Abstract
Odors are often difficult to identify, and can be perceived either via the nose or mouth (“flavor”; not usually perceived as a “smell”). These features provide a unique opportunity to contrast conceptual and perceptual accounts of synesthesia. We presented six olfactory-visual synesthetes with a range of odorants. They tried to identify each smell, evaluate its attributes and illustrate their elicited visual experience. Judges rated the similarity of each synesthetes’ illustrations over time (test-retest reliability). Synesthetic images were most similar from the same odor named consistently, but even inconsistently named same odors generated more similar images than different odors. This was driven by hedonic similarity. Odors presented as flavors only resulted in similar images when consistently named. Thus, the primary factor in generating a reliable synesthetic image is the name, with some influence of odor hedonics. Hedonics are a basic form of semantic knowledge, making this consistent with a conceptual basis for synaesthetic links.
KEYWORDS:
Synesthesia occurs when stimulation in one sense (the inducing stimulus or inducer) induces an involuntary, unusual experience in either the same or a different sense (the elicited experience or concurrent; Grossenbacher & Lovelace, Citation2001). Here, we investigate an unusual form of synesthesia in which odors are reported to induce concurrent visual experiences. The basis of this form of synesthesia in olfaction provides two interesting opportunities. First, in the absence of appropriate visual cues, even common odorants are difficult to name (Cain, Citation1979; Olsson & Friden, Citation2001), allowing us to explore the role of conceptual and perceptual factors in generating reliable synesthetic concurrents. Second, and uniquely among the senses, odors can be perceived via two different routes, orthonasally at the nose, and retronasally as part of flavor via the back of the throat (Hodgson, Linforth, & Taylor, Citation2003). As people generally do not consider smell to be involved in flavor perception (Rozin, Citation1982), we can therefore explore the role of inducer modality awareness in this form of synesthesia.
Odor-induced visual experiences are rare, with only 7% of Sean Day’s sample of 931 synesthetes reporting odor-visual synesthesia (Day, Citation2013). Apart from early reports describing these experiences (Downey, Citation1911; Raines, Citation1909) and Cytowic (Citation1993) reporting case V, there has been no systematic study of odor-induced visual synesthesia (in contrast to where odor, taste, or flavor is the concurrent experience rather than the inducer; e.g. Beeli, Esslen, & Jäncke, Citation2005; Jackson & Sandramouli, Citation2012; Ward & Simner, Citation2003). Here, we present data from six people with odor-induced visual synesthesia, testing the reliability of their experiences and the extent to which different features of the olfactory inducer relate to the visual concurrents.
Despite early claims that synesthesia relies on low-level perceptual mechanisms (e.g., Ramachandran & Hubbard, Citation2001), recent discussions have centered around the role of higher-level conceptual mechanisms (e.g., Chiou & Rich, Citation2014; Nikolić, Jürgens, Rothen, Meier, & Mroczko, Citation2011; Simner, Citation2012). Conceptual theories of synesthesia build on the evidence that grapheme-color synesthesia relies on attention to the inducing stimulus (e.g., Edquist, Rich, Brinkman, & Mattingley, Citation2006; Mattingley, Payne, & Rich, Citation2006; Nijboer & Van der Stigchel, Citation2009; Rich & Mattingley, Citation2003, Citation2010; Sagiv, Heer, & Robertson, Citation2006), and awareness of this inducer’s identity (Mattingley, Rich, Yelland, & Bradshaw, Citation2001), suggesting considerable processing before synesthesia is elicited.
Although attention, identification, and access to semantic memory can be hard to disentangle in vision and audition (e.g., Revonsuo, Citation1999), this is not always the case in olfaction (e.g., Stevenson, Citation2009). First, odors are difficult to identify in the absence of normal contextual cues (Cain, Citation1979). For faces, not knowing the name does not preclude access to semantic memory as long as the face is attended (e.g., Young, Hay, & Ellis, Citation1985). However, for odors, the absence of a name seems to be associated with minimal access to semantic memory (Stevenson & Mahmut, Citation2013b). In fact, the only form of meaning that seems to be reliably accessed without an odor’s name is the degree to which we like or dislike that smell (the “hedonic” information; Stevenson & Mahmut, Citation2013a). Here, we can ask whether an odor that cannot be consistently named still induces a consistent synesthetic concurrent, and what characteristics of the odor determine the visual image. If odors lack access to semantic memory in the absence of a name, and access to meaning is necessary for inducing a synesthetic concurrent, then reliable synesthetic experiences should only be generated by consistently named odors. If, on the other hand, reliable synesthetic concurrents occur regardless of odor names, this suggests some additional aspect of an odor triggers the consistent experience. This may be the strength, irritancy, familiarity, or hedonic information present in a particular odor (e.g., Distel & Hudson, Citation2001; Stevenson, Rich, & Russell, Citation2012).
A second feature of olfactory perception is the presence of two discrete paths to the olfactory receptors: Via the nose (orthonasal) as smell or via the mouth (retronasal) as part of a flavor (Hodgson et al., Citation2003; Rozin, Citation1982). These two pathways have different subjective attributions. Although we clearly attribute something sniffed to the sense modality of smell, when eating and drinking, we are typically unaware that a considerable component of our sensory experience of flavor comes from retronasal olfaction (Stevenson, Citation2014). This provides an opportunity to test whether the odor-induced visual experiences rely on stimulation of the sense of smell (present for both ortho- and retronasal odors) or is influenced by the synesthetes’ awareness of the source (present for ortho- but not for retronasal odors). Thus, we can ask whether the experiences of olfactory synesthetes are less consistent when attention to smell is reduced (i.e., when sampling odors retronasally and in the presence of competing stimulation from taste and oral proprioception) or whether chemical stimulation of the receptors is sufficient, regardless of the pathway, to elicit reliable synesthetic images. In addition, we can compare the features of images generated by olfactory synesthetes with the cross-modal associations generated by non-synesthetic controls in a previous study (Stevenson et al., Citation2012).
We explored odor-visual synesthesia in six individuals focusing on four key questions: (1) Are their synesthetic concurrents reliable?; (2) Do reliable concurrents depend upon identifying (i.e., naming) the odor?; (3) If they do not depend upon identification, what characteristics of the odor drive synesthesia?; (4) Do retronasal odors generate reliable synesthetic concurrents?
METHOD
Participants
Synesthetes
Six self-reported odor-color synesthetes were recruited via an online questionnaire (five female; mean age = 30.5 years, SD = 11.3, range = 19–47; identified as S1–6).
Controls (two-weeks, Stevenson et al., Citation2012)
Eighteen non-synesthetic control participants (seven female, mean age = 22.2 years, SD = 3.6, range 18–32) were tested twice with a two-week interval between sessions. Analyses of these data have been published previously; here, we use these data as a normative dataset for comparison with the synesthetes.
Controls (three-day)
A further group of six non-synesthetic controls (five female, mean age = 29.5 years, SD = 4.5, range = 25–38) were matched to the re-test parameters of the synesthetes (brief test-retest interval, minimum three days) and on age and gender (as females are typically better at odor naming; Cain, Citation1982). Controls completed a questionnaire to confirm they did not have synesthesia.
Judges
Twenty undergraduate psychology students naive to the study were recruited to evaluate the similarity of pairs of images drawn by the synesthetes.
All participants gave informed consent and were paid AUD$15/hour or course credit for their participation. The Macquarie University Human Research Ethics Committee approved the research.
Materials
The participants attended three sessions, described in further detail below. Twenty odors (plus one practice) were used for testing in the two sniffing (orthonasal) sessions (). The odors were selected to vary in terms of hedonics, irritancy, intensity, familiarity, and nameability (see Stevenson et al., Citation2012). Odors were placed on cotton wool balls, which were then placed in identical 250 ml opaque plastic squeezy bottles.
TABLE 1 Stimuli used during the orthonasal test sessions
A third session focused on retronasal delivery of odor within a liquid (flavor). The presence of odor within the liquid was disguised by having blocks of different types of inducing stimuli, to examine the effect of awareness of odor in flavor: Visual (color patches), auditory (musical notes), olfactory (presented in the same kind of squeezy bottles as the olfactory stimuli in the orthonasal sessions), or flavor (liquids presented as colorless 10 ml samples in 25 ml transparent cups covered with aluminum foil). The visual and auditory inducing stimuli were distractors and are not discussed further. We expected participants to realize at some point that the “tastes” were actually odors, but this did not happen. The data obtained from both the olfactory and flavor stimuli were included in analyses presented below. As can be seen in and , some of these chemosensory stimuli were presented in the previous orthonasal sessions (caramel, strawberry, lemon), and some were “new” odors (mint, banana, cherry). All of these odors were presented orthonasally and retronasally. When presented retronasally, they were presented in either sucrose, citric acid, or water. We also included “no odor” control trials, where the squeezy bottle contained just cotton wool balls with no odor (for the orthonasal trials), or where the flavor stimulus included only sucrose, citric acid, or (for all except S3) water, but these just evoked descriptions of the smell of the plastic and so are not discussed further.
Synesthete testing procedures
All six synesthetes attended two sessions where 20 odors () were sniffed at the nose (labelled below as orthonasal sessions: Ortho 1, Ortho 2). Four synesthetes returned for a third session, where stimuli were presented orthonasally as smells and retronasally as part of a flavor (labelled below as flavor session).
Orthonasal session procedure
The orthonasal sessions lasted approximately three hours each, with the following gaps between sessions: S6: three days; S4, S5, and S1: seven days; S3: 12 days; S2: 56 days (based on the synesthetes’ availability).
The synesthetes completed an olfactory screening questionnaire (checking for history indicative of olfactory deficits), the Ishihara color test (Ishihara, Citation1975), and the Smell Identification Test to determine olfactory function (Doty et al., Citation1984). They then smelled 20 odorants in a predetermined random order (different for each synesthete; four synesthetes only smelled 18 odors due to time constraints—butanol and almond were omitted).
Each session started with a practice trial. Participants squeezed the practice odor bottle while holding the spout approximately 7 cm below their nostrils and sniffing. The participants were allowed approximately 20 seconds to identify the odor. This arbitrary limit was imposed in the interest of keeping to session time. The participants were asked to be as specific as possible, but were not prompted with any type of cue (such as a list of odors) and they were told that any response would be considered valid. All participants who named an odor named it almost immediately. When a participant could not immediately name an odor, they were told that they could name the odor later during the trial, but there were no instances where this occurred.
After attempting to identify the odor they were asked to reproduce the synesthetic image, if any, that the odor elicited using a computerized drawing program (GIMP; one participant used crayons and colored pencils on paper instead). We were also interested in whether the act of drawing their elicited image helped the synesthetes to identify an odor that they could not identify during the naming stage, or if the synesthetes changed their odor identification response based on their image. Neither of these events occurred. After a further sniff, the synesthetes used category scales to rate the odor on: Irritancy (1 = not at all, 7 = very); Strength (1 = not at all strong, 7 = very strong); Familiarity (1 = definitely have not smelled before today, 4 = unsure, 7 = definitely have smelled this odor before today); and Liking (1 = dislike, 4 = indifferent, 7 = like). Finally, synesthetes were asked whether the odor evoked any other qualities, such as texture, shape, sound, taste, or emotion. (As this did not generate any unusual responses these data are not further reported.) A three-minute break separated the odors.
Flavor session procedure
The flavor session was at least one month after the Ortho 2 session. Only four synesthetes were available. In the first two steps of the procedure, eight stimuli (those marked as “sniffed” in ) were presented orthonasally in the same manner as the odors in the orthonasal sessions. The remaining stimuli (those marked as “straw” or “sipped” in ) were presented retronasally. The “straw” stimuli were sipped through a straw in order to prevent orthonasal stimulation via sniffing, while the “sipped” stimuli were sipped directly from the cup. In each case they attempted to name the odor/flavor and then drew their elicited image as described above. There was a break of at least two minutes between stimuli. The order of presentation was fixed to make it progressively more obvious that olfaction was involved in flavor perception (i.e., the “sipped” trials involved sniffing the odor prior to sampling it as a flavor in the mouth) but no synesthete realized that the flavor was actually an odor and this progressive procedure had no detectable effect on the synesthetic responses, so it is not further reported.
TABLE 2 Stimuli used during the flavor sessions
Control testing procedures
The three-day control participants were tested with the same orthonasal procedure as the synesthetes. For each odor, they were asked to select colors from a computer palette (or with crayons/color pencils if they preferred) and any other features (e.g., shape, texture, etc.) they felt “went with” the odor. The two-week control participants were also tested with a similar procedure, except that these participants were asked additional questions to probe other features that might “go with” the odor (Stevenson et al., Citation2012).
Consistency rating
All but one of the synesthetes reported visual images of varying complexity (see ; S6 reported single colors). We recruited 20 naive judges to establish the similarity of images from Ortho 1 to Ortho 2. We presented pairs of images and asked the judges to rate the similarity of the two images using a seven-point category scale (1 = not at all similar, 7 = very similar). Each judge rated (in randomized order with counterbalanced presentation of images on the left vs right) between 40 and 110 target pairs per synesthete (not all synesthetes completed all 20 orthonasal odors, and only four synesthetes completed the flavor session). Interspersed randomly were 15 “catch” trials on which the judges had to press a particular number, to help ensure that they maintained attention. Five judges failed one catch trial each but no judge failed more than one.
Figure 1. Responses to smelling two different odors drawn by the synesthetes to illustrate their synesthetic experiences: Caramel (top row L to R: S1, S2, S3; second row L to R: S4, S5, S6) and for the burnt odor (third row L to R: S1, S2, S3; bottom row L to R: S4, S5, S6).
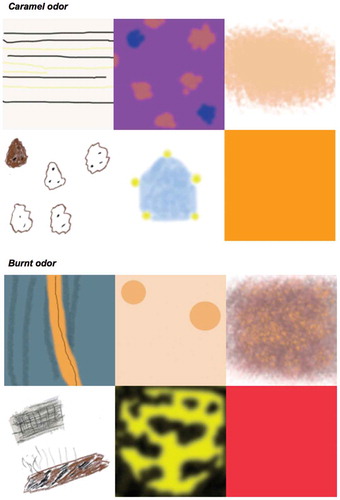
The full set of target pairs for each synesthete—evaluated by all 20 judges—was composed of four different pair types: (1) “Same odor”: 20 pairs of images (one for each odor); one image from Ortho 1 and one from Ortho 2; (2) “Different odors”: 20 randomly selected pairs (using the same fixed random schedule for each judge); one image selected from Ortho 1, and one from Ortho 2, but from different odorants (selected without replacement); (3) “Flavor vs Orthonasal”: 12 pairs of images drawn from the flavor session and Ortho 2; each pair corresponded to the same odor, but the odor had been presented as a flavor in one case and as an odor in the other; and (4) “All stimuli”: 46 pairs of images composed of all possible combinations of odors presented as flavors against different odors drawn from the flavor and Ortho 2 sessions.
Prior to starting the task, judges saw a series of training exemplars. The judges then rated the image pairs, presented side-by-side on a computer monitor, at their own pace, in a single session.
RESULTS
Synesthete screening data
None of the synesthetes were color-blind nor did they report any history of olfactory impairment. shows the smell identification test scores: Four synesthetes were normosmic (i.e., had a normal sense of smell) and two were mildly microsmic (i.e., had a decreased ability to smell; <10th percentile). Given the size of the sample, we cannot make inferences about the number of microsmic synesthetes in the sample, but this might be worth following up in future studies.
TABLE 3 Characteristics of the six synesthetes
Orthonasal data
Consistency of odor naming across time
The synesthetes were generally consistent at naming the odors across time (S6 = 60%, S1 = 61%, S4 = 67%, S3 = 73%, S5 = 75%), with the exception of S2 (28%), who was re-tested following a 56-day interval (the others were re-tested between 3 and 12 days). In contrast, the normative dataset from 18 control participants with a two-week retest interval showed consistent naming for an average of 24.4% (SD = 17.8) of the odors. The six matched controls re-tested at three days could consistently name 39.2% (SD = 13.2). As a group, the synesthetes were significantly more consistent in their odor-naming relative to both groups of controls (two-week controls, t(22) = 4.35, p < .001; three-day controls, t(10) = 2.44, p < .05). Although this could reflect a unique benefit of olfactory synesthesia, it could also represent a non-specific motivational effect. Note that consistent naming is a less biased measure of odor identification than pre-defining a “veridical” name for each odor. A participant who consistently uses the same name for an odor across time is able to identify that odor, even if the name s/he uses differs from that the experimenter would use.
Reliability of odor ratings across time
All of the synesthetes’ intensity, hedonic, irritancy, and familiarity ratings were reliable across time (all rs > .77), and the magnitude of these correlations did not significantly differ from matched controls. This is also consistent with our normative dataset.
Reliability of the synesthetic images across time
For each synesthete, we had ratings from the judges regarding how similar each elicited image was for the same odor presented orthonasally at Ortho 1 and Ortho 2 (“Same odor” trials). This process yielded a similarity rating for each of the 20 odors (or 18 in some cases), for each of the six synesthetes, from each of the 20 judges.
We derived three scores from these similarity ratings: (1) Mean similarity score for image pairs where the eliciting odor was named consistently for Ortho 1 and 2; (2) Mean similarity score for image pairs where the eliciting odor was named inconsistently for Ortho 1 and 2; and (3) Mean similarity ratings derived from images elicited from 20 different odor pairs for each participant (all were named differently), providing a base rate of similarity. Thus each synesthete ended up with three types of mean similarity score from each of the 20 judges: (1) image pairs from the same odor named consistently; (2) image pairs from the same odor named inconsistently; (3) image pairs from different odors.
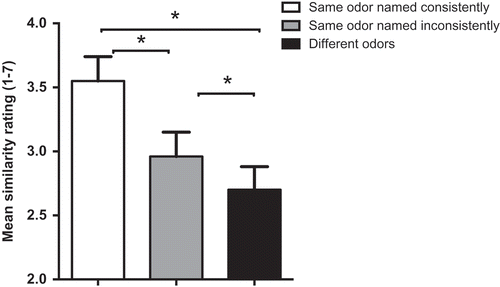
We analyzed the mean similarity of images for (1)–(3), averaged across the six synesthetes, using paired sample t-tests (with alpha set at 0.017 using Bonferonni adjustment). When the odors were the same and were named consistently, the images the synesthetes drew were judged as significantly more similar than: (1) images from the same odor pairs named inconsistently (t(19) = 13.06, p < .017) and (2) images from different odor pairs (t(19) = 16.59, p < .017) (see ). The images from the same odor pairs named inconsistently were judged as significantly more similar than images elicited by different odor pairs (t(19) = 7.20, p < .017).
Figure 2. Mean similarity ratings of image pairs generated from synesthetes’ responses to odors in the Ortho 1 and 2 sessions, divided by consistency of naming. Asterisks show significant differences (p < .017). Error bars are SEM.
We then tested for heterogeneity in the similarity ratings across the six synesthetes, on the three score types. These data were analyzed using a two-way repeated measures ANOVA, with Synesthete as one factor (six levels—one for each synesthete) and Score type (Different, Consistent same, Inconsistent same) as the other. The ANOVA revealed significant main effects (Synesthete, F(5, 190) = 36.2; Score type, F(2, 190) = 181.8) tempered by an interaction between Synesthete and Score type (F(10, 190) = 17.46, MSE = 0.15, p < .001, partial eta-squared = .48) indicating that the synesthetes were heterogeneous across the three score types.
To examine this heterogeneity, we tested whether each of the three comparisons made in the combined analysis (i.e., Same odor named consistently vs. Different odors, etc.) were present for each synesthete (setting alpha at 0.017 for each synesthete to correct for multiple contrasts). The means and outcomes of these tests are presented in . Images elicited by the same odor named consistently were more similar than images elicited by different odors in 5/6 synesthetes (exception: S5). Images elicited by the same odor named inconsistently were more similar than those from different odors for 3/6 synesthetes. Finally, the images from the same odors named consistently were judged as more similar to each other than images from the same odors named inconsistently in 4/6 cases (exceptions: S5 and S6). Thus, the main finding of synesthetic image similarity being driven by the consistent naming of the odor holds for 5/6 synesthetes; the residual similarity from an odor named inconsistently holds for half the individuals.
TABLE 4 Individual data for the judged similarity of synesthetes’ odor-induced images (orthonasal), across time, organized by response type. Mean similarity (SD)
What mediates similarity in synesthetic images without consistent naming?
To explore what factor(s) might support consistent synesthetic images for odors that the synesthetes could not consistently name, we examined the relationships between odor characteristics and image similarity. First, we identified those odors that synesthetes could not consistently name. Second, we selected from these the odors that differed most and least (absolute difference) for ratings of intensity, irritancy, familiarity, and hedonics, across the two orthonasal test sessions. For example, if odors A, B, C, and D could not be named, we looked at the intensity ratings of these four odors. If intensity ratings of A were very different on Ortho 1 and 2, B and C moderately different, and D had the same intensity rating on each occasion, then odors A and D would be selected for this rating, for this synesthete. We then completed the same process for each of the different characteristics (intensity, irritancy, familiarity, and hedonics), identifying pairs of odors for each synesthete that reflected the largest and smallest difference in these features across the two rating occasions.Footnote1 We then determined whether these pairs were associated with more or less similar images. Comparing the similarity scores for odors judged as reliable in intensity, irritancy, familiarity, and hedonics, and as unreliable on these four ratings, allowed us to identify which of these variables is associated with maintaining image similarity in the absence of an odor name.
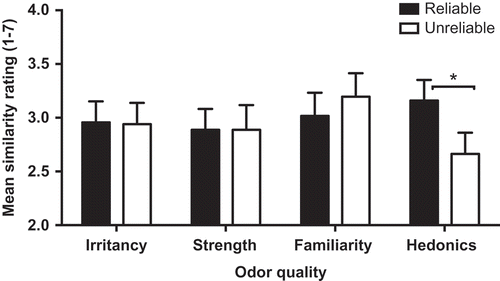
We used a two-way repeated measures ANOVA to analyze data averaged across the six synesthetes (i.e., at the aggregate level). Rating type had four levels (intensity, irritancy, familiarity, and hedonics) and Reliability two levels (reliable across time, unreliable across time). The dependent variable was the image similarity ratings provided by the 20 judges. The ANOVA revealed a main effect of Rating (F = 5.96) tempered by a significant interaction between Reliability and Rating type (F(3, 57) = 9.60, MSE = 0.087, p < .001, partial eta-squared = .34). As can be seen in , mean image similarity ratings were not associated with differences in the reliability of intensity, irritancy, or familiarity ratings (all ts < 1), but there was an effect of differences in hedonics (t(19) = 4.20, p < .001). Pairwise comparisons of the difference scores for each rating type (i.e., reliable across time minus unreliable across time), with alpha set by Bonferonni adjustment, revealed that the hedonic rating difference significantly exceeded all of the other rating differences (all ps < .008). Thus, when an odor received reliable hedonic ratings, the synesthetic images were judged as more similar than when an odor received divergent hedonic ratings on the two occasions.
Figure 3. The association between mean synesthetic image similarity ratings and the reliability of the synesthetes’ odor quality ratings for irritancy, strength, familiarity, and hedonics, for odors that were not consistently named. Error bars are SEM.
We then examined whether the effect of hedonics was consistent across each of the six synesthetes. We repeated the preceding ANOVA, but now with a further within-factor of Synesthete. This revealed an interaction between Synesthete, Reliability, and Rating type (F(15, 285) = 13.45, MSE = 0.58, p < .001, partial eta-squared = .42), indicating heterogeneity in the Reliability by Rating effect between the synesthetes. To further explore this, we constructed a difference score that measured the strength of the hedonic reliability effect relative to all of the other rating types combined. We then tested this value for each synesthete using one-sample t-tests with mu set to 0, to see who showed the hedonic effect identified in the aggregate analysis. Synesthetes S2, S3, S5, and S6 all showed significant hedonic effects (i.e., as with the aggregate analysis) relative to the other rating types (all ts > 3.37), no significant effect was evident for S4, although her mean was in the expected direction, and S1 showed the reverse pattern. Thus, 4/6 synesthetes show hedonic information contributing to similarity of synesthetic images, as shown in the group data.
Flavor data
Reporting of synesthetic images to odor, flavor, and taste
In the initial screening questionnaire, every example given by our synesthetes described visual images evoked by orthonasal smells (the smell of perfume, feces, cooked and raw bacon, vomit, sulphur dioxide, comfort blanket, strawberry, and bread). Participants were also asked if they experienced synesthetic images when exposed to tastes, the term commonly used to describe the sensory experience associated with eating and drinking (Rozin, Citation1982). Three participants (see ) reported synesthetic experiences to tastes.
Image reliability of flavor-odor pairs
The similarity judges also examined the images that the synesthetes drew when they experienced odors retronasally (dissolved in water or various tastants; see Flavor session procedure). Three score types were calculated for the four synesthetes who completed this additional task, each between the images from the second orthonasal session (Ortho 2) and the retronasal (flavor) session. These were the same as our previous analyses: (1) Images from the same odor, named consistently; (2) Images from the same odor, named differently; and (3) Images from different odors. Thus, each synesthete had three similarity scores derived from the responses from each of the 20 judges.
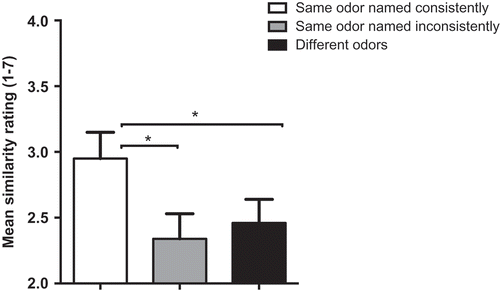
First, we tested for differences in image similarity across the three score types (alpha set at 0.017 using Bonferonni adjustment for multiple comparisons) at a group level. Significantly more similar images () were elicited when an odor was named consistently across presentation orthonasally versus as a flavor than either of the other two alternatives (same odor named inconsistently for orthonasal vs flavor presentation t(19) = 6.95, p < .017); different odors presented orthonasally or as a flavor (t(19) = 8.63, p < .017)). In contrast to the data from the orthonasal reliability analysis, the similarity of an image elicited by a particular odor was completely dependent on the name being consistent. There was no greater similarity of images elicited by inconsistently named orthonasal and flavor presentations of the same odor than those elicited by different odors (t = 1.69).
Figure 4. Mean similarity ratings of image pairs generated from synesthetes’ responses to odors in the Ortho 2 and Flavor sessions, divided by consistency of naming. Asterisks show significant differences (p < .017). Error bars are SEM.
We then tested for heterogeneity in the pattern of response across score types, between the four synesthetes, by conducting a two-way repeated measures ANOVA, with Synesthete as one factor (four levels) and Score type (Different, Consistent same, Inconsistent same) as the other. Although there were significant main effects (Synesthete, F = 5.6; Score type, F = 40.8), there was no interaction between Synesthete and Score type (F = 1.8; see for individual data). Thus, although there was some variability in the overall similarity of images across synesthetes, there was no evidence of heterogeneity in the effect of naming. Synesthetic images from odors presented as flavors are only similar when the odor is named consistently.
TABLE 5 Individual data for the judged similarity of synesthetes’ odor-induced images (comparison of odors experienced as flavors vs odors sniffed at the nose), across time, organized by response type. Mean similarity (SD)
DISCUSSION
The aim of this study was to explore the characteristics of olfactory-visual synesthesia, and to use the unusual aspects of olfaction to examine the contributions of conceptual versus perceptual factors. Our data, from six olfactory-visual synesthetes, reflect the first systematic examination of this phenomenon.
In grapheme-color synesthesia, the elicited images are usually single colors. In contrast, most of our olfactory-visual synesthetes reported complex visual images in which color was only one feature (). As a group, the synesthetes were more consistent than matched non-synesthetes in their consistency of naming odorants on two separate occasions. In addition, there was greater similarity in images elicited by the same odor across test-retest sessions relative to different odors. This similarity was driven primarily by consistent naming, but there was still greater similarity of images than for different odors, even when an odor was given a different name across test sessions. This effect was due to hedonic information. With odors presented to the mouth as part of a flavor on one occasion (retronasal) and as a smell to the nose on another (orthonasal), images were only similar when the odor was named consistently. Overall, then, this suggests that these synesthetes experience reproducible visual experiences when presented with an odorant, analogous to other forms of synesthesia.
Although we discuss the general features of this form of synesthesia below, it is important to note the heterogeneity in synesthetes’ responses. Most, but not all (5/6), synesthetes generated more similar images to the same odor than different odors, but only half the group (3/6) showed similarity based on hedonic information above and beyond similarity based on naming consistency. We could not explore the origins of this variability with our sample size, but our synesthetes varied in age, gender, and olfactory performance score and these differences may account for some of this variability.
The reliability of the elicited images over time was primarily driven by consistent identification of an odor’s name. One possibility is that the odor name alone could evoke these complex visual images, but this does not seem to be the case with our synesthetes. They all reported that the experience occurred on smelling the odorant, not when talking about it. Although all of these synesthetes also have synesthesia related to language, they vary in whether all letters and words, letters alone, or only some words evoke synesthesia. Our data suggest that odor identification is important in supporting the generation of a reliable image, which is consistent with access to meaning being a key driver of synesthetic experience, just as it seems to be for grapheme-color synesthesia (e.g., Chiou & Rich, Citation2014; Dixon, Smilek, Duffy, Zanna, & Merikle, Citation2006; Myles, Dixon, Smilek, & Merikle, Citation2003; Simner, Citation2012; Smilek, Dixon, Cudahy, & Merikle, Citation2001).
We used the difficulty of naming odors to examine the role of perceptual and conceptual factors in the absence of identification. Although we suggest that the absence of a name indicates lack of full access to semantic knowledge about the odor (Stevenson & Mahmut, Citation2013a), it is possible that some fragmentary knowledge might still be available (the “tip of the nose” phenomenon, e.g., Cleary, Konkel, Nomi, & McCabe, Citation2010). In our case, however, we did not find familiarity was a significant predictor of the image similarity; instead, the one aspect of olfaction that contributed to a consistent image in the absence of a consistent name (in the orthonasal data) was hedonics. Whether participants were consistent in their like or dislike of the odor was correlated with the similarity of the images when identification was not consistent. Similar results have been found in studies investigating the relationship between odor and/or taste and visual properties in cross-modal correspondences amongst non-synesthetes (e.g., Schifferstein & Tanudjaja, Citation2004; Seo et al., Citation2010; Velasco, Woods, Deroy, & Spence, Citation2015). Hedonic information has been argued to reflect a form of conceptual knowledge. In an important and still influential series of papers, Osgood and co-workers proposed that the hedonic-evaluative dimension was one of three key components of meaning (Osgood, Citation1952). Indeed, whether something is appraised as good or bad reflects perhaps the most basic kind of concept—whether to approach or avoid a particular stimulus. Thus, we interpret the influence of hedonics to be another form of access to meaning through olfaction.
The aim of the retronasal session was to explore whether odors evoke synesthesia even when they are not perceived as olfactory stimuli and are presented in a situation where they are confounded with taste and oral proprioception. Throughout the retronasal testing session, the participants remained unaware that the stimuli were olfactory rather than “taste.” Despite this, we observed that the images evoked by consistently named retronasal odors were similar to those elicited by the analogous orthonasal odors, but only when the same name was given. This provides more evidence that the name given to an odor is an important determinant of the synesthetic experience, consistent with the effects of identification in grapheme-color synesthesia (e.g., context effects; Dixon et al., Citation2006; Myles et al., Citation2003). Awareness (or lack thereof) of the source of the inducing stimulus therefore plays a role in olfactory synesthesia, as does individuation (identification) of the inducing odor. In addition, to the extent that synesthetic experience requires attention to the inducing stimulus (e.g., Edquist et al., Citation2006; Mattingley et al., Citation2006; Nijboer & Van der Stigchel, Citation2009; Rich & Mattingley, Citation2003, Citation2010; Sagiv et al., Citation2006), these data suggest that attention can be directed towards the olfactory component of flavor in the mouth, a topic that is currently of interest to workers in this field (e.g., Stevenson, Citation2014).
The results of this study of olfactory-visual synesthesia demonstrate that synesthetic experiences from odor depend upon consistent identification and hedonic appraisal of odors. Overall, access to meaning drives olfactory synesthetic experiences, consistent with claims that conceptual-level links may be critical in this phenomenon.
No potential conflict of interest was reported by the authors.
The authors would like to thank Aline Cordonnier and Regine Zopf for assistance with data collection.
Additional information
Funding
Notes
1 One potential concern here is that the absolute difference scores might be larger for one type of rating than for another, but statistical analysis revealed this was not the case.
References
- Beeli, G., Esslen, M., & Jäncke, L. (2005). Synaesthesia: When coloured sounds taste sweet. Nature, 434(7029), 38–38. doi:10.1038/434038a
- Cain, W. S. (1979). To know with the nose: Keys to odor identification. Science, 203(4379), 467–470. doi:10.1126/science.760202
- Cain, W. S. (1982). Odor identification by males and females: Predictions vs performance. Chemical Senses, 7(2), 129–142. doi:10.1093/chemse/7.2.129
- Chiou, R., & Rich, A. N. (2014). The role of conceptual knowledge in understanding synaesthesia: Evaluating contemporary findings from a “hub-and-spokes” perspective. Frontiers in Psychology, 5, 105. doi:10.3389/fpsyg.2014.00105
- Cleary, A. M., Konkel, K. E., Nomi, J. S., & McCabe, D. P. (2010). Odor recognition without identification. Memory & Cognition, 38(4), 452–460. doi:10.3758/MC.38.4.452
- Cytowic, R. E. (1993). The man who tasted shapes. Cambridge, MA: MIT Press.
- Day, S. A. (2013). Types of synaesthesia. Retrieved September 25, 2013, from http://www.daysyn.com/Types-of-Syn.html
- Distel, H., & Hudson, R. (2001). Judgement of odor intensity is influenced by subjects’ knowledge of the odor source. Chemical Senses, 26(3), 247–251. doi:10.1093/chemse/26.3.247
- Dixon, M. J., Smilek, D., Duffy, P. L., Zanna, M. P., & Merikle, P. M. (2006). The role of meaning in grapheme-colour synaesthesia. Cortex, 42(2), 243–252. doi:10.1016/S0010-9452(08)70349-6
- Doty, R. L., Shaman, P., Applebaum, S. L., Giberson, R., Siksorski, L., & Rosenberg, L. (1984). Smell identification ability: Changes with age. Science, 226(4681), 1441–1443. doi:10.1126/science.6505700
- Downey, J. E. (1911). A case of colored gustation. The American Journal of Psychology, 22(4), 528–539.
- Edquist, J., Rich, A. N., Brinkman, C., & Mattingley, J. B. (2006). Do synaesthetic colours act as unique features in visual search? Cortex, 42(2), 222–231. doi:10.1016/S0010-9452(08)70347-2
- GIMP (2.8 and 2.6.6) [Computer software]. http://www.gimp.org/downloads/
- Grossenbacher, P. G., & Lovelace, C. T. (2001). Mechanisms of synesthesia: Cognitive and physiological constraints. Trends in Cognitive Sciences, 5(1), 36–41. doi:10.1016/S1364-6613(00)01571-0
- Hodgson, M., Linforth, R. S., & Taylor, A. J. (2003). Simultaneous real-time measurements of mastication, swallowing, nasal airflow, and aroma release. Journal of Agricultural and Food Chemistry, 51(17), 5052–5057. doi:10.1021/jf030118+
- Ishihara, S. (1975). Ishihara’s tests for colour-blindness. Tokyo: Kanehara Shuppan.
- Jackson, T. E., & Sandramouli, S. (2012). Auditory-olfactory synesthesia coexisting with auditory-visual synesthesia. Journal of Neuro-Ophthalmology, 32(3), 221–223. doi:10.1097/WNO.0b013e31825d3c44
- Mattingley, J. B., Payne, J. M., & Rich, A. N. (2006). Attentional load attenuates synaesthetic priming effects in grapheme-colour synaesthesia. Cortex, 42(2), 213–221. doi:10.1016/S0010-9452(08)70346-0
- Mattingley, J. B., Rich, A. N., Yelland, G., & Bradshaw, J. L. (2001). Unconscious priming eliminates automatic binding of colour and alphanumeric form in synaesthesia. Nature, 410(6828), 580–582. doi:10.1038/35069062
- Myles, K. M., Dixon, M. J., Smilek, D., & Merikle, P. M. (2003). Seeing double: The role of meaning in alphanumeric-colour synaesthesia. Brain and Cognition, 53(2), 342–345. doi:10.1016/S0278-2626(03)00139-8
- Nijboer, T. C., & Van der Stigchel, S. (2009). Is attention essential for inducing synesthetic colors? Evidence from oculomotor distractors. Journal of Vision, 9(6), 21–21. doi:10.1167/9.6.21
- Nikolić, D., Jürgens, U. M., Rothen, N., Meier, B., & Mroczko, A. (2011). Swimming-style synesthesia. Cortex, 47(7), 874–879. doi:10.1016/j.cortex.2011.02.008
- Olsson, M. J., & Friden, M. (2001). Evidence of odor priming: Edibility judgements are primed differently between the hemispheres. Chemical Senses, 26(2), 117–123. doi:10.1093/chemse/26.2.117
- Osgood, C. E. (1952). The nature and measurement of meaning. Psychological Bulletin, 49(3), 197–237. doi:10.1037/h0055737
- Raines, T. H. (1909). Report of a case of psychochromesthesia. The Journal of Abnormal Psychology, 4(4), 249–252. doi:10.1037/h0072856
- Ramachandran, V. S., & Hubbard, E. M. (2001). Synaesthesia: A window into perception, thought and language. Journal of Consciousness Studies, 8(12), 3–34.
- Revonsuo, A. (1999). Binding and the phenomenal unity of consciousness. Consciousness and Cognition, 8(2), 173–185. doi:10.1006/ccog.1999.0384
- Rich, A. N., & Mattingley, J. B. (2003). The effects of stimulus competition and voluntary attention on colour-graphemic synaesthesia. Neuroreport, 14(14), 1793–1798. doi:10.1097/00001756-200310060-00007
- Rich, A. N., & Mattingley, J. B. (2010). Out of sight, out of mind: The attentional blink can eliminate synaesthetic colours. Cognition, 114(3), 320–328. doi:10.1016/j.cognition.2009.10.003
- Rozin, P. (1982). “Taste-smell confusions” and the duality of the olfactory sense. Perception & Psychophysics, 31(4), 397–401. doi:10.3758/BF03202667
- Sagiv, N., Heer, J., & Robertson, L. (2006). Does binding of synesthetic color to the evoking grapheme require attention? Cortex, 42(2), 232–242. doi:10.1016/S0010-9452(08)70348-4
- Schifferstein, H. N., & Tanudjaja, I. (2004). Visualising fragrances through colours: The mediating role of emotions. Perception, 33(10), 1249–1266. doi:10.1068/p5132
- Seo, H. S., Arshamian, A., Schemmer, K., Scheer, I., Sander, T., Ritter, G., & Hummel, T. (2010). Cross-modal integration between odors and abstract symbols. Neuroscience Letters, 478(3), 175–178. doi:10.1016/j.neulet.2010.05.011
- Simner, J. (2012). Defining synaesthesia. British Journal of Psychology, 103(1), 1–15. doi:10.1348/000712610X528305
- Smilek, D., Dixon, M. J., Cudahy, C., & Merikle, P. M. (2001). Synaesthetic photisms influence visual perception. Journal of Cognitive Neuroscience, 13(7), 930–936. doi:10.1162/089892901753165845
- Stevenson, R. J. (2009). The psychology of flavour. London: Oxford University Press.
- Stevenson, R. J. (2014). Flavor binding: Its nature and cause. Psychological Bulletin, 140(2), 487–510. doi:10.1037/a0033473
- Stevenson, R. J., & Mahmut, M. K. (2013a). The accessibility of semantic knowledge for odours that can and cannot be named. The Quarterly Journal of Experimental Psychology, 66(7), 1414–1431. doi:10.1080/17470218.2012.753097
- Stevenson, R. J., & Mahmut, M. K. (2013b). Using response consistency to probe olfactory knowledge. Chemical Senses, 38(3), 237–249. doi:10.1093/chemse/bjs139
- Stevenson, R. J., Rich, A., & Russell, A. (2012). The nature and origin of cross-modal associations to odours. Perception, 41(5), 606–619. doi:10.1068/p7223
- Velasco, C., Woods, A. T., Deroy, O., & Spence, C. (2015). Hedonic mediation of the crossmodal correspondence between taste and shape. Food Quality and Preference, 41, 151–158. doi:10.1016/j.foodqual.2014.11.010
- Ward, J., & Simner, J. (2003). Lexical-gustatory synaesthesia: Linguistic and conceptual factors. Cognition, 89(3), 237–261. doi:10.1016/S0010-0277(03)00122-7
- Young, A. W., Hay, D. C., & Ellis, A. W. (1985). The faces that launched a thousand slips: Everyday difficulties and errors in recognizing people. British Journal of Psychology, 76(Pt 4), 495–523. doi:10.1111/j.2044-8295.1985.tb01972.x
