact. Internal “forward” models are thought to compute dynamic estimates of the body state and to predict the sensory consequences of actions (Wolpert & Ghahramani, Citation2000). In this view, prediction is necessary to compensate for delays associated with the processing of sensory information, but afferent sensory information is critically important for maintaining the accuracy of internal models and is used to monitor and correct for prediction errors, thereby improving future prediction accuracy and movement control. Thus, any discrepancies between the predicted and observed consequences of an action are used to increase or maintain the accuracy of forward models.
Reinforcement learning also involves the evaluation of the outcomes that follow an action. Computational models of reinforcement learning propose that learning can be based upon internal models of the state transitions and action outcomes within an environment, or based upon model-free learning mechanisms (Dayan & Niv, Citation2008). Model-free reinforcement learning involves learning to estimate or predict the likely outcome (value or reward) of a given action (state) given an appropriate action-selection policy. This kind of learning is associated with the formation of “habitual” responses (Graybiel, 2008) and has been particularly linked to the operation of the ventral striatum, the neurotransmitter dopamine acting as a reinforcement signal that codes for reward “prediction error” (Schultz, Dayan, & Montague, Citation1997). By contrast, model-based reinforcement learning has been associated with goal-directed action in which the efficacy of candidate actions is evaluated with reference to an internal model of the task or state space. A key concept associated with this type of learning is that the selection of an appropriate action may involve a “mental simulation” of potential outcomes (Dayan & Niv, Citation2008). We suggest that the formation of “urges-for-action” is probably linked to the operation of the habit-learning system, whereas the formation of “desires-for-action” is probably associated with goal-directed, action-planning mechanisms.
A QUANTITATIVE META-ANALYSIS OF REWARD PREDICTION
In this paper, we have speculated that the insular cortex may accumulate evidence on the outcomes of an action and determine whether the conditions giving rise to the urge for action have been resolved. Within the computational framework offered by the forward model literature, this process might involve a comparison of a “next state estimate” (the output of a forward “sensory” model that provides an estimate of the sensory consequences of a planned action) with afferent sensory information that signals the sensory outcomes of the executed action. Within the computational framework offered by model-free reinforcement learning, this process would involve predicting or representing the likely outcome (value or reward) of an action.
To examine whether the insular cortex plays a key role in the representation of the reward estimates for planned actions, we carried out an ALE meta-analysis of functional brain-imaging studies that have investigated the anticipation or expectation of rewards. Full methodological information is provided in detail in the online supplementary material.
Studies were initially chosen by the following keywords: “reward,” “prediction,” “prediction error,” “fMRI” and “prediction,” “prediction error,” “expectation,” and “anticipation.” The reward modalities used in the studies reported here were monetary or pleasurable taste rewards. Studies were further refined according to the following criteria.
First, events used in the fMRI contrasts should occur prior to the onset of the reward to reflect the outcome of anticipatory processes rather than the processes in response to the actual receipt of a reward.
Second, the contrasts should reflect events in which participants hold an expectation of reward versus a control event such as the expectation of non-rewarding stimuli, or the expectation of loss. The study might include, however, contrasts of high reward probability versus low reward probability. Thus, results that reveal activations by association with the reward probability were explicitly included in the analysis.
Third, participants should expect primary rewards or secondary rewards. Studies that investigated the prediction of non-rewarding events were excluded from this analysis. As a result of these selection criteria, eight studies were included in the meta-analysis. Full details of these studies are provided in the online supplementary material.
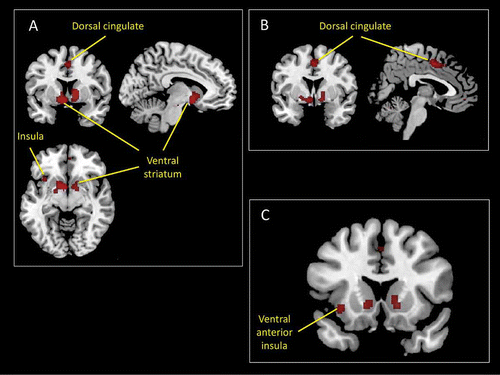
The results of the ALE meta-analysis are illustrated in . They show that the following brain areas were significantly activated for contrasts associated with the prediction or expectation of rewards across the set of fMRI studies included in the analysis: the left ventral anterior insula cortex, the ventral striatum bilaterally, the globus pallidus bilaterally, and the dorsal cingulate cortex.
Figure 8. Main results of an ALE meta-analysis of neuroimaging studies of reward expectation (see the online supplementary material for additional information). This analysis revealed a number of activation foci that survived conservative statistical correction (p < .05 corrected for false discovery rate). Among these were activations within the insular cortex (A and C) and the dorsal mid-cingulate cortex (B).
The above analyses implicate the input and output nuclei of the basal ganglia together with the anterior insula and the dorsal cingulate cortex in the representation of reward estimates. These findings are consistent with recent proposals that the basal ganglia nuclei modulate movement execution according to motivational factors, specifically context-specific cost/reward estimates (Turner & Desmurget, Citation2010); that the anterior insular cortex may represent expectations of both positive and negative action outcomes (e.g., Preuschoff, Quartz, & Bossaerts, Citation2008); and that the anterior cingulate cortex plays a fundamental role in relating actions to their outcomes, and functions, together with the ventral striatum, to mediate cost–benefit decisions over the selection of action (Behrens, Woolrich, Walton, & Rushworth, Citation2007; Rushworth, Mars, & Summerfield, Citation2009; Rushworth, Walton, Kennerley, & Bannerman, Citation2004).
These findings are also consistent with the proposal being advanced here that the anterior insular cortex and the dorsal cingulate motor areas, along with the ventral striatum and thalamus, form core nodes in a motivation-for-action system. Specifically, we propose that pleasant or unpleasant somatosensory or visceral sensations––for instance, the premonitionary sensations that precede the occurrence of tics in TS––are represented within the SII, and in the posterior and mid-insular cortex, and that these sensations will often elicit habitual, overlearned, actions. We also propose that awareness of these bodily sensations, often perceived as an urge for action, is associated more specifically with the activity of the anterior insula cortex. Signals associated with such bodily sensations are relayed from the insula to cingulate motor areas that, together with the ventral striatum, may participate in the selection of a particular action based upon a cost–benefit analysis of the likely “value” of an action given the organism's previous history of action outcomes.
Finally, as illustrated in , we propose that an estimate of the predicted sensory consequences (outcome) of the selected action is then returned to the insula and inferior frontal cortex, where it may be used to determine whether the sensations giving rise to the urge for action are predicted to alter as a result of the intended action, and, if appropriate, to generate a sense that the urge for action has been satisfied. This last proposal is consistent with recent demonstrations that signals from the cingulate motor area participate in the attenuation of somatosensory activity in SII ahead of voluntary movements (Parkinson et al., Citation2011). Such sensory cancellation is thought to be a key function of “forward models” (Wolpert, Citation1997; Wolpert & Ghahramani, Citation2000), and it is suggested that the predicted sensory consequences of self-generated movements can be attenuated in favor of unpredictable, exafferent, somatosensory input (i.e., sensory signals produced by the environment).
Figure 9. (A) GCM effective connectivity analyses of fMRI BOLD activations of yawning revealed a reciprocal pattern of effective connectivity between the anterior insula, the cingulate motor area, and the mid-insula. (B) On the likely function of these connections, we propose that the anterior insula may represent the urge-for-action, that the cingulate motor region may participate in the selection of an appropriate action following a cost–benefit analysis based upon the organism's past action–outcome history, and that the mid-insular cortex may evaluate, based upon a prediction of the likely outcome of the selected action, whether the conditions giving rise to the urge have been resolved, and, if appropriate, may generate a sense that the urge-for-action has been satisfied. CMA: cingulate motor area.
CONCLUSION
In this paper, we have begun to consider the nature and the functional anatomy of “urges-for-action,” both in the context of everyday behaviors such as yawning, swallowing, and micturition, and in relation to clinical disorders such as TS, where urges-for-action are considered pathological, and interfere with activities of daily living. We began by reviewing previous frameworks for thinking about behavioral urges, such as Davenport, Sapienza, and Bolser's (2002) motivation-for-action framework that had been formulated in the context of the urge to cough. While it contains several important insights, we took the view that a core aspect of this framework––that actions depend upon the conversion of an urge-for-action into a conscious desire-for-action––was very likely incorrect. We felt that this was particularly true in the case of individuals with TS, in whom urges-for-action were associated with the occurrence of motor and vocal tics that the individuals found both embarrassing and distressing. In this instance, it is difficult to reconcile such actions with the notion that they spring from a conscious desire for action. Instead we took the view that such actions may be habitual, and as such were highly overlearned and automatic, and could in some cases be executed with very little or no conscious awareness of the sensory stimulation that triggered the action. Importantly, we distinguished between reflex actions and the urge-for-action, which, we argued, occurred when bodily signals gave rise to an action that must be suppressed or deferred.
Then, using quantitative ALE meta-analytic techniques, we investigated the functional anatomy of the urge-for-action in the context of swallowing and micturition, and demonstrated that brain activations associated with these behaviors overlapped in two regions of the brain; the right insula and the dorsal anterior cingulate cortex. Furthermore, we showed that functional activations associated with the urge to tic in individuals with TS and the urge to yawn in neurologically healthy individuals also overlapped within these same two brain areas. These two areas have been conceptualized as the limbic sensory and motor areas respectively (Craig, Citation2002, 2009), and based upon the effective connectivity analyses of our yawning fMRI data, we proposed that they are central in representing the urge-for-action, and together form a neural circuit that represents bodily sensations, generates an urge-for-action (which may or may not reach awareness), selects a particular action based upon a cost–benefit analysis of the likely “value” of that action, accumulates evidence on the outcomes of that action, determines whether the conditions giving rise to the urge have been resolved, and, if appropriate, generates a sense that the urge has been satisfied. Finally, we argue that this circuit is anatomically separate and largely independent of the neural system responsible for the preparation and execution of intentional, goal-directed, actions, although it is possible that these systems may overlap partially with respect to the sense of agency that accompanies intentional action, which has been associated with the activity of the insular cortex of the right hemisphere.
pcns_a_604717_sup_22242066.pdf
Download PDF (59.5 KB)Acknowledgments
This research was supported in part by grants from the Medical Research Council (G0901321), and by the WCU (World Class University) program through the National Research Foundation of South Korea, and funded by the Ministry of Education, Science, and Technology (R31-10008). We are particularly grateful to two anonymous referees for their helpful comments. SBE acknowledges funding by the Human Brain Project (R01-MH074457-01A1), the DFG (IRTG 1328) and the Helmholtz Initiative on Systems-Biology.
References from the Discussion Paper, the commentaries, and the Reply
- Ackermans , L. , Duits , A. , van der Linden , C. , Tijssen , M. , Schruers , K. Temel , Y. 2011 . Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome . Brain , 134 : 832 – 844 .
- Albin , R. L. and Mink , J. W. 2006 . Recent advances in Tourette syndrome research . Trends in Neurosciences , 29 : 175 – 182 .
- Augustine , J. R. 1996 . Circuitry and functional aspects of the insular lobe in primates including humans . Brain research. Brain Research Reviews , 22 : 229 – 244 .
- Baier , B. and Karnath , H. O. 2008 . Tight link between our sense of ownership and self-awareness of actions . Stroke , 39 : 486 – 488 .
- Bancaud , J. , Talairach , J. , Geier , S. , Bonis , A. , Trottier , S. and Manrique , M. 1976 . Behavioral manifestations induced by electric stimulation of the anterior cingulate gyrus in man . Revue Neurologique , 132 : 705 – 724 .
- Behrens , T. E. , Woolrich , M. W. , Walton , M. E. and Rushworth , M. F. 2007 . Learning the value of information in an uncertain world . Nature Neuroscience , 10 : 1214 – 1221 .
- Blakemore , S. , Wolpert , D. and Frith , C. 2002 . Abnormalities in the awareness of action . Trends in Cognitive Science , 6 : 237 – 242 .
- Bohlhalter , S. , Goldfine , A. , Matteson , S. , Garraux , G. , Hanakawa , T. Kansaku , K. 2006 . Neural correlates of tic generation in Tourette syndrome: An event-related functional MRI study . Brain , 129 : 2029 – 2037 .
- Cameron , O. G. 2002 . Visceral sensory neuroscience: Interoception , New York, NY : Oxford University Press .
- Conelea , C. A. and Woods , D. W. 2008 . Examining the impact of distraction on tic suppression in children and adolescents with Tourette syndrome . Behaviour Research and Therapy , 46 : 1193 – 1200 .
- Craig , A. D. 2002 . How do you feel? Interoception: The sense of the physiological condition of the body . Nature Reviews Neuroscience , 3 : 655 – 666 .
- Craig , A. D. 2003 . Pain mechanisms: Labeled lines versus convergence in central processing . Annual Review of Neuroscience , 26 : 1 – 30 .
- Craig , A. D. 2009 . How do you feel – now? The anterior insula and human awareness . Nature Reviews Neuroscience , 10 : 59 – 70 .
- Damasio , A. 1999 . The feeling of what happens: Body, emotion and the making of consciousness , London, , UK : Heinemann .
- Davenport , P. W. 2008 . Urge-to-cough: What can it teach us about cough? . Lung , 186 : S107 – S111 .
- Dayan , P. and Niv , Y. 2008 . Reinforcement learning: The good, the bad and the ugly . Current Opinion in Neurobiology , 18 : 185 – 196 .
- Deecke , L. and Kornhuber , H. H. 1978 . An electrical sign of participation of the mesial “supplementary” motor cortex in human voluntary finger movement . Brain Research , 159 : 473 – 476 .
- Desmurget , M. and Sirigu , A. 2009 . A parietal-premotor network for movement intention and motor awareness . Trends in Cognitive Science , 13 : 411 – 419 .
- Dehaene , S. , Naccache , L. , Cohen , L. , Bihan , D. L. , Mangin , J. F. Poline , J. B. 2001 . Cerebral mechanism of word masking and unconscious repetition priming . Nature Neuroscience , 4 : 752 – 758 .
- Eickhoff , S. B. , Amunts , K. , Mohlberg , H. and Zilles , K. 2006 . The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results . Cerebral Cortex , 16 : 268 – 279 .
- Eickhoff , S. B. , Laird , A. R. , Grefkes , C. , Wang , L. E. , Zilles , K. and Fox , P. T. 2009 . Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty . Human Brain Mapping , 30 : 2907 – 2926 .
- Gerard , E. and Peterson , B. 2003 . Developmental processes and brain imaging studies in Tourette syndrome . Journal of Psychosomatic Research , 55 : 13 – 22 .
- Haggard , P. and Eimer , M. 1999 . On the relation between brain potentials and the awareness of voluntary movements . Experimental Brain Research , 126 : 128 – 133 .
- Jackson , S. R. , Parkinson , A. , Jung , J. , Ryan , S. E. , Morgan , P. S. , Hollis , C. and Jackson , G. M. 2011 . Compensatory neural reorganization in Tourette syndrome . Current Biology , 21 : 580 – 585 .
- Karnath , H. O. , Baier , B. and Nagele , T. 2005 . Awareness of the functioning of one's own limbs mediated by the insular cortex? . Journal of Neuroscience , 25 : 7134 – 7138 .
- Leckman , J. F. 2002 . Tourette's syndrome . Lancet , 360 : 1577 – 1586 .
- Libet , B. , Gleason , C. A. , Wright , E. W. and Pearl , D. K. 1983 . Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act . Brain , 106 : 623 – 642 .
- Lim , S. H. , Dinner , D. S. , Pillay , P. K. , Luders , H. , Morris , H. H. Klem , G. 1994 . Functional anatomy of the human supplementary sensorimotor area: Results of extraoperative electrical stimulation . Electroencephalography and Clinical Neurophysiology , 91 : 179 – 193 .
- Lowell , S. Y. , Poletto , C. J. , Knorr-Chung , B. R. , Reynolds , R. C. , Simonyan , K. and Ludlow , C. L. 2008 . Sensory stimulation activates both motor and sensory components of the swallowing system . NeuroImage , 42 : 285 – 295 .
- Medford , N. and Critchley , H. D. 2010 . Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response . Brain Structure and Function , 214 : 535 – 549 .
- Moore , J. W. , Lagnado , D. , Deal , D. C. and Haggard , P. 2009 . Feelings of control: Contingency determines experience of action . Cognition , 110 : 279 – 283 .
- Naqvi , N. H. and Bechara , A. 2008 . The hidden island of addiction: The insula . Trends in Neurosciences , 32 : 56 – 67 .
- Olausson , H. , Lamarre , Y. , Backlund , H. , Morin , C. , Wallin , B. G. Starck , G. 2002 . Unmyelinated tactile afferents signal touch and project to insular cortex . Nature Neuroscience , 5 : 900 – 904 .
- Ostrowsky , K. , Magnin , M. , Ryvlin , P. , Isnard , J. , Guenot , M. and Mauguiere , F. 2002 . Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation . Cerebral Cortex , 12 : 376 – 385 .
- Parkinson , A. , Condon , L. and Jackson , S. R. 2010 . Parietal cortex coding of limb posture: In search of the body schema . Neuropsychologia , 48 : 3228 – 3234 .
- Parkinson , A. , Plukaard , S. , Pears , S. L. , Newport , R. , Dijkerman , C. and Jackson , S. R. 2011 . Modulation of somatosensory perception by motor intention . Cognitive Neuroscience , 2 : 47 – 56 .
- Pellijeff , A. , Bonilha , L. , Morgan , P. S. , McKenzie , K. and Jackson , S. R. 2006 . Parietal updating of limb posture: An event-related fMRI study . Neuropsychologia , 44 : 2685 – 2690 .
- Penfield , W. and Faulk , M. E. 1955 . The insula: Further observations on its function . Brain , 78 : 445 – 470 .
- Picard , N. and Strick , P. L. 2001 . Imaging the premotor areas . Current Opinion in Neurobiology , 11 : 663 – 672 .
- Preuschoff , K. , Quartz , R. S. and Bossaerts , P. 2008 . Human insula activation reflects risk prediction errors as well as risk . Journal of Neuroscience , 28 : 2745 – 2752 .
- Rushworth , M. F. S. , Mars , R. B. and Summerfield , C. 2009 . General mechanisms for making decisions? . Current Opinion in Neurobiology , 19 : 75 – 83 .
- Rushworth , M. F. S. , Walton , M. E. , Kennerley , S. W. and Bannerman , D. M. 2004 . Action sets and decisions in the medial frontal cortex . Trends in Cognitive Sciences , 8 : 410 – 417 .
- Schüermann , M. , Hesse , M. D. , Stephan , K. E. , Saarela , M. , Zilles , K. Hari , R. 2005 . Yearning to yawn: The neural basis of contagious yawning . NeuroImage , 24 : 1260 – 1264 .
- Schultz , W. , Dayan , P. and Montague , P. R. 1997 . A neural substrate of prediction and reward . Science , 275 : 1593 – 1599 .
- Sirigu , A. , Daprati , E. , Ciancia , S. , Giraux , P. , Nighoghossian , N. , Posada , A. and Haggard , P. 2004 . Altered awareness of voluntary action after damage to the parietal cortex . Nature Neuroscience , 7 : 80 – 84 .
- Talairach , J. , Bancaud , J. , Geier , S. , Bordas-Ferrer , M. , Bonis , A. Szikla , G. 1973 . The cingulate gyrus and human behaviour . Electroencephalography and Clinical Neurophysiology , 34 : 45 – 52 .
- Taylor , K. S. , Seminowicz , D. A. and Davis , K. D. 2009 . Two systems of resting state connectivity between the insula and cingulate cortex . Human Brain Mapping , 30 : 2731 – 2745 .
- Tsakiris , M. 2010 . My body in the brain: A neurocognitive model of body ownership . Neuropsychologia , 48 : 703 – 712 .
- Tsakiris , M. , Hesse , M. D. , Boy , C. , Haggard , P. and Fink , G. 2007 . Neural signatures of body ownership: A sensory network for bodily self-consciousness . Cerebral Cortex , 17 : 2235 – 2244 .
- Turkeltaub , P. E. , Eden , G. F. , Jones , K. M. and Zeffiro , T. A. 2002 . Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation . NeuroImage , 16 : 765 – 780 .
- Turner , R. S. and Desmurget , M. 2010 . Basal ganglia contributions to motor control: A vigorous tutor . Current Opinion in Neurobiology , 20 : 704 – 716 .
- Vallbo , Å. B. , Olausson , H. and Wessberg , J. 1999 . Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin . Journal of Neurophysiology , 81 : 2753 – 2763 .
- Wegner , D. M. 2002 . The illusion of conscious will , Cambridge, MA : MIT Press .
- Wolpert , D. M. 1997 . Computational approaches to motor control . Trends in Cognitive Science , 1 : 209 – 216 .
- Wolpert , D. M. and Ghahramani , Z. 2000 . Computational principles of movement neuroscience . Nature Reviews Neuroscience , 3 : 1212 – 1217 .
- Wolpert , D. M. , Goodbody , S. J. and Husain , M. 1998 . Maintaining internal representations: The role of the human superior parietal lobe . Nature Neuroscience , 1 : 529 – 533 .