?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Agricultural and agro-industrial lignocellulosic residues represent an important renewable resource for the production of fuels and chemicals towards a bio-based economy. Olive pruning, vineyard pruning and almond shells are important residues from agricultural activities in Mediterranean-type ecosystems. In the current work, bioethanol production from the above three types of agro-residues was studied, focusing on the effect of different pretreatment methods on enzymatic saccharrification efficiency of cellulose and production of second-generation bioethanol. Dilute acid, hydrothermal and steam explosion pretreatments were compared in order to remove hemicellulose and facilitate the subsequent enzymatic hydrolysis of the hemicellulose-deficient biomass to glucose. Enzymatic hydrolysis was performed in a free-fall mixing reactor enabling high solids loading of 23% w/w. This allowed hydrolysis of up to 67% of available cellulose in almond shells and close to 50% in olive pruning samples, and facilitated high ethanol production in the subsequent fermentation step; the highest ethanol concentrations achieved were 47.8 g/L for almond shells after steam explosion and 42 g/L for hydrothermally pretreated olive pruning residue.
Introduction
Environmental concerns about greenhouse gas (GHG) emissions from fossil fuels have led to initiatives for their reduction such as the Kyoto Protocol, and the current universal climate change agreement (termed the Paris Agreement) aiming to curb emissions, so that the world temperature can stay below the agreed maximum 2°C rise. Within this context there has been increased interest in the utilisation of biomass as a more environmentally friendly, renewable and alternative source of liquid biofuels, which can also provide energy security and support the local economy. The use of biofuels has been encouraged in the EU through directives and policies such as the 2007 Energy Policy for Europe, and in the US by legislation such as the American Recovery and Reinvestment Act.
One of the most promising biofuels to date is second-generation bioethanol produced from lignocellulosic biomass. All types of plant and wood matter including agricultural, forestry, industrial and municipal wastes and by-products can be used as a source of lignocellulose for the production of bioethanol or other fuels. The agricultural residues with the highest potential for conversion to bioenergy are corn stover, wheat straw, rice straw and sugar cane bagasse [Citation1]. Besides these globally important crop residues there are other residues produced at smaller quantities that are important locally as their use can support the agricultural economy, supply part of the energy demand and enhance regional energy security [Citation2]. Such agricultural byproducts for the Southern Europe and Mediterranean region are olive tree and vineyard pruning residues as well as shells from various nuts. For example, with around 80% of global olive oil production shared between Spain, Italy and Greece [Citation2] the potential for bioethanol production from lignocellulosic wastes of this industry is significant at the local scale [Citation3]. Olive tree biomass, in particular, has received much attention as a source of both chemicals and bioenergy; it was studied as a feedstock for the production of fermentable sugars [Citation4,Citation5] and oligosaccharides [Citation6], antioxidants from both olive stones [Citation7] and olive tree pruning [Citation8], and bioethanol [Citation9,Citation10]. Vineyard pruning residue [Citation11,Citation12] and almond shells [Citation13,Citation14], on the other hand, have not been as thoroughly investigated despite the fact that both are produced in large quantities. These residues could be significant even at a global scale, considering the widespread cultivation of grapes and almonds in Mediterranean-type ecosystems [Citation15] in regions such as South Africa, South and North America, and Australia.
The amount of these lignocellulosic residues produced globally as well as their potential for bioethanol production can be estimated from data available in the literature. More specifically, worldwide olive tree cultivation is based mainly around the Mediterranean basin and amounts to about 8.5 million hectares. From their cultivation in the major olive producing countries (Spain, Italy, Greece and Turkey) around 4 Mt of lignocellulosic residue is produced, with a potential for bioethanol production of around 1206 ML, assuming 300 L ethanol production per ton of lignocellulosic biomass [Citation16]. In the case of vineyards, worldwide cultivation is around 8 million hectares [Citation17], which with an estimated production of 1.2 t per hectare [Citation18] represents a potential of 2800 ML of ethanol using the same assumption for bioethanol yield as above. The world production of almond kernels is estimated at around 1.11 Mt [Citation19]. Assuming a proportion of 50% hull, 25% shell and 25% nut [Citation20], an estimated 3.3 Mt of lignocellulosic residue is produced from almonds with a potential yield of 990 ML of ethanol. Although relatively minor compared to other agricultural byproducts such as wheat straw, these byproducts represent a significant biofuel potential for the established agricultural and agro-industrial economies in these respective fields.
The pretreatment of biomass in the production of second-generation bioethanol is crucial and aims at disrupting the recalcitrant structure of lignocellulose by removing hemicellulose and/or lignin in order to enhance enzymatic cellulose hydrolysis. A variety of pretreatment methods have been applied for ethanol production from lignocellulose in general such as milling [Citation21], dilute acid [Citation22], hydrothermal [Citation23], organosolv [Citation24], wet oxidation [Citation25], steam explosion [Citation26] and ammonia fiber explosion (AFEX) [Citation27]; and from olive pruning residue in particular including hydrothermal [Citation28], dilute acid [Citation29], steam explosion [Citation30], FeCl3 and organosolv [Citation31]. Among the most promising is dilute acid pretreatment as it combines high reaction rates, low cost and low catalyst consumption with a high efficiency in hemicellulose removal and an increase in the enzymatic digestibility of biomass [Citation22]. Hydrothermal pretreatment in neat water is a very attractive alternative to the dilute acid method that reduces cost and environmental hazard while allowing hemicellulose removal with a low formation of inhibitors [Citation23,Citation32]. Steam explosion, where biomass undergoes a rapid decompression at the end of the pretreatment time, combines hemicellulose removal with a reduction of particle size which further enhances enzymatic digestibility [Citation33]. Both hydrothermal and steam pretreatments have been successfully implemented in pilot-scale continuous systems and developed in semi-industrial-scale plants; these significant milestones outline their potential to reach full industrial scale [Citation34,Citation35].
The two most common strategies for bioethanol production at high dry matter content (DM), which reduces water consumption and increases the concentration of sugars/ethanol in the liquid products, are simultaneous saccharification and fermenatation (SSF) and separate hydrolysis and fermentation (SHF) [Citation36]. Both methods are usually applied on pretreated biomass and have shown high efficiency and productivity, but the feasibility of each depends mainly on the enzymatic system that is used [Citation37]. Another interesting approach, which is also used in the current work, is partial enzymatic pre-hydrolysis at higher temperatures, denoted liquefaction, where the viscosity of the substrate (i.e. biomass–water slurry) is decreased. The liquefaction stage can significantly improve the mixing properties of the substrate slurry. This partial hydrolysis can be carried out even with limited enzyme pre-load to avoid the thermal inactivation of the enzymes. However, the above-described thermochemical pretreatment steps always proceed to this enzymatic pre-hydrolysis in order to maximise its efficiency. After this liquefying partial hydrolysis, the saccharification stage using a new enzyme load simultaneously with the fermentation (SSF) can be carried out in a process denoted as PSSF.
The purpose of the current work was to investigate the potential for bioethanol production from three agricultural residues of importance for the Southern Europe/Mediterranean area. These were olive tree and vineyard prunings, which are residues usually left in the field or burned in situ to enrich the soil in minerals and prevent the spread of vegetal diseases, and almond shells which are processing residues from the food industry that are burned for the generation of heat. The three biomass types were pretreated with three different methods, i.e. dilute acid, hydrothermal and steam explosion, at selected process conditions, followed by enzymatic hydrolysis of the pretreated biomass to glucose and fermentation to ethanol. The effects of the different pretreatment methods on the enzymatic hydrolysis efficiency and ethanol production from each biomass type, as well as on the selective recovery of hemicellulose carbohydrates, were identified and are discussed below.
Materials and methods
Feedstock
Three types of lignocellulosic agro-industrial byproducts from the Northern Greece region of Chalkidiki were used in the current work. These were olive tree prunings, comprising branches with diameters 2–5 cm free of leaves and small twigs; grapevine prunings, and almond shells free of the soft outer hull. Both pruning samples contained the bark. The samples were initially air dried in the laboratory and then milled in a Retch SM 300 knife mill with a 1-mm screen. The <1 mm fraction was collected and used for the pretreatment experiments.
Pretreatment
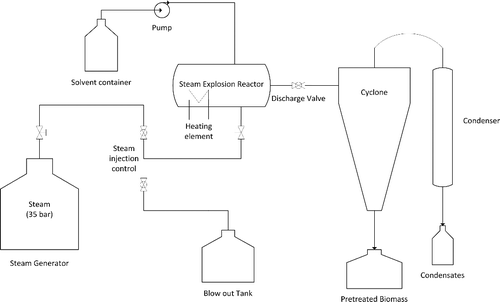
The three biomass types were subjected to three aqueous-based pretreatment methods for comparison. These were hydrothermal (HT), dilute acid (DA), and steam explosion (SE). HT and DA pretreatments were performed in an air-heated multidigester system (AATO) with six 2.5-L batch autoclave reactors. SE pretreatment experiments were performed in a custom-made steam explosion unit with horizontal configuration equipped with a 12-L reaction vessel, a blow tank constructed of stainless steel and a boiler capable of producing saturated steam up to 35 bar (). All operations of steam control valves, reactor temperature and residence time were controlled by a panel-based HMI/SCADA (Human Machine Interface/Supervisory Control and Data Acquisition) system with Programmable Logic Controller (PLCs). Steam introduction into the reactor was controlled by an electric ball valve as well as a mechanical screw-type valve. Purging of condensates into the blowout tank was controlled by an electric ball valve. Pretreatment temperature in the reactor is maintained with the use of an electric heating element. At the end of the desired pretreatment time discharge of the steam and pretreated biomass into the cyclone is done via a manually operated ball valve. The pretreated slurry is collected in a vessel at the bottom of the cyclone and condensates are collected in a vessel at the bottom of the condenser. The pretreatment conditions can be seen in .
Table 1. Conditions of hydrothermal, dilute acid and steam explosion pretreatment experiments.
For the HT and DA pretreatments, 150 g biomass was mixed with 1.5 L of water and 1% w/w H2SO4 aqueous solution and pretreated at the designated temperature (200 and 170°C, respectively) and time (7 and 13 min, respectively). For the SE experiments 300 g of biomass was soaked with 600 g of 1% w/w H2SO4 aqueous solution for 30 min and loaded into the steam explosion reactor. The mixture was heated with steam at 195°C for 10 min, and at the end of the pretreatment time it was exploded through a decompression valve to an air cyclone and collected into a vessel at the bottom. After each pretreatment, the pretreated biomass was separated from the liquid fraction by vacuum filtration and washed with deionised water until neutral pH of the filtrate was obtained; the biomass was then air dried at room temperature and used for further analysis and experiments.
The combined severity factor (CSF) was used and was determined by the following equation (1):(1)
(1) where t = time of pretreatment in min, and T = temperature of pretreatment in °C.
Enzymatic hydrolysis and fermentation
After pretreatment the biomass samples were submitted to PSSF experiments. Initially, enzymatic prehydrolysis was performed in freefall mixing reactors [Citation38] at 50°C for 8 or 24 h; the solids loading of the biomass was 23% w/w in 50 mM citrate-phosphate buffer at pH 5.0. The cellulolytic enzyme cocktail Cellic CTec2® from Novozymes (Bagsærd, Denmark) was used at an enzyme loading of 15 FPU/g biomass. Detailed experimental conditions for the enzymatic prehydrolysis experiments are given in . The biomass slurries obtained from the enzymatic prehydrolysis experiments were then subjected to SSF experiments with the use of the high-ethanol-tolerant industrial Saccharomyces cerevisiae strain Ethanol Red®. The experiments were carried out with and without addition of fresh enzyme (Cellic CTec2®) load of 10 FPU/g biomass at the beginning of the fermentation. Samples of slurries were withdrawn at 24, 48, 72 and 96 h, diluted 1:5 with deionised H2O, incubated for 30 min at room temperature and centrifuged at 8000 rpm for 5 min, and the supernatant was collected and stored at −18°C for analysis.
Table 2. Experimental conditions for enzymatic hydrolysis of hydrothermal-, dilute acid- and steam explosion-pretreated almond shells and olive and vine pruning biomass samples.
Chemical analysis
The chemical composition of the pretreated biomass samples was determined according to the procedure of National Renewable Energy Laboratory (NREL) [Citation39]. In brief, the solid samples were digested with 72% H2SO4 at 30°C for 1 h, followed by dilution with H2O to a concentration of 4% H2SO4, and hydrolysis at 121°C for 1 h. The hydrolysates were filtered and the acid-insoluble lignin (Klason lignin) was determined gravimetrically from the weight of the solid residue. The filtrates were neutralised with CaCO3, passed through 0.2-μm syringe filters and analysed for sugars by High Performance Liquid Chromatography (HPLC) equipped with an Refractive Index (RI) detector and an Aminex HPX-87P column (column characteristics as given below), at 85°C with H2O as mobile phase and a 0.6 mL/min flow rate. The samples from pretreatment liquids, enzymatic hydrolysis and fermentation broths were analysed for glucose, xylose and ethanol with HPLC equipped with an RI detector and an Aminex HPX-87H column (300 mm × 7.8 mm, particle size 9 μm; Bio-Rad, Hercules, CA, USA) at 65°C, with a mobile phase of 5 mM H2SO4 and a flow rate of 0.6 mL/min.
The recovery of carbohydrates (cellulose or hemicellulose) in the pretreated solids and liquids was calculated according to the following equation (2):(2)
(2) where W is the weight.
The recovery of carbohydrates (cellulose or hemicellulose) in the pretreatment liquid was calculated according to the following equation (3):(3)
(3) where carbohydrates in the pretreatment liquid are the total sugars (monomers and oligomers) determined after hydrolysis with 4% H2SO4 at 121°C for 1 h and analysis by HPLC as above.
The sugars were converted to cellulose and hemicellulose carbohydrates by multiplying the concentration with an anhydro correction factor of 0.9 for C6 sugars (glucose, galactose, mannose) and 0.88 for C5 sugars (xylose, arabinose).
Characterisation
X-ray diffraction (XRD) patterns of the biomass samples were obtained using a PANalytical Empyrean diffractometer equipped with a Cu LFF HR X-ray tube and a PIXcel3D detector. The crystallinity index (CRI) of the biomass samples was calculated according to the following equation (4):(4)
(4) where I002 is the intensity of the (002) peak at 2θ = 22.58° and Iam is the intensity of the background at 2θ = 18.3°.
The Fourier Transform Infrared (FTIR) spectra of both the parent biomass and the pretreated biomass solids were recorded on a Bruker VERTEX 80v vacuum FTIR spectrometer at the wavelength region of 400–4000 cm−1.
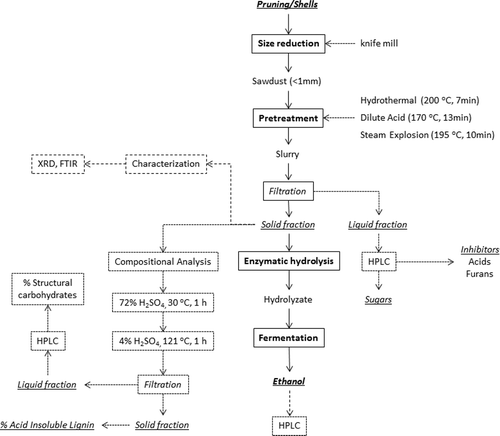
The detailed experimental and analytical protocol that was used in the current work can be seen in .
Results and discussion
Pretreatment
Pretreatment of the three biomass sample was performed as described above (). The conditions were selected based on previous experience with olive pruning lignocellulosic biomass from data available in the literature [Citation10,Citation30]. All three pretreatment methods resulted in the solubilisation of a significant fraction of the biomass, as determined by gravimetric analysis (). Solubilisation was the lowest for HT-pretreated samples (200°C, 7 min) in all cases, reaching 39.3% for almond shells, 41.3% for olive prunings and 34.4% for vineyard prunings. The pH of the HT pretreatment liquids was relatively high, in the range 3.2–3.8, due to the absence of a strong acid catalyst. The mild acidic pH is caused by the release of acetyl units from the hemicellulose fraction of the biomass that was hydrolysed at the employed pretreatment conditions. HT pretreatment, therefore, was the mildest of the three methods with a time and temperature combination resulting in the lowest CSF ().
Table 3. Effect of hydrothermal (HT), dilute acid (DA) and steam explosion (SE) pretreatments on solubilisation, the pH of the process liquids, and the crystallinity index (CRI) of almond shells and olive and vine pruning.
Solubilisation was increased in the DA pretreatment (170°C, 13 min, 1.0% sulphuric acid) to 50.2, 48.5 and 52.4% for almond shells and olive and vineyard prunings, respectively. This increase is caused by the addition of the acidic catalyst in the pretreatment medium leading to an increased CSF, despite the decrease of process temperature (i.e. from 200°C in HT pretreatment to 170°C in DA pretreatment). The use of sulphuric acid leads to a dramatic drop in the pH of the pretreatment liquid (pH values 1.1–1.4). SE leads to even higher solubilisation yields, at least for almond shells and olive prunings (i.e. 53.5, 50.4 and 49.4% solubilisation for almond shells and olive and vineyard prunings, respectively), despite the milder acidic conditions, compared to those in DA pretreatment, due to the lower acid catalyst loading in SE. The corresponding CSF values for the SE pretreatment are between those of HT and DA pretreatments. It would therefore be expected that the percentage of biomass solubilisation in SE would be between those obtained from FT and DA pretreatments. Steam explosion, however, leads to reduction of biomass particle size at the final stage of the treatment which in turn leads to an increased external surface area of the biomass [Citation40]. Since the hydrolytic removal of (mainly) hemicellulose is influenced by the available/exposed surface of the biomass particles, the enhanced solubilisation at the milder acidic conditions of SE pretreatment, compared to DA, can be thus explained.
The effects of pretreatment on the composition of the three biomass feedstocks can be seen in . The composition of the original untreated biomass samples is also provided for comparison. The cellulose content is 22% for almond shells, 26% for olive prunings and 27% for grapevine prunings. Compared to other wood biomass types, i.e. beech, poplar prunings, etc., where cellulose content can be as high as 40%, the values measured for these biomass types are relatively low [Citation23,Citation41]. Hemicellulose content is 20% for almond shells and lower (i.e. 16 and 12%, respectively) for olive and grapevine prunings. Lignin content, on the other hand, is relatively high, i.e. 23–27%, depending on the biomass source. It is also worth noting the relatively high amount of water extractives measured, reaching almost 18% of the total biomass in the case of olive prunings. This high amount of extractives is due to the presence of bark in the olive and grapevine pruning samples. Hardwood bark can contain high levels of water (and organic solvents) extractives as well as lignin, which can explain the relatively high amounts of extractives and lignin and the relatively low amounts of cellulose and hemicellulose in the samples of the current study [Citation42,Citation43]. The small amounts of inorganic ash present in the untreated samples were effectively removed during DA pretreatment due to the low pH of the medium, which enhances their solubilisation. Ash leaching to a lesser extent is observed during HT pretreatment, while in the SE experiments ash is quantitatively almost retained in the biomass ().
Table 4. Composition of untreated and pretreated almond shells, olive pruning solids and vineyard pruning solids, and pretreatment liquids.
It is apparent from that all pretreatment methods can efficiently remove hemicellulose from the different biomass feedstocks at the employed conditions. Almost complete hemicellulose removal was achieved during DA pretreatment due to the lower pH of the reaction medium leading to increased hemicellulose hydrolysis, with minimal 0.1–0.4% hemicellulose remaining in the pretreated samples. In the HT-pretreated samples a slightly higher hemicellulose concentration between 1.0 and 1.7% was observed, while in the SE samples hemicellulose content ranged between 1.4 and 3.6%; these values are a bit higher compared to those in the HT-pretreated samples despite the slightly lower pH of the pretreatment liquid in the SE samples. With regard to cellulose and lignin content of the olive and vineyard pruning pretreated samples, this was around 36–39% and 44–52%, respectively. The pretreated almond shells contained more lignin (47–61%) and less cellulose (26–36%) compared to the other two types of feedstocks. Furthermore, it can be observed that for olive pruning and grapevine pruning the cellulose and lignin contents are comparable across all pretreatment methods. On the other hand, when almond shells were pretreated with dilute acid, significant cellulose hydrolysis led to decreased cellulose content in the solids and, as a consequence, increased lignin content compared to samples from the other pretreatment methods. This is attributed to the enhanced cellulose hydrolysis in this case, in addition to the hydrolysis of hemicellulose, as discussed below.
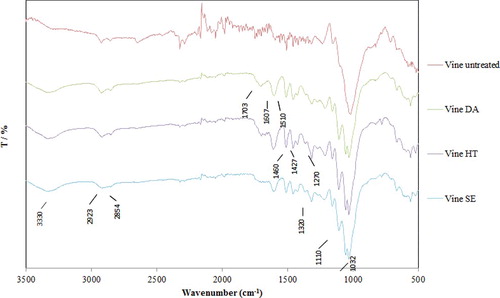
FTIR spectra of untreated vineyard prunings, and of those pretreated with HT, DA and SE, are shown in . The absorption band at 3388 cm−1 is assigned to the stretching vibration of the hydroxyl groups that are present in all three major biomass components, cellulose, hemicellulose and lignin, while the band at 2923 cm−1 is due to the stretching vibration of C-H bonds [Citation44], and that at 2854 cm−1 to CH2 vibrations [Citation45]. The band at 1703 cm−1 is assigned to stretching of the carbonyl group, while absorption bands at 1607 and 1510 cm−1 are due to vibrations of the aromatic ring. These bands, associated with lignin, appear only in the spectra of the pretreated samples and verify the increase in lignin concentration compared to the untreated sample, in accordance with the results of the chemical analysis (). Absorption bands at 1460 and 1427 cm−1 are also attributed to the lignin component of biomass, and in particular to the methoxy-deformation of the aromatic ring. Bands at 1320 and 1270 cm−1 are assigned to syringyl and guaiacyl lignin units, respectively. The band at 1110 cm−1 is assigned to C-O-C stretching of glycosidic bonds, while that at 1032 cm−1 corresponds to the C-O-C ring of hemicelluloses [Citation44]. A reduction in the intensity of the latter and an increase in the intensity of the former, in all pretreated samples compared to the untreated ones, is a clear indication of removal of hemicellulose and increase of cellulose content, respectively. This is, again, in good agreement with the results of the chemical analysis ().
Figure 3. FTIR spectra of vineyard pruning biomass, untreated and pretreated with hydrothermal (HT), dilute acid (DA) and steam explosion (SE).
The XRD patterns of untreated and pretreated solids can be seen in Figure S1 in the online supporting information. Three major peaks were present in all untreated and pretreated samples at approximate 2θ values of 15.5, 22 and 34.6o. Since hemicellulose and lignin are amorphous, the observed peaks are attributed to the cellulose content of the samples. These three peaks correlate to the crystallographic planes (1 1 0) and (1 0), (0 0 2), and (0 2 3) or (0 0 4) of the cellulose crystal [Citation46]. The same peaks are observed in the XRD pattern of the microcrystalline cellulose (Avicel) which, however, exhibit higher intensities due to the lack of the amorphous components present in biomass. An increase in the intensity of the peaks of the pretreated solids compared to that of the untreated biomass can be seen for all biomass types; this can be attributed to the removal of amorphous biomass components, mainly of hemicellulose and extractives, during the pretreatment, leading to an increase of the cellulose content of the pretreated solids. This, again, is in agreement with the chemical analysis of the solids (). From the XRD patterns of the materials the CRI was calculated. In all pretreatment methods the CRI values were increased compared to those of the untreated material (56.7% for almond shells, 59.2% for olive pruning and 52.8% for vine pruning) as an effect of the removal of amorphous materials. The CRI values for the HT-treated samples were in the range of 70.6–77.9%, for SE samples they were 70.6–76.8% and for the DA-treated samples they were 66.4–71.7%.
The contents of cellulose and hemicellulose sugars of the pretreatment liquids (expressed as wt. % of the initial biomass) can also be seen in . The DA pretreatment, being the most severe of the three methods, led to the dissolution of some cellulose with around 5.5 wt. % of this recovered as sugars in the pretreatment liquids. Small amounts of cellulosic sugars between 0.2 and 2.8% depending on the biomass source were also recovered in the SE pretreatment liquids. It appears, therefore, that the presence of sulphuric acid mainly in the DA method at the selected conditions allows for the partial hydrolysis of cellulose. Cellulose hydrolysis does not seem to take place in the HT pretreatment experiments, and the cellulosic sugars recovered in the pretreatment liquid are below 1% for all biomass types.
The content of hemicellulose sugars in the pretreatment liquids is higher compared to that of cellulosic sugars, and is affected by both the type of biomass and the pretreatment method used (). The highest amounts of hemicellulose sugars are again observed in the liquid from the DA pretreatment, with the lowest in the HT pretreatment. The content of soluble sugars in the pretreatment liquids is highly affected by their reactivity and degradation towards the respective furans and acids (i.e. furfural, formic acid etc.), as previously shown [Citation23,Citation41]. Thus, it appears that in the case of HT pretreatment the higher temperature (i.e. 200°C) and accordingly higher heat-up and cooling time, compared to the DA method (i.e. 170°C) are crucial parameters that favour such degradation reactions, leading eventually to higher content of sugars in the liquids of DA pretreatment. In the case of SE, despite the relatively high temperature (i.e. 195°C), the fast cooling and separation of the liquid stream leads also to enhanced sugar contents compared to the HT pretreatment.
The recovery of cellulose and hemicellulose in the pretreatment solids and liquids is very important for the downstream valorisation of these fractions. The recovery of both carbohydrates expressed as percent weight of the initial cellulose and hemicellulose content of the parent biomass samples can be seen in . Cellulose recovery is highest in the HT-pretreated samples and in the region of 90% or more with the majority remaining in the pretreatment solid, since at the employed temperature cellulose hydrolysis in neat water is minimal [Citation23]. In the DA experiments, the total cellulose recovery is in the range 70–85%, indicating a considerable loss of glucose due to degradation from the severe pretreatment conditions. In addition, a significant part of that cellulose, around 20%, is dissolved in the pretreatment liquid and recovered as glucose, limiting the availability of cellulose for enzymatic hydrolysis in the pretreated solid. In SE the total recovery ranges between 75 and 80% and is found in the solids in its majority. Again, here, it seems that the presence of the acid catalyst leads to some degradation of glucose; the majority of cellulose, however, is retained in the solid.
Table 5. Recovery of carbohydrates (cellulose and hemicellulose) in the pretreatment solid and liquid products.
In contrast to cellulose, the recovery of hemicellulose is lowest in the HT experiments (). This again can be attributed on one hand to the limited solubilisation of cellulose with this pretreatment method, as was also previously shown [Citation18,Citation35], and on the other hand to the enhanced degradation of solubilised hemicellulose sugars, as discussed above. The highest hemicellulose recovery in the HT treatment experiments is 38% (29% in the liquids, the rest in the pretreated solid) and is obtained with the grapevine prunings. Higher recoveries are achieved in the pretreatments with DA and SE (21–59%, depending on biomass type). In both methods, the majority of the recovered hemicelluloses are found in the pretreatment liquids. Increased hemicellulose yields have already been observed in the case of other agricultural lignocellulosic materials, such as wheat straw [Citation47].
Enzymatic pre-hydrolysis and simultaneous saccharification and fermentation (PSSF)
The pretreated solids were subjected to saccharification (prehydrolysis) with 15 FPU/g of biomass, of Celic Ctec2 enzyme cocktail at 50°C for 8 h in a free-fall mixing reactor with internal horizontally rotating shafts and an external oil heating jacket. The reactor configuration allows for the handling and enzymatic liquefaction of high concentrations of solids [Citation48] such as the 23% that was used in the current work. The high solid loading is beneficial in achieving high glucose concentrations and consequently high ethanol yields. A high final ethanol concentration also reduces energy consumption during distillation of the fermentation broth. The cellulose conversion in the various prehydrolysate slurries can be seen in . In all experiments glucose was the main product, while cellobiose was also produced with a concentration of up to 30% of total sugars produced, depending on biomass type and pretreatment method. The high glucose concentrations caused by the high solid loading led to b-glucosidase inhibition and cellobiose accumulation, explaining the cellobiose concentrations observed [Citation49]. Small amounts of xylose and mannose due to the residual hemicellulose in the pretreated biomass are also present in all of the prehydrolysis slurries. In almond shells the highest cellulose conversion of 24% (or 35% also taking into account the cellobiose) is achieved by the SE-treated sample, which, however, is relatively low. The olive pruning hydrolysates seem to have benefited more by the various pretreatment methods under the selected conditions, leading to higher cellulose conversions of 33% (39% including cellobiose) for HT treatment, 24% (30% including cellobiose) for DA treatment and 37% (42% including cellobiose) for SE treatment. Contrarily, very poor conversions are observed for vine prunings irrespective of the pretreatment method that was employed. The highest conversion observed for vine was 15% (21% including cellobiose) for the HT-pretreated sample. Among all types of biomass used, vine pruning was the only one where no liquefaction was observed during prehydrolysis. The density of the biomass was so low that all of the liquid phase (consisting of buffer plus enzyme) was absorbed and no liquefaction could be observed even after the end of the 8-h hydrolysis reaction. Even the SE pretreatment, which is well known for its ability to reduce the biomass particle size and increase enzymatic hydrolysis [Citation45], did not enhance liquefaction and yielded only 9% cellulose conversion during 8 h of prehydrolysis.
Figure 4. Enzymatic cellulose hydrolysis of prehydrolysates from hydrothermal (HT), dilute acid (DA), and steam explosion (SE) pretreated biomass samples of (a) almond shells, (b) olive pruning and (c) vineyard pruning, for 8 and 24 h of prehydrolysis.
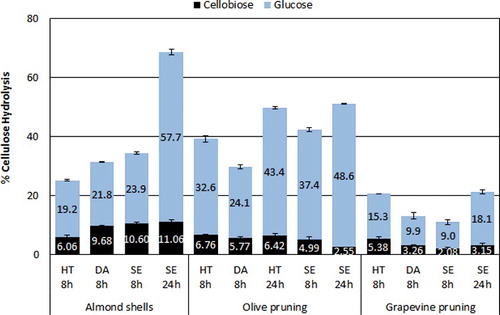
It seems that – with the exception of vineyard prunings where very low glucose concentrations were observed for all pretreatment methods – SE was the most beneficial pretreatment method in terms of facilitating the enzymatic prehydrolysis of the pretreated solids. Despite the fact that it led to a higher percentage of residual hemicellulose than the other preteratment methods (), it also produced solids with relatively high cellulose and low lignin contents, which, together with its known effect on particle size [Citation40], may explain the increased glucose yields.
All prehydrolysates were subsequently subjected to fermentation at 30°C with the ethanol red S. cereviviae strain, and the ethanol concentration was monitored every 24 h for a period of up to 5 days. The ethanol concentrations achieved can be seen in . In the case of almond shell biomass, ethanol concentrations are higher for the SE samples followed by the HT- and the the DA-treated samples. The DA samples yielded around 10 g/L ethanol, which is increased to 15 g/L with the HT samples and 27 g/L with the SE biomass. Ethanol concentrations can be further increased by the addition of 10 FPU/g biomass of Celic Ctec2 at the start of the fermentation, in all types of pretreated biomass; in this way the ethanol concentration was increased to 11, 19 and 36 g/L, respectively. This is a clear indication that more cellulose or cello-oligosacharrides are available for saccharification in the solids, and the addition of cellulases allows it to hydrolyse to a greater extent. In fact, during the fermentation stage cellulose hydrolysis rather than glucose fermentation is the rate-limiting step, and therefore enhancing hydrolysis is critical to achieve a good ethanol yield [Citation50].
Figure 5. Ethanol concentration achieved by 8 and 24 h prehydrolysis and fermentation of almond shells, olive pruning and vine pruning. Dark (black) columns represent experiments without addition of extra enzyme, and light (blue) columns experiments where 10 FPU/g was added to the fermentation broth.
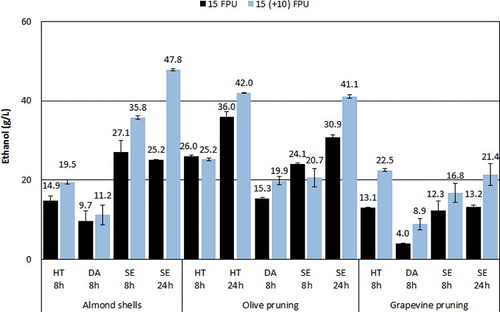
Similar trends in ethanol yields with respect to the pretreatment method are observed for olive pruning biomass after 8 h prehydrolysis. The DA-treated samples yielded the lowest ethanol at around 15 g/L. The HT- and SE-pretreated biomass, however, produced higher and very similar ethanol concentrations at 26 and 24 g/L, respectively. Addition of Celic Ctec2 during fermentation did not have a positive effect on ethanol concentrations, which remained more or less the same. The lowest ethanol production was observed with vine pruning biomass with values for DA, HT and SE biomass at around 4, 13 and 12 g/L, respectively. Addition of cellulases was beneficial and improved ethanol to give a final concentration of 9, 23 and 17 g/L, respectively; however, the concentration of ethanol remained low compared to the other two types of biomass.
Based on the above experimental data, the most promising substrates for ethanol production were the SE almond shells and the HT- and SE-treated olive prunings. In an effort to further improve ethanol production the prehydrolysis time was increased from 8 to 24 h, for these samples. Despite its low ethanol yield, SE vine pruning was also included in this set of experiments for comparison. The results of increased prehydrolysis time on glucose concentration of the prehydrolysates are presented in . A significant increase can be observed for the SE almond shells, with glucose conversion more than doubling from 24 to 58% (or 69% if cellobiose is also included). A smaller but still significant increase can be observed for the HT- and SE-pretreated olive pruning biomass, from 33 to 43% and from 37 to 49%, respectively. In a previously reported work with olive pruning samples pretreated hydrothermally and hydrolysed at 23% solids for 24 h, a concentration of 66 g/L glucose was achieved compared to the 43 g/L achieved in the current work under the same conditions [Citation50]. An improvement was also observed for the SE vineyard pruning biomass by increasing the prehydrolysis time, with glucose concentration doubling from 9 to 18%, but this remained low compared to the other biomass samples. It is apparent that the pretreatment conditions employed were not sufficient to reduce the recalcitrance of vine prunings in contrast to the almond shells and olive prunings, where goods results were obtained.
The effect of increasing prehydrolysis time from 8 to 24 h on the production of ethanol can be seen in . In the case of SE almond shells, increase of prehydrolysis time does not seem to affect the production of ethanol which remains at similar levels, around 25 g/L. With the addition of 10 FPU/g cellulases in the fermentation medium, a significant increase in ethanol concentration from 36 to 48 g/L is achieved. For SE olive prunings an increase in prehydrolysis time leads to increased ethanol production, from 24 to 31 g/L; with the additional cellulase the ethanol concentration is further improved (41 g/L). In the literature olive biomass pretreated by SE at similar conditions, but with phosphoric instead of sulphuric acid as a catalyst, yielded around 30 g/L ethanol in an SSF experiment at 15% solids [Citation30]. The ethanol concentration of the HT-treated olive prunings is improved similarly to the SE sample with increase of prehydrolysis time and additional cellulase, to give a final value of 42 g/L. The ethanol concentration achieved in the literature under the same conditions was 31.1 g/L [Citation10]. A similar improvement is also shown for the SE vineyard prunings; however, the maximum ethanol concentration of 21 g/L remains rather low. The improvement observed in the 24-h prehydrolysis experiments with the increase of enzyme loading during fermentation could be explained by the deactivation of part of the initial enzyme due to non-productive adsorption on the lignin. This deactivation as well as inhibition of enzymes from low-molecular-weight phenols is a well-known effect with a negative impact on hydrolysis yields and on the operational cost of the process [Citation51,Citation52]. This seems to be particularly true for the almond shell biomass which exhibits a dramatic improvement, with hydrolysis yield reaching more than 60% of the available cellulose, when additional enzyme is used.
The ethanol concentrations reported above are quite promising as in many cases the threshold of 4% w/w that is critical for a commercial-scale bioethanol plant is achieved. The yields of ethanol expressed as grams of ethanol per gram of available glucose (Yp/s), as well as in percentage of the maximum theoretical yield, are provided in . The 48 g/L of ethanol achieved from the fermentation of the SE almond shell biomass corresponds to a Yp/s yield of 0.415 that is around 81% of the maximum theoretical (assuming a maximum theoretical yield of 0.511 g ethanol per g of cellulose, expressed as glucose), after 96 h of fermentation time. Assuming part of the glucose is consumed for production of yeast biomass, 81% of theoretical yield is close to the maximum that can be achieved in the selected conditions. In the case of olive prunings the 41 and 42 g/L ethanol concentrations represent Yp/s yields of 0.378 and 0.329 for the SE and HT biomass, respectively; these represent 74 and 64% of theoretical yield for the respective biomass samples, indicating that some small improvement in ethanol yield may be obtainable. These results were achieved at 120 h of fermentation. The 21 g/L of ethanol obtained from the fermentation of the SE grapevine represents a yield of 0.186 g ethanol per gram of cellulose (36% of theoretical); this result is relatively low compared to those from the olive and vine pruning samples.
Table 6. Ethanol yields from 8 and 24 h prehydrolysis and fermentation of almond shell, olive pruning and vine pruning pretreated solids.
Conclusions
The effect of pretreament of various agro-industrial/agricultural residues that are cultivated around the world in Mediterranean-type ecosystems towards improved bioethanol production was investigated in the current work. More specifically, olive and vineyard prunings and almond shells were pretreated by hydrothermal, dilute acid and steam explosion methods. Hydrothermal pretreatment provided high cellulose recovery in the pretreated solids, but led to enhanced enzymatic hydrolysis only in the case of olive prunings. Dilute acid pretreatment showed moderate to high cellulose recovery but also induced relatively high solubilisation of cellulose in the pretreatment liquid; the enzymatic hydrolysis efficiency of the dilute-acid-pretreated biomass samples was low. Steam explosion showed also moderate/high cellulose recovery with most of the cellulose being present in the treated solid, favouring also the enzymatic hydrolysis of cellulose. Overall, when considering the recovery of cellulose in the pretreated solids and the enzymatic hydrolysis activity, steam explosion and hydrothermal pretreatment are of similar efficiency, with the former being slightly better with almond shells and the latter better with olive pruning biomass. Vineyard prunings, on the other hand, performed rather poorly, mainly in terms of enzymatic hydrolysis efficiency, under the employed pretreatment conditions.
The free-fall mixing reactor used for the enzymatic prehydrolysis, which is able to handle a high solids loading of 23%, allowed high glucose concentrations and high cellulose conversion (corresponding to up to 67% hydrolysis of available cellulose), and subsequently high ethanol concentrations. The addition of fresh enzyme load during fermentation was beneficial to cellulose hydrolysis and subsequently to the ethanol yield.
The maximum ethanol concentrations achieved were 41 g/L for steam-exploded olive prunings, 42 g/L for hydrothermally-pretreated olive prunings and 47.8 g/L for steam-exploded almond shells, corresponding to 4.1, 4.2 and 4.8% of ethanol content in the fermentation broth. These values correspond to yields between 65 and 81% of the theoretical ethanol yield for the respective samples. The vineyard prunings gave a maximum ethanol concentration of 21 g/L, corresponding to only 36% of theoretical yield, indicating the more recalcitrant nature of the feedstock; it also suggests that careful optimisation of the pretreatment conditions can greatly improve the ethanol production efficiency of this feedstock. These results were similar or improved compared to data available in the literature. Overall, relatively high ethanol concentrations, above 4% (w/w) which is considered a minimum prerequisite for a feasible large-scale distillation technology, were achieved for two of the three biomass types investigated, namely olive prunings and almond shells.
Supplementary_Data.docx
Download MS Word (952.3 KB)Acknowledgements
This work was supported by an STSM Grant from COST Action FP1306.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bentsen NS, Felby C, Thorsen BJ. Agricultural residue production and potentials for energy and materials services. Prog Energy Combust Sci. 2014;40(1):59–73.
- Hadar Y. Sources for lignocellulosic raw materials for the production of ethanol. In: Faraco V. (eds) Lignocellulose conversion. Springer, Berlin, Heidelberg 2013. pp. 21–38.
- Faraco V, Hadar Y. The potential of lignocellulosic ethanol production in the Mediterranean Basin. Renew Sust Energy Rev. 2011;15(1):252–266.
- Díaz MJ, Huijgen WJJ, Van Der Laan RR, et al. Organosolv pretreatment of olive tree biomass for fermentable sugars. Holzforschung. 2011;65(2):177–183.
- Ballesteros I, Ballesteros M, Cara C, et al. Effect of water extraction on sugars recovery from steam exploded olive tree pruning. Bioresource Technol. 2011;102(11):6611–6616.
- Cara C, Ruiz E, Carvalheiro F, et al. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind Crop Prod. 2012;40:225–231.
- Lama-Muñoz A, Romero-García JM, Cara C, et al. Low energy-demanding recovery of antioxidants and sugars from olive stones as preliminary steps in the biorefinery context. Ind Crop Prod. 2014;60:30–38.
- Conde E, Cara C, Moure A, et al. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2009;114(3):806–812.
- Cara C, Ruiz E, Ballesteros M, et al. Production of fuel ethanol from steam-explosion pretreated olive tree pruning. Fuel. 2008;87(6):692–700.
- Manzanares P, Negro MJ, Oliva JM, et al. Different process configurations for bioethanol production from pretreated olive pruning biomass. J Chem Technol Biotechnol. 2011;86(6):881–887.
- Cotana F, Barbanera M, Foschini D, et al. Preliminary optimization of Alkaline pretreatment for ethanol production from vineyard pruning. Energy Procedia. 2015;82:389–394.
- Buratti C, Barbanera M, Lascaro E. Ethanol production from vineyard pruning residues with steam explosion pretreatment. Environ Prog Sustainable Energy. 2015;34(3):802–809.
- Martinez JM, Granado JM, Montane D, et al. Fractionation of residual lignocellulosics by dilute-acid prehydrolysis and alkaline extraction: Application to almond shells. Bioresource Technol. 1995;52(1):59–67.
- Martínez JM, Reguant J, Montero MÁ, et al. Hydrolytic pretreatment of softwood and almond shells. Degree of polymerization and enzymatic digestibility of the cellulose fraction. Ind Eng Chem Res. 1997;36(3):688–696.
- Nieto-Romero M, Oteros-Rozas E, González JA, et al. Exploring the knowledge landscape of ecosystem services assessments in Mediterranean agroecosystems: Insights for future research. Environ Sci Policy.2014;37:121–133.
- Faraco V, Hadar Y. The potential of lignocellulosic ethanol production in the Mediterranean Basin. Renew Sustainable Energy Rev. 2011;15(1):252–266.
- Spinelli R, Nati C, Pari L, et al. Production and quality of biomass fuels from mechanized collection and processing of vineyard pruning residues. Appl Energy. 2012;89(1):374–379.
- Lapuerta M, Hernández JJ, Pazo A, et al. Gasification and co-gasification of biomass wastes: Effect of the biomass origin and the gasifier operating conditions. Fuel Process Technol. 2008;89(9):828–837.
- International Nut and Dried Fruit Council Foundation (INC). Nuts & dried fruits global statistical review 2015/2016.
- Fadel JG. Quantitative analyses of selected plant by-product feedstuffs, a global perspective. Anim Feed Sci Technol. 1999;79(4):255–268.
- Licari A, Monlau F, Solhy A, et al. Comparison of various milling modes combined to the enzymatic hydrolysis of lignocellulosic biomass for bioenergy production: Glucose yield and energy efficiency. Energy. 2016;102:335–342.
- Mathew AK, Parameshwaran B, Sukumaran RK, et al. An evaluation of dilute acid and ammonia fiber explosion pretreatment for cellulosic ethanol production. Bioresource Technol. 2016;199:13–20.
- Nitsos CK, Matis KA, Triantafyllidis KS. Optimization of hydrothermal pretreatment of Lignocellulosic biomass in the bioethanol production process. ChemSusChem. 2013;6(1):110–122.
- Chen H, Zhao J, Hu T, et al. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: Substrate digestibility, fermentability and structural features. Appl Energy. 2015;150:224–232.
- Sharma A, Ghosh A, Pandey RA, et al. Wet air oxidation pretreatment of mixed lignocellulosic biomass to enhance enzymatic convertibility. Korean Chem Eng Res. 2015;53(2):216–223.
- Capecchi L, Galbe M, Barbanti L, et al. Combined ethanol and methane production using steam pretreated sugarcane bagasse. Ind Crop Prod. 2015;74:255–262.
- Dale BE, Leong CK, Pham TK, et al. Hydrolysis of lignocellulosics at low enzyme levels: Application of the AFEX process. Bioresource Technol. 1996;56(1):111–116.
- Cara C, Romero I, Oliva JM, et al. Liquid hot water pretreatment of olive tree pruning residues. Appl Biochem Biotechnol. 2007;137–140(1–12):379–394.
- Díaz-Villanueva MJ, Cara-Corpas C, Ruiz-Ramos E, et al. Olive tree pruning as an agricultural residue for ethanol production. Fermentation of hydrolysates from dilute acid pretreatment. Span J Agric Res. 2012;10(3):643–648.
- Negro MJ, Alvarez C, Ballesteros I, et al. Ethanol production from glucose and xylose obtained from steam exploded water-extracted olive tree pruning using phosphoric acid as catalyst. Bioresource Technol. 2014;153:101–107.
- Díaz Manuel J, Huijgen Wouter JJ, van der Laan Ron R, et al. Organosolv pretreatment of olive tree biomass for fermentable sugars. Holzforschung. 2011;65(2):177–183.
- Garrote G, Dominguez H, Parajo JC. Hydrothermal processing of lignocellulosic materials. Holz als Roh- und Werkstoff. 1999;57:191–202.
- Kang Y, Bansal P, Realff MJ, et al. SO2-catalyzed steam explosion: The effects of different severity on digestibility, accessibility, and crystallinity of lignocellulosic biomass. Biotechnol Progress. 2013;29(4):909–916.
- Larsen J, Atergaard Petersen M, Thirup L, et al. The IBUS process - Lignocellulosic bioethanol close to a commercial reality. Chem Eng Technol. 2008;31(5):765–772.
- Thomsen MH, Thygesen A, Thomsen AB. Hydrothermal treatment of wheat straw at pilot plant scale using a three-step reactor system aiming at high hemicellulose recovery, high cellulose digestibility and low lignin hydrolysis. Bioresource Technol. 2008;99(10):4221–4228.
- Tomás-Pejó E, Oliva JM, Ballesteros M, et al. Comparison of SHF and SSF processes from steam-exploded wheat straw for ethanol production by xylose-fermenting and robust glucose-fermenting Saccharomyces cerevisiae strains. Biotechnol Bioeng. 2008;100(6):1122–1131.
- Cannella D, Jørgensen H. Do new cellulolytic enzyme preparations affect the industrial strategies for high solids lignocellulosic ethanol production ? Biotechnol Bioeng. 2014;111(1):59–68.
- Paschos T, Xiros C, Christakopoulos P. Simultaneous saccharification and fermentation by co-cultures of Fusarium oxysporum and Saccharomyces cerevisiae enhances ethanol production from liquefied wheat straw at high solid content. Ind Crop Prod. 2015;76:793–802.
- Sluiter A, Hames B, Rui R , et al. Determination of structural carbohydrates and lignin in biomass. Technical report NREL/TP-510-42618, Laboratory analytical procedure. Golden CO: National Renewable Energy Laboratory, 2012.
- Pielhop T, Amgarten J, von Rohr PR, et al. Steam explosion pretreatment of softwood: the effect of the explosive decompression on enzymatic digestibility. Biotechnol Biofuels. 2016;9(1):152.
- Nitsos CK, Choli-Papadopoulou T, Matis KA, et al. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustainable Chem Eng. 2016; 4(9):4529–4544. ( submited for publication) .
- Torget R, Himmel ME, Grohmann K. Dilute sulfuric acid pretreatment of hardwood bark. Bioresource Technol. 1991;35(3):239–246.
- Rowe JW, Conner AH. Extractives in eastern hardwoods: a review. General technical report FPL 18, Forest products laboratory. U.S. Department of Agriculture, Forest service, Madison, Wisconsin. 1979.
- Moretti MMdS, Bocchini-Martins DA, Nunes CdCC, et al. Pretreatment of sugarcane bagasse with microwaves irradiation and its effects on the structure and on enzymatic hydrolysis. Appl Energy.2014;122:189–195.
- Kang S, Xiao L, Meng L, et al. Isolation and structural characterization of lignin from cotton stalk treated in an ammonia hydrothermal system. Int J Mol Sci. 2012;13(11):15209–15226.
- Spinacé MAS, Lambert CS, Fermoselli KKG, et al. Characterization of lignocellulosic curaua fibres. Carbohyd Polym. 2009;77(1):47–53.
- Ballesteros I, Negro MJ, Oliva JM, et al. Ethanol production from steam-explosion pretreated wheat straw. In: McMillan JD, Adney WS, Mielenz JR, Klasson KT, editors. Twenty-seventh symposium on biotechnology for fuels and chemicals. Totowa (NJ): Humana Press; 2006. p. 496–508.
- Matsakas L, Christakopoulos P. Fermentation of liquefacted hydrothermally pretreated sweet sorghum bagasse to ethanol at high-solids content. Bioresource Technol. 2013;127:202–208.
- Karnaouri A, Paschos T, Taouki I, et al. Cloning, expression and characterization of an ethanol tolerant GH3 β-glucosidase from Myceliophthora thermophila. PeerJ. 2013;1:e46.
- Manzanares P, Negro MJ, Oliva JM, et al. Different process configurations for bioethanol production from pretreated olive pruning biomass. J Chem Technol Biotechnol. 2011;86(6):881–887.
- Börjesson J, Engqvist M, Sipos B, et al. Effect of poly(ethylene glycol) on enzymatic hydrolysis and adsorption of cellulase enzymes to pretreated lignocellulose. Enzyme and Microb Technol. 2007;41(1–2):186–195.
- Excoffier G, Toussaint B, Vignon MR. Saccharification of steam-exploded poplar wood. Biotechnol Bioeng. 1991;38(11):1308–1317.